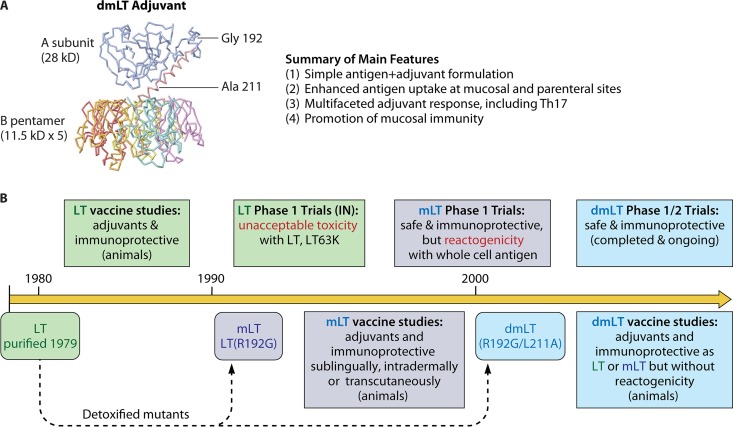
Perhaps the best-studied mucosal adjuvants are the bacterially derived ADP-ribosylating enterotoxins. This adjuvant family includes heat-labile enterotoxin of Escherichia coli (LT), cholera toxin (CT), and mutants or subunits of LT and CT.
KEYWORDS: adjuvant, dmLT, mucosal vaccines
ABSTRACT
Perhaps the best-studied mucosal adjuvants are the bacterially derived ADP-ribosylating enterotoxins. This adjuvant family includes heat-labile enterotoxin of Escherichia coli (LT), cholera toxin (CT), and mutants or subunits of LT and CT. These proteins promote a multifaceted antigen-specific response, including inflammatory Th1, Th2, Th17, cytotoxic T lymphocytes (CTLs), and antibodies. However, more uniquely among adjuvant classes, they induce antigen-specific IgA antibodies and long-lasting memory to coadministered antigens when delivered mucosally or even parenterally. The purpose of this minireview is to describe the general properties, history and creation, preclinical studies, clinical studies, mechanisms of action, and considerations for use of the most promising enterotoxin-based adjuvant to date, LT(R192G/L211A) or dmLT. This review is timely due to completed, ongoing, and planned clinical investigations of dmLT in multiple vaccine formulations by government, nonprofit, and industry groups in the United States and abroad.
INTRODUCTION
The adjuvant dmLT, or more technically LT(R192G/L211A), is an 84-kDa polymeric protein with an AB5 structure composed of an enzymatically active A subunit (28 kDa) noncovalently associated with a pentameric B subunit (consisting of five 11.5-kDa monomers) as shown in Fig. 1A. dmLT is distinguished from its parent molecule heat-labile enterotoxin (LT) by the substitution of two residues in the A subunit, a glycine for an arginine at amino acid 192 (R192G) and an alanine for a leucine at amino acid 211 (L211A). The ribbon diagram of dmLT can be extrapolated from the crystal structure of the partially cleaved LT toxin (1, 2), although there may be as-yet-unresolved changes in three-dimensional (3D) structure due to the amino acid substitutions in the A subunit.
FIG 1 .
dmLT adjuvant structure, summary of main features, and creation timeline. See text for details.
dmLT is an adjuvant that enhances vaccine-specific systemic and mucosal immune responses following mucosal or parenteral delivery, described in detail below (e.g., Table 1 and indicated references). These studies indicate four main features that define dmLT compared with other adjuvant systems.
TABLE 1 .
Preclinical vaccine studies with dmLTa
| Vaccine type and target pathogen | Antigen(s) | Route(s) | Model(s) | Major findings with adjuvant inclusion | Reference(s) |
|---|---|---|---|---|---|
| Subunit vaccines | |||||
| Clostridium difficile | PilA1, PilJ, PilW | p.o. | Mouse | NAO; low levels of antipilin antibodies and no protection from weight loss after oral challenge; no clear difference from other routes/adjuvant combinations | 91 |
| Clostridium difficile and Shigella species | Chimeric toxin A/toxin B; IpaB/IpaD | i.d., i.m., i.p. | Mouse | NAO; route and delivery comparison with microneedles: similar responses in dmLT-adjuvanted i.d. as alum-adjuvanted i.p. groups for antibody responses and response to C. difficile challenge; development of IgG and mucosal IgA after i.d. and i.m. vaccination with dmLT to Shigella | 61 |
| Clostridium tetani | Tetanus toxoid | p.o., i.n. | Mouse | Enhanced serum and mucosal antibodies, Th17 responses | 1, 8 |
| ETEC | EptA | i.d., p.o., s.l. | Mouse | NAO; route evaluation with adjuvanted vaccination: p.o., s.l., or i.d. delivery enhanced mucosal IgG and IgA; however, bacterial burden post-oral challenge reduced only with p.o. and s.l.; antibody avidity reduced in i.d. groups | 92 |
| Nontypeable H. influenzae otitis media | LB1, rsPilA, chimV4 | t.c.i. | Chinchilla | Enhanced skin DC migration to lymphoid organs, serum IgG and IgA, and clearance of bacteria from nasopharynges/middle ears when combined with any antigen vaccine (prophylactic or therapeutic) | 55 |
| chimV4 | t.c.i. | Chinchilla | NAO; enhanced mucosal IgG, IgA, IFN-γ+, and IL-17 CD4 T cells; eradication of bacterial burden and biofilms in middle ears | 22 | |
| chimV4 | t.c.i. | Chinchilla | NAO; enhanced antigen draining to lymphoid organs dependent upon therapeutic t.c.i. patch placement; enhanced Th1, Th17, ASCs, and mucosal IgM and IgG; reduced % of ears with otitis media and bacterial colonization observed with dmLT alone or in combination with antigen | 11 | |
| Polymicrobial otitis media | rsPilA, chimV4, IHC | t.c.i. | Chinchilla | NAO; enhanced antigen-specific ASCs and serum IgG, IgM, and IgA antibodies; reduced incidence of polymicrobial (viral-bacterial) otitis media and disease time course | 93 |
| Helicobacter pylori | Lysate antigens, HpaA, UreB | p.o. | Mouse | Enhanced serum IgG, mucosal IgA, and splenic and stomach Th1 and Th17 responses; reduced bacterial colonization in stomach after oral challenge (equivalent to cholera toxin) | 12 |
| Lysate antigens, freeze-dried | p.o., s.l. | Mouse | Increased activation of ex vivo DCs; enhanced Th1, Th17, and IL-17A+ IFN-γ+ CD4 T cells in DLN or stomach after s.l. but not p.o. (equivalent to cholera toxin) | 13 | |
| Hepatitis B virus | HBsAg (bioencapsulated in maize) | p.o. | Mouse | Nonsignificant enhancement of serum IgG and IgA antibody responses | 54 |
| Vibrio cholerae | Polysaccharide conjugate (OSP-TThc) | i.m. | Mouse | Enhanced level and vibriocidal activity of serum antibodies, formation of memory B cells, and survival by neonatal mice of lethal challenge | 57 |
| Shigella species | IpaB, IpaD, IpgC, IpaB/IpgC | i.n. | Mouse | NAO; antigen evaluation with adjuvanted i.n. vaccination: improvement of serum and mucosal IgG and IgA, ASCs, Th1 responses, and protection from i.n. challenge | 94 |
| IpaB/IpaD | i.d. | Mouse | Enhanced level of serum antibodies, protection from lethal i.n. challenge (antigen dose dependent) with microneedle delivery | 9 | |
| IpaB/IpaD | i.d., s.l. | Mouse | NAO; route evaluation with adjuvanted vaccination: i.d. delivery enhanced mucosal IgG; s.l. delivery enhanced mucosal IgG and IgA; i.d. plus s.l. exhibited maximal mucosal IgG and IgA | 23 | |
| PSSP-1 | i.n., i.d. | Mouse | NAO; enhanced protection to i.n. challenge with multiple Shigella species and serotypes after i.n. but not i.d. immunization | 60 | |
| Whole-killed vaccines | |||||
| ETEC | Whole-killed plus LCTBA (ETVAX) | p.o. | Mouse | Enhanced serum IgG and fecal IgA antibodies (equivalent or better than CT) | 59 |
| Helicobacter pylori | Formalin inactivated, liquid or freeze-dried | p.o. | Mouse | No significant increase in serum IgG responses; however, after oral challenge, reduced stomach urease activity and stomach bacterial burden (equivalent to or better than mLT) | 38 |
| Shigella species | Formalin inactivated trivalent (S. flexneri 2a and 3a and Shigella sonnei) | i.n. | Mouse, guinea pig | In mice, no major adjuvant effect except with low antigen dose (IgA and protection from lethal i.n. challenge); in guinea pig, same or worse response to ocular challenge | 58 |
| Streptococcus pneumoniae | Chloroform-killed nonencapsulated strain RM200 | b.c., s.l. | Mouse | Reduced bacterial burden after i.n. challenge and increased IL-17 secretion (comparison with nonadjuvanted groups performed with only mLT and CT adjuvants) | 10 |
| Poliovirus | Formalin inactivated trivalent (IPV) | s.l. | Mouse | Enhanced antigen dose-sparing, serum IgG, salivary IgA, and virus-neutralizing antibodies with thermoresponsive gel delivery system | 56 |
| i.d., i.m. | Mouse | Enhanced germinal center markers, antigen dose sparing, serum IgG, fecal IgA, and virus-neutralizing antibodies | 21 | ||
| Live-attenuated vaccines | |||||
| Salmonella enteritidis | Strain JOL1087 ± secreted dmLT | i.m., p.o. | Chicken | Biological incorporation of dmLT into vaccine strain increased plasma IgG, intestinal sIgA, PBMC stimulation, and splenic cytokines (IFN-γ, IL-6, and IL-10) and reduced bacterial colonization after oral challenge |
62, 90 |
Abbreviations: ASCs, antibody-secreting cells; DC, dendritic cell; DLN, draining lymph node; IHC, integration host factor; IPV, inactivated polio vaccine; NAO, no antigen-only group without dmLT.
-
(1)
dmLT promotes immunity to antigens that are codelivered after simply admixing dmLT and the antigen in aqueous buffer. Thus, unlike many depot-type adjuvants, such as aluminum hydroxide, no advanced preparation or absorption is required to formulate the antigen/adjuvant vaccine. dmLT can be formulated with the antigen at either the point of manufacture or the point of delivery.
-
(2)
Through the combined action of dmLT’s immunostimulatory properties and universal cell binding, uptake of codelivered antigens is enhanced and mucosal immunity is promoted. This enables the delivery of immunization formulations (most strikingly for subunit vaccines) at previously inaccessible sites, such as in oral (p.o.), sublingual (s.l.), transcutaneous (t.c.i.), etc., delivery. Many of these approaches are needle free and have the potential to increase ease of administration and compliance and lower the risk of disease outbreaks from unsafe injections (3–6).
-
(3)
Unlike other adjuvants, such as aluminum hydroxide or many Toll-like receptor (TLR)-based adjuvants (e.g., monophosphoryl lipid A [MPL] and CpG), dmLT induces strong interleukin-17 (IL-17) recall cytokine secretion and antigen-specific Th17 responses after parenteral or mucosal immunization (7–15). This is a newly appreciated arm of the adaptive immune response that is critical in protection from pathogens, particularly in preventing infections in mucosal tissue and control of bacterial infections (16). In addition, IL-17 secretion enhances the availability of mucosal antibodies by upregulating polymeric Ig receptor levels in epithelial cells, increasing transport of secretory IgA (sIgA) into the lumen of mucosal tissue, and promoting T-independent B-cell differentiation into IgA-secreting cells (17–20).
-
(4)
Last, dmLT promotes the development of mucosal immune responses following parenteral immunization (7, 21–23). While these observations are validated only in preclinical animal models thus far, this is a distinction from most vaccines delivered by parenteral injection, which can induce serum antibodies and cell-mediated immunity but only limited or nonexistent responses at mucosal surfaces. In the remaining sections, we will go into more detail on the history and creation of dmLT protein, preclinical studies, clinical studies, mechanisms of action, and considerations for use.
HISTORY OF LT-BASED PROTEINS AND dmLT CREATION
LT is expressed by enterotoxigenic Escherichia coli (ETEC) strains and was first purified by Clements and Finkelstein in 1979 (24). This effort differed from previous attempts to purify LT by conventional gel-filtration and ion-exchange chromatography as a result of their discovery that LT binds to galactose-containing gel filtration medium (e.g., agarose or immobilized d-galactose) and can be recovered by elution from columns following application of galactose-containing buffers. In addition, unlike cholera toxin (CT) from Vibrio cholerae (which had been purified in 1969), Clements and Finkelstein demonstrated that the majority of LT is not secreted from the bacterium in vitro but rather is in the periplasm in an unactivated form.
In the 1980s, CT was being used to investigate the intestinal IgA response, with Elson and Ealding elegantly demonstrating that CT can abrogate oral tolerance and promote serum IgG and mucosal IgA to p.o.-codelivered antigen (25, 26). Subsequent studies by Clements et al., published in 1988, demonstrated that LT could also prevent induction of oral tolerance and act as an oral adjuvant (27). In a prelude to the current understanding of tolerance and memory regulatory responses, they found that oral tolerance could be prevented if LT was included upon first exposure to an antigen but that tolerance could not be broken once established. In addition, enzymatic activity in the form of the A subunit (LTA) was required since recombinant LT B subunit (LTB) had no effect on induction of tolerance or oral adjuvanticity. A key component in the success and accuracy of this research was the use of recombinant proteins (e.g., LTB) as opposed to the products of dissociation chromatography using the holotoxin, the latter of which commonly resulted in CTB/LTB that was contaminated with a trace amount of holotoxin, which can complicate the interpretation of results in many older publications (28).
Despite contrary evidence in animal studies, clinical trials with human volunteers beginning in the 1990s demonstrated that even low doses (>2.5 µg) of p.o. LT induced diarrhea (29, 30). In parallel, Tamura et al. began testing native LT admixed with LTB as an intranasal (i.n.) adjuvant in humans (31). Subsequent animal studies by a variety of investigators showed excellent promotion of immune responses for multiple antigens delivered by this route. Initial success in clinical studies led to the licensure for an LT-adjuvanted inactivated influenza vaccine in Europe (Nasalflu; Berna Biotech), which was available in Switzerland for the 2000–2001 influenza season. Unfortunately, it soon became clear that the presence of LT was associated with cases of Bell’s palsy or facial paralysis in i.n. vaccine recipients, resulting in a 19-fold-higher risk of developing Bell’s palsy compared to case-controls in a retrospective study (32). Similar results were reported in phase 1 clinical trials with i.n. delivery of mutant LT(S63K) in adjuvanted HIV and tuberculosis subunit vaccines (33). Follow-up research suggests that the risk for Bell’s palsy after i.n. vaccination is a direct consequence of B-subunit neuronal ganglioside binding and retrograde axonal transport combined with the inflammatory response occurring with ADP-ribosyltransferase activity of intact AB5 proteins (34). While investigation into other administration routes continues, use of LT or any AB5 mutant protein for i.n. delivery in humans is unadvised based on these past safety issues (though our studies indicate that the enzymatically active A1 subunit [LTA1] free of any B subunit is a promising adjuvant for i.n. delivery [8]).
A number of investigators have genetically modified LT in an attempt to detoxify the molecule and make it safe for inclusion either as an antigen (in an ETEC vaccine) or for use as an adjuvant. Creation of dmLT was initiated through purposeful stepwise mutations of the LT holotoxin A subunit, based on our understanding of how the holotoxin interacts with mammalian cells. Induction of intestinal fluid secretion by LT and CT occurs after a series of events involving both changes to toxin structure and activation of intracellular signaling pathways, reviewed in reference 28. After B-subunit binding and entry, the subsequent proteolytic cleavage and disulfide bond reduction separate the A subunit into two domains: the enzymatically active A1 subunit and a smaller A2 peptide. Transport of A1 into the cytoplasm results in ADP-ribosylation of Gsα, followed by irreversible activation of adenylate cyclase and increases in intracellular levels of cAMP. In intestinal epithelial cells, this causes a dysregulation of cAMP-sensitive ion transport mechanisms which inhibits intracellular salt absorption, increases electrolyte transport into the gut lumen, and creates an osmotic gradient favoring intestinal water secretion (35).
Clements and Finkelstein had originally shown that proteolytic cleavage at position 192 was essential for activation of the LT molecule (24). Later in the early 1990s, Dickinson and Clements created LT(R192G), or mLT, by substituting a glycine for an arginine at position 192, thereby altering the proteolytically sensitive site in the A subunit that separates A1 and A2 and preventing trypsin cleavage and “activation” (36). In both in vitro assays and animal studies, mLT showed reduced toxicity (36, 37) but maintained adjuvanticity equivalent to LT, inducing a balanced Th1/Th2 cytokine and antibody subclass profile similar to native LT (10, 38–49). The success of these preclinical studies led to several clinical trials with mLT as an adjuvant. In a phase 1 escalating dose-safety study in adults, up to 50 µg of p.o. mLT given twice was well tolerated; however, 16.7% (2 of 12) of volunteers receiving a 100-µg mLT dose reported mild to moderate diarrhea (50). In contrast, >2.5 µg of native LT or CT causes diarrheal secretion in adult volunteers (29, 51). In other clinical studies, 25 µg of p.o. mLT alone was well tolerated, but when it was combined with killed whole-cell bacterial vaccines, 20% of adults receiving killed Campylobacter (52) or killed Helicobacter (53) p.o. vaccines experienced mild diarrhea. Thus, the combination of mLT and a whole-cell antigen delivered p.o. induced enough secretion to overcome the natural resorptive capacity of the intestine, resulting in the observed self-limited, mild diarrhea not seen with mLT alone.
In the early 2000s, in order to further detoxify mLT while preserving adjuvanticity, an additional mutation was added by changing leucine 211 to alanine (L211A) in the A1-A2 activation loop on the A2 domain. This resulted in the adjuvant dmLT. In a 2011 publication, we demonstrated that this extra mutation results in two significant differences: (i) dmLT is more sensitive to trypsin or intracellular proteolytic degradation (as opposed to activation) than either native LT or mLT, and (ii) 250 µg of dmLT completely lacks the ability to induce detectable intestinal secretion after p.o. feeding in a patent mouse assay compared with ≥5 µg LT or ≥125 µg mLT (1).
As summarized in Fig. 1B, dmLT is the product of more than 25 years of research on the use of bacterial ADP-ribosylating enterotoxins as adjuvants. While the adjuvant potential of LT and CT has been known for some time, reducing toxicity while preserving adjuvanticity was challenging. Our efforts to detoxify LT for use as an adjuvant through directed genetic mutations has yielded dmLT, whose mutations make it a subunit more vulnerable to intracellular proteolytic degradation but prevent cleavage into the highly enzymatically active A1 domain. However, dmLT maintains the immunostimulatory properties without associated epithelial cell cAMP intoxication or intestinal fluid secretion of the parent molecule LT or single mutant mLT.
PRECLINICAL STUDIES
Since 2000, a number of preclinical vaccine studies have been published with dmLT adjuvant, as listed in Table 1. Of these, most target bacterial pathogens with subunit antigens, although some have examined dmLT-adjuvanted whole-killed or live-attenuated bacterial and viral vaccines. The target pathogens for many of these vaccine candidates cause predominantly gastrointestinal infections, including Clostridium difficile, ETEC, Helicobacter pylori, poliovirus, Shigella, Salmonella, and V. cholerae. However, other mucosal pathogens—nontypeable Haemophilus influenzae and Streptococcus pneumoniae—or organisms causing systemic infections—Clostridium tetani and hepatitis B virus—have also been evaluated. In addition, there are a large number of unpublished preclinical studies that have been performed by various groups leading to clinical trials.
We and other researchers have documented improvement of parenteral and/or mucosal immunity to bacterial and viral antigens following p.o., buccal (in the mouth by the cheek [b.c.]), s.l. (in the mouth under the tongue), i.n., t.c.i., intradermal (i.d.), and/or intramuscular (i.m.) delivery of dmLT-adjuvanted vaccination (8, 9, 22, 23, 38, 54–59). By far the most common findings after either parenteral or mucosal immunization were improvement of humoral and systemic immunity—including neutralizing antibodies and serum or mucosal IgA and Th17 responses—and protection from lethal challenge or bacterial colonization. Comparative evaluations verified that the adjuvant effects with dmLT are fairly equivalent to those with LT, mLT, or CT adjuvants, including Th17 responses to mucosal pneumococcal vaccine (10), serum IgG and fecal IgA to colonizing factors and LTB after p.o. immunization with an engineered whole-killed ETEC vaccine (59), tetanus toxoid-specific serum IgG and fecal IgA after p.o. or i.n. immunization (1, 8), anti-Shigella PSSP-1 serum IgG1 or IgG2a (60), and immunity and protection against H. pylori infection after s.l. immunization with lysate antigens (12) or p.o. immunization with formalin-inactivated bacteria (38).
Various delivery techniques or devices for i.d. delivery did not impair the adjuvant effect of dmLT, including the use of transdermal silk fibroin microneedles (61) and NanoPass MicronJet 600 needles (9). Similarly, various antigen forms—including freeze-dried forms (13, 38), polysaccharide conjugates (57), and even a live-attenuated strain modified to express dmLT (62)—also demonstrated improved immunity with inclusion of the adjuvant.
In all these studies, only three studies reported lack of significant immunity with dmLT adjuvant with specific formulations. The first was a study immunizing mice p.o. with germ-enriched maize material expressing hepatitis B antigen; however, slightly higher (but nonsignificant) antibody titers were observed in the dmLT-containing group, suggesting that more animals (to better power the study) or an optimized dose may be needed to see a clear adjuvant effect with this vaccine formulation (54). The second study evaluated doses of a highly immunogenic trivalent formalin-inactivated Shigella vaccine that saw improvement only in mucosal IgA and protection from lethal challenge in mice receiving dmLT admixed with the lowest-formulation dose (58). In this same vaccination study, dmLT provided no benefit in a guinea pig model of Shigella keratoconjunctivitis, which may indicate a failure of dmLT to boost eye-specific immunity. Last, in another study with Shigella IpaB/IpaD subunit vaccination, dmLT was unable to skew immunity to a nonprotective high dose of antigen, even though inclusion of dmLT at a medium dose elicited maximal protection from i.n. challenge (9). These studies reveal an important consideration in any vaccine formulation—there is a limit to the adjuvant effect. The antigen formulation, delivery dose, route, and likely other factors continue to play major roles in the overall outcome of responses postvaccination.
An interesting finding reported in several preclinical studies was that the dmLT-only control group provided some nonspecific protection from disease. A t.c.i. band-aid application of 10 µg dmLT significantly reduced the percentage of infected middle ears and bacterial burden of existing nontypeable H. influenzae biofilms in chinchillas despite any observable enhancement of antigen-specific cellular or humoral immunity (11). Similarly, fecal shedding after oral Campylobacter jejuni challenge was decreased (although the percentage of animals colonized was not) in mice who had been treated with three weekly doses of dmLT 1 week prior to infection (63). In this study, there was also no detection of antigen-specific immunity to C. jejuni. Last, mice receiving 5 µg dmLT i.n. on days 0 and 14 exhibited 64% survival of lethal Shigella flexneri 2a pulmonary challenge on day 21 (58).
In several studies, dmLT has been evaluated as a toxoid antigen for ETEC vaccines. dmLT can generate anti-LT antibody responses, detected routinely after p.o. or parenteral immunization (1, 9, 59, 63, 64). It has also been incorporated in a multiepitope fusion antigen for ETEC vaccination to neutralize activity of LT toxin (65). Of note, the existence of preexisting antibodies to dmLT does not impair the ability of subsequent immunization to provide adjuvant effects to a de novo antigen not previously seen by the immune system (E. B. Norton and J. D. Clements, unpublished data).
The consensus of these studies is that dmLT is uniquely able to induce systemic and mucosal responses after parenteral or mucosal immunization. The success of these studies has also led to recent preformulation studies evaluating buffers to promote stability and freeze-dried formulations to maximize dmLT adjuvant and/or antigen responses (66, 67). dmLT has also been used in immunologic studies for mucosal vaccination in order to evaluate how immunity is generated or impacted by infections (68, 69). In addition, as discussed in the next section, there are now several completed and planned clinical studies using dmLT.
CLINICAL STUDIES
As shown in Table 2, dmLT has now been tested for safety and efficacy in several ongoing and recently completed human clinical trials. There have been two dmLT-only phase 1 safety studies. The first trial (NCT01147445) was a dose escalation study with a single dose of 5 to 100 µg p.o. dmLT. Treatment was well tolerated with no reported diarrhea, abdominal pain, anorexia, nausea, vomiting, or fever in any adult subject (70). The second dmLT-only phase 1 safety study (NCT02052934) was conducted to assess the safety and tolerability of dmLT when administered in three 1- to 50-µg s.l. doses compared with three 25-µg p.o. doses of dmLT in healthy adult subjects. This study also assessed long-term safety through 7 months postvaccination. While this study has concluded, no results have been reported to date. An additional phase 1 safety trial (NCT02531685) with dmLT i.d. administration is currently recruiting. The primary objective of this study is to assess the safety and tolerability of dmLT when administered in three i.d. injections over a range of dosages in healthy adult subjects.
TABLE 2 .
Completed and ongoing clinical trials with dmLT adjuvanta
| Pathogen: antigen |
Route | Study design (Clinicaltrials.gov ID [reference {if available}]) |
Population; status: results |
|---|---|---|---|
| None: none | p.o. | Phase 1, escalating dose safety study (NCT01147445 [70]) | U.S. adults; completed: no detectable SAE |
| ETEC: whole-killed (ETVAX) | p.o. | Phase 1, whole cells ± 10 or 25 µg dmLT (EudraCT no. 2011-003228-11 [71]) | Swedish adults; completed: 10 µg dmLT enhanced responses to less immunogenic antigens |
| ETEC: live-attenuated (ACE527) | p.o. | Phase 1 and 2b, 25 µg dmLT with live-attenuated ETEC (NCT01739231 [72]) | U.S. adults; completed: dmLT enhanced protective efficacy from oral challenge 6–7 mo postimmunization |
| None: none | s.l. | Phase 1, escalating dose safety study (NCT02052934) | U.S. adults; completed |
| None: none | i.d. | Phase 1, escalating dose of 0.1, 0.3, 1, or 2 µg dmLT (NCT02531685) | U.S. adults; recruiting |
| ETEC: whole-killed (ETVAX) | p.o. | Phase 1 and 2 escalating dose of ETVAX ± 2.5, 5, or 10 µg dmLT (NCT02531802) | Bangladesh infants, toddlers, children, adults; completed |
| ETEC: subunit (CS6) | i.m. | Phase 1, escalating dose safety study (NCT03404674) | U.S. adults; recruiting |
| ETEC: whole-killed (ETVAX) | p.o. | Phase 2b, ETVAX plus 10 µg dmLT (EudraCT no. 2016-002690-35) | Finnish adult travelers to Benin; ongoing |
Abbreviations: SAE, severe adverse events; ID, identifier.
There have also been a number of phase 1 and 2 trials with dmLT as one component of killed or live-attenuated whole-cell ETEC vaccines that are either completed or ongoing. The killed whole-cell vaccine (ETVAX) is a formalin-inactivated, recombinant E. coli expressing increased levels of ETEC colonization factors (CFs) combined with a recombinant protein (LCTBA), which is a hybrid between the binding subunits of LTB and CT B subunit. The first of these studies (EudraCT no. 2011-003228-11) evaluated the safety of ETVAX administered alone or combined with 10 µg or 25 µg dmLT in two p.o. doses (71). This study observed only minor adverse events and reported no significant differences in adverse events between vaccination groups with and those without dmLT. The vaccine response generated was potent even in the nonadjuvanted group; however, 10 µg dmLT boosted immunity to the least immunogenic antigen evaluated, CS6, and increased the percentage of IgA responders to all five antigens in the vaccine strain to 83% from 74%. In this study, the 10-µg dose of dmLT was optimal. Since none of these studies were performed without the 1-mg dose of LCTBA, the influence of a 25- to 100-fold excess of a B-subunit antigen that competes for receptor binding with dmLT cannot be determined.
A follow-up phase 1 and 2 double-blind, placebo-controlled, dose-escalation study (NCT02531802) evaluating the safety, tolerability, and immunogenicity of ETVAX alone and together with dmLT in descending age groups (45 years to 6 months) was conducted in Bangladesh. This study observed some vomiting in young children when a full adult dose was administered, but the vaccine was tolerable at fractional doses with no significant differences in adverse events in vaccine groups with or without dmLT (F. Qadri, M. Chowdhury, T. Bhuiyan, M. Akhtar, F. Khanam, T. Ahmed, A. Lundgren, L. Bourgeois, R. Walker, N. Maier, A. Fix, T. Wierzba, and A. Svennerholm, presented at the Second International Conference on Vaccines for Shigella and ETEC [VASE], Mexico City, Mexico, 13 to 15 June 2018). An immunologic assessment is under way. The same vaccine (ETVAX plus 10 µg dmLT) is currently being evaluated in a phase 2b trial (EudraCT no. 2016-002690-35) to evaluate safety, immunogenicity, and diagnostic methodology and estimate vaccine efficacy of an oral ETEC vaccine for prevention of clinically significant ETEC diarrhea in healthy adult travelers visiting West Africa.
The use of dmLT with a live-attenuated ETEC vaccine, ACE527, was evaluated in a phase 1 and 2b challenge trial (NCT01739231). For this study, individuals were immunized three times p.o. with ACE527, an admixture of three frozen or lyophilized attenuated ETEC strains expressing CFA/I, CS1, CS2, CS3, CS5, CS6, and LTB, with or without 25 µg dmLT. Six to seven months after the last immunization, individuals were orally challenged with ETEC strain H10407 and monitored for moderate to severe diarrhea (MSD), stool volume, number of stools, and shedding of the challenge strain. Significantly, the protective efficacy of the vaccine against MSD in individuals immunized with ACE527 plus dmLT was 65.9%, compared to 20% in individuals receiving ACE527 alone (72). Addition of dmLT also increased the protective efficacy against diarrhea of any severity to 58.5% compared to −3.7% in individuals receiving ACE527 alone, reduced the mean stool volume (30 g versus 859 g) and total number of stools (1 versus 13), and produced a 13-fold reduction in shedding of the challenge strain compared to individuals receiving ACE527 alone. The mechanism of protection engendered by inclusion of dmLT in the formulation is not clearly defined, but studies to evaluate this are under way.
Recruitment is under way for one additional ETEC vaccine trial (NCT03404674). This will be a phase 1 open-label, dose-escalating study of 5 to 45 µg of a prototype CS6 vaccine (CssBA) administered three times i.m. with and without 0.1 to 0.5 µg of dmLT. The purpose will be to examine reactogenicity of the antigen, adjuvant, and formulation when administered i.m. This will be the first use of dmLT i.m. in humans.
These clinical studies indicate that dmLT is safe and efficacious. More information will become available as study results are reported in the future.
MECHANISM OF ACTION
The broad mechanisms of action for LT, CT, and related proteins have been well established over the past decades and extensively reviewed (28, 73), and slight but significant differences in the immunologic biases between CT- and LT-based adjuvants have consistently been reported in the literature (e.g., references 28 and 74). Still, the subunits of these AB5 proteins contribute uniquely to their mechanisms of action. The B subunit is responsible for receptor binding, leading to cellular entry, and, during mucosal delivery, helping shuttle whole-vaccine antigens across mucosal surfaces (75). The A subunit binds to cytosolic proteins (e.g., ADP-ribosylation factors [ARFs]) and ADP-ribosylates Gsα, resulting in irreversible adenylate cyclase activation and accumulation of intracellular cAMP. The complete immunologic effects of LT appear to require the B subunit, A subunit, and some level of cAMP induction. LT promotes in vitro dendritic cell activation, cytokine secretion, and Th17 cell induction through processes that can be mimicked with cAMP analogs or other cAMP-inducing agents like forskolin (75, 76). However, when isolated B subunit (e.g., LTB) or enzymatically inactive mutants of LT are substituted for native LT as adjuvants, reduced or ablated immunologic effects are observed (37, 77–79). In recent studies, we have demonstrated that purified A subunit of LT has adjuvant properties by itself, inducing a similar quality (e.g., mixed Th1/Th2/Th17) but smaller magnitude of immune responses than native LT, whereas the B subunit alone induces a more Th2/T regulatory cell (Treg)-skewed response (8).
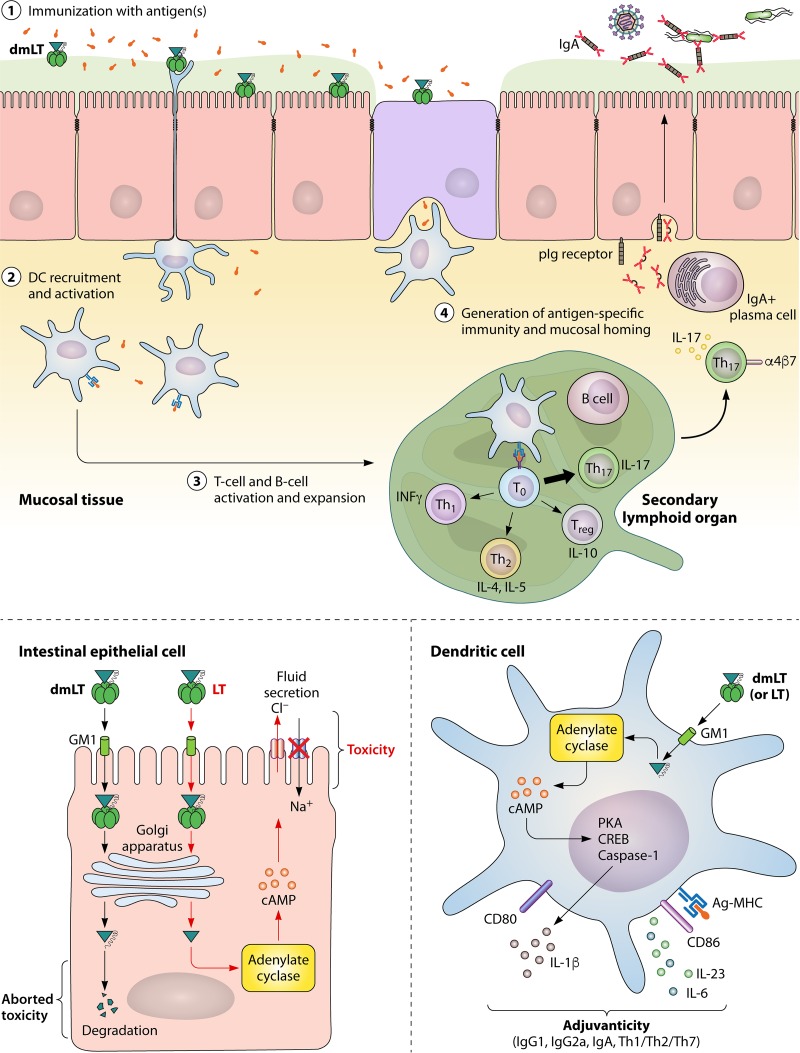
A major question with these adjuvants has always been whether toxicity can be divorced from adjuvanticity. With dmLT, we have determined that detoxification occurs without a reduction in adjuvanticity due to cell-specific effects. dmLT treatment results in no detectable intestinal secretion and over 1,000 times less cAMP in cultured epithelial cells compared to native LT (1), similar to the difference in secretion observed in human volunteers after p.o. treatment with LT versus dmLT (29, 30, 70). In contrast, during comparative vaccine studies dmLT exhibits adjuvanted immunity equivalent to that of LT, mLT, or CT (1, 8, 10, 12, 38, 60). In our newer experiments with murine dendritic cells (DCs), key initiating cells of the immune system, there are surprisingly few differences between cAMP levels, activation, cytokine secretion, and expansion of CD4 T cells between LT, mLT, and dmLT adjuvants (unpublished data). Thus, detoxification of LT into dmLT results in a cellular specific change in the mechanisms of action on epithelial cells responsible for secretion but not DCs responsible for immune stimulation.
The immunologic events that occur with dmLT adjuvant can be summed up in the steps depicted in Fig. 2 (with supporting evidence also described below). First, at the site of immunization, antigen uptake and activation of innate immunity are promoted, including cytokine/chemokine (IL-8, granulocyte colony-stimulating factor [G-CSF]) production by epithelial cells. Second, DCs are recruited to the site of immunization and activated for antigen processing/presentation (including antigen loading of major histocompatibility complex [Ag-MHC]), upregulation of costimulatory molecules (CD80 and CD86), and polarizing cytokine secretion (IL-1, IL-23, IL-6, and G-CSF). Third, these adjuvant-activated and antigen-loaded DCs drain to secondary lymphoid organs and mediate production of antigen-specific T-helper (Th) cells and B-cell differentiation into IgA/IgG antibody-secreting plasma cells (PC). A mixed Th1/Th2/Th17 response is induced after immunization, with particularly strong induction of Th17 cells (1, 10, 15, 69) and mucosal homing markers (7). Th17 cells are recognized as integral in immunity, including promoting germinal center formation in secondary lymphoid organs and enhancing IgA secretion (18–20).
FIG 2 .
Mechanisms of the adjuvant dmLT. See text for details.
The role for DCs as a central orchestrator for immunity has strong scientific evidence. Ex vivo treatment of small intestinal explants with dibutyryl cAMP, mLT, LT, or CT results in recruitment of DCs to lymphoid follicle-associated epithelia for enhanced luminal antigen uptake (75). Depletion of DCs prior to p.o. CT administration inhibits generation of adjuvant-associated CD4 T-cell and antibody responses unless much higher doses of antigen are used (80). Other studies have shown that DC treatment with LT and other cAMP stimulants (including dibutyryl cAMP, forskolin, and phosphodiesterase inhibitors) increases chemokine expression (81), upregulation of maturation markers (76, 82, 83), and antigen presentation for T-cell responses, including Th2 (84, 85), Th1 (86), and Th17 (8, 10, 83, 87, 88). Similarly, after i.d. microneedle injection with dmLT-adjuvanted vaccine, murine CD11c+ DCs in the skin were shown to take up Shigella subunit antigens as soon as 4 h postimmunization (although other antigen-presenting cells, including neutrophils, macrophages, and Langerhans cells, also took up antigen) (9). In a chinchilla model of nontypeable H. influenzae otitis media, carboxyfluorescein succinimidyl ester (CFSE)-labeled dermal DCs (DC-SIGN+ CD11c+ CD207−) were observed in the nasal-associated lymphoid tissue (NALT) after t.c.i. immunization with dmLT and antigen (55). In another paper by this same group, the authors were able to detect labeled dmLT and antigen in CD11c+ secondary lymphoid organs that depended upon the placement of a t.c.i. band-aid vaccine (22).
The activation of DCs with LT and dmLT triggers a strong Th17-biased response due to activation of caspase 1 inflammasome and subsequent secretion of specific secreted factors, including IL-1 and IL-23. Brereton et al. elegantly showed that mouse dendritic cells stimulated with LT secrete IL-1β that is required for generation of Th17 cells and can be inhibited with pretreatment with caspase 1 and NLRP3 inflammasome chemical inhibitors (83). Similarly, restimulation of human peripheral blood mononuclear cells (PBMCs) from recently vaccinated individuals with dmLT and vaccine antigens enhanced IL-17A and IL-13 secretion that was prevented when cultures included anti-IL-1β and anti-IL-23 neutralizing antibodies (15). In a follow-up study, these authors demonstrated that dmLT-stimulated PBMC cytokine expression could be detected through intracellular staining of CD4 T cells and that this adjuvant effect on PBMCs or isolated monocytes could be prevented with inhibition of protein kinase A (PKA), IL-1RA (soluble IL-1 receptor), or caspase 1 (14). The consequences of this antigen-presenting cell (APC) stimulation are immune activation and promotion of mucosal and systemic immunity. Th17 induction and IgA isotype class switching are also important mediators of the effect of dmLT. In addition, using tetramer CD4 T-cell studies in mice, Frederick et al. have also shown upregulation of mucosal homing markers α4β7 on lymphocytes in draining lymph nodes after i.d. or i.m. immunization and corresponding localization of T cells within intestinal tissue (7). This indicates that something in the adjuvant-DC interaction promotes upregulation of gut homing markers, although at this point the exact mechanism is unclear.
Intriguingly, as reported above, several studies now indicate that dmLT can also provide nonspecific protection from disease (11, 58, 63). These reports are similar to older studies done by us and others with related proteins. For example, we have shown that 5 µg CT or mLT improves survival and decreases weight loss from influenza virus pulmonary challenge 24 h after a single or double weekly i.n. treatment (74). Improved survival with this regimen correlated with appearance of inducible bronchus-associated tissue (iBALT) structures in the lung. Similarly, a lower trend of lung CFU after Streptococcus pneumoniae nasopharyngeal challenge was observed in mice who had received their last boost of CT or mLT by i.n. immunization 1 month earlier (10). These studies indicate that activation of innate immunity is likely occurring to mediate these effects. Whether these represent long-term or epigenetic changes like those observed with trained immunity (89) is still unclear and requires further study.
Last, it is important to note that responses generated with dmLT may not be equivalent to all enterotoxins, particularly when specific mechanisms or secretion of specific cytokines is being measured (12, 14). For example, dmLT alteration of gene expression in murine splenic CD11c+ DCs only partially overlaps those of CT, including some notable differences in TSP-1, IL-6, MIP-2α (higher with CT), and gamma interferon (IFN-γ) (higher with dmLT) (12). These slight variations may have important considerations in generation of protective immunity.
In conclusion, studies to date indicate that a precise series of events takes place with dmLT stimulation that results in a protein that cannot induce enterocyte intoxication but is a potent stimulator of APCs and vaccine immunity. Future research into the precise intracellular mechanisms responsible for these adjuvant-induced responses is warranted.
CONSIDERATIONS FOR USE
dmLT adjuvant has the unique potential to promote protective, long-lasting immunity to vaccine antigens when included in vaccination. However, as with any aspect of a vaccine formulation there are several caveats of use that should be carefully scrutinized.
-
(1)
Is dmLT the best adjuvant for anticipated delivery route? dmLT is a good adjuvant to promote dose sparing, mucosal immunity, and/or mucosal delivery. In the last characteristic, dmLT is one of the few adjuvants that can directly assist antigen uptake at mucosal sites through the activity of its B subunit. Current data indicate that dmLT is safe and efficacious in promoting antigen responses with s.l. and p.o. routes but should not be used i.n. Less information is available for i.d. and i.m. routes, but ongoing clinical trials will determine this and also provide clinical data that dmLT may be able to safely promote mucosal immunity even with parenteral delivery routes.
-
(2)
Is dmLT the best adjuvant to promote immunologic biases associated with antigen-specific disease protection? dmLT adjuvant enhances a mixed Th1/Th2/Th17 cellular response and enhances magnitude and longevity of antibody responses. However, the specific outcome of any adjuvanted vaccine responses will be heavily influenced by the nature of the antigen itself. This has been demonstrated recently by Leach et al., examining IL-17 secretion in human cells restimulated with human vaccine antigens and dmLT (15). Immunologic outcomes will need to be evaluated for each antigen(s), which may be complicated by delivery route, dose of antigen or adjuvant, and interactions between vaccine components (e.g., TLR ligands).
-
(3)
Which dose of dmLT adjuvant should be used? Route studies have demonstrated clear differences in doses of dmLT required by each route. For example, dmLT or similar adjuvants are commonly used at a 10- to 25-µg range p.o., 1 to 5 µg s.l., 10 to 50 µg t.c.i., or 0.1 to 5 µg parenterally. However, many studies have indicated that these adjuvant doses may depend upon the nature of the antigen (e.g., subunit, whole-killed, or live-attenuated), as the combination with inflammation induced by antigen alone may play a significant role in immunologic outcomes. Basic immunology studies, furthermore, have clearly indicated that “more is not always better.” Too much inflammation or high doses of antigen can suppress the magnitude of responses and also bias to a stronger antibody/Th2 response. This appeared to be the case in ETVAX clinical trials, where 10 µg p.o. dmLT was superior to 25 µg (71). We also observed higher induction of antibodies and lower development of Th1/Th17 memory responses in mice immunized i.m. or i.d. with 1 µg dmLT and a Mycobacterium tuberculosis subunit antigen at 5 µg compared with 0.5 µg antigen (Norton and Clements, unpublished).
-
(4)
Potential differences between human and animal models. The enterotoxin family of adjuvants has been successfully used in fish, avian, rodent, rabbit, pig, monkey, and human species (e.g., reference 90), often at relatively similar doses between species, pointing to their versatility as an adjuvant family. However, there may be species differences in receptor-ligand interactions or other host cell factors related to dmLT and possibly those pertinent to other vaccine antigens. Therefore, one should be careful in extrapolating or assuming that vaccine formulation research in animal models will perfectly mimic human responses.
CONCLUSION AND FUTURE DIRECTIONS
In conclusion, dmLT is the product of more than 25 years of research. It was the purpose of this review to succinctly describe the history of dmLT and all published studies with dmLT, thereby providing the first review on this adjuvant protein. Both preclinical and clinical data thus far indicate that dmLT is a safe and effective adjuvant that in the right formulation can promote protective immunity. Unanswered questions for future studies include limitation on use of dmLT adjuvant, safety of antigen-adjuvant combinations, and formulation optimizations (including for route and non-cold-chain delivery, such as stability buffers). In addition, the mechanisms of adjuvanticity and generation of protective immunologic outcomes with dmLT adjuvant require more detailed study. Similarly, comparisons of dmLT with older enterotoxin adjuvants (e.g., LTK63 or LTR72) or other adjuvant classes (e.g., alum and TLR agonists) or even in combinatorial adjuvant formulations are warranted.
ACKNOWLEDGMENTS
dmLT is patented by J. D. Clements under U.S. patent no. 6,033,673, “Double mutant enterotoxin for use as an adjuvant.”
We thank Robin L. Baudier for technical editing advice.
Research reported in this publication was supported by the NIAID/NIH under award no. R01AI114697 (principal investigator E. B. Norton) and BAA-NIAID-DAIT-NIH2012146 (principal investigator J. D. Clements). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Biographies
John D. Clements is a Professor of Microbiology and Immunology at Tulane University School of Medicine. After receiving his doctorate from the University of Texas Health Science Center at Dallas, Dr. Clements completed a National Research Council Associateship at Walter Reed Army Institute of Research in Washington, DC. In 1982, Dr. Clements joined the faculty at Tulane University. Dr. Clements served as Professor and Chair of the Department of Microbiology and Immunology from 1999 to 2018. Dr. Clements’ expertise is in vaccine development, adjuvants, delivery systems, and understanding of vaccine-induced immunity. His laboratory has developed novel adjuvants that enhance the immune response to foreign antigens delivered mucosally, parenterally, or dermally. These adjuvants have been shown to enable protection against a number of bacterial and viral pathogens, including Salmonella, Shigella, enterotoxigenic E. coli (ETEC), V. cholerae, M. tuberculosis, and poliovirus.
Elizabeth B. Norton is an Assistant Professor at Tulane University focused on mucosal immunology and clinical therapies. She performed initial training at the CDC in Atlanta, GA, identifying correlates of sepsis in children in sub-Saharan Africa, and later received her M.P.H. in international health and Ph.D. in biomedical sciences at Tulane University. Working with Dr. Clements as a postdoctoral fellow and later as faculty, she has served as project manager and coinvestigator for dmLT-adjuvanted vaccine studies with ETEC, polio, and tuberculosis in mice and nonhuman primates. She has now been investigating LT-based adjuvants for over 15 years. Her lab has continued this inquiry on both optimal use and immunologic mechanisms of LT-based adjuvants (dmLT and LTA1) or related proteins for disease therapies.
REFERENCES
- 1.Norton EB, Lawson LB, Freytag LC, Clements JD. 2011. Characterization of a mutant Escherichia coli heat-labile toxin, LT(R192G/L211A), as a safe and effective oral adjuvant. Clin Vaccine Immunol 18:546–551. doi: 10.1128/CVI.00538-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merritt EA, Pronk SE, Sixma TK, Kalk KH, van Zanten BA, Hol WG. 1994. Structure of partially activated E. coli heat-labile enterotoxin (LT) at 2.6 A resolution. FEBS Lett 337:88–92. doi: 10.1016/0014-5793(94)80635-7. [DOI] [PubMed] [Google Scholar]
- 3.Coleman BL, McGeer AJ, Halperin SA, Langley JM, Shamout Y, Taddio A, Shah V, McNeil SA. 2012. A randomized control trial comparing immunogenicity, safety, and preference for self- versus nurse-administered intradermal influenza vaccine. Vaccine 30:6287–6293. doi: 10.1016/j.vaccine.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Kim YC, Jarrahian C, Zehrung D, Mitragotri S, Prausnitz MR. 2012. Delivery systems for intradermal vaccination. Curr Top Microbiol Immunol 351:77–112. doi: 10.1007/82_2011_123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh J, Bhatia R, Gandhi JC, Kaswekar AP, Khare S, Patel SB, Oza VB, Jain DC, Sokhey J. 1998. Outbreak of viral hepatitis B in a rural community in India linked to inadequately sterilized needles and syringes. Bull World Health Organ 76:93–98. [PMC free article] [PubMed] [Google Scholar]
- 6.Aylward B, Kane M, McNair-Scott R, Hu DJ, Hu DH. 1995. Model-based estimates of the risk of human immunodeficiency virus and hepatitis B virus transmission through unsafe injections. Int J Epidemiol 24:446–452. doi: 10.1093/ije/24.2.446. [DOI] [PubMed] [Google Scholar]
- 7.Frederick DR, Goggins JA, Sabbagh LM, Freytag LC, Clements JD, McLachlan JB. 2018. Adjuvant selection regulates gut migration and phenotypic diversity of antigen-specific CD4(+) T cells following parenteral immunization. Mucosal Immunol 11:549–561. doi: 10.1038/mi.2017.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Norton EB, Lawson LB, Mahdi Z, Freytag LC, Clements JD. 2012. The A-subunit of Escherichia coli heat-labile enterotoxin functions as a mucosal adjuvant and promotes IgG2a, IgA and Th17 responses to vaccine antigens. Infect Immun 80:2426–2435. doi: 10.1128/IAI.00181-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heine SJ, Diaz-McNair J, Andar AU, Drachenberg CB, van de Verg L, Walker R, Picking WL, Pasetti MF. 2014. Intradermal delivery of Shigella IpaB and IpaD type III secretion proteins: kinetics of cell recruitment and antigen uptake, mucosal and systemic immunity, and protection across serotypes. J Immunol 192:1630–1640. doi: 10.4049/jimmunol.1302743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu YJ, Yadav P, Clements JD, Forte S, Srivastava A, Thompson CM, Seid R, Look J, Alderson M, Tate A, Maisonneuve JF, Robertson G, Anderson PW, Malley R. 2010. Options for inactivation, adjuvant, and route of topical administration of a killed, unencapsulated pneumococcal whole-cell vaccine. Clin Vaccine Immunol 17:1005–1012. doi: 10.1128/CVI.00036-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Novotny LA, Clements JD, Bakaletz LO. 2015. Therapeutic transcutaneous immunization with a band-aid vaccine resolves experimental otitis media. Clin Vaccine Immunol 22:867–874. doi: 10.1128/CVI.00090-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sjökvist Ottsjö L, Flach CF, Clements J, Holmgren J, Raghavan S. 2013. A double mutant heat-labile toxin from Escherichia coli, LT(R192G/L211A), is an effective mucosal adjuvant for vaccination against Helicobacter pylori infection. Infect Immun 81:1532–1540. doi: 10.1128/IAI.01407-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sjökvist Ottsjö L, Jeverstam F, Yrlid L, Wenzel AU, Walduck AK, Raghavan S. 2017. Induction of mucosal immune responses against Helicobacter pylori infection after sublingual and intragastric route of immunization. Immunology 150:172–183. doi: 10.1111/imm.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larena M, Holmgren J, Lebens M, Terrinoni M, Lundgren A. 2015. Cholera toxin, and the related nontoxic adjuvants mmCT and dmLT, promote human Th17 responses via cyclic AMP-protein kinase A and inflammasome-dependent IL-1 signaling. J Immunol 194:3829–3839. doi: 10.4049/jimmunol.1401633. [DOI] [PubMed] [Google Scholar]
- 15.Leach S, Clements JD, Kaim J, Lundgren A. 2012. The adjuvant double mutant Escherichia coli heat labile toxin enhances IL-17A production in human T cells specific for bacterial vaccine antigens. PLoS One 7:e51718. doi: 10.1371/journal.pone.0051718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Veldhoen M. 2017. Interleukin 17 is a chief orchestrator of immunity. Nat Immunol 18:612–621. doi: 10.1038/ni.3742. [DOI] [PubMed] [Google Scholar]
- 17.Doreau A, Belot A, Bastid J, Riche B, Trescol-Biemont MC, Ranchin B, Fabien N, Cochat P, Pouteil-Noble C, Trolliet P, Durieu I, Tebib J, Kassai B, Ansieau S, Puisieux A, Eliaou JF, Bonnefoy-Bérard N. 2009. Interleukin 17 acts in synergy with B cell-activating factor to influence B cell biology and the pathophysiology of systemic lupus erythematosus. Nat Immunol 10:778–785. doi: 10.1038/ni.1741. [DOI] [PubMed] [Google Scholar]
- 18.Mitsdoerffer M, Lee Y, Jäger A, Kim HJ, Korn T, Kolls JK, Cantor H, Bettelli E, Kuchroo VK. 2010. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc Natl Acad Sci U S A 107:14292–14297. doi: 10.1073/pnas.1009234107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaffar Z, Ferrini ME, Herritt LA, Roberts K. 2009. Cutting edge: lung mucosal Th17-mediated responses induce polymeric Ig receptor expression by the airway epithelium and elevate secretory IgA levels. J Immunol 182:4507–4511. doi: 10.4049/jimmunol.0900237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaffar Z, Ferrini ME, Girtsman TA, Roberts K. 2009. Antigen-specific Treg regulate Th17-mediated lung neutrophilic inflammation, B-cell recruitment and polymeric IgA and IgM levels in the airways. Eur J Immunol 39:3307–3314. doi: 10.1002/eji.200939498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Norton EB, Bauer DL, Weldon WC, Oberste MS, Lawson LB, Clements JD. 2015. The novel adjuvant dmLT promotes dose sparing, mucosal immunity and longevity of antibody responses to the inactivated polio vaccine in a murine model. Vaccine 33:1909–1915. doi: 10.1016/j.vaccine.2015.02.069. [DOI] [PubMed] [Google Scholar]
- 22.Novotny LA, Clements JD, Bakaletz LO. 2013. Kinetic analysis and evaluation of the mechanisms involved in the resolution of experimental nontypeable Haemophilus influenzae-induced otitis media after transcutaneous immunization. Vaccine 31:3417–3426. doi: 10.1016/j.vaccine.2012.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee S, Picking WL, Tzipori S. 2014. The immune response of two microbial antigens delivered intradermally, sublingually, or the combination thereof. Microbes Infect 16:796–803. doi: 10.1016/j.micinf.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 24.Clements JD, Finkelstein RA. 1979. Isolation and characterization of homogeneous heat-labile enterotoxins with high specific activity from Escherichia coli cultures. Infect Immun 24:760–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elson CO, Ealding W. 1984. Cholera toxin feeding did not induce oral tolerance in mice and abrogated oral tolerance to an unrelated protein antigen. J Immunol 133:2892–2897. [PubMed] [Google Scholar]
- 26.Elson CO, Ealding W. 1984. Generalized systemic and mucosal immunity in mice after mucosal stimulation with cholera toxin. J Immunol 132:2736–2741. [PubMed] [Google Scholar]
- 27.Clements JD, Hartzog NM, Lyon FL. 1988. Adjuvant activity of Escherichia coli heat-labile enterotoxin and effect on the induction of oral tolerance in mice to unrelated protein antigens. Vaccine 6:269–277. doi: 10.1016/0264-410X(88)90223-X. [DOI] [PubMed] [Google Scholar]
- 28.Freytag LC, Clements JD. 2005. Mucosal adjuvants. Vaccine 23:1804–1813. doi: 10.1016/j.vaccine.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 29.Banerjee S, Medina-Fatimi A, Nichols R, Tendler D, Michetti M, Simon J, Kelly CP, Monath TP, Michetti P. 2002. Safety and efficacy of low dose Escherichia coli enterotoxin adjuvant for urease based oral immunisation against Helicobacter pylori in healthy volunteers. Gut 51:634–640. doi: 10.1136/gut.51.5.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michetti P, Kreiss C, Kotloff KL, Porta N, Blanco JL, Bachmann D, Herranz M, Saldinger PF, Corthésy-Theulaz I, Losonsky G, Nichols R, Simon J, Stolte M, Ackerman S, Monath TP, Blum AL. 1999. Oral immunization with urease and Escherichia coli heat-labile enterotoxin is safe and immunogenic in Helicobacter pylori-infected adults. Gastroenterology 116:804–812. doi: 10.1016/S0016-5085(99)70063-6. [DOI] [PubMed] [Google Scholar]
- 31.Tamura S, Asanuma H, Tomita T, Komase K, Kawahara K, Danbara H, Hattori N, Watanabe K, Suzuki Y, Nagamine T, Aizawa C, Oya A, Kurata T. 1994. Escherichia coli heat-labile enterotoxin B subunits supplemented with a trace amount of the holotoxin as an adjuvant for nasal influenza vaccine. Vaccine 12:1083–1089. doi: 10.1016/0264-410X(94)90177-5. [DOI] [PubMed] [Google Scholar]
- 32.Mutsch M, Zhou W, Rhodes P, Bopp M, Chen RT, Linder T, Spyr C, Steffen R. 2004. Use of the inactivated intranasal influenza vaccine and the risk of Bell’s palsy in Switzerland. N Engl J Med 350:896–903. doi: 10.1056/NEJMoa030595. [DOI] [PubMed] [Google Scholar]
- 33.Lewis DJ, Huo Z, Barnett S, Kromann I, Giemza R, Galiza E, Woodrow M, Thierry-Carstensen B, Andersen P, Novicki D, Del Giudice G, Rappuoli R. 2009. Transient facial nerve paralysis (Bell’s palsy) following intranasal delivery of a genetically detoxified mutant of Escherichia coli heat labile toxin. PLoS One 4:e6999. doi: 10.1371/journal.pone.0006999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Ginkel FW, Jackson RJ, Yoshino N, Hagiwara Y, Metzger DJ, Connell TD, Vu HL, Martin M, Fujihashi K, McGhee JR. 2005. Enterotoxin-based mucosal adjuvants alter antigen trafficking and induce inflammatory responses in the nasal tract. Infect Immun 73:6892–6902. doi: 10.1128/IAI.73.10.6892-6902.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Field M. 2003. Intestinal ion transport and the pathophysiology of diarrhea. J Clin Invest 111:931–943. doi: 10.1172/JCI18326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dickinson BL, Clements JD. 1995. Dissociation of Escherichia coli heat-labile enterotoxin adjuvanticity from ADP-ribosyltransferase activity. Infect Immun 63:1617–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng E, Cárdenas-Freytag L, Clements JD. 1999. The role of cAMP in mucosal adjuvanticity of Escherichia coli heat-labile enterotoxin (LT). Vaccine 18:38–49. doi: 10.1016/S0264-410X(99)00168-1. [DOI] [PubMed] [Google Scholar]
- 38.Summerton NA, Welch RW, Bondoc L, Yang HH, Pleune B, Ramachandran N, Harris AM, Bland D, Jackson WJ, Park S, Clements JD, Nabors GS. 2010. Toward the development of a stable, freeze-dried formulation of Helicobacter pylori killed whole cell vaccine adjuvanted with a novel mutant of Escherichia coli heat-labile toxin. Vaccine 28:1404–1411. doi: 10.1016/j.vaccine.2009.10.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uddowla S, Freytag LC, Clements JD. 2007. Effect of adjuvants and route of immunizations on the immune response to recombinant plague antigens. Vaccine 25:7984–7993. doi: 10.1016/j.vaccine.2007.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee S, Belitsky BR, Brinker JP, Kerstein KO, Brown DW, Clements JD, Keusch GT, Tzipori S, Sonenshein AL, Herrmann JE. 2010. Development of a Bacillus subtilis-based rotavirus vaccine. Clin Vaccine Immunol 17:1647–1655. doi: 10.1128/CVI.00135-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quan FS, Yoo DG, Song JM, Clements JD, Compans RW, Kang SM. 2009. Kinetics of immune responses to influenza virus-like particles and dose-dependence of protection with a single vaccination. J Virol 83:4489–4497. doi: 10.1128/JVI.02035-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McNeal MM, Stone SC, Basu M, Clements JD, Choi AH, Ward RL. 2007. IFN-gamma is the only anti-rotavirus cytokine found after in vitro stimulation of memory CD4+ T cells from mice immunized with a chimeric VP6 protein. Viral Immunol 20:571–584. doi: 10.1089/vim.2007.0055. [DOI] [PubMed] [Google Scholar]
- 43.Smiley KL, McNeal MM, Basu M, Choi AH, Clements JD, Ward RL. 2007. Association of gamma interferon and interleukin-17 production in intestinal CD4+ T cells with protection against rotavirus shedding in mice intranasally immunized with VP6 and the adjuvant LT(R192G). J Virol 81:3740–3748. doi: 10.1128/JVI.01877-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Belyakov IM, Kuznetsov VA, Kelsall B, Klinman D, Moniuszko M, Lemon M, Markham PD, Pal R, Clements JD, Lewis MG, Strober W, Franchini G, Berzofsky JA. 2006. Impact of vaccine-induced mucosal high-avidity CD8+ CTLs in delay of AIDS viral dissemination from mucosa. Blood 107:3258–3264. doi: 10.1182/blood-2005-11-4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Glynn A, Roy CJ, Powell BS, Adamovicz JJ, Freytag LC, Clements JD. 2005. Protection against aerosolized Yersinia pestis challenge following homologous and heterologous prime-boost with recombinant plague antigens. Infect Immun 73:5256–5261. doi: 10.1128/IAI.73.8.5256-5261.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu X, Clements JD, Katz JM. 2002. Mutant Escherichia coli heat-labile enterotoxin [LT(R192G)] enhances protective humoral and cellular immune responses to orally administered inactivated influenza vaccine. Vaccine 20:1019–1029. doi: 10.1016/S0264-410X(01)00452-2. [DOI] [PubMed] [Google Scholar]
- 47.Tumpey TM, Renshaw M, Clements JD, Katz JM. 2001. Mucosal delivery of inactivated influenza vaccine induces B-cell-dependent heterosubtypic cross-protection against lethal influenza A H5N1 virus infection. J Virol 75:5141–5150. doi: 10.1128/JVI.75.11.5141-5150.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gerber S, Lane C, Brown DM, Lord E, DiLorenzo M, Clements JD, Rybicki E, Williamson AL, Rose RC. 2001. Human papillomavirus virus-like particles are efficient oral immunogens when coadministered with Escherichia coli heat-labile enterotoxin mutant R192G or CpG DNA. J Virol 75:4752–4760. doi: 10.1128/JVI.75.10.4752-4760.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.DuBois AB, Freytag LC, Clements JD. 2007. Evaluation of combinatorial vaccines against anthrax and plague in a murine model. Vaccine 25:4747–4754. doi: 10.1016/j.vaccine.2007.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oplinger ML, Baqar S, Trofa AF, Clements JD, Gibbs P, Pazzaglia G, Bourgeois AL, Scott DA. 1997. Safety and immunogenicity in volunteers of a new candidate oral mucosal adjuvant, LT(R192G), p 193 Abstr 37th Intersci Conf Antimicrob Agents Chemother. American Society for Microbiology, Washington, DC. [Google Scholar]
- 51.Levine MM, Kaper JB, Black RE, Clements ML. 1983. New knowledge on pathogenesis of bacterial enteric infections as applied to vaccine development. Microbiol Rev 47:510–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tribble DR, Baqar S, Thompson SA. 2008. Development of a human vaccine, p 429–444. In Nachamkin I, Szymanski CM, Blaser MJ. (ed), Campylobacter, 3rd ed ASM Press, Washington, DC. [Google Scholar]
- 53.Kotloff KL, Sztein MB, Wasserman SS, Losonsky GA, DiLorenzo SC, Walker RI. 2001. Safety and immunogenicity of oral inactivated whole-cell Helicobacter pylori vaccine with adjuvant among volunteers with or without subclinical infection. Infect Immun 69:3581–3590. doi: 10.1128/IAI.69.6.3581-3590.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hayden CA, Streatfield SJ, Lamphear BJ, Fake GM, Keener TK, Walker JH, Clements JD, Turner DD, Tizard IR, Howard JA. 2012. Bioencapsulation of the hepatitis B surface antigen and its use as an effective oral immunogen. Vaccine 30:2937–2942. doi: 10.1016/j.vaccine.2012.02.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Novotny LA, Clements JD, Bakaletz LO. 2011. Transcutaneous immunization as preventative and therapeutic regimens to protect against experimental otitis media due to nontypeable Haemophilus influenzae. Mucosal Immunol 4:456–467. doi: 10.1038/mi.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.White JA, Blum JS, Hosken NA, Marshak JO, Duncan L, Zhu C, Norton EB, Clements JD, Koelle DM, Chen D, Weldon WC. 2014. Serum and mucosal antibody responses to inactivated polio vaccine after sublingual immunization using a thermoresponsive gel delivery system. Hum Vaccine Immunother 10:3611–3621. doi: 10.4161/hv.32253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alam MM, Bufano MK, Xu P, Kalsy A, Yu Y, Freeman YW, Sultana T, Rashu MR, Desai I, Eckhoff G, Leung DT, Charles RC, LaRocque RC, Harris JB, Clements JD, Calderwood SB, Qadri F, Vann WF, Kováč P, Ryan ET. 2014. Evaluation in mice of a conjugate vaccine for cholera made from Vibrio cholerae O1 (Ogawa) O-specific polysaccharide. PLoS Negl Trop Dis 8:e2683. doi: 10.1371/journal.pntd.0002683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaminski RW, Wu M, Turbyfill KR, Clarkson K, Tai B, Bourgeois AL, Van De Verg LL, Walker RI, Oaks EV. 2014. Development and preclinical evaluation of a trivalent, formalin-inactivated Shigella whole-cell vaccine. Clin Vaccine Immunol 21:366–382. doi: 10.1128/CVI.00683-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holmgren J, Bourgeois L, Carlin N, Clements J, Gustafsson B, Lundgren A, Nygren E, Tobias J, Walker R, Svennerholm AM. 2013. Development and preclinical evaluation of safety and immunogenicity of an oral ETEC vaccine containing inactivated E. coli bacteria overexpressing colonization factors CFA/I, CS3, CS5 and CS6 combined with a hybrid LT/CT B subunit antigen, administered alone and together with dmLT adjuvant. Vaccine 31:2457–2464. doi: 10.1016/j.vaccine.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 60.Kim JO, Rho S, Kim SH, Kim H, Song HJ, Kim EJ, Kim RY, Kim EH, Sinha A, Dey A, Yang JS, Song MK, Nandy RK, Czerkinsky C, Kim DW. 2015. Shigella outer membrane protein PSSP-1 is broadly protective against Shigella infection. Clin Vaccine Immunol 22:381–388. doi: 10.1128/CVI.00661-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stinson JA, Raja WK, Lee S, Kim HB, Diwan I, Tutunjian S, Panilaitis B, Omenetto FG, Tzipori S, Kaplan DL. 2017. Silk fibroin microneedles for transdermal vaccine delivery. ACS Biomater Sci Eng 3:360–369. doi: 10.1021/acsbiomaterials.6b00515. [DOI] [PubMed] [Google Scholar]
- 62.Lalsiamthara J, Lee JH. 2017. Immunization with Salmonella enteritidis secreting mucosal adjuvant labile toxin confers protection against wild type challenge via augmentation of CD3(+)CD4(+) T-cell proliferation and enhancement of IFN-gamma, IL-6 and IL-10 expressions in chicken. Vaccine 35:767–773. doi: 10.1016/j.vaccine.2016.12.042. [DOI] [PubMed] [Google Scholar]
- 63.Albert MJ, Haridas S, Ebenezer M, Raghupathy R, Khan I. 2015. Immunization with a double mutant (R192G/L211A) of the heat-labile enterotoxin of Escherichia coli offers partial protection against Campylobacter jejuni in an adult mouse intestinal colonization model. PLoS One 10:e0142090. doi: 10.1371/journal.pone.0142090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Norton EB, Branco LM, Clements JD. 2015. Evaluating the A-subunit of the heat-labile toxin (LT) as an immunogen and a protective antigen against enterotoxigenic Escherichia coli (ETEC). PLoS One 10:e0136302. doi: 10.1371/journal.pone.0136302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ruan X, Sack DA, Zhang W. 2015. Genetic fusions of a CFA/I/II/IV MEFA (multiepitope fusion antigen) and a toxoid fusion of heat-stable toxin (STa) and heat-labile toxin (LT) of enterotoxigenic Escherichia coli (ETEC) retain broad anti-CFA and antitoxin antigenicity. PLoS One 10:e0121623. doi: 10.1371/journal.pone.0121623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Toprani VM, Sahni N, Hickey JM, Robertson GA, Middaugh CR, Joshi SB, Volkin DB. 2017. Development of a candidate stabilizing formulation for bulk storage of a double mutant heat labile toxin (dmLT) protein based adjuvant. Vaccine 35:5471–5480. doi: 10.1016/j.vaccine.2017.03.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.White JA, Haghighi C, Brunner J, Estrada M, Lal M, Chen D. 2017. Preformulation studies with the Escherichia coli double mutant heat-labile toxin adjuvant for use in an oral vaccine. J Immunol Methods 451:83–89. doi: 10.1016/j.jim.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nguyen HN, Steede NK, Robinson JE, Landry SJ. 2015. Conformational instability governed by disulfide bonds partitions the dominant from subdominant helper T-cell responses specific for HIV-1 envelope glycoprotein gp120. Vaccine 33:2887–2896. doi: 10.1016/j.vaccine.2015.04.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fonseca DM, Hand TW, Han SJ, Gerner MY, Glatman Zaretsky A, Byrd AL, Harrison OJ, Ortiz AM, Quinones M, Trinchieri G, Brenchley JM, Brodsky IE, Germain RN, Randolph GJ, Belkaid Y. 2015. Microbiota-dependent sequelae of acute infection compromise tissue-specific immunity. Cell 163:354–366. doi: 10.1016/j.cell.2015.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.El-Kamary SS, Cohen MB, Bourgeois AL, Van De Verg L, Bauers N, Reymann M, Pasetti MF, Chen WH. 2013. Safety and immunogenicity of a single oral dose of recombinant double mutant heat-labile toxin derived from enterotoxigenic Escherichia coli. Clin Vaccine Immunol 20:1764–1770. doi: 10.1128/CVI.00464-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lundgren A, Bourgeois L, Carlin N, Clements J, Gustafsson B, Hartford M, Holmgren J, Petzold M, Walker R, Svennerholm AM. 2014. Safety and immunogenicity of an improved oral inactivated multivalent enterotoxigenic Escherichia coli (ETEC) vaccine administered alone and together with dmLT adjuvant in a double-blind, randomized, placebo-controlled phase I study. Vaccine 32:7077–7084. doi: 10.1016/j.vaccine.2014.10.069. [DOI] [PubMed] [Google Scholar]
- 72.Tennant SM, Steele AD, Pasetti MF. 2016. Highlights of the 8th International Conference on Vaccines for Enteric Diseases: the Scottish encounter to defeat diarrheal diseases. Clin Vaccine Immunol 23:272–281. doi: 10.1128/CVI.00082-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lawson LB, Norton EB, Clements JD. 2011. Defending the mucosa: adjuvant and carrier formulations for mucosal immunity. Curr Opin Immunol 23:414–420. doi: 10.1016/j.coi.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 74.Norton EB, Clements JD, Voss TG, Cárdenas-Freytag L. 2010. Prophylactic administration of bacterially derived immunomodulators improves the outcome of influenza virus infection in a murine model. J Virol 84:2983–2995. doi: 10.1128/JVI.01805-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Anosova NG, Chabot S, Shreedhar V, Borawski JA, Dickinson BL, Neutra MR. 2008. Cholera toxin, E. coli heat-labile toxin, and non-toxic derivatives induce dendritic cell migration into the follicle-associated epithelium of Peyer’s patches. Mucosal Immunol 1:59–67. doi: 10.1038/mi.2007.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bagley KC, Abdelwahab SF, Tuskan RG, Fouts TR, Lewis GK. 2002. Cholera toxin and heat-labile enterotoxin activate human monocyte-derived dendritic cells and dominantly inhibit cytokine production through a cyclic AMP-dependent pathway. Infect Immun 70:5533–5539. doi: 10.1128/IAI.70.10.5533-5539.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Negri DR, Pinto D, Vendetti S, Patrizio M, Sanchez M, Riccomi A, Ruggiero P, Del Giudice G, De Magistris MT. 2009. Cholera toxin and Escherichia coli heat-labile enterotoxin, but not their nontoxic counterparts, improve the antigen-presenting cell function of human B lymphocytes. Infect Immun 77:1924–1935. doi: 10.1128/IAI.01559-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lobet Y, Cluff CW, Cieplak W Jr.. 1991. Effect of site-directed mutagenic alterations on ADP-ribosyltransferase activity of the A subunit of Escherichia coli heat-labile enterotoxin. Infect Immun 59:2870–2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lycke N, Tsuji T, Holmgren J. 1992. The adjuvant effect of Vibrio cholerae and Escherichia coli heat-labile enterotoxins is linked to their ADP-ribosyltransferase activity. Eur J Immunol 22:2277–2281. doi: 10.1002/eji.1830220915. [DOI] [PubMed] [Google Scholar]
- 80.Fahlén-Yrlid L, Gustafsson T, Westlund J, Holmberg A, Strömbeck A, Blomquist M, MacPherson GG, Holmgren J, Yrlid U. 2009. CD11c(high)dendritic cells are essential for activation of CD4+ T cells and generation of specific antibodies following mucosal immunization. J Immunol 183:5032–5041. doi: 10.4049/jimmunol.0803992. [DOI] [PubMed] [Google Scholar]
- 81.Hertz AL, Bender AT, Smith KC, Gilchrist M, Amieux PS, Aderem A, Beavo JA. 2009. Elevated cyclic AMP and PDE4 inhibition induce chemokine expression in human monocyte-derived macrophages. Proc Natl Acad Sci U S A 106:21978–21983. doi: 10.1073/pnas.0911684106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ryan EJ, McNeela E, Pizza M, Rappuoli R, O’Neill L, Mills KH. 2000. Modulation of innate and acquired immune responses by Escherichia coli heat-labile toxin: distinct pro- and anti-inflammatory effects of the nontoxic AB complex and the enzyme activity. J Immunol 165:5750–5759. doi: 10.4049/jimmunol.165.10.5750. [DOI] [PubMed] [Google Scholar]
- 83.Brereton CF, Sutton CE, Ross PJ, Iwakura Y, Pizza M, Rappuoli R, Lavelle EC, Mills KH. 2011. Escherichia coli heat-labile enterotoxin promotes protective Th17 responses against infection by driving innate IL-1 and IL-23 production. J Immunol 186:5896–5906. doi: 10.4049/jimmunol.1003789. [DOI] [PubMed] [Google Scholar]
- 84.Hertz AL, Beavo JA. 2011. Cyclic nucleotides and phosphodiesterases in monocytic differentiation. Handb Exp Pharmacol (204):365–390. doi: 10.1007/978-3-642-17969-3_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gagliardi MC, Sallusto F, Marinaro M, Langenkamp A, Lanzavecchia A, De Magistris MT. 2000. Cholera toxin induces maturation of human dendritic cells and licences them for Th2 priming. Eur J Immunol 30:2394–2403. doi:. [DOI] [PubMed] [Google Scholar]
- 86.Hou W, Wu Y, Sun S, Shi M, Sun Y, Yang C, Pei G, Gu Y, Zhong C, Sun B. 2003. Pertussis toxin enhances Th1 responses by stimulation of dendritic cells. J Immunol 170:1728–1736. doi: 10.4049/jimmunol.170.4.1728. [DOI] [PubMed] [Google Scholar]
- 87.Datta SK, Sabet M, Nguyen KP, Valdez PA, Gonzalez-Navajas JM, Islam S, Mihajlov I, Fierer J, Insel PA, Webster NJ, Guiney DG, Raz E. 2010. Mucosal adjuvant activity of cholera toxin requires Th17 cells and protects against inhalation anthrax. Proc Natl Acad Sci U S A 107:10638–10643. doi: 10.1073/pnas.1002348107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee JB, Jang JE, Song MK, Chang J. 2009. Intranasal delivery of cholera toxin induces th17-dominated T-cell response to bystander antigens. PLoS One 4:e5190. doi: 10.1371/journal.pone.0005190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Netea MG, Joosten LA, Latz E, Mills KH, Natoli G, Stunnenberg HG, O’Neill LA, Xavier RJ. 2016. Trained immunity: a program of innate immune memory in health and disease. Science 352:aaf1098. doi: 10.1126/science.aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lalsiamthara J, Kamble NM, Lee JH. 2016. A live attenuated Salmonella enteritidis secreting detoxified heat labile toxin enhances mucosal immunity and confers protection against wild-type challenge in chickens. Vet Res 47:60. doi: 10.1186/s13567-016-0348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Maldarelli GA, Matz H, Gao S, Chen K, Hamza T, Yfantis HG, Feng H, Donnenberg MS. 2016. Pilin vaccination stimulates weak antibody responses and provides no protection in a C57Bl/6 murine model of acute Clostridium difficile infection. J Vaccines Vaccin 7(3):321. doi: 10.4172/2157-7560.1000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Luo Q, Vickers TJ, Fleckenstein JM. 2016. Immunogenicity and protective efficacy against enterotoxigenic Escherichia coli colonization following intradermal, sublingual, or oral vaccination with EtpA adhesin. Clin Vaccine Immunol 23:628–637. doi: 10.1128/CVI.00248-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Novotny LA, Clements JD, Goodman SD, Bakaletz LO. 2017. Transcutaneous immunization with a band-aid prevents experimental otitis media in a polymicrobial model. Clin Vaccine Immunol 24:e00563-16. doi: 10.1128/CVI.00563-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Martinez-Becerra FJ, Kissmann JM, Diaz-McNair J, Choudhari SP, Quick AM, Mellado-Sanchez G, Clements JD, Pasetti MF, Picking WL. 2012. Broadly protective Shigella vaccine based on type III secretion apparatus proteins. Infect Immun 80:1222–1231. doi: 10.1128/IAI.06174-11. [DOI] [PMC free article] [PubMed] [Google Scholar]