Abstract
Inflammatory pseudotumor is a rare benign condition of unknown cause. A 19-year-old female presented with gross hematuria and storage symptoms to the emergency. Contrast-enhanced computed tomography scan revealed a mass of 5 cm × 6 cm involving the dome and anterior wall of the bladder. Urachal carcinoma was kept as a possibility and transurethral biopsy of the aforementioned lesion was performed. The histopathology revealed inflammatory myofibroblastic tumor (IMT) of the bladder. A laparoscopic partial cystectomy was undertaken with adequate resection margins and the histopathology of the lesion confirmed IMT. When evaluating a mass in the genitourinary tract in a young individual, IMT should be considered as a possibility.
Keywords: Inflammatory pseudotumor, laparoscopic partial cystectomy, urachal carcinoma, urinary bladder
INTRODUCTION
Nonepithelial tumors account for 2%–5% of all primary urinary bladder neoplasms, with the most common types being rhabdomyosarcoma. Inflammatory myofibroblastic tumor (IMT) is a rare spindle tumor often mistaken for a sarcoma, particularly when it occurs in the bladder. Since a bladder sarcoma warrants radical cystectomy and IMT of the bladder is generally managed more conservatively, distinguishing between the two tumors is of critical importance. IMT has been described in numerous body sites and tumors with similar morphology have been assigned many names, including plasma cell pseudotumor, inflammatory pseudotumor, xanthomatous pseudotumor, pseudosarcomatous myofibroblastic proliferation, inflammatory myofibrohistiocytic proliferation, atypical fibromyxoid tumor, and atypical myofibroblastic tumor.[1,2,3] Herein, we report on an extremely rare case of an IMT of the urinary bladder.
CASE REPORT
A 19-year-old female presented with gross hematuria and storage symptoms to the emergency department. She denied night sweats or fever and any history of urinary tract infection, trauma, instrumentation, or other urological problems. An ultrasound study of the kidneys and urinary bladder revealed a broad-based mass located in the dome and anterior wall of the bladder. As a part of staging workup patient underwent a contrast-enhanced computed tomography (CECT) scan study of the abdomen. CT scan revealed a mass of 5 cm × 6 cm involving the dome and anterior wall of the bladder [Figure 1]. Clinically, a diagnosis of urachal carcinoma was thought of due to the location of the mass. The patient underwent cystoscopy which confirmed the lesion arising from the dome and anterior wall [Figure 2]. Transurethral biopsy of the aforementioned lesion was performed with hemostatic measures. Hematuria subsided after this exercise. To our surprise, the histopathology of the biopsy revealed it to be IMT of the bladder. With this, the patient was taken up for laparoscopic partial cystectomy. Cystoscopy was done initially and the resection margins were marked with Collins knife. Subsequently, three 12-mm ports were placed at and either side of the umbilicus. Tumor was involving full thickness of urinary bladder and tumor nodule was seen on serosal surface suggesting transmural nature of lesion. A partial cystectomy was performed with adequate resection margins [Figure 2]. The bladder was subsequently closed using absorbable sutures. The patient did well in the postoperative period and was discharged on the 3rd postoperative day. Histopathology confirmed IMT and the patient is doing well in the follow-up [Figures 3 and 4].
Figure 1.
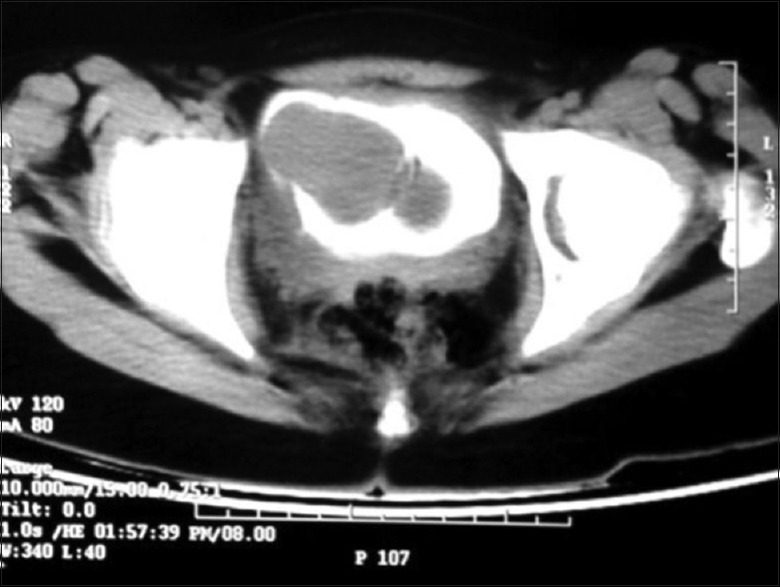
Contrast-enhanced computed tomography demonstrating a heterogeneously enhancing mass in the anterior wall of the urinary bladder
Figure 2.
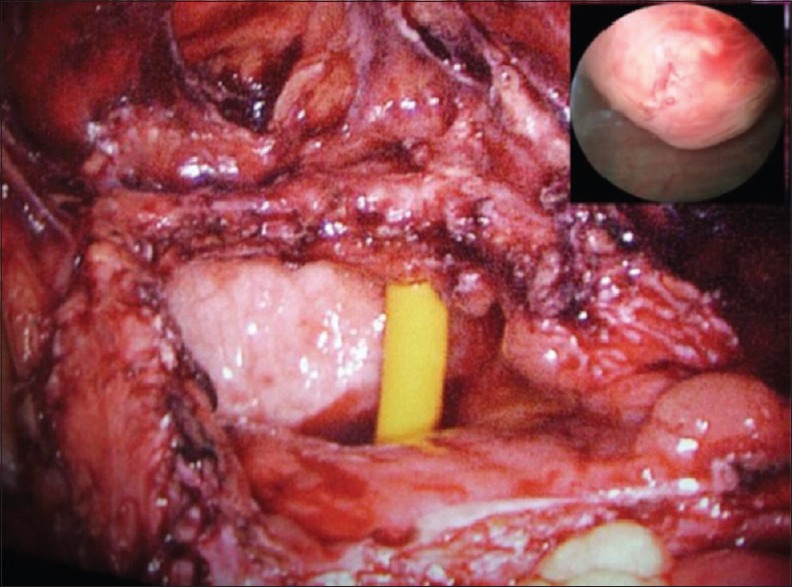
Urinary bladder after resecting the specimen. Inset figure: Cystoscopy finding showing solid-looking mass arising from anterior wall of the urinary bladder
Figure 3.
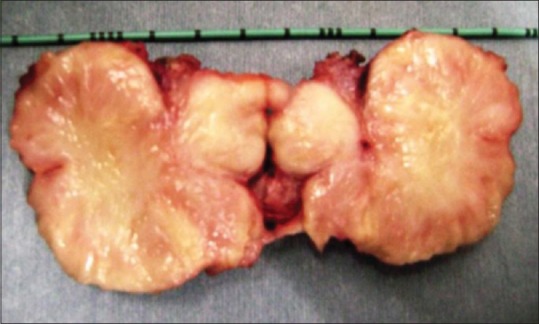
Cut section of the specimen
Figure 4.
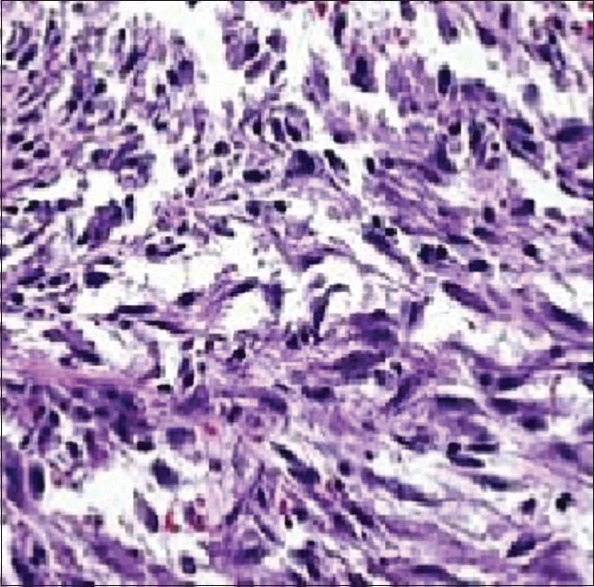
Histopathology slide showing inflammatory cells in between the myofibroblasts
DISCUSSION
Nonepithelial tumors account for 2%–5% of all primary urinary bladder neoplasms, with the most common types being rhabdomyosarcoma. IMT is a rare spindle tumor often mistaken for a sarcoma, particularly when it occurs in the bladder. Since a bladder sarcoma warrants radical cystectomy and IMT of the bladder is generally managed more conservatively, distinguishing between the two tumors at this and other sites is of critical importance.
IMT of the bladder is an uncommon benign tumor of bladder of unknown neoplastic potential characterized by spindle cell proliferation with characteristic fibroinflammatory and pseudosarcomatous appearance. It is also known as plasma cell pseudotumor, inflammatory pseudotumor, xanthomatous pseudotumor, pseudosarcomatous myofibroblastic proliferation, inflammatory myofibrohistiocytic proliferation, atypical fibromyxoid tumor, and atypical myofibroblastic tumor.[1,2,3] No predisposing factors are known and mostly IMT is idiopathic.[4] Since the initial description of its occurrence in the lung, IMT has been reported in a wide variety of anatomical sites, including the abdomen, retroperitoneum, head and neck, brain, and extremities.[5] In the genitourinary tract, IMT has been reported in the kidney, urethra, prostate, ureter, and rete testis but is most frequently observed in the bladder.[3,6] Although the majority of patients with IMT of the bladder are teens or young adults, IMT has also been reported in children and the elderly.[7] IMT is much more likely to occur in males, with a 2:1–3:1 male predominance.[2] The most common symptom is painless hematuria. Less often patients present with dysuria, pelvic pain, or symptoms of urinary tract obstruction or infection, or the lesion may be discernible as a mass lesion during the physical or radiological examination. Other associations with genitourinary IMT include cigarette smoking, bladder instrumentation, and gynecological surgery.[8] Although the radiologic findings of inflammatory pseudotumor are nonspecific, particular findings are observed. Sonographic findings show a variable pattern of echogenicity, and the lesion has been described as hypo- or hyperechogenic with ill-defined or well-defined margins. CECT may show homo- or heterogeneity and hypo-, iso-, or hyperdensity. MRI shows a hypointense lesion on T1- and T2-weighted images (possibly reflecting the fibrotic change) and shows marked gadolinium enhancement. In these gadolinium-enhanced images of inflammatory pseudotumor, delayed enhancement has frequently been observed, probably because of the accumulation Of extravascular contrast media in the fibrotic component within the mass.[9]
The pathogenesis of IMT is enigmatic and numerous theories have been proposed. IMT is slow-growing and rarely exhibits clinically aggressive behavior.[7] High-grade invasive urothelial carcinoma in patients with a history of IMT sometimes exhibits unusually aggressive behavior resulting in death.[2] IMT of the genitourinary tract should be considered a neoplasm of uncertain malignant potential, and routine surveillance and close clinical follow-up are recommended. Aggressive therapy (radical cystectomy, radiation, or chemotherapy) is unwarranted given the indolent and often benign clinical course in the majority of cases.[10] IMT of the genitourinary tract is a rare occurrence and is a neoplasm of uncertain malignant potential. When evaluating a mass in the genitourinary tract in a young individual, IMT should be considered as a possibility. Surgical resection is the treatment of choice and aggressive therapy is unwarranted and close follow-up is recommended.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Jones EC, Clement PB, Young RH. Inflammatory pseudotumor of the urinary bladder. A clinicopathological, immunohistochemical, ultrastructural, and flow cytometric study of 13 cases. Am J Surg Pathol. 1993;17:264–74. doi: 10.1097/00000478-199303000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Montgomery EA, Shuster DD, Burkart AL, Esteban JM, Sgrignoli A, Elwood L, et al. Inflammatory myofibroblastic tumors of the urinary tract: A clinicopathologic study of 46 cases, including a malignant example inflammatory fibrosarcoma and a subset associated with high-grade urothelial carcinoma. Am J Surg Pathol. 2006;30:1502–12. doi: 10.1097/01.pas.0000213280.35413.1b. [DOI] [PubMed] [Google Scholar]
- 3.Lott S, Lopez-Beltran A, Montironi R, MacLennan GT, Cheng L. Soft tissue tumors of the urinary bladder part II: Malignant neoplasms. Hum Pathol. 2007;38:963–77. doi: 10.1016/j.humpath.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 4.Pettinato G, Manivel JC, De Rosa N, Dehner LP. Inflammatory myofibroblastic tumor (plasma cell granuloma).Clinicopathologic study of 20 cases with immunohistochemical and ultrastructural observations. Am J Clin Pathol. 1990;94:538–46. doi: 10.1093/ajcp/94.5.538. [DOI] [PubMed] [Google Scholar]
- 5.Coffin CM, Watterson J, Priest JR, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor).A clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol. 1995;19:859–72. doi: 10.1097/00000478-199508000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Coffin CM, Dehner LP, Meis-Kindblom JM. Inflammatory myofibroblastic tumor, inflammatory fibrosarcoma, and related lesions: An historical review with differential diagnostic considerations. Semin Diagn Pathol. 1998;15:102–10. [PubMed] [Google Scholar]
- 7.Sirvent N, Hawkins AL, Moeglin D, Coindre JM, Kurzenne JY, Michiels JF, et al. ALK probe rearrangement in a t(2;11;2)(p23;p15;q31) translocation found in a prenatal myofibroblastic fibrous lesion: Toward a molecular definition of an inflammatory myofibroblastic tumor family? Genes Chromosomes Cancer. 2001;31:85–90. doi: 10.1002/gcc.1121. [DOI] [PubMed] [Google Scholar]
- 8.Harik LR, Merino C, Coindre JM, Amin MB, Pedeutour F, Weiss SW, et al. Pseudosarcomatous myofibroblastic proliferations of the bladder: A clinicopathologic study of 42 cases. Am J Surg Pathol. 2006;30:787–94. doi: 10.1097/01.pas.0000208903.46354.6f. [DOI] [PubMed] [Google Scholar]
- 9.Nam KJ, Kang HK, Lim JH. Inflammatory pseudotumor of the liver: CT and sonographic findings. AJR Am J Roentgenol. 1996;167:485–7. doi: 10.2214/ajr.167.2.8686633. [DOI] [PubMed] [Google Scholar]
- 10.Cheng L, Foster SR, MacLennan GT, Lopez-Beltran A, Zhang S, Montironi R, et al. Inflammatory myofibroblastic tumors of the genitourinary tract – Single entity or continuum? J Urol. 2008;180:1235–40. doi: 10.1016/j.juro.2008.06.049. [DOI] [PubMed] [Google Scholar]


