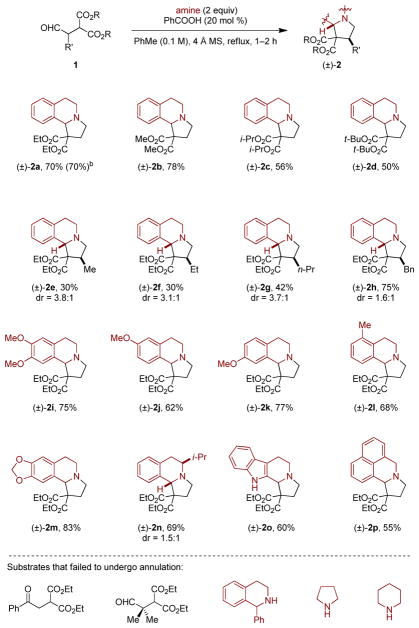
Abstract
Amines such as 1,2,3,4-tetrahydroisoquinoline undergo redox-neutral annulations with 2-(2-oxoethyl)malonates in the presence of catalytic amounts of benzoic acid. These reactions install a fully saturated 5-membered ring and provide access to structures closely related to the natural products crispine A and harmicine.
Graphical abstract
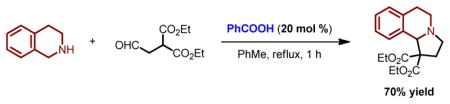
Redox-neutral amine annulation reactions, also referred to as redox-annulations, merge an oxidative α-C–H bond functionalization of an amine with the reductive amination of an aldehyde possessing a pendent (pro)nucleophilic site (Scheme 1, eq 1).1–7 These reactions are ideally suited to install a fused ring onto a cyclic amine in a simple one-step procedure. In the majority of cases, a new six-membered ring is formed. Redox-annulations with formation of a 5-membered ring have been reported,8 an example of which is provided in eq 2.8e,9 Invariably, these reactions install a partially unsaturated 5-membered ring and involve a pericyclic reaction step (e.g., a 1,5-electrocyclization). A possible exception is the recently reported annulation of amines such as 1,2,3,4-tetrahydroisoquinoline (THIQ) with α-ketoamides (eq 3),3k,4e although a pericyclic process may also intervene in the formation of the corresponding ring-fused aminal products. Here we report redox-annulations of cyclic amines with 2-(2-oxoethyl)malonates that lead to the installation of a fully saturated 5-membered ring, a structural motif found in the natural products crispine A and harmicine (eq 4).10
Scheme 1.
Redox-Annulations of Amines
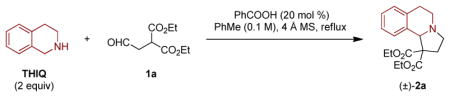
The title reaction was evaluated under a range of conditions, using THIQ and malonate-aldehyde 1a as model substrates (Table 1). Under the optimized conditions (Table 1, entry 1), 1a undergoes a reaction with two equiv of THIQ in the presence of benzoic acid (20 mol %) and 4 Å molecular sieves in toluene under reflux to provide product 2a in 72% yield following a relatively brief reaction time (1.5 h). Other reaction conditions led to inferior results. For instance, in the absence of a carboxylic acid catalyst, only 38% of 2a was formed within a 24 h period with the reaction remaining incomplete (entry 2). Increasing the amount of benzoic acid to 50 mol % led to a slightly faster reaction, but no increase in yield (entry 3). Replacement of benzoic acid for acetic acid or 2-ethylhexanoic acid (2-EHA), led to an increase in reaction time and a slight reduction in yield (entries 4 and 5). An increase or reduction in reactant concentration provided unfavorable results (entries 6 and 7), as did a reduction or an increase in the equiv of THIQ (entries 8 and 9).
Table 1.
Reaction Developmenta
| |||
|---|---|---|---|
|
| |||
| entry | deviation from optimized conditions | time (h) | yield (%) |
| 1 | none | 1.5 | 72 |
| 2 | no PhCOOH | 24 | 38 |
| 3 | 50 mol % of PhCOOH | 1 | 72 |
| 4 | AcOH instead of PhCOOH | 2.5 | 66 |
| 5 | 2-EHA instead of PhCOOH | 2.5 | 67 |
| 6 | 0.2 M conc | 1.5 | 67 |
| 7 | 0.05 M conc | 2.5 | 69 |
| 8 | 1.5 equiv of THIQ | 2 | 46 |
| 9 | 3 equiv of THIQ | 1.5 | 55 |
Reactions were performed on a 0.2 mmol scale. All yields correspond to isolated yields.
2-EHA = 2-ethylhexanoic acid
The scope of the amine annulation with 2-(2-oxoethyl)malonates is outlined in Scheme 2. Aldehydes derived from diethyl-, dimethyl-, diisopropyl-, and ditertbutyl malonate provided the corresponding products 2a–2d in moderate to good yields. A trend in the yields of these products was observed, with more sterically demanding ester substituents resulting in lower yields. Substitution of the α-position of the aldehyde was tolerated, albeit with varying reaction outcomes. The corresponding reaction products 2e–2h were isolated as mixtures of diastereomers. Substitution of the THIQ core was well tolerated, including substitution of the non-benzylic α-position of the amine nitrogen atom (products 2i–2n). In addition, amines other than THIQ underwent reactions with 1a (products 2o and 2p). Ketomalonates and aldehydes with two α-substituents did not participate in annulations with THIQ. 1-Phenyl-THIQ and amines with attenuated reactivities such as pyrrolidine and piperidine also did not undergo the title reaction. At least in the case of the latter two substrates, this is likely due to the higher energy barriers of the C–H functionalization step, which under the current conditions appears to be incompatible with the enolizable nature of the aldehyde substrate.11c, 11e
Scheme 2. Scope of the Redox-Annulationa.
aReactions were performed on a 0.5 mmol scale. All yields correspond to isolated yields. b Value in parentheses corresponds to yield for a reaction conducted on a 2 mmol scale.
2,3,4,5-tetrahydro-1H-benzo[c]azepane (3) did not undergo the expected annulation with malonate-aldehyde 1a under a range of conditions. Instead, regiodivergent annulation led to the formation of product 4, resulting from the substitution of a less activated non-benzylic C–H bond (Scheme 3). Under the optimized conditions using microwave irradiation, product 4 was isolated in 54% yield. This type of regiodivergent annulation has been observed previously,3c,3g,5a although not to the exclusion of the typically dominant reaction pathway. As was established in analogous redox-annulations of THIQ with 4-nitrobutyraldehydes, the primary site of C–H functionalization is almost certainly the benzylic position. Subsequent isomerization of an intermediate N,O-acetal via the corresponding azomethine ylide ultimately results in the final product 4.3g
Scheme 3.
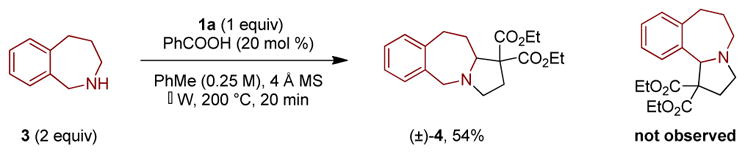
Redox-Annulation with Divergent Regioselectivity
Decarboxylative annulations of proline and pipecolic acid have been shown in some cases to provide products not readily or not efficiently accessible from redox-annulations of pyrrolidine and piperidine.11–13 Given the failure of pyrrolidine and piperidine in the present case, a decarboxylative variant of the title reaction was also explored (Scheme 4). Gratifyingly, condensation of 1a with proline in the presence of excess acetic acid under reflux in mesitylene provided pyrrolizidine 5 in 81% yield. Application of identical conditions to a reaction with pipecolic acid and 1a furnished indolizidine 6 in 69% yield.
Scheme 4. Decarboxylative Annulationsa.
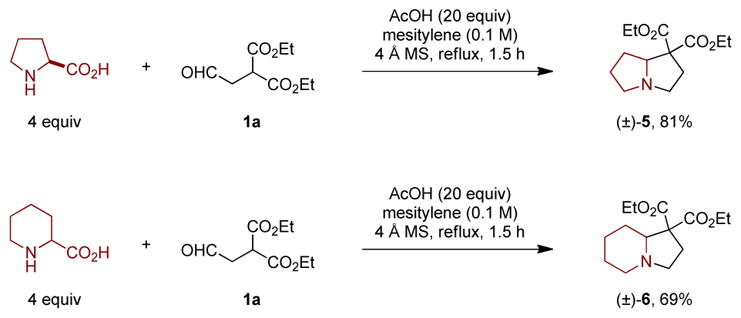
aReactions were performed on a 0.5 mmol scale. The aldehyde was added slowly over 1 h, followed by an additional 0.5 h reaction time. Yields correspond to isolated yields.
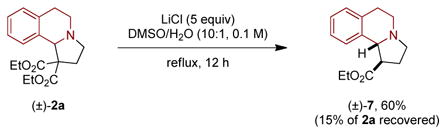
As shown in eq 5, annulation product 2a was subjected to Krapcho dealkoxycarbonylation conditions,14 resulting in the formation of a single diastereomer of monoester 7 in 60% yield. Under the indicated conditions, starting material 2a was recovered in 15% yield. Attempts to increase the conversion led to a reduction in the yield of product 7.
 |
(5) |
In summary, we have achieved redox-annulations of amines with 2-(2-oxoethyl)malonates, reactions that rapidly form the core structures of natural products such as crispine A and harmicine. These reactions likely represent the first examples of redox-annulations leading to five-membered ring formation without intervention of pericyclic processes.
Supplementary Material
Acknowledgments
Financial support from the NIH–NIGMS (Grant R01GM101389) is gratefully acknowledged. We thank Dr. Tom Emge (Rutgers University) for X-ray crystallographic analysis.
Footnotes
Experimental procedures and characterization data, including the X-ray crystal structure of product 2l (PDF, CIF). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Selected reviews on amine C–H functionalization, including redox-neutral approaches: Murahashi SI. Angew Chem, Int Ed Engl. 1995;34:2443.Matyus P, Elias O, Tapolcsanyi P, Polonka-Balint A, Halasz-Dajka B. Synthesis. 2006:2625.Campos KR. Chem Soc Rev. 2007;36:1069. doi: 10.1039/b607547a.Murahashi SI, Zhang D. Chem Soc Rev. 2008;37:1490. doi: 10.1039/b706709g.Li CJ. Acc Chem Res. 2009;42:335. doi: 10.1021/ar800164n.Jazzar R, Hitce J, Renaudat A, Sofack-Kreutzer J, Baudoin O. Chem Eur J. 2010;16:2654. doi: 10.1002/chem.200902374.Yeung CS, Dong VM. Chem Rev. 2011;111:1215. doi: 10.1021/cr100280d.Pan SC. Beilstein J Org Chem. 2012;8:1374. doi: 10.3762/bjoc.8.159.Mitchell EA, Peschiulli A, Lefevre N, Meerpoel L, Maes BUW. Chem Eur J. 2012;18:10092. doi: 10.1002/chem.201201539.Zhang C, Tang C, Jiao N. Chem Soc Rev. 2012;41:3464. doi: 10.1039/c2cs15323h.Jones KM, Klussmann M. Synlett. 2012;23:159.Peng B, Maulide N. Chem Eur J. 2013;19:13274. doi: 10.1002/chem.201301522.Platonova AY, Glukhareva TV, Zimovets OA, Morzherin YY. Chem Heterocycl Compd. 2013;49:357.Prier CK, Rankic DA, MacMillan DWC. Chem Rev. 2013;113:5322. doi: 10.1021/cr300503r.Girard SA, Knauber T, Li CJ. Angew Chem Int Ed. 2014;53:74. doi: 10.1002/anie.201304268.Haibach MC, Seidel D. Angew Chem Int Ed. 2014;53:5010. doi: 10.1002/anie.201306489.Wang L, Xiao J. Adv Synth Catal. 2014;356:1137.Vo CVT, Bode JW. J Org Chem. 2014;79:2809. doi: 10.1021/jo5001252.Seidel D. Org Chem Front. 2014;1:426. doi: 10.1039/C4QO00022F.Qin Y, Lv J, Luo S. Tetrahedron Lett. 2014;55:551.Seidel D. Acc Chem Res. 2015;48:317. doi: 10.1021/ar5003768.Beatty JW, Stephenson CRJ. Acc Chem Res. 2015;48:1474. doi: 10.1021/acs.accounts.5b00068.Mahato S, Jana CK. Chem Rec. 2016;16:1477. doi: 10.1002/tcr.201600001.Qin Y, Zhu L, Luo S. Chem Rev. 2017;117:9433. doi: 10.1021/acs.chemrev.6b00657.Cheng MX, Yang SD. Synlett. 2017;28:159.
- 2.Selected reviews on various types of redox-neutral transformations: Burns NZ, Baran PS, Hoffmann RW. Angew Chem Int Ed. 2009;48:2854. doi: 10.1002/anie.200806086.Mahatthananchai J, Bode JW. Acc Chem Res. 2014;47:696. doi: 10.1021/ar400239v.Ketcham JM, Shin I, Montgomery TP, Krische MJ. Angew Chem Int Ed. 2014;53:9142. doi: 10.1002/anie.201403873.Huang H, Ji X, Wu W, Jiang H. Chem Soc Rev. 2015;44:1155. doi: 10.1039/c4cs00288a.
- 3.Selected examples of redox-annulations from our laboratory: Zhang C, De CK, Mal R, Seidel D. J Am Chem Soc. 2008;130:416. doi: 10.1021/ja077473r.Zhang C, Das D, Seidel D. Chem Sci. 2011;2:233.Dieckmann A, Richers MT, Platonova AY, Zhang C, Seidel D, Houk KN. J Org Chem. 2013;78:4132. doi: 10.1021/jo400483h.Richers MT, Deb I, Platonova AY, Zhang C, Seidel D. Synthesis. 2013;45:1730.Richers MT, Breugst M, Platonova AY, Ullrich A, Dieckmann A, Houk KN, Seidel D. J Am Chem Soc. 2014;136:6123. doi: 10.1021/ja501988b.Jarvis CL, Richers MT, Breugst M, Houk KN, Seidel D. Org Lett. 2014;16:3556. doi: 10.1021/ol501509b.Kang Y, Chen W, Breugst M, Seidel D. J Org Chem. 2015;80:9628. doi: 10.1021/acs.joc.5b01384.Ma L, Seidel D. Chem Eur J. 2015;21:12908. doi: 10.1002/chem.201501667.Chen W, Seidel D. Org Lett. 2016;18:1024. doi: 10.1021/acs.orglett.6b00151.Zhu Z, Seidel D. Org Lett. 2017;19:2841. doi: 10.1021/acs.orglett.7b01047.Zhu Z, Lv X, Anesini JE, Seidel D. Org Lett. 2017;19:6424. doi: 10.1021/acs.orglett.7b03309.
- 4.Selected examples of redox-annulations by others: Zheng L, Yang F, Dang Q, Bai X. Org Lett. 2008;10:889. doi: 10.1021/ol703049j.Mahato S, Haque MA, Dwari S, Jana CK. RSC Adv. 2014;4:46214.Li J, Qin C, Yu Y, Fan H, Fu Y, Li H, Wang W. Adv Synth Catal. 2017;359:2191.Li J, Fu Y, Qin C, Yu Y, Li H, Wang W. Org Biomol Chem. 2017;15:6474. doi: 10.1039/c7ob01527e.Liu Y, Wu J, Jin Z, Jiang H. Synlett. 2018;29:1061.
- 5.Selected examples of related intermolecular redox-transformations: Ma L, Chen W, Seidel D. J Am Chem Soc. 2012;134:15305. doi: 10.1021/ja308009g.Das D, Sun AX, Seidel D. Angew Chem Int Ed. 2013;52:3765. doi: 10.1002/anie.201300021.Das D, Seidel D. Org Lett. 2013;15:4358. doi: 10.1021/ol401858k.Zheng QH, Meng W, Jiang GJ, Yu ZX. Org Lett. 2013;15:5928. doi: 10.1021/ol402517e.Chen W, Kang Y, Wilde RG, Seidel D. Angew Chem Int Ed. 2014;53:5179. doi: 10.1002/anie.201311165.Chen W, Seidel D. Org Lett. 2014;16:3158. doi: 10.1021/ol501365j.Chen W, Wilde RG, Seidel D. Org Lett. 2014;16:730. doi: 10.1021/ol403431u.Lin W, Ma S. Org Chem Front. 2014;1:338.Lin W, Cao T, Fan W, Han Y, Kuang J, Luo H, Miao B, Tang X, Yu Q, Yuan W, Zhang J, Zhu C, Ma S. Angew Chem Int Ed. 2014;53:277. doi: 10.1002/anie.201308699.Haldar S, Mahato S, Jana CK. Asian J Org Chem. 2014;3:44.Li J, Wang H, Sun J, Yang Y, Liu L. Org Biomol Chem. 2014;12:2523. doi: 10.1039/c3ob42431f.Haldar S, Roy SK, Maity B, Koley D, Jana CK. Chem Eur J. 2015;21:15290. doi: 10.1002/chem.201502297.Shao G, He Y, Xu Y, Chen J, Yu H, Cao R. Eur J Org Chem. 2015;2015:4615.Mandal S, Mahato S, Jana CK. Org Lett. 2015;17:3762. doi: 10.1021/acs.orglett.5b01744.Zhu Z, Seidel D. Org Lett. 2016;18:631. doi: 10.1021/acs.orglett.5b03529.Huang J, Li L, Xiao T, Mao Z-w, Zhou L. Asian J Org Chem. 2016;5:1204.Zhou S, Tong R. Chem Eur J. 2016;22:7084. doi: 10.1002/chem.201601245.Kumar M, Kaur BP, Chimni SS. Chem Eur J. 2016;22:9948. doi: 10.1002/chem.201601222.Hu G, Chen W, Ma D, Zhang Y, Xu P, Gao Y, Zhao Y. J Org Chem. 2016;81:1704. doi: 10.1021/acs.joc.5b02625.Yan JM, Bai QF, Xu C, Feng G. Synthesis. 2016;48:3730.Lin W, Ma S. Org Chem Front. 2017;4:958.Zhou S, Tong R. Org Lett. 2017;19:1594. doi: 10.1021/acs.orglett.7b00414.Yu J, Zhang Z, Zhou S, Zhang W, Tong R. Org Chem Front. 2018;5:242.Yi CB, She ZY, Cheng YF, Qu J. Org Lett. 2018;20:668. doi: 10.1021/acs.orglett.7b03807.
- 6.For detailed discussions on the mechanisms of these transformations, see references 1u, 3c, 3e–g and the following reports: Xue X, Yu A, Cai Y, Cheng J-P. Org Lett. 2011;13:6054. doi: 10.1021/ol2025247.Ma L, Paul A, Breugst M, Seidel D. Chem Eur J. 2016;22:18179. doi: 10.1002/chem.201603839.
- 7.Recent examples of mechanistically distinct, redox-neutral amine α-C–H bond functionalization reactions: Suh CW, Kwon SJ, Kim DY. Org Lett. 2017;19:1334. doi: 10.1021/acs.orglett.7b00184.Zhen L, Wang J, Xu QL, Sun H, Wen X, Wang G. Org Lett. 2017;19:1566. doi: 10.1021/acs.orglett.7b00378.Ramakumar K, Maji T, Partridge JJ, Tunge JA. Org Lett. 2017;19:4014. doi: 10.1021/acs.orglett.7b01752.Li SS, Zhou L, Wang L, Zhao H, Yu L, Xiao J. Org Lett. 2018;20:138. doi: 10.1021/acs.orglett.7b03492.Zhu S, Chen C, Xiao M, Yu L, Wang L, Xiao J. Green Chem. 2017;19:5653.Idiris FIM, Majeste CE, Craven GB, Jones CR. Chem Sci. 2018;9:2873. doi: 10.1039/c8sc00181b.Mori K, Isogai R, Kamei Y, Yamanaka M, Akiyama T. J Am Chem Soc. 2018;140:6203. doi: 10.1021/jacs.8b02761.
- 8.Examples of redox-neutral α-C–H bond annulations of secondary amines that result in the formation of 5-membered rings: Grigg R, Nimal Gunaratne HQ, Henderson D, Sridharan V. Tetrahedron. 1990;46:1599.Soeder RW, Bowers K, Pegram LD, Cartaya-Marin CP. Synth Commun. 1992;22:2737.Grigg R, Kennewell P, Savic V, Sridharan V. Tetrahedron. 1992;48:10423.Deb I, Seidel D. Tetrahedron Lett. 2010;51:2945.Kang Y, Richers MT, Sawicki CH, Seidel D. Chem Commun. 2015;51:10648. doi: 10.1039/c5cc03390j.Cheng YF, Rong HJ, Yi CB, Yao JJ, Qu J. Org Lett. 2015;17:4758. doi: 10.1021/acs.orglett.5b02298.Yang Z, Lu N, Wei Z, Cao J, Liang D, Duan H, Lin Y. J Org Chem. 2016;81:11950. doi: 10.1021/acs.joc.6b01781.Rong HJ, Cheng YF, Liu FF, Ren SJ, Qu J. J Org Chem. 2017;82:532. doi: 10.1021/acs.joc.6b02562.Purkait A, Roy SK, Srivastava HK, Jana CK. Org Lett. 2017;19:2540. doi: 10.1021/acs.orglett.7b00832.
- 9.Examples of redox-neutral α-C–H bond functionalizations of secondary amines in the context of (3+2) cycloadditions: Ardill H, Grigg R, Sridharan V, Surendrakumar S, Thianpatanagul S, Kanajun S. J Chem Soc, Chem Commun. 1986:602.Ardill H, Dorrity MJR, Grigg R, Leon-Ling MS, Malone JF, Sridharan V, Thianpatanagul S. Tetrahedron. 1990;46:6433.Ardill H, Fontaine XLR, Grigg R, Henderson D, Montgomery J, Sridharan V, Surendrakumar S. Tetrahedron. 1990;46:6449.Wang B, Mertes MP, Mertes KB, Takusagawa F. Tetrahedron Lett. 1990;31:5543.Wittland C, Arend M, Risch N. Synthesis. 1996:367.Marx MA, Grillot AL, Louer CT, Beaver KA, Bartlett PA. J Am Chem Soc. 1997;119:6153.Grigg R, Sridharan V, Thornton-Pett M, Wang J, Xu J, Zhang J. Tetrahedron. 2002;58:2627.Parmar NJ, Pansuriya BR, Labana BM, Kant R, Gupta VK. RSC Adv. 2013;3:17527.Rahman M, Bagdi AK, Mishra S, Hajra A. Chem Commun. 2014;50:2951. doi: 10.1039/c4cc00454j.Mantelingu K, Lin Y, Seidel D. Org Lett. 2014;16:5910. doi: 10.1021/ol502918g.Pavan Kumar CS, Harsha KB, Mantelingu K, Rangappa KS. RSC Adv. 2015;5:61664.Safaei-Ghomi J, Masoomi R. RSC Adv. 2015;5:15591.Yang HT, Tan YC, Ge J, Wu H, Li JX, Yang Y, Sun XQ, Miao CB. J Org Chem. 2016;81:11201. doi: 10.1021/acs.joc.6b02193.Zheng KL, Shu WM, Ma JR, Wu YD, Wu AX. Org Lett. 2016;18:3526. doi: 10.1021/acs.orglett.6b01369.Du Y, Yu A, Jia J, Zhang Y, Meng X. Chem Commun. 2017;53:1684. doi: 10.1039/c6cc08996h.Zheng KL, You MQ, Shu WM, Wu YD, Wu AX. Org Lett. 2017;19:2262. doi: 10.1021/acs.orglett.7b00769.
- 10.Original isolation paper of crispine A: Zhang Q, Tu G, Zhao Y, Cheng T. Tetrahedron. 2002;58:6795.Original isolation paper of harmicine: Kam TS, Sim KM. Phytochemistry. 1998;47:145.For a recent synthesis of both natural products and further references, see: Fandrick DR, Hart CA, Okafor IS, Mercadante MA, Sanyal S, Masters JT, Sarvestani M, Fandrick KR, Stockdill JL, Grinberg N, Gonnella N, Lee H, Senanayake CH. Org Lett. 2016;18:6192. doi: 10.1021/acs.orglett.6b03253.
- 11.Cohen N, Blount JF, Lopresti RJ, Trullinger DP. J Org Chem. 1979;44:4005.Tang M, Tong L, Ju L, Zhai W, Hu Y, Yu X. Org Lett. 2015;17:5180. doi: 10.1021/acs.orglett.5b02484.Kang Y, Seidel D. Org Lett. 2016;18:4277. doi: 10.1021/acs.orglett.6b02020.Wu J-s, Jiang H-j, Yang J-g, Jin Z-n, Chen D-b. Tetrahedron Lett. 2017;58:546.Paul A, Thimmegowda NR, Galani Cruz T, Seidel D. Org Lett. 2018;20:602. doi: 10.1021/acs.orglett.7b03721. See also references 3a, 3b, 3d, 3j, and 4a.
- 12.Selected examples of mechanistically related decarboxylative α-amino acid functionalizations: Bi HP, Zhao L, Liang YM, Li CJ. Angew Chem Int Ed. 2009;48:792. doi: 10.1002/anie.200805122.Bi HP, Chen WW, Liang YM, Li CJ. Org Lett. 2009;11:3246. doi: 10.1021/ol901129v.Bi HP, Teng Q, Guan M, Chen WW, Liang YM, Yao X, Li CJ. J Org Chem. 2010;75:783. doi: 10.1021/jo902319h.Zhang C, Seidel D. J Am Chem Soc. 2010;132:1798. doi: 10.1021/ja910719x.Yang D, Zhao D, Mao L, Wang L, Wang R. J Org Chem. 2011;76:6426. doi: 10.1021/jo200981h.Das D, Richers MT, Ma L, Seidel D. Org Lett. 2011;13:6584. doi: 10.1021/ol202957d.Firouzabadi H, Iranpoor N, Ghaderi A, Ghavami M. Tetrahedron Lett. 2012;53:5515.Kaboudin B, Karami L, Kato JY, Aoyama H, Yokomatsu T. Tetrahedron Lett. 2013;54:4872.Manjappa KB, Jhang WF, Huang SY, Yang DY. Org Lett. 2014;16:5690. doi: 10.1021/ol5027574.Dighe SU, KSAK, Srivastava S, Shukla P, Singh S, Dikshit M, Batra S. J Org Chem. 2015;80:99. doi: 10.1021/jo502029k.Jin Z-n, Jiang H-j, Wu J-s, Gong W-z, Cheng Y, Xiang J, Zhou Q-Z. Tetrahedron Lett. 2015;56:2720.Samala S, Singh G, Kumar R, Ampapathi RS, Kundu B. Angew Chem Int Ed. 2015;54:9564. doi: 10.1002/anie.201504429.Zhou J, Liu H, Li Z, Jin C, Su W. Tetrahedron Lett. 2017;58:3174.Guo J, Xie Y, Wu QL, Zeng WT, Chan ASC, Weng J, Lu G. RSC Adv. 2018;8:16202. doi: 10.1039/c8ra02340a.
- 13.Selected reviews on decarboxylative coupling reactions not limited to amino acids: Rodriguez N, Goossen LJ. Chem Soc Rev. 2011;40:5030. doi: 10.1039/c1cs15093f.Xuan J, Zhang ZG, Xiao WJ. Angew Chem Int Ed. 2015;54:15632. doi: 10.1002/anie.201505731.Patra T, Maiti D. Chem Eur J. 2017;23:7382. doi: 10.1002/chem.201604496.Wei Y, Hu P, Zhang M, Su W. Chem Rev. 2017;117:8864. doi: 10.1021/acs.chemrev.6b00516.
- 14.Krapcho AP, Ciganek E. Organic Reactions. Vol. 81 John Wiley & Sons, Inc; 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.