Abstract
A number of studies have indicated that disorders of consciousness result from multifocal injuries as well as from the impaired functional and anatomical connectivity between various anterior forebrain regions. However, the specific causal mechanism linking these regions remains unclear. In this study, we used spectral dynamic causal modeling to assess how the effective connections (ECs) between various regions differ between individuals. Next, we used connectome-based predictive modeling to evaluate the performance of the ECs in predicting the clinical scores of DOC patients. We found increased ECs from the striatum to the globus pallidus as well as from the globus pallidus to the posterior cingulate cortex, and decreased ECs from the globus pallidus to the thalamus and from the medial prefrontal cortex to the striatum in DOC patients as compared to healthy controls. Prediction of the patients’ outcome was effective using the negative ECs as features. In summary, the present study highlights a key role of the thalamo-basal ganglia-cortical loop in DOCs and supports the anterior forebrain mesocircuit hypothesis. Furthermore, EC could be potentially used to assess the consciousness level.
Keywords: Mesocircuit, Basal ganglia, Posterior cingulate cortex, Spectral dynamic causal modeling, Connectome-based predictive modeling
Introduction
Disorders of consciousness (DOCs) have always been difficult to understand; yet studies have advanced our understanding of both responsiveness and consciousness after severe brain injuries. Recent neuroimaging studies have made much progress in this field and have consistently revealed that impaired consciousness is associated with damaged mid-line regions of the frontal and parietal cortices [1–4] as well as abnormal thalamus- and basal ganglia-modulated connectivity [5–7]. Based on the evidence that traumatic brain injuries in these subcortical and cortical regions as well as in their complex interactions may cause impaired consciousness, a mesocircuit hypothesis [8] has been proposed to predict the dynamic process that occurs in the anterior forebrain during the restoration of consciousness. According to the circuit-level mechanism, DOCs can be attributed to widespread reductions in the thalamocortical and thalamostriatal outflows followed by a failure in the firing state of the striatum. These events result in excessive inhibition of the thalamus and then the cortex [9].
The thalamus acts as a transfer station that transmits information through its broad connectivity [10] between the cortex and subcortical nuclei (the basal ganglia, for example) [11–15]. The thalamo-frontal circuit [16–18] as well as the cortico-thalamo-basal ganglia-cortical circuit [8, 16] are crucial in the recovery from DOCs, and atrophy of the thalamus has been found to be proportional to the severity of DOCs [8]. In addition to its function in DOCs, some studies have also shown that the thalamus is important in many aspects of forebrain function through various projection patterns, such as those that control alertness, attention, and awareness, and those that regulate sleep-wake rhythms [19, 20].
In contrast, several studies in both humans and animals have indicated that the thalamo-cortical loop may have a lesser, or only an indirect, effect on limited wakefulness after brain lesions [21] and in propofol-induced unconsciousness [22–24]. According to the mesocircuit hypothesis, other subcortical structures (e.g. the striatum and globus pallidus) in the basal ganglia also contribute to consciousness through interactions with the thalamus [8], and may be controlled by the thalamus [25]. Reciprocal connections between the thalamus and basal ganglia as well as other basal ganglia connections may jointly contribute to arousal, attention [26–28] and movement [29, 30]. Another study suggested that regions in the basal ganglia can mediate consciousness, bypassing the thalamus [21].
While subcortical regions including the thalamus and basal ganglia have been shown to be important in DOCs, how they interact with cortical regions and the exact locations of these regions remain under debate. Crone et al. [31] have compared various existing models and inferred that recovery of consciousness is a dynamic process involving changes in the cortico-thalamo-basal ganglia-cortical connectivity, and cortical regions such as the medial prefrontal cortex (mPFC) and posterior cingulate cortex (PCC) are particularly emphasized. These two regions have also been frequently found to be important in impaired consciousness in multimodal imaging studies [32–34] and literature reviews [4, 35] on DOCs.
Apart from the specific regions, the mechanisms and systems underlying consciousness, including the subcortico-cortical and cortico-cortical connectivity, have gained increasing attention [19, 36, 37]. Structural [13] and functional [31] imaging studies have both shown that pathways connecting the thalamus, basal ganglia, and PCC jointly contribute to consciousness. Despite the revealed impairments in fiber tracts and functional connectivity in DOC patients, a mechanistic understanding of the specific effective couplings (ECs) and their directionality in DOCs remains unclear.
The present study was designed to investigate how regions of the anterior forebrain are related to DOCs. We first compared the ECs among the thalamus, striatum, globus pallidus, and two midline regions in the default mode network (DMN) between DOC patients and healthy controls using spectral dynamic causal modeling (spDCM). Then, a machine-learning method was used to inspect the indexes that performed best in predicting the Coma Recovery Scale-Revised (CRS-R) scores.
Materials and Methods
Participants
A total of 40 patients with severe brain injury (30 M/10 F, aged 20–64 years, mean ± SD, 40.9 ± 13.9) were recruited to this study. The inclusion criteria before the scanning were as follows: (1) disease course <1 year; (2) no history of psychological disorders; (3) no previous alcohol or drug abuse; and (4) either in a vegetative state/unresponsive wakefulness syndrome (VS/UWS) or a minimally conscious state (MCS). The clinical severity was evaluated based on the Coma Recovery Scale-Revised [38]. To decrease diagnostic error, each patient was evaluated by 2–3 doctors together on the day of scanning. During data processing, 8 patients who had excessive head motion (translation >3 mm in any of the planes or rotation >3° in any of the x, y, and z axes) and 7 with severe brain lesions were excluded. Thus, 19 DOC patients (8 in MCS and 11 in VS/UWS) were included for further analyses. The detailed clinical information of these patients are listed in Table 1.
Table 1.
Demographic and clinical characteristics of the patients with disorders of consciousness.
| Patient index | Gender | Age (years) | Months post-icuts | Etiology | Diagnosis | CRS-R scores | |
|---|---|---|---|---|---|---|---|
| Au/V/M/O/C/Ar | Total | ||||||
| P01 | M | 36 | 2 | HIE | VS/UWS | 1/0/0/0/0/1 | 2 |
| P02 | M | 62 | 1 | HIE | VS/UWS | 0/0/1/0/0/2 | 3 |
| P03 | M | 48 | 1 | HIE | VS/UWS | 0/0/1/0/0/2 | 3 |
| P04 | M | 43 | 1 | HIE | VS/UWS | 0/0/1/1/0/2 | 4 |
| P05 | M | 64 | 2 | TBI | VS/UWS | 0/0/1/1/0/2 | 4 |
| P06 | M | 21 | 1 | HIE | VS/UWS | 1/0/2/1/0/1 | 5 |
| P07 | M | 39 | 2 | TBI | VS/UWS | 0/0/2/1/0/2 | 5 |
| P08 | M | 39 | 1 | HIE | VS/UWS | 0/0/2/1/0/2 | 5 |
| P09 | M | 32 | 9 | HIE | VS/UWS | 1/0/1/1/0/2 | 5 |
| P10 | M | 36 | 9 | TBI | VS/UWS | 0/0/1/2/0/2 | 5 |
| P11 | M | 51 | 1 | TBI | VS/UWS | 1/0/2/1/0/2 | 6 |
| P12 | M | 47 | 3 | HIE | MCS | 1/3/0/1/0/2 | 7 |
| P13 | M | 41 | 3 | TBI | MCS | 1/1/3/0/0/2 | 7 |
| P14 | F | 46 | 2 | TBI | MCS | 1/0/3/1/0/2 | 7 |
| P15 | M | 41 | 3 | HIE | MCS | 2/3/2/1/0/1 | 9 |
| P16 | F | 59 | 1 | TBI | MCS | 1/0/5/1/0/2 | 9 |
| P17 | F | 27 | 1 | TBI | MCS | 1/0/5/1/0/2 | 9 |
| P18 | M | 30 | 1 | TBI | MCS | 1/1/3/2/0/2 | 9 |
| P19 | F | 20 | 2 | TBI | MCS | 2/3/3/1/0/2 | 11 |
MCS, minimally conscious state; VS/UWS, vegetative state/unresponsive wakefulness syndrome; TBI, traumatic brain injury; HIE, hypoxic-ischemic encephalopathy; M, male; F, female; CRS-R, Coma Recovery Scale-Revised; Au, auditory; V, visual; M, motor; O, oromotor; C, communication; Ar, arousal.
We also recruited 19 gender- and age-matched healthy participants (13 M/6 F, aged 20–50 years, 35.6 ± 10.0) as controls (Table 2). None of them had a history of neurological or psychiatric illnesses or brain injury. Written informed consent was given by each healthy participant and by the legal surrogate of each patient. The protocols were approved by the Research Review Board of The General Hospital of Guangzhou Military Command of The PLA.
Table 2.
Demographic and clinical characteristics of the patients with disorders of consciousness (DOCs) and the healthy controls (HCs).
| Characteristics | DOCs | HCs | Statistics | P-value |
|---|---|---|---|---|
| Gender (male/female) | 15/4 | 13/6 | χ2 = 0.71 | 0.71a |
| Age (years) | 42.3 ± 13.0 | 35.6 ± 10.0 | t = 1.78 | 0.08b |
| Months post-ictus | 1.1 ± 0.3 | – | – | – |
| Etiology (HIE/TBI) | 9/10 | – | – | – |
| Diagnosis (MCS,VS/UWS) | 8,11 | – | – | – |
| CRS-R scores | 6.1 ± 2.5 | – | – | – |
HIE, hypoxic ischemic encephalopathy; TBI, traumatic brain injury; MCS, minimally conscious state; VS/UWS, vegetative state/unresponsive wakefulness syndrome; CRS-R, Coma Recovery Scale-Revised.
aχ2-test.
bTwo sample t-test.
Data Acquisition
All MRI data were acquired on a 3T GE MRI scanner with an eight-channel phased-array head coil. Two hundred and forty resting-state functional volumes were acquired for each participant. Resing state fMRI data were obtained using a gradient-echo echo-planar imaging sequence with the following parameters: repetition time (TR) = 2,000 ms, echo time (TE) = 26 ms, flip angle (FA) = 90°, field of view (FOV) = 240 × 240 mm2, data matrix = 64 × 64, voxel size = 3.75 × 3.75 × 3.6 mm3, 36 sequential slices, and 240 volumes obtained in ~8 min. In addition, high-resolution structural images were acquired using a T1-weighted 3D fast spoiled gradient recalled sequence (TR = 8.86 ms, TE = 3.52 ms, FA = 90°, FOV = 240 × 240 mm2, data matrix = 256 × 256, voxel size = 0.94 × 0.94 × 1 mm3, and 176 sagittal slices).
Data Preprocessing
Functional images were preprocessed using statistical parametric mapping (SPM12, http://www.fil.ion.ucl.ac.uk/spm) and data preprocessing and analysis for brain imaging (DPABI, http://rfmri.org/dpabi). First, we removed the initial 10 volumes of functional images and then performed slice-timing correction and motion correction. Afterwards, the high-resolution structural and functional images were both manually reoriented to the standard position parallel to the anterior commissure-posterior commissure line. Functional images were individually co-registered with the high-resolution structural images and then spatially normalized to the group template, which was made using diffeomorphic anatomical registration through exponentiated Lie algebra [39]. Finally, the resulting data were spatially smoothed with a Gaussian kernel of 4 mm full-width at half-maximum.
Region selection
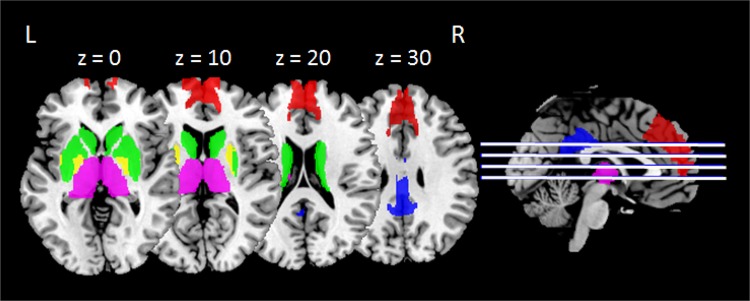
The ROIs were determined according to the Brainnetome atlas (http://atlas.brainnetome.org/bnatlas.html), which divides the whole brain into 105 cortical regions and 18 subcortical regions in each hemisphere [40]. The ROIs in our study were directly extracted and combined using these segmentations implemented in MarsBaR (http://marsbar.sourceforge.net). The posterior part of the cingulate cortex (BA 23) was extracted as the ROI for the PCC. Two parts of the basal ganglia (caudate and putamen) were combined as the striatum. Segmentation of the thalamus and mPFC (8 individual parts for the thalamus and 2 parts for mPFC) were separately combined into one ROI. The globus pallidus was also extracted directly from the atlas. Every ROI was further checked visually using the Automated Anatomical Labeling [41] and Brodmann atlases. These regions have been previously described in other consciousness-related studies using spDCM [31, 42]. Figure 1 illustrates the distributions of these ROIs.
Fig. 1. Regions of interest selected in the current study.
Red, medial prefrontal cortex; blue, posterior cingulate cortex; green, striatum; yellow, globus pallidus; purple, thalamus. L, left hemisphere; R, right hemisphere.
Spectral Dynamic Causal Modeling
Using DCM12 implemented in SPM12, we performed spDCM analysis. The specific calculation steps were as follows: (1) General linear model (GLM) definition: A GLM was defined using the preprocessed images and the confounding time-series from the white matter and cerebrospinal fluid were regressed out as nuisance variables. (2) Time series extraction: The first eigenvectors for the 5 ROIs were extracted for each participant. (3) Model specification and estimation: The fully and reciprocally connected models, including the self-connections, between the 5 ROIs were specified. Thus 25 = 32 free parameters were produced and then estimated for each model. (4) Bayesian post-hoc model selection routine: A post-hoc model optimization routine [43] was used to determine the model that had the best fit for each group.
Machine-Learning Approach in Brain-Behavior Prediction
To predict the clinical performance of the DOC patients from their brain connectivity, we used a recently launched machine-learning approach termed connectome-based predictive modeling [44]. The calculation included the following steps: (1) Feature selection. The correlation between the effective connectivity and the total CRS-R score for each patient was analyzed using Spearman’s correlation. By taking the correlation coefficients as matrix elements, we produced a 19-by-19 matrix, in which each element ranged from −1 to 1. Only the significant coefficients (P < 0.05) were selected as features. Positive and negative correlations were analyzed separately. All 19 patients were divided into a training set and a testing set to perform leave-one-subject-out cross-validation. (2) Feature summarization. The significant features (coefficients) were simply summed for further computations. In the calculations, we separately analyzed the positive and negative EC values. (3) Model building and evaluation. The summary value of the features and the total CRS-R score for each participant in the training set were built into a linear model using the least squares method. The predicted CRS-R scores were generated by applying this linear model to the testing set. Finally, we evaluated the predictive performance by computing the Spearman’s correlation between the predicted CRS-R scores and the real scores. In this way, we produced the correlation coefficients and their P-values.
Statistics
Comparisons of Effective Connections
Differences in EC strength between patients and controls were determined with permutation tests. The ECs were also compared among patients with different etiologies (HIE and TBI) to calculate the influence of the heterogeneity. All the parameters of intrinsic connectivity (shown as DCM.Ep.A in Matlab files) were calculated. In addition, the connection strengths for the corresponding winning models within each group were analyzed using one-sample t-tests. All the resulting P-values were corrected for a false-discovery rate (FDR < 0.05), and another significance threshold, P < 1/(number of comparisons) [42], was also taken into consideration when analyzing the differences between the groups. Of note, positive values of the EC indicate excitatory influence from one region to another, and negative values indicate inhibitory influence, whereas self-connections of regions are assumed to be always inhibitory [42].
Brain-Behavior Prediction
The predictive significance was assessed using permutation testing. By preserving the connectivity matrices but randomly reassigning the behavioral scores a thousand times, an empirical null distribution was generated. The resulting P-value was the proportion of sampled permutations that are greater than or equal to the real predictive correlation.
Results
Dynamic Causal Modeling
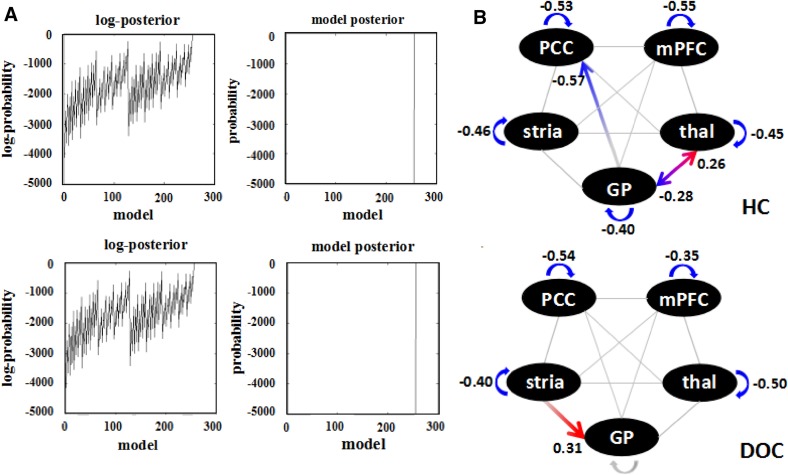
The Bayesian optimization procedure searched all the reduced versions of the full model for each group. The fully-connected model was the winning one for both the patients and controls and had a posterior probability of almost 1 (Fig. 2A). After the optimized model was selected, the parameter inference of connection strength within the same group was compared using one-sample t-tests. The connectivity strengths and directions between the 5 ROIs are shown in Fig. 2B. In the healthy controls, all the self-connections within the nodes were significant and the thalamo-globus pallidus-posterior cingulate cortex circuit was also significantly connected. In the patient group, however, almost all the reciprocal connections between regions disappeared except for a driving influence from the striatum to the globus pallidus. The self-connection within the globus pallidus also disappeared, but the self-inhibition of the other 4 regions remained. The ECs were not influenced by etiology.
Fig. 2. Results of DCM post-hoc selection and one-sample t-tests.
A Bayesian model optimization. The full model was optimized for both the patients and the controls with a posterior probability of almost one. B Results of one-sample t-tests. Red arrows, significant positive inputs or outputs; blue arrows, significant negative values (P < 0.05, FDR-corrected for multiple comparisons); and grey, insignificant values. The digits represent the mean EC within the group. mPFC, medial prefrontal cortex; PCC, posterior cingulate cortex; thal, thalamus; GP, globus pallidus; stria, striatum; HC, healthy control; DOCs, disorders of consciousness.
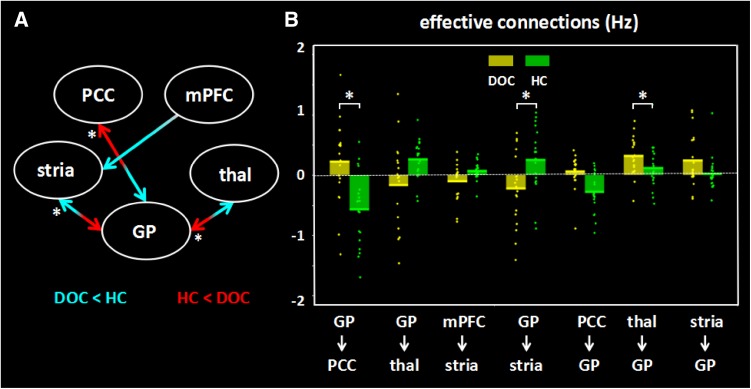
Significant changes in effective connectivity strength were found in the DOC patients compared to the controls (Fig 3). Specifically: (1) The patients showed a significant increase in the influence from the striatum to the globus pallidus (P = 0.039) and also a significant decrease in the opposite direction (P = 0.006). This went along with a reduction in the influence from the globus pallidus to the thalamus (P = 0.011) and an increase in the opposite direction (P = 0.0007). (2) In the DOC patients, the PCC negatively influenced the globus pallidus (P = 0.032) and received a positive influence (P = 0.0006). There was also a significant reduction in the driving influence from the mPFC to the striatum (P = 0.021). The effective connectivity strengths and P-values for both groups are listed in Table 3.
Fig. 3.
Significant changes in effective connections (Hz) in the DOC patients. A Differences between patients and controls derived from permutation tests. Red arrows, connections with significant increases; blue arrows, connections with significant decreases (P < 0.04 = 1/25 uncorrected); asterisks, significant results after FDR correction (P < 0.006). B Mean effective connections of significantly changed pairs of regions. PCC, posterior cingulate cortex; mPFC, medial prefrontal cortex; thal, thalamus; GP, globus pallidus; stria, striatum.
Table 3.
Effective connectivity strengths in patients and controls
| Regions of interest | Within groups | Between groups | ||||
|---|---|---|---|---|---|---|
| DOC | HC | DOC-HC | ||||
| Mean ± SD | P-value | Mean ± SD | P-value | Mean differences | P-value | |
| From mPFC to | ||||||
| mPFC | − 0.35 ± 0.38* | 0.001 | − 0.55 ± 0.41* | 0.000 | 0.20 | 0.065 |
| PCC | − 0.08 ± 0.54 | 0.555 | 0.09 ± 0.27 | 0.152 | − 0.17 | 0.126 |
| Thalamus | 0.01 ± 0.33 | 0.900 | 0.12 ± 0.82 | 0.536 | − 0.11 | 0.299 |
| Striatum | − 0.17 ± 0.49 | 0.157 | − 0.13 ± 0.79 | 0.489 | 0.04 | 0.435 |
| Globus pallidus | − 0.09 ± 0.54 | 0.467 | − 0.13 ± 0.67 | 0.399 | 0.04 | 0.424 |
| From PCC to | ||||||
| mPFC | 0.24 ± 0.44 | 0.028 | 0.02 ± 0.29 | 0.753 | 0.22 | 0.041 |
| PCC | − 0.54 ± 0.34* | 0.000 | − 0.53 ± 0.28* | 0.000 | − 0.01 | 0.450 |
| Thalamus | − 0.06 ± 0.19 | 0.225 | 0.10 ± 0.65 | 0.503 | − 0.16 | 0.165 |
| Striatum | − 0.10 ± 0.41 | 0.324 | − 0.21 ± 0.54 | 0.101 | 0.12 | 0.228 |
| Globus pallidus | 0.22 ± 0.68 | 0.180 | − 0.57 ± 0.61* | 0.001 | 0.79# | 0.001 |
| From thalamus to | ||||||
| mPFC | − 0.03 ± 0.34 | 0.686 | 0.01 ± 0.07 | 0.597 | − 0.04 | 0.324 |
| PCC | − 0.02 ± 0.45 | 0.877 | 0.00 ± 0.11 | 0.909 | − 0.01 | 0.455 |
| Thalamus | − 0.50 ± 0.26* | 0.000 | − 0.45 ± 0.25* | 0.000 | − 0.05 | 0.277 |
| Striatum | 0.08 ± 0.64 | 0.599 | − 0.03 ± 0.45 | 0.794 | 0.11 | 0.279 |
| Globus pallidus | − 0.17 ± 0.70 | 0.317 | 0.26 ± 0.32 | 0.002 | − 0.43 | 0.011 |
| From striatum to | ||||||
| mPFC | − 0.10 ± 0.30 | 0.164 | 0.06 ± 0.15 | 0.091 | − 0.16 | 0.020 |
| PCC | − 0.03 ± 0.28 | 0.612 | 0.01 ± 0.14 | 0.873 | − 0.04 | 0.305 |
| Thalamus | 0.04 ± 0.43 | 0.652 | − 0.13 ± 0.26 | 0.037 | 0.17 | 0.064 |
| Striatum | − 0.40 ± 0.39* | 0.000 | − 0.46 ± 0.32* | 0.000 | 0.06 | 0.304 |
| Globus pallidus | − 0.23 ± 0.60 | 0.113 | 0.25 ± 0.54 | 0.054 | − 0.48# | 0.006 |
| From globus pallidus to | ||||||
| mPFC | 0.03 ± 0.30 | 0.623 | 0.02 ± 0.18 | 0.691 | 0.02 | 0.418 |
| PCC | − 0.12 ± 0.33 | 0.131 | 0.04 ± 0.17 | 0.310 | − 0.16 | 0.032 |
| Thalamus | 0.06 ± 0.28 | 0.389 | − 0.28 ± 0.33 | 0.002 | 0.34# | 0.001 |
| Striatum | 0.31 ± 0.32* | 0.001 | 0.11 ± 0.37 | 0.201 | 0.20 | 0.039 |
| Globus pallidus | − 0.22 ± 0.25 | 0.001 | − 0.40 ± 0.39* | 0.000 | 0.18 | 0.042 |
*Significant effective connectivity at the group level (P < 0.05, FDR-corrected); #significant differences between groups (P < 0.05, FDR-corrected); significant differences (P < 0.04, uncorrected) between groups are shown in bold. PCC, posterior cingulate cortex; mPFC, medial prefrontal cortex
Brain-Behavior Prediction
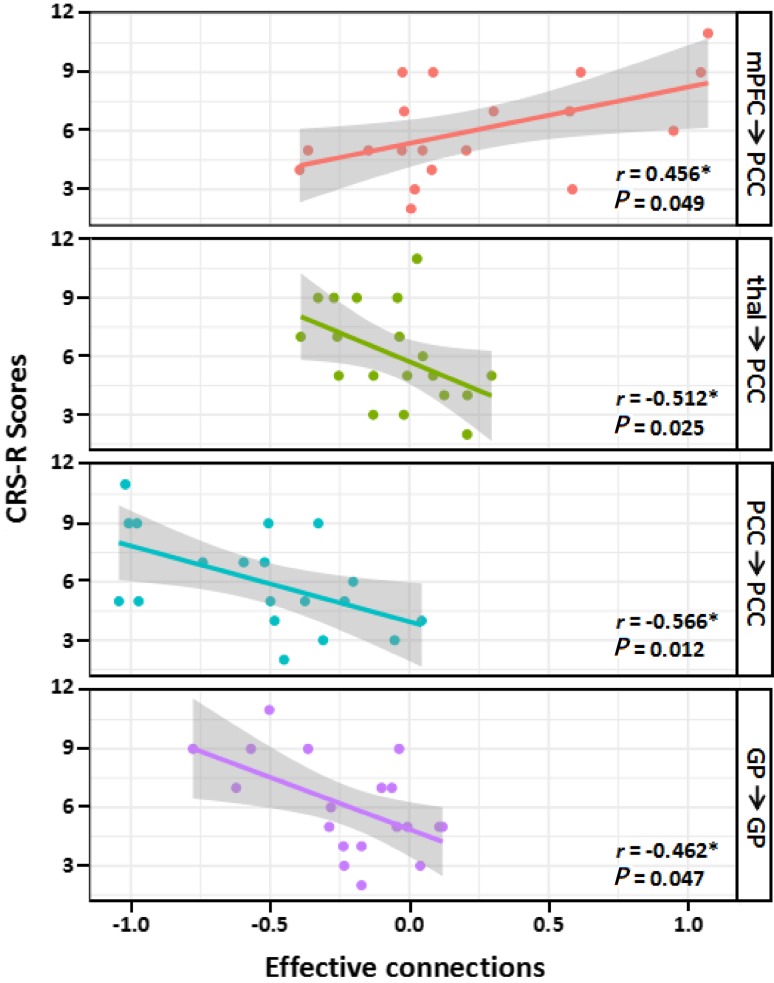
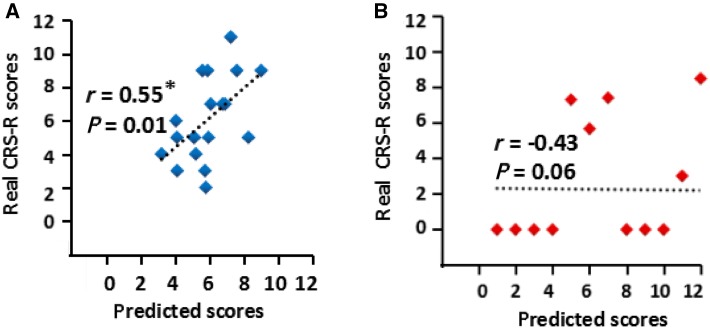
The valid features used in the predictive procedures are shown in Fig. 4. Given the 25 ECs in the optimized model (that is, 20 inter-regional connectivities between the 5 ROIs and 5 recurrent connectivities within the 5 ROIs) and using a threshold of P < 0.05, four out of 25 features (correlations) were retained. These four consisted of only one positive feature, a significantly positive correlation between the CRS-R scores and the mPFC-to-PCC EC (r = 0.456, P = 0.049), and three negative features. The latter were significantly negative correlations between the CRS-R scores and self-connections within the PCC (r = – 0.566, P = 0.012), self-connections within the GP (r = – 0.462, P = 0.047), and the thalamus-to-PCC EC (r = – 0.512, P = 0.025). When taking the negative effective connections as features, we found a significant correlative relationship (r = 0.55, P = 0.014) between the real CRS-R scores and the predicted performance. The nonparametric permutation testing showed that the statistical significance of this performance was P = 0.018. No significant correlation was found when taking the positive effective connections as features (Fig. 5).
Fig. 4.
Significant Spearman’s correlation between the CRS-R scores and the effective connections (EC) in the DOC patients. Scatter plots showing the linear fits between ECs and CRS-R scores. Each dot represents a patient. Each colored line represents the fitted line in each correlation. The grey shaded areas show the 95% confidence interval (P < 0.05). mPFC, medial prefrontal cortex; PCC, posterior cingulate cortex; thal, thalamus; GP, globus pallidus.
Fig. 5.
Spearman’s correlation between real and predicted CRS-R scores using machine learning. A Features selected only from negative effective connections. B Features selected only from positive effective connections. Each dot represents a patient (*P < 0.05).
Discussion
In this work, we investigated the relationships among 5 key regions within the anterior forebrain using spDCM. We identified disrupted ECs in the thalamo-basal ganglia-posterior cingulate cortex circuit of a group of DOC patients compared to the healthy group. Using connectome-based predictive modeling, we found that the predicted CRS-R scores had a significant correlation with the real scores when negative correlation coefficients were selected as features. Importantly, the PCC and the globus pallidus appeared to play a key role in this brain-behavior prediction.
Changes in Effective Connections within the Mesocircuit in DOC Patients
Our results indicated that, compared to controls, patients with DOCs had disrupted connectivity between key regions within the anterior forebrain mesocircuit. In these patients, the striatum first seemed to lose its inhibition of the globus pallidus, then the globus pallidus had a decrease in self-inhibition, and finally the globus pallidus had an inhibitory influence on the thalamus (see Figs 2 and 3). Our results are in line with the mesocircuit hypothesis in that the globus pallidus was released from striatal inhibition and was overactive, resulting in excessive inhibition of the thalamus [8]. Another DCM study [31] also reported the loss of striatal output to the globus pallidus in healthy individuals under propofol-induced sedation, and this influence returned after the recovery of consciousness, suggesting that the striatopallidal inhibition is modulated by the level of consciousness. We found a significantly decreased and negative influence from the globus pallidus to the thalamus in the DOC patients. Several current models suggest that striatal projection neurons modulate the inhibitory pallido-thalamic connectivity [8, 45, 46] in both direct and indirect pathways [47–49]. These existing models have been proposed to be linked to basic motor functions essential for survival [50], an aspect which might explain the impaired motor function of DOC patients.
Differences in the Connectivity between the Basal Ganglia and the Cortex between the Two Groups
In addition to the disrupted subcortico-subcortical connections (the striato-pallido-thalamic loop) in the DOC patients, two significantly changed subcortico-cortical pathways were found between the basal ganglia and cortical regions. First, we detected a significantly decreased and negative influence from the mPFC to the striatum in the DOC patients. Previous studies [42, 51–53] have suggested that, as an important part of the DMN, the mPFC is engaged in modulating consciousness levels. In addition, the striatum receives frontal influence from the mPFC through different types of connectivity, which are proposed to influence information processing [54], sensorimotor functions [55], self-related social cognition [56, 57], and decreased dopamine [58, 59]. Second, we found a significant difference between the two groups in the connection between the globus pallidus and the PCC. Our results agree with previous findings implying that pallido-cortical connectivity [31] is modulated by the level of consciousness. Significant differences in the mPFC-striatum-globus pallidus loop between groups are also in line with a previous study [5], implying that the basal ganglia might regulate consciousness and cortical activation via the cortico-striato-pallidal loop. Taking these studies together, we can reasonably infer that the basal ganglia-cortical loop is a prominent neural circuit in regulating consciousness. Animal models have shown that the basal ganglia are critical for motion with its direct and indirect neural circuits [29, 30]. This evidence may explain the movement disorders in DOC patients.
Impaired Thalamic and Extrathalamic Circuits in Disorders of Consciousness
In contrast to the widespread deafferentation across the thalamo-cortical system that is hypothesized in the mesocircuit theory [8], we did not find a significant difference in thalamo-cortical connectivity between the patients and the controls. A growing body of literature has suggested that the direct connectivity of the thalamo-cortical loop may be less involved in loss of consciousness [7, 31, 60, 61] and that thalamic input may be neither necessary nor sufficient to produce wakefulness [21]. Our results might support those studies indicating that thalamo-cortical deafferentation is mainly associated with higher cognitive functions and motor responsiveness [8], whereas the basal ganglia-cortical loop seems to play a more important role in regulating consciousness [5, 21, 25]. Although no significant difference was detected in thalamo-cortical connectivity, the thalamo-globus pallidus-posterior cingulate cortex loop was found to differ between the two groups. Our results support the view that, at least in DOCs, different aspects of consciousness can be mediated by both thalamic and extrathalamic circuits including the basal ganglia-cortical circuit [25].
Negative Correlations Predicted CRS-R Scores
From the machine-learning analysis based on the correlative relationship between the patients’ ECs and their clinical assessments, we found that negative correlations predicted the patients’ CRS-R scores (Fig. 5A). Three significant features played a joint role in this prediction (Fig. 4). First, as the level of consciousness decreased, self-connection within the PCC approached zero. This suggests that self-inhibition of the PCC is related to consciousness levels. A close relationship between the activity of the PCC and the levels of DOCs has consistently been found across different kinds of imaging studies [1, 3, 62, 63]. Similarly, PCC activity is an important index for distinguishing different types of DOC, such as between MCS and VS/UWS [3]. Second, patients with higher CRS-R scores also had increased self-inhibition in the globus pallidus. This seems to support the mesocircuit hypothesis [8] by implying that dynamic changes in the globus pallidus occur during recovery from DOCs. Third, the EC from the thalamus to the PCC was negatively correlated with the CRS-R scores. Previous evidence [35, 64] has shown that functional connectivity between the thalamus and the PCC is associated with the degree of impaired consciousness. Our findings indicate that the EC might be a useful marker of a patient’s state of impairment.
Although the positive feature made a non-significant prediction of the CRS-R scores, a significant correlation was detected before the prediction step. We found a positive correlation between the CRS-R scores and the EC from the mPFC to the PCC. A previous study [65] has implied that functional coupling within the midline DMN may be useful for detecting different levels of DOCs. The DMN also has particularly interesting implications for consciousness-level studies ranging from pathological alterations of consciousness [1–3, 32, 33, 63, 66] to sleep-wake cycles [60, 67, 68] and pharmacological changes (e.g., anesthesia) [69–71]. Our results support a previous study [65] that suggested that the decoupling of these two spontaneous, synchronized regions of the DMN could account for the varied states of consciousness. Notably, this positive feature (correlation) failed to build a significant model for the DOC patients in the subsequent prediction step. This supports previous studies [65, 72] showing that patients with extreme consciousness disruption can maintain a preserved DMN network. More interestingly, none of the four effective connections with a significant correlation with the CRS-R scores of the DOC patients showed any group difference from the healthy controls. Our results imply that the EC-based predictive model is able to predict patient outcome. However, the changes within the midline DMN, the self-inhibition of the globus pallidus, and the thalamo-PCC connectivity may not be a definite diagnostic biomarker for DOC patients.
Limitations
A number of limitations should be considered when interpreting our results. First, although we recruited 40 patients, we discarded more than half based on relatively strict procedures of quality control. A larger cohort is needed to validate our results, especially the cross-validation results. Second, previous studies [73–75] have shown that specific losses of neurons in the thalamus are associated with different kinds of disease (e.g. the central thalamus with DOCs [76]). In our study, to fully utilize this region, the entire thalamus was extracted; therefore, further studies are needed to correlate different parts of the thalamus with brain or behavioral functions. Third, because our signals were acquired from fMRI rather than neuron-level experiments, our interpretation of the results must be limited to the meso-system level. Particularly importantly, the positive or negative signs of the ECs should be kept in mind when explaining our findings. We may not conclude that the EC is an inhibitory or an excitatory signal in the biological sense. Last, we performed an FDR correction to correct for multiple comparisons, and we also expected <1 false-positive at P <1/25 uncorrected for the between-group differences, which accordingly increased the probability of type-I (false-positive) errors. In this way, we were able to investigate more specific influences between each pair of regions. Importantly, our main findings among the thalamus, the basal ganglia, and the PCC persisted after correction. Further studies with a finer-grained hypothesis for every brain region, unlike this one that used the fully-connected model in an exploratory manner, could reduce the number of connections in the model and the false-positive error rate.
Conclusion
In the current study, we investigated the effective connectivity between the anterior forebrain regions in DOC patients and matched controls. Our findings were primarily in line with the mesocircuit hypothesis, showing a dysfunctional thalamo-basal ganglia-cortical connection loop in the DOC patients. Self-connections within the PCC and the globus pallidus seemed to be less variable in the DOC patients and were highly effective when used to predict the patients’ clinical scores. The current findings suggest that the effective couplings among anterior forebrain regions have potential value for the clinical assessment of DOCs.
Acknowledgements
This work was supported by National Natural Science Foundation of China (81471654, 81428013, 81371535, and 81271548), the Natural Science Foundation of Guangdong Province, China (2015A030313609), Planned Science and Technology Project of Guangzhou Municipality, China (20160402007 and 201604020184) and the Innovation Project of The Graduate School of South China Normal University. The authors express appreciation to Drs. Rhoda E. and Edmund F. Perozzi for editing assistance.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Ping Chen and Qiuyou Xie have contributed equally to this work.
Contributor Information
Ronghao Yu, Email: gesund@21cn.com.
Ruiwang Huang, Email: ruiwang.huang@gmail.com.
References
- 1.Koenig MA, Holt JL, Ernst T, Buchthal SD, Nakagawa K, Stenger VA, et al. MRI default mode network connectivity is associated with functional outcome after cardiopulmonary arrest. Neurocrit Care. 2014;20:348–357. doi: 10.1007/s12028-014-9953-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Norton L, Hutchison RM, Young GB, Lee DH, Sharpe MD, Mirsattari SM. Disruptions of functional connectivity in the default mode network of comatose patients. Neurology. 2012;78:175–181. doi: 10.1212/WNL.0b013e31823fcd61. [DOI] [PubMed] [Google Scholar]
- 3.Vanhaudenhuyse A, Noirhomme Q, Tshibanda LJF, Bruno MA, Boveroux P, Schnakers C, et al. Default network connectivity reflects the level of consciousness in non-communicative brain-damaged patients. Brain. 2009;133:161–171. doi: 10.1093/brain/awp313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laureys S, Schiff ND. Coma and consciousness: paradigms (re)framed by neuroimaging. Neuroimage. 2012;61:478–491. doi: 10.1016/j.neuroimage.2011.12.041. [DOI] [PubMed] [Google Scholar]
- 5.Qiu MH, Vetrivelan R, Fuller PM, Lu J. Basal ganglia control of sleep-wake behavior and cortical activation. Eur J Neurosci. 2010;31:499–507. doi: 10.1111/j.1460-9568.2009.07062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laudes T, Meis S, Munsch T, Lessmann V. Impaired transmission at corticothalamic excitatory inputs and intrathalamic GABAergic synapses in the ventrobasal thalamus of heterozygous BDNF knockout mice. Neuroscience. 2012;222:215–227. doi: 10.1016/j.neuroscience.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Mhuircheartaigh RN, Rosenorn-Lanng D, Wise R, Jbabdi S, Rogers R, Tracey I. Cortical and subcortical connectivity changes during decreasing levels of consciousness in humans: a functional magnetic resonance imaging study using propofol. J Neurosci. 2010;30:9095–9102. doi: 10.1523/JNEUROSCI.5516-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schiff ND. Recovery of consciousness after brain injury: a mesocircuit hypothesis. Trends Neurosci. 2010;33:1–9. doi: 10.1016/j.tins.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giacino JT, Fins JJ, Laureys S, Schiff ND. Disorders of consciousness after acquired brain injury: the state of the science. Nat Rev Neurol. 2014;10:99–114. doi: 10.1038/nrneurol.2013.279. [DOI] [PubMed] [Google Scholar]
- 10.Yd VDW, Witter MP, Groenewegen HJ. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Rev. 2002;39:107–140. doi: 10.1016/S0165-0173(02)00181-9. [DOI] [PubMed] [Google Scholar]
- 11.Fridman EA, Beattie BJ, Broft A, Laureys S, Schiff ND. Regional cerebral metabolic patterns demonstrate the role of anterior forebrain mesocircuit dysfunction in the severely injured brain. Proc Natl Acad Sci U S A. 2014;111:6473–6478. doi: 10.1073/pnas.1320969111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fridman EA, Schiff ND. Neuromodulation of the conscious state following severe brain injuries. Curr Opin Neurobiol. 2014;29:172–177. doi: 10.1016/j.conb.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lant ND, Gonzalez-Lara LE, Owen AM, Fernandez-Espejo D. Relationship between the anterior forebrain mesocircuit and the default mode network in the structural bases of disorders of consciousness. Neuroimage Clin. 2016;10:27–35. doi: 10.1016/j.nicl.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J, Lee HJ, Weitz AJ, Fang Z, Lin P, Choy M, et al. Frequency-selective control of cortical and subcortical networks by central thalamus. Elife. 2015;4:e09215. doi: 10.7554/eLife.09215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schiff ND. Central thalamic deep brain stimulation to support anterior forebrain mesocircuit function in the severely injured brain. J Neural Transm (Vienna) 2016;123:797–806. doi: 10.1007/s00702-016-1547-0. [DOI] [PubMed] [Google Scholar]
- 16.Schiff ND. Central thalamic contributions to arousal regulation and neurological disorders of consciousness. Ann N Y Acad Sci. 2008;1129:105. doi: 10.1196/annals.1417.029. [DOI] [PubMed] [Google Scholar]
- 17.Laureys S. The neural correlate of (un)awareness: Lessons from the vegetative state. Trends in Cognitive Sciences. 2005;9:556. doi: 10.1016/j.tics.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 18.Laureys S, Faymonville ME, Luxen A, Lamy M, Franck G, Maquet P. Restoration of thalamocortical connectivity after recovery from persistent vegetative state. Lancet. 2000;355:1790. doi: 10.1016/S0140-6736(00)02271-6. [DOI] [PubMed] [Google Scholar]
- 19.Laureys S, Gosseries O, Tononi G. The Neurology of Consciousness: Cognitive Neuroscience and Neuropathology. Cambridge: Academic Press; 2015. [Google Scholar]
- 20.Wu W, Cui L, Fu Y, Tian Q, Liu L, Zhang X, et al. Sleep and cognitive abnormalities in acute minor thalamic infarction. Neurosci Bull 2016: 341–348. [DOI] [PMC free article] [PubMed]
- 21.Fuller PM, Sherman D, Pedersen NP, Saper CB, Lu J. Reassessment of the structural basis of the ascending arousal system. J Comp Neurol. 2011;519:933–956. doi: 10.1002/cne.22559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boly M, Moran R, Murphy M, Boveroux P, Bruno MA, Noirhomme Q, et al. Connectivity changes underlying spectral EEG changes during propofol-induced loss of consciousness. J Neurosci. 2012;32:7082–7090. doi: 10.1523/JNEUROSCI.3769-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monti MM, Lutkenhoff ES, Rubinov M, Boveroux P, Vanhaudenhuyse A, Gosseries O, et al. Dynamic change of global and local information processing in propofol-induced loss and recovery of consciousness. PLoS Comput Biol. 2013;9:e1003271. doi: 10.1371/journal.pcbi.1003271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Constantinople CM, Bruno RM. Effects and mechanisms of wakefulness on local cortical networks. Neuron. 2011;69:1061–1068. doi: 10.1016/j.neuron.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lutkenhoff ES, Chiang J, Tshibanda L, Kamau E, Kirsch M, Pickard JD, et al. Thalamic and extrathalamic mechanisms of consciousness after severe brain injury. Ann Neurol. 2015;78:68. doi: 10.1002/ana.24423. [DOI] [PubMed] [Google Scholar]
- 26.Dreher JC, Grafman J. The roles of the cerebellum and basal ganglia in timing and error prediction. Eur J Neurosci. 2002;16:1609–1619. doi: 10.1046/j.1460-9568.2002.02212.x. [DOI] [PubMed] [Google Scholar]
- 27.Häger F, Volz HP, Gaser C, Mentzel HJ, Kaiser WA, Sauer H. Challenging the anterior attentional system with a continuous performance task: a functional magnetic resonance imaging approach. Eur Arch Psychiatry Clin Neurosci. 1998;248:161–170. doi: 10.1007/s004060050034. [DOI] [PubMed] [Google Scholar]
- 28.Ring H, Serra-Mestres J. Neuropsychiatry of the basal ganglia. J Neurol Neurosurg Psychiatry. 2002;72:12–21. doi: 10.1136/jnnp.72.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calabresi P, Picconi B, Tozzi A, Ghiglieri V, Di Filippo M. Direct and indirect pathways of basal ganglia: a critical reappraisal. Nat Neurosci. 2014;17:1022–1030. doi: 10.1038/nn.3743. [DOI] [PubMed] [Google Scholar]
- 30.Cui G, Jun SB, Jin X, Pham MD, Vogel SS, Lovinger DM, et al. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature. 2013;494:238–242. doi: 10.1038/nature11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crone JS, Lutkenhoff ES, Bio BJ, Laureys S, Monti MM. Testing proposed neuronal models of effective connectivity within the cortico-basal ganglia-thalamo-cortical loop during loss of consciousness. Cereb Cortex. 2017;27:2727–2738. doi: 10.1093/cercor/bhw112. [DOI] [PubMed] [Google Scholar]
- 32.Rosazza C, Andronache A, Sattin D, Bruzzone MG, Marotta G, Nigri A, et al. Multimodal study of default-mode network integrity in disorders of consciousness. Ann Neurol. 2016 doi: 10.1002/ana.24634. [DOI] [PubMed] [Google Scholar]
- 33.Di Perri C, Bahri MA, Amico E, Thibaut A, Heine L, Antonopoulos G, et al. Neural correlates of consciousness in patients who have emerged from a minimally conscious state: a cross-sectional multimodal imaging study. Lancet Neurol. 2016;15:830–842. doi: 10.1016/S1474-4422(16)00111-3. [DOI] [PubMed] [Google Scholar]
- 34.Demertzi A, Gómez F, Crone JS, Vanhaudenhuyse A, Tshibanda L, Noirhomme Q, et al. Multiple fMRI system-level baseline connectivity is disrupted in patients with consciousness alterations. Cortex. 2014;52:35–46. doi: 10.1016/j.cortex.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 35.Hannawi Y, Lindquist MA, Caffo BS, Sair HI, Stevens RD. Resting brain activity in disorders of consciousness: A systematic review and meta-analysis. Neurology. 2015;84:1272–1280. doi: 10.1212/WNL.0000000000001404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laureys S, Gosseries O, Tononi G. The Neurology of Conciousness. 2. Cambridge: Academic Press; 2016. [Google Scholar]
- 37.Dehaene S, Changeux JP. Experimental and theoretical approaches to conscious processing. Neuron. 2011;70:200–227. doi: 10.1016/j.neuron.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 38.Giacino JT, Kalmar K, Whyte J. The JFK Coma Recovery Scale-Revised: Measurement characteristics and diagnostic utility. Arch Phys Med Rehabil. 2004;85:2020–2029. doi: 10.1016/j.apmr.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 39.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 40.Fan L, Li H, Zhuo J, Zhang Y, Wang J, Chen L, et al. The human Brainnetome Atlas: A new brain atlas based on connectional architecture. Cereb Cortex. 2016;26:3508–3526. doi: 10.1093/cercor/bhw157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 42.Crone JS, Schurz M, Holler Y, Bergmann J, Monti M, Schmid E, et al. Impaired consciousness is linked to changes in effective connectivity of the posterior cingulate cortex within the default mode network. Neuroimage. 2015;110:101–109. doi: 10.1016/j.neuroimage.2015.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Friston K, Penny W. Post hoc Bayesian model selection. Neuroimage. 2011;56:2089–2099. doi: 10.1016/j.neuroimage.2011.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shen X, Finn ES, Scheinost D, Rosenberg MD, Chun MM, Papademetris X, et al. Using connectome-based predictive modeling to predict individual behavior from brain connectivity. Nat Protoc. 2017;12:506–518. doi: 10.1038/nprot.2016.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-X. [DOI] [PubMed] [Google Scholar]
- 46.Saunders A, Oldenburg IA, Berezovskii VK, Johnson CA, Kingery ND, Elliott HL, et al. A direct GABAergic output from the basal ganglia to frontal cortex. Nature. 2015;521:85. doi: 10.1038/nature14179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Freeze BS, Kravitz AV, Hammack N, Berke JD, Kreitzer AC. Control of basal ganglia output by direct and indirect pathway projection neurons. J Neurosci. 2013;33:18531. doi: 10.1523/JNEUROSCI.1278-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kravitz AV, Freeze BS, Parker PRL, Kay K, Thwin MT, Deisseroth K, et al. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;67:28–29. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith Y, Bevan MD, Shink E, Bolam JP. Microcircuitry of the direct and indirect pathways of the basal ganglia. Neuroscience. 1998;86:353. doi: 10.1016/S0306-4522(97)00608-8. [DOI] [PubMed] [Google Scholar]
- 50.Grillner S, Hellgren J, Ménard A, Saitoh K, Wikström MA. Mechanisms for selection of basic motor programs – roles for the striatum and pallidum. Trends Neurosci. 2005;28:364. doi: 10.1016/j.tins.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 51.Horovitz SG, Braun AR, Carr WS, Picchioni D, Balkin TJ, Fukunaga M, et al. Decoupling of the brain’s default mode network during deep sleep. Proc Natl Acad Sci U S A. 2009;106:11376–11381. doi: 10.1073/pnas.0901435106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Horovitz SG, Fukunaga M, de Zwart JA, Van GP, Fulton SC, Balkin TJ, et al. Low frequency BOLD fluctuations during resting wakefulness and light sleep: a simultaneous EEG-fMRI study. Hum Brain Mapp. 2008;29:671–682. doi: 10.1002/hbm.20428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fernándezespejo D, Junque C, Cruse D, Bernabeu M, Roigrovira T, Fábregas N, et al. Combination of diffusion tensor and functional magnetic resonance imaging during recovery from the vegetative state. BMC Neurol. 2010;10:77. doi: 10.1186/1471-2377-10-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haber SN, Fudge JL, Mcfarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chikama M, Mcfarland NR, Amaral DG, Haber SN. Insular cortical projections to functional regions of the striatum correlate with cortical cytoarchitectonic organization in the primate. J Neurosci. 1997;17:9686. doi: 10.1523/JNEUROSCI.17-24-09686.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Somerville LH, Jones RM, Ruberry EJ, Dyke JP, Glover G, Casey BJ. The medial prefrontal cortex and the emergence of self-conscious emotion in adolescence. Psychol Sci. 2013;24:1554–1562. doi: 10.1177/0956797613475633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Izuma K, Saito DN, Sadato N. The roles of the medial prefrontal cortex and striatum in reputation processing. Soc Neurosc. 2010;5:133. doi: 10.1080/17470910903202559. [DOI] [PubMed] [Google Scholar]
- 58.Brodnik Z, Double M, Jaskiw GE. Presynaptic regulation of extracellular dopamine levels in the medial prefrontal cortex and striatum during tyrosine depletion. Psychopharmacology. 2013;227:363–371. doi: 10.1007/s00213-013-2977-0. [DOI] [PubMed] [Google Scholar]
- 59.Middleton LS, Cass WA, Dwoskin LP. Nicotinic receptor modulation of dopamine transporter function in rat striatum and medial prefrontal cortex. J Pharmacol Exp Ther. 2004;308:367–377. doi: 10.1124/jpet.103.055335. [DOI] [PubMed] [Google Scholar]
- 60.Boly M, Perlbarg V, Marrelec G, Schabus M, Laureys S, Doyon J, et al. Hierarchical clustering of brain activity during human nonrapid eye movement sleep. Proc Natl Acad Sci U S A. 2012;109(15):5856–5861. doi: 10.1073/pnas.1111133109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Monti MM, Lutkenhoff ES, Rubinov M, Boveroux P, Vanhaudenhuyse A, Gosseries O, et al. Dynamic change of global and local information processing in propofol-induced loss and recovery of consciousness. PLoS Comput Biol. 2013;9:e1003271. doi: 10.1371/journal.pcbi.1003271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Soddu A, Vanhaudenhuyse A, Bahri MA, Bruno MA, Boly M, Demertzi A, et al. Identifying the default-mode component in spatial IC analyses of patients with disorders of consciousness. Hum Brain Mapp. 2012;33:778–796. doi: 10.1002/hbm.21249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fernandez-Espejo D, Soddu A, Cruse D, Palacios EM, Junque C, Vanhaudenhuyse A, et al. A role for the default mode network in the bases of disorders of consciousness. Ann Neurol. 2012;72:335–343. doi: 10.1002/ana.23635. [DOI] [PubMed] [Google Scholar]
- 64.Crone JS, Soddu A, Höller Y, Vanhaudenhuyse A, Schurz M, Bergmann J, et al. Altered network properties of the fronto-parietal network and the thalamus in impaired consciousness. Neuroimage Clin. 2013;4:240–248. doi: 10.1016/j.nicl.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stein S, Francesco DP, Corine V, Beatrice R, Isabelle L, Thomas G, et al. Disruption of posteromedial large-scale neural communication predicts recovery from coma. Neurology. 2015;85:2036. doi: 10.1212/WNL.0000000000002196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Qin P, Wu X, Huang Z, Duncan NW, Tang W, Wolff A, et al. How are different neural networks related to consciousness? Ann Neurol. 2015;78:594–605. doi: 10.1002/ana.24479. [DOI] [PubMed] [Google Scholar]
- 67.Laird AR, Fox PM, Eickhoff SB, Turner JA, Ray KL, Mckay DR, et al. Behavioral interpretations of intrinsic connectivity networks. J Cogn Neurosci. 2011;23:4022–4037. doi: 10.1162/jocn_a_00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Larsonprior LJ, Zempel JM, Nolan TS, Prior FW, Snyder AZ, Raichle ME. Cortical network functional connectivity in the descent to sleep. Proc Natl Acad Sci U S A. 2009;106:4489–4494. doi: 10.1073/pnas.0900924106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Martuzzi R, Ramani R, Qiu M, Rajeevan N, Constable RT. Functional connectivity and alterations in baseline brain state in humans. Neuroimage. 2010;49:823. doi: 10.1016/j.neuroimage.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boveroux P, Vanhaudenhuyse A, Bruno MA, Noirhomme Q, Lauwick S, Luxen A, et al. Breakdown of within- and between-network resting state functional magnetic resonance imaging connectivity during propofol-induced loss of consciousness. Anesthesiology. 2010;113:1038–1053. doi: 10.1097/ALN.0b013e3181f697f5. [DOI] [PubMed] [Google Scholar]
- 71.Schrouff J, Perlbarg V, Boly M, Marrelec G, Boveroux P, Vanhaudenhuyse A, et al. Brain functional integration decreases during propofol-induced loss of consciousness. Neuroimage. 2011;57:198–205. doi: 10.1016/j.neuroimage.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 72.Boly M, Tshibanda L, Vanhaudenhuyse A, Noirhomme Q, Schnakers C, Ledoux D, et al. Functional connectivity in the default network during resting state is preserved in a vegetative but not in a brain dead patient. Hum Brain Mapp. 2009;30:2393–2400. doi: 10.1002/hbm.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Henderson JM, Carpenter K, Cartwright H, Halliday GM. Loss of thalamic intralaminar nuclei in progressive supranuclear palsy and Parkinson’s disease: clinical and therapeutic implications. Brain. 2000;123(Pt 7):1410. doi: 10.1093/brain/123.7.1410. [DOI] [PubMed] [Google Scholar]
- 74.Xuereb JH, Perry RH, Candy JM, Perry EK, Marshall E, Bonham JR. Nerve cell loss in the thalamus in Alzheimer’s disease and Parkinson’s disease. Brain. 1991;114(Pt 3):1363. [PubMed] [Google Scholar]
- 75.Popken GJ, Bunney W, Jr, Potkin SG, Jones EG. Subnucleus-specific loss of neurons in medial thalamus of schizophrenics. Proc Natl Acad Sci U S A. 2000;97:9276–9280. doi: 10.1073/pnas.150243397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maxwell WL, Mary Anne M, Smith DH, Mcintosh TK, Graham DI. Thalamic nuclei after human blunt head injury. J Neuropathol Exp Neurol. 2006;65:478–488. doi: 10.1097/01.jnen.0000229241.28619.75. [DOI] [PubMed] [Google Scholar]