PRESENTATION OF CASE
Dr. Molly E. Wolf (Medicine): A 64-year-old man was admitted to this hospital because of progressive leg weakness, recurrent falls, and anemia.
The patient had been in his usual state of health until 8 months before this admission, when fatigue on exertion and subjective leg weakness developed. During the next 3 months, the distance he was able to walk became progressively shorter, because he frequently needed to stop and rest owing to fatigue and leg weakness; he had previously been able to walk long distances. Five months before this admission, intermittent hypoesthesias and paresthesias developed in the feet. Four months before this admission, two lower teeth became loose and fell out, with no preceding trauma.
Four months before this admission, the patient fell in his garage and attributed the fall to tripping over an object on the ground. He did not hit his head, lose consciousness, or need assistance to stand or walk after the fall. He was evaluated by his primary care provider. On evaluation, he reported 3 months of increased alcohol consumption (up to 6 or 7 glasses of wine each night) and several months of voluntarily restricted food intake to achieve weight loss. On physical examination, the vital signs were normal. The height was 166.6 cm, and the weight was 128.4 kg (6 months earlier, the weight had been 140.6 kg); the body-mass index (the weight in kilograms divided by the square of the height in meters) was 46. Ecchymoses were present on the knees. Two lower front teeth were absent, and there were areas of gingival bleeding; the remainder of the physical examination was normal. The patient was counseled on unhealthy alcohol use, but he declined a referral to an outpatient alcohol-cessation program.
During the next 4 months, fatigue and leg weakness gradually progressed, and the patient needed to use his arms to rise from a seated position. He had three additional falls, which he described as his legs “giving out” while he was walking. Two days before admission, the patient fell while he was carrying a bag of groceries and struck the left side of his face on the ground. With each fall, there was no loss of consciousness or associated light-headedness, dizziness, chest pain, dyspnea, palpitations, nausea, or incontinence. The patient rented a wheelchair to avoid walking long distances and presented to his primary care clinic at this hospital for evaluation.
On evaluation in the clinic, the patient reported mild pain above the left eye but no headache, neck pain, back pain, urinary retention, fecal incontinence, fever, chills, morning stiffness, muscle pain, muscle swelling, dark stools, melena, or hematemesis. He had a history of atrial fibrillation, hypertension, gout, osteoarthritis of the knees, gastroesophageal reflux disease, and Barrett’s esophagus, which had been diagnosed by means of biopsy 7 years before this admission. Roux-en-Y gastric bypass had been performed 6 years before this admission. Medications were allopurinol, amlodipine, furosemide, indomethacin, losartan, metoprolol, omeprazole, rivaroxaban, bupropion, cyanocobalamin, ergo-calciferol, and a thiamine supplement. The patient was divorced, lived alone, and worked as a store manager. He did not smoke tobacco. His father and brother had both died of esophageal cancer.
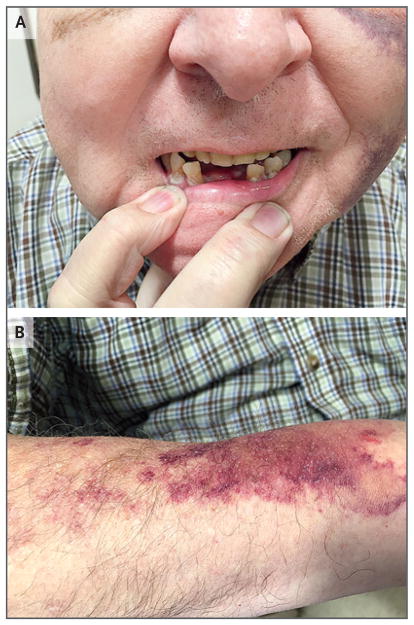
On evaluation in the clinic, the temperature was 36.3°C, the blood pressure 139/81 mm Hg, the pulse 98 beats per minute, the respiratory rate 20 breaths per minute, and the oxygen saturation 98% while the patient was breathing ambient air. The weight was 124.3 kg. Purple ecchymoses were present on the left side of the face and left flank, and brown ecchymoses were present around the right eye. Two lower front teeth were absent (Fig. 1A). The first and second heart sounds were normal, without murmurs. The breath sounds were normal bilaterally, without wheezing or rhonchi. Bowel sounds were present, and the abdomen was soft, nondistended, and nontender on palpation. The edge of the liver was not palpable, and the spleen was not enlarged. Motor strength was 3 out of 5 bilaterally on hip flexion and 4 out of 5 bilaterally on hip extension; the patient could not rise from a seated position, even when he used his arms for assistance. The remainder of the motor examination was normal. There was no muscle atrophy, swelling, or tenderness on palpation. Perception of pinprick was diminished in the legs from the toes to above the knees. Perception of light touch was diminished on the plantar surface of the feet. Proprioception was decreased in the big toes. Reflexes were normal, as were the results of finger–nose–finger testing. An evaluation for the Babinski sign and a Romberg test were not performed. Hair was thin and fragile on the arms and absent on the legs. The stool was brown and negative for occult blood. The remainder of the physical examination was normal. The patient was referred to the emergency department of this hospital for further evaluation.
Figure 1. Clinical Photographs.
On admission, the patient was missing two lower teeth and had bleeding gums (Panel A). He also had thinning arm hair and bruised easily (Panel B).
In the emergency department, laboratory evaluation revealed normal blood levels of calcium, phosphorus, magnesium, total protein, albumin, troponin T, creatine kinase, vitamin B12, 1,25-dihy-droxyvitamin D, thyrotropin, and glycated hemoglobin. Urinalysis revealed a specific gravity of 1.009 (reference range, 1.001 to 1.035), a pH of 5 (reference range, 5 to 9), and ketones, with no protein, glucose, bilirubin, or blood. Blood tests for human immunodeficiency virus (HIV) type 1 p24 antigen and HIV type 1 and type 2 antibodies were negative; other laboratory test results are shown in Table 1. Imaging studies were obtained.
Table 1.
Laboratory Data.*
| Variable | Reference Range, Adults† | On Presentation |
|---|---|---|
| Hematocrit (%) | 41–53 | 36.1 |
| Hemoglobin (g/dl) | 13.5–17.5 | 12.7 |
| White-cell count (per mm3) | 4500–11,000 | 6090 |
| Differential count (%) | ||
| Neutrophils | 40–70 | 69 |
| Lymphocytes | 22–44 | 18 |
| Monocytes | 4–11 | 12 |
| Eosinophils | 0–8 | 0.5 |
| Basophils | 0–3 | 0.3 |
| Platelet count (per mm3) | 150,000–400,000 | 236,000 |
| Red-cell count (per mm3) | 4,500,000–5,900,000 | 3,970,000 |
| Mean corpuscular volume (fl) | 80–100 | 90.9 |
| Mean corpuscular hemoglobin (pg) | 26–34 | 32 |
| Mean corpuscular hemoglobin level (g/dl) | 31–37 | 35.2 |
| Red-cell distribution width (%) | 11.5–14.5 | 14.5 |
| Reticulocyte count (%) | 0.5–2.5 | 2.1 |
| Erythrocyte sedimentation rate (mm/hr) | 0–13 | 36 |
| Sodium (mmol/liter) | 135–145 | 126 |
| Potassium (mmol/liter) | 3.4–5.0 | 3.9 |
| Chloride (mmol/liter) | 98–108 | 83 |
| Carbon dioxide (mmol/liter) | 23–32 | 23 |
| Glucose (mg/dl) | 70–110 | 95 |
| Urea nitrogen (mg/dl) | 8–25 | 14 |
| Creatinine (mg/dl) | 0.6–1.5 | 1.1 |
| Prothrombin time (sec) | 11–14 | 15.2 |
| Prothrombin-time international normalized ratio | 0.9–1.1 | 1.2 |
| Alkaline phosphatase (U/liter) | 45–115 | 142 |
| Bilirubin (mg/dl) | ||
| Total | 0–1.0 | 1.3 |
| Direct | 0–0.4 | 0.5 |
| Alanine aminotransferase (U/liter) | 10–55 | 58 |
| Aspartate aminotransferase (U/liter) | 10–40 | 79 |
| Osmolality in blood (mOsm/kg of water) | 280–296 | 263 |
| Osmolality in random urine (mOsm/kg of water) | 150–1150 | 153 |
| Sodium in random urine (mmol/liter) | NA | <10 |
To convert the values for glucose to millimoles per liter, multiply by 0.05551. To convert the values for urea nitrogen to millimoles per liter, multiply by 0.357. To convert the values for creatinine to micromoles per liter, multiply by 88.4. To convert the values for bilirubin to micromoles per liter, multiply by 17.1. NA denotes not available.
Reference values are affected by many variables, including the patient population and the laboratory methods used. The ranges used at Massachusetts General Hospital are for adults who are not pregnant and do not have medical conditions that could affect the results. They may therefore not be appropriate for all patients.
Computed tomography of the head, performed without the administration of intravenous contrast material, revealed soft-tissue swelling on the left anterior side of the face, with no evidence of fracture, intracranial hemorrhage, infarction, hydrocephalus, or mass lesion. A chest radiograph showed degenerative changes of the bony thorax, with no evidence of pneumonia or pulmonary edema.
The patient was admitted to the hospital, and a high-dose thiamine infusion, a folate supplement, and a multivitamin were administered, in addition to his usual medications. One hour after admission, the blood pressure was 89/47 mm Hg and the heart rate was 90 beats per minute while the patient was sitting; after he stood up, the blood pressure was 75/40 mm Hg and the heart rate was 100 beats per minute. Amlodipine, furosemide, losartan, and metoprolol were discontinued, and fluids were administered intravenously. Two days later, the blood pressure was 124/72 and the heart rate was 91 beats per minute while the patient was sitting; after he stood up, the blood pressure was 80/50 mm Hg and the heart rate was 89 beats per minute. He could rise from a seated position with minimal use of his arms. An area of bruising (10 cm in diameter) developed on the right forearm, where a peripheral intravenous catheter had been placed (Fig. 1B).
A diagnostic test was performed.
DIFFERENTIAL DIAGNOSIS
Dr. Susan E. Bennett: In this 64-year-old man, fatigue on exertion and leg weakness developed, followed by hypoesthesias and paresthesias in the feet. During a routine clinic visit with his primary care physician, he reported that he had fallen at home but had not lost consciousness. Falls are a commonly encountered problem in primary care; 30% of adults older than 65 years of age fall at least once a year.1,2 When evaluating a patient who has fallen, it is important to determine whether the event was an accident or had an identifiable underlying cause.
FALLS IN OLDER PATIENTS
This patient attributed his fall to tripping over an object in his garage. After a fall, many patients develop a narrative or explanation to normalize the event. Falling is always a surprise to a person with a lifetime of experience remaining upright, despite tripping or walking on slippery surfaces. Maintaining balance after tripping is dependent on strength in the hip flexor and gluteal muscles, and such strength decreases gradually with aging. When patients describe a fall as “just tripping,” I ask whether they think they would have fallen if the same event had occurred 10 or 20 years earlier. Testing for core strength with the “get up and go” test, an assessment of mobility that requires static and dynamic balance, can help to determine whether a patient would benefit from a referral to physical therapy for a strength-training program; the test measures the time it takes to stand up from a chair, walk 3 m, turn around, return to the chair, and sit down. This patient had osteoarthritis in the knees, and pain in weight-bearing joints can also increase the risk of falling in older adults. Tests of this patient’s vision, vestibular function, and proprioception might be informative, since age-related decline in these sensory systems can contribute to postural instability.
ORTHOSTATIC HYPOTENSION
Could this patient’s recurrent falls have been due to orthostatic (postural) hypotension? An assessment for orthostatic hypotension would be useful, since he was receiving antihypertensives and diuretics, and medication side effects such as orthostatic hypotension are another common cause of falls in older adults. On admission to the hospital, he was given his usual medications, as well as a standard multivitamin, a folate supplement, and a thiamine infusion. Within 1 hour after the thiamine infusion, he became profoundly hypotensive. Antihypertensives were discontinued, and intravenous fluids were administered; test results ruled out gastrointestinal bleeding, myocardial infarction, and sepsis. Although his blood pressure improved over the next 48 hours, he had persistent and substantial orthostatic hypotension for days. Rapid intravenous infusion of thiamine can cause hypotension, but such hypotension is transient. However, although this patient had potential age-related risk factors for falling, his alcohol use was the most worrisome risk factor.
FALLS IN PATIENTS WITH ALCOHOL USE
If I had seen this patient in the clinic, I would have strongly suspected that his first fall had been related to alcohol intoxication, since he had reported drinking up to 6 or 7 glasses of wine daily during the 3 months before the evaluation. Because he had undergone Roux-en-Y gastric bypass, he had a predisposition to the development of a high blood alcohol level within seconds after ingesting alcohol.3 Alcohol-use disorder can occur after Roux-en-Y gastric bypass in patients who have no history of excessive drinking.4 Alcoholic cerebellar degeneration can also lead to a gait disorder and falls but typically occurs after at least a decade of a high level of daily alcohol ingestion.5 Alcohol-induced peripheral neuropathy can cause paresthesias and hypoesthesias in the feet, and rapid weight loss can lead to leg weakness; each of these conditions increases the risk of falling.
FALLS IN PATIENTS WITH NUTRITIONAL DEFICIENCIES
When the patient returned to the clinic 4 months after the initial fall, he reported falling multiple times, with injuries, and had rented a wheelchair in order to safely negotiate his activities of daily living. His primary care physician performed a detailed neurologic examination that revealed weakness on hip flexion and hip extension and changes in sensation and proprioception, findings that raised concerns about subacute combined degeneration related to a nutritional deficiency, such as vitamin B12 deficiency. The patient was at high risk for nutritional deficiencies resulting from both decreased intake of vitamins and minerals in the context of alcohol use and decreased absorption of vitamins and minerals in the context of previous gastric bypass. He reported taking prescribed vitamin B12 and vitamin D supplements daily, and he had normal levels of those vitamins. However, he was not taking the daily multivitamin that is recommended after bariatric surgery, and he could have had other nutritional deficiencies. Ingested copper is stabilized by gastric acid and absorbed by the stomach and proximal small intestine. Copper deficiency after Roux-en-Y gastric bypass is increasingly recognized as a cause of myeloneuropathy that is similar to vitamin B12 deficiency.6,7
The patient was at risk for deficiencies in all B vitamins, but he reported adhering to thiamine supplementation and had no evidence of encephalopathy. For example, his decision to rent a wheelchair showed high executive functioning. He was not taking folic acid supplements, and isolated folic acid deficiency is an underappreciated cause of a slowly progressive and sensory-dominant pattern of peripheral neuropathy.8
Laboratory testing that was performed on admission ruled out other causes of peripheral neuropathy, such as diabetes, neurosyphilis, HIV infection, and Lyme disease. The creatine kinase level was normal, which ruled out a purely myopathic process.
I would have been confused by the fact that this patient’s deep-tendon reflexes were normal, because this finding argues against most causes of peripheral neuropathy. It is possible that his reflexes appeared to be normal because of the concomitant presence of peripheral neuropathy (due to alcohol use or folate deficiency) and subacute combined degeneration (due to copper or vitamin B12 deficiency), a condition that is usually associated with hyperreflexia. In one study, 32% of patients with chronic alcoholism had evidence of peripheral neuropathy and 24% had evidence of autonomic neuropathy on electro-physiological testing.9 In patients with alcohol-use disorder, the most common nutritional deficiencies are in folic acid, thiamine, and vitamin B6.
Within 2 days after vitamin supplementation was initiated, with no other intervention, the patient could stand without assistance. His fatigue and strength improved dramatically. There is only one cause of slowly progressive fatigue on exertion and weakness that responds so quickly to vitamin supplementation, and that is scurvy, a condition caused by vitamin C (ascorbic acid) deficiency.
SCURVY
Vitamin C is present in many fruits and vegetables, but proper food preparation is necessary to avoid degrading the vitamin C content. This patient is the prototypical person who is at risk for the development of “bachelor scurvy,” a condition that occurs among unpartnered men who prepare their own meals or eat out frequently, drink heavily, and eat virtually no fruits or vegetables.10 The third National Health and Nutrition Examination Survey estimated that 14% of men and 10% of women in the United States have vitamin C deficiency.11
In contrast with most other mammals, humans do not synthesize vitamin C, an essential nutrient that is necessary for many enzymatic reactions in its role as an electron donor and is critical in the synthesis of catecholamines.12 In addition, in vitro experiments have shown that vitamin C binds to the alpha-adrenergic receptor, enhancing its activation by epinephrine.13 Vasomotor instability with shock is thought to be one of the primary causes of sudden death in patients with scurvy.14
In this patient, the orthostatic hypotension, bruising, gingival bleeding with loss of teeth, progressive fatigue, and rapid reversal of weakness after the initiation of multivitamin supplementation were all consistent with the diagnosis of scurvy. In addition, heavy alcohol consumption decreases the absorption of vitamin C, further increasing the likelihood that scurvy will develop.15
Scurvy has not been associated with peripheral neuropathy, but other factors could explain the peripheral neuropathy in this patient, including folic acid deficiency and the toxic effects of alcohol on the peripheral and autonomic nervous system. Although a patient with scurvy would be expected to have a more pronounced macrocytic anemia, this patient had hyponatremia and a low urine sodium level on admission, findings that most likely suggested volume contraction. Also, he most likely had both iron deficiency and folate deficiency, resulting in a falsely normal mean corpuscular volume. The borderline high red-cell distribution width supports the possibility that he had red cells with varying volumes. Iron absorption is facilitated by vitamin C and is also limited by Roux-en-Y gastric bypass. Although the patient did not have cutaneous manifestations of impaired collagen synthesis, which is a hallmark of scurvy, overwhelming evidence suggests that scurvy would be the most likely diagnosis in this case. Since vitamin C deficiency would not explain all the features of this patient’s presentation, I assume that he had other vitamin deficiencies that were caused by the same underlying problem that had led to vitamin C deficiency. To establish the diagnosis of scurvy, I would obtain the blood vitamin C level.
Dr. Meridale V. Baggett (Medicine): Dr. Schmitt, what was your clinical impression when you initially evaluated this patient?
Dr. William P. Schmitt: When this patient presented to my primary care office after his first fall, I thought he had just stumbled in the night. I was most worried about his drinking, and we spent most of the visit talking about his alcohol intake and its effects on his health. I offered him outpatient treatment for alcohol-use disorder, which he declined.
The patient returned to my clinic 4 months later in a wheelchair and reported three additional falls. He could no longer walk and had extensive facial bruising. I took him to the emergency department for an evaluation of head trauma and new leg weakness that had been detected on neurologic examination. After 2 days in the hospital, the patient’s condition had rapidly improved with vitamin repletion. At the time, I was reading Patrick O’Brian’s series that begins with Master and Commander, and when I visited the patient in the hospital, I realized that he had spongy gums, loose teeth, bruising, and hair loss on the arms and legs, symptoms seen in Dr. Maturin’s patients with scurvy on Desolation Island. Scurvy could also explain the patient’s profound orthostatic hypotension and loss of vascular tone.
CLINICAL DIAGNOSIS
Vitamin C deficiency (scurvy).
DR. SUSAN E. BENNET T’S DIAGNOSES
Scurvy causing progressive weakness and falling.
Micronutrient deficiencies causing peripheral neuropathy and anemia.
DIAGNOSTIC TESTING
Dr. Jason M. Baron: Laboratory testing revealed deficiencies in multiple vitamins and minerals. The patient had markedly low blood levels of folate (2 ng per milliliter; normal range, >4.7), vitamin B6 (<2 μg per liter; normal range, 5 to 50), and vitamin C (<0.1 mg per deciliter [<6 μmol per liter]; normal range, 0.4 to 2.0 mg per deciliter [23 to 114 μmol per liter]). The zinc level was mildly low, and the copper and selenium levels were at the low end of the normal range. The levels of vitamin B1 (thiamine), vitamin A, vitamin E, and 25-hydroxyvitamin D were not obtained.
In addition to the low serum folate level, this patient had a low red-cell folate level. However, the American Society for Clinical Pathology Choosing Wisely guidelines recommend against obtaining the red-cell folate level.16 The serum folate level can vary according to recent folate intake,17 and thus a normal serum folate level may not rule out folate deficiency in a patient with recent supplementation or dietary intake. In theory, the red-cell folate level has an advantage over the serum folate level in that it may reflect a patient’s longer-term folate level (over the period of the red-cell life span) and may be less sensitive to recent folate intake. However, assays for red-cell folate are typically less precise than assays for serum folate,18 and thus in practical terms, obtaining the red-cell folate level usually offers little or no diagnostic advantage over obtaining the serum folate level.16–18
Although the level of 25-hydroxyvitamin D was not obtained, the level of 1,25-dihydroxyvita-min D, which represents the active form of vitamin D, was normal. However, it is appropriate to obtain the 25-hydroxyvitamin D level to test for vitamin D deficiency.19 Since the 1,25-dihy-droxyvitamin D level may be normal even in the context of marked vitamin D deficiency,20 it is not appropriate to obtain the 1,25-dihydroxyvita-min D level to test for vitamin D deficiency. The Endocrine Society Choosing Wisely guidelines recommend against obtaining the 1,25-dihydroxy-vitamin D level, except for certain limited indications, such as the evaluation of some patients with hypercalcemia or renal disease.20 Taken together, the final pathologic diagnoses in this case were vitamin C deficiency (scurvy), as well as folate, vitamin B6, and zinc deficiencies.
DISCUSSION OF MANAGEMENT
Dr. Fatima C. Stanford: Essential vitamins and nutrients are absorbed in the stomach, small intestine, and large intestine, and patients who have undergone bariatric surgery are at risk for vitamin deficiencies.21 After bariatric surgery, patients receive lifelong daily vitamin supplementation, which includes a multivitamin, vitamin B12, and calcium citrate with vitamin D3, as well as vitamin D3 alone. After bariatric surgery, the most common deficiencies are in calcium, vitamin B12, vitamin D, and iron, and the least common deficiencies are in vitamin B1 (thiamine), copper, and zinc.22
Roux-en-Y gastric bypass, which this patient had undergone, was once the most common bariatric surgery, but it has been surpassed by sleeve gastrectomy.22,23 When evaluating a patient who has undergone bariatric surgery, the clinician would note the type of procedure, since the associated vitamin and micronutrient deficiencies vary according to procedure type. For example, copper deficiency occurs in 10 to 20% of patients who have undergone Roux-en-Y gastric bypass, but there is only one case report of copper deficiency occurring after sleeve gastrectomy.24 This patient had been taking vitamin D and vitamin B12 supplements, and a thiamine supplement was added after alcohol use was discovered. However, he had not been taking a daily multivitamin and was at risk for other deficiencies, such as deficiencies in vitamin C and folate. This case highlights the need for all members of the medical team to reiterate the importance of all vitamin supplementation as part of routine care after bariatric surgery.
Dr. Schmitt: My experience with this patient highlights for me the importance of routinely asking patients about their alcohol use — especially patients I have known for a long time. This patient had been sober for years but started to drink alcohol again in the context of the stress of his divorce. I could have recognized this sooner had I repeated a validated screening test, such as the Alcohol Use Disorders Identification Test–Concise (AUDIT-C), during follow-up visits.25 I screen all new patients using the AUDIT-C, but I do not rescreen during every follow-up visit with established patients. In patients with previous alcohol-use disorders or stressful life events, rescreening can allow recognition of problematic alcohol use, which can lead to an assessment of severity and appropriate interventions. A short intervention, administered by a caring professional, can reduce harmful drinking practices and help to identify patients with more severe alcohol-use disorder who would benefit from counseling or medication.26
After this patient was discharged from the hospital, I saw him in my office monthly for 3 months. At 3 months after discharge, he had made an excellent recovery and was maintaining sobriety. He was walking without a cane and had returned to working full-time. The orthostatic hypotension and anemia had resolved, and the hair on his arms had grown back. Unfortunately, 3 months after this outpatient visit, he had a relapse of his alcohol use, despite engaging in treatment for alcohol-use disorder. During this relapse, he fell while he was intoxicated and died from a cerebral hemorrhage.
FINAL DIAGNOSES
Vitamin C deficiency (scurvy).
Vitamin B6, folate, zinc, and selenium deficiencies.
Alcohol-use disorder.
Footnotes
This case was presented at the Medical Case Conference.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
Dr. Baron reports receiving grant support, paid to his institution, from IBM. No other potential conflict of interest relevant to this article was reported.
References
- 1.Chang JT, Morton SC, Rubenstein LZ, et al. Interventions for the prevention of falls in older adults: systematic review and meta-analysis of randomised clinical trials. BMJ. 2004;328:680. doi: 10.1136/bmj.328.7441.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988;319:1701–7. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- 3.Steffen KJ, Engel SG, Wonderlich JA, Pollert GA, Sondag C. Alcohol and other addictive disorders following bariatric surgery: prevalence, risk factors and possible etiologies. Eur Eat Disord Rev. 2015;23:442–50. doi: 10.1002/erv.2399. [DOI] [PubMed] [Google Scholar]
- 4.Spadola CE, Wagner EF, Dillon FR, Trepka MJ, De La Cruz-Munoz N, Messiah SE. Alcohol and drug use among postoperative bariatric patients: a systematic review of the emerging research and its implications. Alcohol Clin Exp Res. 2015;39:1582–601. doi: 10.1111/acer.12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicolás JM, Fernández-Solà J, Robert J, et al. High ethanol intake and malnutrition in alcoholic cerebellar shrinkage. QJM. 2000;93:449–56. doi: 10.1093/qjmed/93.7.449. [DOI] [PubMed] [Google Scholar]
- 6.Kumar P, Hamza N, Madhok B, et al. Copper deficiency after gastric bypass for morbid obesity: a systematic review. Obes Surg. 2016;26:1335–42. doi: 10.1007/s11695-016-2162-8. [DOI] [PubMed] [Google Scholar]
- 7.Case Records of the Massachusetts General Hospital (Case 35–2017) N Engl J Med. 2017;377:1977–84. [Google Scholar]
- 8.Koike H, Takahashi M, Ohyama K, et al. Clinicopathologic features of folate-deficiency neuropathy. Neurology. 2015;84:1026–33. doi: 10.1212/WNL.0000000000001343. [DOI] [PubMed] [Google Scholar]
- 9.Monforte R, Estruch R, Valls-Solé J, Nicolás J, Villalta J, Urbano-Marquez A. Autonomic and peripheral neuropathies in patients with chronic alcoholism: a dose-related toxic effect of alcohol. Arch Neurol. 1995;52:45–51. doi: 10.1001/archneur.1995.00540250049012. [DOI] [PubMed] [Google Scholar]
- 10.Connelly TJ, Becker A, McDonald JW. Bachelor scurvy. Int J Dermatol. 1982;21:209–11. doi: 10.1111/j.1365-4362.1982.tb02075.x. [DOI] [PubMed] [Google Scholar]
- 11.Hampl JS, Taylor CA, Johnston CS. Vitamin C deficiency and depletion in the United States: the Third National Health and Nutrition Examination Survey, 1988 to 1994. Am J Public Health. 2004;94:870–5. doi: 10.2105/ajph.94.5.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.May JM, Qu ZC, Meredith ME. Mechanisms of ascorbic acid stimulation of nor-epinephrine synthesis in neuronal cells. Biochem Biophys Res Commun. 2012;426:148–52. doi: 10.1016/j.bbrc.2012.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dillon PF, Root-Bernstein RS, Lieder CM. Antioxidant-independent ascorbate enhancement of catecholamine-induced contractions of vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2004;286:H2353–H2360. doi: 10.1152/ajpheart.00968.2003. [DOI] [PubMed] [Google Scholar]
- 14.Zipursky JS, Alhashemi A, Juurlink D. A rare presentation of an ancient disease: scurvy presenting as orthostatic hypotension. BMJ Case Rep. 2014;2014 doi: 10.1136/bcr-2013-201982. bcr2013201982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fazio V, Flint DM, Wahlqvist ML. Acute effects of alcohol on plasma ascorbic acid in healthy subjects. Am J Clin Nutr. 1981;34:2394–6. doi: 10.1093/ajcn/34.11.2394. [DOI] [PubMed] [Google Scholar]
- 16.Choosing wisely: twenty things physicians and patients should question. Chicago: American Society for Clinical Pathology; ( http://www.choosingwisely.org/wp-content/uploads/2015/02/ASCP-Choosing-Wisely-List.pdf) [Google Scholar]
- 17.Yetley EA, Pfeiffer CM, Phinney KW, et al. Biomarkers of folate status in NHANES: a roundtable summary. Am J Clin Nutr. 2011;94:303S–312S. doi: 10.3945/ajcn.111.013011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Westra B. Red cell folate testing — unwarranted and overutilized in the era of folic acid supplementation. Insights. 2010 Nov 1; ( https://webcache.googleusercontent.com/search?q=cache:vtV8jJwbB9MJ:https://news.mayomedicallaboratories.com/2010/11/01/red-cell-folate-testing-unwarranted-and-overutilized-in-the-era-of-folic-acid-supplementation-hot-topic/)
- 19.Risteli J, Winters WE, Kleerekoper M, Risteli L. Bone and mineral metabolism. In: Brutis CA, Ashwood ER, Bruns DE, editors. Tietz textbook of clinical chemistry and molecular diagnosis. 5. St. Louis: Saunders; 2012. pp. 1768–9. [Google Scholar]
- 20.Choosing wisely: five things physicians and patients should question. Washington, DC: Endocrine Society; 2013. ( http://www.choosingwisely.org/wp-content/uploads/2015/02/ES-Choosing-Wisely-List.pdf) [Google Scholar]
- 21.Hansen EP, Metzsche C, Henningsen E, Toft P. Severe scurvy after gastric bypass surgery and a poor postoperative diet. J Clin Med Res. 2012;4:135–7. doi: 10.4021/jocmr726w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parrott J, Frank L, Rabena R, Craggs-Dino L, Isom KA, Greiman L. American Society for Metabolic and Bariatric Surgery integrated health nutritional guidelines for the surgical weight loss patient 2016 update: micronutrients. Surg Obes Relat Dis. 2017;13:727–41. doi: 10.1016/j.soard.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 23.Gropper SS. Advanced nutrition and human metabolism. 7. Boston: Cen-gage Learning; 2016. [Google Scholar]
- 24.Oliveira YS, Iba Ba J, Mba Angoué JM, Emery Itoudi Bignoumba P, Nzenze JR. Copper deficiency and peripheral neuropathy as an outcome of gastrectomy. Rev Med Interne. 2013;34:234–6. doi: 10.1016/j.revmed.2012.12.019. (In French) [DOI] [PubMed] [Google Scholar]
- 25.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Arch Intern Med. 1998;158:1789–95. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 26.Curry SJ, Ludman EJ, Grothaus LC, Donovan D, Kim E. A randomized trial of a brief primary-care-based intervention for reducing at-risk drinking practices. Health Psychol. 2003;22:156–65. [PubMed] [Google Scholar]