Abstract
Obesity is one of the major risk factors for cardiovascular diseases and its prevalence is increasing in all age groups, with the biggest impact observed in middle-aged and older adults. A critical mechanism by which obesity promotes vascular pathologies in these patients involves impairment of endothelial function. While endothelial dysfunction in large vessels promotes atherogenesis, obesity-induced microvascular endothelial dysfunction impairs organ perfusion and thereby is causally related to the pathogenesis of ischemic heart disease, chronic kidney disease, intermittent claudication, exercise intolerance, and exacerbates cognitive decline in aging. Reduction of weight via calorie-based diet and exercise in animal models of obesity results in significant improvement of endothelial function both in large vessels and in the microcirculation, primarily due to attenuation of oxidative stress and inflammation. Clinical data on the protective effects of weight loss on endothelial function is limited to studies of flow-mediated dilation assessed in brachial arteries. Currently, there is no guideline on testing the effects of different weight management strategies on microvascular endothelial function in obese patients. Here, we provide proof-of-concept that weight loss-induced improvement of microvascular endothelial function can be reliably assessed in the setting of a geriatric outpatient clinic using a fast, reproducible, non-invasive method: laser speckle contrast imaging-based measurement of endothelium-dependent microvascular responses during post-occlusive reactive hyperemia tests. Our study also provides initial evidence that short-term weight loss induced by consumption of a low-carbohydrate low-calorie diet can reverse microvascular endothelial dysfunction associated with obesity.
Keywords: Weight loss, Obesity, Endothelial function, Aging
Introduction
Obesity is national and global epidemic among older adults (Ogden et al. 2006, 2015). According to recent statistics, over 35% of the US population are considered obese and over 69% are considered either overweight or obese (Flegal et al. 2012). Among these, the rates of obesity are significantly higher in middle age (40–59 years of age, 40.2%) and older adults (> 60 years of age, 37%) than in younger adults (20–39 years of age, 32.3%) (Ogden et al. 2015). Recent data indicate that obesity is associated with high financial burden, and only in the USA the per capita medical costs associated with obesity have increased from $2741 in 2005 to $6899 in 2011 (Tremmel et al. 2017).
The literature is replete with evidence that obesity accelerates the aging processes (Baur et al. 2006; Bernier et al. 2016; Mattison et al. 2014; Minor et al. 2011; Pearson et al. 2008a, b) resulting in decreased life expectancy both in humans and laboratory animals (Abdelaal et al. 2017; Bailey-Downs et al. 2013; Ozanne and Hales 2004; Tucsek et al. 2014a). In many cases, such a dramatic decrease in lifespan is attributed to co-morbidities that accompany obesity in middle age, which are then carried into the advanced age. In addition to its adverse effects on metabolism, the musculoskeletal system, systemic inflammatory processes, sleep apnea, carcinogenesis, and mental illness, obesity is known to exert multifaceted deleterious effects on vascular health. Obesity is a critical risk factor for atherosclerotic cardiovascular and cerebrovascular diseases (Hubert et al. 1983) and also significantly contributes to the development of other risk factors for cardiovascular disease including hypertension, hypercholesterolemia and type 2 diabetes (Din-Dzietham et al. 2007; Hubert et al. 1983). A critical mechanism by which obesity promotes vascular pathologies involves impairment of endothelial function (Steinberg et al. 1996). There is substantial evidence from clinical (Perticone et al. 2001) and pre-clinical (Galili et al. 2007; Pearson et al. 2008a; Tucsek et al. 2014a; Ungvari et al. 2010a, 2011) studies demonstrating that obesity impairs bioavailability of NO by promoting oxidative stress in endothelial cells.
It is increasingly recognized that obesity is also a significant risk factor for microvascular disease, promoting both adverse structural and functional alterations in the microcirculation in a variety of tissues, including heart, brain, kidneys, lungs, adipose tissue, and skeletal muscle (Sorop et al. 2017). These microvascular pathological changes involve inflammatory processes, metabolic alterations, impaired barrier and transport functions. Importantly, obesity-induced global impairment of endothelium-mediated dilation of resistance arterioles impairs organ perfusion and thereby is causally related to the pathogenesis of ischemic heart disease, heart failure, pulmonary hypertension, chronic kidney disease, intermittent claudication and exercise intolerance. Recently, the view has emerged that obesity also promotes cognitive decline (Elias et al. 2003; Elias et al. 2005; Roriz-Cruz et al. 2007; Whitmer et al. 2008), at least in part, due to its adverse effects on the cerebral microcirculation (Alosco et al. 2012; Kim et al. 2012; Letra and Sena 2017; Li et al. 2013; Tucsek et al. 2014a; Tucsek et al. 2014b).
Successful approach to weight management in obese people includes evidence-based lifestyle modification approaches (diet, physical activity, and/or behavior change therapies), pharmacological treatments and bariatric surgery (American College of Cardiology/American Heart Association Task Force on Practice Guidelines 2014; Yumuk et al. 2015). It is predicted that these weight loss strategies may confer microvascular protection in obese patients, contributing to the prevention of a wide range of diseases, from hypertension to vascular cognitive impairment. To test this prediction, it is essential to evaluate the effects of different weight loss approaches on microvascular endothelial function in various patient populations. Despite its clinical importance, there is no guideline on testing the impact of weight loss on microvascular endothelial function in obese patients.
In this case report study, we tested the hypothesis that weight loss-induced improvement of microvascular endothelial function in the setting of a geriatric outpatient clinic can be assessed using a fast, repeatable, and non-invasive method: laser speckle contrast imaging-based measurement of endothelium-dependent microvascular responses during post-occlusive reactive hyperemia tests (Barcelos et al. 2017; Cordovil et al. 2012). As proof-of-concept, we report the assessment of the effects of short-term, voluntary weight loss achieved by consumption of a low-carbohydrate low-calorie diet on microvascular endothelial function in a middle-aged man.
Methods
Study participant
This study has been performed under an approved Institutional Review Board protocol in the Translational Geroscience Laboratory, Reynolds Oklahoma Center on Aging, Department of Geriatric Medicine, at the University of Oklahoma Health Sciences Center. A study participant (45 years of age, male, Caucasian) was enrolled into current study with a BMI of 31.8 (obesity class 1), history of controlled arterial hypertension, and hypercholesterolemia before starting a voluntary weight loss program based on a low-carbohydrate low-calorie diet (1200 cal/day) for 30 days. The study participant was taking lisinopril (10 mg/day p.o.) and rosuvastatin (10 mg/day p.o.), did not smoke and conducted a sedentary lifestyle throughout the study period. A complete blood metabolic panel was performed prior to and after the weight loss program.
Assessment of microvascular endothelial function
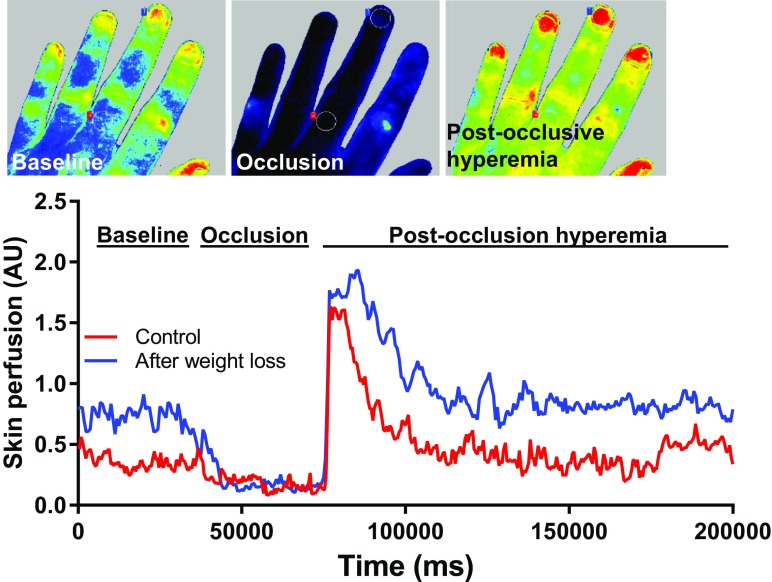
To assess microvascular endothelial function, a post-occlusive reactive hyperemia tests were performed after a 10-min rest with the patient being in a temperature-controlled room (22 ± 1 °C). Blood pressure was measured on the left arm prior the microvascular testing. Reactivity of microvessels was evaluated using a laser speckle contrast imaging system equipped with a 785 nm wavelength laser (Perimed PSI System, Perimed, Jӓrfӓlla, Sweden). The left hand was placed on a black background mat, the distance from the imaging camera was set to 20 cm and the sampling rate was set to 19 images/second. Occlusion was performed via a sphygmomanometer cuff (Welch Allyn, Skaneateles Falls, NY, USA) inflated to 220 mmHg for 3 min on the upper arm, above the antecubital fossa (Fig. 1). Skin temperature was measured from a distance of less than 10 mm with a non-contact, laser-based thermometer (Thermoworks TW2, Thermoworks, American Fork, UT, USA) after removal of the occlusion cuff on the back of hands, and on the last phalanx of the middle finger. Recordings were analyzed offline with the manufacturer’s software (PIMSoft, Pedimed, Jӓrfӓlla, Sweden), and normalized microvascular perfusion units were measured. Two measurement areas were selected on the middle finger avoiding skin pigmentation, visible veins, skin irritation, and wounds. Two regions of interest (10 mm in diameter each) were selected above the nail bed and above the first phalanx of middle finger. A 30-s average of basal perfusion was considered the baseline perfusion. Perfusion during occlusion was also evaluated to assess the minimal perfusion rate. Images were recorded for 3 min after release of occlusion and maximal perfusion, the time-perfusion integral of the reactive hyperemia were evaluated. Reactive hyperemia was calculated based on relative changes of maximal perfusion over the baseline perfusion. We have also calculated the acute reperfusion rate based on the perfusion characteristics during the first 4 s after the arterial cuff deflation in the nail beds. Assessment of microvascular endothelial function was performed on four consecutive days before and after the weight loss program.
Fig. 1.
Assessment of changes in skin perfusion and microvascular endothelial function induced by short-term weight loss using laser speckle contrast imaging. To assess microvascular endothelial function, we have occluded the blood flow to the hand using arterial cuff inflated to 220 mmHg for 3 min and measured changes in skin reactive hyperemia (arbitrary units) during post-occlusion test
Statistical analysis
Data were analyzed by two-tailed t test. A p value less than 0.05 was considered statistically significant. Data are expressed as mean ± S.E.M.
Results
The low-carbohydrate low-calorie diet-based weight loss program resulted in a significant reduction in body mass of 14 kg (from 103 to 89 kg) and improvement of BMI index from 31.8 (obesity class 1) to 27.5 (overweight) over the period of 30 days. This weight loss was accompanied by improved cholesterol, HDL, LDL, and triglycerides plasma levels (Table 1).
Table 1.
Changes in the blood metabolic panel in case study participant before and after weight loss program
| Before weight loss | After weight loss | |
|---|---|---|
| Body mass (kg) | 103 | 89* |
| BMI | 31.8 | 27.5* |
| Systolic blood pressure (mmHg) | 128 | 122 |
| Diastolic blood pressure (mmHg) | 84 | 78 |
| Cholesterol, < 200 mg/dL | 259 | 189* |
| HDL, 40–59 mg/dL | 52 | 61* |
| LDL Calculated, < 100 mg/dL | 169 | 100* |
| Non-HDL cholesterol, < 130 mg/dL | 207 | 128* |
| Triglyceride, < 150 mg/dL | 191 | 141* |
| Albumin, 3.5–5.2 g/dL | 4.8 | 4.9 |
| Alkaline phosphatase, 34–132 U/L | 66 | 58 |
| ALT, 0–41 U/L | 74 | 23 |
| Anion GAP, 0–16 mmol/L | 13 | 16 |
| AST, 0–40 U/L | 30 | 14 |
| Bilirubin total, 0.0–1.2 mg/dL | 0.6 | 0.6 |
| Bun, 6–20 mg/dL | 15 | 18 |
| Calcium, 8.4–10.4 mg/dL | 10.1 | 10.2 |
| Chloride, 98–107 mmol/L | 98 | 102 |
| CO2, 22–29 mmol/L | 29 | 26 |
| Creatinine, 0.60–1.30 mg/dL | 1.06 | 1.02 |
| GFR, African American, ≥ 60 mL/min/1.73 m2 | > 60 | > 60 |
| GFR, ≥ 60 mL/min/1.73 m2 | > 60 | > 60 |
| Glucose, 74–106 mg/dL | 85 | 89 |
| Potassium, 3.5–5.1 mmol/L | 4.3 | 4.1 |
| Total protein, 6.4–8.3 g/dL | 7.4 | 7.1 |
| Sodium, 136–145 mmol/L | 140 | 144 |
| Hematocrit, 39.0–50.0% | 45.4 | 44.8 |
| Hemoglobin, 13.1–17.2 g/dL | 15.7 | 15.3 |
| Lymphocyte absolute, 1.00–4.80 K/uL | 2.10 | 1.94 |
| Lymphocytes, 24–44% | 28 | 28 |
| MCH, 27.0–35.0 pg | 30.5 | 30.8 |
| MCHC, 32.0–36.0 g/dL | 34.6 | 34.2 |
| MCV, 81.0–101.0 fL | 88.2 | 90.1 |
| Monocyte absolute, 0.00–0.80 K/uL | 0.72 | 0.64 |
| Monocytes, 0–10% | 10 | 9 |
| Neutrophil absolute, 1.80–7.70 K/uL | 4.57 | 4.14 |
| Neutrophils, 36–78% | 61 | 60 |
| Platelets, 150–450 K/uL | 297 | 281 |
| RBC, 4.20–5.60 M/uL | 5.15 | 4.97 |
| RDW, 11.0–16.0% | 12.8 | 13.2 |
| RDW-STDEV, 37.0–54.0 fL | 41.4 | 43.6 |
| WBC, 4.5–11.0 K/uL | 7.5 | 7.0 |
HDL, high-density lipoprotein; LDL, low-density lipoprotein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CO2, carbon dioxide; GFR, glomerular filtration rate; MCH, mean corpuscular hemoglobin; MCHC; mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; RBC, red blood cells; RDW, red cell distribution width; STDEV, standard deviation; WBC, white blood cells. *sign indicates values that were improved with the weight loss
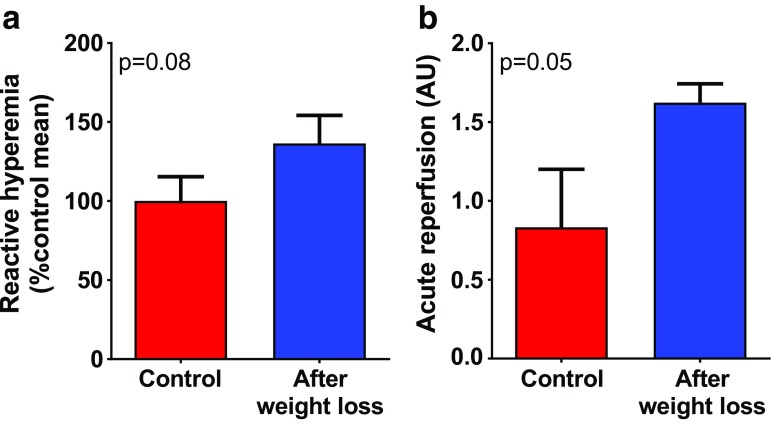
No differences in skin temperature were detected during the endothelial function measurements before and after weight loss (32.7 ± 5.8 °C vs 30.2 ± 2.4 °C, p = 0.33), indicating that temperature differences did not confound the functional measurements of endothelial function. After weight loss, a trend toward improved endothelial function was discernible, as measured by post-occlusive reactive hyperemia using laser speckle contrast imaging in the skin (Fig. 2a). In addition, we have assessed the reperfusion of the microvasculature in the nail beds over the first 4 s after the arterial cuff deflation. Our data showed that weight loss also tended to improve reperfusion rate (Fig. 2b).
Fig. 2.
Demonstration of improved microvascular endothelial function and acute reperfusion induced by short-term weight loss in a middle-aged obese man using laser speckle contrast imaging. a Changes in reactive hyperemia during post-occlusion test before and after weight loss. b Changes in acute reperfusion rate (see “Methods” section). Data presented are mean ± SEM
Discussion
In the present study, we have utilized a Laser Speckle Contrast Imaging (LSCI)-based method to assess microvascular endothelial function. There are several advantages of this approach. LSCI is a non-invasive, non-contact, and fast technique for measuring microvascular blood perfusion. The protocol used is also significantly easier to implement than measurement of brachial arterial flow in FMD studies, particularly in the setting of a geriatric outpatient clinic. Our study lays the foundation for further studies on larger cohorts of geriatric patients on different weight loss programs. Ongoing studies will also compare flow-mediated dilation (FMD) in the brachial artery and the LSCI-based microvascular perfusion data to demonstrate how endothelial functional changes in the macro- and microvasculature correlate.
Abundant preclinical data demonstrate that obesity induces microvascular endothelial dysfunction in animal models (Elmarakby and Imig 2010; Erdei et al. 2006; Henderson et al. 2004; Lynch et al. 2013; Park et al. 2012; Sweazea et al. 2010; Tarantini et al. 2018; Ungvari et al. 2010a; Ungvari et al. 2011) and that these effects are exacerbated in aging (Tucsek et al. 2014b). The available clinical data agrees with the preclinical findings, showing that even moderate obesity is associated with significant endothelial dysfunction in humans (Mohler et al. 2013; Romero-Corral et al. 2010; Williams et al. 2005). This view is also supported by ex vivo data demonstrating impaired acetylcholine-induced endothelium-dependent relaxation in subcutaneous arterioles isolated from obese subjects compared to lean individuals (Grassi et al. 2010). On the basis of these observations and the known role of endothelial dysfunction in the pathogenesis of age-related vascular diseases (Ungvari et al. 2010b), it has been predicted that weight loss in obese individuals should confer important cardiovascular benefits. Our results show that short-term significant weight loss in middle-aged obese man (reduction of BMI from 31.8 to 27.5) improves microvascular endothelial function, extending the findings of previous studies assessing FMD in brachial arteries (Bigornia et al. 2010; Joris et al. 2015; Romero-Corral et al. 2010; Rudofsky et al. 2011; Williams et al. 2005). For example, 16 weeks of combined aerobic/resistance training and diet-induced weight loss was shown to improve endothelial function in overweight and obese women (Cotie et al. 2014). Similar findings were reported by other investigators as well (Bigornia et al. 2010). Importantly, the benefits of weight loss seem to be manifested as early as 1 week after initiation of dietary intervention that resulted in a ~ 4% reduction of BMI (Mavri et al. 2011). Improvement in FMD induced by weight loss in obese subjects have been attributed to a decline in circulating inflammatory mediators/adipokines, blood pressure and insulin (Williams et al. 2005). We predict that the abovementioned factors would improve microvascular endothelial function as well. Interestingly, some clinical studies have reported mixed results on the effects of weight loss on endothelial function in human subjects. For example, no significant improvement in endothelial function was found in a 2-year prospective study in humans after significant weight loss achieved by either a low-carbohydrate or a low-fat diet (Mohler et al. 2013). A recent meta-analysis concluded that the protective effects of weight loss on flow-mediated vasodilation of the brachial artery may depend on subject characteristics, type of weight-loss treatment, and dietary composition. In general, weight loss-mediated endothelial protection tends to be more pronounced when participants have coexisting obesity-related morbidities or when subjects receive low-fat diets or weight-reduction regimens including exercise therapy or weight-loss medication (Joris et al. 2015). Future studies should determine how these factors influence microvascular endothelial function.
Previous studies demonstrate a beneficial effect of weight reduction on central arterial function in obese subjects. For example, low-calorie diet-induced weight reduction in obese middle-aged men, who were similar to the study participant reported here (age, ~ 45 years; BMI, ~ 30 kg/m2), resulted in a significant improvement of central arterial distensibility (carotid arterial compliance significantly increased and b-stiffness index and aortic pulse-wave velocity significantly decreased) (Miyaki et al. 2009). Our data also suggest that weight loss may result in the improvement of vascular stiffness, evidenced by a faster reperfusion of superficial arteries of the hand during the first 4 s after the arterial cuff deflation.
Taken together, our study provides proof-of-concept that weight loss-induced improvement of microvascular endothelial function can be reliably assessed in the setting of a geriatric outpatient clinic using LSCI-based measurement of endothelium-dependent microvascular responses during post-occlusive reactive hyperemia tests. Our study also provides initial evidence that short-term weight loss induced by consumption of a low-carbohydrate low-calorie diet can reverse microvascular endothelial dysfunction associated with obesity.
Funding information
This work was supported by the National Institutes of Health (NIH) Grants R01-AT-006526, R01-AG047879, R01-AG038747, and R01-NS056218, the Geroscience Training Program in Oklahoma (NIH Grant T32-AG-052363), the Oklahoma Nathan Shock Center (NIH Grant 3-P30-AG050911-02S1), the Oklahoma Shared Clinical and Translational Resources (NIH Grant U54-GM-104938), the Oklahoma Center for the Advancement of Science and Technology (HR17-070), the College of Medicine Alumni Association, the Presbyterian Health Foundation, and the EU-funded grant EFOP-3.6.1-16-2016-00008. The paper was published as part of the “Translational Geroscience” initiative of the Journal of the American Aging Association (Ashpole et al. 2017; Bennis et al. 2017; Callisaya et al. 2017; Csiszar et al. 2017; Deepa et al. 2017; Grimmig et al. 2017; Hancock et al. 2017; Kane et al. 2017; Kim et al. 2017; Konopka et al. 2017; Liu et al. 2017; Meschiari et al. 2017; Perrott et al. 2017; Podlutsky et al. 2017; Shobin et al. 2017; Sierra and Kohanski 2017; Tarantini et al. 2017a; Tarantini et al. 2017b; Tenk et al. 2017; Tucsek et al. 2017; Ungvari et al. 2017a, b ; Urfer et al. 2017a, b).
Footnotes
Tamas Csipo and Gabor A. Fulop contributed equally to this work.
References
- Abdelaal M, le Roux CW, Docherty NG. Morbidity and mortality associated with obesity. Ann Transl Med. 2017;5:161. doi: 10.21037/atm.2017.03.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alosco ML, Spitznagel MB, Raz N, Cohen R, Sweet LH, Colbert LH, Josephson R, van Dulmen M, Hughes J, Rosneck J, Gunstad J. Obesity interacts with cerebral hypoperfusion to exacerbate cognitive impairment in older adults with heart failure. Cerebrovasc Dis Extra. 2012;2:88–98. doi: 10.1159/000343222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American College of Cardiology/American Heart Association Task Force on Practice Guidelines OEP (2014) Executive summary: guidelines (2013) for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society published by the Obesity Society and American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Based on a systematic review from the The Obesity Expert Panel, 2013 Obesity (Silver Spring) 22 Suppl 2:S5–39. 10.1002/oby.20821 [DOI] [PubMed]
- Ashpole NM, Logan S, Yabluchanskiy A, Mitschelen MC, Yan H, Farley JA, Hodges EL, Ungvari Z, Csiszar A, Chen S, Georgescu C, Hubbard GB, Ikeno Y, Sonntag WE. IGF-1 has sexually dimorphic, pleiotropic, and time-dependent effects on healthspan, pathology, and lifespan. Geroscience. 2017;39:129–145. doi: 10.1007/s11357-017-9971-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey-Downs LC, Tucsek Z, Toth P, Sosnowska D, Gautam T, Sonntag WE, Csiszar A, Ungvari Z. Aging exacerbates obesity-induced oxidative stress and inflammation in perivascular adipose tissue in mice: a paracrine mechanism contributing to vascular redox dysregulation and inflammation. J Gerontol A Biol Sci Med Sci. 2013;68:780–792. doi: 10.1093/gerona/gls238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcelos A, Lamas C, Tibirica E. Evaluation of microvascular endothelial function in patients with infective endocarditis using laser speckle contrast imaging and skin video-capillaroscopy: research proposal of a case control prospective study. BMC Res Notes. 2017;10:342. doi: 10.1186/s13104-017-2660-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennis MT, Schneider A, Victoria B, Do A, Wiesenborn DS, Spinel L, Gesing A, Kopchick JJ, Siddiqi SA, Masternak MM. The role of transplanted visceral fat from the long-lived growth hormone receptor knockout mice on insulin signaling. Geroscience. 2017;39:51–59. doi: 10.1007/s11357-017-9957-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier M, Wahl D, Ali A, Allard J, Faulkner S, Wnorowski A, Sanghvi M, Moaddel R, Alfaras I, Mattison J, Tarantini S, Tucsek Z, Ungvari Z, Csiszar A, Pearson K, de Cabo R. Resveratrol supplementation confers neuroprotection in cortical brain tissue of nonhuman primates fed a high-fat/sucrose diet. Aging (Albany NY) 2016;8:899–916. doi: 10.18632/aging.100942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigornia SJ, Mott MM, Hess DT, Apovian CM, McDonnell ME, Duess MA, Kluge MA, Fiscale AJ, Vita JA, Gokce N. Long-term successful weight loss improves vascular endothelial function in severely obese individuals. Obesity (Silver Spring) 2010;18:754–759. doi: 10.1038/oby.2009.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callisaya ML, Launay CP, Srikanth VK, Verghese J, Allali G, Beauchet O. Cognitive status, fast walking speed and walking speed reserve-the gait and Alzheimer interactions tracking (GAIT) study. Geroscience. 2017;39:231–239. doi: 10.1007/s11357-017-9973-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordovil I, Huguenin G, Rosa G, Bello A, Kohler O, de Moraes R, Tibirica E. Evaluation of systemic microvascular endothelial function using laser speckle contrast imaging. Microvasc Res. 2012;83:376–379. doi: 10.1016/j.mvr.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Cotie LM, Josse AR, Phillips SM, MacDonald MJ. Endothelial function increases after a 16-week diet and exercise intervention in overweight and obese young women. Biomed Res Int. 2014;2014:327395. doi: 10.1155/2014/327395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Tarantini S, Fülöp GA, Kiss T, Valcarcel-Ares MN, Galvan V, Ungvari Z, Yabluchanskiy A. Hypertension impairs neurovascular coupling and promotes microvascular injury: role in exacerbation of Alzheimer’s disease. Geroscience. 2017;39:359–372. doi: 10.1007/s11357-017-9991-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deepa SS, Bhaskaran S, Espinoza S, Brooks SV, McArdle A, Jackson MJ, van Remmen H, Richardson A. A new mouse model of frailty: the Cu/Zn superoxide dismutase knockout mouse. Geroscience. 2017;39:187–198. doi: 10.1007/s11357-017-9975-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Din-Dzietham R, Liu Y, Bielo MV, Shamsa F. High blood pressure trends in children and adolescents in national surveys, 1963 to 2002. Circulation. 2007;116:1488–1496. doi: 10.1161/CIRCULATIONAHA.106.683243. [DOI] [PubMed] [Google Scholar]
- Elias MF, Elias PK, Sullivan LM, Wolf PA, D’Agostino RB. Lower cognitive function in the presence of obesity and hypertension: the Framingham heart study. Int J Obes Relat Metab Disord. 2003;27:260–268. doi: 10.1038/sj.ijo.802225. [DOI] [PubMed] [Google Scholar]
- Elias MF, Elias PK, Sullivan LM, Wolf PA, D’Agostino RB. Obesity, diabetes and cognitive deficit: the Framingham heart study. Neurobiol Aging. 2005;26(Suppl 1):11–16. doi: 10.1016/j.neurobiolaging.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Elmarakby AA, Imig JD. Obesity is the major contributor to vascular dysfunction and inflammation in high-fat diet hypertensive rats. Clin Sci (Lond) 2010;118:291–301. doi: 10.1042/CS20090395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdei N, Toth A, Pasztor ET, Papp Z, Edes I, Koller A, Bagi Z. High-fat diet-induced reduction in nitric oxide-dependent arteriolar dilation in rats: role of xanthine oxidase-derived superoxide anion. Am J Physiol Heart Circ Physiol. 2006;291:H2107–H2115. doi: 10.1152/ajpheart.00389.2006. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- Galili O, Versari D, Sattler KJ, Olson ML, Mannheim D, McConnell JP, Chade AR, Lerman LO, Lerman A. Early experimental obesity is associated with coronary endothelial dysfunction and oxidative stress. Am J Physiol Heart Circ Physiol. 2007;292:H904–H911. doi: 10.1152/ajpheart.00628.2006. [DOI] [PubMed] [Google Scholar]
- Grassi G, Seravalle G, Scopelliti F, Dell'Oro R, Fattori L, Quarti-Trevano F, Brambilla G, Schiffrin EL, Mancia G. Structural and functional alterations of subcutaneous small resistance arteries in severe human obesity. Obesity (Silver Spring) 2010;18:92–98. doi: 10.1038/oby.2009.195. [DOI] [PubMed] [Google Scholar]
- Grimmig B, Kim SH, Nash K, Bickford PC, Douglas Shytle R. Neuroprotective mechanisms of astaxanthin: a potential therapeutic role in preserving cognitive function in age and neurodegeneration. Geroscience. 2017;39:19–32. doi: 10.1007/s11357-017-9958-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock SE, Friedrich MG, Mitchell TW, Truscott RJ, Else PL. The phospholipid composition of the human entorhinal cortex remains relatively stable over 80 years of adult aging. Geroscience. 2017;39:73–82. doi: 10.1007/s11357-017-9961-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson KK, Turk JR, Rush JW, Laughlin MH. Endothelial function in coronary arterioles from pigs with early-stage coronary disease induced by high-fat, high-cholesterol diet: effect of exercise. J Appl Physiol (1985) 2004;97:1159–1168. doi: 10.1152/japplphysiol.00261.2004. [DOI] [PubMed] [Google Scholar]
- Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham heart study. Circulation. 1983;67:968–977. doi: 10.1161/01.CIR.67.5.968. [DOI] [PubMed] [Google Scholar]
- Joris PJ, Zeegers MP, Mensink RP. Weight loss improves fasting flow-mediated vasodilation in adults: a meta-analysis of intervention studies. Atherosclerosis. 2015;239:21–30. doi: 10.1016/j.atherosclerosis.2014.12.056. [DOI] [PubMed] [Google Scholar]
- Kane AE, Gregson E, Theou O, Rockwood K, Howlett SE. The association between frailty, the metabolic syndrome, and mortality over the lifespan. Geroscience. 2017;39:221–229. doi: 10.1007/s11357-017-9967-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CK, Kwon HM, Lee SH, Kim BJ, Ryu WS, Kwon HT, Yoon BW. Association of obesity with cerebral microbleeds in neurologically asymptomatic elderly subjects. J Neurol. 2012;259:2599–2604. doi: 10.1007/s00415-012-6546-y. [DOI] [PubMed] [Google Scholar]
- Kim S, Myers L, Wyckoff J, Cherry KE, Jazwinski SM. The frailty index outperforms DNA methylation age and its derivatives as an indicator of biological age. Geroscience. 2017;39:83–92. doi: 10.1007/s11357-017-9960-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka AR, Laurin JL, Musci RV, Wolff CA, Reid JJ, Biela LM, Zhang Q, Peelor FF, Melby CL, Hamilton KL, Miller BF. Influence of Nrf2 activators on subcellular skeletal muscle protein and DNA synthesis rates after 6 weeks of milk protein feeding in older adults. Geroscience. 2017;39:175–186. doi: 10.1007/s11357-017-9968-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letra L, Sena C. Cerebrovascular disease: consequences of obesity-induced endothelial dysfunction. Adv Neurobiol. 2017;19:163–189. doi: 10.1007/978-3-319-63260-5_7. [DOI] [PubMed] [Google Scholar]
- Li W, Prakash R, Chawla D, du W, Didion SP, Filosa JA, Zhang Q, Brann DW, Lima VV, Tostes RC, Ergul A. Early effects of high-fat diet on neurovascular function and focal ischemic brain injury. Am J Physiol Regul Integr Comp Physiol. 2013;304:R1001–R1008. doi: 10.1152/ajpregu.00523.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Bhatt T, Wang S, Yang F, Pai YC. Retention of the "first-trial effect" in gait-slip among community-living older adults. Geroscience. 2017;39:93–102. doi: 10.1007/s11357-017-9963-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch CM, Kinzenbaw DA, Chen X, Zhan S, Mezzetti E, Filosa J, Ergul A, Faulkner JL, Faraci FM, Didion SP. Nox2-derived superoxide contributes to cerebral vascular dysfunction in diet-induced obesity. Stroke. 2013;44:3195–3201. doi: 10.1161/STROKEAHA.113.001366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison JA, Wang M, Bernier M, Zhang J, Park SS, Maudsley S, An SS, Santhanam L, Martin B, Faulkner S, Morrell C, Baur JA, Peshkin L, Sosnowska D, Csiszar A, Herbert RL, Tilmont EM, Ungvari Z, Pearson KJ, Lakatta EG, de Cabo R. Resveratrol prevents high fat/sucrose diet-induced central arterial wall inflammation and stiffening in nonhuman primates. Cell Metab. 2014;20:183–190. doi: 10.1016/j.cmet.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavri A, Poredos P, Suran D, Gaborit B, Juhan-Vague I, Poredos P. Effect of diet-induced weight loss on endothelial dysfunction: early improvement after the first week of dieting. Heart Vessel. 2011;26:31–38. doi: 10.1007/s00380-010-0016-1. [DOI] [PubMed] [Google Scholar]
- Meschiari CA, Ero OK, Pan H, Finkel T, Lindsey ML. The impact of aging on cardiac extracellular matrix. Geroscience. 2017;39:7–18. doi: 10.1007/s11357-017-9959-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor RK, Baur JA, Gomes AP, Ward TM, Csiszar A, Mercken EM, Abdelmohsen K, Shin YK, Canto C, Scheibye-Knudsen M, Krawczyk M, Irusta PM, Martín-Montalvo A, Hubbard BP, Zhang Y, Lehrmann E, White AA, Price NL, Swindell WR, Pearson KJ, Becker KG, Bohr VA, Gorospe M, Egan JM, Talan MI, Auwerx J, Westphal CH, Ellis JL, Ungvari Z, Vlasuk GP, Elliott PJ, Sinclair DA, de Cabo R (2011) SRT1720 improves survival and healthspan of obese mice. Sci Rep 1. 10.1038/srep00070http://www.nature.com/srep/2011/110818/srep00070/abs/srep00070.html#supplementary-information [DOI] [PMC free article] [PubMed]
- Miyaki A, Maeda S, Yoshizawa M, Misono M, Saito Y, Sasai H, Endo T, Nakata Y, Tanaka K, Ajisaka R. Effect of weight reduction with dietary intervention on arterial distensibility and endothelial function in obese men. Angiology. 2009;60:351–357. doi: 10.1177/0003319708325449. [DOI] [PubMed] [Google Scholar]
- Mohler ER, 3rd, et al. Endothelial function and weight loss: comparison of low-carbohydrate and low-fat diets. Obesity (Silver Spring) 2013;21:504–509. doi: 10.1002/oby.20055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Fryar CD, Flegal KM (2015) Prevalence of obesity among adults and youth: United States, 2011–2014. NCHS Data Brief:1–8 [PubMed]
- Ozanne SE, Hales CN. Lifespan: catch-up growth and obesity in male mice. Nature. 2004;427:411–412. doi: 10.1038/427411b. [DOI] [PubMed] [Google Scholar]
- Park Y, Booth FW, Lee S, Laye MJ, Zhang C. Physical activity opposes coronary vascular dysfunction induced during high fat feeding in mice. J Physiol. 2012;590:4255–4268. doi: 10.1113/jphysiol.2012.234856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson KJ, Lewis KN, Price NL, Chang JW, Perez E, Cascajo MV, Tamashiro KL, Poosala S, Csiszar A, Ungvari Z, Kensler TW, Yamamoto M, Egan JM, Longo DL, Ingram DK, Navas P, de Cabo R. Nrf2 mediates cancer protection but not prolongevity induced by caloric restriction. Proc Natl Acad Sci U S A. 2008;105:2325–2330. doi: 10.1073/pnas.0712162105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrott KM, Wiley CD, Desprez PY, Campisi J. Apigenin suppresses the senescence-associated secretory phenotype and paracrine effects on breast cancer cells. Geroscience. 2017;39:161–173. doi: 10.1007/s11357-017-9970-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perticone F, Ceravolo R, Candigliota M, Ventura G, Iacopino S, Sinopoli F, Mattioli PL. Obesity and body fat distribution induce endothelial dysfunction by oxidative stress: protective effect of vitamin C. Diabetes. 2001;50:159–165. doi: 10.2337/diabetes.50.1.159. [DOI] [PubMed] [Google Scholar]
- Podlutsky A, Valcarcel-Ares MN, Yancey K, Podlutskaya V, Nagykaldi E, Gautam T, Miller RA, Sonntag WE, Csiszar A, Ungvari Z. The GH/IGF-1 axis in a critical period early in life determines cellular DNA repair capacity by altering transcriptional regulation of DNA repair-related genes: implications for the developmental origins of cancer. Geroscience. 2017;39:147–160. doi: 10.1007/s11357-017-9966-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Corral A, Sert-Kuniyoshi FH, Sierra-Johnson J, Orban M, Gami A, Davison D, Singh P, Pusalavidyasagar S, Huyber C, Votruba S, Lopez-Jimenez F, Jensen MD, Somers VK. Modest visceral fat gain causes endothelial dysfunction in healthy humans. J Am Coll Cardiol. 2010;56:662–666. doi: 10.1016/j.jacc.2010.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roriz-Cruz M, Rosset I, Wada T, Sakagami T, Ishine M, de Sá Roriz-Filho J, Cruz TRS, Hosseinkhani M, Rodrigues RP, Sudoh S, Arai H, Wakatsuki Y, Souza AC, Nakagawa M, Kita T, Matsubayashi K. Cognitive impairment and frontal-subcortical geriatric syndrome are associated with metabolic syndrome in a stroke-free population. Neurobiol Aging. 2007;28:1723–1736. doi: 10.1016/j.neurobiolaging.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Rudofsky G, Roeder E, Merle T, Hildebrand M, Nawroth PP, Wolfrum C. Weight loss improves endothelial function independently of ADMA reduction in severe obesity. Horm Metab Res. 2011;43:343–348. doi: 10.1055/s-0031-1271778. [DOI] [PubMed] [Google Scholar]
- Shobin E, Bowley MP, Estrada LI, Heyworth NC, Orczykowski ME, Eldridge SA, Calderazzo SM, Mortazavi F, Moore TL, Rosene DL. Microglia activation and phagocytosis: relationship with aging and cognitive impairment in the rhesus monkey. Geroscience. 2017;39:199–220. doi: 10.1007/s11357-017-9965-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra F, Kohanski R. Geroscience and the trans-NIH geroscience interest group. GSIG Gerosci. 2017;39:1–5. doi: 10.1007/s11357-016-9954-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorop O, Olver TD, van de Wouw J, Heinonen I, van Duin RW, Duncker DJ, Merkus D. The microcirculation: a key player in obesity-associated cardiovascular disease. Cardiovasc Res. 2017;113:1035–1045. doi: 10.1093/cvr/cvx093. [DOI] [PubMed] [Google Scholar]
- Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest. 1996;97:2601–2610. doi: 10.1172/JCI118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweazea KL, Lekic M, Walker BR. Comparison of mechanisms involved in impaired vascular reactivity between high sucrose and high fat diets in rats. Nutr Metab (Lond) 2010;7:48. doi: 10.1186/1743-7075-7-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Fulop GA, Kiss T, Farkas E, Zölei-Szénási D, Galvan V, Toth P, Csiszar A, Ungvari Z, Yabluchanskiy A. Demonstration of impaired neurovascular coupling responses in TG2576 mouse model of Alzheimer's disease using functional laser speckle contrast imaging. Geroscience. 2017;39:465–473. doi: 10.1007/s11357-017-9980-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Yabluchanksiy A, Fülöp GA, Hertelendy P, Valcarcel-Ares MN, Kiss T, Bagwell JM, O’Connor D, Farkas E, Sorond F, Csiszar A, Ungvari Z. Pharmacologically induced impairment of neurovascular coupling responses alters gait coordination in mice. Geroscience. 2017;39:601–614. doi: 10.1007/s11357-017-0003-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S et al. (2018) Nrf2 deficiency exacerbates obesity-induced oxidative stress, neurovascular dysfunction, blood brain barrier disruption, neuroinflammation, amyloidogenic gene expression and cognitive decline in mice, mimicking the aging phenotype J Gerontol A Biol Sci Med Sci:in press [DOI] [PMC free article] [PubMed]
- Tenk J, Rostás I, Füredi N, Mikó A, Solymár M, Soós S, Gaszner B, Feller D, Székely M, Pétervári E, Balaskó M. Age-related changes in central effects of corticotropin-releasing factor (CRF) suggest a role for this mediator in aging anorexia and cachexia. Geroscience. 2017;39:61–72. doi: 10.1007/s11357-017-9962-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremmel M, Gerdtham UG, Nilsson PM, Saha S (2017) Economic burden of obesity: a systematic literature review. Int J Environ Res Public Health 14. 10.3390/ijerph14040435 [DOI] [PMC free article] [PubMed]
- Tucsek Z, Toth P, Sosnowska D, Gautam T, Mitschelen M, Koller A, Szalai G, Sonntag WE, Ungvari Z, Csiszar A. Obesity in aging exacerbates blood-brain barrier disruption, neuroinflammation, and oxidative stress in the mouse hippocampus: effects on expression of genes involved in beta-amyloid generation and Alzheimer’s disease. J Gerontol A Biol Sci Med Sci. 2014;69:1212–1226. doi: 10.1093/gerona/glt177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucsek Z, Toth P, Tarantini S, Sosnowska D, Gautam T, Warrington JP, Giles CB, Wren JD, Koller A, Ballabh P, Sonntag WE, Ungvari Z, Csiszar A. Aging exacerbates obesity-induced cerebromicrovascular rarefaction, neurovascular uncoupling, and cognitive decline in mice. J Gerontol A Biol Sci Med Sci. 2014;69:1339–1352. doi: 10.1093/gerona/glu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucsek Z, Noa Valcarcel-Ares M, Tarantini S, Yabluchanskiy A, Fülöp G, Gautam T, Orock A, Csiszar A, Deak F, Ungvari Z. Hypertension-induced synapse loss and impairment in synaptic plasticity in the mouse hippocampus mimics the aging phenotype: implications for the pathogenesis of vascular cognitive impairment. Geroscience. 2017;39:385–406. doi: 10.1007/s11357-017-9981-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Bagi Z, Feher A, Recchia FA, Sonntag WE, Pearson K, de Cabo R, Csiszar A. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol. 2010;299:H18–H24. doi: 10.1152/ajpheart.00260.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Kaley G, de Cabo R, Sonntag WE, Csiszar A. Mechanisms of vascular aging: new perspectives. J Gerontol A Biol Sci Med Sci. 2010;65:1028–1041. doi: 10.1093/gerona/glq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari ZI, Bailey-Downs L, Gautam T, Jimenez R, Losonczy G, Zhang C, Ballabh P, Recchia FA, Wilkerson DC, Sonntag WE, Pearson K, de Cabo R, Csiszar A. Adaptive induction of NF-E2-related Factor-2-driven antioxidant genes in endothelial cells in response to hyperglycemia. Am J Physiol Heart Circ Physiol. 2011;300:H1133–H1140. doi: 10.1152/ajpheart.00402.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Tarantini S, Hertelendy P, Valcarcel-Ares MN, Fülöp GA, Logan S, Kiss T, Farkas E, Csiszar A, Yabluchanskiy A. Cerebromicrovascular dysfunction predicts cognitive decline and gait abnormalities in a mouse model of whole brain irradiation-induced accelerated brain senescence. Geroscience. 2017;39:33–42. doi: 10.1007/s11357-017-9964-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Valcarcel-Ares MN, Tarantini S, Yabluchanskiy A, Fulop GA, Kiss T, Csiszar A. Connective tissue growth factor (CTGF) in age-related vascular pathologies. Geroscience. 2017;39:491–498. doi: 10.1007/s11357-017-9995-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urfer SR, Kaeberlein TL, Mailheau S, Bergman PJ, Creevy KE, Promislow DE, Kaeberlein M. Asymptomatic heart valve dysfunction in healthy middle-aged companion dogs and its implications for cardiac aging. Geroscience. 2017;39:43–50. doi: 10.1007/s11357-016-9956-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urfer SR, Kaeberlein TL, Mailheau S, Bergman PJ, Creevy KE, Promislow DE, Kaeberlein M. A randomized controlled trial to establish effects of short-term rapamycin treatment in 24 middle-aged companion dogs. Geroscience. 2017;39:117–127. doi: 10.1007/s11357-017-9972-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K. Central obesity and increased risk of dementia more than three decades later. Neurology. 2008;71:1057–1064. doi: 10.1212/01.wnl.0000306313.89165.ef. [DOI] [PubMed] [Google Scholar]
- Williams IL, Chowienczyk PJ, Wheatcroft SB, Patel AG, Sherwood RA, Momin A, Shah AM, Kearney MT. Endothelial function and weight loss in obese humans. Obes Surg. 2005;15:1055–1060. doi: 10.1381/0960892054621134. [DOI] [PubMed] [Google Scholar]
- Yumuk V, Tsigos C, Fried M, Schindler K, Busetto L, Micic D, Toplak H, Obesity Management Task Force of the European Association for the Study of Obesity European guidelines for obesity management in adults. Obes Facts. 2015;8:402–424. doi: 10.1159/000442721. [DOI] [PMC free article] [PubMed] [Google Scholar]