Abstract
Working memory abilities significantly decrease with advancing age; hence, the search for factors that may increase or mitigate this decline is critical. Several factors have been identified that influence working memory; however, their effects have been mainly assessed separately and rarely together with other factors in the same sample. We examined 120 variables to search for factors that jointly act as mediators of working memory decay across the adult life span. A sample of 1652 healthy adults was assessed in spatial and verbal working memory domains. Structural equation modeling analyses were conducted to search for potential mediators that intervened between age and working memory. Only 14 and 10 variables reliably mediated spatial and verbal working memory, respectively. Factors from several domains remained in the models, such as individual characteristics, physiological traits, consumption habits, and regular activities. These factors are sufficiently powerful to influence working memory decline when they jointly interact, as in everyday living.
Electronic supplementary material
The online version of this article (10.1007/s11357-018-0031-1) contains supplementary material, which is available to authorized users.
Keywords: Spatial working memory, Verbal working memory, Mediators, Adult life span, Structural equation modeling
In everyday living, we are challenged to use our working memory abilities to solve numerous situations that require manipulating and transforming information in our mind. Working memory is conceived as the memory we use for the execution of our plans (Miller et al. 1960), and for that reason, it is crucial for our cognitive functionality. However, working memory abilities are highly vulnerable to the effects of age. The information processed in working memory may pertain to the spatial or verbal domain. Working memory in each domain can be equivalently examined by means of the n-back task (Kirchner 1958). This procedure consists of judging whether the current item is equal or not equal to the one presented n trials previously. The two-back level of difficulty is suitable for assessing working memory because requires using the processes that have been outlined to describe this memory system: storage, binding each item to its temporal order, retrieval, updating the item and its temporal order, monitoring, and interference control from items that do not correspond to the lag under evaluation. Using this procedure, it has been observed that working memory in both domains, spatial and verbal, diminishes gradually with advancing age across the adult life span (Cansino et al. 2013).
The search for factors that lessen or increase cognitive decay has been investigated over the last three decades. One of the first pieces of evidence emerged from experiments on older rats that were housed in enriched environments filled with toys and showed increased dendritic branching compared to rats housed in empty enclosures (Black et al. 1987). However, young rats benefited more than old rats from these environments, suggesting that the old brain has fewer resources to sustain neuroplasticity changes, such as reduced metabolic energy. This finding led to the search for factors that may improve metabolic processes, such as glucose, oxygen, and nutrients. For example, some of the nutrients that have been associated with cognitive function are fatty acids. The consumption of polyunsaturated fatty acids is associated with higher memory performance, whereas the intake of saturated fatty acids is related to lower cognitive function and even to neurological diseases (e.g., Kaplan and Greenwood 1998; Beilharz et al. 2015). Therefore, assessing the effects that each type of fatty acid may exert on working memory is essential; this approach has been adopted in the present study.
Several factors have been found to be related to or have an effect on working memory. However, their effects are rarely analyzed on working memory exclusively but instead combined with scores of other memory types or other cognitive functions (e.g., Santos et al. 2014). Additionally, the influence of these variables has mostly been analyzed separately (e.g., Zahodne et al. 2011) and rarely together with other factors in the same sample, and most of these studies have been conducted in older adults (e.g., Hansson and Hagber 2005). However, in everyday life, these factors occur simultaneously, and it is unknown whether they would still have an effect on working memory when they act together. Therefore, the purpose of this study was to examine which of these factors continues to influence working memory even in the presence of other factors.
To achieve this goal, we assessed 120 variables in a healthy adult life span sample comprised of 1652 individuals between 21 and 80 years old; age and sex were equally distributed across the age range (see Supplementary Material Table 1). The present study is the first to include almost all variables that have been empirically documented to be positive or negative predictors of working memory, memory, or cognition in general. Moreover, we examined these factors in an adult life span sample to be able to identify predictors of working memory performance that are relevant across adulthood and not only for an exclusive age group. Furthermore, we assessed the effects of these variables exclusively in working memory and separately in the spatial and verbal domains. We used structural equation modeling (SEM) to identify the most plausible models that included only reliable mediators of the effect of age on spatial and verbal working memory; all mediators were observable variables, which allow the direct comparison with everyday measurements of these variables and with other studies. The factors measured (Supplementary Material Table 2) included demographic variables; diseases with which participants had been diagnosed; biological, physical, and physiological measures; nutrient consumption; medication intake; tobacco, drug, and alcohol consumptions; lifestyle variables such as physical, mental, social, and cultural activities; experience of stressful events; metamemory (participants’ affects and beliefs about their own memory); depression; and general intellectual and cognitive state.
Methods
Participants
A sample of 1652 healthy adults between 21 and 80 years of age participated in the study. From each decade included in the age range, approximately the same number of men and women participated (Supplementary Material Table 1). We recruited 1657 participants; however, 5 individuals were excluded because their data from the working memory task were lost due to technical problems. Participants were recruited through appeals to community groups, advertisements, flyers, and word of mouth. The inclusion criteria were a minimum of 8 years of education, normal, or corrected-to-normal vision, a score ≤ 20 on the Beck Depression Inventory (BDI) (Beck 1987), a score ≥ 24 on the Mini-Mental State Exam (MMSE) (Folstein et al. 1975), and a score ≥ 26 on the vocabulary subtest of the Wechsler Adult Intelligence Scale-Revised (WAIS-R) (Wechsler 1981). These performance scores were required to ensure that participants were not suffering from depression, dementia, or intellectual difficulties. Supplementary Material Table 1 displays the participants’ scores on these tests by decade. The exclusion criteria were addiction to drugs or alcohol, consumption of medication that acts on the nervous system in the previous 6 months, neurological or psychiatric diseases, or head trauma.
All participants provided informed consent and received a monetary reward for his/her participation. The study was approved by the Bioethics Committee of the School of Medicine at the National Autonomous University of Mexico. All experiments were performed in accordance with the Declaration of Helsinki. Because the goodness-of-fit statistic has a chi-square distribution when the model adequately fits the assumption of multivariate normality of the data, it is possible to obtain the power of the test of good fit given the sample size and degrees of freedom of the model, as has been proposed by MacCallum et al. (1996). We estimated the statistical power of the models we fit by testing the covariance structure model using RMSEA. We performed these estimations using the Preacher and Coffman (2006) web utility. All models were found to have a power of 1 when the alpha level was set to 0.05, the null RMSEA to 0.05, and the alternative RMSEA to 0.08.
Measures
The different measurement methods used in the current study are described in detail in the Supplementary Material. The metamemory dimensions were examined with the Metamemory in Adulthood scale (Dixon et al. 1988). Food consumption was assessed through the Food Frequency Questionnaire (FFQ) (Hernández-Ávila et al. 1998), an instrument developed according to the method proposed by Willett et al. (1985) through several stages to confirm its reproducibility and validity (Hernández et al. 1983; Hernández-Ávila et al. 1998; Romieu et al. 1999). Further information about how the Food Frequency Questionnaire was developed is provided in the Supplementary Material. Nutrient intake was estimated using the Evaluation System of Nutritional Habits and Nutrient Consumption (SNUT) software (Hernández-Ávila et al. 2000). The nutrient content for each food item is based on the US Department of Agriculture (1963–1997, USDA) and complemented by a database from the National Institute of Nutrition (Chávez et al. 1996). The experience of positive and negative stressful life events was evaluated by means of the Social Readjustment Rating Scale (SRRS) (Holmes and Rahe 1967). To examine education; occupation; income; health status; medication intake; tobacco, drug, and alcohol consumptions; and cultural, social, mental, and physical activities, a Lifestyle Questionnaire was created specifically for the current study. Scores obtained on the screening tests the Beck Depression Inventory (BDI) (Beck 1987), the Mini-Mental State Exam (MMSE) (Folstein et al. 1975), and the vocabulary subtest of the Wechsler Adult Intelligence Scale-Revised (WAIS-R) (Wechsler 1981) were also included in the structural equation modeling analyses.
Stimuli
We used 12 uppercase letters (B, F, G, K, L, N, P, Q, R, S, T, and X) for the verbal version of the n-back task. The letters subtended vertical and horizontal visual angles of 1.5° and 1°, respectively. The letters were presented in a dark gray color at the center of a white screen to maintain a low contrast. For the spatial n-back task, we used a dark gray circle with a diameter of a visual angle of 1.5°. The screen was white in color, and a black cross (vertical and horizontal visual angles of 0.5°) was continuously displayed at the center of the screen. The circle was displayed in one of 12 possible positions around the center of the screen. The distance between the circle and the center of the screen was 4°. The letters for the verbal task and the positions for the spatial task were selected randomly and with the same probability.
Procedure
The study lasted 6.5 years. Approximately, the same number of participants from each decade in the age range was evaluated each year. Participants attended two sessions that were approximately 2 h each. Graduate psychology collaborators conducted the experimental sessions and applied the instruments after several months of training. The training consisted of learning how to interview, apply psychological tests, evaluate visual acuity, and administer memory tasks. To ensure that the collaborators had acquired the necessary skills before being allowed to formally carry out the study, they were observed several times and evaluated through a Gesell chamber while conducting interviews and applying psychological tests. The collaborators were also supervised and evaluated while they administered the memory tasks to guarantee that the procedure was applied consistently to all of the participants. Potential participants were assessed through prescreening questions to determine if they fulfilled the inclusion and exclusion criteria prior to being invited to attend the first session. The first session occurred in a silent room in which only the participant and the experimenter were present. At the beginning of this session, participants were further interviewed to confirm that they satisfied the inclusion criteria. Afterwards, participants were tested with the WAIS-R vocabulary subtest, the MMSE, and the BDI, and their vision was tested. Participants that were eligible for the study were asked to provide their informed consent. Afterwards, participants completed the Lifestyle Questionnaire, followed by the Food Frequency Questionnaire and the SRRS, which were completed in a counterbalanced order. Then, the Annett Hand Preference Questionnaire (Annett 1970) was administered. At the end of the session, participants had the Metamemory in Adulthood questionnaire explained, which was then handed to them to be answered at home. Participants were also asked to record all foods they ate for 3 days in special formats (data not analyzed here). Finally, participants’ weight and height were measured.
In the second session, participants’ glucose, cholesterol, and triglycerides were measured in a non-fasting state with the Accutrend Plus System, Roche Diagnostics, Rotkreuz, Switzerland. These measurements were taken in a counterbalanced order. In this session, participants performed the working memory tasks and a source memory task (data not shown) in a sound-dampened chamber. The participants were seated in a high-back armchair 100 cm away from the monitor screen. Blood pressure and heart rate were measured with a digital upper arm sphygmomanometer Hem-712C, Omron, Kyoto, Japan. Mean arterial pressure (MAP) was estimated as [(2 × diastolic blood pressure) + systolic blood pressure]/3. Skin conductance responses were recorded by placing electrodes on the ring and middle fingers of the non-dominant hand (data not presented here). Participants responded by utilizing two keys from a response panel located on a platform on the left or right armchair according to the participant’s handedness. The verbal and spatial tasks were performed by the participants in a counterbalanced order, and within each domain, participants performed the task at two levels of difficulty (one-back and two-back), also in a counterbalanced order. Data from the low difficulty tasks (one-back) are not shown. Prior to performing each of the n-back tasks, participants carried out brief versions of each task as training. The stimulus presentations and response recordings were controlled by E-Prime Software v1.0, Psychological Software Tools, Pittsburgh, PA, USA.
Working memory paradigm
For both the verbal and spatial n-back tasks, each trial started with the presentation of the stimulus (letter or circle) for 300 ms, followed by a period of 2700 ms. After this time, the next stimulus was displayed. Participants were allowed to provide their response during the 3000-ms period following the onset of the stimulus. In the verbal task, participants were requested to indicate whether the current letter was equal or not equal to the one displayed two trials prior. In the spatial version, participants were required to indicate whether the current circle was presented in the same position as the one displayed two trials before. Participants performed 72 trials from each version of the task, of which 33% of the trials were target (letters or positions equal to the one of the current trial).
Data analysis
Based on previous empirical findings that have demonstrated which variables have an effect on working memory, memory in general, or cognition, two hypothetical models were formulated a priori. The models comprised only one exogenous variable, participant’s age, and only one outcome variable, discrimination in the spatial working memory task for one model and discrimination in the verbal working memory task for the other model. Discrimination levels were determined by using d-prime (d′) values, which are not affected by the participants’ criterion for completing the tasks. The rest of the variables were considered endogenous mediator variables that intervened between age and working memory in a causal sequence. We estimated the magnitude of the direct effect of age on spatial and verbal working memory not mediated by other variables in each model separately. In addition, we calculated the indirect effect of age mediated by the other variables in each model and the indirect effect of each mediator variable by multiplying the coefficients for the two linked direct effects. Only observable variables were included in the models because they could be directly interpreted and compared with predictors from other studies.
A set of 120 variables were initially considered as potential mediator variables, none of which had missing values. All variables were examined with descriptive analyses, and those with skewness exceeding ± 3 were natural log-transformed. Afterwards, linear regression analyses between all variables and discrimination in the spatial and verbal working memory domains were conducted. Those variables with a significant (P < .05) effect on spatial or verbal working memory were selected to be included in the first models for each domain. After the paths were fit with SEM analyses, the models were fine-tuned by eliminating endogenous variables, whose paths had t values that were no longer significant (P > .05). The SEM analyses were repeated with only the endogenous variables that remained significant in each model. Goodness-of-fit was assessed with the χ2 likelihood ratio with degrees of freedom, relative or normed χ2 (χ2/degrees of freedom), the comparative fit index (CFI), the root mean square error of approximation (RMSEA), and the standardized root mean squared residual (SRMS). Finally, we performed a bootstrapped test of mediation (Preacher and Hayes 2008) with 20,000 replications to estimate the 95% bias-corrected confidence intervals for each mediator variable and the total indirect effect. SEM analyses were conducted with Stata v.13, Texas, USA, using the maximum likelihood estimation procedure.
Results
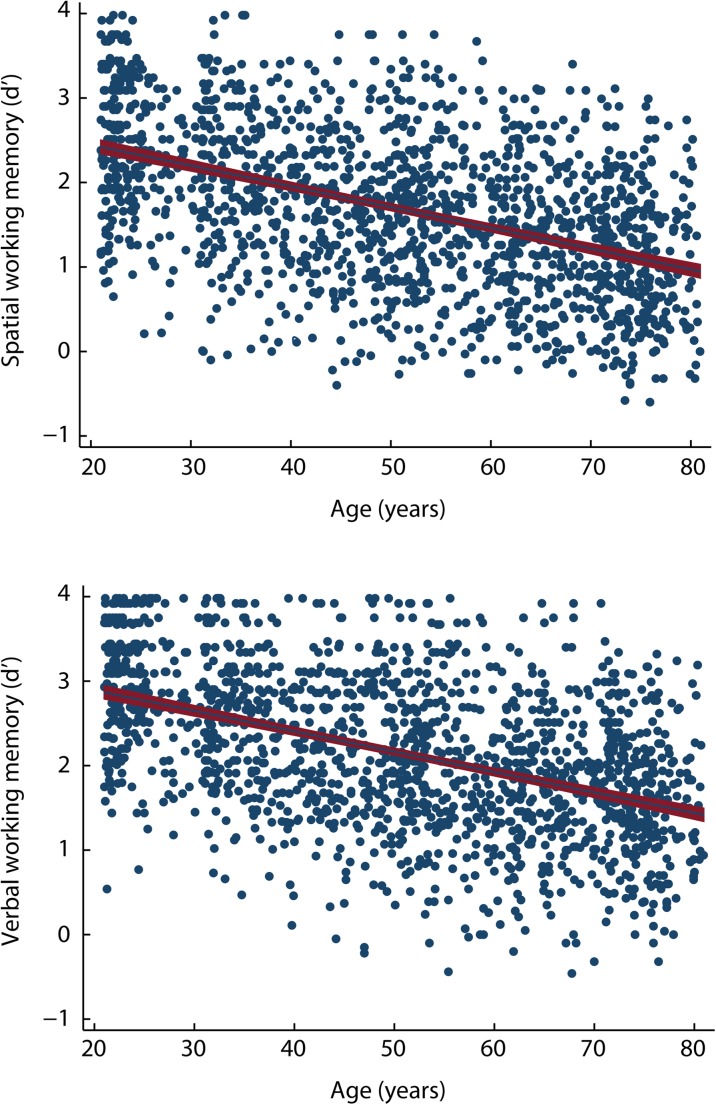
Participants’ discrimination levels (d′) in the spatial and verbal working memory tasks as a function of age are displayed in Fig. 1. The descriptive analyses conducted on all variables are depicted in Supplementary Material Table 2. Because skewness for some variables increased after log-transformation, no transformed data were used for these variables. The results of the linear regression analyses computed between all variables and discrimination in the spatial working memory task are displayed in Supplementary Material Table 3, and those conducted between all variables and discrimination in the verbal working memory domain are shown in Supplementary Material Table 4.
Fig. 1.
Discrimination level (d′) in the spatial and verbal working memory domains, examined through a two-back task, as a function of participant’s age in a life span sample of 1652 adults between 21 and 80 years of age. The solid line represents the linear regression fit, and the red band (gray) represents the 95% confidence interval
Spatial working memory
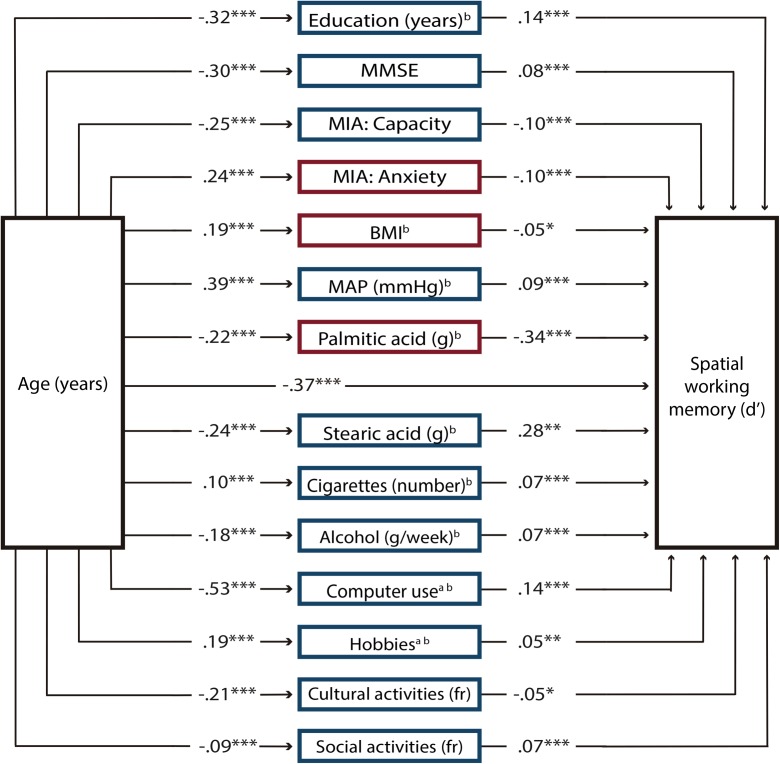
Linear regression analyses revealed 54 variables that had a significant effect on discrimination in the spatial domain and were included in the first SEM analysis as potential mediators. After the model was fit, only the paths of 14 endogenous variables had significant t values (P < .05); thus, the model was fine-tuned by eliminating the mediator variables that had paths that were no longer significant. The SEM estimation was repeated, and results revealed that the path coefficients for all 14 mediator variables in the model were significant (Fig. 2). The correlation matrix of this model is presented in Supplementary Material Table 5, and the SEM results are depicted in Supplementary Material Table 7; for reference, we present in this table the coefficients from the model fit with variables that were not log-transformed. The inspection of the modification indices showed some model misspecifications due to related variables. Misspecifications were observed between education and the scores from the Capacity and Anxiety scales of the MIA, computer use, and cultural activities; between the anxiety scale and the capacity scale of the MIA and computer use; between cultural activities and computer use, hobbies, and social activities; between alcohol and cigarettes and social activities; between body mass index (BMI) and MAP; and between palmitic acid and stearic acid. Because these variables are strongly theoretically related, we added covariance terms between these variables. The goodness-of-fit for this final model was χ2 (78) = 279.89, P < .0001; relative χ2 = 3.59; CFI = 0.98; RMSEA = 0.040; and SRMS = 0.036. The coefficient of determination (CD) of the model was 0.53. The indirect effects of each mediator variable were significant as revealed by bootstrapping (Fig. 4). In addition, the total indirect effect of age through all mediator variables was significant [β (95% CI) = − 0.005 (− 0.007, − 0.003)]. Because the direct effect of age on spatial working memory was β = − 0.019, the proportion of the total effect mediated was 0.207.
Fig. 2.
Structural equation model for spatial working memory. The model comprises only variables that significantly mediated the effects of age on discrimination (d′) decline in the spatial working memory domain across the adult life span. Path coefficients are standardized estimates. Positive predictors of spatial working memory performance are presented in blue (dark gray) squares and negative predictors in red (light gray) squares. Note that two factors that have positive effects on spatial working memory (Capacity of the Metamemory in Adulthood Scale [MIA] and cultural activities) had opposite effects when mediating the effects of age on working memory. These factors decreased with age to the extent that their effects were negative. MMSE Mini-Mental State Scale, BMI body mass index, MAP mean arterial pressure, fr frequency. aTotal intake or time = frequency × duration. bLog-transformed variable. *P < 0.05, **P < 0.01, ***P < 0.001 (color figure online)
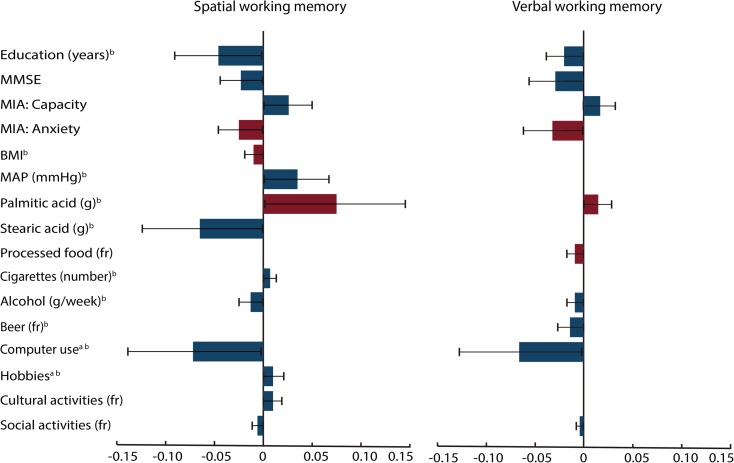
Fig. 4.
Estimates of the indirect effects in the spatial and verbal working memory structural equation models. The indirect effects (β) and 95% bias-corrected confidence intervals for each mediator variable were calculated using a bootstrap procedure with 20,000 samples. Indirect effects were significant for all mediator variables. However, note that some positive (blue or dark gray) and negative predictors (red or light gray) had opposite indirect effects when mediating the effects of age on working memory. The indirect effects are products of the effects of age on each mediator and the effects of each mediator on working memory. Thus, if both effects are positive or negative, then the indirect effects will be positive. However, if they have opposite effects, then their indirect effects will be negative. MMSE Mini-Mental State Scale, MIA Metamemory in Adulthood Scale, BMI body mass index, MAP mean arterial pressure, fr frequency. aTotal intake or time = frequency × duration. bLog-transformed variable (color figure online)
Verbal working memory
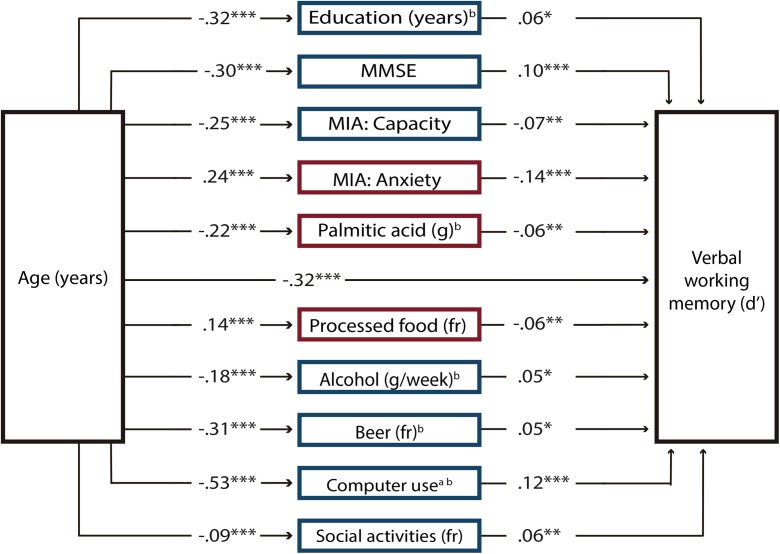
Linear regression analyses were significant for 53 variables, which were included in the first SEM analysis as potential mediators of discrimination in the verbal working memory domain. The results revealed that the paths of 10 endogenous variables had significant t values (P < .05); therefore, mediator variables that had no significant paths were eliminated from the model. The estimated second model showed that the path coefficients for the 10 mediator variables in the model were significant (Fig. 3). Supplementary Material Table 6 displays the correlation matrix of this model, and the SEM results are shown in Supplementary Material Table 8. The modification indices revealed some misspecifications because some variables were related. We added covariance terms between the following variables because they were strongly theoretically related: between education and the scores from the capacity and anxiety scales of the MIA, computer use, and cultural activities; between the anxiety scale and the capacity scale of the MIA and computer use; between social activities and alcohol and beer; between alcohol and beer; and between palmitic acid and processed food. The final model had the following goodness-of-fit: χ2 (36) = 147.76, P < .0001; relative χ2 = 4.10; CFI = 0.96; RMSEA = 0.043; and SRMS = 0.033. The CD of the model was 0.47. Bootstrapping analyses revealed that the indirect effects of each mediator variable were significant (Fig. 4). The total indirect effect of age through all mediator variables was also significant [β (95% CI) = − 0.008 (− 0.010, − 0.006)]. Given that the direct effect of age on verbal working memory was β = − 0.016, the proportion of the total effect mediated was 0.320.
Fig. 3.
Structural equation model for verbal working memory. The model comprises only variables that significantly mediated the effects of age on discrimination (d′) decline in the verbal working memory domain across the adult life span. Path coefficients are standardized estimates. Positive predictors of verbal working memory performance are presented in blue (dark gray) squares and negative predictors in red (light gray) squares. Note that the positive effects of the Capacity of the Metamemory in Adulthood Scale (MIA) on verbal working memory had opposite effects when mediating the effects of age on working memory. The scores in this scale decreased with age to the extent that their effects were negative. MMSE Mini-Mental State Scale, fr frequency. aTotal intake or time = frequency × duration. bLog-transformed variable. *P < 0.05, **P < 0.01, ***P < 0.001 (color figure online)
Discussion
The final models showed that only 14 and 10 predictors out of 54 and 53 potential variables significantly mediated the effects of age on spatial and verbal working memory, respectively. These results confirm that when several factors occur concurrently, as in real life, only a few of them are sufficiently powerful to remain influential in working memory when other competitive factors are present.
Individual characteristics such as years of education, general cognitive status measured by means of the MMSE, and participants’ beliefs about their own memory capacity (MIA: capacity) and the anxiety experienced when they employ their memory (MIA: anxiety) significantly mediated the effects of age on working memory in both domains. Education and other challenging life experiences are thought to be inducers of cognitive reserve, the capacity to more effectively use brain networks and cognitive processes (Stern 2009) such as memory. Whether accurate or not, an individual’s metamemory influences actual working memory performance. This is because metamemory is a form of metacognition, which is the ability to control various thinking strategies, such as organizing and monitoring (Flavell 1979), which also belong to working memory.
The spatial working memory model comprised both physical, BMI, and physiological, MAP, predictors that were absent in the verbal model. BMI had a negative effect on spatial working memory, whereas MAP had a positive effect. Although there is evidence that individuals with high BMI values in the range of obesity perform poorer in working memory tasks (Elias et al. 2003) and show less total gray matter volume and whole brain volume (Gunstad et al. 2008) than non-obese persons, the mechanisms through which BMI impacts cognition and the brain are still under investigation; the robust association of BMI with cardiovascular and metabolic diseases is one of the strongest explanations. MAP is a measurement of blood perfusion pressure delivery to all organs, including the brain, which explains its positive influence on memory.
The consumption of two long-chain saturated fatty acids, palmitic acid (16:0) and stearic acid (18:0), impacted working memory discrimination, while the former impacted both memory domains; however, their effects are opposite. Palmitic acid, found mainly in animal fats and palm oil, increases low-density lipoprotein (LDL), which, in turn, is associated with cardiovascular diseases. Conversely, stearic acid, found predominantly in animal fats and cocoa butter, has neutral effects on serum LDL and total cholesterol concentration (Grande et al. 1970). In the current study, stearic acid effects were not neutral, given that spatial working memory was positively mediated by this fatty acid. Post-mortem brains of Alzheimer’s disease patients, compared to healthy individuals, show a significant reduction in stearic acid composition in frontal and temporal cortices (e.g., Fraser et al. 2010); thus, it is possible that the positive influence of stearic acid may be through its functions within neuronal membranes. Additionally, the consumption of processed food negatively mediated the effects of age on verbal working memory. Processed food is the major source of trans fatty acids formed by partially hydrogenated vegetable oils, which increase LDL concentration in the plasma, a condition highly correlated with coronary artery disease (Filip et al. 2010).
The finding that cigarette smoking was associated with higher spatial working memory is not unexpected given that numerous studies have found that nicotine enhances attention and memory (for a meta-analysis, see Heishman et al. 2010). Nicotine binds to presynaptic nicotinic acetylcholine receptors in the brain, inducing the release of acetylcholine and other neurotransmitters in regions such as the prefrontal cortex (dos Santos Coura and Granon 2012), which is crucial for working memory. Contrary to the adverse effects of high alcohol intake, moderate alcohol consumption is associated with a reduced risk of dementia (Ruitenberg et al. 2002), lower levels of systemic inflammatory markers (Imhof et al. 2004), increased high density lipoprotein (HDL) cholesterol (Rimm et al. 1999), and higher cognitive performance (e.g., Britton et al. 2004). In the present study, we now provide additional evidence that moderate alcohol consumption positively mediates the effects of age on both spatial and verbal working memory. In addition to the indirect effects of alcohol on cognition through its influence on the cardiovascular system, it has been proposed that ethanol may benefit memory because it enhances the expression of N-methyl-d-aspartate receptor (NMDAR), which is highly involved in the control of synaptic plasticity and memory (Kalev-Zylinska and During 2007). The favorable effects of alcohol are independent of the type of alcoholic beverage (e.g., Ruittenbeg et al. 2002; Rimm et al. 1999), indicating that alcohol itself is the major protective agent in all alcoholic beverages. However, in the current study, beer in particular had a positive impact on verbal working memory, a finding that may be related, in addition to its alcohol content, to the fact that beer is a rich source of type B vitamins, minerals, and antioxidants (Brenner et al. 2001).
Both models showed that the use of the computer and the engagement in social activities positively influenced spatial and verbal working memory discrimination. Likewise, hobbies and cultural activities benefited spatial working memory. However, because cultural activities greatly diminish with advancing age, the mediating effect of this variable on memory did not reach positive values. Note that all these activities were currently performed by the participants, suggesting that the maintenance of highly demanding activities seems crucial for working memory performance. Moreover, activities that were significant mediators in the models essentially involved working memory processes, indicating that the continuous use of working memory benefits its functionality. There is evidence that mentally and socially active individuals perform better in general cognitive assessments and have reduced risk of suffering from dementia (Fratiglioni et al. 2004); we now demonstrate that working memory in particular benefits from these activities.
Because some of our variables were assessed through participants’ reports, one limitation of the study is the possibility that the findings were affected by random measurement errors due to participants’ inaccuracies. This examination method was necessary to obtain information on a wide range of variables. This is the case for nutrient intake estimations obtained through the FFQ, which relies on participants’ exactness in their food consumption reporting, even though dietary consumption is relativity stable, thereby reducing participant inaccuracy. However, because the FFQ allows for the examination of dietary intake over a period of 1 year, it is more suitable to examine the effects that nutrients may have on memory because their influence is the consequence of long-term dietary exposure, information on which is not available through other precise methods that only provide short-term intake information. Observational studies, such as this study, have the disadvantage that participants’ responses may not be genuine due to social desirability bias; however, this method has the advantage of allowing the assessment of individuals in real-life situations. Moreover, the estimation of the effects of numerous factors on memory, as in the present study, may only be suitable through observational methods. Another limitation is that SEM analyses only indicate whether the models fit and supported by the data are plausible, but this does not mean that the models are correct or true. Despite this disadvantage, SEM has the power to identify influential factors within several predictors because it accounts for all possible relationships among the variables included in the model.
The models allow the identification of factors that have enough power to affect spatial and verbal working memory when they interact together, as they do in real life. The indirect effects of each mediator and the joint effects of all mediators significantly accounted for the effects of age on spatial and verbal working memory. However, these effects were low in comparison with the direct effect of age on each type of memory. The models include predictors from four main fields: factors that describe individual characteristics such as education, cognitive integrity, and metamemory awareness; variables that represent physical or physiological traits (BMI and MAP); factors that express consumption habits (palmitic and stearic fatty acids, processed food, tobacco, alcohol and beer); and mediators that illustrate individual activities (the use of the computer and the engagement in hobbies and cultural and social activities).
Several of the factors (BMI, MAP, and the intake of palmitic and stearic fatty acids, processed food, alcohol and beer) mediate the effects of age on working memory indirectly through the cardiovascular system, which consequently influences brain resources. However, some mediators also (palmitic acid, stearic acid, alcohol and beer consumptions) or exclusively (cigarette consumption) have direct effects on brain function. The models distinctly reveal that working memory functionality is directly influenced by its customary usage through several activities involving working memory mechanisms (computer use, hobbies, cultural and social activities) and cognitive control processes (metamemory: capacity and anxiety). Likewise, cognitive integrity in all areas and years of education provide support for the mitigation of the effects of age on working memory.
Electronic supplementary material
(DOCX 114 kb)
Funding information
This work was supported by the National Council of Science and Technology (CONACYT) (grant number 238826) and the National Autonomous University of Mexico, General Direction of Academic personal Affairs (DGAPA) (grant numbers IN304202, IN300206, IN300309, ID300312, IG300115, IG300618).
Compliance with ethical standards
All participants provided informed consent and received a monetary reward for his/her participation. The study was approved by the Bioethics Committee of the School of Medicine at the National Autonomous University of Mexico. All experiments were performed in accordance with the Declaration of Helsinki.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s11357-018-0031-1) contains supplementary material, which is available to authorized users.
References
- Annett M. A classification of hand preference by association analysis. Br J Psychol. 1970;61:303–321. doi: 10.1111/j.2044-8295.1970.tb01248.x. [DOI] [PubMed] [Google Scholar]
- Beck AT. Beck depression inventory. San Antonio: The Psychological Corporation; 1987. [Google Scholar]
- Beilharz JE, Maniam J, Morris MJ. Diet-induced cognitive deficits: the role of fat and sugar, potential mechanisms and nutritional interventions. Nutrients. 2015;7:6719–1638. doi: 10.3390/nu7085307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JE, Greenough WT, Anderson BJ, Isaacs KR. Environment and the aging brain. Can J Psychol. 1987;41:111–130. [PubMed] [Google Scholar]
- Brenner H, Rothenbacher D, Bode G, März W, Hoffmeister A, Koenig W. Coronary heart disease risk reduction in a predominantly beer-drinking population. Epidemiology. 2001;12:390–395. doi: 10.1097/00001648-200107000-00008. [DOI] [PubMed] [Google Scholar]
- Britton A, Singh-Manoux A, Marmot M. Alcohol consumption and cognitive function in the Whitehall II Study. Am J Epidemiol. 2004;160:240–247. doi: 10.1093/aje/kwh206. [DOI] [PubMed] [Google Scholar]
- Cansino S, Hernández-Ramos E, Estrada-Manilla C, Torres-Trejo F, Martínez-Galindo JG, Ayala-Hernández M, Gómez-Fernández T, Osorio D, Cedillo-Tinoco M, Garcés-Flores L, Beltrán-Palacios K, García-Lázaro HG, García-Gutiérrez F, Cadena-Arenas Y, Fernández-Apan L, Bärtschi A, Rodríguez-Ortiz MD. The decline of verbal and visuospatial working memory across the adult life span. AGE. 2013;35:2283–2302. doi: 10.1007/s11357-013-9531-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chávez MM, Chávez A, Pérez-Gil F, Roldán JA, Ledesma JA, Mendoza E, Hernández SL, Chaparro AG. Tablas de Valor Nutritivo de los Alimentos de Mayor Consumo en México. Pax México: Edición Internacional Español-Inglés; 1996. [Google Scholar]
- Dixon RA, Hultsch DF, Hertzog C. The Metamemory in Adulthood (MIA) questionnaire. Psychopharmacol Bull. 1988;24:671–688. [PubMed] [Google Scholar]
- dos Santos Coura R, Granon S. Prefrontal neuromodulation by nicotinic receptors for cognitive processes. Psychopharmacology. 2012;221:1–18. doi: 10.1007/s00213-011-2596-6. [DOI] [PubMed] [Google Scholar]
- Elias MF, Elias PK, Sullivan LM, Wolf PA, D’Agostino RB. Lower cognitive function in the presence of obesity and hypertension: the Framingham heart study. Int J Obes Relat Metab Disord. 2003;27:260–268. doi: 10.1038/sj.ijo.802225. [DOI] [PubMed] [Google Scholar]
- Filip S, Fink R, Hribar J, Vidrih R. Trans fatty acids in food and their influence on human health. Food Technol Biotechnol. 2010;48:135–142. [Google Scholar]
- Flavell JH. Metacognition and cognitive monitoring: a new area of cognitive-developmental inquiry. Am Psychol. 1979;34:906–911. doi: 10.1037/0003-066X.34.10.906. [DOI] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fraser T, Tayler H, Love S. Fatty acid composition of frontal, temporal and parietal neocortex in the normal human brain and in Alzheimer’s disease. Neurochem Res. 2010;35:503–513. doi: 10.1007/s11064-009-0087-5. [DOI] [PubMed] [Google Scholar]
- Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 2004;3:343–353. doi: 10.1016/S1474-4422(04)00767-7. [DOI] [PubMed] [Google Scholar]
- Grande F, Anderson JT, Keys A. Comparison of effects of palmitic and stearic acids in the diet on serum cholesterol in man. Am J Clin Nutr. 1970;23:1184–1193. doi: 10.1093/ajcn/23.9.1184. [DOI] [PubMed] [Google Scholar]
- Gunstad J, Paul RH, Cohen RA, Tate DF, Spitznagel MB, Grieve S, Gordon E. Relationship between body mass index and brain volume in healthy adults. Int J Neurosci. 2008;118:1582–1593. doi: 10.1080/00207450701392282. [DOI] [PubMed] [Google Scholar]
- Hansson JA, Hagber B. Determinant factors contributing to variations in memory performance in centenarians. Int J Aging Hum Dev. 2005;60:19–51. doi: 10.2190/WFUP-2J25-LWQF-PQ3W. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Kleykamp BA, Singleton EG. Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology. 2010;210:453–469. doi: 10.1007/s00213-010-1848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández M, Aguirre J, Serrano L (1983) Alimentación de obreros y sus familias. División de Nutrición de Comunidades, Publicación L-61. Instituto Nacional de la Nutrición “Salvador Zubirán”, Mexico
- Hernández-Ávila M, Romieu I, Parra S, Hernández-Ávila J, Madrigal H, Willett W. Validity and reproducibility of a food frequency questionnaire to assess dietary intake of women living in Mexico City. Salud Publica Mex. 1998;39:133–140. doi: 10.1590/S0036-36341998000200005. [DOI] [PubMed] [Google Scholar]
- Hernández-Ávila JE, González-Aviles L, Rosales-Mendoza E. Manual de usuario. SNUT Sistema de evaluación de hábitos nutricionales y consumo de nutrimentos. Cuernavaca: Instituto Nacional de Salud Pública; 2000. [Google Scholar]
- Holmes TH, Rahe RH. The social readjustment rating scale. J Psychosom Res. 1967;11:213–318. doi: 10.1016/0022-3999(67)90010-4. [DOI] [PubMed] [Google Scholar]
- Imhof A, Woodward M, Doering A, Helbecque N, Loewel H, Amouyel P, Lowe GD, Koenig W. Overall alcohol intake, beer, wine, and systemic markers of inflammation in western Europe: results from three MONICA samples (Augsburg, Glasgow, Lille) Eur Heart J. 2004;25:2092–2100. doi: 10.1016/j.ehj.2004.09.032. [DOI] [PubMed] [Google Scholar]
- Kalev-Zylinska ML, During MJ. Paradoxical facilitatory effect of low-dose alcohol consumption on memory mediated by NMDA receptors. J Neurosci. 2007;27:10456–10467. doi: 10.1523/JNEUROSCI.2789-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan RJ, Greenwood CE. Dietary saturated fatty acids and brain function. Neurochem Res. 1998;23:615–626. doi: 10.1023/A:1022478503367. [DOI] [PubMed] [Google Scholar]
- Kirchner WK. Age differences in short-term retention of rapidly changing information. J Exp Psychol. 1958;55:352–358. doi: 10.1037/h0043688. [DOI] [PubMed] [Google Scholar]
- MacCallum RC, Browne MW, Sugawara HM. Power analysis and determination of sample size for covariance structure modeling. Psychol Methods. 1996;1:130–149. doi: 10.1037/1082-989X.1.2.130. [DOI] [Google Scholar]
- Miller GA, Galanter E, Pribram KH. Plans and the structure of behavior. New York: Holt, Rinehart & Winston; 1960. [Google Scholar]
- Preacher KJ, Coffman DL (2006) Computing power and minimum sample size for RMSEA [Computer software]. Retrieved from http://quantpsy.org/
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40:879–891. doi: 10.3758/BRM.40.3.879. [DOI] [PubMed] [Google Scholar]
- Rimm EB, Williams P, Fosher K, Criqui M, Stampfer MJ. Moderate alcohol intake and lower risk of coronary heart disease: meta-analysis of effects on lipids and haemostatic factors. BMJ. 1999;319:1523–1528. doi: 10.1136/bmj.319.7224.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romieu I, Parra S, Hernández JF, Madrigal H, Willet W, Hernández M. Questionnaire assessment of antioxidants and retinol intakes in Mexican women. Arch Med Res. 1999;30:224–239. doi: 10.1016/S0188-0128(99)00017-2. [DOI] [PubMed] [Google Scholar]
- Ruitenberg A, van Swieten JC, Witteman JC, Mehta KM, van Duijn CM, Hofman A, Breteler MM. Alcohol consumption and risk of dementia: the Rotterdam Study. Lancet. 2002;359:281–286. doi: 10.1016/S0140-6736(02)07493-7. [DOI] [PubMed] [Google Scholar]
- Santos NC, Costa PS, Cunha P, Portugal-Nunes C, Amorim L, Cotter J, Cerqueira JJ, Palha JA, Sousa N. Clinical, physical and lifestyle variables and relationship with cognition and mood in aging: a cross-sectional analysis of distinct educational groups. Front Aging Neurosci. 2014;6:21. doi: 10.3389/fnagi.2014.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of Agriculture (1963–1997) Composition of foods—raw, processed, and prepared. Agricultural handbook no. 8. Government Printing Offices, Washington, D.C
- Wechsler D. WAIS-R manual. New York: The Psychological Corporation; 1981. [Google Scholar]
- Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- Zahodne LB, Glymour MM, Sparks C, Bontempo D, Dixon RA, MacDonald SW, Manly JJ. Education does not slow cognitive decline with aging: 12-year evidence from the Victoria Longitudinal Study. J Int Neuropsychol Soc. 2011;17:1039–1146. doi: 10.1017/S1355617711001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 114 kb)