Abstract
Calorie restriction (CR) without malnutrition increases life span and health span in multiple model organisms. In non-human and human primates, CR causes changes that protect against several age-related pathologies, reduces inflammation, and preserves or improves cell-mediated immunity. However, CR has also been shown to exhibit adverse effects on certain organs and systems, including the immune system, and to impact genetically different organisms of the same species differentially. Alternately, short periods of fasting followed by refeeding may result in the proliferation of bone marrow stem cells, suggesting a potential rejuvenation effect that could impact the hematopoietic compartment. However, the global consequences of CR followed by refeeding on the immune system have not been carefully investigated. Here, we show that individuals practicing long-term CR with adequate nutrition have markedly lower circulating levels of total leukocytes, neutrophils, lymphocytes, and monocytes. In 10-month-old mice, short-term CR lowered lymphocyte cellularity in multiple lymphoid tissues, but not in bone marrow, which appears to be a site of influx, or a “safe haven” for B, NK, and T cells during CR. Cellular loss and redistribution was reversed within the first week of refeeding. Based on BrdU incorporation and Ki67 expression assays, repopulating T cells exhibited high proliferation in the refeeding group following CR. Finally, we demonstrated that the thymus was not essential for T cell repopulation following refeeding. These findings are of potential relevance to strategies to rejuvenate the immune system in mammals and warrant further investigation.
Electronic supplementary material
The online version of this article (10.1007/s11357-018-0022-2) contains supplementary material, which is available to authorized users.
Keywords: Leukocytes, Lymphocytes, Rejuvenation, Calorie restriction, Aging, Cellular immunology
Introduction
Aging is associated with an increased risk of morbidity and mortality from infection, due to a dysfunction affecting both the innate and adaptive arms of the immune system (Fontana et al. 2010). Susceptibility to emerging pathogens and decreased efficacy of vaccines are driven in part by this age-related immune decline, termed immune senescence (Parmigiani et al. 2013; Ortman et al. 2014; Nikolich-Žugich 2014). The earliest age-related change that partially sets the stage for a subsequent immune decline is the involution of the thymus that results in a decreased output of naïve T cells into the periphery as early as before puberty in humans (Koup et al. 1998; Sempowski et al. 2002; Rudd et al. 2011). This mandates long-term peripheral maintenance of the naïve T cell pool (Naylor et al. 2005; Čičin-Šain et al. 2007) that, while initially successful, also fails in the last third of life (Naylor et al. 2005; Ferrando-Martínez et al. 2011, 2013; Rezzani et al. 2014; Vescovini et al. 2014). Moreover, pathogen exposure across the life span selectively converts antigen-specific naïve T cells into memory T cells (Rudd et al. 2011), further pronouncing the relative dominance of memory lymphocytes with aging. Similar changes are seen in B-lymphocytes. In addition to these cell population changes, cell intrinsic defects, as well as decline in environmental/stromal and soluble factor-driven coordination of immune homeostasis and immune responses, result in the exacerbation of age-related immune decline (Becklund et al. 2016).
Calorie restriction (CR) without malnutrition is the most robust intervention known to increase life span and health span in most model organisms investigated to date (Fontana et al. 2010; Mattison et al. 2012; Colman et al. 2014). In humans, it causes changes that protect against multiple age-associated chronic disease and powerfully reduces inflammation without impairing the delayed-type hypersensitivity skin response or the antibody response to vaccines (Heilbronn and Ravussin 2003; Meydani et al. 2016; Most et al. 2017). However, experimental animal investigation of the consequences of CR on the immune system has revealed potentially beneficial, as well as detrimental, effects. In the mouse model, CR reduced the extent of thymic involution in old animals (Yang et al. 2009); and in both murine and non-human primate models, the proportion of naïve peripheral T cells was increased (Messaoudi et al. 2006). These observations seemingly indicate that CR could be used as a therapeutic regimen in the elderly to prevent age-related immune senescence. On the other hand, studies of infectious challenge in vivo have produced cautionary results. Several groups, including ours, have demonstrated that mice living in specific pathogen-free facilities on lifelong CR regimen displayed increased mortality compared to ad libitum fed counterparts in response to sepsis (Sun et al. 2001), influenza A (Gardner 2005), parasitic infections (Kristan 2007), and West Nile virus infection (Goldberg et al. 2015). These studies suggested that in the face of microbial challenge, both innate and adaptive immune responses require access to energy to optimally defend the organism. Collectively, these results suggest that it is necessary to evaluate modalities of CR that provide increased longevity and rejuvenating effects in non-immune tissues, can be adhered to, and do not compromise the functional integrity of the immune system.
A variety of CR modalities have been reported to be associated with lymphopenia (Weindruch et al. 1997; Meydani et al. 2016) presenting another potential adverse immune effect of CR. As this effect has remained largely unexplored, we investigated changes in numbers of a broad array of leukocytes within multiple lymphoid tissues (lymph nodes, thymus, bone marrow, blood, and spleens) during 2 months of CR induction. We then returned animals to ad libitum (AL) feeding and monitored alterations to populations of leukocytes within these same tissues and tested whether any of the observed changes may be due to de novo cell production or to potential cellular redistribution between peripheral reservoirs.
We here demonstrate that CR resulted in global leukopenia in both rodents and humans, which could be reversed following return to “normal” or AL feeding. We further show that after a return to AL feeding, these cells exhibited rapid proliferation driven by homeostatic and peripheral factors, without a significant input from the thymus. We discuss the results in light of potential modulation of the functional ability of the immune system during aging.
Results
Caloric restriction in humans results in blood leukopenia
We analyzed multiple health parameters of healthy volunteers practicing long-term CR with adequate nutrition for an average of 10 years (range 3–20 years) and age-matched sedentary individuals consuming typical Western diets (WD) (Fryar et al. 2012). Weight, BMI, and body fat percentages of people on CR versus WD were significantly lower in both men and women. We also measured multiple subsets of leukocytes by complete blood count (CBC) test and found that men and women practicing long-term CR have a decrease in total leukocyte count when compared to WD control subjects. These observations were then broken down to analyze neutrophils, lymphocytes, and monocytes and all subsets were significantly reduced in comparison to the WD cohort (Online Resource 1). From these data, we conclude that CR drives a reduction in total leukocyte cellularity, either by sequestration in tissues or a contraction/elimination during periods of reduced calories. As these questions cannot be ethically answered in human CR, we employed a mouse model of CR in which we induced, in a stepwise manner, a caloric deficit in 10-month-old C57BL/6 mice for approximately 2 months and immediately returned mice to AL feeding for an additional 2 months.
Caloric restriction results in weight loss that is rapidly reversed
To address the above questions in mice, we elected to initiate CR at 10 months of age. This age was chosen as it is analogous to mid-adulthood in humans, based on studies showing that the effects of calorie restriction are most beneficial when initiated in early to middle adulthood (Messaoudi et al. 2006). Reduction in caloric intake resulted in weight loss. As our model of acute calorie restriction has not been fully characterized in mid-adulthood mice, we first sought to determine the consequences of restriction on the weight profiles of mice during and after the reduction of calories. Animals were weighed prior to feeding, at least twice a week during induction, maintenance, and cessation of CR. To calorically restrict mice, AL food was replaced by 3-g pellets of irradiated NIH-31 chow and administered to mice once a day. Total food intake was reduced by 40% of ad libitum diet over 3 weeks, which in our hands is equal to a total reduction of 1.4 g of food. CR diet resulted in a steady, gradual, and modest daily loss of weight to no greater than 20% of starting weight that was immediately, within 1 day, reversible following return to AL food access (Online Resource 2) (Mahoney et al. 2006; Hambly et al. 2015).
Two months of calorie restriction results in lymphopenia and lymphocyte redistribution across multiple lymphoid organs
To determine how CR affects the distribution of cells of the immune system, we compared the effect of our 2-month bout of CR to age-matched littermate AL control male C57BL/6 mice. We sought to address the basis of leukopenia observed in CR human cohort. Our primary hypothesis was that CR induces a redistribution of leukocytes within the host, resulting in lower number of leukocytes within lymphoid tissues. We further hypothesized that this lost cellularity is reversible following return to adequate nutrition. We specifically investigated changes in the lymphocyte compartments: B cells, natural killer (NK) cells, and T cells, as well as of T cell subsets (naïve and memory, as defined by CD44 and CD62L expression), of both CR and AL fed mice across the blood, bone marrow, spleen, and brachial and inguinal lymph nodes.
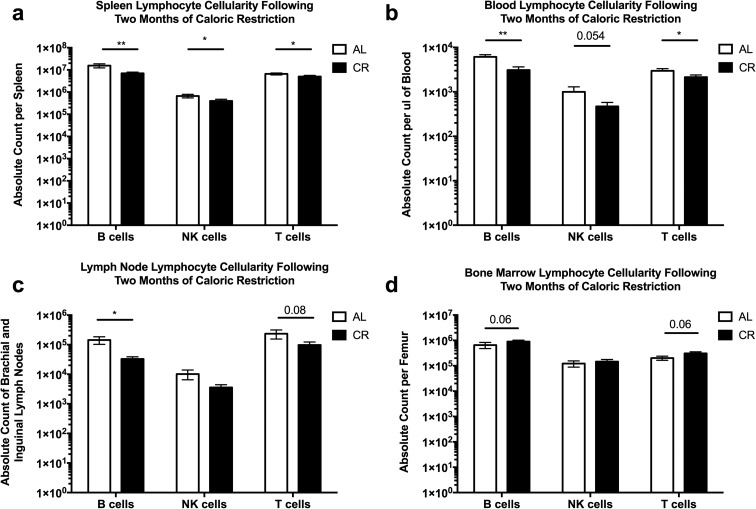
We found that CR resulted in a statistically significant decrease of all three of these lymphocyte subsets across the spleen, blood, and pooled brachial and inguinal lymph nodes (Fig. 1a–c). Furthermore, the CR mice all had spleens that were visibly smaller when compared to their AL fed counterparts (Online Resource 3). By contrast, we observed a trend of an increase in the B cells and T cells within the bone marrow of CR mice when compared to AL controls (Fig. 1d), although that trend did not reach significance with the group size used in our experiments. Thus, in line with our hypothesis and modeling the results from humans, these data suggest that the induction of CR over a period of 2 months results in an alteration of the cellularity of multiple lymphoid tissues. The data also confirm, in our hands, that lymphoid organ size is affected by the nutritional state of an animal, as previously demonstrated by others (Wing et al. 1988; Weindruch and Sohal 1997; Colman et al. 2009).
Fig. 1.
Lymphoid organ cellularity is decreased after caloric restriction. Ten-month-old B6 mice were calorically restricted, as described in the “Methods,” for 2 months and lymphocyte subsets were analyzed by flow cytometry from the a spleen, b blood, c brachial and inguinal lymph nodes, and d bone marrow of the femur. Statistical differences were calculated by one-tailed Mann Whitney U tests by comparing cellularity to age-matched, AL fed mice. *P < 0.05; **P < 0.01. Data are representative of three independent experiments with 2–8 mice per treatment group
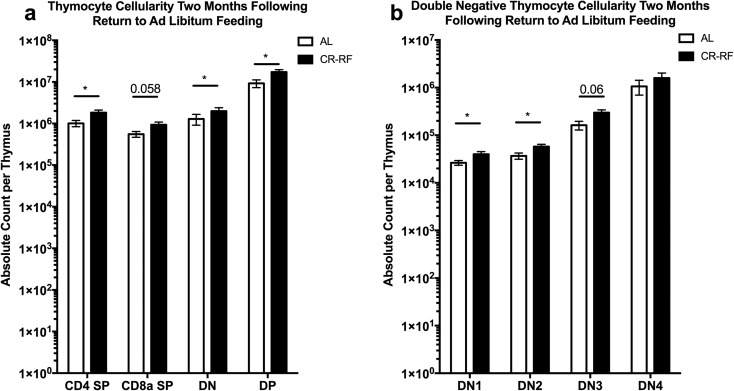
Thymic size and cellularity is reduced following 2 months of calorie restriction
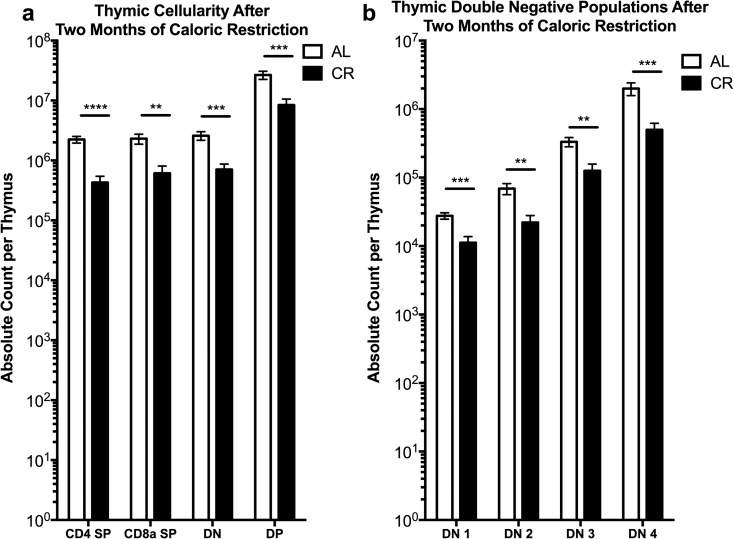
Lifelong CR has been shown to improve cellularity of the old thymus and to reduce its accumulation of adipocytes (Yang et al. 2009). Moreover, thymic output was improved and the peripheral T cell compartment contained a higher frequency of naïve T cells in lifelong CR mice compared to AL controls (Yang et al. 2009). Therefore, we wondered whether a 2-month period of CR would induce similar changes in the thymus as seen in the lifelong model. We found significant decreases in the absolute numbers of CD4+CD8+ double positive, CD4+ and CD8+ single positive, and double negative (DN) thymocytes within the thymi of CR mice in comparison to AL fed mice (Fig. 2a). Furthermore, the main subsets of DN thymocytes (DN1-4, as delineated by the expression of CD25 and CD44 (Nikolić-Žugić 1991; Godfrey et al. 1993)) were also universally decreased in comparison to AL fed controls (Fig. 2b) and the size of the thymus of CR mice was visibly smaller than that of AL counterparts (Online Resource 4). These data demonstrate that a short period of CR results in a loss of both size and cellularity of the thymus. The lost cellularity of the thymus is seen in all subsets of the αβ T lymphocyte lineage analyzed.
Fig. 2.
Thymic cellularity is decreased after caloric restriction. Ten-month-old B6 mice treated by CR as in Fig. 1 were analyzed for thymus lymphocyte subset cellularity by flow cytometry. a Absolute counts of CD4 single positive, CD8 single positive, double negative, and CD4/CD8 double positive thymocytes. b Absolute count of CD4/CD8 double negative thymocyte subsets (DN1-4), based on CD44 and CD25 expression. Statistical differences were calculated by one-tailed Mann Whitney U tests by comparing cellularity to age-matched, AL fed mice. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. Data are representative of three independent experiments with 2–8 mice per treatment group
Return to ad libitum feeding results in increased blood cellularity and T cell cycling
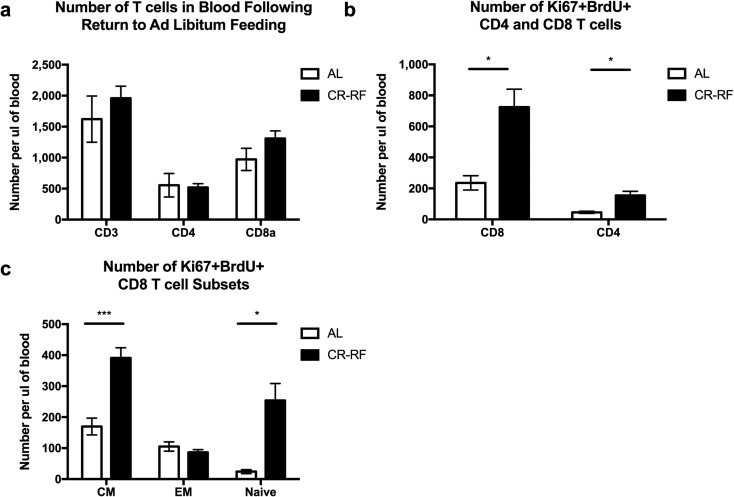
We next wished to determine whether the cells lost from blood and lymphoid organs were capable of rebounding back to baseline levels following a return to AL feeding. To that effect, following the completion of the 2-month period of CR, we returned mice to AL food. At the same time, mice were given a thymidine analog, BrdU in drinking water, to label dividing cells and thereby estimate their turnover. Following 1 week of refeeding and BrdU treatment, we determined the fraction of cells in the blood that had incorporated BrdU and also expressed the cell cycle marker Ki67 (actively cycling cells within the last 2–3 days of analysis) as in our prior studies (Čičin-Šain et al. 2007). After 1 week of CR reversal with AL refeeding (CR-RF), both CD4 and CD8 T cells in the blood rebounded to or above control levels (Fig. 3a). Furthermore, both the CD4 and CD8 T cell compartments contained significantly more cells that were double positive for BrdU and Ki67 relative to the AL controls that did not undergo CR (Fig. 3b). These data suggest that when mice are returned to AL feeding after CR, cells enter into cell cycle and undergo a proliferative burst as indicated by the increased number of T cells in the blood in CR-RF mice when compared to AL counterparts. To evaluate potential mechanisms of this rebound, we analyzed CD8 T cell subsets (effector memory, central memory, and naïve; based on expression of CD62L and CD44) and found that CD8 central memory (CD62L+CD44+) and naïve (CD62L+CD44−) T cells contained significantly more actively cycling (Ki67+BrdU+) cells following refeeding relative to AL controls (Fig. 3c). These data are supportive of the hypothesis that during CR, T lymphocyte populations contract, redistribute, and then, following return to AL nutrition, undergo homeostatic proliferation, presumably to fill the void left by redistributing cells in response to homeostatic cytokines. Based on the fact that CD8 naïve and central memory cells, but not CD8 effector memory cells, proliferated, we would speculate that this proliferation is most likely to be driven by IL-7 and not by IL-15.
Fig. 3.
Peripheral T lymphocytes enter cell cycle after 1 week of refeeding. Calorically restricted mice were returned to AL food access and treated with BrdU in their drinking water. The T cell compartment was analyzed by flow cytometry. a Absolute count of T cells per μl of blood. b Absolute count of Ki67+BrdU+ CD8a and c CD4 T cells. d–f Absolute count of Ki67+BrdU+ central memory (CD44+CD62L+), naïve (CD44−CD62L+), and effector memory (CD44+CD62L−) cells in blood. Statistical differences were calculated by two-tailed Mann Whitney U tests by comparing cellularity to age-matched, AL fed mice. *P < 0.05. Data are representative of two independent experiments with 3–7 mice per treatment group
Lymphoid tissue cellularity returns to AL levels 2 months following return to AL feeding
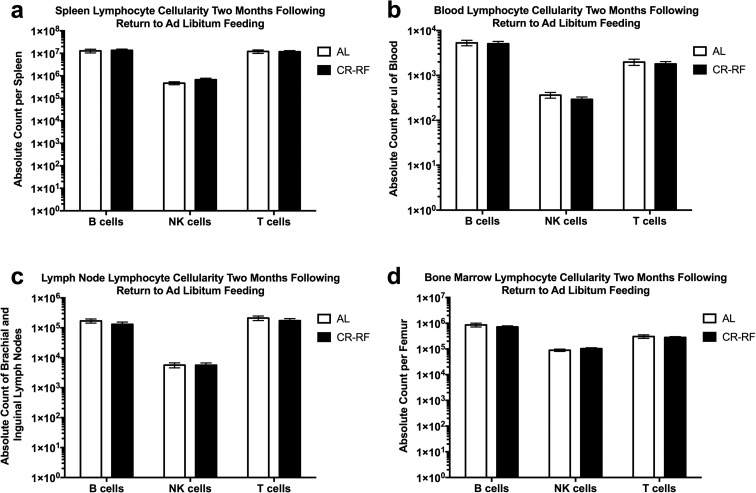
During the early time point after return to AL feeding, T cells appeared to have entered cell cycle based upon BrdU incorporation and Ki67 expression. This observation led us to ask whether organ size and cellularity lost during the period of CR return to control sizes? Mice that were reverted back to AL feeding were maintained on the AL diet for 2 months and once again, we analyzed lymphocyte subsets within the blood, bone marrow, spleen, and brachial and inguinal lymph nodes. We found that absolute cell counts of B, NK, and T cells were not statistically different across tissues between AL and CR-RF animals (Fig. 4). Similar results were obtained with thymus cellularity after 2 months of refeeding. We found that CD4 single positive, double negative, and double positive thymocytes exhibited an absolute increase within the CR-RF groups when compared to AL controls (Fig. 5a). When the double negative population was analyzed further, we found a statistically significant increase in the DN1 and DN2 populations (Fig. 5b). However, we found no statistically significant differences between the DN3 and DN4 populations in CR-RF and AL mice. Therefore, following contraction in both size and cellularity during CR, lymphoid organ cellularity equilibrated at pre-intervention steady state following 2 months of AL feeding.
Fig. 4.
Lymphoid organ cellularity returns to AL levels following 2 months of refeeding. Calorically restricted mice were maintained on AL food for 2 months after CR treatment. Lymphocyte subsets were then analyzed by flow cytometry from the a spleen, b blood, c brachial and inguinal lymph nodes, and d bone marrow of the femur. Statistical differences were calculated by two-tailed Mann Whitney U tests by comparing cellularity to age-matched, AL fed mice. We found no statistical difference in numbers between the groups. Data are representative of three independent experiments with 2–8 mice per treatment group
Fig. 5.
Thymic cellularity returns to AL levels following 2 months of refeeding. Calorically restricted mice were maintained on AL food for 2 months after CR treatment. Thymus subsets were analyzed by flow cytometry. a Absolute counts of CD4 single positive, CD8 single positive, double negative, and CD4/CD8 double positive thymocytes. b Absolute count of CD4/CD8 double negative thymocyte subsets, based on CD44 and CD25 expression. Statistical differences were calculated by two-tailed Mann Whitney U tests by comparing cellularity to age-matched, AL fed mice. *P < 0.05. Data are representative of three independent experiments with 2–8 mice per treatment group
Thymus is dispensable for the refeeding-triggered T cell rebound after CR
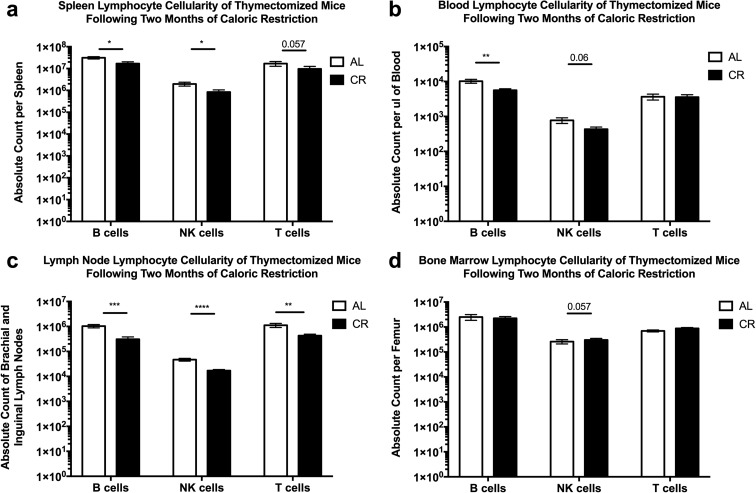
Our results were consistent with the possibility that during a relatively short period of CR, lymphocytes may leave secondary lymphoid tissues and that at least some of them may reside in the bone marrow, so that upon refeeding, they can repopulate the same lymphoid tissues to reestablish homeostasis. However, because thymus also rebounds post-refeeding, it was possible that some or even all of the rebounding cells were originating from the regenerated thymus. To formally and stringently test this possibility, we performed adult thymectomy (ATX) on 3-month-old C57BL/6 mice and then aged them to 10 months of age before inducing our model of CR and compared results to sham-thymectomized (Sh-TX) littermates.
ATX mice exhibited weight loss changes in response to CR induction and return to AL feeding indistinguishable from either their sham controls or surgically intact animals from our initial experiments. Furthermore, following 2 months of CR, ATX mice displayed similar losses of lymphocytes across multiple tissues. We observed a significant decrease in B cells and NK cells in the spleen, whereas T cell data trended lower but did not reach significance (Fig. 6a). In the blood, B cells were significantly reduced, but NK and T cells were not (Fig. 6b). Brachial and inguinal lymph nodes displayed the most significantly decreased number of B, T, and NK cells (Fig. 6c). As in the case of thymus-sufficient wild-type mice, there were no significant changes in the cellularity of B, T, or NK cells in the bone marrow (Fig. 6d). Therefore, from these data, we conclude that loss of the thymus does not alter CR-induced lymphopenia across the spleen and lymph nodes.
Fig. 6.
Thymectomized mice display CR-induced lymphopenia. B6 mice were thymectomized in house or at the Jackson Laboratory (Bar Harbor, ME) and then subjected to 2 months of CR and lymphocyte subsets were analyzed by flow cytometry from the a spleen, b blood, c brachial and inguinal lymph nodes, and d bone marrow of the femur. Statistical differences were calculated by one-tailed Mann Whitney U tests by comparing cellularity to age-matched, AL fed mice. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. Data are representative of two independent experiments with 4–8 mice per treatment group
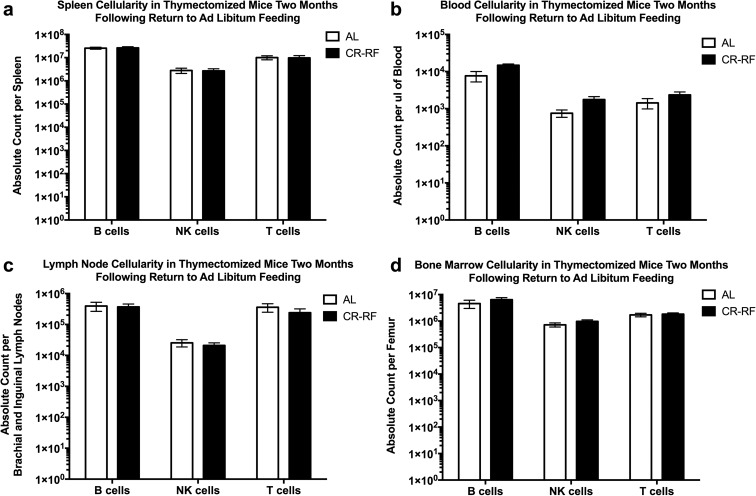
We next reverted mice back to AL food. Two months post-refeeding of ATX animals, we observed that B, NK, and T lymphocyte cellularity across the blood, spleen, lymph nodes, and bone marrow rebounded to pre-CR levels and was indistinguishable from the AL fed ATX controls (Fig. 7). These data demonstrate that T cells return to the organs and restoration of T cell homeostasis is reestablished by 2 months post-refeeding regardless of the presence of the thymus. We interpret these data to suggest that T cells contract and/or redistribute to bone marrow and/or other tissues, not surveyed by our study, during periods of a caloric deficit, and “come out of hiding” when nutritional supply is reestablished, independent from central (thymic) production. It remains to be seen to what extent homeostatic proliferation following refeeding is necessary for full restoration of homeostasis in this model of CR and refeeding.
Fig. 7.
Lymphocyte recovery is not dependent upon the thymus. Calorically restricted thymectomized mice treated as in Fig. 6 (return to AL food for 2 months after CR) were analyzed exactly as in Fig. 6. a Spleen. b Blood. c Brachial and inguinal lymph nodes. d Bone marrow of the femur. Statistical differences were calculated by two-tailed Mann Whitney U tests by comparing cellularity to age-matched, AL fed mice. Data are representative of two independent experiments with 4–8 mice per treatment group
Discussion
In this study, we report that individuals practicing long-term CR with adequate nutrition have significantly lower circulating levels of total leukocytes, neutrophils, lymphocytes, and monocytes. In middle-aged 10-month-old mice, we found that during periods of caloric restriction, the absolute number of lymphocytes within the blood, inguinal and brachial lymph nodes, and spleen is reduced in comparison to AL fed counterparts. By contrast, the bone marrow of CR animals appeared to be a site of influx, or a “safe haven” for B, NK, and T cells, the numbers of which were at least intact, if not elevated in bone marrow. Lymphocyte cellularity within all organs returned to AL levels as early as a week following refeeding. Repopulating T cells exhibited high proliferation in the CR-RF group, based on BrdU incorporation and Ki67 expression. Finally, we demonstrated that thymus was not essential for T cell repopulation following refeeding.
Based on the above observations, we propose the following model of lymphocyte migration following CR and subsequent refeeding: (1) Upon CR induction, lymphocytes leave secondary lymphoid organs (SLO) and migrate into niches such as the bone marrow that are known to protect and maintain different lymphocyte subsets (dominantly memory T and B cells) in the course of normal homeostasis. (2) The thymus also drastically loses cellularity, because early steps in the αβ T cell development (proliferation at the DN2 and DN3b/4 stages) require enormous energy expenditure. (3) Following refeeding, there is an almost instantaneous redistribution of cells back to SLO from the bone marrow and perhaps other niches. (4) In addition to this redistribution, cells arriving into SLO undergo homeostatic proliferation, presumably because the SLO environment has a relative surplus of homeostatic cytokines, chiefly IL-7, that were not used in situ due to CR-induced lymphopenia. (5) Thymus cellularity also rebounds, but thymic production of new T cells is not essential to the repopulation of SLO.
What mechanisms may be operating behind lymphocyte redistribution in the course of different phases of CR-RF? Emigration out of LN is typically mandated by downregulation of S1P1 receptor on lymphocytes and is usually associated with acquisition of chemokine receptors and integrins such as CXCR3, CCR5, and VLA-4 (Nolz et al. 2011) that target T cells to inflamed tissues. While micronutrients, and in particular vitamins A and D, have been shown to be involved in programming of lymphocytes for residence in different tissues (gut vs. skin, etc.), so far, there are no information on how a paucity of calories would influence lymphocyte migration. However, it is likely that the same mechanism is operating in the reversal of the initial redistribution and the release of lymphocytes back to the SLO, and for both of these phases, GLUT-1 or other glucose transporters and/or amino acid transporters (like CD98) could very well be involved. An alternative, and not mutually exclusive, mechanism would have it that upon refeeding, SLO stromal elements (Thompson et al. 2017) might secrete chemokines to reattract lymphocytes to SLO. Studies are currently in progress in our laboratory to discriminate between these possibilities.
An obvious question of interest in this model relates to the functional consequences of CR-induced lymphopenia on immune defense. We and others have shown that systemic sepsis (Sun et al. 2001), epithelial (Gardner 2005), and cutaneous (Goldberg et al. 2015) infections all produce substantially higher mortality in old mice on lifelong CR. Less is known about vulnerability of mid-life animals in the course of CR induction, or for that matter, animals that are not held within specific pathogen free environments (Beura et al. 2016). Certainly, hypocellular LN would delay initial priming and expansion of effector lymphocytes and that would have the potential to adversely impact protective immunity. This issue deserves urgent experimental attention and currently, we are investigating the role of broader antigen experience in functional immunity during aging.
The power of CR in extending life span has been shown in many models for nearly a century. However, difficult compliance regimens, reports of uneven effects of CR in different genetic backgrounds (Liao et al. 2010), and reports of potential adverse effects of CR upon certain organ systems in certain strains of mice, including the immune system, have all spurred intense search for other modalities of nutritional manipulation, including intermittent dieting (Johnson et al. 2013; Martin-Montalvo et al. 2013; Cheng et al. 2014; Ehninger et al. 2014; Brandhorst et al. 2015). In light of these efforts and our results above, it will be important to understand the impact of these manipulations on functional immunity, to validate appropriate modalities of CR, and to be able to properly manage induction phases of any dietary regimens that may adversely impact functional immunity.
Methods
Humans
Thirty-four individuals had been on CR for an average of 10 years (3–20 years). Subjects were instructed by an experienced research dietician to record all food and beverages consumed, preparation methods, and approximate portion sizes for 7 consecutive days. Food records were analyzed by using the NDS-R program (version 4.03_31), which is the Nutrition Data System for research from the Nutrition Coordinating Center at the University of Minnesota. CR subjects consumed a variety of nutrient-dense unprocessed foods (i.e., vegetables, fruits, nuts, egg whites, fish, poultry, low-fat dairy products, whole grains, and beans) which supplied > 100% of the Recommended Daily Intake for all essential nutrients. Refined foods rich in empty calories and trans-fatty acids were avoided. Energy intake was 30% lower in the CR group (1790 ± 225 kcal/d) than in the Western diet (WD) group (n = 58) (2540 ± 358 kcal/d) (P ≤ 0.0001). The percentage of total energy intake derived from protein, carbohydrate, fat, and alcohol was 22, 51, 27, and 0.2%, respectively, in the CR group and 17, 48, 34, and 1% in the WD group. Height was measured without shoes to the nearest 0.1 cm. Body weight and venous blood samples were taken after subjects had fasted for at least 12 h. Total body fat mass and fat free mass were determined by dual-energy X-ray absorptiometry (DXA) (QDR 4500, Hologic, Waltham, MA). The human study was approved by the Human Studies Committee of Washington University School of Medicine, and all participants gave informed consent before their participation.
Mice
All mice were C57BL/6 and were purchased from Jackson Laboratories and held under specific pathogen-free conditions in the animal facility at the University of Arizona (UA) for life. All experiments were conducted by guidelines set by the UA Institutional Animal Care and Use Committee. Thymectomies were carried out in-house (cohort 1) or at the Jackson Laboratories (cohort 2), with superimposable results between the cohorts. Upon necropsy, we validated lack of thymus in all ATX animals.
Calorie restriction
Mice were introduced to caloric restriction by first switching to the NIH-31 fortified formula in the form of 3 g pellets. In a stepwise manner, mice were acclimated to two pellets per day to allow for acclimation to the change in food access (6 g/mouse), followed by a second and third week reduction to 4.5 g/mouse and 3 g respectively. Ultimately, mice were reduced to 2.4 g a day, resulting in approximately a 40% reduction of food intake (in our hands mice averaged ~ 3.5 g of ad libitum chow a day) that translated to approximately 20% weight loss. This diet was maintained until approximately 20% weight loss, over a period of 2 months.
Leukocyte isolation
At the end of study or at selected time points, mice were euthanized by isoflurane. Spleens, brachial and inguinal lymph nodes, and thymi were collected into ice cold RPMI and accutase treated for 30 min at 37 °C and mechanically disassociated through 40 μm nylon filters and re-suspended in RPMI supplemented with 5% fetal bovine serum (FBS). Bone marrow was obtained from femur, tibia, and fibula, by clipping the ends of the bones and pushing PBS through to dislodge marrow. Marrow was then pushed through a 40-μm nylon filter with a rubber plunger of a 3-cm3 syringe and re-suspended in RPMI supplemented with 5% FBS. Blood was obtained by retro-orbital bleeding from living, anesthesia-free mice. Red blood cells were hypotonically lysed. Isolated leukocytes were then quantified on a Hemavet 950 (Drew Scientific, USA) and put into 96-well plates for flow cytometric staining.
Flow cytometry
Prior to each collection, voltages were manually calibrated to a common template using Rainbow Beads (BD Biosciences, San Jose, CA, USA), to insure accurate MFI tracking over time. Cells were treated with antibody for CD16/CD32 (BD Biosciences) and then against antibodies for CD3 (17A2), CD4 (RM4-5), CD8a (53-6.7), CD19 (6D5), Mouse I-1/I-E (M5/114.15.2), Ki67 (16A8), CD49b (DX5), CD25 (PC61) (BioLegend, USA), NK1.1 (PK136), CD44 (IM7), and CD62L (MEL-14) (ThermoFisher, USA). Staining occurred at 4 °C followed by fixation and permeabilization (BD Cytofix). Fluorescence minus one (FMO) controls were conducted to determine gating schema. Samples were run on a Fortessa Flow Cytometer equipped with four lasers and using DiVa software (BD Biosciences). Compensation and analysis were performed using FlowJo software (Tree Star, Ashland, OR, USA).
Statistics
Statistics were performed in Prism 7.0 (GraphPad Software, La Jolla, CA, USA). During experiments, analyzing the effects of CR, one-tailed Mann Whitney U tests with equal SD were carried out between cell types of CR and AL groups based upon the hypothesis that CR groups would have lower values compared to AL counterparts. Two-tailed Mann Whitney U tests with equal SD were carried out during refeeding experiments. Significance is noted as follows throughout: ns = not significant, ****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05. All error bars shown are SEM. In all cases, groups compared for significance are overlaid by a bar, and significance is denoted by the stars as described above. There were no statistically significant differences between AL and RF groups for any parameters measured.
Electronic supplementary material
Diet Modulates Leukocyte Cellularity in the Blood of Humans. Humans that were self-reported as caloric restrictors compared to age matched self-reported western diet. (XLSX 9 kb)
Body Weight Change in CR Treatment Mice. (PNG 1.14 mb)
CR-mediated Reduction in Murine Spleen Size. Spleen from CR mouse in comparison to AL controls. Representative organ images are shown. (PNG 3.09 mb)
CR-mediated Reduction in Murine Thymic Size. Thymus from CR mouse in comparison to AL controls. Representative organ images are shown. (PNG 13.9 mb)
Funding information
This study is supported by the grant AG045734 from the USPS (National Institute of Aging, NIH) to J.N-Z and grants from the Bakewell Foundation, the Longer Life Foundation (an RGA/Washington University Partnership), and the National Center for Research Resources (UL1 RR024992) to L.F. This research was also supported in part by the National Institute on Aging-Intramural Research Program; the funding agencies had no role in the analysis or interpretation of the data or in the decision to submit the report for publication.
Compliance with ethical standards
The human study was approved by the Human Studies Committee of Washington University School of Medicine, and all participants gave informed consent before their participation.
Footnotes
Nico A. Contreras and Luigi Fontana contributed equally to this work.
Electronic supplementary material
The online version of this article (10.1007/s11357-018-0022-2) contains supplementary material, which is available to authorized users.
Contributor Information
Luigi Fontana, Email: lfontana@wustl.edu.
Janko Nikolich-Žugich, nikolich@email.arizona.edu.
References
- Becklund BR, Purton JF, Ramsey C, Favre S, Vogt TK, Martin CE, Spasova DS, Sarkisyan G, LeRoy E, Tan JT, Wahlus H, Bondi-Boyd B, Luther SA, Surh CD. The aged lymphoid tissue environment fails to support naïve T cell homeostasis. Sci Rep. 2016;6:30842. doi: 10.1038/srep30842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beura LK, Hamilton SE, Bi K, Schenkel JM, Odumade OA, Casey KA, Thompson EA, Fraser KA, Rosato PC, Filali-Mouhim A, Sekaly RP, Jenkins MK, Vezys V, Haining WN, Jameson SC, Masopust D. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature. 2016;532:512–516. doi: 10.1038/nature17655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandhorst S, Choi IY, Wei M, Cheng CW, Sedrakyan S, Navarrete G, Dubeau L, Yap LP, Park R, Vinciguerra M, di Biase S, Mirzaei H, Mirisola MG, Childress P, Ji L, Groshen S, Penna F, Odetti P, Perin L, Conti PS, Ikeno Y, Kennedy BK, Cohen P, Morgan TE, Dorff TB, Longo VD. A periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and healthspan. Cell Metab. 2015;22:86–99. doi: 10.1016/j.cmet.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CW, Adams GB, Perin L, Wei M, Zhou X, Lam BS, da Sacco S, Mirisola M, Quinn DI, Dorff TB, Kopchick JJ, Longo VD. Prolonged fasting reduces IGF-1/PKA to promote hematopoietic-stem-cell-based regeneration and reverse immunosuppression. Cell Stem Cell. 2014;14:810–823. doi: 10.1016/j.stem.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Čičin-Šain L, Messaoudi I, Park B, Currier N, Planer S, Fischer M, Tackitt S, Nikolich-Zugich D, Legasse A, Axthelm MK, Picker LJ, Mori M, Nikolich-Žugich J. Dramatic increase in naive T cell turnover is linked to loss of naive T cells from old primates. Proc Natl Acad Sci. 2007;104:19960–19965. doi: 10.1073/pnas.0705905104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, et al (2009) recommendations. Ongoing full genome sequencing will monitor for the possibility of future reassortment events (39). Science (80) 201–204
- Colman RJ, Beasley TM, Kemnitz JW, Johnson SC, Weindruch R, Anderson RM. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat Commun. 2014;5:3557. doi: 10.1038/ncomms4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehninger D, Neff F, Xie K. Longevity, aging and rapamycin. Cell Mol Life Sci. 2014;71:4325–4346. doi: 10.1007/s00018-014-1677-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrando-Martínez S, Ruiz-Mateos E, Hernández A, Gutiérrez E, Rodríguez-Méndez MM, Ordoñez A, Leal M. Age-related deregulation of naive T cell homeostasis in elderly humans. Age (Omaha) 2011;33:197–207. doi: 10.1007/s11357-010-9170-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrando-Martínez S, Romero-Sánchez MC, Solana R, Delgado J, de la Rosa R, Muñoz-Fernández MÁ, Ruiz-Mateos E, Leal M. Thymic function failure and C-reactive protein levels are independent predictors of all-cause mortality in healthy elderly humans. Age (Omaha) 2013;35:251–259. doi: 10.1007/s11357-011-9341-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Partridge L, Longo VD. Extending healthy life span—from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryar CD, Carroll MD, Ogden CL (2012) Prevalence of overweight, obesity, and extreme obesity among adults: United States, trends 1960–1962 through 2009–2010
- Gardner EM. Caloric restriction decreases survival of aged mice in response to primary influenza infection. J Gerontol A Biol Sci Med Sci. 2005;60:688–694. doi: 10.1093/gerona/60.6.688. [DOI] [PubMed] [Google Scholar]
- Godfrey DI, Kennedy J, Suda T, Zlotnik A. A developmental pathway involving four phenotypically and functionally distinct subsets of CD3-CD4-CD8-triple-negative adult mouse thymocytes defined by CD44 and CD25 expression. J Immunol. 1993;150:4244–4252. [PubMed] [Google Scholar]
- Goldberg EL, Romero-Aleshire MJ, Renkema KR, Ventevogel MS, Chew WM, Uhrlaub JL, Smithey MJ, Limesand KH, Sempowski GD, Brooks HL, Nikolich-Žugich J. Lifespan-extending caloric restriction or mTOR inhibition impair adaptive immunity of old mice by distinct mechanisms. Aging Cell. 2015;14:130–138. doi: 10.1111/acel.12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambly C, Rauw W, Speakman JR (2015) Mice that gorged during dietary restriction increased foraging related behaviors and differed in their macronutrient preference when released from restriction. doi: 10.7717/peerj.1091 [DOI] [PMC free article] [PubMed]
- Heilbronn LK, Ravussin E. Calorie restriction and aging: review of the literature and implications for studies in humans. Am J Clin Nutr. 2003;78:361–369. doi: 10.1093/ajcn/78.3.361. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature. 2013;493:338–345. doi: 10.1038/nature11861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koup RA, Douek DC, McFarland RD, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- Kristan DM. Chronic calorie restriction increases susceptibility of laboratory mice (Mus musculus) to a primary intestinal parasite infection. Aging Cell. 2007;6:817–825. doi: 10.1111/j.1474-9726.2007.00345.x. [DOI] [PubMed] [Google Scholar]
- Liao C-Y, Rikke BA, Johnson TE, Diaz V, Nelson JF. Genetic variation in the murine lifespan response to dietary restriction: from life extension to life shortening. Aging Cell. 2010;9:92–95. doi: 10.1111/j.1474-9726.2009.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney LB, Denny CA, Seyfried TN. Caloric restriction in C57BL/6J mice mimics therapeutic fasting in humans. Lipids Health Dis. 2006;5:13. doi: 10.1186/1476-511X-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, Gomes AP, Ward TM, Minor RK, Blouin MJ, Schwab M, Pollak M, Zhang Y, Yu Y, Becker KG, Bohr VA, Ingram DK, Sinclair DA, Wolf NS, Spindler SR, Bernier M, de Cabo R. Metformin improves healthspan and lifespan in mice. Nat Commun. 2013;4:2192. doi: 10.1038/ncomms3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison JA, Roth GS, Beasley TM, et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489:318–321. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoudi I, Warner J, Fischer M, Park B, Hill B, Mattison J, Lane MA, Roth GS, Ingram DK, Picker LJ, Douek DC, Mori M, Nikolich-Žugich J. Delay of T cell senescence by caloric restriction in aged long-lived nonhuman primates. Proc Natl Acad Sci. 2006;103:19448–19453. doi: 10.1073/pnas.0606661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meydani SN, Das SK, Pieper CF, Lewis MR, Klein S, Dixit VD, Gupta AK, Villareal DT, Bhapkar M, Huang M, Fuss PJ, Roberts SB, Holloszy JO, Fontana L. Long-term moderate calorie restriction inhibits inflammation without impairing cell-mediated immunity: a randomized controlled trial in non-obese humans. Aging (Albany NY) 2016;8:1416–1431. doi: 10.18632/aging.100994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Most J, Tosti V, Redman LM, Fontana L. Calorie restriction in humans: an update. Ageing Res Rev. 2017;39:36–45. doi: 10.1016/j.arr.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor K, Li G, Vallejo AN, Lee WW, Koetz K, Bryl E, Witkowski J, Fulbright J, Weyand CM, Goronzy JJ. The influence of age on T cell generation and TCR diversity. J Immunol. 2005;174:7446–7452. doi: 10.4049/jimmunol.174.11.7446. [DOI] [PubMed] [Google Scholar]
- Nikolich-Žugich J. Aging of the T cell compartment in mice and humans: from no naive expectations to foggy memories. J Immunol. 2014;193:2622–2629. doi: 10.4049/jimmunol.1401174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolić-Žugić J. Phenotypic and functional stages in the intrathymic development of αβ T cells. Immunol Today. 1991;12:65–70. doi: 10.1016/0167-5699(91)90160-U. [DOI] [PubMed] [Google Scholar]
- Nolz JC, Starbeck-Miller GR, Harty JT. Naive, effector and memory CD8 T-cell trafficking: parallels and distinctions. Immunotherapy. 2011;3:1223–1233. doi: 10.2217/imt.11.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortman BJM, Velkoff VA, Hogan H (2014) An aging nation: the older population in the United States Curr Popul Reports 1964:
- Parmigiani A, Alcaide ML, Freguja R, Pallikkuth S, Frasca D, Fischl MA, Pahwa S. Impaired antibody response to influenza vaccine in HIV-infected and uninfected aging women is associated with immune activation and inflammation. PLoS One. 2013;8:e79816. doi: 10.1371/journal.pone.0079816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezzani R, Nardo L, Favero G, Peroni M, Rodella LF. Thymus and aging: morphological, radiological, and functional overview. Age (Omaha) 2014;36:313–351. doi: 10.1007/s11357-013-9564-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd BD, Venturi V, Li G, Samadder P, Ertelt JM, Way SS, Davenport MP, Nikolich-Žugich J. Nonrandom attrition of the naive CD8+ T-cell pool with aging governed by T-cell receptor: pMHC interactions. Proc Natl Acad Sci U S A. 2011;108:13694–13699. doi: 10.1073/pnas.1107594108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sempowski GD, Gooding ME, Liao HX, le PT, Haynes BF. T cell receptor excision circle assessment of thymopoiesis in aging mice. Mol Immunol. 2002;38:841–848. doi: 10.1016/S0161-5890(01)00122-5. [DOI] [PubMed] [Google Scholar]
- Sun D, Muthukumar AR, Lawrence RA, Fernandes G. Effects of calorie restriction on polymicrobial peritonitis induced by cecum ligation and puncture in young C57BL/6 mice. Clin Diagn Lab Immunol. 2001;8:1003–1011. doi: 10.1128/CDLI.8.5.1003-1011.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson HL, Smithey MJ, Surh CD, Nikolich-Žugich J. Functional and homeostatic impact of age-related changes in lymph node stroma. Front Immunol. 2017;8:706. doi: 10.3389/fimmu.2017.00706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vescovini R, Fagnoni FF, Telera AR, Bucci L, Pedrazzoni M, Magalini F, Stella A, Pasin F, Medici MC, Calderaro A, Volpi R, Monti D, Franceschi C, Nikolich-Žugich J, Sansoni P. Naïve and memory CD8 T cell pool homeostasis in advanced aging: impact of age and of antigen-specific responses to cytomegalovirus. Age (Dordr) 2014;36:625–640. doi: 10.1007/s11357-013-9594-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weindruch R, Sohal RS. Caloric intake and aging. N Engl J Med. 1997;337:986–994. doi: 10.1056/NEJM199710023371407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weindruch R, Lane MA, Ingram DK, Ershler WB, Roth GS. Dietary restriction in rhesus monkeys: lymphopenia and reduced mitogen-induced proliferation in peripheral blood mononuclear cells. Aging (Milano) 1997;9:304–308. doi: 10.1007/BF03341833. [DOI] [PubMed] [Google Scholar]
- Wing EJ, Magee DM, Barczynski LK. Acute starvation in mice reduces the number of T cells and suppresses the development of T-cell-mediated immunity. Immunology. 1988;63:677–682. [PMC free article] [PubMed] [Google Scholar]
- Yang H, Youm Y-H, Dixit VD. Inhibition of thymic adipogenesis by caloric restriction is coupled with reduction in age-related thymic involution. J Immunol. 2009;183:3040–3052. doi: 10.4049/jimmunol.0900562. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Diet Modulates Leukocyte Cellularity in the Blood of Humans. Humans that were self-reported as caloric restrictors compared to age matched self-reported western diet. (XLSX 9 kb)
Body Weight Change in CR Treatment Mice. (PNG 1.14 mb)
CR-mediated Reduction in Murine Spleen Size. Spleen from CR mouse in comparison to AL controls. Representative organ images are shown. (PNG 3.09 mb)
CR-mediated Reduction in Murine Thymic Size. Thymus from CR mouse in comparison to AL controls. Representative organ images are shown. (PNG 13.9 mb)