Abstract
Background
Hyperkalemia in neonatal diarrheic calves can potentially result in serious cardiac conduction abnormalities and arrhythmias.
Objectives
To document electrocardiographic (ECG) findings and the sequence of ECG changes that are associated with increasing plasma potassium concentrations (cK+) in a large population of neonatal diarrheic calves.
Animals
One hundred and thirty neonatal diarrheic calves (age ≤21 days).
Methods
Prospective observational study involving calves admitted to a veterinary teaching hospital.
Results
Hyperkalemic calves (cK+: 5.8‐10.2, blood pH: 6.55‐7.47) had significantly (P < .05) longer QRS durations as well as deeper S wave, higher T wave, and higher ST segment amplitudes in lead II than calves, which had both venous blood pH and cK+ within the reference range. The first ECG changes in response to an increase in cK+ were an increase in voltages of P, Ta, S, and T wave amplitudes. Segmented linear regression indicated that P wave amplitude decreased when cK+ >6.5 mmol/L, S wave amplitude voltage decreased when cK+ >7.4 mmol/L, QRS duration increased when cK+ >7.8 mmol/L, J point amplitude increased when cK+ >7.9 mmol/L, and ST segment angle increased when cK+ >9.1 mmol/L. P wave amplitude was characterized by a second common break point at cK+ = 8.2 mmol/L, above which value the amplitude was 0.
Conclusions and Clinical Importance
Hyperkalemia in neonatal diarrheic calves is associated with serious cardiac conduction abnormalities. In addition to increased S and T wave amplitude voltages, alterations of P and Ta wave amplitudes are early signs of hyperkalemia, which is consistent with the known sensitivity of atrial myocytes to increased cK+.
Keywords: acidemia, calves, electrocardiography, hyperkalemia, hypokalemia
1. INTRODUCTION
Neonatal diarrhea in calves can result in metabolic derangements including electrolyte and acid‐base imbalances. Acidemia and metabolic acidosis are frequently evident in diarrheic calves and are typically characterized by a low strong ion difference as a result of hyponatremia (accompanied by normochloremia or hyperchloremia) and an increase of unmeasured anions such as d‐lactate.1, 2, 3 Although neonatal diarrheic calves have a negative potassium balance because of intestinal potassium losses and low milk intake,4 they usually have normokalemic or hyperkalemic plasma concentrations in the presence of acidemia, with hypokalemia being infrequently observed.5, 6
Hyperkalemia is a clinically relevant electrolyte imbalance in diarrheic calves7 that has historically been attributed to an acidemic state with intracellular buffering of hydrogen ions and impaired Na+/K+‐ATPase activity with transcellular movement of potassium ions into the extracellular space as the proposed underlying mechanism.8, 9 A potentially viable mechanism for acidemia‐induced hyperkalemia is activation of a cell membrane potassium channel called TREK‐1 by low intracellular pH, resulting in potassium efflux from the cell.10, 11 The presence of a hyperkalemic state in diarrheic calves strictly depends on the nature of an existing acidosis but not on acidemia per se, as d‐lactic acidosis is only rarely associated with increased plasma potassium concentrations (cK+).5 More importantly, increased plasma cK+ in diarrheic calves is closely associated with severe dehydration, indicating that decreased glomerular filtration rate plays a central pathophysiological role in the development of hyperkalemia.5, 7, 12
Abnormal plasma cK+ can have a profound effect on excitable tissues because the ratio of the extra‐ to intracellular potassium concentration is a major determinant of the resting membrane potential, resulting in skeletal muscle weakness and cardiac conduction abnormalities.13 Moreover, the cardiotoxic effects of hyperkalemia are exacerbated by the presence of hyponatremia and metabolic acidosis,14, 15 conditions, which are usually present in neonatal diarrheic calves. Electrocardiographic (ECG) manifestations in hyperkalemic human patients typically include tall and symmetric T waves, widening of the QRS complex, progressive flattening and eventually disappearance of P waves, and life threatening dysrhythmias or ventricular escape rhythms.16, 17 Similar findings occur in calves with experimentally induced hyperkalemia,18 and experimentally induced8 or naturally acquired diarrhea.19, 20, 21, 22 However, knowledge on ECG findings in calves with naturally acquired diarrhea is based on case reports,22 case series,19 or small study populations of calves with marked increases in cK+.21 Moreover, retrospective studies on the presence of ECG changes in hyperkalemic human patients reported a low sensitivity of the ECG for diagnosing the presence of hyperkalemia.23, 24 Also, ECG abnormalities such as tall and tent‐shaped appearing T waves have been observed in acidemic human patients without hyperkalemia.25 Consequently, the aim of the present prospective observational study was to determine how frequent ECG abnormalities can be found in a large study population of calves with a broad range of cK+ values and other metabolic disorders and to assess what kind of ECG findings are associated with certain levels of increased cK+. Another aim of our study was to evaluate the association of metabolic imbalances, such as acidemia and dysnatremias, with the presence of ECG abnormalities in neonatal diarrheic calves.
2. MATERIALS AND METHODS
2.1. Calves
Between January 2015 and March 2017 a prospective study was conducted involving a convenience sample of 130 calves up to an age of 21 days that were admitted to the Clinic for Ruminants with Ambulatory and Herd Health Services, LMU Munich, with a clinical diagnosis of diarrhea. A subset of included hyperkalemic calves was also used in a recent study focusing on the potassium‐lowering effect of different hypertonic infusion solutions.26 According to previous publications,2, 27 diarrhea was defined as fecal consistency that permitted feces to run through slightly open fingers. The presence of concurrent problems was not an exclusion criteria for study enrollment; however, calves were not included if diarrhea was not considered to be the main problem on admission. Specifically 2 calves with acute respiratory distress, 4 calves with acute abdominal emergencies (gastrointestinal ileus or peritonitis), 3 calves with neurologic abnormalities and a postmortem diagnosis of meningitis, and 2 calves with complicated navel infections requiring surgical intervention were therefore excluded from the study. Our investigation was approved by the Ethics committee of the Center of Veterinary Clinical Medicine, LMU Munich (permit No. 84‐21‐09−2016; 29‐04‐06−2014).
2.2. Electrocardiographic examinations
Electrocardiographic examinations were performed using a PC based ECG system (PC‐EKG 2000, Eickemeyer, Tuttlingen, Germany) after the initial clinical examination was completed on admission to the hospital. Electrocardiograms were recorded in a standardized body position by placing calves in sternal recumbency with the legs positioned parallel to the long axis and folded normally at the carpal and tarsal joints (Figure 1). Electrocardiography electrodes were attached to the skin using alligator clips over the olecranon on the caudal aspect of the forelimbs, over the patellar ligament on the cranial aspect of the hindlimbs, and at the 8th intercostal space of the left thorax close to the costochondral junction (the neutral electrode was placed over the right patellar ligament). For placement of electrodes, a small area of the skin was clipped, cleaned with alcohol (70% solution) and ECG gel applied. An ECG was recorded at least for a 5 minutes period and monitored for the presence of arrhythmias.
Figure 1.

Electrocardiographic examination in a diarrheic calf. Electrocardiograms were recorded in a standardized body position by placing calves in sternal recumbency with the legs positioned parallel to the long axis and folded normally at the carpal and tarsal joints
The ECG software program digitized the signal at 500 Hz at a band width of 0.05–120 Hz and amplitude error measurement <1%. Digital filters were not applied. The digitization rate and low‐frequency cutoff filter of 0.05 Hz met current American Heart Association recommendations.28 The high‐frequency cutoff filter of 120 Hz was less than the recommended filter of 150 Hz; however, the maximal measured Q, R, or S wave amplitude was 3.6 mV, which at an amplitude error measurement of <1% corresponded to <36 μV. Amplitudes and durations of the ECG complexes were determined using the bipolar limb lead II from a software program that averaged the amplitude and duration of the complexes during a selected 32 seconds period, thereby producing a single template of P, QRS, and T waves.
The software program required placement of markers for the start and end points of the P, QRS, and T waves. The markers were placed as follows. The start of the P wave was identified as the 1st deviation from a stable plateau of the TP line, which was defined as the isoelectrical line.29 In case of an absent P wave, the TR line was considered to be the isoelectrical line. Because of the presence of Ta waves, the end of the P wave and the start of the QRS complex was identified by means of abrupt slope changes in respect to the PR line. As ST‐segment elevations and slope changes were observed in a large proportion of calves, a tangent was drawn from the ST segment centered on the approximate mid part of the QT interval, and the first and second deviations from this tangent defined as the J‐point (junction point) and start of the T‐wave, respectively.30, 31 The end of the T wave was defined as the point where the tangent of the steepest downslope of the T wave intersected with the isoelectrical line.29, 30, 32 Representative graphical displays of the placement of markers for software based ECG analyses are provided by Figure 2.
Figure 2.
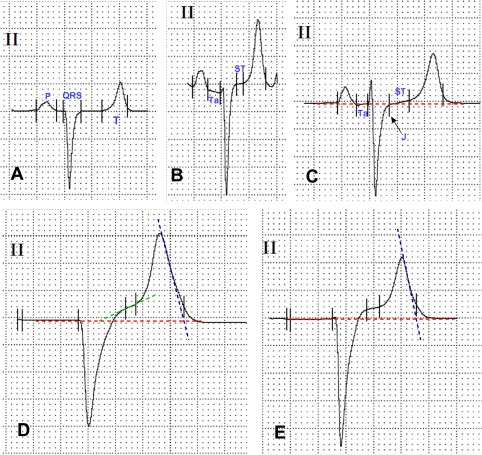
Representative graphical display of software‐based ECG analysis, which required placement of markers for the start and end points of the P, QRS, and T waves (paper speed 50 mm/s, sensitivity 10 mm/mV). A, ECG with horizontal ST segment. B, ECG with a prominent Ta wave and an almost horizontal ST segment but increased ST segment amplitude. C, ECG showing a Ta wave and J point depression (arrow) with ascending ST segment. D, E, ECGs showing missing P waves and increased ST segment amplitudes. Note the endpoints for T wave, which were defined as the points where the tangent of the steepest downslope of the T wave (dashed blue line) intersects with the isoelectric line (dashed red lines). ST‐angle was defined as the angle formed by the baseline and the tangent of the ST segment (dashed green line)
Heart rate, durations and amplitudes of P, QRS, and T waves as well as the duration of the PR interval, QT interval, and ST segment were then measured by the ECG analysis software program. All amplitude measurements were performed by referring to the isoelectrical line, except of measurement of the R wave, which was determined as deviation from the PR line.
The amplitude of the T wave was indexed to the modulus of S wave amplitude to provide a relative measure of changes in T wave amplitude. Furthermore, the ratio of T wave amplitude to P wave amplitude was calculated. The angle of the ST segment above the isoelectric line, deviations of the J point and start of T wave from the isoelectric line (J respective ST segment amplitude), as well as the intervals from the J point to the peak of the T wave (JT max) and to the end of the T wave (JT end) were also determined.30 The value for JT end is equivalent to that of QT interval minus QRS duration, and corrects the QT interval for prolongation of the QRS duration.33
The measured QT interval was corrected for heart rate (QTcorrected [QTc]) using the formula according to Fridericia with the RR interval measured in seconds:
| (1) |
Tall and peak‐shaped appearing T waves were defined as the presence of T wave amplitude ≥0.75 mV and a “peakedness index” ≥8.0 μV/ms, which was calculated by dividing T wave amplitude by T wave duration. Arrhythmias were defined as a deviation of 2 consecutive RR intervals of more than 10%.34 Based on an established reference interval for heart rate of 90–110 beats/min for calves,35 bradyarrhythmias were defined as the presence of arrhythmias and a heart rate <90 beats/min. Analysis of ECG measurements could not be masked as clinical and laboratory examinations were performed by the same investigator.
2.3. Determination of repeatability of ECG measurements
Additionally, in a total of 20 consecutively admitted calves, the repeatability of bipolar limb lead II measurements was assessed by taking the electrodes on and off and recording the ECG measurements twice. In those 20 calves the coefficients of variation, amplitudes, and duration from the duplicate measurements of limb lead II were compared to those of limb leads I and III and a base‐apex lead. Base‐apex leads were recorded by attaching the positive electrode of lead I to the skin of the left thorax in the vicinity of the apex beat at the 5th to 6th intercostal space immediately caudal to the olecranon, and the negative electrode on the skin over the jugular furrow in the caudal 3rd of the right neck.36
2.4. Laboratory examinations
Within 15 minutes after recording the ECG, a lithium‐heparinized blood sample was anaerobically collected from the jugular vein and blood pH, partial pressure of carbon dioxide (pCO2) and oxygen (pO2), sodium (cNa+), chloride (cCl–), potassium (cK+), and ionized calcium concentrations (cCa2+) determined using a blood pH, gas, and electrolyte analyzer with ion‐selective electrodes (Rapidpoint 405, Siemens Healthcare Diagnostics, Tarrytown). Further detailed information concerning the determination of acid‐base variables can be found elsewhere.26
An automated analyzing system (Cobas c 311, Roche Diagnostics, Mannheim, Germany) was used for the biochemical analysis including determination of magnesium (Xylidyl blue), total protein (biuret), albumin (bromocresol green), and creatinine (picric acid) from serum samples and glucose (hexokinase) as well as d‐lactate (d‐lactate dehydrogenase) and l‐Lactate (l‐lactate dehydrogenase) from heparinized blood samples containing potassium fluoride as glycostatic agent.
2.5. Statistical analysis
Commercially available software programs were used for the statistical analysis of the results (SPSS, version 23, IBM, New York; GraphPad Prism, version 7.01, GraphPad Software, La Jolla; SAS, version 9.4, SAS Inc, Cary North Carolina) and P values of <.05 were considered to be statistical significant. Data are presented as medians and interquartile ranges (Q 1/Q 3) because a large proportion of the variables were not considered to be normally distributed as indicated by the results of a Shapiro‐Wilk test and visual examination of QQ‐plots.
A subset of calves with cK+ and venous blood pH within the reference range was used to compare results of ECG measurements to the remaining calves of our study population, which were assigned to one of the groups according to the measured cK+ (hypokalemia, normokalemia, and hyperkalemia). For this purpose, a reference range for plasma cK+ of 3.9 to 5.8 mmol/L and for venous blood pH of 7.35‐7.50 was used.36 Categorized variables were compared using a chi‐square test or Fisher's exact test if the expected frequency in one or more of the cells of the contingency table was <5. Comparisons of continuous variables between the defined groups were made using a non‐parametric Kruskal‐Wallis test. For the subsequent pair‐wise comparisons, a Mann‐Whitney U test was used and the level of significance adjusted using the Bonferroni method (P ≤ .0167). The same approach was also used to compare limb lead II measurements with those of limb l and III and a base apex lead. Repeatability for each lead were determined by calculating a variability index which was defined as the ratio between the intraquartile interval and the median.
Spearman's coefficients of correlation (r s) were also used to characterize associations between ECG measurements and laboratory variables. Using stepwise forward linear regression analysis, models for the P and T amplitudes, QRS duration, T wave peakedness index, and ST segment duration, angle, and amplitude were constructed including variables of clinical pathology significantly correlated to the dependent variable or considered relevant from a biological standpoint of view. To minimize the effects of collinearity, when 2 variables were closely correlated to each other (r s > 0.70), only the variable that had the highest r s in the preliminary univariable analysis was entered into the model. The relative importance of the included variables was assessed by the order of entry into the model as well as by the change of the model R 2 value (ΔR 2). Standardized residual plots of each multivariable model were examined to confirm an approximately normal distribution of residuals. Based on the examination of residual plots, the duration of the QRS interval and ST segment had to be log transformed to the base of 10 and a quadratic transformation of plasma cK+ was used in all models that incorporated a linear value for cK+ to prevent a nonhierarchical analysis.37 Additionally, interaction terms for plasma cK+ and cNa+ as well as venous blood pH were calculated and included into the models as centered variables by multiplying the differences from the mean of each variable.
Univariable logistic regression was also used to determine the associations between the presence of arrhythmias (yes = 1; no = 0), bradyarrhythmias (yes = 1; n = 0), tall and peak shaped appearing T waves (yes = 1; no = 0) as well as the absence of P waves (yes = 1; no = 0) and plasma cK+, cNa+, the K+/Na+‐ratio (calculated by dividing cK+ with cNa+ and multiplying values with 100), venous blood pH, plasma glucose and serum magnesium concentration, and rectal temperature as continuous variables (determined as part of the clinical examination on hospital admission). This was conducted by calculation of Odds ratios (OR) and associated 95% confidence intervals (95% CI).
Additionally segmented linear regression38, 39 was used to characterize the relationship between each ECG variable of interest and cK+ by sequentially modeling the ECG variable‐plasma cK+ relationship using 3 models. Because the focus of the study was on the ECG changes in hyperkalemia, data from calves with hypokalemia (plasma cK+ <3.9 mmol/L)6 were not included in this statistical analysis.
The first model used ordinary linear regression to apply a linear regression equation to the data, such that y = b 0 + b 1 × [K]. The second model used nonlinear regression to apply 2 sequential linear regression equations with different slopes and a common break point ([K]1) to the data, such that if x ≤ [K]1 then y = b 0 + b 1 × x; if x > [K]1 then y = b 0 + b 1 × [K]1 + b 2 × (x ‐ [K]1). The 3rd model used nonlinear regression to apply 3 sequential linear regression equations with different slopes (the 3rd was constrained to a slope = 0) and 2 common break points ([K]1, [K]2) to the data, such that if x ≤ [K]1 then y = b 0 + b 1 × x; if [K]1 < x ≤ [K]2 then y = b 0 + b 1 × [K]1 + b 2 × (x – [K]1), and if x > [K]2 then y = b 0 + b 1 × [K]1 + b 2 × ([K]2 – [K]1). The last equation assumes that y = 0 when plasma [K] > [K]2. When 1 or 2 break points were identified, the estimated cK+ for the break point and the 95% confidence interval for the estimate were calculated to characterize the sequence of ECG changes as plasma cK+ increased. Model fit was evaluated using residual plots and summarized as R 2 from ordinary linear regression or pseudo R 2 from nonlinear regression, where pseudo R 2 = (Model SS – Mean SS)/(Total SS – Mean SS), where Mean SS is the sum of squares due to the mean, Model SS is the sum of squares due to the model, and Total SS is the total (uncorrected) sum of squares of Y (the dependent variable).
3. RESULTS
3.1. General conditions
The median value (and interquartile range) for age was 9 (7–12) days. Because of regional preferences, 90% (n = 117) of calves belonged to the Simmental breed (German Fleckvieh). Figure 3 illustrates individual values for venous blood pH and plasma cK+ of calves of the present study population. Hyperkalemia was present in 44 calves (34%), normokalemia in 73 calves (56%), and hypokalemia in 13 calves (10%). A total of 16 out of 73 normokalemic calves had venous blood pH values in the range of 7.35‐7.50 and were therefore used for comparison of ECG measurements. Selected laboratory findings in the defined groups of calves are given in Table 1.
Figure 3.
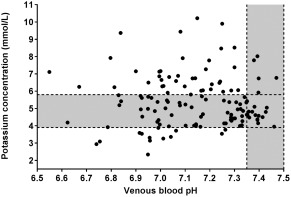
Venous blood pH and plasma potassium concentrations of calves of our study population (n = 130). Gray‐shaded areas represent the reference ranges for plasma potassium concentration (3.9‐5.8 mmol/L) and venous blood pH (7.35‐7.50)
Table 1.
Selected findings of acid‐base and electrolyte status in 130 neonatal diarrheic calves
| Variable | Group I | Group II | Group III | P value | |
|---|---|---|---|---|---|
| Normokalemia | Hypokalemia | Normokalemia | Group IV | ||
| pH ≥ 7.35 | pH < 7.35 | pH < 7.35 | Hyperkalemia a | ||
| n = 16 | n = 13 | n = 57 | n = 44 | ||
| Plasma/serum electrolyte concentration | |||||
| K+ (mmol/L) | 4.62 (4.40/4.82) | 3.36 (3.12/3.66)* | 4.85 (4.25/5.37) | 6.75 (6.26/7.68)* | <.001 |
| Na+ (mmol/L) | 137.9 (135.9/142.6) | 148.6 (141.8/158.2)* | 136.1 (131.1/144.9) | 135.6 (125.8/143.2) | .002 |
| K+/Na+‐ratio | 3.33 (3.15/3.59) | 2.34 (2.01/2.45)* | 3.45 (3.02/3.96) | 5.02 (4.61/5.81)* | <.001 |
| Cl– (mmol/L) | 98 (95/102) | 118 (111/124)* | 105 (98/112)* | 96 (91/103) | <.001 |
| Ca2+ (mmol/L) | 1.19 (1.14/1.24) | 1.43 (1.35/1.49)* | 1.30 (1.22/1.40)* | 1.23 (1.14/1.35) | <.001 |
| Mg2+ (mmol/L) b | 0.80 (0.74/0.85) | 0.88 (0.86/1.27)* | 0.93 (0.83/1.14)* | 1.31 (1.10/1.51)* | <.001 |
| Acid‐base status | |||||
| Venous blood pH | 7.389 (7.359/7.410) | 6.957 (6.920/7.053)* | 7.144 (6.988/7.288)* | 7.136 (7.005/7.249)* | <.001 |
| pCO2 (mm Hg) | 51.9 (48.6/56.0) | 35.7 (28.6/41.0)* | 44.7 (34.3/51.5)* | 49.9 (40.8/61.1) | <.001 |
| pO2 (mm Hg) c | 32.7 (30.8/38.9) | 47.1 (38.1/53.8)* | 38.8 (33.8/43.9) | 30.7 (25.3/37.2) | <.001 |
| (mmol/L) | 31.4 (26.4/34.7) | 8.4 (5.5/10.4)* | 13.0 (7.6/23.6)* | 16.1 (10.8/22.9)* | <.001 |
| Base Excess (mmol/L) | 5.6 (1.5/8.7) | −22.3 (–26.5/–19.1)* | −15.6 (–22.7/–2.5)* | −13.2 (–19.3/–5.1)* | <.001 |
| Anion gap (mEq/L) | 12.8 (11.8/16.6) | 26.1 (22.9/28.0)* | 22.1 (16.1/27.1)* | 26.4 (22.7/31.7)* | <.001 |
| d‐lactate (mmol/L) | 0.8 (0.3/1.7) | 9.7 (7.8/11.6)* | 5.2 (1.5/9.2)* | 1.9 (1.3/6.1)* | <.001 |
| l‐lactate (mmol/L) | 1.9 (1.4/2.7) | 0.9 (0.7/1.1)* | 1.4 (1.0/2.4) | 4.4 (2.4/7.1)* | <.001 |
| Hydration status | |||||
| Creatinine (μmol/L) | 91 (80/152) | 166 (112/235) | 119 (87/160) | 365 (236/570)* | <.001 |
| Total protein (g/L) | 51.7 (47.4/58.3) | 55.7 (51.7/62.0) | 54.8 (48.8/62.5) | 63.8 (58.7/69.1*) | <.001 |
| Albumin (g/L) | 26.8 (23.8/28.4) | 29.1 (27.7/30.2) | 30.1 (26.9/33.1)* | 33.4 (30.8/35.5)* | <.001 |
A subset of 16 calves with venous blood pH and plasma potassium concentrations in the reference range (group I) was used to compare respective findings to the remaining calves of our study population, which were assigned to one of the groups II‐IV according to categories of plasma potassium concentrations.
Values are reported as median and interquartile ranges. P values indicate a statistically significant difference between groups and asterisks indicate values, which are significantly different from group I (P ≤ .0167).
Five calves of group IV had venous blood pH in the reference range.
Information was missing in 1 calf of group III.
Information was missing in 2 calves of group III.
3.2. Specific ECG findings
Table 2 compares the results of lead II ECG measurements between the 4 defined groups of calves with statistically significant differences being found for the majority of ECG variables. Selected ECG findings of calves are shown in Figure 4. Ta waves were detectable in a total of 121 calves and Q waves in 9 calves. P waves were not detectable in 8 markedly hyperkalemic calves (Figure 4H‐J). These calves had a median plasma cK+ of 8.9 mmol/L (range: 7.8–10.2) and a median cNa+ of 124 mmol/L (range: 120–147 mmol/L).
Table 2.
Lead II ECG findings and measurements in 130 neonatal diarrheic calves
| Variable | Group I | Group II | Group III | P value | |
|---|---|---|---|---|---|
| Normokalemia | Hypokalemia | Normokalemia | Group IV | ||
| pH ≥ 7.35 | pH < 7.35 | pH < 7.35 | Hyperkalemia a | ||
| n = 16 | n = 13 | n = 57 | n = 44 | ||
| Heart rate (beats/min) | 115 (100/144) | 106 (83/121) | 107 (96/126) | 122 (95/148) | .19 |
| Bradycardia (<90 beats/min) | 2/16 | 4/13 | 9/57 | 9/44 | .55 |
| Arrhythmias b | 1/16 | 2/13 | 11/57 | 12/44 | .35 |
| Bradyarrhythmias c | 1/16 | 0/13 | 5/57 | 7/44 | .44 |
| Absence of P‐waves | 0/16 | 0/13 | 0/57 | 8/44 | .001 |
| Tall and peaked T waves | 1/16 | 1/13 | 13/57 | 22/44* | .001 |
| P amplitude (mV) | 0.17 (0.15/0.24) | 0.24 (0.16/0.29) | 0.19 (0.15/0.25) | 0.21 (0.14/0.25) | .60 |
| R amplitude (mV) | 0.06 (0.02/0.20) | 0.05 (0.02/0.09) | 0.04 (0.02/0.17) | 0.03 (0.02/0.06) | .12 |
| S amplitude (mV) | −1.31 (−1.53/−1.14) | −1.90 (−2.29/−1.62)* | −1.58 (−1.88/−1.31)* | −1.93 (−2.18/−1.72)* | <.001 |
| T amplitude (mV) | 0.53 (0.40/0.61) | 0.68 (0.51/0.95) | 0.63 (0.48/0.83) | 0.91 (0.64/1.16)* | <.001 |
| J point amplitude (mV) | 0.00 (−0.02/0.01) | 0.03 (0.02/0.05)* | 0.00 (−0.01/0.02) | 0.03 (−0.01/0.06)* | <.001 |
| Ta amplitude (mV) | −0.05 (−0.08/−0.03) | −0.05 (−0.07/−0.04) | −0.06 (−0.07/−0.04) | −0.05 (−0.07/−0.03) | .78 |
| P wave duration d (ms) | 58 (56/76) | 74 (66/83) | 66 (61/74) | 66 (60/78) | .098 |
| PR interval d (ms) | 116 (108/138) | 136 (129/157)* | 120 (113/136) | 115 (102/134) | .003 |
| QRS duration (ms) | 55 (52/64) | 76 (69/84)* | 66 (61/72)* | 74 (67/92)* | <.001 |
| QT interval (ms) | 242 (212/274) | 292 (258/329)* | 260 (230/288) | 247 (212/279) | .009 |
| QTc interval Fridericia (ms) | 301 (285/330) | 350 (324/369)* | 316 (290/331) | 311 (283/327) | .002 |
| ST segment duration (ms) | 90 (72/125) | 96 (70/134) | 92 (67/111) | 64 (39/78)* | <.001 |
| ST segment amplitude (mV) | 0.05 (0.02/0.06) | 0.09 (0.05/0.14)* | 0.05 (0.04/0.08) | 0.07 (0.04/0.12)* | .003 |
| ST angle (°) | 2.5 (0.5/4.0) | 4.0 (2.0/5.5) | 3.0 (2.0/4.5) | 5.5 (3.0/9.0)* | .004 |
| JT max (ms) | 152 (131/185) | 180 (144/201) | 160 (132/187) | 126 (100/152)* | <.001 |
| JT end (ms) | 181 (158/219) | 222 (183/249) | 192 (163/221) | 163 (137/193) | <.001 |
| T wave peakedness index (μV/mS) | 5.2 (3.6/6.6) | 6.3 (4.2/7.3) | 6.0 (4.6/7.9) | 8.9 (5.7/11.4)* | <.001 |
| T to P amplitude ratio d | 3.0 (1.8/3.6) | 3.3 (2.9/4.3) | 3.2 (2.1/4.4) | 3.5 (2.4/5.0) | .27 |
| T to S amplitude ratio | 0.41 (0.31/0.44) | 0.36 (0.30/0.48) | 0.39 (0.27/0.52) | 0.44 (0.34/0.58) | .21 |
A subset of 16 calves with venous blood pH and plasma potassium concentrations in the reference range (group I) was used to compare respective findings to the remaining calves of our study population, which were assigned to one of the groups II‐IV according to categories of plasma potassium concentrations.
Values are reported as ratios or median and interquartile ranges. P values indicate a statistically significant difference between groups and asterisks indicate values, which are significantly different from group I (P ≤ .0167).
Five calves of group IV had venous blood pH in the reference range.
Including ventricular or supraventricular premature complexes.
Heart rate < 90 beats/min and presence of arrhythmias.
Values from 8 hyperkalemic calves with missing P waves were not included in the analysis.
Figure 4.
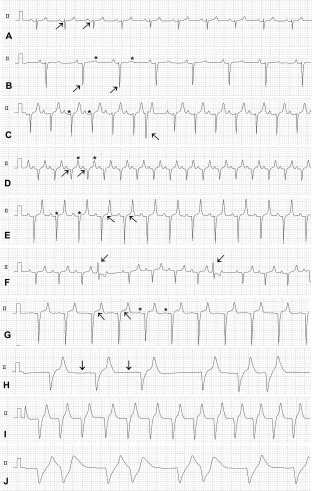
Selected lead II ECG findings in calves with diarrhea (paper speed 25 mm/s, sensitivity 5 mm/mV). A, Normal appearance of an ECG in a diarrheic calf (cK+: 4.5 mmol/L, cNa+: 137 mmol/L, pH 7.41) with a Ta wave present (arrows). B, ECG of a severely acidemic and hypokalemic diarrheic calf (cK+: 2.9 mmol/L, cNa+: 147 mmol/L, blood pH 6.74; rectal temperature: 36.7°C; plasma glucose concentration: 4.2 mmol/L) showing sinus bradycardia (atrial and ventricular rate, 76 beats/min), deep and prominent S waves (arrows), and decreased T wave amplitudes (asterisks). C, Prolonged PR intervals (asterisks) and a supraventricular or junctional premature complex (arrow) in a hypokalemic and severely acidemic diarrheic calf (cK+: 3.1 mmol/L, cNa+: 157 mmol/L, blood pH: 6.75). D, Sinus tachycardia (atrial and ventricular rate, 170 beats/min), Ta waves (arrows) and increased amplitude and slightly peaked T waves (asterisks) in a hyperkalemic diarrheic calf (cK+: 6.3 mmol/L; cNa+: 150 mmol/L, blood pH: 7.01). E, Large amplitude “tented” symmetric T waves and prominent S waves in a hyperkalemic diarrheic calf (cK+: 6.8 mmol/L, cNa+: 135 mmol/L, blood pH: 7.04). Also note the increased QRS duration (asterisks) and ST amplitude (arrows) when compared with A. F, Unifocal premature ventricular complexes (arrows) in a hyperkalemic diarrheic calf (cK+: 6.3 mmol/L, cNa+: 126 mmol/L, blood pH: 7.10). G, Almost disappeared P waves (asterisks), marked ST segment elevation (arrows) and prominent S wave amplitudes in a hyperkalemic diarrheic calf (cK+: 7.8 mmol/L, cNa+: 118 mmol/L, blood pH 7.38). H, Bradycardia (ventricular rate, 56 beats/min) and missing P waves (arrows) and arrhythmias in a severely hyperkalemic diarrheic calf (cK+: 9.4 mmol/L, cNa+: 124 mmol/L, blood pH: 7.08). I, Ventricular tachycardia (ventricular rate, 133 beats/min) in a severely hyperkalemic diarrheic calf (cK+: 10.2 mmol/L, cNa+: 147, blood pH 7.15). J, Irregular wide‐QRS rhythm in a severely hyperkalemic diarrheic calf (cK+: 9.9 mmol/L, cNa+: 139 mmol/L, blood pH 7.25)
Bradycardia (<90 beats/min) was observed in 24 calves of which 4 calves suffered from hypokalemia and 9 calves from hyperkalemia. Arrhythmias were seen in 26 calves of which 12 were presented in a hyperkalemic state with a median cK+ of 7.5 mmol/L (range: 6.0–9.9 mmol/L). An irregular sinus rhythm was evident in all 14 nonhyperkalemic calves of which 6 calves were also bradycardic. In 1 hypokalemic calf with 1st degree A‐V block, arrhythmias were related to supraventricular or junctional premature complexes (Figure 4C). Six out of the 12 hyperkalemic calves with arrhythmias had an irregular sinus rhythm, which was related to premature ventricular complexes in one of them (Figure 4F). The remaining 6 hyperkalemic calves had a slow irregular escape rhythm with missing P waves (Figure 4H).
Of the 8 calves with missing P waves, 2 had ventricular tachycardia (Figure 4I) and 1 calf a R‐on‐T configuration of ECG complexes (Figure 4J).
Tall (>0.75 mV) and peak‐shaped appearing T waves (Figure 4D,E,H,I) were observed in 37 calves (28%) of which 1 calf was hypokalemic, 14 calves normokalemic, and 22 calves hyperkalemic, respectively. Those 37 calves had a median venous blood pH of 7.01 (range: 6.55‐7.43).
3.3. Associations between ECG findings and variables of clinical pathology
Results of a univariable logistic regression analysis for the prediction of the presence of arrhythmias, bradyarrhythmias, tall and peak‐shaped appearing T waves and absence of P waves are presented in Table 3. The K+/Na+‐ratio was a statistically significant predictor in all models, but the absence of P waves and the presence of arrhythmias was not significantly associated with cK+.
Table 3.
Univariable binary logistic regression analysis for the prediction of specific ECG findings in a study population of 130 neonatal diarrheic calves
| Variable | OR | 95% CI for OR | P value |
|---|---|---|---|
| Presence of arrhythmias (n = 26) | |||
| Na+ | 0.956 | 0.916‐0.997 | .034 |
| K+/Na+‐ratio | 1.52 | 1.082‐2.135 | .016 |
| Presence of bradyarrhythmias (n = 13) | |||
| K+ | 1.70 | 1.18‐2.43 | .004 |
| K+/Na+‐ratio | 2.03 | 1.31‐3.16 | .002 |
| Presence of tall and peak‐shaped T waves (n = 37) | |||
| K+ | 2.0 | 1.43‐2.70 | <.001 |
| K+/Na+‐ratio | 2.03 | 1.42‐2.90 | <.001 |
| Mg2+ | 20.4 | 4.7–87.8 | <.001 |
| Venous blood pH | 0.02 | 0.002‐0.174 | <.001 |
| Absence of P waves (n = 8) | |||
| K+/Na+‐ratio | 66.5 | 3.0–1501.0 | .008 |
| Mg2+ | 9.82 | 1.15–83.84 | .037 |
Abbreviations: OR, odds ratio; 95% CI for OR, 95% confidence interval for odds ratio.
Plasma concentrations of potassium and sodium as well as venous blood pH, the K+/Na+‐ratio, plasma glucose and serum magnesium concentrations, and rectal temperature were used as independent variables. Only statistically significant associations are shown.
As shown in Figure 5, no statistically significant association (r s = 0.14, P = .12) was found between plasma cK+ and heart rate. Also no or weak to moderate correlations were found between other ECG measurements and laboratory variables (Supporting Information Table S1). Multivariable stepwise linear regression analysis indicated that a quadratic transformation of plasma cK+ was the most important predictor for the log10 transformed QRS and ST segment duration, P, T, and ST amplitude, and the ST angle (Table 4). Serum magnesium concentration was the most important predictor for S wave amplitude and venous blood pH was identified as a statistically significant predictor in the models for T wave amplitude and T wave peakedness index.
Figure 5.
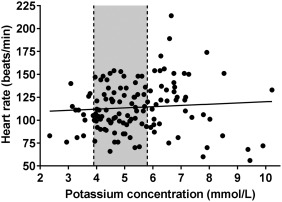
Heart rate and plasma potassium concentrations of calves of the present study population (n = 130). Gray‐shaded areas represent the reference range for plasma potassium concentration (3.9‐5.8 mmol/L) and the line the result of simple linear regression analysis
Table 4.
Results of a stepwise forward linear regression analysis for predicting the log10 transformed duration of the QRS interval, P, T, S, and ST segment amplitude as well as a T wave peakedness index, ST angle and the log10 transformed duration of the ST segment by means of variables of clinical pathology in calves of the study population
| Order of entry | Variable | Coefficient | ± SE | P value | ΔR 2 | Model R 2 |
|---|---|---|---|---|---|---|
| Log10 transformed QRS duration a | ||||||
| Constant | 3.294 | 0.246 | <.001 | |||
| 1 | K × K | 0.015 | 0.002 | <.001 | .402 | .402 |
| 2 | K | −0.154 | 0.022 | <.001 | .141 | .543 |
| 3 | pH | −0.127 | 0.030 | <.001 | .055 | .598 |
| 4 | Creatinine | 0.0001 | 0.00004 | .001 | .028 | .626 |
| 5 | Na | −0.001 | 0.001 | .005 | .023 | .649 |
| P amplitude b | ||||||
| Constant | 0.004 | 0.069 | .949 | |||
| 1 | K × K | −0.011 | 0.002 | <.001 | .122 | .122 |
| 2 | Log Mg | 0.348 | 0.062 | <.001 | .200 | .322 |
| 3 | K | 0.095 | 0.023 | <.001 | .081 | .403 |
| T amplitude c | ||||||
| Constant | 5.725 | 0.883 | <.001 | |||
| 1 | K × K | 0.025 | 0.008 | .001 | .250 | .250 |
| 2 | pH | −0.663 | 0.122 | <.001 | .152 | .402 |
| 3 | K | −0.198 | 0.094 | .037 | .021 | .422 |
| S amplitude d | ||||||
| Constant | −5.966 | 1.604 | <.001 | |||
| 1 | Log Mg | −1.840 | 0.383 | <.001 | .298 | .298 |
| 2 | pH | 0.595 | 0.224 | .009 | .037 | .335 |
| T peakedness index e | ||||||
| Constant | 32.705 | 13.455 | .016 | |||
| 1 | AG | 0.102 | 0.050 | <.043 | .240 | .240 |
| 2 | K | 0.867 | 0.196 | <.001 | .078 | .318 |
| 3 | pH | −4.580 | 1.808 | .013 | .033 | .352 |
| Log10 transformed ST segment duration f | ||||||
| Constant | 2.080 | 0.032 | <.001 | |||
| 1 | K × K | −0.006 | 0.001 | <.001 | .266 | .266 |
| ST segment amplitude g | ||||||
| Constant | 0.496 | 0.049 | <.001 | |||
| 1 | K × K | 0.017 | 0.001 | <.001 | .341 | .341 |
| 2 | K | −0.175 | 0.017 | <.001 | .302 | .644 |
| ST segment angle h | ||||||
| Constant | 22.516 | 3.625 | <.001 | |||
| 1 | K × K | 0.844 | 0.099 | <.001 | .341 | .341 |
| 2 | K | −8.639 | 1.194 | <.001 | .168 | .509 |
| 3 | K × Na | 0.054 | 0.019 | .005 | .028 | .537 |
| 4 | AG | 0.107 | 0.041 | .010 | .024 | .561 |
The variables K × pH, pH, and pCO2 were not retained in the final model.
The variables Na and K × Na, K × pH, and pH were not retained in the final model.
The variables Na, K × Na, K × pH, Creatinine, and AG were not retained in the final model.
The variables K × K, K, K × pH, K × Na, and Na were not retained in the final model.
The variables Na, K × K, K × pH, K × Na, and Creatinine were not retained in the final model.
The variables Na, K, pH, K × pH, and K × Na, pH, Creatinine, and AG were not retained in the final model.
The variables pH, Na, K × pH, and K × Na, Creatinine, and AG were not retained in the final model.
The variables pH, Na, K × pH, and Creatinine were not retained in the final model.
Figures 6 and 7 illustrate the association between cK+ and further selected ECG findings. An interesting and previously unreported finding was that the first ECG changes associated with an increase in plasma cK+ were increased voltages of P, Ta, S, and T wave amplitudes (Figure 6). As also shown in Figures 6 and 7, 8 ECG indices were characterized by 2 linear regression equations with different slopes and a different break point of cK+ as listed in Table 5. Of these 8 indices, P wave amplitude and Ta wave amplitude were also characterized by a second common break point at cK+ = 8.2 and 8.4 mmol/L, respectively, above which value the amplitude was 0.
Figure 6.
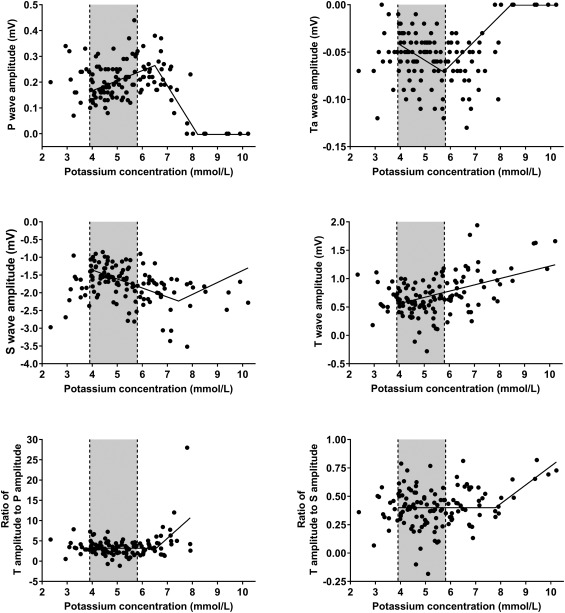
Scatterplots illustrating the association between plasma potassium concentration and P amplitude, Ta amplitude, S amplitude, T amplitude, ratio of T amplitude to P amplitude and the ratio of T amplitude to S amplitude. The lines represent the result of simple linear regression (T amplitude) and segmented regression analysis (P amplitude, Ta amplitude, S amplitude, ratios of T amplitude to P amplitude and T amplitude to S amplitude). Gray‐shaded areas represent the reference range for plasma potassium concentration (3.9‐5.8 mmol/L)
Figure 7.
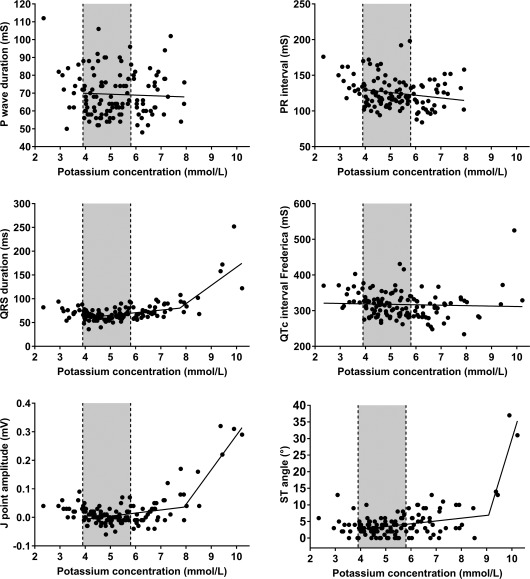
Scatterplots illustrating the association between plasma potassium concentration and P wave duration, PR interval, QRS duration, corrected QT interval according to Fridericia, J point amplitude, and ST angle. The lines represent the result of simple linear regression (P wave duration, PR interval, QTc interval) and segmented regression analysis (QRS duration, J point amplitude, ST angle). Gray‐shaded areas represent the reference range for plasma potassium concentration (3.9‐5.8 mmol/L)
Table 5.
Results of a segmented linear regression analysis for modeling the relationship between 8 ECG variables and plasma potassium concentrations in calves of our study population
| Variable | n | Intercept | Slope 1 | Break point [K]1 | 95% CI for [K]1 | Slope 2 | Break point [K]2 | 95% CI for [K]2 |
|---|---|---|---|---|---|---|---|---|
| Ta wave amplitude | 117 | 0.02 | −0.02 | 5.7 | 5.0–6.4 | 0.03 | 8.4 | 6.7–10.1 |
| P wave amplitude | 117 | 0.01 | 0.04 | 6.5 | 6.1–6.9 | −0.16 | 8.2 | 7.5–8.9 |
| T to P amplitude ratio | 109 | 3.18 | 0 | 6.6 | 6.2–6.9 | 5.53 | – | – |
| S wave amplitude | 117 | −0.34 | −0.25 | 7.4 | 6.0–8.9 | 0.34 | – | – |
| QRS duration | 117 | 38.1 | 5.44 | 7.8 | 7.4–8.1 | 38.27 | – | – |
| J point amplitude | 117 | −0.05 | 0.01 | 7.9 | 7.5–8.3 | 0.12 | – | – |
| T to S amplitude ratio | 117 | 0.40 | 0 | 7.8 | 6.8‐8.7 | 0.16 | – | – |
| ST segment angle | 117 | −0.74 | 0.84 | 9.1 | 8.8–9.3 | 24.9 | – | – |
The slope in the 3rd model for P and Ta wave amplitude was 0.
Data from 13 hypokalemic calves were not included in the analysis.
3.4. Comparison of ECG measurements between bipolar limb leads and base apex lead
A comparative analysis of bipolar limb lead and base‐apex lead measurements is given in Supporting Information Table S2. The values for cK+ and venous blood pH in those 20 calves ranged from 3.3 to 8.0 mmol/l and 6.55 to 7.40, respectively. Statistically significant differences were found for the P, T, S, and ST segment amplitudes, T duration as well as for values of the ST angle and T to S amplitude ratio. However, pair‐wise comparisons did not reveal a statistically significant difference between lead II and base‐apex lead measurements. Coefficients of variation indicated a higher variation and poorer repeatability of lead I and III measurements when compared to lead II and base‐apex lead measurements. Similar coefficients of variation were however observed for base‐apex and lead II measurements, but values indicated a smaller variation for S and T amplitudes and the ST angle in lead II.
3.5. Outcome of therapy
A total of 90% of calves (n = 118) of our study population recovered and were discharged after a median duration of 11 days of hospitalization.
4. DISCUSSION
Our study confirms that hyperkalemia is a clinically relevant electrolyte imbalance in neonatal diarrheic calves that is associated with typical ECG manifestations such as increased QRS duration, tall and peak‐shaped appearing T waves, and findings of atrial standstill and life‐threatening arrhythmias, that have also been previously described in single reports of affected neonatal diarrheic calves19, 20, 22 or experimental investigations.8, 18 However, to the author's knowledge, this is the first study to statistically determine the sequence of ECG changes as plasma cK+ increases in domestic animals or humans with naturally acquired hyperkalemia. All previous studies have been descriptive studies and have not employed objective quantitative analytical techniques.
We applied segmented regression instead of quantile regression or restricted cubic splines regression for 4 reasons. First, the focus of the study was to identify and statistically prioritize cK+ break points for commonly observed ECG abnormalities in hyperkalemia. Segmented regression provides 95% confidence intervals for an objectively identified break point,40 whereas quantile regression and cubic splines fail to provide this desired information38, 39; in fact, the researcher typically assigns values for the knots in the last 2 statistical methods.41 Second, segmented regression typically requires fewer data points than quantile regression.41 Third, the use of polynomial modelling is appropriately criticized in biology because a polynomial model is not relevant for many biological systems. Finally, examination of the scatterplots with the fitted segmented regression model indicated good model fit to the data.
Segmented regression analysis indicated that the first ECG evidence of hyperkalemia were increased voltages of P, Ta, S, and T wave amplitudes. Although increased T wave amplitudes have been well documented in hyperkalemia,17, 42, 43 we are unaware of any studies documenting an increase of voltages of P, Ta, and S wave amplitudes associated with early stages of hyperkalemia. These ECG changes are consistent with the well documented higher sensitivity of atrial myocytes to increased cK+ when compared with ventricular myocytes.14, 44 This also explains the absence of P waves, as an ECG manifestation of atrial standstill, which was seen in 8 calves of the present study. Missing atrial activity was also reported in markedly hyperkalemic (and hyponatremic) diarrheic calves.19, 20, 22 and in markedly hyperkalemic human patients.15, 17, 42 In our study, the plasma potassium to sodium ratio, was significantly associated with the absence of P waves in the ECG. Low sodium concentrations also decrease the amplitude and the rate of rise of phase 0 of the cardiac action potential and therefore probably exaggerate the cardiotoxic effect of hyperkalemia.14 This interrelation between increased cK+ and low cNa+ is of some therapeutic usefulness because the cardiotoxic effects of hyperkalemia can be rapidly reversed by increasing plasma cNa+, which has been demonstrated for hypertonic saline in hyperkalemic humans, dogs, and a diarrheic calf.15, 20, 45
Hyperkalemia results in a lower transmembrane potassium gradient and therefore a less negative resting membrane potential. This magnitude of the transmembrane resting potential is a major determinant of upstroke velocity of the cardiac action potential (phase 0) and therefore the speed of cardiac conduction. Consequently hyperkalemia can slow depolarization of the myocardium which can manifest in the ECG as prolongation of the PR interval (representing the time interval between the onset of atrial and ventricular depolarization) and more commonly as an increased QRS duration (representing the duration of ventricular depolarization).14, 43, 46
Another effect of hyperkalemia is an increased membrane permeability of potassium ions and an increase in the velocity of phase 3 of the cardiac action potential and therefore shortening of the action potential and acceleration of repolarization.42, 46 The latter produces the typical changes of T wave morphology (representing ventricular repolarization) with tall, spiked (symmetric) or peak‐shaped appearance which is considered to be the earliest ECG sign of hyperkalemia in humans.17, 42, 43 More pronounced hyperkalemia increases the risk for a further depression of sinoatrial and atrioventricular conduction which has been described to manifest in the appearance of escape rhythms and complexes17 as also seen in calves of the study reported here (Figure 4H and J). Along with a further prolongation of QRS duration, a fusion of the QRS complex with the T wave can occur,16, 17 leading to a sine wave appearance of the ECG, which was also observed in a calf of our study population (Figure 4J).
Increased cK+ was also associated with alterations of Ta wave amplitude of calves in the study reported here. Ta waves represent the atrial repolarization phase and are usually broader, of lower amplitude, and in the opposite direction to the P wave. Studies about Ta waves are scarce as they are difficult to identify because the Ta amplitude is usually small and they merge with the QRS complex under normal conditions.47, 48 Therefore studies about Ta wave characteristics are usually based on patients with atrioventricular blocks.47, 48 A small increase in Ta wave amplitude was reported as an early sign of experimentally induced hyperkalemia in mice,49 which was similar to the findings of the study reported here.
Increased voltages of S wave amplitude (manifest as increased QRS peak to peak amplitude) was an early sign of hyperkalemia in calves of the present study population. Our finding was consistent with previous observations in calves with diarrhea21 and critically ill humans with hyperkalemia, acidemia, and diarrhea because of cholera.50 Dehydration and hemoconcentration are common in hyperkalemic diarrheic calves5, 7, 12 and the resultant hypovolemia decreases ventricular chamber volumes. The effect of heart chamber volume on the amplitude of Q, R, and S waves is called the Brody phenomenon,51 whereby the intracavitary blood mass augments the radial components and reduces the tangential components of the cardiac dipole. The Brody phenomenon explains why decreased ventricular chamber volumes result in alterations of Q, R, and S wave amplitudes.52, 53, 54, 55 However, the Brody phenomenon would predict that Q, R, and S wave amplitude voltages in diarrheic dehydrated calves would decrease as plasma cK+ increased because of the decrease in plasma volume and consequently chamber volume, which is opposite to the relationship identified in the study reported here and elsewhere.21 We found a negative association between hypermagnesemia and S wave amplitude, which at r s = −0.59 represented the strongest association between a serum biochemical analyte and an ECG variable in our study. Stepwise multivariable regression identified that the 2 independent predictors of S wave amplitude were log10(serum magnesium concentration) and blood pH. Marked hypermagnesemia has been associated with ECG abnormalities such as tall T waves, prolonged PR intervals, and increased QRS duration and amplitude18, 56, 57 and consequently hypermagnesemia might contribute to the observed ECG abnormalities in neonatal diarrheic calves. Similarly, low blood pH can produce ECG abnormalities similar to those seen with hyperkalemia,58 and consequently acidemia might also contribute to the observed ECG abnormalities in neonatal diarrheic calves.
A recent study59 reported ST segment elevation in a high proportion of neonatal diarrheic calves with hyperkalemia, which was also commonly seen in calves of the present study (Table 2, Figure 4C‐H). ST segment elevation was also described as a rare manifestation of hyperkalemia in humans (most frequently in patients with diabetic ketoacidosis) where the ECG findings can resemble acute myocardial infarction and have therefore been described as pseudoinfarct or pseudoinjury ECG pattern.60, 61 The mechanisms for hyperkalemia associated ST segment elevation in humans are not well understood, but repolarization abnormalities and other concurrent metabolic abnormalities such as acidosis have been discussed.62
Although typical ECG abnormalities were seen in hyperkalemic calves of our study population, bradycardia, or bradyarrhythmia was a rare finding in calves with increases in cK+. There was also no association between heart rate and cK+, which is in agreement to previous clinical studies in diarrheic calves as well as dogs and cats who also found no significant relationship63, 64 or even a slight positive association.7, 65 Arrhythmias were detectable in 26 calves of the present study population of which 20 calves had an irregular sinus rhythm. However, it should be considered that the presence of sinus arrhythmias does not necessarily represent a pathologic alteration as it was reported to be detectable in 10% of healthy Holstein calves in a recent study using 24‐h ECG Holter monitoring.34
Nonsignificant or only weak to moderately significant coefficients of correlation between cK+ and specific ECG measurements such as QRS duration or T amplitude were found in our study (Supporting Information Table S1). Studies on the frequency of ECG changes in hyperkalemic dogs and cats64 and human patients23, 24 reported a high variability of ECG manifestations and a low sensitivity of the ECG for diagnosing a hyperkalemic state. Also reports on cases of severe hyperkalemia with minimal or no ECG manifestations in humans have been published.66, 67 This indicates that an increase of extracellular potassium concentration per se is not the single most important factor for the development of ECG manifestations associated with hyperkalemia. There is some evidence that the rate of rise of cK+ is also playing a role in this respect68 and it is conceivable that some calves of our study experienced a slow increase of cK+ such that compensatory mechanisms probably allowed to stabilize the transmembrane resting potential. However, an important finding of our study was that ECG abnormalities such as tall and peak‐shaped appearing T waves, deep S waves, widening of QRS complex or ST segment elevation were also seen in acidemic calves that were normo‐ or even hypokalemic. Similar findings were reported in a study25 where tall and tent shaped appearing T‐waves in acidemic humans without hyperkalemia have been documented. Experimental and in‐vitro studies additionally suggested a direct effect of acidemia on cardiac conduction. In dogs, ECG abnormalities were observed during different types of experimentally induced acidosis that were similar to those of induced hyperkalemia, even in the absence of a hyperkalemic response.58 Also a delayed atrioventricular conduction was reported after exposing heart preparations in an acidic extracellular solution.69, 70
However, in calves of the present study another issue needs to be considered, as the transmembrane resting potential is not only dependent from alterations of extracellular but also from alterations of intracellular cK+. Neonatal diarrheic calves have a negative potassium balance because of intestinal losses and low milk intake,4 which is likely enhanced by alterations of internal potassium balance because of an acidemic state and resulting intracellular losses of potassium ions. It is therefore conceivable that in some calves an intracellular depletion of potassium stores and concomitant alterations of internal potassium balance because of acidemia (leading to normokalemia) resulted in a similar extracellular to intracellular potassium ratio than in calves with marked increases in cK+ but normal or only slightly decreased intracellular K+. This would explain the presence of ECG abnormalities in acidemic but non‐hyperkalemic calves.
Although our study documented ECG findings in a large study population of diarrheic calves with a broad range of acid‐base and potassium balance disorders, our analysis has also some limitations. A potential limitation of our study represents the choice of bipolar limb lead II for analysis, which was selected as it is a frequently used ECG lead in small animal medicine. A previous study on ECG parameters in healthy lactating Holstein cows, however, revealed lower amplitudes and greater wave form variability of limb lead measurements (including lead II) when compared to standard base‐apex lead analyses.71 Those differences can be explained by the positions of the electrodes relative to the position of the heart in the thorax of the bovine species, a higher susceptibility of limb leads to movement artefacts, and species specific characteristics of the distribution of the conduction system within the myocardium, which may cause cancellation of wave fronts in limb lead measurements.71, 72, 73, 74 However, in our study limb lead II measurements produced clear waveforms and a comparative analysis of limb lead II and base‐apex lead did not reveal major differences in respect to amplitudes, durations and variability of ECG parameters (Supporting Information Table S2). In addition, analysis of ECG measurements in a nonblinded manner might be considered as a limitation although the use of a software program provided a standardized method of measuring the amplitudes and durations of selected ECG complexes. Another limitation of our study was the lack of a healthy control group. Although calves of the reference groups had venous blood pH and plasma cK+ in the reference range it is possible that other metabolic conditions might have affected the ECG measurements in those calves.
5. CONCLUSIONS
Results of our study confirm that hyperkalemia is a clinically relevant and potentially cardiotoxic electrolyte imbalance in neonatal diarrheic calves. However, increased plasma cK+ only partly explained the observed ECG abnormalities in our study population. Especially the findings that venous blood pH was significantly associated with the presence of specific ECG abnormalities such as tall and peak‐shaped appearing T waves, and that those abnormalities were also seen in acidemic but nonhyperkalemic calves, indicate that acidemia is a contributing factor that predisposes to cardiac conduction abnormalities in diarrheic calves. We anticipate that application of the segmented regression approach used in the critically ill calves in the study reported here will provide additional insight into the sequence of ECG changes and facilitate the diagnosis and treatment of hyperkalemia in critically ill domestic animals and humans.
CONFLICT OF INTEREST DECLARATION
Peter Constable has received funding from Boehringer‐Ingelheim related to the treatment of hypokalemia in adult dairy cattle.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
This study was approved by the Ethics committee of the Center of Veterinary Clinical Medicine, LMU Munich.
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information 1
Supporting Information 2
ACKNOWLEDGMENTS
This work was done at the Clinic for Ruminants with Ambulatory and Herd Health Services, LMU Munich, Oberschleißheim, Germany. This work was financed by a research grant (TR 1321/1‐1) of the German Research Foundation (Deutsche Forschungsgemeinschaft, Bonn, Germany).
Trefz FM, Lorenz I, Constable PD. Electrocardiographic findings in 130 hospitalized neonatal calves with diarrhea and associated potassium balance disorders. J Vet Intern Med. 2018;32:1447–1461. 10.1111/jvim.15220
Funding information German Research Foundation (Deutsche Forschungsgemeinschaft, Bonn, Germany), Grant/Award Number: TR 1321/1‐1
REFERENCES
- 1. Constable PD, Stämpfli HR, Navetat H. Use of a quantitative strong ion approach to determine the mechanism for acid‐base abnormalities in sick calves with or without diarrhea. J Vet Intern Med. 2005;19:581–589. [DOI] [PubMed] [Google Scholar]
- 2. Trefz FM, Constable PD, Lorenz I. Quantitative physicochemical analysis of acid‐base balance and clinical utility of anion gap and strong ion gap in 806 neonatal calves with diarrhea. J Vet Intern Med. 2015;29:678–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lorenz I. Influence of D‐lactate on metabolic acidosis and on prognosis in neonatal calves with diarrhoea. J Vet Med A Physiol Pathol Clin Med. 2004;51:425–428. [DOI] [PubMed] [Google Scholar]
- 4. Lewis LD, Phillips RW. Water and electrolyte losses in neonatal calves with acute diarrhea. A complete balance study. Cornell Vet. 1972;62:596–607. [PubMed] [Google Scholar]
- 5. Trefz FM, Constable PD, Sauter‐Louis C, Lorch A, Knubben‐Schweizer G, Lorenz I. Hyperkalemia in neonatal diarrheic calves depends on the degree of dehydration and the cause of the metabolic acidosis but does not require the presence of acidemia. J Dairy Sci. 2013;96:7234–7244. [DOI] [PubMed] [Google Scholar]
- 6. Trefz FM, Lorch A, Zitzl J, Kutschke A, Knubben‐Schweizer G, Lorenz I. Risk factors for the development of hypokalemia in neonatal diarrheic calves. J Vet Intern Med. 2015;29:688–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Trefz FM, Lorch A, Feist M, Sauter‐Louis C, Lorenz I. The prevalence and clinical relevance of hyperkalaemia in calves with neonatal diarrhoea. Vet J. 2013;195:350–356. [DOI] [PubMed] [Google Scholar]
- 8. Lewis LD, Phillips RW. Diarrheic induced changes in intracellular and extracellular ion concentrations in neonatal calves. Ann Rech Vétér. 1973;4:99–111. [Google Scholar]
- 9. Sweeney RW. Treatment of potassium balance disorders. Vet Clin North Am Food Anim Pract. 1999;15:609–617. [DOI] [PubMed] [Google Scholar]
- 10. Maingret F, Patel AJ, Lesage F, Lazdunski M, Honoré E. Mechano‐ or acid stimulation, two interactive modes of activation of the TREK‐1 potassium channel. J Biol Chem. 1999;274:26691–26696. [DOI] [PubMed] [Google Scholar]
- 11. Constable PD, Grünberg W. Hyperkalemia in diarrheic calves: Implications for diagnosis and treatment. Vet J. 2013;195:271–272. [DOI] [PubMed] [Google Scholar]
- 12. Trefz FM, Lorenz I. Plasma potassium concentrations in neonatal diarrhoeic calves are correlated with serum aldosterone concentrations but not with insulin concentrations. Vet J. 2017;230:41–44. [DOI] [PubMed] [Google Scholar]
- 13. Weisberg LS. Management of severe hyperkalemia. Crit Care Med. 2008;36:3246–3251. [DOI] [PubMed] [Google Scholar]
- 14. Fisch C. Relation of electrolyte disturbances to cardiac arrhythmias. Circulation. 1973;47:408–419. [DOI] [PubMed] [Google Scholar]
- 15. Garcia‐Palmieri MR. Reversal of hyperkalemic cardiotoxicity with hypertonic saline. Am Heart J. 1962;64:483–488. [DOI] [PubMed] [Google Scholar]
- 16. Diercks DB, Shumaik GM, Harrigan RA, Brady WJ, Chan TC. Electrocardiographic manifestations: Electrolyte abnormalities. J Emerg Med. 2004;27:153–160. [DOI] [PubMed] [Google Scholar]
- 17. Mattu A, Brady WJ, Robinson DA. Electrocardiographic manifestations of hyperkalemia. Am J Emerg Med. 2000;18:721–729. [DOI] [PubMed] [Google Scholar]
- 18. Bergman E, Sellers A. Studies on intravenous administration of calcium, potassium, and magnesium to dairy calves. II. Some cardiac and respiratory effects. Am J Vet Res. 1954;15:25. [PubMed] [Google Scholar]
- 19. Weldon AD, Moise NS, Rebhun WC. Hyperkalemic atrial standstill in neonatal calf diarrhea. J Vet Intern Med. 1992;6:294–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Constable PD. Hypertonic saline. Vet Clin North Am Food Anim Pract. 1999;15:559–585. [DOI] [PubMed] [Google Scholar]
- 21. Özkan C, Altuğ N, Yüksek N, et al. Assessment of electrocardiographic findings, serum nitric oxide, cardiac troponins and some enzymes in calves with hyperkaliemia related to neonatal diarrhoea. Revue Méd Vét. 2011;162:171–176. [Google Scholar]
- 22. Basoglu A, Aydogdu U. Terminal atrial standstill with ventricular escape rhythm in a neonatal calf with acute diarrhea. Turk J Vet Anim Sci. 2013;37:362–365. [Google Scholar]
- 23. Montague BT, Ouellette JR, Buller GK. Retrospective review of the frequency of ECG changes in hyperkalemia. Clin J Am Soc Nephrol. 2008;3:324–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wrenn KD, Slovis CM, Slovis BS. The ability of physicians to predict hyperkalemia from the ECG. Ann Emerg Med. 1991;20:1229–1232. [DOI] [PubMed] [Google Scholar]
- 25. Dreyfuss D, Jondeau G, Couturier R, Rahmani J, Assayag P, Coste F. Tall T waves during metabolic acidosis without hyperkalemia: A prospective study. Crit Care Med. 1989;17:404–408. [DOI] [PubMed] [Google Scholar]
- 26. Trefz FM, Constable PD, Lorenz I. Effect of intravenous small‐volume hypertonic sodium bicarbonate, sodium chloride, and glucose solutions in decreasing plasma potassium concentration in hyperkalemic neonatal calves with diarrhea. J Vet Intern Med. 2017;31:907–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Trefz FM, Lorenz I, Lorch A, Constable PD. Clinical signs, profound acidemia, hypoglycemia, and hypernatremia are predictive of mortality in 1,400 critically ill neonatal calves with diarrhea. PLoS One. 2017;12:e0182938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kligfield P, Gettes LS, Bailey JJ, et al. Recommendations for the standardization and interpretation of the electrocardiogram. Part I: The electrocardiogram and its technology. A scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2007;49:1109–1127. [DOI] [PubMed] [Google Scholar]
- 29. Velagapudi V, O'Horo JC, Vellanki A, et al. Computer‐assisted image processing 12 lead ECG model to diagnose hyperkalemia. J Electrocardiol. 2017;50:131–138. [DOI] [PubMed] [Google Scholar]
- 30. Bidoggia H, Maciel JP, Capalozza N, et al. Sex‐dependent electrocardiographic pattern of cardiac repolarization. Am Heart J. 2000;140:430–436. [DOI] [PubMed] [Google Scholar]
- 31. Melgaard J, Struijk JJ, Kanters JK, et al. Automatic J‐point location in subjects with electrocardiographic early repolarization. Comput Cardiol. 2014;41:585–588. [Google Scholar]
- 32. Uberoi A, Stein R, Perez MV, et al. Interpretation of the electrocardiogram of young athletes. Circulation. 2011;124:746–757. [DOI] [PubMed] [Google Scholar]
- 33. Rautaharju PM, Surawicz B, Gettes LS, et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram. Part IV: The ST segment, T and U waves, and the QT interval. A scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009;53:982–991. [DOI] [PubMed] [Google Scholar]
- 34. Pessoa RB, Batista CF, Santos KR, et al. Holter monitoring (24‐h electrocardiography) of Holstein calves. Acta Sci Vet. 2018;44:5. [Google Scholar]
- 35. Rosenberger G. Die klinische Untersuchung des Rindes. Berlin: Verlag Paul Parey; 1990. [Google Scholar]
- 36. Constable PD, Hinchcliff KW, Done SH, Grünberg W. Veterinary Medicine: A textbook of the diseases of cattle, horses, sheep, pigs and goats. 11th ed St. Louis: Elsevier Health Sciences; 2017. [Google Scholar]
- 37. McCullagh P, Nelder JA. Generalized linear models. 2nd ed. New York: Chapman & Hall; 1989. [Google Scholar]
- 38. Vieth E. Fitting piecewise linear regression functions to biological responses. J Appl Physiol. 1989;67:390–396. [DOI] [PubMed] [Google Scholar]
- 39. Bang H, Mazumdar M, Spence D. Tutorial in biostatistics: Analyzing associations between total plasma homocysteine and B vitamins using optimal categorization and segmented regression. Neuroepidemiology. 2006;27:188–200. [DOI] [PubMed] [Google Scholar]
- 40. Jones RH, Molitoris BA. A statistical method for determining the breakpoint of two lines. Anal Biochem. 1984;141:287–290. [DOI] [PubMed] [Google Scholar]
- 41. Marrie RA, Dawson NV, Garland A. Quantile regression and restricted cubic splines are useful for exploring relationships between continuous variables. J Clin Epidemiol. 2009;62:511–517. [DOI] [PubMed] [Google Scholar]
- 42. Dittrich KL, Walls RM. Hyperkalemia: ECG manifestations and clinical considerations. J Emerg Med. 1986;4:449–455. [DOI] [PubMed] [Google Scholar]
- 43. Weiner ID, Wingo CS. Hyperkalemia: A potential silent killer. J Am Soc Nephrol. 1998;9:1535–1543. [DOI] [PubMed] [Google Scholar]
- 44. De Mello WC, Hoffman BF. Potassium ions and electrical activity of specialized cardiac fibers. Am J Physiol. 1960;199:1125–1130. [DOI] [PubMed] [Google Scholar]
- 45. Kaplan JL, Eynon CA, Dalsey WC, et al. Hypertonic saline treatment of severe hyperkalemia in nonnephrectomized dogs. Acad Emerg Med. 2000;7:965–973. [DOI] [PubMed] [Google Scholar]
- 46. Ettinger PO, Regan TJ, Oldewurtel HA. Hyperkalemia, cardiac conduction, and the electrocardiogram: A review. Am Heart J. 1974;88:360–371. [DOI] [PubMed] [Google Scholar]
- 47. Perego M, Skert S, Santilli RA. Analysis of the atrial repolarization wave in dogs with third‐degree atrioventricular block. Am J Vet Res. 2014;75:54–58. [DOI] [PubMed] [Google Scholar]
- 48. Hayashi H, Okajima M, Yamada K. Atrial T(Ta) wave and atrial gradient in patients with A‐V block. Am Heart J. 1976;91:689–698. [DOI] [PubMed] [Google Scholar]
- 49. Chawla KK, Harris WS. The atrial repolarization wave: A newly described finding in the electrocardiogram of the mouse (Mus musculus). J Electrocardiol. 1970;3:317–324. [DOI] [PubMed] [Google Scholar]
- 50. Carpenter CC, Biern RO, Mitra PP, et al. Electrocardiogram in Asiatic cholera. Separated studies of effects of hypovolaemia, acidosis, and potassium loss. Br Heart J. 1967;29:103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Brody DA. A theoretical analysis of intracavitary blood mass influence on the heart‐lead relationship. Circ Res. 1956;4:731–738. [DOI] [PubMed] [Google Scholar]
- 52. Ker J, Webb EC, Van Papendorp D. The Brody effect induced by premature ventricular complexes in the ovine heart. Onderstepoort J Vet Res. 2009;76:443–448. [DOI] [PubMed] [Google Scholar]
- 53. Della Torre PK, Zaki S, Govendir M, Church DB, Malik R. Effect of acute haemorrhage on QRS amplitude of the lead II canine electrocardiogram. Aust Vet J. 1999;77:298–300. [DOI] [PubMed] [Google Scholar]
- 54. Manoach M, Gitter S, Grossman E, Varon D, Gassner S. Influence of hemorrhage on the QRS complex of the electrocardiogram. Am Heart J. 1971;82:55–61. [DOI] [PubMed] [Google Scholar]
- 55. McManus JG, Convertino VA, Cooke WH, Ludwig DA, Holcomb JB. R‐wave amplitude in lead II of an electrocardiograph correlates with central hypovolemia in human beings. Acad Emerg Med. 2006;13:1003–1010. [DOI] [PubMed] [Google Scholar]
- 56. Swaminathan R. Magnesium metabolism and its disorders. Clin Biochem Rev. 2003;24:47–66. [PMC free article] [PubMed] [Google Scholar]
- 57. Mordes JP, Wacker WE. Excess magnesium. Pharmacol Rev. 1977;29:273–300. [PubMed] [Google Scholar]
- 58. Roberts KE, Magida MG. Electrocardiographic alterations produced by a decrease in plasma pH, bicarbonate and sodium as compared with those produced by an increase in potassium. Circ Res. 1953;1:206–218. [DOI] [PubMed] [Google Scholar]
- 59. Chalmeh A, Pourjafar M, Naghib M. Comparison of ST‐segment duration and morphology between clinically healthy and diarrheic Holstein dairy calves. J Fac of Vet Med Istanbul Univ. 2014;40:162–167. [Google Scholar]
- 60. Bellazzini MA, Meyer T. Pseudo‐myocardial infarction in diabetic ketoacidosis with hyperkalemia. J Emerg Med. 2010;39:e139–e141. [DOI] [PubMed] [Google Scholar]
- 61. Simon BC. Pseudomyocardial infarction and hyperkalemia: a case report and subject review. J Emerg Med. 1988;6:511–515. [DOI] [PubMed] [Google Scholar]
- 62. Ziakas A, Basagiannis C, Stiliadis I. Pseudoinfarction pattern in a patient with hyperkalemia, diabetic ketoacidosis and normal coronary vessels: a case report. J Med Case Rep. 2010;4:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Klee W. Serumkaliumgehalt und Herzbefund bei der Kälberdiarrhoe‐retrospektive Analyse. Tierärztl Umschau. 1982;495–495. [Google Scholar]
- 64. Tag TL, Day TK. Electrocardiographic assessment of hyperkalemia in dogs and cats. J Vet Emerg Crit. 2008;18:61–67. [Google Scholar]
- 65. Constable PD, Berchtold JF, Walker PG, et al. Effect of serum potassium concentration on heart rate in dehydrated calves with diarrhea. J Vet Intern Med. 1999;13:233. [Google Scholar]
- 66. Martinez‐Vea A, Bardají A, Garcia C, Oliver JA. Severe hyperkalemia with minimal electrocardiographic manifestations: A report of seven cases. J Electrocardiol. 1999;32:45–49. [DOI] [PubMed] [Google Scholar]
- 67. Szerlip HM, Weiss J, Singer I. Profound hyperkalemia without electrocardiographic manifestations. Am J Kidney Dis. 1986;7:461–465. [DOI] [PubMed] [Google Scholar]
- 68. Surawicz B, Chlebus H, Mazzoleni A. Hemodynamic and electrocardiographic effects of hyperpotassemia. Differences in response to slow and rapid increases in concentration of plasma K. Am Heart J. 1967;73:647–664. [DOI] [PubMed] [Google Scholar]
- 69. Aberra A, Komukai K, Howarth FC, Orchard CH. The effect of acidosis on the ECG of the rat heart. Exp Physiol. 2001;86:27–31. [DOI] [PubMed] [Google Scholar]
- 70. Nisbet AM, Burton FL, Walker NL, et al. Acidosis slows electrical conduction through the atrio‐ventricular node. Front Physiol. 2014;5:233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. DeRoth L. Electrocardiographic parameters in the normal lactating Holstein cow. Can Vet J. 1980;21:271–277. [PMC free article] [PubMed] [Google Scholar]
- 72. Rezakhani A, Paphan AA, Shekarfroush S. Analysis of base apex lead electrocardiograms of normal dairy cows. Vet Arhiv. 2004;74:351–358. [Google Scholar]
- 73. Smith CR, Hamlin RL, Crocker HD. Comparative electrocardiography. Ann NY Acad Sci. 1965;127:155–169. [DOI] [PubMed] [Google Scholar]
- 74. Hamlin RL, Smith CR. Categorization of common domestic mammals based upon their ventricular activation process. Ann NY Acad Sci. 1965;127:195–203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information 1
Supporting Information 2


