Copper intoxication triggers both specific and nonspecific responses in Salmonella. The scs locus, which codes for periplasmic thiol/disulfide-oxidoreductase/isomerase-like proteins, has been the focus of attention because it is necessary for copper resistance, oxidative stress responses, and virulence and because it is not present in nonpathogenic Escherichia coli. Still, the conditions under which the scs locus is expressed and the roles of its individual components remain unknown. In this report, we examine the contribution of each Scs factor to survival under H2O2 and copper stress. We establish that the scs genes form a copper-activated operon controlled by the CpxR/CpxA signal transduction system, and we provide evidence of its conserved gene arrangement and regulation in other bacterial pathogens.
KEYWORDS: copper resistance, CpxRA, envelope homeostasis, oxidative stress, ScsABCD, thioredoxin-like proteins
ABSTRACT
Periplasmic thiol/disulfide oxidoreductases participate in the formation and isomerization of disulfide bonds and contribute to the virulence of pathogenic microorganisms. Among the systems encoded in the Salmonella genome, the system encoded by the scsABCD locus was shown to be required to cope with Cu and H2O2 stress. Here we report that this locus forms an operon whose transcription is driven by a promoter upstream of scsA and depends on CpxR/CpxA and on Cu. Furthermore, genes homologous to scsB, scsC, and scsD are always detected immediately downstream of scsA and in the same genetic arrangement in all scsA-harboring enterobacterial species. Also, a CpxR-binding site is detected upstream of scsA in most of those species, providing evidence of evolutionarily conserved function and regulation. Each individual scs gene shows a different role in copper and/or H2O2 resistance, indicating hierarchical contributions of these factors in the defense against these intoxicants. A protective effect of Cu preincubation against H2O2 toxicity and the increased Cu-mediated activation of cpxP in the ΔscsABCD mutant suggest that the CpxR/CpxA-controlled transcription of the ScsABCD system contributes to prevent Cu toxicity and to restore the redox balance at the Salmonella envelope.
IMPORTANCE Copper intoxication triggers both specific and nonspecific responses in Salmonella. The scs locus, which codes for periplasmic thiol/disulfide-oxidoreductase/isomerase-like proteins, has been the focus of attention because it is necessary for copper resistance, oxidative stress responses, and virulence and because it is not present in nonpathogenic Escherichia coli. Still, the conditions under which the scs locus is expressed and the roles of its individual components remain unknown. In this report, we examine the contribution of each Scs factor to survival under H2O2 and copper stress. We establish that the scs genes form a copper-activated operon controlled by the CpxR/CpxA signal transduction system, and we provide evidence of its conserved gene arrangement and regulation in other bacterial pathogens.
INTRODUCTION
Copper is required in trace amounts as a cofactor or structural component of several enzymes, mainly linked to aerobic metabolism, but is very harmful in excess (1, 2). Free Cu ions can displace other essential metals, such a Fe from Fe-S clusters on enzymes, or catalyze redox cycling reactions with oxygen or nitrogen species, promoting the formation of reactive radicals and resulting in cell death (3, 4). The envelope of Gram-negative bacteria is the primary barrier against external injuries and, in consequence, is the first target of Cu toxicity. Cu(II)/Cu(I) cycling in this compartment was proposed to increase the formation of nonspecific disulfide bonds on proteins, affecting redox homeostasis (5–7).
Recent evidence indicates that eukaryotic cells use the biocidal properties of Cu to defend themselves against microbial pathogens (8–10). Macrophages actively deliver the metal ion to specific compartments where the pathogen resides, contributing to its intoxication (11). Therefore, the ability to handle and to eliminate the incoming toxic metal rapidly and actively or to repair Cu-induced damage is crucial for the survival of intracellular pathogens such as Salmonella enterica. This Gram-negative species contains a dedicated copper resistance system controlled at the transcriptional level by the cytoplasmic Cu(I) sensor CueR (1, 12). In the presence of Cu, CueR induces the expression of the membrane-bound P1B-type ATPase CopA, which removes Cu(I) from the cytoplasm, and of two periplasmic proteins, namely, the multicopper oxidase CueO and the Salmonella-specific Cu(II)-binding protein CueP, which contribute to avoidance of further toxic reactions and back-diffusion of the metal ion into the cytoplasm (13–15). Recently, we demonstrated that, in contrast to other CueR-controlled genes, the expression of CueP also depends on CpxR/CpxA (16), an ancestral envelope stress-responding two-component system that activates gene expression in response to Cu excess (7, 17, 18). Unlike Escherichia coli and a number of Gram-negative species, and with the exception of new strains isolated from copper-fed cattle (19, 20), S. enterica does not harbor in its core genome genes that encode the CusCFBA efflux pump to remove Cu ions from the cell envelope (14). Although CueP was found to partially restore the copper resistance of an E. coli Δcus mutant (14, 21), it is currently not known how Salmonella eliminates the excess metal ion from this compartment to counteract its toxic effects.
Protein cysteine SH groups are likely to oxidize at the periplasmic redox potential (22). A set of dedicated systems of oxidoreductases of the thioredoxin superfamily are required to promote the correct S-S formation and to preserve specific functional SH groups in this compartment, particularly under stress (23–25). These systems are composed of periplasmic proteins that oxidize or reduce thiol groups using electrons transferred from the cytoplasm by membrane-integrated components. Salmonella harbors the widely distributed DsbA/DsbB pair, which is responsible for de novo S-S formation, and two isomerase/reductase activity complexes, DsbC/DsbD and DsbG/DsbD, which fix improper S-S bonds or keep S groups reduced on different Cys-containing substrates (26–28). Also present in the pathogen are a DsbA homologue, SrgA, a substrate-specific DsbA/DsbB paralogue, DsbL/DsbI, which is essential for virulence (29), and the ScsC/ScsB pair, which was initially identified as part of the Salmonella-specific scsABCD locus, which suppresses the copper sensitivity of E. coli mutants after overexpression (30), with no identified substrates. The periplasmic component ScsC displays structural similarities to DsbA and DsbG; it forms monomers in solution like DsbA, but its catalytic domain is typical of the disulfide isomerases and is almost identical to that of DsbG (31). ScsB shows similarities to Caulobacter crescentus and Proteus mirabilis ScsB proteins, members of the DsbD superfamily that were shown to provide electrons to the specific ScsC homologues and to an envelope peroxide reduction pathway (32, 33). All Scs proteins, including ScsC, ScsB, and the other two inner-membrane-associated proteins with unknown function, ScsD and ScsA, contain Cys-X-X-Cys motifs (a hallmark of the oxidoreductase-thioredoxin superfamily) and a putative Cu-binding site (10, 23). Mutants with deletions of scsC, scsB, or scsD or the whole scs locus but not scsA showed equally decreased Cu resistance (34). Only the ΔscsA strain was affected by H2O2, however, and enhanced protein carboxylation in the periplasmic space in the presence of H2O2 was reported for the ΔscsABCD strain (34). The scs locus was also found to be required for SPI1-mediated secretion of SipB and for bacterial proliferation inside cortisol-activated macrophages (34, 35).
In this work, we report that scsABCD transcription is induced by Cu and depends on CpxR/CpxA (36, 37). The contributions of the Scs components, together with those of the DsbC-DsbG/DsbD systems, to Cu tolerance, as well as their roles in oxidative stress resistance, are evaluated. Our results indicate that the scsABCD operon is part of the Cpx regulon, which increases Salmonella survival under severe Cu and oxidative stress, hostile conditions encountered by the pathogen during its intracellular survival.
RESULTS
Transcription of the scs genes is induced by Cu.
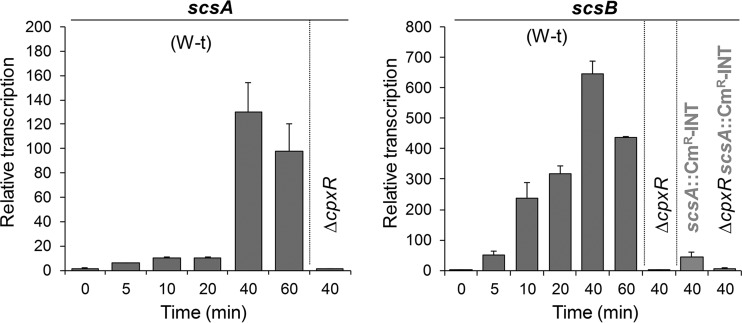
A genome-wide transcriptome analysis of the response of Salmonella after a 10-min shock with Cu or Zn salts (7) revealed that the scs locus was specifically upregulated in the presence of CuSO4 when cells were grown in either minimal or rich medium (see Fig. S1A at http://www.ibr-conicet.gov.ar/investigacion/publicaciones). Under these conditions, no activation of other genes encoding proteins of the oxidoreductase-thioredoxin superfamily, i.e., dsbA, dsbB, dsbC, dsbG, dsbD, dsbL, dsbI, or srgA, was observed (see Fig. S1B at http://www.ibr-conicet.gov.ar/investigacion/publicaciones). Cu-mediated activation of the scs genes was verified using real-time quantitative reverse transcription-PCR (qRT-PCR). Transcription of both scsA and scsB increased with time and reached a maximum 40 min after Cu addition (Fig. 1), although with differences in the magnitude of the response achieved at different times after metal addition, particularly at times shorter than 20 min. This and the 48-bp spacing between scsA and the rest of the partially overlapping scs genes (see Fig. S2A at http://www.ibr-conicet.gov.ar/investigacion/publicaciones) might suggest that transcription of these genes could originate from two separate promoters, one located upstream of scsA and the other upstream of scsB, as prior reports proposed (30, 34, 35). Insertion of a chloramphenicol resistance (Cmr) cassette 100 bp downstream of the translation start site of scsA and in the opposite orientation (scsA::Cmr-INT), leaving a 311-bp region upstream of scsB, decreased by >10-fold the maximal Cu-promoted induction of scsB transcription (Fig. 1), indicating that transcription of the whole scs locus under copper stress is driven by the scsA promoter.
FIG 1.
Transcription of scsABCD is induced by Cu and depends on CpxR/CpxA. The relative scsA and scsB mRNA levels were determined by real-time qRT-PCR using LB medium cultures obtained 0, 5, 10, 20, 40, and 60 min after challenge of the wild-type (W-t), ΔcpxR, scsA::Cmr-INT, or ΔcpxR scsA::Cmr-INT strain with 1 mM CuSO4. At each time point, transcription levels were first normalized to the expression of rnpB and then relativized to the levels obtained in the absence of metal. Data correspond to the mean values from three independent experiments performed in triplicate. Error bars depict standard deviations (SDs).
Transcription of the scs locus is stimulated by the CpxR/CpxA regulatory system.
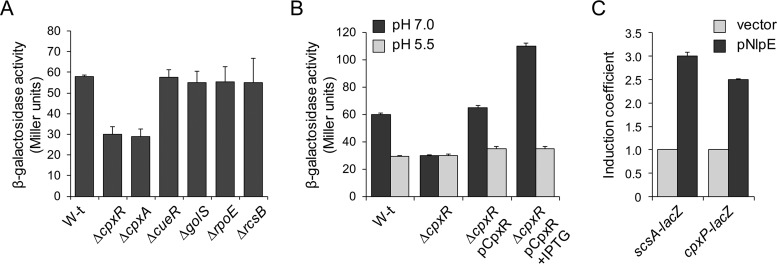
The Cu-mediated induction of scs transcription (Fig. 1) and the role of the Scs proteins in alleviating the damage caused by Cu and oxidative stress (34) prompted us to investigate whether transcription of the scs locus is controlled by regulatory factors involved in preserving the Cu or envelope homeostasis, such as CueR, the CueR paralogue GolS, the CpxR/CpxA and Rcs two-component systems, and the extracytoplasmic sigma E factor (7, 13, 14, 16, 36). A chromosomal lacZ reporter fusion to the promoter upstream of scsA (Pscs-lacZ) was introduced into cells with deletions of cueR, golS, cpxR-cpxA, rcsB, or rpoE. As shown in Fig. 2A, only deletion of the genes encoding the sensor kinase CpxA or its cognate response regulator CpxR decreased Pscs-lacZ expression. CpxR-mediated regulation was verified by qRT-PCR (Fig. 1). Deletion of CpxR abrogated the Cu-induced transcription of both scsA and scsB, including the remnant scsB transcription observed in the scsA::Cmr-INT strain. Wild-type expression of the reporter construct was restored by complementing the ΔcpxR strain with cpxR expressed in trans under the control of an inducible promoter (Fig. 2B). As expected for a CpxR/CpxA-regulated gene, expression was reduced at acidic pH (Fig. 2B), a condition under which the CpxA kinase is not active (38), and was increased by NlpE overexpression (Fig. 2C), a condition that is known to activate the kinase (38, 39).
FIG 2.
CpxR/CpxA controls the expression of the scs locus. (A) β-Galactosidase activity from a scsA::lacZ transcriptional fusion expressed in wild-type (W-t), ΔcpxR, ΔcpxA, ΔcueR, ΔgolS, ΔrpoE, and ΔrcsB cells grown overnight in LB broth. (B) β-Galactosidase activity determined for the wild-type strain, the ΔcpxR strain, and the ΔcpxR strain complemented with pCpxR (ΔcpxR/pCpxR), all carrying the scsA::lacZ reporter fusion. Cells were grown overnight in LB medium with 100 mM MES buffer to adjust the pH value to 7.0 or 5.5, without (−IPTG) or with (+IPTG) the addition of 100 μM IPTG as indicated. The data shown in panels A and B correspond to mean values from four independent experiments performed in duplicate. Error bars represent SDs. (C) β-Galactosidase activity determined for wild-type cells carrying scsA::lacZ (scsA-lacZ) or cpxP::lacZ (cpxP-lacZ) (included as a CpxR-regulated positive control) transcriptional fusions and transformed either with the empty vector pUHE21-2lacIq (vector) or with pNlpE and then grown in LB medium with the addition of 100 μM IPTG. (It should be noted that the lacZ in the cpxP::lacZ construction was introduced after the 3′ end of cpxP, in order to avoid undesirable disturbance of the CpxR/CpxA signal transduction pathway [36, 37, 54].) All values were normalized to the average activity obtained for cells with the control vector. Bars represent the average normalized values from at least three separate experiments. Error bars represent SDs.
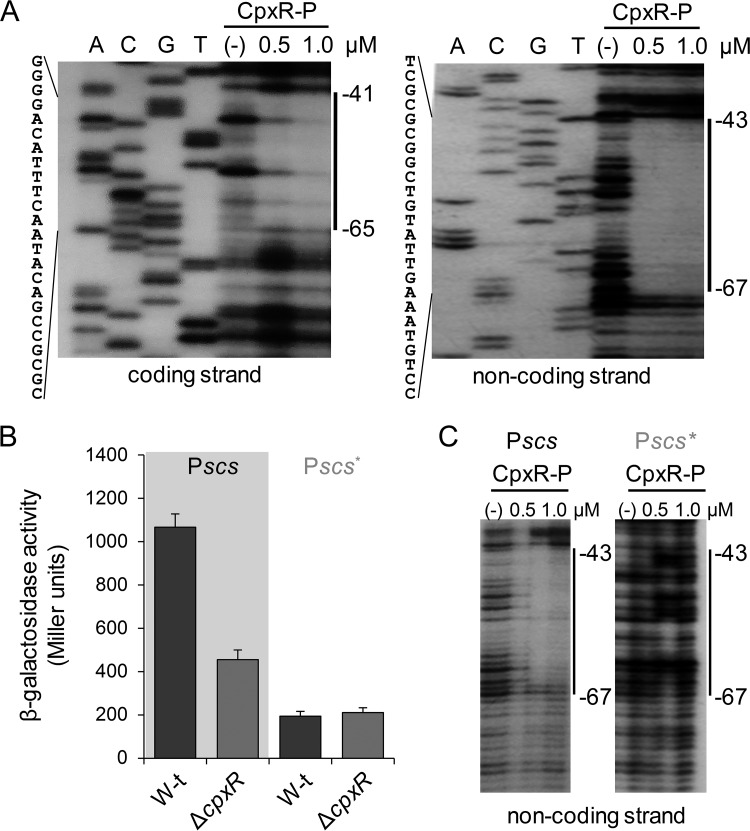
An in silico analysis of the promoter region upstream of scsA revealed the presence of a putative CpxR-binding site between position −46 and position −60, relative to the scsABCD transcriptional start site in the intergenic scsA-cbpA region (see Fig. S2A at http://www.ibr-conicet.gov.ar/investigacion/publicaciones). This sequence differs in 3 bases from the consensus 5′-GTAAAN5GTAAA-3′ CpxR-binding site (40). No putative CpxR-binding sequence was identified within the scsA gene or in the intergenic scsA-scsB region. To confirm CpxR interaction with the predicted binding site on the scsA promoter (Pscs), electrophoretic mobility shift assays (EMSAs) and DNase I footprinting assays were performed using increasing amounts of phosphorylated CpxR protein (CpxR-P) (Fig. 3A; also see Fig. S2B at http://www.ibr-conicet.gov.ar/investigacion/publicaciones). The regulator provided protection from position −41 to position −65, relative to the transcription start site of scsA in the coding strand, and from position −43 to position −67 in the noncoding strand (Fig. 3A), encompassing the predicted CpxR-binding sequence (see Fig. S2A at http://www.ibr-conicet.gov.ar/investigacion/publicaciones).
FIG 3.
CpxR interacts with the scsA promoter at the predicted CpxR binding site. (A) DNase I footprinting analysis of the promoter region of scsABCD, performed on both end-labeled coding and noncoding strands. Purified and acetyl-phosphate-preincubated CpxR (CpxR-P), at the final concentrations of 0.5 and 1 μM, was added to the DNA fragments. Solid vertical lines on the right and sequences on the left indicate the CpxR-protected region. (B) β-Galactosidase activity of wild-type (W-t) and ΔcpxR strains carrying reporter plasmids in which expression of the lacZ gene was directed by the native scsABCD promoter (Pscs) or by the promoter harboring mutations at the CpxR-binding site (Pscs*). The activity was determined on overnight cultures grown in LB medium with 100 mM MES (pH 7.0). The data correspond to mean values from three independent experiments performed in duplicate. Error bars correspond to SDs. (C) DNase I footprinting analysis of the native (Pscs) or mutant (Pscs*) promoter regions performed on the noncoding strand. CpxR-P was added at the same final concentrations as in panel A. The predicted CpxR-protected region is shown with solid vertical lines.
We modified, by site-directed mutagenesis, 2 key bases of the consensus CpxR-binding site identified at the Pscs promoter, yielding the Pscs* promoter (5′-CGCCGACATAACTTcAgAGG-3′, in which the modified bases are underlined and lowercase). Both modified and native (5′-CGCCGACATAACTTTACAGG-3′) Pscs promoters were cloned upstream of the promoterless lacZ gene in the pMC1871 plasmid. As shown in Fig. 3B, the mutation reduced the levels of β-galactosidase activity measured in wild-type cells, and it was not affected by the cpxR deletion, in contrast to the strains harboring the native Pscs-lacZ plasmid. Furthermore, CpxR-P was unable to interact with the modified Pscs* promoter (Fig. 3C), confirming the role of the CpxR/CpxA system in scsABCD transcriptional regulation.
Primary role of the ScsC/ScsB pair and ScsD in the defense of the cell envelope against Cu stress.
It was reported previously that the absence of scsB, scsC, or scsD, but not scsA, produced moderate and identical effects on the susceptibility to Cu (34). In fact, it was shown that a strain with deletions of all four genes, ΔscsABCD, was as sensitive to Cu as the individual ΔscsB, ΔscsC, and ΔscsD strains. We reexamined the contribution of each Scs protein in the defense against Cu stress by recording the optical density at 600 nm (OD600) of the cultures for 15 h and by assessing the development of colonies on Luria-Bertani (LB) agar plates containing increasing amounts of CuSO4, a more direct and accurate method to detect small differences between strains. With both methods, we confirmed that ScsA, although coregulated with the other scs genes, was not involved in copper resistance. Importantly, we observed that ScsB, ScsC, and ScsD contributed differently to Cu tolerance (Table 1; also see Fig. S3 at http://www.ibr-conicet.gov.ar/investigacion/publicaciones) (34). Only the strain with the deletion of scsB was as sensitive to Cu as the ΔscsABCD mutant, which could not form colonies at concentrations higher than 3.25 mM CuSO4 (see Fig. S3B at http://www.ibr-conicet.gov.ar/investigacion/publicaciones). Identical Cu-sensitive phenotypes were observed for the strain with a polar cat cassette inserted in scsA, i.e., scsA::Cmr-INT, and the ΔscsBCD mutant (see Fig. S3 at http://www.ibr-conicet.gov.ar/investigacion/publicaciones), providing further support for the presence of a major Cu-induced promoter upstream of scsA controlling scsB, scsC, and scsD transcription. Under these conditions, single ΔscsC or ΔscsD mutants were less sensitive to Cu than were strains with deletions of scsB or the whole scs locus (Table 1). As shown in Fig. S3B at http://www.ibr-conicet.gov.ar/investigacion/publicaciones, the absence of ScsC or ScsD impaired colony formation at copper concentrations higher than 3.5 or 3.75 mM, respectively. These results highlight hierarchical contributions of the components of the scs locus in copper resistance, with the membrane-associated reductase ScsB being the most important factor in this phenotype, followed by its putative periplasmic partner ScsC and the inner-membrane-associated protein ScsD. As expected (32, 33), the strain lacking both ScsB and ScsC was as sensitive to Cu as the ΔscsB strain (Table 1). In contrast, the ΔscsCD strain was more sensitive to the metal than were the mutants with individual deletions of scsC or scsD, resembling the ΔscsB phenotype (see Fig. S3B at http://www.ibr-conicet.gov.ar/investigacion/publicaciones), which suggests that ScsB could provide electrons not only to its periplasmic ScsC partner but also to the membrane-bound ScsD.
TABLE 1.
Contributions of ScsC/ScsB and DsbC-DsbG/DsbD to copper tolerance
| Strain description | MIC (mM)a |
|---|---|
| Wild type | 5.00 |
| ΔscsA | 5.00 |
| ΔscsB | 3.50 |
| ΔscsC | 3.75 |
| ΔscsD | 3.75 |
| ΔscsBC | 3.50 |
| ΔscsCD | 3.50 |
| ΔscsBCD | 3.50 |
| ΔscsABCD | 3.50 |
| scsA::Cmr-INT | 3.50 |
| ΔdsbC | 5.00b |
| ΔdsbG | 5.00 |
| ΔscsC ΔdsbC ΔdsbG | 3.00 |
| ΔdsbD (dipZ) | 5.00b |
| ΔscsB ΔdsbD | 3.00 |
MIC values were determined on LB plates containing increasing amounts of CuSO4, under aerobic conditions (see Materials and Methods for details). The data correspond to mean values from three independent experiments performed in duplicate.
Smaller colonies were observed, compared with the wild-type strain.
The periplasmic Cu(II)-binding protein CueP was recently reported to be a substrate of DsbC (28), a disulfide isomerase that, although not induced by Cu in Salmonella (see Fig. S1B at http://www.ibr-conicet.gov.ar/investigacion/publicaciones), contributes to Cu tolerance in E. coli (5). We analyzed the sensitivity to Cu of strains with deletions of dsbC, dsbG, or the associated membrane-bound reductase gene dsbD (also known as dipZ in Salmonella), in the presence or absence of a functional ScsC/ScsB system. Unlike the ΔscsB or ΔscsC mutants, deletion of dsbC, dsbG, or dsbD had little or no effect on Cu resistance (Table 1). All of those mutants exhibited similar MICs for CuSO4, compared with the wild-type strain, although smaller colonies were observed for the ΔdsbC and ΔdsbD strains at 4.75 mM CuSO4 (data not shown). Interestingly, the simultaneous deletion of dsbC, dsbG, and scsC or of dsbD and scsB severely affected Cu resistance, decreasing the MIC for CuSO4 to 3.0 mM, lower than the MICs exhibited by the ΔscsC and ΔscsB single mutants (Table 1). These results indicate that, in the absence of a functional ScsC/ScsB system, DsbC-DsbG/DsbD also contributes to Cu resistance, which supports overlapping roles for different oxidoreductase pairs under conditions of severe redox imbalance, as proposed previously (9, 23).
Role of the Scs proteins in response to H2O2.
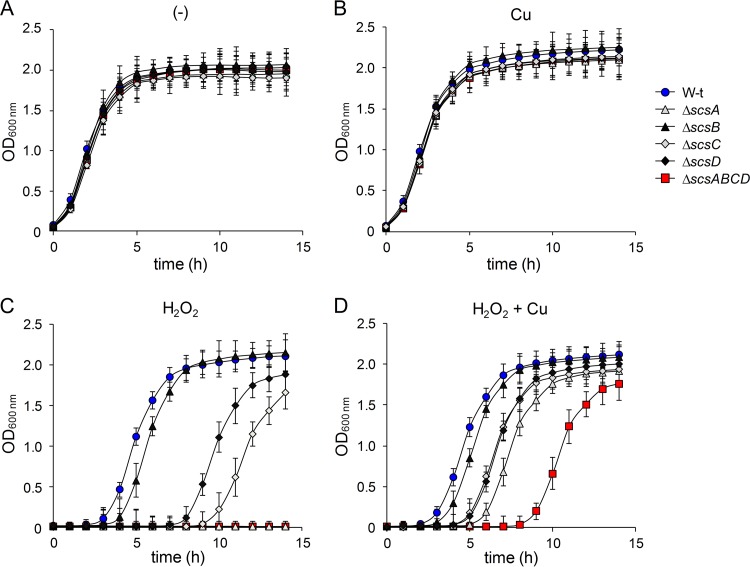
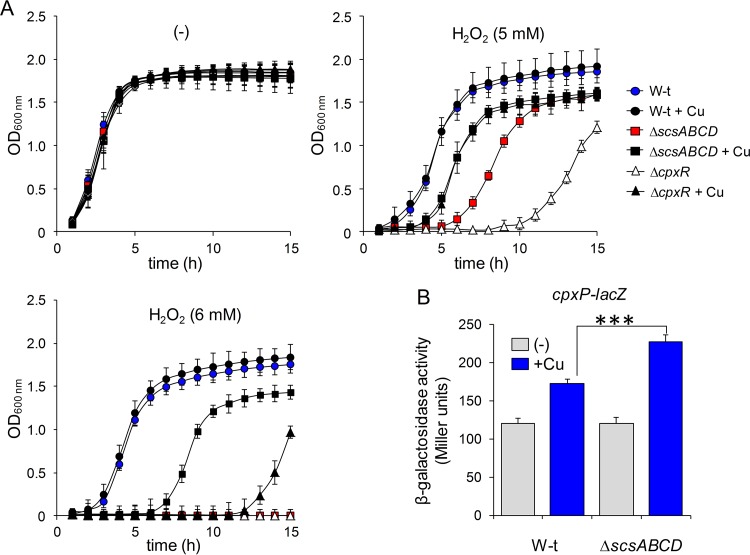
ScsA was reported to affect survival after 2 h of incubation with 2 or 4 mM H2O2 (34). Surprisingly, under those conditions, Anwar et al. reported that strains with deletions of the other three scs genes or the whole scs locus exhibited wild-type sensitivity to the oxidant, although the ΔscsABCD strain showed enhanced protein carboxylation in the periplasmic space (34), a hallmark of oxidative damage. In view of these somewhat contradictory results, we first compared the tolerance to H2O2 of the wild-type, ΔscsABCD, and ΔscsBCD strains, by recording their growth (OD600) in LB broth for 15 h. A mutant in tpx, which codes for a periplasmic peroxiredoxin with low H2O2 tolerance (41), was used as a control. All of the strains, but particularly the Δtpx strain, exhibited an extended lag phase as the concentration of H2O2 added to the medium increased (see Fig. S4 at http://www.ibr-conicet.gov.ar/investigacion/publicaciones). The differences in susceptibility to H2O2 between the wild-type strain and the scs mutants, as well as between the ΔscsABCD and ΔscsBCD strains, were more evident at 6 mM H2O2. At that concentration, the ΔscsABCD mutant was unable to grow (see Fig. S4 at http://www.ibr-conicet.gov.ar/investigacion/publicaciones), while the ΔscsBCD mutant showed a very extended lag phase, indicative of severe damage. (It should be noted that the growth observed for the ΔscsBCD strain after 8 h of incubation with 6 mM H2O2 could be attributed only to some surviving cells after H2O2 treatment and not to the appearance of suppressor mutations, because reincubation with 6 mM H2O2 of mutant cells recovered from the culture treated with peroxide for 15 h showed the same behavior.)
The results describe above confirm the requirement for ScsA in the defense against oxidative stress and demonstrate that the rest of the Scs proteins are also required for full H2O2 resistance. The analysis of the single scs mutants supports this suggestion (Fig. 4). The ΔscsA strain exhibited the same H2O2 sensitivity as the ΔscsABCD mutant at 6 mM H2O2. However, deletion of either scsC or scsD also increased the sensitivity to the oxidant (Fig. 4C), highlighting the importance of these proteins against oxidative stress. Finally, a small but significant increase in the lag phase, compared with the wild-type strain, was observed for the ΔscsB strain (Fig. 4C). These results indicate that all Scs proteins, not just ScsA, are involved in balancing oxidative stress at the Salmonella enterica serovar Typhimurium envelope.
FIG 4.
The Scs proteins are involved in balancing oxidative stress at the Salmonella envelope. Wild-type (W-t), ΔscsA, ΔscsB, ΔscsC, ΔscsD, and ΔscsABCD cells were grown under aerobic conditions in LB medium, without (−) or with the addition of 6 mM H2O2 and/or 1 mM CuSO4, as indicated. The OD600 of the cultures was recorded every 1 h for 15 h. Results are the means and SDs from four independent experiments, each performed in duplicate.
Because expression of the Scs proteins is induced by Cu, we tested the effects of the simultaneous addition of 6 mM H2O2 and 1 mM CuSO4, a metal concentration that does not affect the growth of the wild-type strain or the mutant strains tested in this study (Fig. 4B; also see Fig. S5 at http://www.ibr-conicet.gov.ar/investigacion/publicaciones). The addition of Cu partially reversed the susceptibility to H2O2 of all scs mutants, with the exception of the ΔscsB strain (compare Fig. 4C and D). The Cu-mediated protection was not caused by Cu-catalyzed elimination of the oxidant (42), since it did not improve the resistance to H2O2 of the wild-type strain (see Fig. S5 at http://www.ibr-conicet.gov.ar/investigacion/publicaciones). Most probably, it could be caused by nonspecific Cu-catalyzed oxidation of thiol groups at the envelope of the mutant cells, as proposed previously (5), or Cu-induced expression of protecting factors in these strains. To distinguish between these possibilities, we preincubated both the wild-type strain and the ΔscsABCD mutant for 60 min with 1 mM CuSO4 and then removed the metal ion prior to H2O2 exposure. Under these conditions, only minimal nonspecific thiol-oxidizing activity of the metal was expected. As shown in Fig. 5A, preincubation with Cu markedly decreased the lag phase of the mutant in the presence of H2O2, while it had no effect on the wild-type strain, supporting the hypothesis of Cu-induced expression of factors that protect against and/or repair redox damage in cells lacking a functional Scs system. We tested whether these putative factors were also under CpxR control. Indeed, the ΔcpxR strain had increased sensitivity to H2O2 (Fig. 5A), supporting our observations and confirming the importance of the Cpx response in coping with this oxidative damage. However, preincubation of the ΔcpxR strain with copper increased its resistance to peroxide (although not to the ΔscsABCD level), suggesting that other unidentified factors, besides those controlled by CpxR, are involved in the Cu-induced protection against H2O2.
FIG 5.
Cu protects mutant scs cells from oxidative stress by activating the expression of protecting factors. (A) Preincubation with copper protects scsABCD-deficient mutants from stress caused by H2O2. The S. Typhimurium wild-type (W-t) strain and mutant strains with deletion of scsABCD (ΔscsABCD) or cpxR (ΔcpxR) were grown to the early log phase (60 min) in LB medium without (−) or with 1 mM CuSO4. The metal was then removed, and cultures were continued or challenged with the addition of 5 or 6 mM H2O2, as indicated. The growth was monitored as described above. Results are averages from three independent assays performed in duplicate, and error bars correspond to SDs. (B) The CpxR/CpxA response is enhanced by Cu in the absence of the scs locus. β-Galactosidase activity from a cpxP-lacZ transcriptional fusion expressed in wild-type or ΔscsABCD cells grown in LB medium with 100 mM MES (pH 7.0) was determined after 180 min of exposure to 1 mM CuSO4. Data correspond to mean values from three independent experiments performed in triplicate. Error bars depict SDs. The Cu induction of the PcpxP reporter in the ΔscsABCD strain differed significantly from that in the wild-type strain. ***, P < 0.001.
Deletion of the scs locus augments the CpxR/CpxA response in the presence of Cu.
The contribution of the Scs system in alleviating Cu and oxidative stress (Fig. 4; also see Fig. S3 at http://www.ibr-conicet.gov.ar/investigacion/publicaciones), its CpxR/CpxA dependence (Fig. 1, 2, and 3), and the CpxR-dependent protective effect of Cu against oxidative stress observed in the scs mutants (Fig. 5A) prompted us to evaluate whether the absence of the Scs system affected envelope homeostasis and consequently CpxR/CpxA activity. To test this, we determined the expression of a chromosomal lacZ gene fusion to cpxP, the archetypal Cpx-regulated factor (37), in the wild-type strain and the ΔscsABCD mutant, grown in the presence or absence of 1 mM CuSO4. As shown in Fig. 5B, addition of Cu increased transcription from the PcpxP promoter in the wild-type strain, as expected for a Cpx-regulated gene (1, 16–18). Remarkably, in the absence of a functional scsABCD locus, Cu-mediated cpxP induction showed a significant increase (Fig. 5B), suggesting a role for the Scs proteins in restoring envelope homeostasis after a surge of the metal ion, thus preventing overstimulation of the Cpx system.
DISCUSSION
The scs (suppressor of copper sensitivity) locus was initially identified for its ability to restore the copper tolerance of E. coli strains carrying mutations in cutF (nlpE) or cutC, as well as in cutA (dsbD), lnt, or lgt (which code for a periplasmic disulfide isomerase, an inner membrane apolipoprotein N-acyltransferase, and a phosphatidylglycerol-prolipoprotein diacylglyceryl transferase, respectively) (30, 43). Because of the homology of the scs gene products with thioredoxin-like proteins involved in oxidative disulfide folding and disulfide isomerization at the cell envelope of Gram-negative bacteria, the gene products were tested for their roles in redox biology and were found to alleviate the stress caused by copper or H2O2 (30, 31, 34). Their presence also reduces H2O2-mediated protein carbonylation in the periplasm (34), a common type of damage produced by reactive oxygen species (ROS)-generating agents such as copper (44). Several reports suggested that the locus contains two separate and independent transcriptional units, i.e., scsA and scsBCD, with different biological functions, with the first being required for oxidative stress responses and for scsBCD regulation and the second being involved in copper resistance (30, 31, 34, 35). Deletion of scsA but not the rest of the scs genes decreased H2O2 tolerance (34). In addition, a ΔscsA mutant showed increased transcription of scsB, scsC, and scsD, suggesting a role for ScsA in repressing the expression of scsBCD (35). Finally, deletions of scsB, scsC, or scsD but not scsA were shown to affect copper resistance (31, 34).
In contrast to these observations, here we establish that transcription of all four scs genes is driven by a Cu-activated promoter located upstream of scsA (Fig. 1). We demonstrated that the insertion of a polar chloramphenicol resistance cassette downstream from the scsA translational start site decreased the Cu-mediated activation of scsB transcription >10-fold (Fig. 1). We also showed that Cu induction of scsABCD depended on CpxR/CpxA (Fig. 1, 2, and 3), a two-component system essential for preserving and/or repairing periplasmic or inner membrane proteins damaged by different physical or chemical agents, including metals such as Cu, Zn, and Au (36, 37, 45). Our results indicate that, like scsA transcription, transcription of scsB, scsC, and scsD is driven by the CpxR-dependent scsA promoter, at least during copper stress. The identification of a single transcription start site located upstream of scsA in a S. Typhimurium global gene expression study (46, 47) gives further support to our observation. In addition, we observed that, in all scsA-harboring enterobacterial species genomes, the scsA gene is always followed by homologues of scsB, scsC, and scsD, in that order (see Fig. S6 at http://www.ibr-conicet.gov.ar/investigacion/publicaciones), suggesting that the four scs products operate in an integrated biological pathway. In fact, the identification of a putative Cpx-binding sequence at the Pscs promoter in most of those species (see Table S3 at http://www.ibr-conicet.gov.ar/investigacion/publicaciones) also provides evidence of conserved regulation. However, the presence of an alternative transcription start site upstream of scsB, promoting transcription of scsBCD independent of scsA under currently unidentified conditions, cannot be ruled out, as a short intergenic region separating scsA and scsB was observed in all of the analyzed genomes (see Fig. S6 at http://www.ibr-conicet.gov.ar/investigacion/publicaciones).
Anwar et al. reported that deletion of either scsB, scsC, or scsD had the same effect on Cu tolerance in Salmonella (34), suggesting that their products form part of a single detoxification complex. By performing a more detailed analysis of the contributions of these Scs proteins to copper resistance, here we establish that each of them plays a distinct role in alleviating the stress produced by the metal ion. We showed that the absence of ScsB produced the most dramatic effect on Cu tolerance under the conditions tested, followed in relevance by the mutant with deletion of the gene encoding its putative coupled periplasmic oxidoreductase, ScsC, and the strain with deletion of scsD, coding for a still uncharacterized integral membrane protein with a putative periplasmic thioredoxin-like domain (Table 1; also see Fig. S3 at http://www.ibr-conicet.gov.ar/investigacion/publicaciones). In addition, we demonstrated that other envelope thioredoxin-like proteins, such as DsbC, DsbG, and DsbD, are dispensable for Cu tolerance, except in the absence of a functional ScsC/ScsB system (Table 1), highlighting the importance of ScsB and its putative partner proteins ScsC and ScsD in Cu resistance. Our results also suggest that the Scs proteins and the DsbC/DsbD and DsbG/DsbD pairs act on different substrates, and we provide evidence regarding the functional cross talk between different Dsb-like systems to favor Salmonella survival under stressful conditions.
ScsC and ScsD are important not only in alleviating the damage caused by Cu but also in the defense against oxidative stress. Together with the major H2O2 detoxification factor of the system, ScsA (34), deletion of either scsC or scsD severely decreased growth at high concentrations of H2O2 (Fig. 4; also see Fig. S4 at http://www.ibr-conicet.gov.ar/investigacion/publicaciones). In fact, we observed that Cu contributed to protect the Δscs mutant strain from H2O2 damage not only by stimulating nonspecific corrections of misformed S-S bonds on periplasmic proteins, as suggested previously for E. coli (5), but also by triggering the expression of damage-correcting factors. In this sense, the induction of transcription of the canonical CpxR/CpxA-regulated gene cpxP in the ΔscsABCD strain under Cu stress (Fig. 5B) suggests the existence of a feedback loop between Scs and CpxR/CpxA to restore envelope homeostasis after severe Cu and/or redox injury. The Cu-mediated induction of Cpx-independent oxidation-protecting factors (Fig. 5A) strengthens the relevance of the Scs system in restoring envelope homeostasis after severe Cu and/or redox injury.
The Cu-dependent transcriptional activation of the scs operon and the role of ScsB, ScsC, and ScsD in Cu resistance (Fig. 1 and Table 1; also see Fig. S3 at http://www.ibr-conicet.gov.ar/investigacion/publicaciones) predict a role for these proteins in the folding of periplasmic Cu-resistant determinants. CueP is of particular relevance, among the two periplasmic factors controlled by the copper sensor CueR (1); it is the major periplasmic Cu-binding protein required for Cu tolerance under anaerobic conditions (14, 21), and it was shown to deliver Cu to the periplasmic Cu,Zn-superoxide dismutase SodCII (48), linking Cu and oxidative stress. Three cysteine residues involved in metal binding and intrachain/interchain interactions in CueP are essential for its biological function (49). Like the Scs system, CueP is present in Salmonella and in a small set of bacterial species but not in E. coli (14), and its gene's transcription is also dependent on CpxR/CpxA (16). However, a recent report indicates that CueP is a DsbC substrate (28). Current work is under way in our laboratory to determine the Scs target factors and their roles in copper and H2O2 tolerance.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The S. enterica serovar Typhimurium strains and plasmids used in this study are listed in Table S1 at http://www.ibr-conicet.gov.ar/investigacion/publicaciones. Oligonucleotides are listed in Table S2 at http://www.ibr-conicet.gov.ar/investigacion/publicaciones. Cells were routinely grown at 37°C in LB broth or on LB agar plates, except when indicated. Ampicillin, tetracycline, kanamycin, and chloramphenicol were used, when necessary, at 100, 15, 50, and 20 μg ml−1, respectively. All reagents and chemicals were from Sigma, except for the LB culture media, which were from Difco, and oligonucleotides and enzymes that were from Life Technologies.
Genetic and molecular biological techniques.
The strains carrying gene deletions or a lacZ reporter fusion to a promoter on the chromosome, all derivatives of strain ATCC 14028s, were generated by Lambda Red-mediated recombination followed by P22-mediated transduction, using previously described protocols (16, 50, 51) and the primers listed in Table S2 at http://www.ibr-conicet.gov.ar/investigacion/publicaciones. When necessary, the antibiotic resistance cassette inserted at the deletion point was removed using FLP-mediated recombination (52). A similar procedure was employed to construct the scsA::Cmr-INT strain, harboring the resistance cassette inserted 100 bp from the translational start site of scsA. DNA fragments as well as plasmids were introduced into bacterial cells by electroporation using a Bio-Rad device, following the manufacturer's recommendations. All constructs were verified by DNA sequencing.
The pPB1334 plasmid carrying the transcriptional fusion of the native Salmonella Pscs promoter to lacZ (see Table S1 at http://www.ibr-conicet.gov.ar/investigacion/publicaciones) was constructed by cloning a 303-bp PCR-amplified product into the XmaI site of pMC1871 (Amersham), using previously described protocols (51). The reporter pPB1477 plasmid carrying the modified, CpxR-independent Pscs promoter, Pscs* (see Table S1 at http://www.ibr-conicet.gov.ar/investigacion/publicaciones), was constructed by PCR-mediated site-directed mutagenesis. First, we amplified a 119-bp fragment using oligonucleotides Pscs*-Fw and PscsA-Rv (XmaI) (see Table S2 at http://www.ibr-conicet.gov.ar/investigacion/publicaciones), with pPB1334 (see Table S1 at http://www.ibr-conicet.gov.ar/investigacion/publicaciones) as the template. This fragment was then used as a primer along with PscsA-Fw (XmaI) to generate the final product carrying the mutant promoter for cloning into XmaI-digested pMC1871.
Induction and inhibition assays.
β-Galactosidase activity was measured in total extracts from cells cultured for 18 h at 37°C in LB broth adjusted to either pH 7.0 or pH 5.5 by the addition of 100 mM 2-(N-morpholino)ethanesulfonic acid (MES) and 1 mM CuSO4, essentially as described previously (51). When indicated, 100 μM isopropyl-β-thiogalactopyranoside (IPTG) was added to induce expression of cpxR or nlpE from plasmids.
Real-time qRT-PCR assays were performed basically as described previously (7). Total RNA was prepared from wild-type, ΔcpxR, scsA::Cmr-INT, or scsA::Cmr-INT ΔcpxR cells grown to mid-exponential phase (OD620 of 0.4 to 0.7) after incubation for 0, 10, 20, 40, and 60 min with or without 1 mM CuSO4, as indicated in the figures, using the RNAzol RT reagent (Molecular Research Center). After RQ1 DNase (Promega) treatment to improve quality, cDNA was obtained using Super Script II reverse transcriptase (Invitrogen), deoxynucleoside triphosphates (dNTPs), and specific sets of oligonucleotides (listed in Table S2 at http://www.ibr-conicet.gov.ar/investigacion/publicaciones) to amplify scsA, scsB, or the rnpB gene, which was used as a housekeeping gene to normalize transcription levels. Relative transcription levels were calculated as the ratio of normalized expression levels obtained after incubation in the presence or absence of Cu ions.
Copper sensitivity assays in liquid media were performed by recording the OD600 of cultures grown under aerobic conditions in LB broth without or with the indicated concentrations of CuSO4. Metal sensitivity assays were performed on LB agar plates containing increasing concentrations of CuSO4. To estimate the MIC values by CFU determination, overnight cultures of each strain were diluted to 10−6 in phosphate-buffered saline (PBS) and 10 μl of the indicated dilution was applied to the top of the plate. Colonies were allowed to develop for 24 h at 37°C before photographic recording. The MIC values were determined as the minimal concentration of CuSO4 at which no growth was observed.
Sensitivity to H2O2 was tested by recording OD620 values, with a BioTek Synergy 2 multimode microplate reader, every 60 min for 15 h at 37°C. Overnight cultures of the indicated strains were diluted 1:100 in LB broth and applied in duplicate to a sterile 96-well microplate (Greiner Bio-One) containing fresh H2O2, at the indicated final concentration, and/or 1 mM CuSO4 (see figure legends for details). When indicated, the metal salt was used to treat the cultures before sensitivity testing.
Protein-DNA interaction assays.
EMSAs and DNase I footprinting assays were performed using 6 fmol of a 32P-labeled DNA fragment containing the scsABCD promoter and CpxR-P, basically as described previously (16, 45). CpxR-P was obtained by incubating purified His-tagged CpxR with 25 mM acetyl phosphate for 1 h at 30°C. Protein concentrations were routinely determined with the Bradford assay, using bovine serum albumin as the standard. A DNA sequence ladder was generated in parallel by using the reverse primer and a Sequenase DNA sequencing kit (Affymetrix). After electrophoresis, the gels were dried and exposed for autoradiography.
In silico analysis.
The Seed tool (http://pubseed.theseed.org) was used to search for scsA homologues in other bacterial genomes (53).
Statistical analysis.
One-way analysis of variance and the Tukey-Kramer multiple-comparison test, with an overall significance level of 0.05, were used.
ACKNOWLEDGMENTS
We thank Julian Mendoza and M. Ayelen Carabajal for technical assistance.
This work was supported by a grant from Agencia Nacional de Promoción Científica y Tecnológica (grant PICT-2013-1513). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication. C.L. was a fellow of CONICET. S.K.C. and F.C.S. are career investigators of CONICET; F.C.S. is also a career investigator of the Rosario National University Research Council.
C.L. made major contributions to the design of the study and to the acquisition, analysis, and interpretation of the data. S.K.C. and F.C.S. made major contributions to the conception of the study, the interpretation of the data, and the writing of the manuscript.
We declare no conflicts of interest.
REFERENCES
- 1.Pontel LB, Checa SK, Soncini FC. 2015. Bacterial copper resistance and virulence, p 1–19. In Saffarini D (ed), Bacteria-metal interactions. Springer International Publishing, Basel, Switzerland. [Google Scholar]
- 2.Rensing C, Alwathnani HA, McDevitt SF. 2016. The copper metallome in prokaryotic cells, p 161–173. In de Bruijn FJ (ed), Stress and environmental regulation of gene expression and adaptation in bacteria. John Wiley & Sons, Hoboken, NJ. [Google Scholar]
- 3.Macomber L, Imlay JA. 2009. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc Natl Acad Sci U S A 106:8344–8349. doi: 10.1073/pnas.0812808106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Macomber L, Rensing C, Imlay JA. 2007. Intracellular copper does not catalyze the formation of oxidative DNA damage in Escherichia coli. J Bacteriol 189:1616–1626. doi: 10.1128/JB.01357-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hiniker A, Collet J-F, Bardwell JCA. 2005. Copper stress causes an in vivo requirement for the Escherichia coli disulfide isomerase DsbC. J Biol Chem 280:33785–33791. doi: 10.1074/jbc.M505742200. [DOI] [PubMed] [Google Scholar]
- 6.Pontel LB, Pezza A, Soncini FC. 2010. Copper stress targets the rcs system to induce multiaggregative behavior in a copper-sensitive Salmonella strain. J Bacteriol 192:6287–6290. doi: 10.1128/JB.00781-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pontel LB, Scampoli NL, Porwollik S, Checa SK, McClelland M, Soncini FC. 2014. Identification of a Salmonella ancillary copper detoxification mechanism by a comparative analysis of the genome-wide transcriptional response to copper and zinc excess. Microbiology 160:1659–1669. doi: 10.1099/mic.0.080473-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Becker KW, Skaar EP. 2014. Metal limitation and toxicity at the interface between host and pathogen. FEMS Microbiol Rev 38:1235–1249. doi: 10.1111/1574-6976.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Besold AN, Culbertson EM, Culotta VC. 2016. The yin and yang of copper during infection. J Biol Inorg Chem 21:137–144. doi: 10.1007/s00775-016-1335-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu Y, Chang FM, Giedroc DP. 2014. Copper transport and trafficking at the host-bacterial pathogen interface. Acc Chem Res 47:3605–3613. doi: 10.1021/ar500300n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ladomersky E, Khan A, Shanbhag V, Cavet JS, Chan J, Weisman GA, Petris MJ. 2017. Host and pathogen copper-transporting P-type ATPases function antagonistically during Salmonella infection. Infect Immun 85:e00351-. doi: 10.1128/IAI.00351-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osman D, Cavet JS. 2011. Metal sensing in Salmonella: implications for pathogenesis. Adv Microb Physiol 58:175–232. doi: 10.1016/B978-0-12-381043-4.00005-2. [DOI] [PubMed] [Google Scholar]
- 13.Espariz M, Checa SK, Audero ME, Pontel LB, Soncini FC. 2007. Dissecting the Salmonella response to copper. Microbiology 153:2989–2997. doi: 10.1099/mic.0.2007/006536-0. [DOI] [PubMed] [Google Scholar]
- 14.Pontel LB, Soncini FC. 2009. Alternative periplasmic copper-resistance mechanisms in Gram negative bacteria. Mol Microbiol 73:212–225. doi: 10.1111/j.1365-2958.2009.06763.x. [DOI] [PubMed] [Google Scholar]
- 15.Lim SY, Joe MH, Song SS, Lee MH, Foster JW, Park YK, Choi SY, Lee IS. 2002. cuiD is a crucial gene for survival at high copper environment in Salmonella enterica serovar Typhimurium. Mol Cells 14:177–184. [PubMed] [Google Scholar]
- 16.Pezza A, Pontel LB, Lopez C, Soncini FC. 2016. Compartment and signal-specific codependence in the transcriptional control of Salmonella periplasmic copper homeostasis. Proc Natl Acad Sci U S A 113:11573–11578. doi: 10.1073/pnas.1603192113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kershaw CJ, Brown NL, Constantinidou C, Patel MD, Hobman JL. 2005. The expression profile of Escherichia coli K-12 in response to minimal, optimal and excess copper concentrations. Microbiology 151:1187–1198. doi: 10.1099/mic.0.27650-0. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto K, Ishihama A. 2005. Transcriptional response of Escherichia coli to external copper. Mol Microbiol 56:215–227. doi: 10.1111/j.1365-2958.2005.04532.x. [DOI] [PubMed] [Google Scholar]
- 19.Hao X, Luthje FL, Qin Y, McDevitt SF, Lutay N, Hobman JL, Asiani K, Soncini FC, German N, Zhang S, Zhu YG, Rensing C. 2015. Survival in amoeba: a major selection pressure on the presence of bacterial copper and zinc resistance determinants? Identification of a “copper pathogenicity island.” Appl Microbiol Biotechnol 99:5817–5824. [DOI] [PubMed] [Google Scholar]
- 20.Petrovska L, Mather AE, AbuOun M, Branchu P, Harris SR, Connor T, Hopkins KL, Underwood A, Lettini AA, Page A, Bagnall M, Wain J, Parkhill J, Dougan G, Davies R, Kingsley RA. 2016. Microevolution of monophasic Salmonella Typhimurium during epidemic, United Kingdom, 2005–2010. Emerg Infect Dis 22:617–624. doi: 10.3201/eid2204.150531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osman D, Waldron KJ, Denton H, Taylor CM, Grant AJ, Mastroeni P, Robinson NJ, Cavet JS. 2010. Copper homeostasis in Salmonella is atypical and copper-CueP is a major periplasmic metal complex. J Biol Chem 285:25259–25268. doi: 10.1074/jbc.M110.145953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ezraty B, Gennaris A, Barras F, Collet J-F. 2017. Oxidative stress, protein damage and repair in bacteria. Nat Rev Microbiol 15:385–396. doi: 10.1038/nrmicro.2017.26. [DOI] [PubMed] [Google Scholar]
- 23.Hatahet F, Boyd D, Beckwith J. 2014. Disulfide bond formation in prokaryotes: history, diversity and design. Biochim Biophys Acta 1844:1402–1414. doi: 10.1016/j.bbapap.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kojer K, Riemer J. 2014. Balancing oxidative protein folding: the influences of reducing pathways on disulfide bond formation. Biochim Biophys Acta 1844:1383–1390. doi: 10.1016/j.bbapap.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Arts IS, Gennaris A, Collet J-F. 2015. Reducing systems protecting the bacterial cell envelope from oxidative damage. FEBS Lett 589:1559–1568. doi: 10.1016/j.febslet.2015.04.057. [DOI] [PubMed] [Google Scholar]
- 26.Anwar N, Rouf SF, Römling U, Rhen M. 2014. Modulation of biofilm-formation in Salmonella enterica serovar Typhimurium by the periplasmic DsbA/DsbB oxidoreductase system requires the GGDEF-EAL domain protein STM3615. PLoS One 9:e106095. doi: 10.1371/journal.pone.0106095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miki T, Okada N, Danbara H. 2004. Two periplasmic disulfide oxidoreductases, DsbA and SrgA, target outer membrane protein SpiA, a component of the Salmonella pathogenicity island 2 type III secretion system. J Biol Chem 279:34631–34642. doi: 10.1074/jbc.M402760200. [DOI] [PubMed] [Google Scholar]
- 28.Yoon BY, Kim JS, Um SH, Jo I, Yoo JW, Lee K, Kim YH, Ha NC. 2014. Periplasmic disulfide isomerase DsbC is involved in the reduction of copper binding protein CueP from Salmonella enterica serovar Typhimurium. Biochem Biophys Res Commun 446:971–976. doi: 10.1016/j.bbrc.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 29.Lin D, Kim B, Slauch JM. 2009. DsbL and DsbI contribute to periplasmic disulfide bond formation in Salmonella enterica serovar Typhimurium. Microbiology 155:4014–4024. doi: 10.1099/mic.0.032904-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta SD, Wu HC, Rick PD. 1997. A Salmonella typhimurium genetic locus which confers copper tolerance on copper-sensitive mutants of Escherichia coli. J Bacteriol 179:4977–4984. doi: 10.1128/jb.179.16.4977-4984.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shepherd M, Heras B, Achard MES, King GJ, Argente MP, Kurth F, Taylor SL, Howard MJ, King NP, Schembri MA, McEwan AG. 2013. Structural and functional characterization of ScsC, a periplasmic thioredoxin-like protein from Salmonella enterica serovar Typhimurium. Antioxid Redox Signal 19:1494–1506. doi: 10.1089/ars.2012.4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cho S-H, Parsonage D, Thurston C, Dutton RJ, Poole LB, Collet J-F, Beckwith J. 2012. A new family of membrane electron transporters and its substrates, including a new cell envelope peroxiredoxin, reveal a broadened reductive capacity of the oxidative bacterial cell envelope. mBio 3:e00291-. doi: 10.1128/mBio.00291-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Furlong EJ, Choudhury HG, Kurth F, Duff AP, Whitten AE, Martin JL. 2018. Disulfide isomerase activity of the dynamic, trimeric Proteus mirabilis ScsC protein is primed by the tandem immunoglobulin-fold domain of ScsB. J Biol Chem 293:5793–5805. doi: 10.1074/jbc.RA118.001860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anwar N, Sem XH, Rhen M. 2013. Oxidoreductases that act as conditional virulence suppressors in Salmonella enterica serovar Typhimurium. PLoS One 8:e64948. doi: 10.1371/journal.pone.0064948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verbrugghe E, Dhaenens M, Leyman B, Boyen F, Shearer N, Van Parys A, Haesendonck R, Bert W, Favoreel H, Deforce D, Thompson A, Haesebrouck F, Pasmans F. 2016. Host stress drives Salmonella recrudescence. Sci Rep 6:20849. doi: 10.1038/srep20849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grabowicz M, Silhavy TJ. 2017. Envelope stress responses: an interconnected safety net. Trends Biochem Sci 42:232–242. doi: 10.1016/j.tibs.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raivio TL. 2014. Everything old is new again: an update on current research on the Cpx envelope stress response. Biochim Biophys Acta 1843:1529–1541. doi: 10.1016/j.bbamcr.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 38.Danese PN, Silhavy TJ. 1998. CpxP, a stress-combative member of the Cpx regulon. J Bacteriol 180:831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Snyder WB, Davis LJ, Danese PN, Cosma CL, Silhavy TJ. 1995. Overproduction of NlpE, a new outer membrane lipoprotein, suppresses the toxicity of periplasmic LacZ by activation of the Cpx signal transduction pathway. J Bacteriol 177:4216–4223. doi: 10.1128/jb.177.15.4216-4223.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Wulf P, McGuire AM, Liu X, Lin ECC. 2002. Genome-wide profiling of promoter recognition by the two-component response regulator CpxR-P in Escherichia coli. J Biol Chem 277:26652–26661. doi: 10.1074/jbc.M203487200. [DOI] [PubMed] [Google Scholar]
- 41.Horst SA, Jaeger T, Denkel LA, Rouf SF, Rhen M, Bange F-C. 2010. Thiol peroxidase protects Salmonella enterica from hydrogen peroxide stress in vitro and facilitates intracellular growth. J Bacteriol 192:2929–2932. doi: 10.1128/JB.01652-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pham AN, Xing G, Miller CJ, Waite TD. 2013. Fenton-like copper redox chemistry revisited: hydrogen peroxide and superoxide mediation of copper-catalyzed oxidant production. J Catalysis 301:54–64. doi: 10.1016/j.jcat.2013.01.025. [DOI] [Google Scholar]
- 43.Gupta SD, Lee BT, Camakaris J, Wu HC. 1995. Identification of cutC and cutF (nlpE) genes involved in copper tolerance in Escherichia coli. J Bacteriol 177:4207–4215. doi: 10.1128/jb.177.15.4207-4215.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levine RL. 2002. Carbonyl modified proteins in cellular regulation, aging, and disease. Free Radic Biol Med 32:790–796. doi: 10.1016/S0891-5849(02)00765-7. [DOI] [PubMed] [Google Scholar]
- 45.Cerminati S, Giri GF, Mendoza JI, Soncini FC, Checa SK. 2017. The CpxR/CpxA system contributes to Salmonella gold-resistance by controlling the GolS-dependent gesABC transcription. Environ Microbiol 19:4035–4044. doi: 10.1111/1462-2920.13837. [DOI] [PubMed] [Google Scholar]
- 46.Colgan AM, Kröger C, Diard M, Hardt W-D, Puente JL, Sivasankaran SK, Hokamp K, Hinton JCD. 2016. The impact of 18 ancestral and horizontally-acquired regulatory proteins upon the transcriptome and sRNA landscape of Salmonella enterica serovar Typhimurium. PLoS Genet 12:e1006258. doi: 10.1371/journal.pgen.1006258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Srikumar S, Kröger C, Hébrard M, Colgan A, Owen SV, Sivasankaran SK, Cameron ADS, Hokamp K, Hinton JCD. 2015. RNA-seq brings new insights to the intra-macrophage transcriptome of Salmonella Typhimurium. PLoS Pathog 11:e1005262. doi: 10.1371/journal.ppat.1005262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Osman D, Patterson CJ, Bailey K, Fisher K, Robinson NJ, Rigby SE, Cavet JS. 2013. The copper supply pathway to a Salmonella Cu,Zn-superoxide dismutase (SodCII) involves P1B-type ATPase copper efflux and periplasmic CueP. Mol Microbiol 87:466–477. doi: 10.1111/mmi.12107. [DOI] [PubMed] [Google Scholar]
- 49.Abriata LA, Pontel LB, Vila AJ, Dal Peraro M, Soncini FC. 2014. A dimerization interface mediated by functionally critical residues creates interfacial disulfide bonds and copper sites in CueP. J Inorg Biochem 140:199–201. doi: 10.1016/j.jinorgbio.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 50.Ibanez MM, Cerminati S, Checa SK, Soncini FC. 2013. Dissecting the metal selectivity of MerR monovalent metal ion sensors in Salmonella. J Bacteriol 195:3084–3092. doi: 10.1128/JB.00153-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perez Audero ME, Podoroska BM, Ibanez MM, Cauerhff A, Checa SK, Soncini FC. 2010. Target transcription binding sites differentiate two groups of MerR-monovalent metal ion sensors. Mol Microbiol 78:853–865. doi: 10.1111/j.1365-2958.2010.07370.x. [DOI] [PubMed] [Google Scholar]
- 52.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Overbeek R, Begley T, Butler RM, Choudhuri JV, Chuang H-Y, Cohoon M, de Crécy-Lagard V, Diaz N, Disz T, Edwards R, Fonstein M, Frank ED, Gerdes S, Glass EM, Goesmann A, Hanson A, Iwata-Reuyl D, Jensen R, Jamshidi N, Krause L, Kubal M, Larsen N, Linke B, McHardy AC, Meyer F, Neuweger H, Olsen G, Olson R, Osterman A, Portnoy V, Pusch GD, Rodionov DA, Rückert C, Steiner J, Stevens R, Thiele I, Vassieva O, Ye Y, Zagnitko O, Vonstein V. 2005. The subsystems approach to genome annotation and its use in the project to annotate 1000 genomes. Nucleic Acids Res 33:5691–5702. doi: 10.1093/nar/gki866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hunke S, Keller R, Müller VS. 2012. Signal integration by the Cpx-envelope stress system. FEMS Microbiol Lett 326:12–22. doi: 10.1111/j.1574-6968.2011.02436.x. [DOI] [PubMed] [Google Scholar]