 Sensitisation of the respiratory tract to chemicals resulting in respiratory allergy and allergic asthma is an important occupational health problem, and presents toxicologists with no shortage of challenges.
Sensitisation of the respiratory tract to chemicals resulting in respiratory allergy and allergic asthma is an important occupational health problem, and presents toxicologists with no shortage of challenges.
Abstract
Sensitisation of the respiratory tract to chemicals resulting in respiratory allergy and allergic asthma is an important occupational health problem, and presents toxicologists with no shortage of challenges. A major issue is that there are no validated or, even widely recognised, methods available for the identification and characterisation of chemical respiratory allergens, or for distinguishing respiratory allergens from contact allergens. The first objective here has been review what is known (and what is not known) of the mechanisms through which chemicals induce sensitisation of the respiratory tract, and to use this information to construct a hybrid Adverse Outcome Pathway (AOP) that combines consideration of both skin and respiratory sensitisation. The intention then has been to use the construction of this hybrid AOP to identify areas of commonality/confluence, and areas of departure/divergence, between skin sensitisation and sensitisation of the respiratory tract. The hybrid AOP not only provides a mechanistic understanding of how the processes of skin and respiratory sensitisation differ, buy also a means of identifying areas of uncertainty about chemical respiratory allergy that benefit from a further investment in research.
General introduction
There are at the disposal of toxicologists a variety of tools for the identification and characterisation of skin sensitising chemicals, and for the conduct of effective risk assessments. The situation that pertains with allergic sensitisation of the respiratory tract and occupational asthma is very different, and here there are available no validated methods for hazard assessment. This lack of suitable methods creates challenges for the toxicologist charged with identifying health risks associated with respiratory allergy.
In this article we will explore areas of commonality and areas of divergence between the responses induced by contact allergens and respiratory allergens, using for this purpose the vehicle of a hybrid Adverse Outcome Pathway (AOP). The construct of this AOP is intended to address three main objectives:
(a) to identify areas of commonality/confluence between skin sensitisation and sensitisation of the respiratory tract that will aid in the development of novel methods for the identification and characterisation of chemical respiratory allergens,
(b) to identify points of departure/divergence between the processes involved in skin sensitisation and sensitisation of the respiratory tract to enhance further understanding of such differences, and to create new opportunities for distinguishing between contact allergens and chemical respiratory allergens,
(c) to identify areas of uncertainty that would benefit from an investment in further research.
In developing and exploiting such an AOP it is necessary first to review briefly the differences between skin sensitisation and sensitisation of the respiratory tract, and to identify some of the toxicological challenges.
Introduction to chemical allergy
Allergy resulting from exposure to chemicals is an important occupational health issue, and can take a variety of forms. Those of greatest relevance are allergic contact dermatitis (ACD) resulting from skin sensitisation, and occupational rhinitis and asthma caused by sensitisation of the respiratory tract.1
It is important to appreciate that chemical allergens display selectivity with respect to the type of sensitisation that they will induce, and the form that subsequent allergic reactions will take. Thus, many chemical allergens, either preferentially, or often exclusively, induce skin sensitisation and ACD. In contrast, other chemicals (substantially fewer in number) selectively induce allergic sensitisation of the respiratory tract and occupational asthma. Some chemicals have the potential, in different subjects, to induce different forms of allergy, but most chemical allergens display some degree of selectivity.1
Skin sensitisation
Many thousands of chemicals have been implicated as contact allergens. In common with all forms of allergic disease, ACD develops in 2 phases. In the first phase skin exposure of an inherently susceptible subject to a threshold or greater concentration of a contact allergen causes priming of the immune system. If the induced immune response is sufficiently vigorous then systemic sensitisation is acquired such that future skin contact with the same chemical allergen will provoke an accelerated and more aggressive secondary immune response resulting in a cutaneous inflammatory reaction that is recognised clinically as ACD.1
Although our understanding is incomplete, much is known of the mechanisms through which skin sensitisation is acquired. The processes involved can be summarised briefly as follows. For an immune response to be induced, and for skin sensitisation to be acquired, the contact allergen must gain access across the stratum corneum and reach the viable epidermis and beyond. Here a variety of important events take place, including the formation of immunogenic complexes following the stable association of the chemical with proteins. These hapten-protein complexes are recognised, internalised and processed by populations of cutaneous dendritic cells (DC), including epidermal Langerhans cells (LC), that play pivotal roles in the initiation and regulation of skin sensitisation. Migratory DC (some at least of which carry antigen), travel from the skin, via afferent lymphatics, to regional lymph nodes draining the site of exposure. Here the hapten complex is presented to responsive T lymphocytes. The central event in the development of skin sensitisation is the antigen-induced activation of responsive T lymphocytes and their division and differentiation resulting in immunological primining.1–4
It is essential to appreciate that skin sensitisation is a complex biological process that requires directed interactions between a variety of cells and molecules to elicit responses that are tightly regulated in time and space.1 Some of these complexities will been considered when the AOP for skin sensitisation is addressed later in this article.
A general appreciation of the mechanisms involved has facilitated the development and application of methods for the identification of skin sensitisation hazards. In the first instance such methods were developed in Guinea pigs, the most widely used being the Guinea Pig Maximisation Test (GPMT)5 and the occluded patch test of Buehler.6 These test methods were later largely superseded by the mouse Local Lymph Node Assay (LLNA).7–9 More recently still attention has turned to the development of alternative approaches that obviate the use of experimental animals, and some in vitro methods have now been validated and have been translated into OECD (Organisation for Economic Cooperation and Development) guidelines.10–13
In addition to the alternative tests that have now been validated, there are other approaches (both in vitro methods and those based on structure–activity relationships) that are at various stages of development and evaluation, and some of these will no doubt be strong candidates for validation in the future.14–17 Moreover, consideration has been given to whether integrated testing strategies, that combine data from two or more individual alternative test methods, can be used to improve the accuracy of hazard identification.18–26
Finally, it is clear that, in addition to effective hazard identification, there is a need – for the purposes of accurate risk assessment and human health protection – to have information about potency. This is particularly important for skin sensitisation because it is known that contact allergens vary by up to 5 orders of magnitude with respect to their relative sensitising potency.1 For this purpose the LLNA has proven to be of considerable value. The end point used for hazard identification in the LLNA is the proliferation of draining lymph node cells (LNC) induced by topical exposure of mice to the test chemical. Importantly, it is known that LNC proliferation is not only causally linked with the development of skin sensitisation, but also correlates quantitatively with the extent to which sensitisation is acquired. It has therefore been possible to use the LLNA for the purpose of measuring the relative sensitising potency of contact allergens.27–30 The authors are aware that currently work is being undertaken to determine whether some of the non-animal approaches that have recently been developed for hazard identification might also provide some information about relative potency.
Thus, the position with skin sensitisation can be summarised as follows. An increasingly sophisticated understanding of the cellular and molecular events and processes that are required for the acquisition of skin sensitisation has provided a sound scientific basis for the development of effective methods for hazard identification, hazard characterisation and risk assessment.
This situation differs significantly from sensitisation of the respiratory tract and chemical respiratory allergy.
Sensitisation of the respiratory tract
Occupational asthma resulting from allergic sensitisation of the respiratory tract to chemicals is associated with significant morbidity, and is clearly an important health concern.31–35 Compared with contact allergens, far fewer chemicals have been confirmed as respiratory allergens; the total number being somewhat less than 80. Among those commonly implicated are diisocyanates, acid anhydrides, chloroplatinate salts and some reactive dyes.36–38
Our understanding of the mechanistic bases for the development of sensitisation of the respiratory tract to chemicals is much less complete than it is for skin sensitisation, and as a consequence there remain a variety of significant toxicological challenges. Many of the problems have been the subject of recent review articles.13,39–47 Although inevitably there are debates about the fine detail of biologic processes, the main areas of uncertainty and controversy with respect to chemical respiratory allergy are: (a) the route(s) of exposure though which sensitisation of the respiratory tract can be achieved, and (b) the relevance of, and requirement for, IgE antibody. The continuing lack of a consensus about these issues, and in particular uncertainty about the need for IgE antibody for the acquisition of sensitisation, has been a major factor in preventing the development of approaches for the identification and characterisation of chemical respiratory allergens. Thus, there are currently no validated, or even widely used or accepted, methods for assessment of the respiratory sensitising potential of chemicals. This does not reflect a lack of endeavour. In fact over several decades there have been a variety of approaches proposed,48–58 but it remains the case that there is no accepted test available for predictive testing or hazard characterisation.
The major uncertainties associated with chemical respiratory allergy
Routes of exposure
The phrase ‘respiratory sensitisation’ has commonly led to the assumption that inhalation exposure is a mandatory requirement for the development of sensitisation of the respiratory tract to chemical allergens. (In fact, a better descriptor is ‘sensitisation of the respiratory tract’, and this is the notation that the authors have favoured in this article.) This has resulted in a view that only methods incorporating exposure via the respiratory tract (or in vitro tests incorporating appropriate tissue or cells derived from the respiratory tract) will be suitable for the identification of chemical respiratory allergens.
However, there is good reason to believe that, based on both studies in experimental animals, and clinical experience, effective sensitisation of the respiratory tract can be acquired by skin exposure to the relevant chemical allergen.59–66 This should come as no real surprise. It is clear that many chemicals are able to again access across the skin and trigger an immune response, and indeed even high molecular weight proteins can induce adaptive immune responses following skin exposure.67 There is, moreover, some direct evidence from studies conducted in Guinea pigs that skin exposure is considerably more effective than inhalation exposure at inducing sensitisation of the respiratory tract to a known chemical respiratory allergen, diphenylmethane diisocyanate (MDI).61 Given that background, there is no reason why, following skin exposure, chemical respiratory allergens should not provoke the quality and vigour of immune response required for effective sensitisation of the respiratory tract. The nature of such responses is addressed below. The effectiveness of skin exposure for the acquisition of sensitisation of the respiratory tract to chemicals has two important implications.
The first of these is that the differences between chemical allergens with respect to the type of sensitisation they will preferentially induce, and the form that allergic reactions will take, cannot be a function solely of the route through which exposure occurred.
The second is that there is no reason why there should be a need to necessarily base proposed predictive test methods on inhalation exposure, or on the interaction of chemicals with respiratory tract cells or tissue. That is, a suitable method for identification of chemical respiratory allergens could just as legitimately be based on skin exposure, or events and responses induced following interaction of test chemicals with skin or skin cells.
The relevance of, and requirements for, IgE antibody
The main area of continuing controversy with respect to chemical respiratory allergy is the mechanism(s) through which allergic sensitisation of the respiratory tract is acquired. By definition, the development of allergy is dependent upon the elicitation of an adaptive immune response. The question here is what is the nature of that response?
In sensitisation of the respiratory tract to proteins, IgE antibodies play a central, and mandatory, role. In protein respiratory allergy, and other forms of allergy to proteins (such as for instance in most types of food allergy), it is IgE antibody that induces sensitisation. Allergen-specific IgE antibody associates with local tissue mast cells and basophils. Following subsequent exposure, the inducing allergenic protein binds to, and cross-links, IgE antibody displayed at the mast cell surface. This cross-linking in turn causes mast cell activation and degranulation, and the release of both pre-formed and newly synthesised mediators (such as histamine) that together induce a local inflammatory reaction associated with vasodilation, induration and oedema.
It appears reasonable, therefore, to speculate that IgE performs a similarly pivotal role in the development of sensitisation of the respiratory tract to chemicals. Indeed, it is the case that for all chemical respiratory allergens that have been investigated in any detail there is evidence in each instance for at least some cases of confirmed occupational asthma being associated with IgE antibody specific for the inducing chemical allergen.1 Although this confirms that chemical respiratory allergens are able to elicit IgE responses, the difficulty is that it is not always possible to demonstrate an association with detectable IgE antibody. That is, cases of chemical allergen-induced occupational asthma in which there is no detectable serum IgE antibody of the appropriate specificity. In some instances, with for example the acid anhydrides and chloroplatinate salts, there can be a fairly close association between clinical symptoms and IgE antibody.68–70 With other chemical respiratory allergens, and in particular with the diisocyanates, the picture is rather different. In the case of diisocyanates only a fraction, and sometimes only a small fraction, of symptomatic subjects have been shown to have discernible levels of serum IgE antibody.71–79
In the case of diisocyanates, therefore, the available data suggest that IgE antibody has a low predictive sensitivity for occupational asthma. However, it is important to note that even in the case of diisocyanates, where detection of IgE has proven the most problematic, there is a general view that the presence of serum IgE antibody is highly predictive of occupational asthma.71,74,76,80–82
Nevertheless, the absence of a demonstrably close association between IgE antibody and clinical symptoms has led to the suggestion that diisocyanate-induced occupational asthma (in particular) is an IgE-independent disease;83,84 the implication being that allergic sensitisation of the respiratory tract to chemical allergens can be achieved via immunological mechanisms other than IgE antibody.
Before exploring what those other mechanisms might be, it is appropriate to consider critically the view that IgE antibody is not an important – or indeed mandatory – requirement for chemical respiratory allergy. It is the view of the present authors that in fact IgE is more closely associated with occupational asthma to chemical allergens than has been recognised previously. That view is based upon two main considerations.
The first of these, and arguably the most important, is the fact that it is notoriously difficult to prepare appropriate hapten-protein conjugates that can be used as substrates in detection systems for the measurement of anti-hapten antibodies.62,76,81,82,85–87 Of particular importance in creating an appropriate conjugate are the molar ratio of the hapten : protein reaction mixture, and the nature of the carrier protein.76 It is possible, and even probable, therefore, that in many instances hapten-specific IgE antibody (which in any event will be present at only low levels in plasma) will be missed with a false negative result.
The second consideration is the persistence of detectable IgE antibody. The half-life of unbound plasma IgE is low at approximately 2 days,88 and for this reason the detection of hapten-specific IgE antibody becomes less successful as the period from previous exposure increases.74
To summarise, it is clear that chemical respiratory allergens do have an inherent potential to induce IgE antibody, and that with some classes of allergen that potential is frequently realised, such that there is a relatively close association between IgE and symptoms of occupational asthma. Although in the case of diisocyanates (in particular) it has frequently been difficult to show such an association, there are good reasons (described above) to suppose that the technical difficulties in detecting hapten-specific IgE antibody results in a significant underestimation of the correlation between serum IgE and occupational asthma to chemical allergens. (In this context it is possible that the development of effective diisocyanate hapten-protein conjugates is particularly problematic.76)
However, even if there is a serious underestimation of the correlation between hapten-specific IgE antibody and chemical respiratory allergy, the uncertainties described above have resulted in the scientific community having little confidence in, or enthusiasm for, the use of methods in which identification of respiratory sensitising potential is predicated on the elicitation of IgE antibody responses. It is this uncertainty about the pivotal events in the development of sensitisation of the respiratory tract to chemical allergens that has proven the major hurdle in designing suitable (and widely acceptable) predictive test methods.
Of course, this same uncertainty also creates difficulties in fashioning an AOP for chemical respiratory allergy. Attempts have been made to develop such AOPs,89–91 but there remains a need to find a solution to the conundrum of the relevant immunological effector mechanism(s).
The mechanistic basis for chemical respiratory allergy: a proposed way forward
In an AOP described relatively recently,89 an attempt was made to solve this issue by consideration of the relevant cellular effector mechanisms, which in turn is based on an appreciation of the essential differences between skin sensitisation and sensitisation of the respiratory tract (see next section).
A focus on the role played by T lymphocytes is an attractive option for two main reasons. The first is that, by definition, allergy – including allergy to chemicals – is dependent upon the elicitation of an adaptive immune response, and almost all adaptive immune responses are associated with T lymphocyte responses. The second is that the induction of IgE antibody production is highly dependent on an appropriate T lymphocyte response.
The proposal is that the development of sensitisation of the respiratory tract to chemicals, and the elicitation of respiratory allergy and occupational asthma, is associated with, and dependent upon, the initiation of selective T helper 2 (Th2)-type immune responses. Th2 cells are the source of cytokines (interleukins 4, 5 and 13) that promote the development of allergic responses and the production of IgE antibody.1 The argument is that a selective Th2 response will favour both the elaboration of IgE antibody and respiratory sensitisation, and that even if IgE antibody is (for whatever reason) not produced, then a preferential stimulation of a Th2 response will be sufficient for sensitisation of the respiratory tract to be acquired.89
The attractiveness of this proposal is that a common mechanism for chemical respiratory allergy is identified based upon the preferential elicitation of selective Th2 cell responses, and that this is consistent with both IgE-dependent and IgE-independent sensitisation of the respiratory tract.
The evidence for chemical respiratory allergens inducing selective Th2-type immune responses is reviewed briefly below.
The key difference between skin sensitisation and sensitisation of the respiratory tract
The induction by chemical allergens of preferential forms of sensitisation poses an intriguing question. Why is it that some contact allergens are never associated with sensitisation of the respiratory tract, while some chemical respiratory allergens are never, or only rarely, associated with skin sensitisation? Phthalic anhydride, a chemical known to cause occupational asthma, provides an illustrative example of the latter; a chemical respiratory allergen that only very rarely has been reported to cause skin sensitisation and ACD.92
The key difference between skin sensitisation and respiratory sensitisation is the nature and quality of the adaptive immune responses required for their acquisition. That is, contact allergens and chemical respiratory allergens elicit different qualities of immune response that result in different forms of sensitisation. The quality of an adaptive immune response is governed largely by the balance between discrete functional subpopulations of T lymphocytes.1
A detailed survey of cellular immunology is not required here, and for the purposes of this article it is necessary only to identify the main subpopulations of T lymphocytes that are relevant for the initiation and regulation of allergic sensitisation to chemicals. It is now recognised that there exists a variety of important functional subsets of CD4+ T lymphocytes, including Th1, Th2 and Th17 cells, and regulatory T cells (Treg).93–95 These cells, together with CD8+ T lymphocytes, have responsibility for orchestrating differentiated adaptive immune responses that are tailored to meet the challenges posed to host defence by specific antigenic threats. These same cell types are involved in the initiation and regulation of immune responses to chemical allergens.2,3,96,97
It has become clear from experimental studies in rodents that T lymphocyte subsets, and their cytokine products, govern the form that allergic reactions will take. In most experimental systems, and based primarily on cytokine secretion patterns, it has been reported that contact allergens induce selective Th1-type immune responses, whereas chemical respiratory allergens elicit Th2-type responses.40,41,53,98–105 Thus, these animal data are consistent with the proposal described above that a common feature of immune responses to chemical respiratory allergens, and the acquisition of sensitisation of the respiratory tract, is the elicitation of a selective Th2 response and the production of Th2 cytokines such as IL-4, IL-5 and IL-13.
Evidence from human studies also points to an important role for Th2-type responses in the development of respiratory sensitisation to chemical allergens and occupational asthma.106 It is known that, irrespective of a central role for IgE antibody, occupational asthma is associated with T lymphocyte responses.107–109 In addition, it has been shown that in subjects with occupational asthma to diisocyanates, the interferon γ (IFN-γ) gene promoter is hypermethylated resulting in down-regulated IFN-γ gene expression.110 It is known that a reduced potential to produce IFN-γ favours the development of selective Th2 responses. Another intriguing observation was reported by Newell et al. (2013).111 Volunteers with or without atopic dermatitis (a Th2-biased disease) were sensitised with 2,4-dinitrochlorobenzene (DNCB), a contact allergen that fails to induce sensitisation of the respiratory tract or respiratory allergy. In normal subjects, as expected, sensitisation to DNCB was associated with a systemic Th1 response. In patients with atopic dermatitis the Th1 response was reduced with a significant skewing of immune responses to DNCB towards a Th2 phenotype.111 These data provide an elegant demonstration of the influence of the immune phenotype of the subject, and the balance between Th1- and Th2-type responses, on the form immune responses to chemical allergens will take in humans. Under normal circumstances contact allergens will preferentially elicit selective Th1 responses, whereas chemical respiratory allergens will favour the development of Th2 responses.106
Therefore, based upon both experimental data and observations in humans, it is proposed that, for the purposes of this article at least, and for the development of a hybrid AOP, the induction of preferential Th2 responses is acknowledged as being an essential requirement for effective sensitisation of the respiratory tract, and as a distinguishing characteristic of chemical respiratory allergens.
Having established this unifying characteristic of chemical respiratory allergens (and the basis for the elicitation by contact and respiratory allergens of divergent forms of sensitisation), the next step is to examine the pathways to the dual adverse outcomes of ACD and chemical respiratory allergy, and to identify common events, and events and processes that differ between skin sensitisation and sensitisation of the respiratory tract. To this end it is appropriate to use as a template the AOP that has been developed for skin sensitisation.
Responses to chemical allergens in the context of the AOP for skin sensitisation
Adverse outcome pathways are analytical constructs that describe series of linked events (key events) that culminate in an adverse health or environmental effect (outcome).112–114 Since the concept was introduced, possibly the most fully developed AOP is that describing skin sensitisation,115–117 and as indicated above this will be used as a basis for considering areas of commonality and areas of divergence between skin sensitisation and sensitisation of the respiratory tract. There is no requirement here to rehearse in full detail aspects of the skin sensitisation AOP; for that purpose readers are referred to the relevant OECD (Organisation for Economic Cooperation and Development) monograph,115 and to detailed reviews of the cellular and molecular processes through which skin sensitisation is acquired that are available elsewhere.1,3,4
The AOP for skin sensitisation recognises a number of steps along the pathway from: chemical exposure, creation of an immunogenic complex, internalisation, processing, transport (to regional lymph nodes) and presentation of that complex, the elicitation of danger signals that act as important cofactors for the initiation of adaptive immune responses, and the activation, division and differentiation of T lymphocytes. This results in the acquisition of sensitisation. Following that, the steps to the adverse outcome are: subsequent exposure of the sensitised subject to the inducing chemical allergen, the elicitation of a secondary T lymphocyte response, and the induction of a cutaneous inflammatory reaction that is recognised clinically as ACD.115
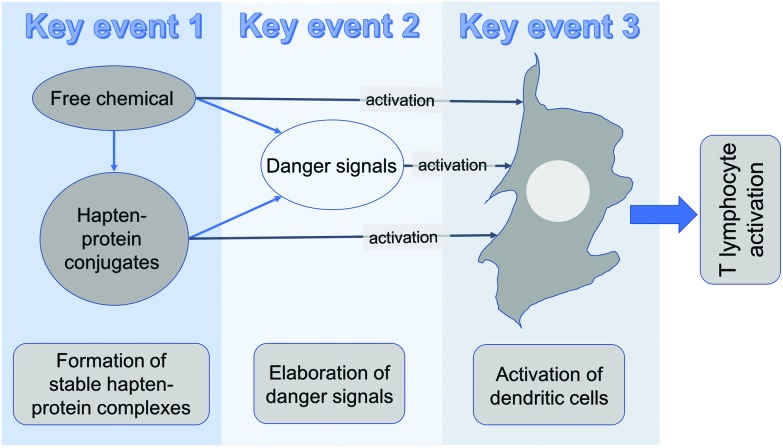
From these steps the AOP identifies 4 key events in the development of skin sensitisation. These key events are those considered to be essential for the effective induction of sensitisation and are as follows: Key event 1 (Molecular Initiating Event): The formation of stable (covalent) hapten-protein complexes (sensitising chemicals must be naturally electrophilic, or converted to an electrophilic species). Key event 2: The stimulation of a local inflammatory response, involving keratinocytes, that results in the elicitation of danger signals and cofactors that promote and support adaptive immune responses. Key event 3: The activation of DC. Key event 4: The activation, division and differentiation of T lymphocytes that results in skin sensitisation.115
For both skin sensitisation and sensitisation of the respiratory tract the stimulation of a T lymphocyte response is a mandatory step. However, as reviewed above, the important observation is that these different forms of sensitisation are driven by discrete immune responses. The question is, therefore, why is it that these two classes of allergen, that are both of low molecular weight, both naturally (or potentially) protein reactive, and both inherently immunogenic, stimulate qualitatively divergent T lymphocyte responses. In this article we examine each of these 4 Key Events from the perspective of possible similarities and differences between contact allergens and chemical respiratory allergens that might account for their ultimate stimulation of divergent immune responses resulting in different forms of sensitisation. In addition, and in advance of considering in turn each Key Event, structural aspects of respiratory sensitisation are reviewed.
Structural features of contact and respiratory chemical allergens
One important consideration that needs to be addressed here is the potential impact of chemistry on exposure. It is appreciated that for a chemical to induce skin sensitisation is must transit effectively across the stratum corneum and gain access to the viable epidermis. This process is influenced, among other factors, by lipophilicity and molecular weight.118 However, as discussed previously, there is good reason to suppose that the development of respiratory sensitisation to a chemical allergen can be acquired via skin contact. Thus, in the context of this exercise, it appears unreasonable to suppose that the differences between contact allergens and chemical respiratory allergens are predicated on either differential access to the viable epidermis following skin exposure, or on a requirement for different routes of exposure to effect sensitisation. Therefore, for the purposes of this article the potential impact of different routes of exposure, or of differential toxicokinetics in the skin, will not be explored.
There have been many attempts to characterise structure–activity relationships for chemical respiratory allergens. The objectives being to identify structural alerts that might serve as a basis for hazard identification, and to determine whether there exists a ‘chemical’ basis for distinguishing between contact and respiratory allergens.40,57,90,119–123 These studies have met with various degrees of success. One problem has been the inclusion criteria for such analyses, with some investigators choosing to include both true chemical respiratory allergens (where the mode of action necessarily involves the elicitation of an adaptive immune response), together with chemical asthmagens where the adverse health effect is mediated by non-immunologic mechanisms, such as for instance irritation. (The inappropriate pooling of true respiratory allergens and non-immunologic asthmagens for the purposes of classification and labelling has been a long-standing subject of debate.124,125) Notwithstanding this, there are two features that are commonly identified as being associated with chemical respiratory allergens. The first is ‘hard’ electrophilic activity, and the second is an ability to cross-link proteins.126,127 These are factors that will influence the interaction of chemical allergens with host proteins, and could therefore potentially impact on the formation of hapten-protein complexes and the way that they are recognised and processed by elements of the immune system.
Defining the structural characteristics of chemical respiratory allergens is of considerable value, particularly if such understanding can be translated into methods that will support hazard identification and characterisation. However, in the context of this article the important consideration is the effect that structural properties might have on downstream biological and immunological events that drive sensitisation of the respiratory tract, and which distinguish chemical respiratory allergens from contact allergens. For this we turn to an exploration of the Key Events identified in the AOP for skin sensitisation.
Key event 1 (molecular initiating event). Formation of stable hapten-protein complexes
Low molecular weight chemicals are too small to engage effectively with the immune system and elicit an immune response. To acquire immunogenic (and allergenic) potential they must form stable associations with proteins and create complexes that can be processed and presented to responsive T lymphocytes. The first formal recognition of the need for an association of chemicals with proteins to induce sensitisation was reported over 80 years ago by Landsteiner and Jacobs in 1935.128 The formation of hapten-protein conjugates is recognised as being an essential requirement for the development of sensitisation, and therefore all contact allergens are naturally protein-reactive, or can be converted to electrophilic species either by air oxidation and/or within the skin. Naturally, the requirement for the formation of hapten-protein conjugates applies equally to contact allergens and chemical respiratory allergens.
The interesting question is whether the nature of the conjugates required for skin sensitisation and sensitisation of the respiratory tract differ. There is evidence that this might indeed be the case. For instance, it has been reported that the nature of antigen can influence the characteristics of induced T lymphocyte responses, and the balance between Th1 and Th2 cells.129 It seems self-evident that the characteristics of an antigen should have an important impact on the induced immune response; this would enable such responses to be tailored to meet effectively the nature of the threat. What is not clear, however, is how the formation of a hapten-protein conjugate might be fashioned in such a way as to influence the nature of an immune response. One possibility is that some forms of protein modification resulting from interaction with chemical allergens may mimic post-translational modifications, and as such have the potential to influence the localisation and processing of the haptenated protein that will in turn impact on the quality of downstream immune responses.
Arguably one of the most interesting developments regarding this question has been the observation that some chemicals, including chemical respiratory allergens, associate selectively with lysine residues130,131 (consistent with ‘hard’ electrophilic activity126,127). This raised the possibility that contact allergens and respiratory allergens might display differential amino acid selectivity during interaction with proteins.132 The ability of some protein-reactive chemicals to favour lysine (Lys) rather than cysteine (Cys) has been demonstrated using the Direct Peptide Reactivity Assay (DPRA), an alternative (non-animal) method for the identification of skin sensitising chemicals.10 It was found that chemical respiratory allergens showed a preference for association with Lys, whereas contact allergens in most cases favoured Cys.133
These data are intriguing, but care is needed in their interpretation. For instance, the amino acid selectivity reported using the DPRA133 measured peptide binding under highly defined experimental conditions, and it is uncertain whether in other situations, that perhaps more closely resemble biological reality, the Lys/Cys binding preferences would be as clear-cut.134 Moreover, it is possible that not all classes of chemical respiratory allergens show the same preference for Lys.135
Despite some uncertainties, it would be unwise currently to discount the possibility that amino acid selectivity plays a role in influencing the evolution of selective T lymphocyte responses, and this is certainly an area that would benefit from further research.
Another possibility is that preferential association of chemicals with proteins at the level of the whole molecule plays a role in directing the nature of immune responses. In a series of investigations conducted some years ago the selectivity of contact allergens and chemical respiratory allergens for cell-associated and soluble (serum) proteins was characterised. Although both types of chemical allergen displayed an inherent potential to bind to cell-associated or soluble proteins when mixed with cells or serum alone, a different picture emerged when the chemicals were incubated with cells and serum together. Under those conditions it was found that some contact allergens bound selectively to cellular proteins, whereas chemical respiratory allergens favoured association with serum proteins.136 One speculation prompted by these observations was that the apparent preference of respiratory allergens for serum proteins might, in part, arise simply as a result of albumin being an abundant serum protein that is comprised of a large number of lysine residues.136 Such an interpretation is consistent with the peptide selectivity data summarised above, and with the observation that albumin appears to be a major target protein for the respiratory allergen hexahydrophthalic anhydride (HHPA).137 However, other factors are also at play because it has been reported that although HHPA favours association with albumin, it forms adducts with only a relatively small fraction of lysine residues available on this protein.138
Working from first principles, it seems self-evident that the nature of hapten associations with protein should have an important impact on the form that induced immune responses to chemical allergens will take. Despite there being some intriguing indications that this might be the case, the picture remains unclear. A detailed characterisation of the hapten-protein adducts that drive the eventual emergence of preferential T lymphocyte responses would be great value and of considerable interest. However, such investigations will be extremely demanding technically and a resolution may be some way off, although it must be acknowledged that some progress has been made in the characterisation of skin sensitisation adducts. In the meantime there are other factors, including the elicitation of danger signals, that are known to influence the evolution of immune responses.
Key event 2. Danger signals and cofactors
In 1935 Landsteiner and Jacobs made the observation that a common feature of contact allergens is that they display some potential to induce irritation.128 More recently it has become accepted that skin sensitisation requires, or is at least most effective when there is, some local trauma.1 In recent years the nature of the signals that result from that trauma, and the ways in which they influence innate and adaptive immune function, has attracted considerable attention.
The term ‘danger signal’ was first introduced by Matzinger.139,140 The concept, which is now well-established, is that for the deployment of a full adaptive immune response molecular signals, in addition to antigen, have to be available (so called ‘danger signals’). That is, the launch of an adaptive immune response is dependent upon recognition of signals that indicate that there has been an incursion by a pathogenic microorganism, and/or tissue disruption/inflammation that might indicate a threat. In practice these signals represent the interface between the innate and adaptive immune systems, and provide – in principle – a mechanism through which elements of innate immunity can influence the trajectory of immune responses. Danger signals are known to play important roles in the acquisition of skin sensitisation.141–147
Danger signals fall into 2 main groups; pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs). As the term implies, DAMPs are endogenous non-microbial signals that are generated as the result of cell/tissue trauma. These signals can be of a variety of types and sources that include, among others, heat shock proteins, extracellular matrix proteins, uric acid crystals and hyaluronic acid.148–150 It is DAMPS that, in most circumstances, are of greatest relevance to the development of allergic sensitisation. The signals provided by PAMPs and DAMPs are delivered via pattern recognition receptors (PRR) that are expressed by a variety of cell types. Among the most important PRR are the Toll-like receptors (TLR) that are membrane-associated, and the nucleotide-binding oligomerisation domain-like (NOD-like) receptors (NLR) that are cytosolic.151,152
Collectively, ligand activation of these PRR initiates a series of events and processes, including notably inflammasome activation and inflammatory responses.153,154 The molecular mechanisms involved are complex. For instance, the NLR family of proteins comprises a large number of intracellular sensors,155 the best studied of which is NLRP3 which has been shown to be required for normal immune responses to skin sensitising chemicals.156
The inflammatory reactions induced by the elicitation of danger signals will have a significant impact on the development of immune responses to chemical allergens, not least through the elevated expression of cytokines and chemokines, that in turn will influence the behaviour of DC146 (see next section). It is tempting to speculate that contact allergens and chemical respiratory allergens may induce different types of cell trauma, and elicit different patterns of danger signals, such that the characteristics of downstream immune responses are affected. A systematic comparison of the patterns of DAMPs induced by exposure to contact and respiratory allergens has not yet (as far as these authors are aware) been undertaken. However, such research may prove profitable in characterising early molecular events that influence the subsequent development of differentiated immune responses.
Another signalling mechanism that may be relevant for the discrimination between different classes of chemical allergen is the Nrf2-Keap1-ARE pathway. It has been shown that the nuclear factor erythroid 2-related factor 2 (Nrf2)-Kelch-like ECH-associated protein-1 (Keap1)-antioxidant response element (ARE) pathway plays an important role in the acquisition of sensitisation.157,158 The Keap1 protein can be regarded as a sensor that is equipped with Cys residues. Under normal conditions, in the steady state, Keap1 is closely associated with the Nrf2 transcription factor. However, when there is covalent interaction between electrophiles and these Cys residues then Nrf2 disassociates from Keap1 and accumulates in the nucleus triggering the expression of genes that have ARE in their promoter regions.158 It has been proposed previously that if contact allergens favour interaction with Cys residues then their activation of the Nrf2-Keap1-ARE pathway might result in patterns of gene transcription that will favour the development of Th1-type responses and the acquisition of skin sensitisation. The argument is that, in contrast, chemical respiratory allergens will induce the transcription of other genes that favour the elicitation of Th2 responses and sensitisation of the respiratory tract.158 Although this is an attractive hypothesis, this distinction between contact and respiratory allergens in this regard may not be so clear-cut. Thus, some chemical respiratory allergens have been found to elicit positive response in predictive test methods based on the activation of Nrf2.13
It seems highly likely that the innate immune system will be affected by the nature of cell trauma and the danger signals released following exposure to chemical allergens of different types. It is reasonable also to expect that this in turn will influence the characteristics of downstream adaptive immune responses and the type of sensitisation that will be acquired. However, what is required is a detailed analysis of patterns of DAMPs elicited by chemical allergens to determine whether danger signals truly represent a pivotal decision point in determining the form allergic responses will take. If danger signals do indeed govern the characteristics of adaptive immune responses then it is likely that their influence will be mediated through the agency of DC.
Key event 3. The activation of dendritic cells
Epidermal DC (Langerhans cells; LC) were first identified by Paul Langerhans in 1868.159 However, the first full systematic descriptions of the phenotypic characteristics and functional properties of DC in lymphoid organs were published by Steinman and Cohn over a century later.160,161 Dendritic cells are now known to be orchestrators of adaptive immune responses having responsibility for the recognition, internalisation, processing and presentation of antigen to responsive T lymphocytes.162,163
It is clear too that DC are heterogeneous in nature, and a variety of phenotypic subsets have been described that display different functional properties.164–167 This is true also in the skin where both epidermal LC and dermal DC play roles in the initiation and regulation of cutaneous immune responses, including skin sensitisation.168–173
The question we address here is whether this family of DC, either individually or collectively, is able to determine the form that immune responses to chemical allergens take, and the type of sensitisation that will be acquired. We propose that DC will ‘read’ chemical allergens, or rather hapten-protein complexes, and tailor the quality of immune responses accordingly – favouring either Th1- or Th-2 type responses. It is likely that how the antigen is read will, in this case, be governed by the nature of the haptenated protein, and by the characteristics of danger signals and the down-stream immunological milieu they create. In this way DC may trigger responses that will result in sensitisation of the respiratory tract.
Certainly there is evidence that DC can induce Th2-type responses, and that there exist specialised subsets of DC that preferentially promote the development of Th2 cells.164,174–178 In some instances selective Th2 responses may be triggered by discrete functional sub-populations of DC, in other circumstances the ability to promote Th2 cell development may be conferred on plastic DC by DAMPs and the cytokines and other stimuli resulting from their recognition.179–188
The exact mechanisms through which DC acquire and express a Th1 or Th2 bias is not clear, although the cytokine interleukin 12 (IL-12) may be one important influence. The induction of selective Th1 responses is dependent on the availability of this cytokine, and if it is unavailable, or if production is compromised, then Th2-type responses will be favoured.189–191
Another factor that may affect the polarisation of DC function is IL-10, an anti-inflammatory cytokine. Evidence for this derives from experimental studies that examined the migration of epidermal LC from the skin of mice following topical exposure to DNCB (a potent contact allergen), or to trimellitic anhydride (TMA; a known human respiratory allergen). It was observed that the migration of LC from the skin towards regional lymph nodes was considerably faster following treatment of mice with DNCB compared with TMA, even though the concentrations of DNCB and TMA used displayed the same levels of overall immunogenicity. Subsequent experiments revealed that the slower tempo of LC migration in response to TMA was due to the stimulation by this chemical of local IL-10 production that resulted in the reduced expression of the proinflammatory cytokines required for the normal mobilisation and migration of LC.192 The potential significance of this difference is that a slower tempo of migration (as seen with TMA) might confer on LC a Th2 bias because while in transit the cells have longer to mature before reaching the draining lymph node.193 In addition to the effect of LC maturity at the time of arrival in draining lymph nodes, it has been suggested that the ratio of DC to T lymphocytes in nodes can impact on the selectivity of Th1/Th2 responses.188
Although other factors may be relevant also, the conclusion drawn is that DC are likely to play pivotal roles in shaping immune responses to chemical allergens. This might be dictated by discrete functional subsets of DC pre-committed to favouring either Th1 or Th2 cell development, and/or by plastic DC where a Th1 or Th2 bias might be acquired by responses to DAMPs and other microenvironmental stimuli such as IL-10 and IL-12. This appears to be a critical checkpoint in the development of sensitisation to chemical allergens, and the medium through which the nature of hapten-protein complexes, and patterns of danger signals and other molecular signals, will influence subsequent immune responses and the outcome of exposure. This is clearly an area where a continued investment in research is warranted, with an emphasis on characterising the molecular events and signals that drive DC to elicit selective Th cell responses to chemical allergens, and that in turn determine the form allergic sensitisation will take.
Before looking at the final Key Event, it is appropriate to summarise briefly the potential interplay between Key Events 1, 2 and 3. This is illustrated in diagrammatic form in Fig. 1.
Fig. 1. Interplay between key events 1, 2 and 3.
The end result of these key events is to engage with relevant DC and to equip them with the ability to process antigen, and to migrate to draining lymph nodes where presentation of that antigen to responsive T lymphocytes occurs. Chemical allergens could, in theory at least, act directly on DC to effect the necessary changes. This could occur. There is some experimental evidence to support this,194 and there are some novel test methods for the identification of skin sensitisation potential, such as the human Cell Line Activation Test (h-CLAT), that are predicated on the ability of chemicals to cause phenotypic changes (consistent with cellular activation) on surrogate DC in vitro.12 However, it is very difficult to be certain if it is truly the free chemical that acts on DC, or whether the chemical first has to form a stable association with protein. So, as illustrated in Fig. 1, it is possible chemical alone, or chemical associated with protein, can at least in part stimulate the necessary activation of DC required for development of an immune response. However, there can be little doubt that, in addition, there is usually a critical need for the chemical itself, or hapten-protein complexes, to stimulate the elicitation of DMAPs and other danger signals to drive the full deployment of activated DC. It is these activated DC that, when they reach the draining lymph nodes, present antigen to responsive T lymphocytes and initiate Key Event 4.
Key event 4. The activation, division and differentiation of T lymphocytes
This is the final Key Event on the road to the acquisition of both skin sensitisation and sensitisation of the respiratory tract. Both forms of sensitisation are dependent upon the antigen-driven activation of responsive T lymphocytes, their division, and differentiation into effector and memory populations. There is no need here to provide a commentary on the complex cellular processes that characterise the activation of T lymphocytes. Suffice it to say that the effective acquisition of sensitisation is dependent upon an adequate vigour and appropriate quality of response. The vigour of the response will be dependent upon both the extent of cell division (that determines the degree of clonal expansion of allergen-responsive T lymphocytes), and the repertoire diversity of responsive T lymphocytes (the number of clones that are able to recognise and respond to presented antigen).96,195,196 As has been described above, the quality of response will be determined by the balance achieved between various functional subpopulations of T lymphocytes. Thus, both skin sensitisation and sensitisation of the respiratory tract rely on the activation, division and differentiation of allergen responsive T lymphocytes. Where they differ is in the quality of that response with respect to T lymphocyte subsets.
The development of skin sensitisation and the subsequent elicitation of ACD are known to involve both Th1-type CD4+ and CD8+ T lymphocytes. In fact, the most important effector cells in eliciting ACD are antigen-specific CD8+ T lymphocytes.2–4,197–199 There are also other cells that play a part in this process, including for example Th17 cells and natural killer (NK) cells.147,200 It is beyond the scope of this article to consider in any greater detail the cell types that promote and regulate the elicitation of ACD (reviews are available elsewhere2–4,147), the point to make is that much less is known about the T lymphocytes that contribute to the development of respiratory reactions to chemical allergens. The evidence is that Th2-type cells play a central role, but the possibility that other cells may also contribute cannot be excluded.
In summary, the sequences of events that have been tracked here result in the provocation by contact allergens and chemical respiratory allergens of discrete T lymphocyte responses, and it is these divergent responses that eventually result in the elicitation of different forms of allergic reaction.
The aim now is to synthesise what is known of this pathway to sensitisation and allergic reactions into a hybrid AOP that uses as its foundations the AOP that has been developed previously for skin sensitisation.115
A hybrid AOP
Before considering the construct of a hybrid AOP for skin and respiratory sensitisation it is appropriate to reflect briefly on some more general aspects of the AOP as a tool for describing toxicological modes of action. There is little doubt that AOPs have had a positive impact on toxicology, and have provided a new model and a new lexicon for describing the sequence of events that will result in adverse health effects or environmental insults. However, AOPs in their current manifestation are not without limitations, and some of those limitations are relevant to the process of sensitisation.
For instance, it might not always be the case, in the AOP for sensitisation at least, that each Key Event necessarily plays an essential role. Thus, for instance, as described above, and as illustrated in Fig. 1, it is possible that activation of DC might result from the direct effect of the inducing chemical itself, or a of hapten-protein complex. Although the elicitation of danger signals will usually occur, and will likely have the greatest impact on the activation of DC, it might not always be a mandatory step – the necessary signals being provided by direct contact of DC with the chemical allergen or with the hapten-protein complex.
Another issue is the fact that AOPs, including the AOP for skin sensitisation, do not readily accommodate considerations of thresholds and to do so remains a substantial challenge, despite recent proposals.201 The acquisition of sensitisation is a threshold phenomenon, and throughout the course of the AOP for skin sensitisation thresholds can be discerned for each key event. Thus, the successful acquisition of sensitisation is dependent upon there being a sufficient level of exposure to chemical, the formation of adequate numbers of hapten-protein conjugates, a level of trauma necessary to deliver costimulatory signals, and a sufficient number of activated antigen-bearing DC to trigger an immune response of the vigour necessary to result in sensitisation. These quantitative aspects are not readily captured in a conventional AOP, and it is possible, in the context of this exercise, that quantitative factors play a role on determining the quality of immune response that will develop.
It is also potentially relevant that, to date at least, most AOPs have not considered the question of mixtures. This is certainly true for sensitisation. It is possible that components in mixtures that themselves are not able to induce sensitisation could impact on the vigour or quality of sensitisation induced by other components that are chemical allergens. One example will serve to illustrate the point. In an experimental system it has been shown that the admixture of DNCB with a non-sensitising skin irritant (sodium lauryl sulphate; SLS), while having no impact on skin penetration, reduced the concentration of DNCB required to elicit a significant T lymphocyte in draining lymph nodes.202
Finally, the responses that ultimately result in an adverse outcome will commonly be subject to internal checks and balances and regulatory interventions that might serve to modify or weaken the response, or even halt its progress altogether. For example, it is known that responses to skin sensitisers are subject to endogenous immunoregulatory mechanisms that serve to dampen immune responses and thereby prevent the development of potential damaging levels of immune reactivity.203,204 In this instance the immunoregulatory influence may be a function of CD4+ Tregs.97,197–199,205
Against this background, and acknowledging that in the above respects the current model of AOPs may have some limitations in reflecting all aspects of pathways to toxicity, the plan here is to illustrate a hybrid AOP for sensitisation to chemicals. As discussed above, the AOP for skin sensitisation provides an excellent foundation for this exercise, not least because the key events described there are in general terms identical to those needed for the acquisition of respiratory sensitisation. That is, for sensitisation of the respiratory tract there are requirements for exposure (at the required level and at a relevant tissue site), the formation of immunogenic hapten-protein complexes, the elicitation of danger signals to support initiation of an adaptive immune response, the activation of local DC, and the proliferation and differentiation of specific T lymphocytes. The differences are of fine detail rather than of separate pathways or key events.
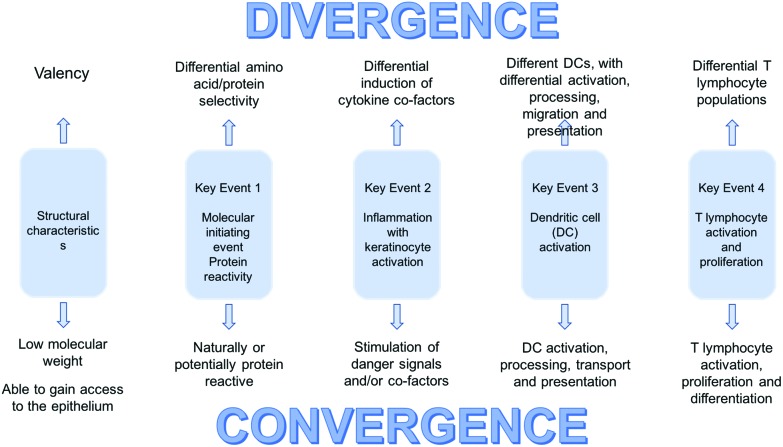
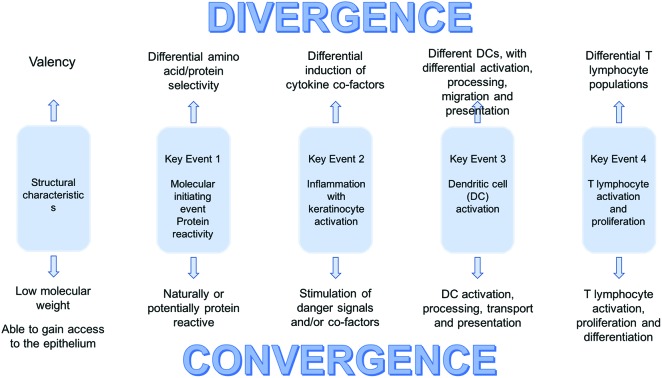
A hybrid AOP is illustrated in diagrammatic form in Fig. 2. The Key Events for the development of skin and respiratory sensitisation remain the same. As indicated above, the differences are small, but clearly of considerable importance in determining the form immune responses will take.
Fig. 2. The hybrid sensitisation AOP concept.
Before attempting to catalogue those differences it may be helpful to put immune responses to chemical allergens into the broader context of host defence and protective immunity. As discussed previously, the immune system has important qualitative aspects such that responses can be tailored to address particular challenges. Maintenance of human health demands that the immune system is able to deal effectively with a very wide range of pathogenic microorganisms; viruses, bacteria, fungi, protozoa and multicellular parasites. No single type of immune response would able to counter such very diverse infectious agents, and for this reason there has evolved the capacity to ensure that responses are properly aligned with particular antigenic threats. The ways in which the relevant information is acquired by the immune system is only incompletely understood, but it is clear that the nature of the antigen(s) encountered, the danger signals that are elicited (PAMPs) by their incursion, and the way in which antigen is processed and presented, will all undoubtedly influence the nature of the induced T cell response and the form that protective immune responses will take. Thus, small differences in the early events during immune responses can have profound effects on protective immunity. The point is that similarly small differences associated with the early Key Events in the acquisition of sensitisation to chemical allergens will have equally significant effects on the form that allergic sensitisation will take. With an appreciation that small variations in those early events will likely determine whether skin sensitisation or sensitisation of the respiratory tract predominates, the likely most important variables are identified in Fig. 2.
Fig. 2 serves to illustrate the fact that there are areas of convergence where the main events on the pathway to sensitisation are broadly the same for contact and respiratory chemical allergens. It also identifies areas of potential divergence where there exist nodes of activity where small variations may, individually or collectively, drive the downstream immune response in different directions. Some of the possible factors that might be responsible to the induction of divergent responses have been discussed above, and include: structural properties, amino acid selectivity, differential induction of DAMPs, other cofactors and cytokines, and engagement with DC with inherent or acquired differences in functional properties.
Pursuing the argument that small differences in the early stages of the pathway might result in profound effects on the characteristics of the developing immune response, it is appropriate to ask whether the sum of those small differences may be associated with variable patterns of gene transcription that cut across recognised Key Events in the AOP. It is early days, but there is reason to believe that immune responses to contact and chemical respiratory display differential patterns of gene expression and of epigenetic signatures.58,206–208
The differences in the AOPs to skin sensitisation and sensitisation of the respiratory tract do not immediately offer the basis for the development of novel approaches for the selective identification of respiratory allergens, or for distinguishing between contact and respiratory allergens. However, an appreciation of nodes in the pathway where differences may originate does suggests areas that are worthy of an investment (or a continued investment) in research. These research needs/opportunities are shown in Table 1.
Table 1. Research needs and opportunities: differentiation between chemical contact and respiratory allergens.
| Event/characteristics | Potential points of divergence | Research needs and opportunities |
| Structural characteristics/chemistry | • Valency | Systematic comparison of contact allergens and chemical respiratory allergens |
| • Differing electrophilic reaction mechanisms | ||
| KEY EVENT 1 Protein reactivity | • Differential amino acid selectivity | A more detailed analysis of the Lys selectivity of respiratory allergens |
| KEY EVENT 2 Inflammation/elicitation of danger signals | • Differential danger signal/DAMPS | Systematic survey of DAMP expression by contact and respiratory allergens. |
| • Differential induction of cytokines and chemokines | Do IL-10 or IL-12 provide selective biomarkers | |
| KEY EVENT 3 DC activation | • Engagement with different DC populations. | Characterisation of phenotypic property of DC associated with immune responses of contact and respiratory allergy |
| • Differential activation and/or mobilisation of DC | ||
| • Differential processing | ||
| KEY EVENT 4 T lymphocyte activation/proliferation | • Induction of qualitatively divergent immune responses | Identification of allergen-specific T lymphocyte sub-populations in human chemical allergy |
Recommendations for future research
Before discussing briefly possible future research opportunities that are identified on consideration of the hybrid AOP for sensitisation, it is important to make clear that other approaches may prove to be of equal or greater productivity. Thus, it might be that robust gene expression and/or epigenetic signatures will in the future reveal clear differences between contact and respiratory allergens, that reflect the divergent immune responses they elicit, and will form the basis for a new generation approach to hazard characterisation.
In tandem, opportunities exist for more focused research predicated on the hybrid AOP, and these are highlighted in Table 1. The present authors recommend that the areas likely to be most productive include: a more detailed comparison of the structural characteristics of contact and respiratory chemical allergens, further exploration of the observation that in some circumstances chemical allergens of different types display variable amino acid selectivity, a comparative analysis of DAMPs, other cofactors and cytokines associated with skin and respiratory sensitisation, and an assessment of the impact of different subsets of DC on the development of sensitisation. In addition, it would be very valuable to identify in humans the functional subpopulations of T lymphocytes that are stimulated by different classes of chemical allergen.
It is our view that these areas have the potential to provide a basis for the design of new methods for the identification of chemical respiratory allergens, and for further characterisation of the differences between skin sensitisation and sensitisation of the respiratory tract.
Conclusions
Considering the challenges and uncertainties associated with sensitisation of the respiratory tract by chemicals has been instructive in formulating a hybrid AOP for skin and respiratory sensitisation. The hybrid AOP does not itself solve those challenges, or resolve those uncertainties, nor does it reveal a previously unrecognised approach to hazard characterisation of chemical respiratory allergy. What it has achieved, however, is to highlight those areas where the pathways to skin sensitisation and sensitisation of the respiratory tract might diverge. A focus on these nodes may provide an important step forward in our understanding of the mechanisms through which chemical allergens are able to cause different forms of allergy, and offer opportunities for the design of effective methods for the identification of chemical respiratory allergens.
Conflicts of interest
All authors were remunerated by ECETOC for the preparation of this review.
Acknowledgments
The authors wish to acknowledge here the remarkable contributions made to the science and practice of toxicology by Iain Purchase and Cliff Elcombe. They are both sorely missed by the international scientific community.
One author (IK) would like to record his enormous personal gratitude to Iain Purchase for his kindness, encouragement and guidance. He was an extremely able Director of the Central Toxicology Laboratory, an excellent manager, a fine scientist, and a true gentleman. Iain was also instrumental in forging a collaboration that – for better or worse – first brought two of the present authors (IK and DAB) together in what has turned into a longstanding partnership.
References
- Kimber I., Basketter D. A., Gerberick G. F., Ryan C. A., Dearman R. J. Toxicol. Sci. 2011;120(Suppl. 1):S238–S268. doi: 10.1093/toxsci/kfq346. [DOI] [PubMed] [Google Scholar]
- Kimber I., Dearman R. J. Contact Dermatitis. 2002;46:1–5. doi: 10.1034/j.1600-0536.2002.460101.x. [DOI] [PubMed] [Google Scholar]
- Kimber I., Basketter D. A., Gerberick G. F., Dearman R. J. Int. Immunopharmacol. 2002;2:201–211. doi: 10.1016/s1567-5769(01)00173-4. [DOI] [PubMed] [Google Scholar]
- Rustemeyer T., Van Hoogstraten I. M. W., Von Blomberg B. M. A., Gibbs S. and Scheper R. J., Mechanisms of irritant and allergic contact dermatitis, In Textbook of Contact Dermatitis, ed. J. D. Johansen, P. J. Frosch and J.-P. Lepoittevin, Springer, Berlin, 5th edn., 2011, pp. 43–90. [Google Scholar]
- Magnusson B., Kligman A. M. J. Invest. Dermatol. 1969;52:268–276. doi: 10.1038/jid.1969.42. [DOI] [PubMed] [Google Scholar]
- Buehler E. V. Arch. Dermatol. 1965;92:171–177. doi: 10.1001/archderm.1965.01600080079017. [DOI] [PubMed] [Google Scholar]
- Kimber I., Weisenberger C. Arch. Toxicol. 1989;63:274–282. doi: 10.1007/BF00278640. [DOI] [PubMed] [Google Scholar]
- Kimber I., Dearman R. J., Basketter D. A., Ryan C. A., Gerberick G. F. Contact Dermatitis. 2002;47:315–328. doi: 10.1034/j.1600-0536.2002.470601.x. [DOI] [PubMed] [Google Scholar]
- Basketter D. A., Scholes E. W., Kimber I. Food Cosmet. Toxicol. 1994;32:543–547. doi: 10.1016/0278-6915(94)90112-0. [DOI] [PubMed] [Google Scholar]
- Gerberick G. F., Vassallo J. D., Bailey R. E., Chaney J. G., Morrall S. W., Lepoittevin J-P. Toxicol. Sci. 2004;81:332–343. doi: 10.1093/toxsci/kfh213. [DOI] [PubMed] [Google Scholar]
- Emter R., Ellis G., Natsch A. Toxicol. Appl. Pharmacol. 2010;245:281–290. doi: 10.1016/j.taap.2010.03.009. [DOI] [PubMed] [Google Scholar]
- Ashikaga T., Sakaguchi H., Sono S., Kosaka N., Ishikawa M., Nukada Y., Miyazawa M., Ito Y., Nishiyama N., Itagaki H. ATLA, Altern. Lab. Anim. 2010;38:275–284. doi: 10.1177/026119291003800403. [DOI] [PubMed] [Google Scholar]
- Basketter D. A., Poole A., Kimber I. Regul. Toxicol. Pharmacol. 2017;86:101–106. doi: 10.1016/j.yrtph.2017.03.002. [DOI] [PubMed] [Google Scholar]
- Dearden J. C., Hewitt M., Roberts D. W., Enoch S. J., Rowe P. H., Pryzbylak K. R., Vaughan-Williams G. D., Smith M. L., Pillai G. G., Katritzky A. R. Chem. Res. Toxicol. 2015;28:1975–1986. doi: 10.1021/acs.chemrestox.5b00197. [DOI] [PubMed] [Google Scholar]
- Johansson H., Gradin R., Forreryd A., Agemark M., Zeller K., Jonansson A., Larne O., Van Vliet E., Borrebaeck C., Lindstedt M. ALTEX. 2017;34:515–523. doi: 10.14573/altex.1701121. [DOI] [PubMed] [Google Scholar]
- Galbati V., Papale A., Marinovich M., Gibbs S., Roggen E., Corsini E. Toxicol. Lett. 2017;271:1–11. doi: 10.1016/j.toxlet.2017.01.016. [DOI] [PubMed] [Google Scholar]
- Cottrez F., Boitel E., Ourlin J. C., Peiffer J. L., Henaoui I. S., Mari B., Vallauri A., Paquet A., Auriault C., Aeby P., Groux H. Toxicol. In Vitro. 2016;32:248–260. doi: 10.1016/j.tiv.2016.01.007. [DOI] [PubMed] [Google Scholar]
- Tsujita-Inoue K., Hirota M., Ashikaga T., Atobe T., Kouzuki H., Aiba S. Toxicol. In Vitro. 2014;28:626–639. doi: 10.1016/j.tiv.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Van der Veen J. W., Rorije E., Emter R., Natsch A., van Loveren H., Ezendam J. Regul. Toxicol. Pharmacol. 2014;69:371–379. doi: 10.1016/j.yrtph.2014.04.018. [DOI] [PubMed] [Google Scholar]
- Jaworska J. S., Natsch A., Ryan C., Strickland J., Ashikaga T., Miyazawa M. Arch. Toxicol. 2015;89:2355–2383. doi: 10.1007/s00204-015-1634-2. [DOI] [PubMed] [Google Scholar]
- Macmillan D. S., Canipa S. J., Chilton M. L., Williams R. V., Barber C. G. Regul. Toxicol. Pharmacol. 2016;76:30–38. doi: 10.1016/j.yrtph.2016.01.009. [DOI] [PubMed] [Google Scholar]
- Strickland J., Zang Q., Kleinstreuer N., Paris M., Lehmann D. M., Choksi N., Matheson J., Jacobs A., Lowit A., Allen D., Casey W. J. Appl. Toxicol. 2016;36:1150–1162. doi: 10.1002/jat.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezendam J., Brackhuis H. M., Vandebriel R. H. Arch. Toxicol. 2016;90:2861–2883. doi: 10.1007/s00204-016-1842-4. [DOI] [PubMed] [Google Scholar]
- Roberts D. W., Patlewicz G. J. Appl. Toxicol. 2017;38:41–50. doi: 10.1002/jat.3479. [DOI] [PubMed] [Google Scholar]
- Casati S. Curr. Opinion Toxicol. 2017;5:1–5. [Google Scholar]
- Johansson H., Gradin R. Toxicol. Sci. 2017;159:3–5. doi: 10.1093/toxsci/kfx115. [DOI] [PubMed] [Google Scholar]
- Kimber I., Dearman R. J. Food Cosmet. Toxicol. 1991;29:125–129. [Google Scholar]
- Kimber I., Basketter D. A. Hum. Ecol. Risk Assess. 1997;3:385–395. [Google Scholar]
- Basketter D. A., Blaikie L., Dearman R. J., Kimber I., Ryan C. A., Gerberick G. F., Harvey P., Evans P., White I. R., Rycroft R. J. G. Contact Dermatitis. 2000;42:244–348. doi: 10.1034/j.1600-0536.2000.042006344.x. [DOI] [PubMed] [Google Scholar]
- Gerberick G. F., Robinson M. K., Ryan C. A., Dearman R. J., Kimber I., Basketter D. A., Wright Z., Marks J. G. Am. J. Contact Dermat. 2001;12:156–161. doi: 10.1053/ajcd.2001.23926. [DOI] [PubMed] [Google Scholar]
- Kimber I., Wilks M. F. Hum. Exp. Toxicol. 1995;14:735–736. doi: 10.1177/096032719501400907. [DOI] [PubMed] [Google Scholar]
- Mapp C. E., Boschetto P., Maestrelli P., Fabbri L. M. Am. J. Respir. Crit. Care Med. 2005;172:280–305. doi: 10.1164/rccm.200311-1575SO. [DOI] [PubMed] [Google Scholar]
- Bakerly N. D., Moore V. C., Jaakkola M. S., Robertson A. S., Burge P. S. Occup. Med. 2008;58:169–174. doi: 10.1093/occmed/kqn007. [DOI] [PubMed] [Google Scholar]
- Kenyon N. J., Morrisey B. M., Schivo M., Albertson T. E. Clin. Rev. Allergy Immunol. 2012;43:3–13. doi: 10.1007/s12016-011-8272-0. [DOI] [PubMed] [Google Scholar]
- Feary J., Pinnock H., Cullinan P. Br. Med. J. 2016;353:i2658. doi: 10.1136/bmj.i2658. [DOI] [PubMed] [Google Scholar]
- Kimber I. and Dearman R. J., Chemical respiratory allergy: an introduction, in Toxicology of Chemical Respiratory Hypersensitivity, ed. I. Kimber and R. J. Dearman, Taylor & Francis, London, 1997, pp. 1–6. [Google Scholar]
- Baur X. J. Occup. Med. Toxicol. 2013;8:1–8. doi: 10.1186/1745-6673-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur X., Bakehe P. Int. Arch. Occup. Environ. Health. 2014;87:339–363. doi: 10.1007/s00420-013-0866-9. [DOI] [PubMed] [Google Scholar]
- Holsapple M. P., Jones D., Kawabata T. T., Kimber I., Sarlo K., Selgrade M. K., Shah J., Woolhiser M. R. Toxicol. Sci. 2006;91:4–13. doi: 10.1093/toxsci/kfj074. [DOI] [PubMed] [Google Scholar]
- Kimber I., Agius R., Basketter D. A., Corsini E., Cullinan P., Dearman R. J., Gimenez-Arnau E., Greenwell L., Hartung T., Kuper F., Maestrelli P., Roggen E., Rovida C. ATLA, Altern. Lab. Anim. 2007;35:243–265. doi: 10.1177/026119290703500212. [DOI] [PubMed] [Google Scholar]
- Boverhof D. R., Billington R., Gollapudi B. B., Hotchkiss J. A., Krieger S. M., Poole A., Wiescinski C. M., Woolhiser M. R. Toxicol. Appl. Pharmacol. 2008;226:1–13. doi: 10.1016/j.taap.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Isola D., Kimber I., Sarlo K., Lalko J., Sipes I. G. J. Appl. Toxicol. 2008;28:249–253. doi: 10.1002/jat.1336. [DOI] [PubMed] [Google Scholar]
- Basketter D. A., Kimber I. Regul. Toxicol. Pharmacol. 2011;61:365–372. doi: 10.1016/j.yrtph.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Kimber I., Dearman R. J., Basketter D. A. J. Appl. Toxicol. 2014;34:1073–1077. doi: 10.1002/jat.3041. [DOI] [PubMed] [Google Scholar]
- Cochrane S. A., Arts J. H., Hindle S., Hollnagel H. M., Poole A., Suto H., Kimber I. Toxicology. 2015;333:179–194. doi: 10.1016/j.tox.2015.04.010. [DOI] [PubMed] [Google Scholar]
- Dotson G. S., Maier A., Siegel P. D., Anderson S. E., Green B. J., Stefaniak A. B., Codispoti C. P., Kimber I. J. Occup. Environ. Hyg. 2015;12(Suppl. 1):S82–S98. doi: 10.1080/15459624.2015.1072277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North C. M., Ezendam J., Hotchkiss J. A., Maier C., Ayotama K., Enoch S., Goetz A., Graham C., Kimber I., Karljalainen A., Pauluhn J., Roggen E. L., Selgrade M., Chen C. L. Regul. Toxicol. Pharmacol. 2016;80:295–309. doi: 10.1016/j.yrtph.2016.06.006. [DOI] [PubMed] [Google Scholar]
- Botham P. A., Hext P. M., Rattray N. J., Walsh S. T., Woodcock D. R. Toxicol. Lett. 1988;41:159–173. doi: 10.1016/0378-4274(88)90089-6. [DOI] [PubMed] [Google Scholar]
- Griffths-Johnson D. A., Karol M.M. H.H. Toxicology. 1991;65:283–294. doi: 10.1016/0300-483x(91)90087-h. [DOI] [PubMed] [Google Scholar]
- Sarlo K., Clark E. D. Fundam. Appl. Toxicol. 1992;18:107–114. doi: 10.1016/0272-0590(92)90202-s. [DOI] [PubMed] [Google Scholar]
- Satoh T., Kramarik J. A., Tollerud D. J., Karol M. H. Toxicol. Lett. 1995;78:57–66. doi: 10.1016/0378-4274(94)03234-x. [DOI] [PubMed] [Google Scholar]
- Hilton J., Dearman R. J., Boylett M. S., Fielding I., Basketter D. A., Kimber I. J. Appl. Toxicol. 1996;16:165–170. doi: 10.1002/(SICI)1099-1263(199603)16:2<165::AID-JAT325>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Dearman R. J., Kimber I. J. Appl. Toxicol. 2001;21:153–163. doi: 10.1002/jat.743. [DOI] [PubMed] [Google Scholar]
- Arts J. H., de Jong W. H., van Triel J. J., Schijf M. A., de Klerk A., van Loveren H., Kuper C. F. Toxicol. Sci. 2008;106:423–434. doi: 10.1093/toxsci/kfn199. [DOI] [PubMed] [Google Scholar]
- Pauluhn J. Regul. Toxicol. Pharmacol. 2008;50:57–66. doi: 10.1016/j.yrtph.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Lalko J. F., Kimber I., Dearman R. J., Gerberick G. F., Sarlo K., Api A. M. Toxicol. In Vitro. 2011;25:433–445. doi: 10.1016/j.tiv.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Enoch S. J., Seed M. J., Roberts D. W., Cronin M. T., Stocks S. J., Agius R. M. Chem. Res. Toxicol. 2012;25:2490–2498. doi: 10.1021/tx3003092. [DOI] [PubMed] [Google Scholar]
- Forreryd A., Johansson H., Albrekt A. S., Borrebaeck C. A., Lindstedt M. PLoS One. 2015;10(3):e0118808. doi: 10.1371/journal.pone.0118808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karol M. H., Hauth B. A., Riley E. J., Magreni C. M. Toxicol. Appl. Pharmacol. 1981;58:221–230. doi: 10.1016/0041-008x(81)90426-9. [DOI] [PubMed] [Google Scholar]
- Botham P. A., Rattray N. J., Woodcock D. R., Walsh S. T., Hext P. M. Toxicol. Lett. 1989;47:25–39. doi: 10.1016/0378-4274(89)90083-0. [DOI] [PubMed] [Google Scholar]
- Rattray N. J., Botham P. A., Hext P. M., Woodcock D. R., Fielding I., Dearman R. J., Kimber I. Toxicology. 1994;88:15–30. doi: 10.1016/0300-483x(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Kimber I., Dearman R. J. Toxicology. 2002;181–182:311–315. doi: 10.1016/s0300-483x(02)00299-8. [DOI] [PubMed] [Google Scholar]
- Tarlo S., Malo J. L. Eur. Respir. J. 2006;27:607–614. doi: 10.1183/09031936.06.00062105. [DOI] [PubMed] [Google Scholar]
- Bello D., Herrick C. A., Smith T. J., Woskie S. R., Streicher R. P., Cullen M. R., Liu Y., Redlich C. A. Environ. Health Perspect. 2007;115:328–335. doi: 10.1289/ehp.9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redlich C. A., Herrick C. A. Curr. Opin. Allergy Clin. Immunol. 2008;8:115–119. doi: 10.1097/ACI.0b013e3282f85a31. [DOI] [PubMed] [Google Scholar]
- Redlich C. A. Proc. Am. Thorac. Soc. 2010;7:134–137. doi: 10.1513/pats.201002-025RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimber I., Griffiths C. E. M., Basketter D. A., McFadden J. P., Dearman R. J. Eur. J. Dermatol. 2014;24:10–14. doi: 10.1684/ejd.2013.2187. [DOI] [PubMed] [Google Scholar]
- Howe W., Venables K. M., Topping M. D., Dally M. B., Hawkins R., Law S. J., Newman Taylor A. J. J. Allergy Clin. Immunol. 1983;71:5–11. doi: 10.1016/0091-6749(83)90539-0. [DOI] [PubMed] [Google Scholar]
- Murdoch R. D., Pepys J., Hughes E. G. Br. J. Ind. Med. 1986;43:37–43. doi: 10.1136/oem.43.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J., Welinder H., Ottoson H., Bensryd I., Venge P., Skerfving S. Clin. Exp. Allergy. 1994;24:440–449. doi: 10.1111/j.1365-2222.1994.tb00932.x. [DOI] [PubMed] [Google Scholar]
- Cartier A., Grammer L. C., Malo J.-L., Lagier F., Ghezzo H., Harris K., Patterson R. J. Allergy Clin. Immunol. 1989;84:507–514. doi: 10.1016/0091-6749(89)90364-3. [DOI] [PubMed] [Google Scholar]
- Vandenplas O., Cartier A., Lesage L., Cloutier Y., Perreault G., Grammer L. C., Shaughnessy M. A., Malo J.-L. J. Allergy Clin. Immunol. 1993;91:850–861. doi: 10.1016/0091-6749(93)90342-d. [DOI] [PubMed] [Google Scholar]
- Cullinan P. Clin. Exp. Allergy. 1998;28:688–690. doi: 10.1046/j.1365-2222.1998.00292.x. [DOI] [PubMed] [Google Scholar]
- Tee R. D., Cullinan P., Welch J., Burge P. S., Newman – Taylor A. J. J. Allergy Clin. Immunol. 1998;101:709–715. doi: 10.1016/S0091-6749(98)70181-2. [DOI] [PubMed] [Google Scholar]
- Tarlo S. M. J. Allergy Clin. Immunol. 1999;103:739–741. doi: 10.1016/s0091-6749(99)70413-6. [DOI] [PubMed] [Google Scholar]
- Ott A., Jolly A. T., Burkert A. L., Brown W. E. Crit. Rev. Toxicol. 2007;37:567–585. doi: 10.1080/10408440701419553. [DOI] [PubMed] [Google Scholar]
- Pronk A., Preller L., Raulf-Helmsoth M., Jonkers I. C. L., Lammers J.-W., Woulters I. M., Doekes G., Wisnewski A. V., Heederik D. Am. J. Respir. Crit. Care Med. 2007;176:1090–1097. doi: 10.1164/rccm.200702-215OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur G-Y., Koh D-H., Choi G-S., Park H-J., Choi S-J., Ye Y-M, Kim K-S, Park H-S. Clin. Exp. Allergy. 2008;38:586–593. doi: 10.1111/j.1365-2222.2008.02935.x. [DOI] [PubMed] [Google Scholar]
- Swierczynska-Machura D., Breznicki S., Nowakowska-Swirta E., Walusiak-Skorupa J., Wittczak T., Dudek W., Boncwarowska M., Wesolowski W., Czerczak S., Palczynski C. Int. J. Occup. Med. Environ. Health. 2015;28:985–998. doi: 10.13075/ijomeh.1896.00284. [DOI] [PubMed] [Google Scholar]
- Malo J.-L., L'Archeveque J. L., Lummus Z., Bernstein D. J. Allergy Clin. Immunol. 2006;118:530–533. doi: 10.1016/j.jaci.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Wisnewski A. V. Curr. Opin. Allergy Clin. Immunol. 2007;7:138–145. doi: 10.1097/ACI.0b013e3280895d22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budnik L. T., Preisser A. M., Permentir H., Baur X. Int. Arch. Occup. Environ. Health. 2013;86:417–430. doi: 10.1007/s00420-012-0772-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapp C. E., Boschetto P., Miotti D., De Rosa E. Am. J. Respir. Crit. Care Med. 2005;172:280–285. doi: 10.1164/rccm.200311-1575SO. [DOI] [PubMed] [Google Scholar]
- Jones M. G., Floyd A., Nouri-Aria K. T., Jacobson M. R., Durham S. R., Newman Taylor A., Cullinan P. J. Allergy Clin. Immunol. 2006;117:663–669. doi: 10.1016/j.jaci.2005.09.053. [DOI] [PubMed] [Google Scholar]
- Kimber I., Warbrick E. V., Dearman R. J. Hum. Exp. Toxicol. 1998;17:537–540. doi: 10.1177/096032719801701002. [DOI] [PubMed] [Google Scholar]
- Wisnewski A. V., Stowe M. H., Cartier A., Liu Q., Chem L., Redlich C. A. J. Allergy Clin. Immunol. 2004;113:1178–1184. doi: 10.1016/j.jaci.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Campo R., Wisnewski A. V., Lummus Z., Cartier A., Malo J.-L., Boulet L. P., Bernstein D. I. Clin. Exp. Allergy. 2007;37:1095–1102. doi: 10.1111/j.1365-2222.2007.02745.x. [DOI] [PubMed] [Google Scholar]
- Hellman L. Biomed. Pharmacother. 2007;61:34–39. doi: 10.1016/j.biopha.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Kimber I., Dearman R. J., Basketter D. A., Boverhof D. R. Toxicology. 2014;318:32–39. doi: 10.1016/j.tox.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Mekenyan O. G., Patliewicz G. Y., Kuseva C., Popova I., Mehmed A., Kotov S., Zhechev T., Pavlov T., Temelkov S., Roberts D. W. Chem. Res. Toxicol. 2014;27:219–239. doi: 10.1021/tx400345b. [DOI] [PubMed] [Google Scholar]
- Sullivan K., Enoch S. J., Ezendam J., Sewald K., Roggen E. L., Cochrane S. Appl. In Vitro Toxicol. 2017;3:213–226. [Google Scholar]
- Basketter D. A., Kimber I. J. Immunotoxicol. 2016;13:767–769. doi: 10.1080/1547691X.2016.1177149. [DOI] [PubMed] [Google Scholar]
- Romagnani S. J. Allergy Clin. Immunol. 2004;113:395–400. doi: 10.1016/j.jaci.2003.11.025. [DOI] [PubMed] [Google Scholar]
- O'Shea J. J., Paul W. E. Science. 2010;327:1098–1102. doi: 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geginat J., Paroni M., Facciotti F., Kastirr I., Caprioli F., Pagani M., Abrignani S. Semin. Immunol. 2013;25:252–262. doi: 10.1016/j.smim.2013.10.012. [DOI] [PubMed] [Google Scholar]
- Kimber I., Maxwell G., Gilmour N., Dearman R. J., Friedmann P. S., Martin S. F. Toxicology. 2012;291:18–24. doi: 10.1016/j.tox.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Kimber I., Travis M. A., Martin S. F., Dearman R. J. Contact Dermatitis. 2012;67:179–183. doi: 10.1111/j.1600-0536.2012.02148.x. [DOI] [PubMed] [Google Scholar]
- Dearman R. J., Kimber I. Immunology. 1991;72:563–570. [PMC free article] [PubMed] [Google Scholar]
- Dearman R. J., Kimber I. Clin. Exp. Allergy. 1992;22:241–250. doi: 10.1111/j.1365-2222.1992.tb03079.x. [DOI] [PubMed] [Google Scholar]
- Dearman R. J., Kimber I. Methods. 1999;19:56–63. doi: 10.1006/meth.1999.0827. [DOI] [PubMed] [Google Scholar]
- Dearman R. J., Filby A., Humphreys I. R., Kimber I. J. Appl. Toxicol. 2002;22:317–325. doi: 10.1002/jat.865. [DOI] [PubMed] [Google Scholar]
- Kimber I., Dearman R. J. Curr. Opin. Allergy Clin. Immunol. 2005;5:119–124. doi: 10.1097/01.all.0000162302.82233.93. [DOI] [PubMed] [Google Scholar]
- Vento K. L., Dearman R. J., Kimber I., Basketter D. A., Coleman J. W. Cell. Immunol. 1996;172:246–253. doi: 10.1006/cimm.1996.0239. [DOI] [PubMed] [Google Scholar]
- Vandebriel R. J., De Jong W. H., Spiekstra S. W., Van Dijk M., Fluitman A., Garssen J., Van Loveren H. Toxicol. Appl. Pharmacol. 2000;162:77–85. doi: 10.1006/taap.1999.8841. [DOI] [PubMed] [Google Scholar]
- Van Och F. M. M., Van Loveren H., De Jong W. H., Vandebriel R. J. Toxicol. Appl. Pharmacol. 2002;184:46–56. [PubMed] [Google Scholar]
- Kimber I., Basketter D. A., Thyssen J. P., Dearman R. J., McFadden J. P. J. Immunotoxicol. 2014;11:203–204. doi: 10.3109/1547691X.2013.833661. [DOI] [PubMed] [Google Scholar]
- Bentley A. M., Maestrelli P., Saetta M., Fabbri L. M., Robinson D., Bradley B. L., Jeffrey P. K., Durham S. R., Kay A. B. J. Allergy Clin. Immunol. 1992;89:821–829. doi: 10.1016/0091-6749(92)90437-7. [DOI] [PubMed] [Google Scholar]
- Sastre J., Vandenplas O., Park H.-S. Eur. Respir. J. 2003;22:364–373. doi: 10.1183/09031936.03.00045103. [DOI] [PubMed] [Google Scholar]
- Mamessier E., Milhe F., Guillot C., Birnbaum J., Dupuy P., Lorec A. M., Vervloet D., Magnan A. Allergy. 2007;62:162–169. doi: 10.1111/j.1398-9995.2006.01288.x. [DOI] [PubMed] [Google Scholar]
- Ouyang B., Bernstein D. I., Lummus Z. L., Yinng J., Boulet L.-P., Cartier A., Gautrin D., Ho S.-M. Toxicol. Sci. 2013;133:218–224. doi: 10.1093/toxsci/kft079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell L., Polak M. E., Perera J., Owen C., Boyd P., Pickard C., Howarth P. H., Healy E., Holloway J. W., Friedmann P. S., Arden-Jones M. R. J. Invest. Dermatol. 2013;133:2372–2380. doi: 10.1038/jid.2013.148. [DOI] [PubMed] [Google Scholar]
- Ankley G. T., Bennett R. S., Erikson R. J., Hoff D. J., Hornung M. W., Johnson R. D., Mount D. R., Nichols J. W., Russom C. L., Schmieder P. K., Serrano J. A., Tietage J. E., Villeneuve D. L. Environ. Toxicol. Chem. 2010;29:730–741. doi: 10.1002/etc.34. [DOI] [PubMed] [Google Scholar]
- Vinken M. Toxicology. 2013;312:158–165. doi: 10.1016/j.tox.2013.08.011. [DOI] [PubMed] [Google Scholar]
- Organisation for Economic Cooperation and Development (OECD) .
- Organisation for Economic Cooperation and Development (OECD) .
- MacKay C., Davies M., Summerfield V., Maxwell G. ALTEX. 2013;30:473–486. doi: 10.14573/altex.2013.4.473. [DOI] [PubMed] [Google Scholar]
- Maxwell G., MacKay C., Cubberley R., Davies M., Gellatly N., Glavin S., Gouin T., Jacquilleot S., Moore C., Pendlington R., Saib O., Sheffield D., Stark R., Summerfield V. Toxicol. In Vitro. 2014;28:8–12. doi: 10.1016/j.tiv.2013.10.013. [DOI] [PubMed] [Google Scholar]
- Potts R. O., Guy R. H. Pharm. Res. 1992;9:663–669. doi: 10.1023/a:1015810312465. [DOI] [PubMed] [Google Scholar]
- Jarvis J., Seed M. J., Elton R., Sawyer L., Agius R. Occup. Environ. Med. 1995;62:243–250. doi: 10.1136/oem.2004.016402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham C., Rosenkranz H. S., Karol M. H. Regul. Toxicol. Pharmacol. 1997;26:296–306. doi: 10.1006/rtph.1997.1170. [DOI] [PubMed] [Google Scholar]
- Seed M., Cullinan P., Agius R. M. Curr. Opin. Allergy Clin. Immunol. 2008;8:103–109. doi: 10.1097/ACI.0b013e3282f4cadd. [DOI] [PubMed] [Google Scholar]
- Seed M., Agius R. M. Occup. Med. 2010;60:115–120. doi: 10.1093/occmed/kqp168. [DOI] [PubMed] [Google Scholar]
- Seed M. J., Agius R. M. Curr. Opin. Allergy Clin. Immunol. 2017;17:64–71. doi: 10.1097/ACI.0000000000000355. [DOI] [PubMed] [Google Scholar]
- Kimber I., Basketter D. A., Roggeband R. Toxicology. 2001;167:159–162. doi: 10.1016/s0300-483x(01)00433-4. [DOI] [PubMed] [Google Scholar]
- Arts J., Kimber I. Regul. Toxicol. Pharmacol. 2017;89:268–278. doi: 10.1016/j.yrtph.2017.07.018. [DOI] [PubMed] [Google Scholar]
- Enoch S. J., Roberts D. W., Cronin M. T. D. Chem. Res. Toxicol. 2009;22:1447–1453. doi: 10.1021/tx9001463. [DOI] [PubMed] [Google Scholar]
- Enoch S. J., Roberts D. W., Cronin M. T. D. Chem. Res. Toxicol. 2010;23:1547–1555. doi: 10.1021/tx100218h. [DOI] [PubMed] [Google Scholar]
- Landsteiner K., Jacobs J. J. Exp. Med. 1925;61:643–656. doi: 10.1084/jem.61.5.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constant S. L., Bottomly K. Annu. Rev. Immunol. 1997;15:297–322. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- Banks P. R., Paquette D. M. Bioconjugate Chem. 1995;6:447–458. doi: 10.1021/bc00034a015. [DOI] [PubMed] [Google Scholar]
- Jonsson B. A., Wishnok J. S., Skipper P. L., Stillwell W. G., Tannenbaum S. R. Toxicol. Appl. Pharmacol. 1995;135:156–162. doi: 10.1006/taap.1995.1218. [DOI] [PubMed] [Google Scholar]
- Lalko J. F., Kimber I., Dearman R. J., Gerberick G. F., Sarlo K., Api A. M. Toxicol. In Vitro. 2011;25:433–445. doi: 10.1016/j.tiv.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Lalko J. F., Kimber I., Gerberick G. F., Foertsch L. M., Api A. M., Dearman R. J. Toxicol. Sci. 2012;129:421–431. doi: 10.1093/toxsci/kfs205. [DOI] [PubMed] [Google Scholar]
- Lalko J. F., Kimber I., Dearman R. J., Api A. M., Gerberick G. F. J. Immunotoxicol. 2013;10:292–301. doi: 10.3109/1547691X.2012.725784. [DOI] [PubMed] [Google Scholar]
- Raffray M., Personal communication, 2017.
- Hopkins J. E., Naisbitt D. J., Kitteringham N. R., Dearman R. J., Kimber I., Park B. K. Chem. Res. Toxicol. 2005;18:375–381. doi: 10.1021/tx049688+. [DOI] [PubMed] [Google Scholar]
- Johannesson G., Rosqvist S., Lindh C. H., Welinder H., Jonsson B. A. Clin. Exp. Allergy. 2001;31:1021–1030. doi: 10.1046/j.1365-2222.2001.01109.x. [DOI] [PubMed] [Google Scholar]
- Kristiansson M. H., Lindh C. H., Jonsson B. A. Biomarkers. 2003;8:343–359. doi: 10.1080/13547500310001607836. [DOI] [PubMed] [Google Scholar]
- Matzinger P. Annu. Rev. Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- Matzinger P. Semin. Immunol. 1998;10:399–415. doi: 10.1006/smim.1998.0143. [DOI] [PubMed] [Google Scholar]
- McFadden J. P., Basketter D. A. Contact Dermatitis. 2000;42:123–127. doi: 10.1034/j.1600-0536.2000.042003123.x. [DOI] [PubMed] [Google Scholar]
- Kimber I., Cumberbatch M., Dearman R. J., Griffiths C. E. M. Br. J. Dermatol. 2002;147:613–614. doi: 10.1046/j.1365-2133.2002.48776.x. [DOI] [PubMed] [Google Scholar]
- Martin S. F., Jakob T. Curr. Opin. Allergy Clin. Immunol. 2008;8:289–293. doi: 10.1097/ACI.0b013e3283088cf9. [DOI] [PubMed] [Google Scholar]
- Martin S. F. Allergologie. 2010;33:66–70. [Google Scholar]
- Martin S. F., Esser P. R., Weber F. C., Jakob T., Freudenberg M. A., Schmidt M., Goebeler M. Allergy. 2011;66:1152–1163. doi: 10.1111/j.1398-9995.2011.02652.x. [DOI] [PubMed] [Google Scholar]
- Ainscough J. S., Gerberick G. F., Dearman R. J., Kimber I. J. Immunotoxicol. 2013;10:223–234. doi: 10.3109/1547691X.2012.711782. [DOI] [PubMed] [Google Scholar]
- Martin S. F. Curr. Opin. Allergy Clin. Immunol. 2015;15:124–130. doi: 10.1097/ACI.0000000000000142. [DOI] [PubMed] [Google Scholar]
- Seong S. Y., Matzinger P. Nat. Rev. Immunol. 2004;4:469–478. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]
- Oppenhein J. J., Tewary P., de la Rosa G., Yang D. Adv. Exp. Med. Biol. 2007;601:185–194. doi: 10.1007/978-0-387-72005-0_19. [DOI] [PubMed] [Google Scholar]
- Vance R. E., Isberg R. R., Portnoy D. A. Cell Host Microbe. 2009;6:10–21. doi: 10.1016/j.chom.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Shaw M. H., Kim Y. G., Nunez G. Annu. Rev. Phytopathol. 2009;4:365–398. doi: 10.1146/annurev.pathol.4.110807.092239. [DOI] [PubMed] [Google Scholar]
- Kawai T., Akira S. Nat. Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- Martinon F., Mayor A., Tschopp J. Annu. Rev. Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- Fukata M., Vamadevan A. S., Abreu M. T. Semin. Immunol. 2009;21:242–253. doi: 10.1016/j.smim.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Franchi L., Eigenbrod T., Munoz-Planillo R., Nunez G. Nat. Immunol. 2009;10:241–247. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H., Gaide O., Petrilli V., Martinon F., Contassot E., Roques S., Kummer J. A., Tschopp J., French L. E. J. Invest. Dermatol. 2007;127:1956–1963. doi: 10.1038/sj.jid.5700819. [DOI] [PubMed] [Google Scholar]
- Natsch A., Emter R. Toxicol. Sci. 2008;102:110–119. doi: 10.1093/toxsci/kfm259. [DOI] [PubMed] [Google Scholar]
- Natsch A. Toxicol. Sci. 2010;113:284–292. doi: 10.1093/toxsci/kfp228. [DOI] [PubMed] [Google Scholar]
- Langerhans P. Virchows Arch. Pathol. Anat. Physiol. 1868;44:325–337. [Google Scholar]
- Steinman R. M., Cohn Z. A. J. Exp. Med. 1973;137:1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Cohn Z. A. J. Exp. Med. 1974;139:380–397. doi: 10.1084/jem.139.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M., Sallusto F., Lanzavecchia A. Curr. Opin. Immunol. 1997;9:10–16. doi: 10.1016/s0952-7915(97)80153-7. [DOI] [PubMed] [Google Scholar]
- Banchereau J., Steinman R. M. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Liu Y.-J., Kanzler H., Soumelis V., Gilliet M. Nat. Immunol. 2001;2:585–589. doi: 10.1038/89726. [DOI] [PubMed] [Google Scholar]
- Murphy K. M. Adv. Immunol. 2013;120:239–267. doi: 10.1016/B978-0-12-417028-5.00009-0. [DOI] [PubMed] [Google Scholar]
- O'Keeffe M., Mok W. H., Radford K. J. Cell. Mol. Life Sci. 2015;72:4309–4325. doi: 10.1007/s00018-015-2005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Uto T., Kukaya T., Takagi H. Curr. Top. Microbiol. Immunol. 2017;410:47–71. doi: 10.1007/82_2017_60. [DOI] [PubMed] [Google Scholar]
- Kimber I., Cumberbatch M. Toxicol. Appl. Pharmacol. 1992;117:137–146. doi: 10.1016/0041-008x(92)90230-p. [DOI] [PubMed] [Google Scholar]
- Kimber I., Dearman R. J., Cumberbatch M., Huby R. J. D. Curr. Opin. Immunol. 1998;10:614–619. doi: 10.1016/s0952-7915(98)80078-2. [DOI] [PubMed] [Google Scholar]
- Kimber I., Cumberbatch M., Dearman R. J. J. Invest. Dermatol. 2009;129:1852–1853. doi: 10.1038/jid.2009.54. [DOI] [PubMed] [Google Scholar]
- Kaplan D. H. Trends Immunol. 2010;31:446–451. doi: 10.1016/j.it.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan D. H., Igyarto B. Z., Gaspari A. A. Nat. Rev. Immunol. 2012;12:114–124. doi: 10.1038/nri3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashem S. W., Haniffa M., Kaplan D. H. Annu. Rev. Immunol. 2017;35:469–499. doi: 10.1146/annurev-immunol-051116-052215. [DOI] [PubMed] [Google Scholar]
- Stumbles P. A., Thomas J. A., Pimm C. L., Lee P. T., Venaille T. J., Proksch S., Holt P. G. J. Exp. Med. 1998;188:2019–2031. doi: 10.1084/jem.188.11.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulendran B., Smith J. L., Caspary G., Brasel K., Pettit D., Maraskovsky E., Maliszewski E. Proc. Natl. Acad. Sci. U. S. A. 1999;96:1036–1041. doi: 10.1073/pnas.96.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-Lopez R., De Smedt T., Michel P., Godfroid J., Pajak B., Heirman C., Thielemans K., Leo O., Urbain J., Moser M. J. Exp. Med. 1999;189:587–592. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klechesvsky E., Morita R., Liu M., Cao Y., Coquery S., Thompson-Snipes L., Briere F., Chaussabel D., Zurawski G., Palucka A. K., Reier Y., Banchereau J., Ueno H. Immunity. 2008;29:497–510. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vroman H., Hendriks R. W., Kool M. Front. Immunol. 2017;8:941. doi: 10.3389/fimmu.2017.00941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ostiani C. F., Sero G., Bracci A., Montagnoli C., Spreca A., Menacci A., Ricciardi-Castagnoli P., Romani L. J. Exp. Med. 2000;191:1661–1674. doi: 10.1084/jem.191.10.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan M., Narnett M. M., Houston K. M., Patel V., Harnett W., Rigley K. P. J. Immunol. 2000;164:6453–6400. doi: 10.4049/jimmunol.164.12.6453. [DOI] [PubMed] [Google Scholar]
- Caron G., Delnest Y., Roelandts E., Duez C., Bonnefoy J. Y., Pestel J., Jeannin P. J. Immunol. 2001;167:3682–3686. doi: 10.4049/jimmunol.167.7.3682. [DOI] [PubMed] [Google Scholar]
- De Jong E. C., Viera P. L., Kalinski P., Schultemaker J. H., Tanaka Y., Wierenga E. A., Yazdanbakhsh M., Kapsenberg M. L. J. Immunol. 2002;168:1704–1709. doi: 10.4049/jimmunol.168.4.1704. [DOI] [PubMed] [Google Scholar]
- Edwards A. D., Manickasingham S. P., Sporri R., Diebold S. S., Schulz O., Sher A., Kaisho T., Akira S., Resi e Sousa C. J. Immunol. 2002;169:3652–3660. doi: 10.4049/jimmunol.169.7.3652. [DOI] [PubMed] [Google Scholar]
- Soumelis V., Reche P., Kanzler H., Yuan W., Edward G., Homey B., Gillet M., Ho S., Antonenko S., Lauerma A., Smith K., Gorman D., Zurawski S., Abrams J., Menon S., McClanahan T., de Waal-Malefyt R. D., Bazan R. D., Kastelein R. A., Liu Y. J. Nat. Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- Tang H., Cao W., Kasturi S. P., Ravindran R., Nakaya H. I., Kundu K., Murthy N., Kepler T. B., Malissen B., Pulendran B. Nat. Immunol. 2010;11:608–617. doi: 10.1038/ni.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell B. D., Kitajima M., Larson R. P., Stoklasek T. A., Dang K., Sakamoto K., Wagner K.-U., Kaplan D. H., Reizis B., Hennighausen L., Ziegler S. F. Nat. Immunol. 2013;14:364–371. doi: 10.1038/ni.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durai V., Murphy K. M. Immunity. 2016;45:719–736. doi: 10.1016/j.immuni.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbarth S. C., Piggott D. A., Bottomly K. Curr. Opin. Immunol. 2003;15:620–626. doi: 10.1016/j.coi.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Ghaemmaghami A. M., Gough L., Sewell H. F., Shakib F. Clin. Exp. Allergy. 2002;32:1468–1475. doi: 10.1046/j.1365-2745.2002.01504.x. [DOI] [PubMed] [Google Scholar]
- Traidl-Hoffmann C., Mariani V., Hochrein H., Karg K., Wagner H., Ring J., Mueller M. J., Jakob T., Behrendt H. J. Exp. Med. 2005;201:627–636. doi: 10.1084/jem.20041065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulendran B., Tang H., Manicassamy S. Nat. Immunol. 2010;11:647–655. doi: 10.1038/ni.1894. [DOI] [PubMed] [Google Scholar]
- Cumberbatch M., Clelland K., Dearman R. J., Kimber I. J. Immunol. 2005;175:43–50. doi: 10.4049/jimmunol.175.1.43. [DOI] [PubMed] [Google Scholar]
- Langenkamp A., Messi M., Lanzavecchia A., Sallusto F. Nat. Immunol. 2000;1:311–316. doi: 10.1038/79758. [DOI] [PubMed] [Google Scholar]
- Cumberbatch M., Gould S. J., Peters S. W., Basketter D. A., Dearman R. J., Kimber I. J. Invest. Dermatol. 1992;9:107S–108S. doi: 10.1111/1523-1747.ep12669992. [DOI] [PubMed] [Google Scholar]
- Esser P. R., Kimber I., Martin S. F. EXS. 2014;104:101–114. doi: 10.1007/978-3-0348-0726-5_8. [DOI] [PubMed] [Google Scholar]
- Oakes T., Popple A. L., Williams J., Best K., Heather J. M., Ismail M., Maxwell G., Gellatly N., Dearman R. J., Kimber I., Chain B. Front. Immunol. 2017;8:1–11. doi: 10.3389/fimmu.2017.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabbe S., Schwarz T. Immunol. Today. 1998;19:37–44. doi: 10.1016/s0167-5699(97)01186-9. [DOI] [PubMed] [Google Scholar]
- Cavani A., Albanesi C., Traidl C., Sebastiani S., Girolomoni G. Trends Immunol. 2001;22:118–120. doi: 10.1016/s1471-4906(00)01815-9. [DOI] [PubMed] [Google Scholar]
- Saint-Mezard P., Berard F., Dubois B., Kaiserlian D., Nicolas J. F. Eur. J. Dermatol. 2004;14:131–138. [PubMed] [Google Scholar]
- Peiser M. Clin. Dev. Immunol. 2013 doi: 10.1155/2013/261037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conolly R.B, Ankley G.T, Cheng W., Mayo M.L, Miller D.H, Perkins E.J, Villeneuve D.L, Watanabe K.H. Environ. Sci. Technol. 2017;51(8):4661–4672. doi: 10.1021/acs.est.6b06230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumberbatch M., Scott R. C., Basketter D. A., Scholes E. W., Hilton J., Dearman R. J., Kimber I. Toxicology. 1993;77:181–191. doi: 10.1016/0300-483x(93)90148-l. [DOI] [PubMed] [Google Scholar]
- Friedman P. S., Haddadeen C., Lai C., Healy E. Contact Dermatitis. 2017;76:235–255. doi: 10.1111/cod.12697. [DOI] [PubMed] [Google Scholar]
- Mose K. F., Andersen F., Skov L., Ropke M. A., Litman T., Friedmann P. S., Andersen K. E. Br. J. Dermatol. 2017;176:1095–1097. doi: 10.1111/bjd.14949. [DOI] [PubMed] [Google Scholar]
- Vocanson M., Hennino A., Rozieres A., Poyet G., Nicolas J. F. Allergy. 2009;64:1699–1714. doi: 10.1111/j.1398-9995.2009.02082.x. [DOI] [PubMed] [Google Scholar]
- Moggs J. G., Terranova R., Kammuller M. E., Chibout S. D., Chapman V., Dearman R. J., Kimber I. Toxicol. Sci. 2012;130:60–69. doi: 10.1093/toxsci/kfs207. [DOI] [PubMed] [Google Scholar]
- Adenuga D., Woolhiser M. R., Gollapudi B. B., Boverhof D. R. Toxicol. Sci. 2012;126:413–425. doi: 10.1093/toxsci/kfs071. [DOI] [PubMed] [Google Scholar]
- Chapman V. L., Zollinger T., Terranova R., Moggs J., Kimber I., Dearman R. J. Toxicol. Sci. 2014;139:350–361. doi: 10.1093/toxsci/kfu047. [DOI] [PubMed] [Google Scholar]