A cardiogenic shock requirement was recently added to the now accepted 6-Status Heart Allocation System (Figure 1).1 The criteria, derived from the American Heart Association standards for cardiogenic shock, were designed to prevent non-urgent candidates from qualifying for high priority Status based on therapies alone.1 Although the original proposal has undergone extensive simulation, those prediction models were estimated before the addition of the cardiogenic shock criteria.2 Therefore, the number of candidates the shock criteria would cause to list at a lower priority Status was not quantified, and the effect of the shock criteria on overall allocation was not estimated. We aimed to determine the proportion of candidates impacted by the requirement and examine the ability of the shock criteria to predict transplant-free waitlist survival.
Figure 1.
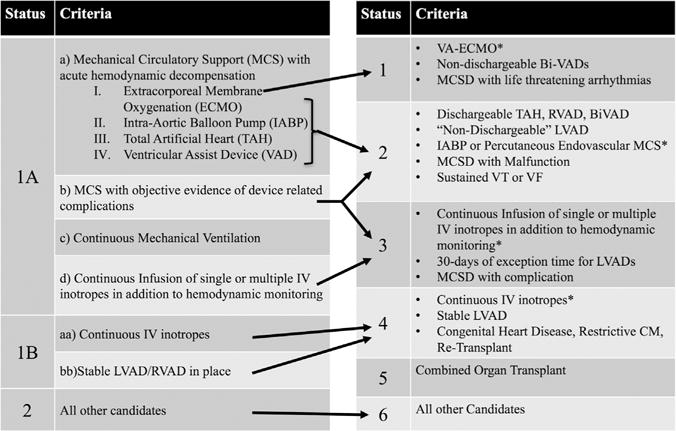
Current and future adult heart allocation. Schematic depiction of the shift from the current adult heart allocation system to the modified system. *Cardiogenic shock requirement applies. (Constructed with permission directly from the policy details in Organ Procurement and Transplantation Network.1).
The registrations of all adult heart-only candidates listed during the years 2010–2015 were analyzed using the Scientific Registry of Transplant Recipients (SRTR) data set. Candidates subject to the shock criteria include candidates supported with venoarterial extracorporeal membrane oxygenation (VA-ECMO) for Status 1, percutaneous endovascular support devices for Status 2, intra-aortic balloon pump (IABP) for Status 2, high-dose/multiple inotropes for Status 3, and low-dose inotropes for Status 4. The proportion of candidates meeting the cardiogenic shock criteria by cardiac index was calculated for each group. Patients listed with VA-ECMO and percutaneous support devices were conservatively categorized as “in shock” owing to high rates of missing hemodynamic data. We then analyzed the ability of the shock criteria to predict candidate death or delisting using both unadjusted Kaplan-Meier survival functions and competing risks models (to provide adjusted differences in transplant-free waitlist survival).
The registrations of 19,924 adult heart-alone candidates were analyzed. The cardiogenic shock criteria would have applied to 1,330 candidates per year on average (40% of all candidates listed in 2010–2015). We identified an average of 630 candidates per year (19% of yearly listings) that would have had their Status level reduced by the cardiogenic shock criteria (Table 1 and Figure 2). Of candidates, 40% of IABP candidates (Status 2), 62% of high-dose/multiple inotropes candidates (Status 3), and 47% of low-dose inotropes candidates (Status 4) would be listed at a lower priority Status. Candidates receiving multiple inotropes had a higher cardiac index (mean 2.24 liters/min/m2 vs 2.16 liters/min/m2; p = 0.018) and were more likely to be ineligible by shock criteria for Status 3 than candidates receiving high-dose inotropes (74% vs 40%; p < 0.001).
Table 1.
Potential Impact of Shock Criteria
| VA-ECMO | Percutaneous Endovascular support device | IABP | High-dose single inotrope | Multiple inotropes | Low-dose inotropes | |
|---|---|---|---|---|---|---|
| Average yearly listings subject to shock requirement, n | 40 | 4 | 118 | 119 | 190 | 862 |
| Potential Status (new system) | 1 | 2 | 2 | 3 | 3 | 4 |
| Hemodynamics available, n (%) | 23 (56) | 3 (79) | 109 (92) | 102 (86) | 175 (92) | 830 (96) |
| Ineligible by shock criteria, n (%) | —a | —a | 47 (40) | 48 (40) | 141 (74) | 393 (47) |
| Disqualified by high cardiac index, n (%)b | — | — | 47 (40) | 45 (37) | 55 (29) | 393 (47) |
| Disqualified by low inotrope dose, n (%)d | — | — | N/A | 2 (2) | 29 (15) | —c |
| Disqualified by both high cardiac index and low inotrope dose, n (%) | — | — | N/A | 1 (1) | 57 (30) | —c |
| Cardiac index >3.0 liters/min/m2, n (%) | — | — | 8 (7) | 26 (22) | 34 (18) | 64 (8) |
IABP, intra-aortic balloon pump; N/A, not applicable; VA-ECMO, venoarterial extracorporeal membrane oxygenation.
Average number of candidates per year displayed for 2010–2015, rounded to nearest whole candidate. Percentages are by qualifying therapy group. Bolded candidates were candidates who would have been rendered ineligible by cardiogenic shock criteria. Candidates receiving high-dose inotropes and candidates receiving multiple inotropes can be disqualified by either high cardiac index or low inotrope dose.
Disqualifications not calculated for VA-ECMO and percutaneous endovascular support devices owing to lack of hemodynamic data before mechanical circulatory support.
Maximum cardiac index is defined as 1.8 liters/min/m2 for candidates without inotropic support or 2.2 liters/min/m2 for candidates with inotropic support.
Candidates receiving low-dose inotropes can also be disqualified by low inotrope dose; however, inotrope dose data were unavailable for this group.
Minimum inotrope requirements are (1) 1 high-dose intravenous inotrope (dobutamine ≥7.5 μg/kg/min or milrinone ≥0.50 μg/kg/min) or (2) at least 2 intravenous inotropes (dobutamine ≥3 μg/kg/min, milrinone ≥0.25 μg/kg/min, or dopamine ≥3 μg/kg/min).
Figure 2.
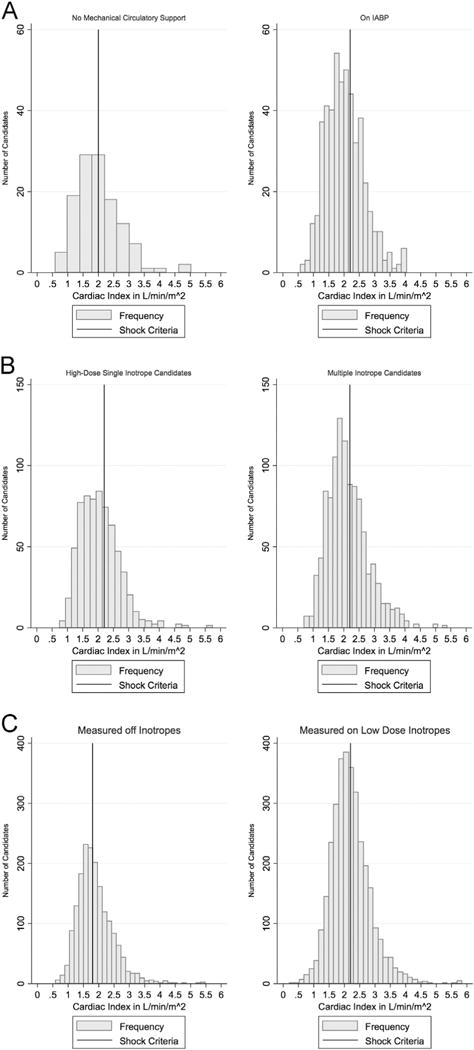
(A) Cardiac index of candidates supported by an IABP at the time of listing. The histogram on the left is of cardiac indices for candidates without circulatory support at the time of hemodynamic measurement. The histogram on the right is of cardiac indices of candidates supported with the listing IABP at the time of hemodynamic measurement. The vertical lines represent the cutoff for cardiogenic shock, 2.0 liters/min/m2 for candidates without mechanical support at the time of listing and 2.2 liters/min/m2 for candidates supported by IABP. Candidates to the right of the line would be ineligible for Status 2 listing. (B) Cardiac index of candidates supported by high-dose single inotropes or multiple inotropes at the time of listing. The cardiogenic shock cardiac index criterion (<2.2 liters/min/m2 when supported by inotropes) is marked with a vertical line. Candidates to the right of the line would be ineligible for Status 3 listing. Candidates supported by multiple inotropes had a higher cardiac index (mean 2.24 liters/min/m2 vs 2.16 liters/min/m2; p = 0.018) and were more likely to be ineligible by shock criteria for Status 3 than candidates supported by high-dose inotropes (74% vs 40%; p< 0.001). (C) Cardiac index of candidates supported by low-dose inotropes at the time of listing. The histogram on the left is of cardiac indices for candidates not on inotropes at the time of hemodynamic measurement. The histogram on the right is of cardiac indices of candidates receiving low-dose inotropes at the time of measurement. Candidates to the right of the line would be ineligible for Status 4 listing. For candidates with hemodynamics measured before inotrope initiation, the mean cardiac index was 1.88 liters/min/m2, and the proportion of candidates disqualified by shock criteria was 49%. For candidates with hemodynamics measured on the listed inotrope, the mean cardiac index was 2.21 liters/min/m2, and the proportion disqualified was 46% (p = 0.027 for shock proportion comparison).
The presence of the shock criteria at listing did not affect the Kaplan-Meier estimated waitlist survival for any tested candidate group (p > 0.18 by log-rank test). We found a borderline significant difference in adjusted transplant-free survival based on shock criteria for IABP candidates (subhazard ratio for death delisting 1.50, 95% confidence interval 1.00–2.26) but no significant difference by the shock criteria for high-dose/multiple inotropes candidates and low-dose inotropes candidates (p = 0.27 and p = 0.31) (supplementary data, available in the online version of this article at www.jhltonline.org).
In this analysis of the SRTR database, we demonstrated that the cardiogenic shock criteria will likely reduce the priority for transplantation of >600 candidates a year—19% of all candidates listed in the United States. The major driver of disqualifications will likely be multiple inotropes and low-dose inotropes candidates. We also found that the presence of shock criteria at listing does not predict waitlist survival in any of the candidate groups subject to the shock requirement.
The consequences for the candidates who will not meet the shock criteria will be profound. Transplant programs will be forced either to list the candidates at substantially lower priority Status (presumably Status 6) or choose a support therapy that does not require the cardiogenic shock criteria. The therapies that are exempt from the shock criteria are typically surgically placed devices, such as a “Non-dischargeable, Surgically Implanted, Non-Endovascular Left Ventricular Assist Device (LVAD)” for Status 2 listing.1 We are concerned that programs will be incentivized to choose surgical ventricular assist device support options over percutaneous or inotropic support strategies to circumvent the cardiogenic shock criteria and permit listing at higher priority Statuses.
The results of our waitlist survival analysis are consistent with previous work that demonstrated that mean pulmonary capillary wedge pressure, not cardiac index, is the important hemodynamic explanatory variable for predicting waitlist survival in heart transplant candidates.3 Perhaps this is due to the unreliability of clinically obtained cardiac output measurements compared with gold standard measurements.4,5 The lack of a significant transplant-free survival difference between candidates with and without the shock criteria also has important policy implications. If the intention of the policy is to prioritize candidates with a higher chance of dying on the waitlist without transplant, our results demonstrate that the shock criteria are unlikely to do this.
Limitations to our findings are related to the inherent limitations of hemodynamic data in the SRTR data set. Exact hemodynamics timing and blood pressure measurements are unavailable. We did not have enough pre–circulatory support hemodynamic data to reliably estimate the shock portion of the VA-ECMO and percutaneous support device candidates. Finally, we did not have inotrope dosage data for low-dose inotropes candidates. Overall, we believe these limitations led to an underestimation in our calculated disqualification portions, and the magnitude of the shock requirement impact will likely be greater than reported here.
In conclusion, the cardiogenic shock criteria added to the proposal to modify adult heart allocation would reduce the priority for transplantation for >600 adult heart transplant candidates a year nationally. These disqualifications may lead to increased use of surgically placed devices to circumvent the shock requirement, which would place candidates at increased and unnecessary risks while awaiting organ transplantation. Furthermore, the cardiogenic shock criteria, as currently defined, do not predict transplant-free waitlist survival and therefore are unlikely to lead to increased transplantation of more medically urgent candidates. Although well intentioned, the cardio-genic shock requirement is likely to have far-reaching unintended consequences and should be simulated formally with the Thoracic Simulation Allocation Model before implementation.
Supplementary Material
Acknowledgments
This study was supported in part by institutional Clinical and Translational Science Award Grant No. UL1 RR024999 (Principal Investigator Dr. Julian Solway). W.F.P. is supported by National Institutes of Health T32 Training Grant No. 5T32HL007605-32. M.M.C. is supported by National Institutes of Health K08 Grant No. HL121080-03.
The data reported here have been supplied by the Minneapolis Medical Research Foundation as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the SRTR or the US Government.
Footnotes
Disclosure statement
None of the authors has a financial relationship with a commercial entity that has an interest in the subject of the presented manuscript or other conflicts of interest to disclose.
Supporting information
Supplementary data associated with this article can be found in the online version at jhltonline.org.
References
- 1.Organ Procurement and Transplantation Network. Modify adult heart allocation 2016 2nd round. Available at: https://optn.transplant.hrsa.gov/governance/public-comment/modify-adult-heart-allocation-2016-2nd-round/. Accessed December 15, 2016.
- 2.Colvin M, Bolch C, Pyke J, Skeans M, Wang X, Zeglin J. Analysis report: data request from the Heart Subcommittee of the OPTN Thoracic Organ Transplantation Committee. 2015 Oct; Data Request ID: HR2015_01.\. [Google Scholar]
- 3.Singh TP, Almond CS, Taylor DO, Graham DA. Decline in heart transplant wait list mortality in the United States following broader regional sharing of donor hearts. Circ Heart Fail. 2012;5:249–58. doi: 10.1161/CIRCHEARTFAILURE.111.964247. [DOI] [PubMed] [Google Scholar]
- 4.Narang N, Thibodeau JT, Levine BD, et al. Inaccuracy of estimated resting oxygen uptake in the clinical setting. Circulation. 2014;129:203–10. doi: 10.1161/CIRCULATIONAHA.113.003334. [DOI] [PubMed] [Google Scholar]
- 5.Balik M, Pachl J, Hendl J, Martin B, Jan P, Jan H. Effect of the degree of tricuspid regurgitation on cardiac output measurements by thermodilution. Intensive Care Med. 2002;28:1117–21. doi: 10.1007/s00134-002-1352-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


