Abstract
In this study, we aimed to (1) identify white matter (WM) deficits underlying the consciousness level in patients with disorders of consciousness (DOCs) using diffusion tensor imaging (DTI), and (2) evaluate the relationship between DTI metrics and clinical measures of the consciousness level in DOC patients. With a cohort of 8 comatose, 8 unresponsive wakefulness syndrome/vegetative state, and 14 minimally conscious state patients and 25 patient controls, we performed group comparisons of the DTI metrics in 48 core WM regions of interest (ROIs), and examined the clinical relevance using correlation analysis. We identified multiple abnormal WM ROIs in DOC patients compared with normal controls, and the DTI metrics in these ROIs were significantly correlated with clinical measures of the consciousness level. Therefore, our findings suggested that multiple WM tracts are involved in the impaired consciousness levels in DOC patients and demonstrated the clinical relevance of DTI for DOC patients.
Electronic supplementary material
The online version of this article (10.1007/s12264-018-0253-3) contains supplementary material, which is available to authorized users.
Keywords: Disorder of consciousness, White matter, Diffusion tensor imaging, Brain injury
Introduction
With the great advances in intensive care, more and more patients survive after severe brain injuries such as traumatic brain injury (TBI), spontaneous intracerebral hemorrhage, and ischemic-hypoxic injury. This has resulted in a large population of patients with disorders of consciousness (DOCs) who exhibit varied levels of consciousness [from low to high: coma (COMA), unresponsive wakefulness syndrome/vegetative state (UWS/VS), and minimally conscious state (MCS)]. This DOC population provides a natural model in which to study the basic question of what structural neural substrates support the maintenance of a normal level of consciousness. Recent exciting advances in neuroimaging techniques allow us to tackle this question. Neuroimaging findings have consistently revolutionized our understanding of consciousness [1], and have shown the value of neuroimaging biomarkers for aiding clinical diagnosis [2].
The altered consciousness level in DOC patients is associated with disruption of the functional connections in the brain. Several functional MRI studies have revealed that the level of consciousness in DOC patients is associated with deficiencies in the functional connectivity between the default mode network (DMN) and the thalamus [3–5]. Whole-brain functional graph-theoretical network analysis has identified abnormal connectivity patterns in DOC patients [6], and the strength of functional connectivity can predict the consciousness level and recovery outcome of DOC patients [7]. Together, all this evidence suggests that consciousness in humans is an outcome of functional integration across the brain rather than local functional activity.
As WM is the structural basis of the functional connections in the brain, impairments of WM in DOC patients might provide valuable insights into the structure of WM substrates that support the maintenance of consciousness. Clinically, diffuse axonal injury patients with DOCs are often found to have WM damage that is detectable by computed tomography/MRI. Diffusion tensor imaging (DTI) is a quantitative MRI technique that has been widely used in clinical WM studies [8]. A case study of one MCS patient using DTI found an increase of WM anisotropy, which was associated with the recovery of expressive language [9]. Several cross-sectional DTI studies have shown that fiber tracts connecting the thalamus and DMN regions are associated with the consciousness levels in DOC patients [10–13]. But there has still not been a whole-brain search for WM substrates underlying the reduced consciousness levels in DOC patients. Moreover, these studies also had limitations such as (1) recruiting only a small number of individuals (making it difficult to interpret the clinical relevance of their findings) or a relatively large sample but from different scanning centers (introducing the new confounding factor of scanning center); (2) mainly covering part of the spectrum of the consciousness level in DOC patients, including UWS/VS and MCS patients but not COMA patients; (3) the absence of lesion patients with full consciousness; and (4) a focus on a limited number of pre-defined WM tracts/ ROIs or the WM as a whole.
Therefore, we aimed to (1) identify the impaired WM regions associated with the impaired consciousness level in DOC patients using DTI, and (2) explore the clinical relevance of DTI in DOC patients. Given previous findings, we hypothesized that distributed WM injuries, rather than a local WM injury, contribute to the abnormal levels of consciousness in these patients. To test this hypothesis, an atlas-based whole-brain search for abnormal WM regions using DTI was applied to a large cohort of 30 DOC patients, covering the entire spectrum of consciousness levels (COMA, UWS/VS, and MCS) as well as 25 patient controls (PCs) from a single center. We then evaluated the relationship between DTI metrics and clinical measures of the consciousness levels in DOC patients.
Materials and Methods
Participants
Ninety-one patients with acquired brain injuries in the Huashan Hospital of Fudan University in Shanghai participated in our study. Their etiology was either TBI (77 patients) or non-TBI (spontaneous intracerebral hemorrhage or ischemic-hypoxic injury; 14 patients). All patients had an MRI scan during the sub-acute or chronic stage, and they were followed up either in the rehabilitation hospital or at re-admission for a ventricular peritoneal shunt with hydrocephalus and/or cranioplasty. Informed consent was given by each patient or his/her family. Ethical approval for this study was granted by the Ethics Committee of Huashan Hospital.
The patients were clinically diagnosed as COMA, UWS/VS, MCS, or PC on the day of their MRI scan. The COMA patients were characterized by the absence of arousal responses and awareness [14]. The UWS/VS patients were in a state of arousal, including a sleep/wake cycle, but were unaware of themselves or their global environment [15]. The MCS patients retained a low level of consciousness but exhibited inconsistent and non-reflexive behavior [16]. The PCs were characterized by (1) a conscious state and communicability; (2) a brain injury confirmed by MRI and computed tomography scans; and (3) the absence of locked-in syndrome. The consciousness level of each patient was quantified using two standardized scales: the Glasgow Coma Scale (GCS) [17] and the Coma Recovery Scale-Revised (CRS-R) [18].
After manually checking the quality of the widely-used DTI metric—fractional anisotropy (FA) images after normalization (exclusion examples are shown in Fig. S1) from 55 patients (8 COMA, 8 UWS/VS, 14 MCS, and 25 PC) were included in the statistical analysis. The detailed demographic and clinical characteristics are summarized in Table S1 and Fig. S2.
Diffusion Weighted MR Image Acquisition
All diffusion MRI images were acquired on the 3T SIEMENS MRI scanner in the Huashan Hospital, Shanghai. We used a single-shot echoplanar imaging sequence, and the acquisition protocol consisted of 12 non-linear diffusion-weighted directions with b = 1000 s/mm2 and one additional image without diffusion weighting (i.e., b = 0 s/mm2). The scanning parameters were set as follows: 3.5 mm slice thickness, no gap between slices, 38 slices covering the whole brain, echo time = 82 ms, repetition time = 8400 ms, acquisition matrix = 128 × 128, non-interpolated voxel size = 1.8 × 1.8 × 3.5 mm3, flip angle = 90°, and field of view = 230 × 230 mm2.
DTI Post-processing
The diffusion-weighted images were processed with a PANDA pipeline toolbox [19] called the FMRIB Software Library (version 4.1.9) [20] for skull stripping, eddy-current correction, tensor fitting, and the calculation of diffusion tensor metrics as well as spatial normalization. Common DTI metrics—FA, axial diffusivity (AD), and radial diffusivity (RD)—were chosen for subsequent analysis. Specifically, FA is the fraction of anisotropic diffusion, and a breakdown in WM integrity typically results in a lower FA [8]. AD and RD are thought to be selectively sensitive to specific micro-structural changes: an abnormal AD is thought to reflect axonal damage or loss, whereas an increased RD reflects demyelination [21]. Another common diffusion parameter, mean diffusivity, was not included because it is linearly dependent on AD and RD. After nonlinear normalization of the FA, AD, and RD maps to the FMRIB58 FA template, we extracted the FA, AD, and RD of the 48 WM regions of interest (ROIs) defined in the standard space of the ICBM-DTI-81 WM atlas [22].
In the present study, we chose the ROI-based analysis of DTI metrics primarily for two reasons. First, ROI-based analysis has a higher tolerance for inaccurate normalization than voxel-based analysis [23]. Second, it is biologically plausible to assume diffuse WM impairment following traumatic or ischemic/hypoxic injury rather than a very local and concentrated impairment. In addition, because outer brain tissues may be severely damaged in TBI patients, we defined the WM regions according to the “core white matter” atlas [22]. The ICBM-DTI-81 WM atlas consists of 48 core WM tracts in MNI152 space that are located relatively deep inside the brain and therefore are less deformed in DOC patients (Fig. S3). In all, an ROI-based analysis not only provided adequate spatial specificity and but also improved the statistical power of our study.
Statistical Analysis
All statistical analyses were performed with IBM SPSS 2.0 software. We first compared the demographic and clinical data across the four patient groups. One-way analysis of variance (ANOVA) was used for the continuous variables (age and days post-ictus) and the χ2 test for the categorical variables (etiology and gender).
To explore the association between WM deficits and consciousness level, we first identified WM deficits using one-way analysis of covariance (ANCOVA) on the mean FA of each ROI. Specifically, group was a between-subjects factor of interest, and age, gender, and days post-ictus were included as covariates. The Bonferroni method was applied to correct for multiple comparisons across the WM regions, and a corrected P < 0.05 was considered to be significant. To identify whether the FA changes were attributed to AD and/or RD changes, we applied the same ANCOVA model to the mean AD and RD of the WM deficits detected by FA ANCOVA.
Among the WM deficits detected by FA ANCOVA, we further explored the relationship between DTI metrics and clinical measures of the consciousness level in DOC patients. We first calculated Pearson correlations between the mean FA and the clinical scores of consciousness level (GCS and CRS-R) after controlling for age, gender, and days post-ictus. Similar correlation analyses were applied to the AD and RD of the WM deficits that had significant group effects in AD or RD. Notably, a substantial proportion of the patients (mainly the PC and UWS/VS patients) received ceiling-level scores (GCS score = 15 or CRS-R score = 23), which might bias the correlation results. To exclude this effect (the correlation might be driven by the high GCS and CRS-R scores at the upper end), we further calculated the correlations after excluding the patients with ceiling-level scores.
Results
Clinical Samples
There were no differences among the four patient groups (COMA, UWS/VS, MCS, and PC) in age, gender, or etiology distribution (Table 1). The duration of illness (days post-ictus) was significantly different (P = 0.009).
Table 1.
Demographics and clinical characteristics of all DOC patients.
| Diagnostic categories | COMA (n = 8) |
UWS/VS (n = 8) |
MCS (n = 14) |
PC (n = 25) |
Statistic | P |
|---|---|---|---|---|---|---|
| Age: mean ± SD | 42.3 ± 8.7 | 46.4 ± 13.9 | 43.1 ± 16.7 | 38.2 ± 14.5 | F3,51 = 0.79 | 0.506 |
| Gender: male/female | 6/2 | 3/5 | 11/3 | 18/7 | χ2 = 4.56 | 0.207 |
| Etiology: TBI/non-TBI | 8/0 | 6/2 | 13/1 | 22/3 | χ2 = 2.85 | 0.416 |
| Days post-ictus: median (range) | 20 (13–42) | 96 (10–182) | 20.5 (15–98) | 58 (4–167) | F3,51 = 4.33 | 0.009 |
There were no differences among the four DOC groups in age at scan, gender, or etiology distribution. The days post-ictus differed among the four groups. The etiology of each non-TBI patient was either spontaneous intracerebral hemorrhage or ischemic-hypoxic injury.
Group Differences in Diffusion Parameters Across DOC Patients
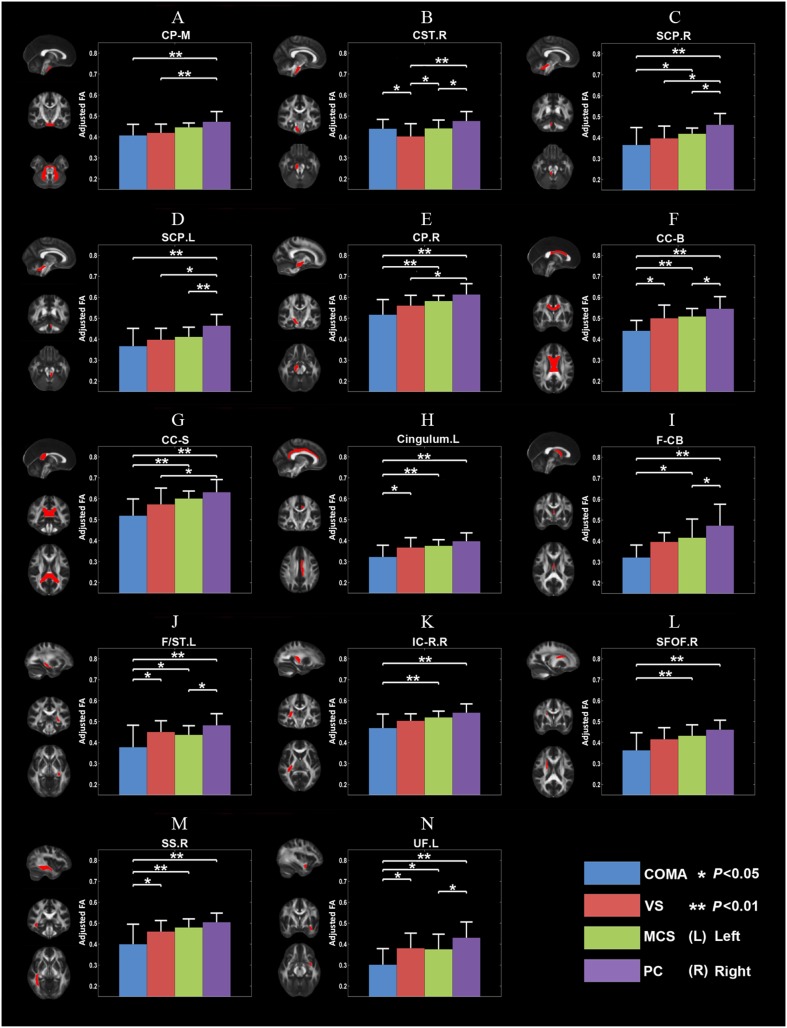
The ANCOVA of FA revealed significant group differences in 14 WM ROIs (Table 2 and Fig. 1). For most of these ROIs, post hoc comparisons indicated that FA decreased as the consciousness level declined. Notably, the significant group effect might be mainly driven by the differences between the COMA and PC patients (e.g., the body of the corpus callosum).
Table 2.
WM deficits in DOCs using ANCOVA.
| DTI metric | WM region of interest | F 3,48 | P |
|---|---|---|---|
| FA | Middle cerebellar peduncle | 6.348 | 0.001 |
| Right corticospinal tract | 6.549 | 0.0008 | |
| Right superior cerebellar peduncle | 7.640 | 0.0003 | |
| Left superior cerebellar peduncle | 7.626 | 0.0003 | |
| Right cerebral peduncle | 8.413 | 0.0001 | |
| Body of corpus callosum | 8.675 | 0.0001 | |
| Splenium of corpus callosum | 7.332 | 0.0004 | |
| Left cingulum | 7.051 | 0.0005 | |
| Fornix (column and body) | 6.642 | 0.0008 | |
| Left stria terminalis | 6.569 | 0.0008 | |
| Right internal capsule (retrolenticular part) | 6.543 | 0.0008 | |
| Right superior fronto-occipital fasciculus | 6.893 | 0.0006 | |
| Right sagittal stratum | 7.885 | 0.0002 | |
| Left uncinate fasciculus | 6.834 | 0.0006 | |
| AD | Right cerebral peduncle | 2.830 | 0.048 |
| Splenium of corpus callosum | 2.976 | 0.041 | |
| Left stria terminalis | 3.888 | 0.014 | |
| RD | Right superior cerebellar peduncle | 5.270 | 0.003 |
| Left superior cerebellar peduncle | 4.435 | 0.008 | |
| Right cerebral peduncle | 4.041 | 0.012 | |
| Body of corpus callosum | 5.305 | 0.003 | |
| Splenium of corpus callosum | 5.512 | 0.002 | |
| Left cingulum | 5.287 | 0.003 | |
| Fornix (column and body) | 3.529 | 0.022 | |
| Left stria terminalis | 5.136 | 0.004 | |
| Right internal capsule (retrolenticular part) | 4.386 | 0.008 | |
| Right sagittal stratum | 6.910 | 0.001 | |
| Left uncinate fasciculus | 5.003 | 0.004 |
Fig. 1.
Fourteen abnormal WM ROIs detected with FA ANCOVA. The mean FA differed across the levels of consciousness identified by the group comparisons (corrected P < 0.05, Bonferroni correction). In each subfigure, the left column shows a WM ROI in standard space, with the corresponding brain areas indicated in red, and the graph on the right shows the adjusted mean and standard deviation of the FA of the corresponding WM ROI for the four DOC subgroups. *P < 0.05, **P < 0.01. CC-B, body of corpus callosum; CC-S, splenium of corpus callosum; Cingulum L, left cingulum; CP-M, middle cerebellar peduncle; CP.R, right cerebral peduncle; CST.R, right corticospinal tract; F/ST.L, left fornix/stria terminalis; F-CB, fornix (column and body part); IC-R.R, right retrolenticular part of internal capsule; MPFC, medial prefrontal cortex; SCP.L, left superior cerebellar peduncle; SCP.R, right superior cerebellar peduncle; SFOF.R, right superior fronto-occipital fasciculus; SS.R, right sagittal stratum; UF.L, left uncinate fasciculus.
Among the 14 WM ROIs detected by FA ANCOVA, there were significant group differences in AD in only three WM ROIs (Table 2 and Fig. S4), while RD significantly differed in 11 (Table 2 and Fig. S5). As the level of consciousness decreased, RD in the 3/11 WM ROIs increased. In the 3 WM ROIs that had AD differences across DOC subgroups, AD decreased as the level of consciousness declined in both the right cerebellar peduncle and the splenium of the corpus callosum but increased as the level of consciousness declined in the left fornix/stria terminalis.
Correlations Between DTI Metrics and GCS/CRS-R Clinical Scores
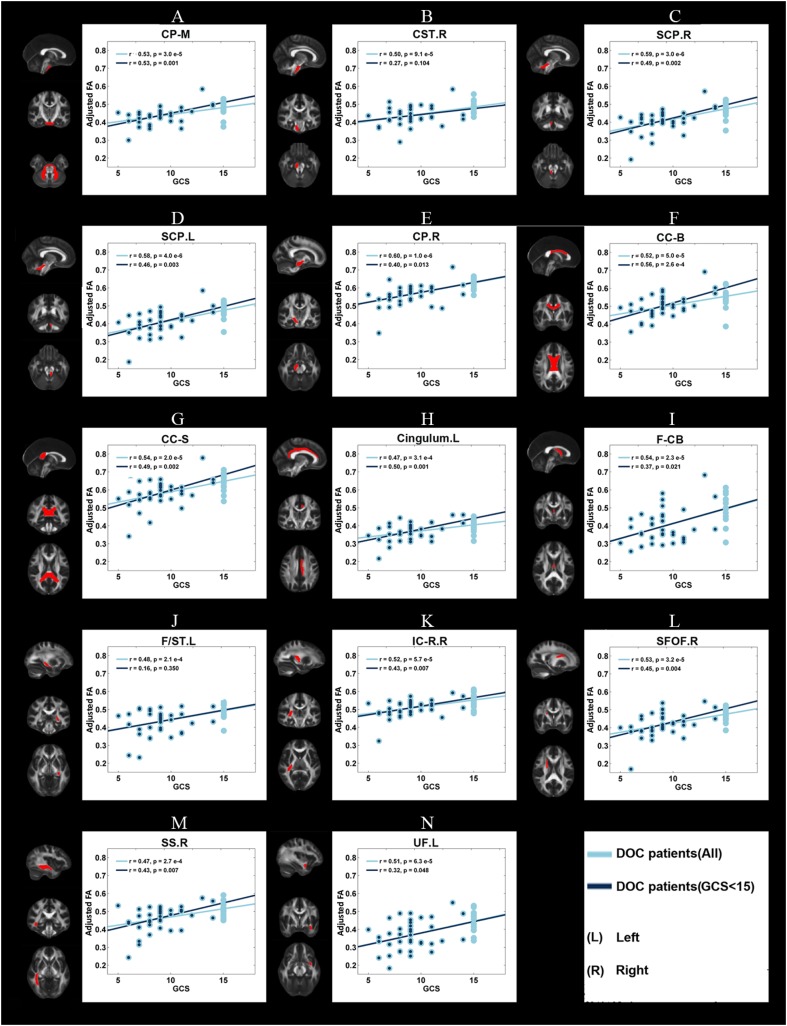
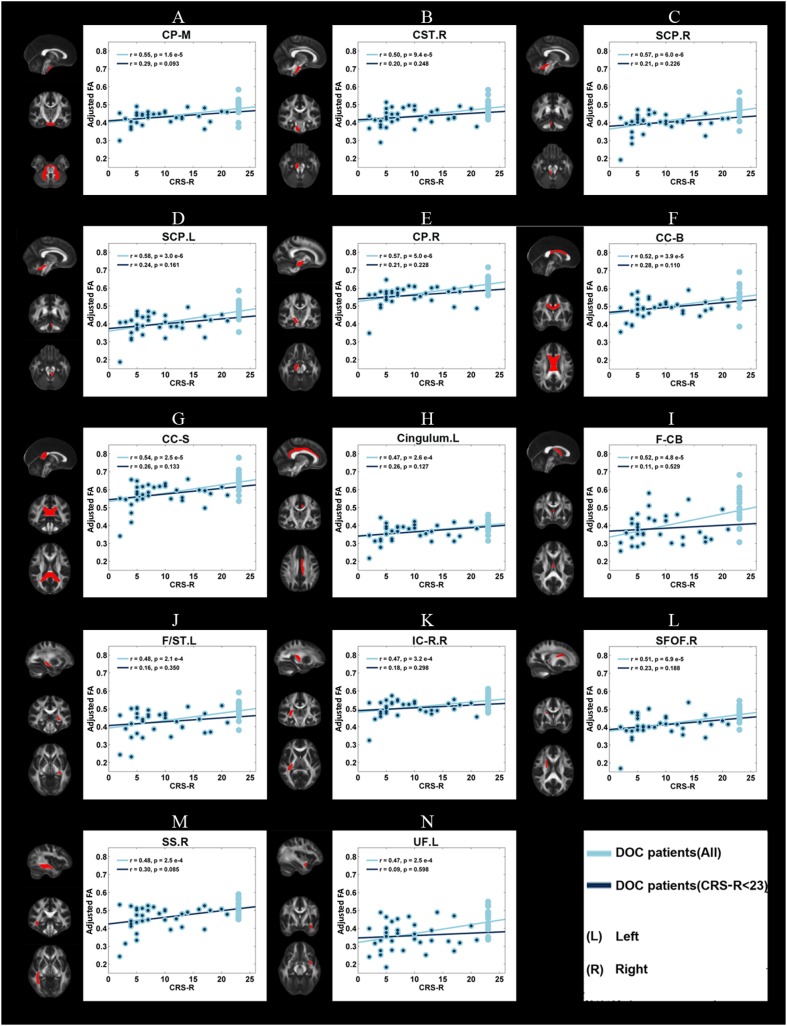
In DOC patients, significant correlations (P < 0.05) were found between the GCS/CRS-R scores and the mean FA of the 14 WM ROIs (as described above) (Figs. 2, 3). Even after the patients with GCS scores of 15 were excluded, the mean FA in 12 WM ROIs was still correlated with GCS scores. In contrast, no correlations were found between the mean FA of these regions and CRS-R scores after the patients with a CRS-R score of 23 were excluded.
Fig. 2.
Correlations between the adjusted mean FA and the clinical measure of consciousness level, GCS score, for each of the 14 WM ROIs. Light blue dots, adjusted mean FA of all DOC patients; dark blue dots, adjusted mean FA of DOC patients after exclusion of those with a GCS score of 15 (see legend of Fig. 1 for abbreviations).
Fig. 3.
Correlations between the adjusted mean FA and the clinical measure of consciousness level, CRS-R score, for each of the 14 WM ROIs. Light blue dots, adjusted mean FA of all DOC patients; dark blue dots, mean adjusted FA of DOC patients after exclusion of those with a CRS-R score of 23 (see legend of Fig. 1 for abbreviations).
For the three WM ROIs with AD differences, the mean AD was correlated with the GCS and CRS-R scores across all patients, but no correlation remained after the patients with ceiling-level scores (GCS or CRS-R) were excluded (Fig. S6 and Fig. S7). In the 11 WM ROIs with RD differences, the mean RD correlated with the GCS and CRS-R scores. After exclusion of the patients with maximum GCS and CRS-R scores, the mean RD of eight WM ROIs [body of the corpus callosum, splenium of the corpus callosum, left cingulum, fornix (column and body), left stria terminalis, right internal capsule (retrolenticular portion), right sagittal stratum, and left uncinate fasciculus] were still correlated with the GCS score. However, the CRS-R score did not significantly correlate with the mean RD of any region (Fig. S8 and Fig. S9).
Taken together, our data confirmed the value of DTI metrics in WM deficits for aiding the clinical evaluation of the consciousness level from the lowest (COMA) to the medium (UWS/VS), higher (MCS), and highest (PC). Even after controlling for the ceiling effect, most of the correlations remained the same, confirming that they were driven by the differences across the spectrum of consciousness, from COMA to PC.
Discussion
To our knowledge, this research is the first to demonstrate the involvement of widespread WM deficits in alterations of the consciousness level in DOC patients. The DTI metrics of these WM deficits were correlated with the clinical assessments of the consciousness level (GCS and CRS-R scores). All these together suggested an important role of WM in the maintenance of normal consciousness and the clinical value of DTI in aiding assessment of the consciousness levels in DOC patients.
Widespread White Matter Deficits in DOC Patients
By searching the entire “core WM” in the brain, we identified 14 WM regions in which the FA differed across levels of consciousness using ANCOVA. The lower the FA is, the worse the reduction in the consciousness level is. FA is often referred to as a marker of fiber integrity in WM. Therefore, the abnormalities in FA suggested a widespread structural WM substrate associated with the impairment of consciousness in DOC patients. Moreover, in most of these WM deficits, RD increased as the consciousness level declined across the DOC subgroups, which suggested that axonal demyelination [24] is the dominant process in the WM of DOC patients.
In terms of biological validity, the right internal capsule (retrolenticular part) originates from the lateral geniculate nucleus of the thalamus, and the cingulum is a key fiber tract within the DMN because it connects the posterior cingulate cortex/precuneus and medial prefrontal cortex. The sagittal stratum is a major cortico-subcortical WM bundle between the parietal, occipital, cingulate, and temporal regions with the thalamus. All these findings are compatible with a recent study showing significant differences between WM tracts of the thalamus and DMN brain regions in VS and MCS patients [10], and the abnormalities in these WM tracts may provide anatomical substrates for the deficiencies in thalamo-cortical functional connectivity in DOCs. In terms of the relationship with functional activity, the WM deficits revealed in our study are also in accordance with the deficits of functional connections in DOC studies [3–5] that are mainly located within the DMN and thalamus-cortical connectivity.
In addition to these tracts, we identified abnormalities in parts of the callosal commissure, the splenium and body of the corpus callosum, suggesting that the impaired consciousness in DOCs is associated with the disruption of interactions between the two hemispheres. This provides a structural basis for the disrupted functional connectivity between the hemispheres demonstrated in a previous study using functional MRI [13].
Moreover, the abnormalities in the uncinate fasciculus and fornix as well as in the stria terminalis suggested a potential role of the hippocampus and amygdala in the maintenance of consciousness. The uncinate fasciculus is the WM tract connecting the hippocampus and the amygdala [25]; the fornix is the major pathway for the output of the hippocampus, and carries some afferent fibers to the hippocampus from structures in the diencephalon and the basal forebrain; and the stria terminalis is the fiber pathway between the amygdala and the septal nuclei, hypothalamic, and thalamic areas. The findings in our study matched well with the known roles of the hippocampus, amygdala, and hypothalamus/thalamus in consciousness. The fornix has also been shown to be severely impaired in DOC patients [26].
The abnormalities in the superior cerebellar peduncle were unexpected. The superior cerebellar peduncle is a paired WM structure that connects the cerebellum and the midbrain. It consists of efferent fibers (cerebellothalamic and cerebellorubral tracts), and an afferent tract (ventral spinocerebellar tract). The role of the superior cerebellar peduncle in consciousness remains unclear.
Roles of the White Matter Around the Brainstem in Consciousness
The brainstem is crucial for regulating the sleep cycle, respiratory function, cardiovascular activities, and alertness. All these are essential for the maintenance of consciousness in humans [27–30]. Intriguingly, our results showed that four WM tracts around the brainstem were associated with the impairment of consciousness in DOC patients: the middle cerebellar peduncle, right corticospinal tract, right superior cerebellar peduncle, and right cerebral peduncle. This was also reported in a TBI study showing that brainstem WM integrity is associated with loss of consciousness [31]. Our findings provide further evidence of the importance of the brainstem in maintaining normal consciousness. Since the WM around the brainstem serves as the main pathway linking peripheral organs and the cerebellum with the cerebral cortex, our findings suggest that the disruption of information flow might occur not only within the brain but also between the brain and the rest of the body. A plausible explanation would be that the interactions between the central and peripheral nervous systems concerning the surrounding environment are disrupted in DOC patients. This suggests a key role of these non-cerebral structures in consciousness, and deserves further investigation.
Correlations Between DTI Metrics and Consciousness Scales
With increasing numbers of patients surviving after severe brain injuries, it is important to accurately assess the level of consciousness in DOC patients. GCS and CRS-R are two of the most popular clinical scales for assessing the consciousness level. In clinical practice, the accuracy of evaluating these consciousness scales is greatly limited by the experience of the person who conducts the evaluations. Improving the accuracy of assessing the consciousness level in DOC patients is key to making timely therapeutic plans and legal or ethical decisions [32]. Therefore, sensitive and robust neuroimaging biomarkers will greatly benefit and aid clinical practice [33].
Here, we demonstrated correlations between DTI metrics and consciousness scales (both GCS and CRS-R) in DOC patients. Even after excluding those participants with the highest value on the consciousness scales, most of the correlations between GCS and DTI metrics still survived. This highlighted the value of DTI in aiding the accurate assessment of the consciousness level in DOC patients. However, it is worth mentioning that most of the correlations between CRS-R and DTI metrics did not survive after we excluded the participants who had the highest CRS-R score (CRS-R = 23). This might be caused by the distribution of the CRS-R, which was much less even than that of the GCS in our cohort. Due to the much wider value range of CRS-R than GCS (maximum GCS = 15), even though our study had a relatively large cohort of DOC patients, we may still need a much larger cohort with a more evenly distributed CRS-R to understand this ceiling effect on correlations between DTI metrics and CRS-R. Nevertheless, these correlations between consciousness scales and DTI metrics in those WM deficits identified in FA ANCOVAs further confirmed our findings that DOC patients had widespread WM deficits.
Limitations and Future Directions
There are a few issues that should be addressed in future. First, although the sample size of DOC patients in the ANCOVA was relatively large, further studies with more patients in each group are needed to validate and confirm our findings. Second, the DTI technique is incapable of quantifying complex micro-structural changes in voxels with crossing fibers or partial volume effects and therefore may provide false-negative or false-positive results. Although the tensor model only needs 6 non-linear gradient directions plus 1 non-diffusion-weighting image to be estimated, the number of diffusion-weighted gradient directions in this study was relatively small for advanced analysis like tractography. To overcome these issues, a more up-to-date diffusion MRI acquisition protocol and advanced diffusion MRI techniques such as diffusion spectrum imaging [34] should be considered. Also, it might be useful to eliminate the potential free-water contamination in the fornix with high-resolution data using post-processing methods, such as super-resolution track density imaging. This method has been validated in animal data and provides enhanced tissue contrast [35]. In addition, the clinical diagnosis of DOC patients is still largely based on behavioral observations, and this can lead to a high misdiagnosis rate (up to 40%) [36]. Therefore, an interesting follow-up question would be “Can these structural substrates be used as potential targets for aiding the clinical diagnosis and even predicting the recovery outcomes in DOC patients?”. Finally, in the current study, we only identified abnormal WM regions and it would be interesting to quantify the contribution of the abnormalities in each specific WM tract to the impaired consciousness level in DOC patients.
Conclusions
By comparing different subgroups of DOC patients and a PC group with preserved consciousness using DTI, we identified widespread WM deficits in DOC patients, and DTI metrics in the abnormal WM areas correlated with clinical assessments of the consciousness level. These findings provide novel insights into the roles of WM in consciousness and indicate that DTI might be a useful addition to the clinical assessment of the consciousness level in DOC patients in the future.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the Natural Science Foundation of China (81571025), International Cooperation Project from Shanghai Science Foundation (18410711300), the National Science Foundation for Distinguished Young Scholars of China (81025013), National Basic Research Development Program (973 Program) of China (2012CB720700, 2010CB945500, 2012CB966300, and 2009CB941100), the National Natural Science Foundation of China (81322021), the Beijing Nova Program (Z121110002512032), the Project for National 985 Engineering of China (985III-YFX0102), the “Dawn Tracking” Program of Shanghai Education Commission (10GG01), the Shanghai Natural Science Foundation (08411952000 and 10ZR1405400), the National Natural Science Young Foundation in China (81201033), the grants of Shanghai Health Bureau (20114358), the National High-Technology Development Project (863 Project) of China (2015AA020501), the Program for New Century Excellent Talents in University of China (NCET-10-0356), and the National Program for the Support of Top-Notch Young Professionals. Dr. Georg Northoff is supported by the Michael Smith Foundation, the CRC, and the CIHR. Jiaying Zhang is supported by the China Scholarship Council.
Compliance with ethical standards
Conflict of interest
All authors claim that there are no conflicts of interest.
Footnotes
Xuehai Wu and Jiaying Zhang have contributed equally to this work.
Electronic supplementary material
The online version of this article (10.1007/s12264-018-0253-3) contains supplementary material, which is available to authorized users.
Contributor Information
Gaolang Gong, Email: gaolang.gong@bnu.edu.cn.
Ying Mao, Email: maoying@fudan.edu.cn.
References
- 1.Owen AM, Coleman MR, Boly M, Davis MH, Laureys S, Pickard JD. Detecting awareness in the vegetative state. Science. 2006;313:1402. doi: 10.1126/science.1130197. [DOI] [PubMed] [Google Scholar]
- 2.Giacino JT, Fins JJ, Laureys S, Schiff ND. Disorders of consciousness after acquired brain injury: the state of the science. Nat Rev Neurol. 2014;10:99–114. doi: 10.1038/nrneurol.2013.279. [DOI] [PubMed] [Google Scholar]
- 3.Boly M, Tshibanda L, Vanhaudenhuyse A, Noirhomme Q, Schnakers C, Ledoux D, et al. Functional connectivity in the default network during resting state is preserved in a vegetative but not in a brain dead patient. Hum Brain Mapp. 2009;30:2393–2400. doi: 10.1002/hbm.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boly M, Faymonville M-E, Schnakers C, Peigneux P, Lambermont B, Phillips C, et al. Perception of pain in the minimally conscious state with PET activation: an observational study. Lancet Neurol. 2008;7:1013–1020. doi: 10.1016/S1474-4422(08)70219-9. [DOI] [PubMed] [Google Scholar]
- 5.Vanhaudenhuyse A, Noirhomme Q, Tshibanda LJ-F, Bruno M-A, Boveroux P, Schnakers C, et al. Default network connectivity reflects the level of consciousness in non-communicative brain-damaged patients. Brain. 2010;133:161–171. doi: 10.1093/brain/awp313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qin P, Wu X, Huang Z, Duncan NW, Tang W, Wolff A, et al. How are different neural networks related to consciousness? Ann Neurol. 2015;78:594–605. doi: 10.1002/ana.24479. [DOI] [PubMed] [Google Scholar]
- 7.Wu X, Zou Q, Hu J, Tang W, Mao Y, Gao L, et al. Intrinsic functional connectivity patterns predict consciousness level and recovery outcome in acquired brain injury. J Neurosci. 2015;35:12932–12946. doi: 10.1523/JNEUROSCI.0415-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- 9.Voss HU, Uluğ AM, Dyke JP, Watts R, Kobylarz EJ, McCandliss BD, et al. Possible axonal regrowth in late recovery from the minimally conscious state. J Clin Invest. 2006;116:2005–2011. doi: 10.1172/JCI27021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernández-Espejo D, Soddu A, Cruse D, Palacios EM, Junque C, Vanhaudenhuyse A, et al. A role for the default mode network in the bases of disorders of consciousness. Ann Neurol. 2012;72:335–343. doi: 10.1002/ana.23635. [DOI] [PubMed] [Google Scholar]
- 11.Fernández-Espejo D, Bekinschtein T, Monti MM, Pickard JD, Junque C, Coleman MR, et al. Diffusion weighted imaging distinguishes the vegetative state from the minimally conscious state. Neuroimage. 2011;54:103–112. doi: 10.1016/j.neuroimage.2010.08.035. [DOI] [PubMed] [Google Scholar]
- 12.Lant ND, Gonzalez-Lara LE, Owen AM, Fernández-Espejo D. Relationship between the anterior forebrain mesocircuit and the default mode network in the structural bases of disorders of consciousness. Neuroimage Clin. 2015;10:27–35. doi: 10.1016/j.nicl.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newcombe VFJ, Williams GB, Scoffings D, Cross J, Carpenter TA, Pickard JD, et al. Aetiological differences in neuroanatomy of the vegetative state: insights from diffusion tensor imaging and functional implications. J Neurol Neurosurg Psychiatr. 2010;81:552–561. doi: 10.1136/jnnp.2009.196246. [DOI] [PubMed] [Google Scholar]
- 14.Posner JB, Plum F. Plum and Posner’s Diagnosis of Stupor and Coma. 4. New York: Oxford University Press; 2007. [Google Scholar]
- 15.Multi-Society Task Force on PVS Medical aspects of the persistent vegetative state. New Engl J Med. 1994;330:1572–1579. doi: 10.1056/NEJM199406023302206. [DOI] [PubMed] [Google Scholar]
- 16.Giacino JT, Ashwal S, Childs N, Cranford R, Jennett B, Katz DI, et al. The minimally conscious state: definition and diagnostic criteria. Neurology. 2002;58:349–353. doi: 10.1212/WNL.58.3.349. [DOI] [PubMed] [Google Scholar]
- 17.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–84. doi: 10.1016/S0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 18.Giacino JT, Kalmar K, Whyte J. The JFK coma recovery scale-revised: measurement characteristics and diagnostic utility. Arch Phys Med Rehabil. 2004;85:2020–2029. doi: 10.1016/j.apmr.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 19.Cui Z, Zhong S, Xu P, Gong G, He Y. PANDA: a pipeline toolbox for analyzing brain diffusion images. Front Hum Neurosci. 2013;7:42. doi: 10.3389/fnhum.2013.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 21.Winklewski PJ, Sabisz A, Naumczyk P, Jodzio K, Szurowska E, Szarmach A. Understanding the physiopathology behind axial and radial diffusivity changes—What do we know? Front Neurol. 2018;9:92. doi: 10.3389/fneur.2018.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage. 2008;40:570–582. doi: 10.1016/j.neuroimage.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faria AV, Zhang J, Oishi K, Li X, Jiang H, Akhter K, et al. Atlas-based analysis of neurodevelopment from infancy to adulthood using diffusion tensor imaging and applications for automated abnormality detection. Neuroimage. 2010;52:415–428. doi: 10.1016/j.neuroimage.2010.04.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20:1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Kier EL, Staib LH, Davis LM, Bronen RA. MR imaging of the temporal stem: anatomic dissection tractography of the uncinate fasciculus, inferior occipitofrontal fasciculus, and Meyer’s loop of the optic radiation. Am J Neuroradiol. 2004;25:677–691. [PMC free article] [PubMed] [Google Scholar]
- 26.Burzynska AZ, Jiao Y, Knecht AM, Fanning J, Awick EA, Chen T, et al. White matter integrity declined over 6-months, but dance intervention improved integrity of the fornix of older adults. Front Aging Neurosci. 2017;9:59. doi: 10.3389/fnagi.2017.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol. 1949;1:455–473. doi: 10.1016/0013-4694(49)90219-9. [DOI] [PubMed] [Google Scholar]
- 28.Northoff G. Unlocking the Brain: Volume 2: Consciousness. New York: Oxford University Press; 2014. [Google Scholar]
- 29.Parvizi J, Damasio AR. Neuroanatomical correlates of brainstem coma. Brain. 2003;126:1524–1536. doi: 10.1093/brain/awg166. [DOI] [PubMed] [Google Scholar]
- 30.Starzl TE, Taylor CW, Magoun HW. Ascending conduction in reticular activating system, with special reference to the diencephalon. J Neurophysiol. 1951;14:461–477. doi: 10.1152/jn.1951.14.6.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delano-Wood L, Bangen KJ, Sorg SF, Clark AL, Schiehser DM, Luc N, et al. Brainstem white matter integrity is related to loss of consciousness and postconcussive symptomatology in veterans with chronic mild to moderate traumatic brain injury. Brain Imaging Behav. 2015;9:500–512. doi: 10.1007/s11682-015-9432-2. [DOI] [PubMed] [Google Scholar]
- 32.Monti MM, Vanhaudenhuyse A, Coleman MR, Boly M, Pickard JD, Tshibanda L, et al. Willful modulation of brain activity in disorders of consciousness. New Engl J Med. 2010;362:579–589. doi: 10.1056/NEJMoa0905370. [DOI] [PubMed] [Google Scholar]
- 33.Laureys S, Schiff ND. Coma and consciousness: Paradigms (re)framed by neuroimaging. Neuroimage. 2012;61:478–491. doi: 10.1016/j.neuroimage.2011.12.041. [DOI] [PubMed] [Google Scholar]
- 34.Wedeen VJ, Wang RP, Schmahmann JD, Benner T, Tseng WYI, Dai G, et al. Diffusion spectrum magnetic resonance imaging (DSI) tractography of crossing fibers. Neuroimage. 2008;41:1267–1277. doi: 10.1016/j.neuroimage.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 35.Dai JK, Wang SX, Shan D, Niu HC, Lei H. Super-resolution track-density imaging reveals fine anatomical features in tree shrew primary visual cortex and hippocampus. Neurosci Bull. 2017;1:1–11. doi: 10.1007/s12264-017-0199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schnakers C, Vanhaudenhuyse A, Giacino J, Ventura M, Boly M, Majerus S, et al. Diagnostic accuracy of the vegetative and minimally conscious state: clinical consensus versus standardized neurobehavioral assessment. BMC Neurol. 2009;9:35. doi: 10.1186/1471-2377-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.