 This paper includes new information on the effect of endosulfan and/or its commercial formulations on some aspects of sperm function.
This paper includes new information on the effect of endosulfan and/or its commercial formulations on some aspects of sperm function.
Abstract
Endosulfan is an organochloride insecticide extensively used in several countries to protect crops from pests. As several studies indicate that endosulfan can affect human and animal development, the aim of this study was to analyse whether sperm parameters and the process of chromatin decondensation could be altered by endosulfan in mice sperm. Spermatozoa from cauda epididymis were obtained from mature male mice and incubated in the presence of two commercial formulations (CFs) of endosulfan (Master® and Zebra Ciagro®) or the active ingredient (AI) alone. A significant decrease in the percentage motility and viability of spermatozoa with respect to controls was found. In vitro decondensation was performed in the presence of glutathione and heparin. Spermatozoa incubated with the AI, endosulfan Master® and endosulfan Zebra Ciagro® showed an increase in chromatin decondensation. In addition, the TUNEL assay showed that DNA fragmentation was significantly higher when sperm were incubated with either one of the CFs when compared to the AI or controls. The ultrastructure analysis of sperm cells showed evident changes in the structure of the plasma and acrosome membranes of sperm incubated with endosulfan AI or the CFs. These results suggest that endosulfan can affect sperm integrity and in vitro chromatin decondensation as well as DNA fragmentation.
Introduction
Organochlorine pesticides top the list of pesticides which have proved detrimental to human health, most likely due to their high transport potential and their physicochemical properties.1 Endosulfan (6,7,8,9,10,10-hexachloro-1,5,5a,6,9,9a-hexahydro-6,9-ethylenedioxy bridge-2,4,3-hept-3-benzo sulfur dioxide-oxide) is a highly toxic broad-spectrum organochloride insecticide and miticide.2 Since its introduction in 1954 by Farbwerke Hoechst, Germany,3 endosulfan has become the agrochemical and pest control agent of choice.4 Because of its properties, endosulfan is applied to a wide number of crop types (cotton, cereals, fruit trees, tea and coffee). Due to its semi-volatility and relative persistence, it appears as a ubiquitous contaminant found in many environmental compartments. Although its use seems to have declined in the northern hemisphere, it has increased in the southern hemisphere (e.g., South America, Australia) over the last few years.4,5 Endosulfan is probably one of the most hazardous organochlorine pesticides, and was included in the list of Persistent Organic Chemicals by the Stockholm Convention in 2011 (United Nations C.N.703.2011.TREATIES-8). Even though it has almost been phased out worldwide, endosulfan is still widely used in several countries, with India and China as the largest consumers, with the presence of endosulfan being reported in the last years in different regions of the planet.6–9
It has been extensively shown that endosulfan could be responsible for several reproductive disorders. The results from experiments performed in rats suggested that endosulfan could affect sperm production, testis size and Sertoli cell function, as well as inhibit spermatogenesis and steroidogenesis.10–14 Other authors have suggested that endosulfan might produce lipid peroxidation and oxidative damage in rat testis, thus impairing reproductive function. Decreased sperm count and motility, lower circulating testosterone levels, reduced expression of the androgen receptor mRNA in testes and increased plasma LH have also been reported in male mice administered endosulfan.15 Endosulfan also induced apoptosis, oxidative stress and mitochondrial dysfunction in spermatogenic cells in mice testis,1,16 thus explaining why vitamins E and C have been used as antioxidants to reverse the effects of endosulfan.17 Contrarily, it was found that endosulfan had no effects on daily sperm production, epididymal sperm count, sperm morphology, and male fertility. Although there are some discrepancies among the published data, most of the studies indicate that endosulfan can cause reproductive toxicity.15 Despite this, little is known about the mechanism of endosulfan action on reproductive functions in mice.2
Most studies regarding the effects of pesticides on non-target species focus on either the active ingredient or a commercial formulation without analysing the possible differences between them. Coadjuvants can contribute to the overall toxicity of the formulation and may not have the same effects as those caused by the active ingredient. Both antagonistic and synergistic effects between the active ingredient and other components present in commercial formulations have been reported in terms of both genotoxicity and reproductive toxicity.18 Considering the relatively scarce information that is available, the aim of this study was to evaluate the effects of the active ingredient and two of the currently used commercial formulations of endosulfan on mice sperm viability and motility, with special interest in sperm chromatin decondensation, as the main event occurring after sperm penetration of the oocyte prior to syngamy, and DNA fragmentation.
Materials and methods
Chemicals
Endosulfan active ingredient (AI; α : β isomer ratio 70 : 30; chromatographic purity 95%) was donated by SENASA (Buenos Aires, Argentina). Commercial formulations of ES used (35% AI): Zebra Ciagro® (Ciagro SA, Argentina) and Master® (Cheminova, Argentina) were purchased from local suppliers. All chemicals and reagents used were obtained from Sigma Chemical Co. (St Louis, MO), unless otherwise stated.
Animals
Hybrid F1 (C57BL/6xBalb/C) mature (10–12-week-old) male mice were used in the present study. Animals were obtained from the animal facility center of the Instituto de Biología y Medicina Experimental (IByME – CONICET), Buenos Aires, Argentina. They were maintained at 23 °C with a 12 : 12 h light/dark cycle. All mice were provided food and drinking water ad libitum. Animal experimental procedures were performed in strict accordance with the Guide for Care and Use of Laboratory Animals approved by the National Institutes of Health, reviewed and approved by the Ethical Committee of IByME (Protocol CE/003-1/2011).
Sperm collection
The mice were sacrificed by cervical dislocation and epididymis from each mouse was removed and transferred to a dish containing 300 μL of In Vitro Fertilization Medium (IVFM: 99.3 mM NaCl, 2.70 mM KCl, 0.50 mM MgSO4·6H2O, 1 mg mL–1 glucose, 0.31 mM Na2HPO4·2H2O, 1.80 mM CaCl2·H2O). Medium pH was adjusted to 7.3 with 25.07 mM NaOH, and 0.0055 mg sodium pyruvate and 0.35 mL l-Na-lactate (60% syrup) were added to a final volume of 100 mL. Spermatozoa were recovered by cutting the isolated caudae into fragments and allowing mature sperm to ‘swim out’ into IVFM supplemented with 0.3% bovine serum albumin (BSA) (IVFM ±3B) at 37 °C for 10 min. Tissue fragments were removed and the remaining sperm suspension was incubated under capacitating conditions for 1 h at a concentration of 8–10 × 106 spermatozoa per mL.19
Viability and motility rate of sperm
To evaluate the effect of the pesticide on the sperm viability and motility rate, after capacitation, mice sperm were exposed to different concentrations of the AI or the two commercial formulations of endosulfan (0.0001 μM, 0.01 μM, 1 μM, 100 μM) for 15 minutes at 37 °C. Serial dilutions were made in dimethylsulfoxide (DMSO) so the negative control of the experiments consisted of DMSO alone in the incubation in IVFM. The sperm motility rate was evaluated on a 10 μL aliquot of the sperm suspension, placed on a pre-heated slide. Samples were observed using a phase contrast microscope (Nikon Eclipse E200). To evaluate sperm viability, a volume of 5 μL of 0.05% Eosin Y was added to an equal volume of sperm suspension and slides were observed by using a light microscope. Live sperm remained colourless following staining, whereas dead sperm showed pink or red coloration. At least a total of 200 sperm cells were counted in each case and the percentage of viability and motility was determined.
In vitro sperm nuclear decondensation assay
To evaluate the effect of endosulfan on sperm chromatin decondensation, capacitated sperm were preincubated for 15 minutes in the presence of increasing concentrations of the AI or the two CFs of endosulfan (0.0001 μM, 0.01 μM, 1 μM, 100 μM) in DMSO. After the preincubation with the pesticide, sperm were decondensed in the presence of 10 mM GSH and 4.6 mM heparin (H) (13 500 Da, 170 IU mg–1) in IVFM ±3B at 37 °C.19 Controls consisted of parallel incubations with H or GSH alone (data not shown). After 30 minutes of incubation, a 20 mL aliquot was fixed with an equal volume of 2.5% glutaraldehyde in phosphate-buffered saline (PBS). Two 5 mL aliquots were transferred onto a microscope slide, and the nuclear status was assessed under a phase contrast microscope (Nikon Eclipse E200 microscope). Sperm were classified20 as unchanged (U), moderately decondensed (M) or grossly decondensed (G). At least 200 sperm cells were evaluated per aliquot. The total decondensation percentage achieved was determined as the sum of M and G (%(M + G)). The results were analyzed and graphically represented using the GraphPad Prism 7 software (GraphPad Software, Inc.).
DNA fragmentation (terminal deoxynucleotidyl transferase dUTP nick end labelling; TUNEL) assay
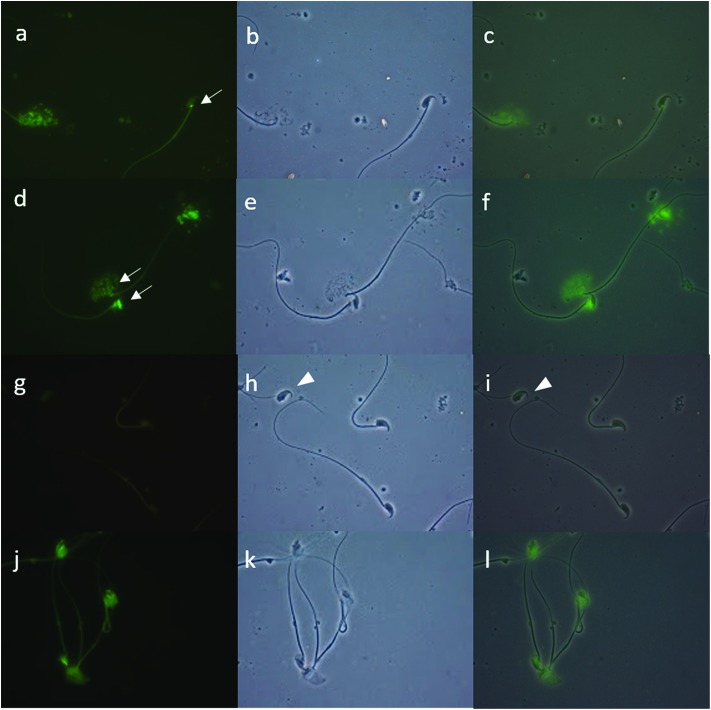
To evaluate the possible effect of endosulfan on DNA fragmentation, capacitated sperm were preincubated in the presence of endosulfan AI or both CFs for 15 minutes; only the 100 μM concentration was used based on the previously observed effects. After this, sperm from each condition were decondensed and DNA fragmentation was analysed performing the TUNEL assay. For this experiment, a lower concentration of H was used in order to obtain a readable fluorescence signal. All fractions of mice sperm were fixed in 2% formaldehyde (Sigma) in 1× phosphate-buffered saline solution (PBS; pH 7.4; Gibco) for at least 1 hour. Each sample was placed into one well of a multiwell (4 mm diameter) Teflon-printed slide (Electron Microscopy Sciences) and allowed to settle. After 1 hour of drying, each well was washed with 1× PBS (three times, 5 minutes each), and the cells were permeabilized with cold methanol (Merck). Before incubation with the TUNEL solution, each well was washed again with 1× PBS. For each sample, one extra well was incubated with DNase (1 U mL–1; Sigma) for 30 minutes at 37 °C as a positive control, and in another well the TUNEL enzyme solution was omitted as a negative control. Then, all samples were incubated in TUNEL solution (Roche) for 1 hour at 37 °C. Finally, all samples were washed with 1× PBS (three times, 5 minutes each), and mounted in Vectashield H-1000 medium (Vector Laboratories). A total of 500 spermatozoa were counted by fluorescence microscopy for each fraction. TUNEL staining was evaluated using a green filter (fluorescein isothiocyanate, 488 nm). Fig. 1 shows light and dark field images of the negative (omission of enzyme solution; Fig. 1A, A′ and A′′) and positive controls (incubated with DNase so that all spermatozoa had fluorescent green heads; Fig. 1B, B′ and B′′) of the TUNEL assay. Intact sperm with intense fluorescence were considered as positive (Fig. 1C and D, arrow). Only whole spermatozoa were considered for percentage estimates; heads without tails were not considered (Fig. 1A′ and A′′, arrowhead).21
Fig. 1. Terminal deoxynucleotide transferase-mediated dUTP nick-end labelling (TUNEL) assay. DNA fragmentation was evaluated by performing the TUNEL assay in decondensed mice sperm that were previously preincubated with endosulfan AI or the control conditions. A: The negative control was performed by omitting the “enzyme” solution [under green fluorescence (A), phase contrast (A′), and merge (A′′)]. B: A DNase-treated sample was used as the positive control [green fluorescence, 100% of positive cells (B), phase contrast (B′), and merge (B′′)]. C: Intact sperm with intense fluorescence considered as positive (arrow). D: Moderately (M) and grossly (G) decondensed sperm with positive staining (arrows). Only complete spermatozoa were considered in the evaluation of TUNEL; sperm heads without tails were not counted for this purpose (A′, A′′, arrowhead).
Oxidative stress evaluation
In order to evaluate alterations in the antioxidant system (glutathione-S-transferase and superoxide dismutase activities, glutathione content) and/or oxidative damage to macromolecules (lipid peroxidation) caused by endosulfan, previously capacitated sperm (7.5 × 106 sperm per mL) were incubated in the presence of the AI or the CFs of endosulfan (as stated before, only the 100 μM concentration was used, consistent with the observed effect) for 15 minutes at 37 °C. Sperm were frozen at 0 °C, sonicated three times for 5 seconds at 40 V (allowing for sperm membrane breakage without the loss of cytoplasm content) and aliquots were centrifuged at 12 000 rpm for 15 minutes. The results are expressed as a percentage of the control (considered as 100%).
Glutathione-S-transferase
Glutathione-S-transferase (EC 2.5.1.18) activity was measured by using the Habig technique.22 Briefly, the standard assay mixture contained the enzymatic sample, 100 mM GSH solution and 100 mM 1-chloro-2,4-dinitrobenzene (CDNB) in ethanol, to a final volume of 0.8 mL with 100 mM phosphate buffer (pH 6.5). After adding CDNB, the change in absorbance at 340 nm was recorded for 120 seconds. One GST unit was defined as the amount of enzyme that catalyses the formation of 1 mmol of GS-DNB per minute at 25 °C; the results are expressed as a percentage of the control.23
Superoxide dismutase
Superoxide dismutase (EC 1.15.1.1) activity was measured using a modified Beauchamp and Fridovich procedure in a microplate.24 The standard assay mixture contained the enzymatic sample, 0.1 mM EDTA, 13 mM dl-methionine, 75 mM nitroblue tetrazolium, and 2 mM riboflavin, to a final volume of 0.3 mL with 50 mM phosphate buffer (pH 7.9). Samples were exposed for 5 minutes to intense cool white light. One SOD unit was defined as the amount of enzyme necessary to cause a 50% inhibition of the reaction rate. Samples were measured at 560 nm in a BIO-RAD Benchmark microplate reader (BIO-RAD Laboratories), and the results are expressed as a percentage of the control.23
Glutathione equivalent content
Glutathione (GSH) levels were measured in mice sperm cells following the Anderson procedure,25 with some modifications. Briefly, samples were deproteinised with 10% sulphosalicylic acid in a 2 : 1 ratio. Samples were centrifuged for 10 minutes at 7000–10 000 rpm and the supernatant was used to perform the determinations. Deproteinized samples were incubated with 6 mM dinitrothiocyanobenzene (DNTB) in 0.143 mM sodium phosphate buffer (pH 7.5 containing 6.3 mM EDTA) for 30 minutes at room temperature. At the same time, different aliquots of a standard solution of 1 μM of GSH (0, 5, 10, 15, 20 μL) were incubated to a final volume of 250 μL to obtain the standard curve. The samples and standard curve were measured at 412 nm in a Benchmark microplate reader (BIO-RAD Laboratories). The results are expressed as a percentage of the control.23
Lipid peroxidation
Lipid peroxidation was measured through thiobarbituric acid reactive substances (TBARs). MDA contents, the final product of polyunsaturated fatty acid oxidation, were measured as a lipid peroxidation indicator. Sample aliquots of 175 μL were mixed with 1 mL MDA reactive substance. The preparation of the TBA reactive substance was done by dissolving 0.375% p/v MDA in an acid solution of 15% TCA and 0.25 N hydrochloric acid by shaking at a temperature no higher than 60 °C. Once the solution was homogenized and was at room temperature, BHT in ethanol was added to a final concentration of 0.68 mM. To prepare the MDA reactive substance, 0.37 g of TBA, 2 mL of HCl, 30 mL of 50% TCA and 680 mL of 100 mM BHT were added, and the final volume was attained with distilled water. Finally, the samples were heated for 45 minutes and centrifuged. The absorbance of the supernatant was determined at 535 nm and the results are expressed as moles of MDA per mL of the sample.
Transmission electron microscopy (TEM)
To analyze the effect of endosulfan on the ultrastructure of murine spermatozoa by TEM, capacitated sperm were incubated in the presence of endosulfan AI or the CFs (100 μM) at 37 °C in 5% CO2/95% air. Samples were diluted 1 : 4 in 0.1 mol L–1 PBS (pH 7.4) at room temperature, and centrifuged at 2000 rpm for 10 min. Pellets (5 × 106 spermatozoa) were fixed using 3% glutaraldehyde in PBS, at 4 °C. After 18 h, the fixed samples were treated with osmium tetroxide (1.3%), dehydrated with increasing concentrations of ice-cold ethanol and washed with propylene oxide at room temperature. Pellets were embedded in Eponate 12 – Araldite (Pelco), sectioned in an ultramicrotome and analyzed with a Zeiss EM 109 T electron microscope after double staining with uranyl acetate and lead citrate.
Statistical analysis
All quantitative data are presented as mean ± SEM. One-way analysis of variance (ANOVA) followed by Tukey's test was performed to determine the statistical significance between different groups using GraphPad software (GraphPad Software, Inc.) and p < 0.05 was considered significant. The analysis of correlation between the TUNEL assay and in vitro sperm chromatin decondensation assay was determined by using the Spearman Rank correlation test.
Results
Effect of endosulfan on mice sperm viability and motility
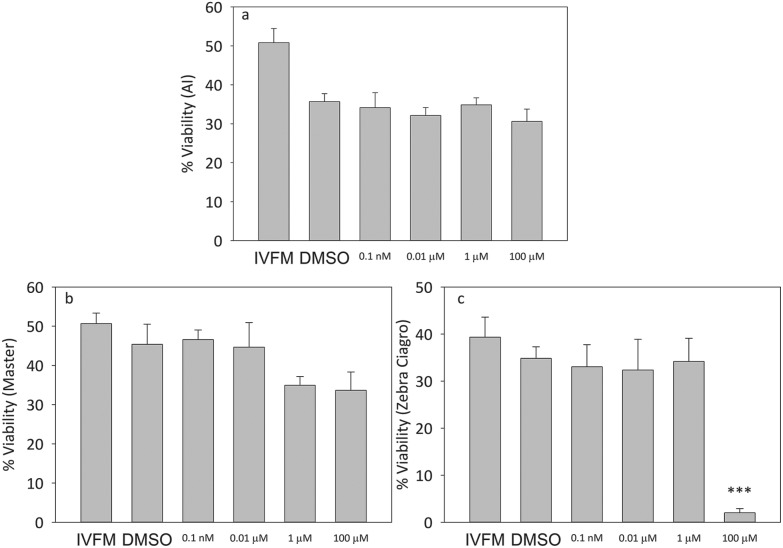
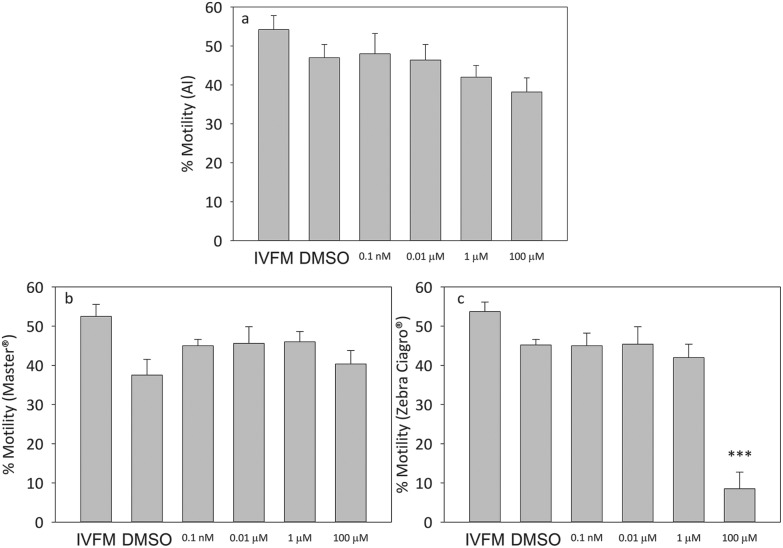
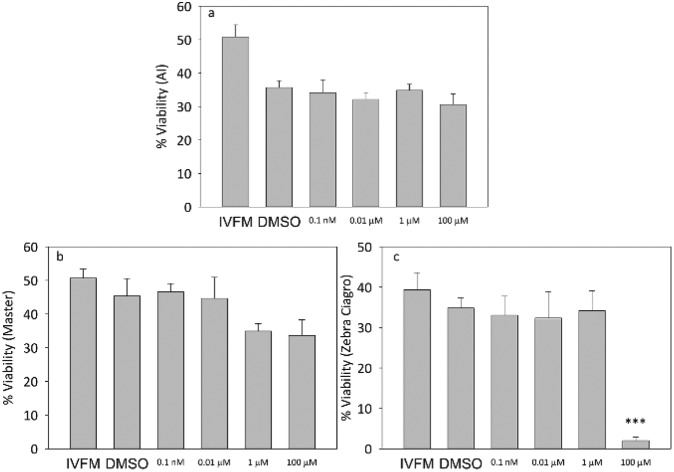
When capacitated mice sperm were incubated in the presence of increasing concentrations of the pesticide, there were no significant differences in viability at any of the doses tested for the AI or Master® CF (Fig. 2). When sperm were incubated in the presence of the highest concentration of endosulfan Zebra Ciagro® CF, the percentage of viability was significantly lower than the DMSO control (p < 0.0001, Fig. 2). No significant differences were found in motility when sperm were incubated with endosulfan AI or Master® when compared to the DMSO control, but there was a significant decrease in the percentage of motility when sperm were incubated with endosulfan Zebra Ciagro® (Fig. 3).
Fig. 2. Effect of endosulfan on sperm viability. Percentage of viable capacitated sperm after treatment with different concentrations of endosulfan (0.0001 μM, 0.01 μM, 1 μM, 100 μM). A: AI, B: endosulfan Master®, C: endosulfan Zebra Ciagro®. IVFM (In Vitro Fertilization Medium) or the DMSO control. The results are expressed as mean ± SEM (Zebra Ciagro®: n = 4, ***p < 0.0001).
Fig. 3. Effect of endosulfan on sperm motility. Percentage of motile capacitated sperm after treatment with different concentrations of endosulfan (0.0001 μM, 0.01 μM, 1 μM, 100 μM). A: AI, B: endosulfan Master®, C: endosulfan Zebra Ciagro®. IVFM (In Vitro Fertilization Medium) or the DMSO control. The results are expressed as mean ± SEM (Zebra Ciagro®: n = 4, ***p < 0.0001).
Effect of endosulfan on mice sperm chromatin decondensation
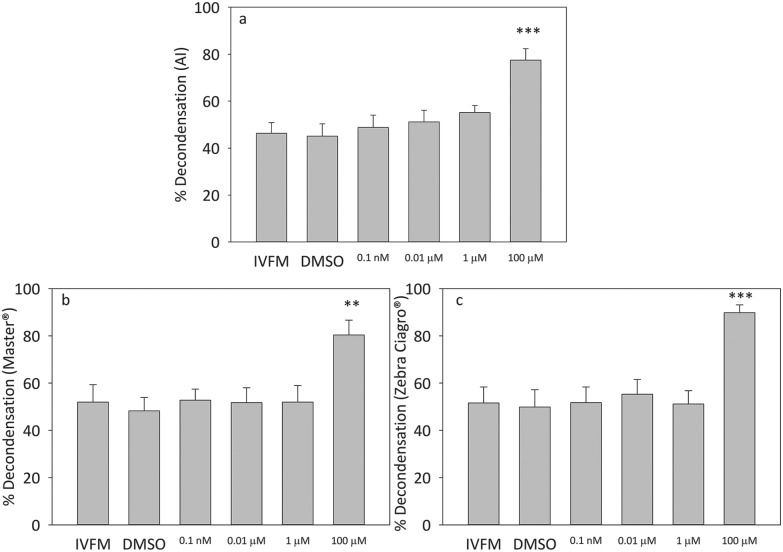
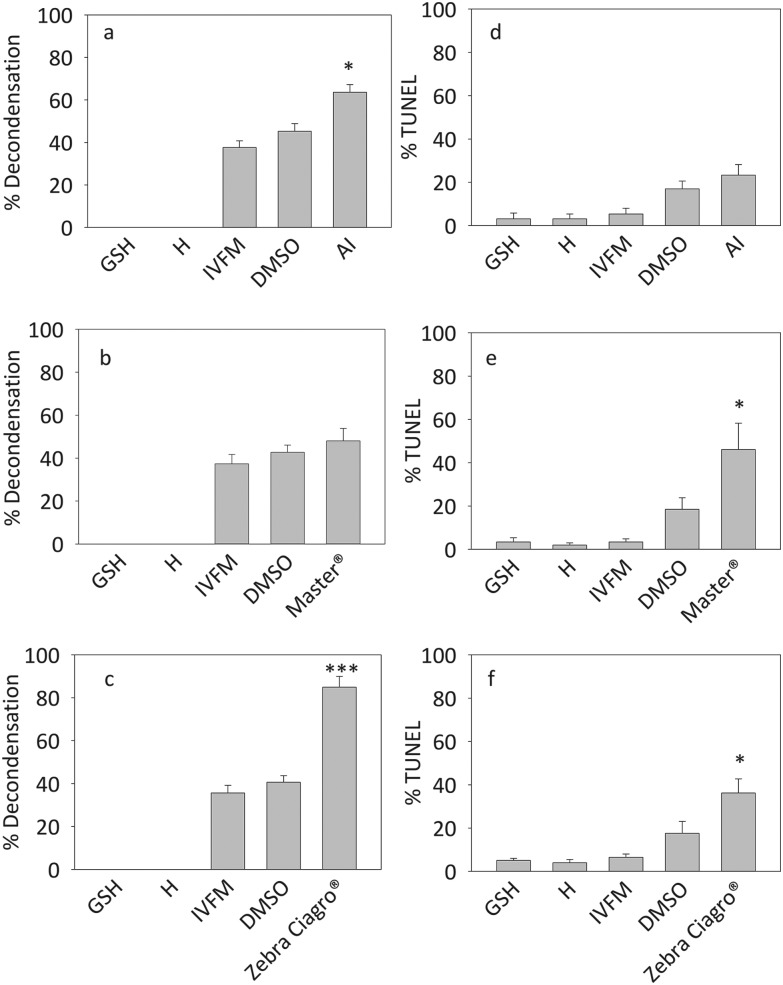
The exposure of sperm to the highest concentration of pesticide, either the AI or the CFs Master® and Zebra Ciagro®, significantly increased sperm in vitro chromatin decondensation (p < 0.005, p < 0.0001) (Fig. 4). No significant differences were found with the other concentrations tested. Consequently, 100 μM of endosulfan was used in all experiments hereafter.
Fig. 4. Effects of endosulfan on sperm chromatin decondensation. Percentage of decondensed capacitated sperm after treatment with different concentrations of endosulfan (100 pM, 10 nM, 1 μM, 100 μM) for 15 minutes. A: AI, B: endosulfan Master®, C: endosulfan Zebra Ciagro®. Controls consisted of capacitated sperm incubated in the presence of IVFM or DMSO alone. The results are expressed as mean ± SEM (n = 5, Master®: **p < 0.001, Zebra Ciagro®: ***p < 0.0001).
Effect of endosulfan on DNA fragmentation
The levels of in vitro decondensation with GSH (10 mM) + H (2.3 μM) before performing the TUNEL assay were significantly higher when sperm were preincubated with endosulfan AI or Zebra Ciagro®, but it was not significantly different from the control when sperm were preincubated with Master® (*p < 0.05, ***p < 0.0001, Fig. 5A– C). The average TUNEL levels significantly increased when mice sperm were decondensed after being exposed to the highest concentration of endosulfan Master® and Zebra Ciagro® (p < 0.05, Fig. 5–F).
Fig. 5. Effect of endosulfan on sperm DNA fragmentation. Mice sperm previously capacitated were preincubated with endosulfan AI or CFs and decondensed in the presence of H (2.3 μM) and GSH (10 mM) (A, B, C). Sperm DNA fragmentation was evaluated performing the TUNEL assay (D, E, F). A and D: mice sperm preincubated with endosulfan AI. B and E: sperm preincubated with endosulfan Master®. C and F: sperm preincubated with endosulfan Zebra Ciagro®. H: heparin, GSH: glutathione. The results are expressed as mean ± SEM (AI: n = 5, Master®: n = 4, Zebra Ciagro®: n = 5; *p < 0.05, ***p ≤ 0.0001).
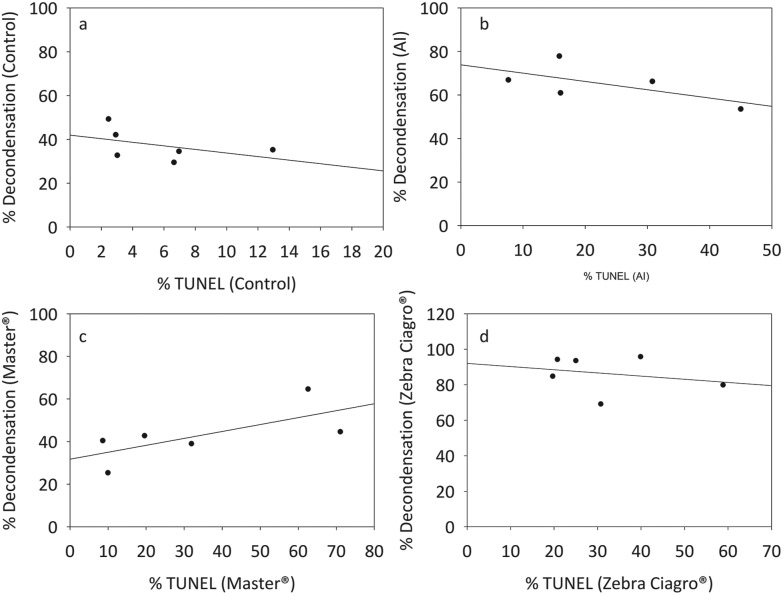
To understand whether DNA fragmentation levels were a possible consequence of the in vitro chromatin decondensation or simply related to the effects of the pesticide, the correlation between these two parameters was evaluated. No significant correlation was found for any of the conditions tested (Fig. 6).
Fig. 6. Correlation between in vitro sperm chromatin decondensation and DNA fragmentation. Mice sperm previously capacitated were preincubated with endosulfan or IVFM (control) and decondensed in the presence of GSH (10 mM) + H (2.3 μM). After that, the TUNEL assay was performed on each sample and the correlation was evaluated. A: Capacitated sperm incubated in the presence of IVFM and decondensed with GSH + H (r2 = –0.4857, p = 0.3556). B: Capacitated sperm incubated in the presence of endosulfan AI and decondensed with GSH + H (r2 = –0.5508, p = 0.2972). C: Capacitated sperm incubated in the presence of endosulfan Master® and decondensed with GSH + H (r2 = 0.6571, p = 0.1750). D: Capacitated sperm incubated in the presence of endosulfan Zebra Ciagro® and decondensed with GSH + H (r2 = –0.1160, p = 0.8028) (Spearman correlation, NS, n = 6).
Effect of endosulfan on oxidative stress
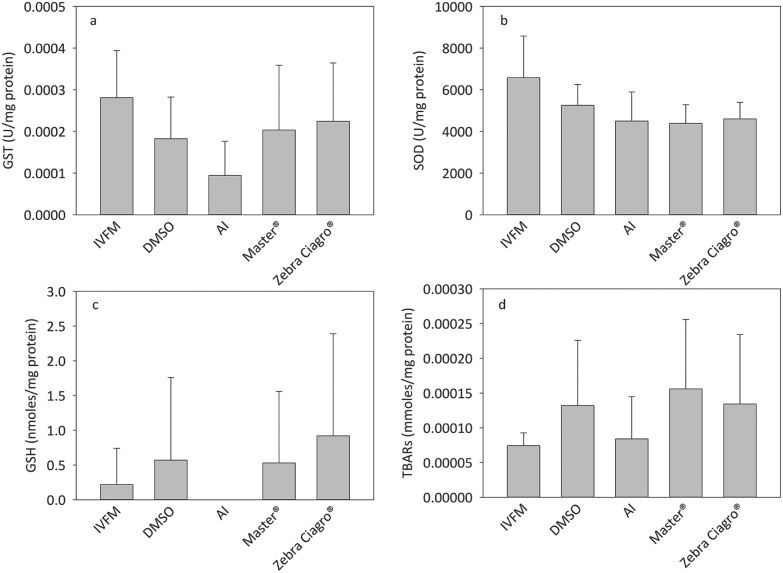
No significant differences were found between the different conditions evaluated for the activities of GST and SOD, as well as for the content of GSH and TBARs (Fig. 7).
Fig. 7. Effect of endosulfan on oxidative stress. Capacitated sperm were incubated in the presence of IVFM, DMSO or endosulfan for 15 minutes. Samples were frozen, thawed and sonicated before performing each analysis. A: GSH activity, B: SOD activity, C: GSH content, D: TBARs’ content.
Ultrastructure of mice spermatozoa exposed to endosulfan
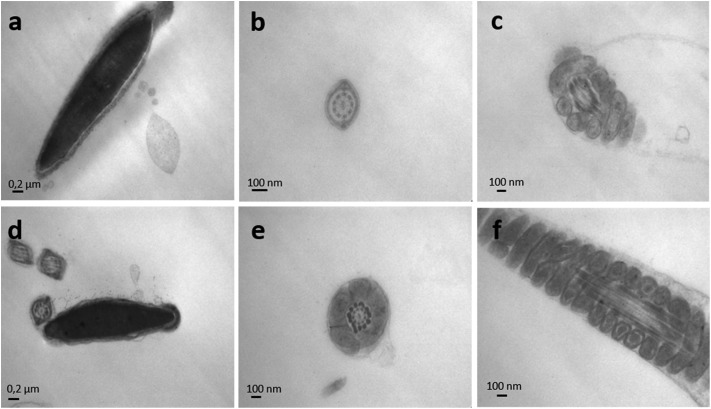
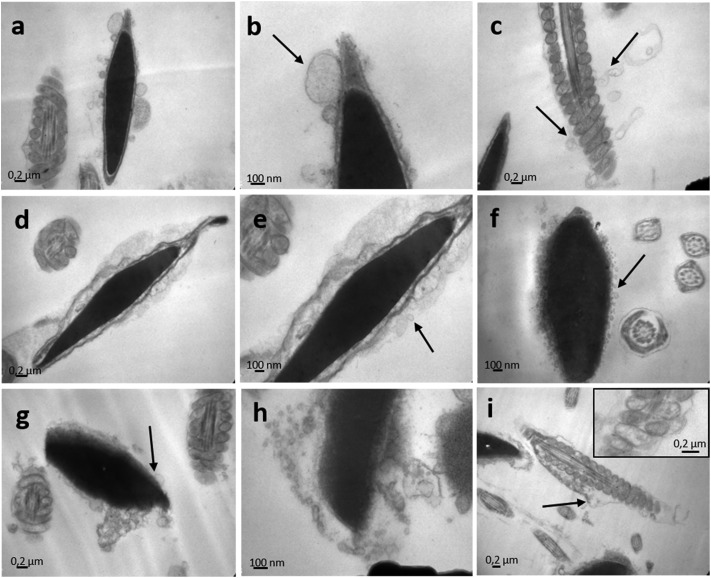
The effect of endosulfan on the ultrastructure of murine spermatozoa was analysed by TEM (Fig. 8 and 9). Following incubation with IVFM, fully condensed sperm heads were distinguishable, showing an adequate nuclear envelope conformation. Acrosome and nuclear membranes were intact in control samples (Fig. 8a). Mitochondrial membranes of the middle piece had a normal structure, as well as the flagellum membranes of the principal piece (Fig. 8b and c). The same morphology was observed when sperm were incubated with DMSO (Fig. 9a–c). Contrarily, acrosome and nuclear membrane disarray became apparent following incubation with endosulfan AI (Fig. 9a–c), Master® (Fig. 9d–f) or Zebra Ciagro® (Fig. 9g–i). In addition, the presence of vacuoles in the plasma membrane was observed. The mitochondrial content often appeared clear as if empty (Fig. 9i and the inset). Chromatin morphology in treated spermatozoa appeared to be as compact as in control samples.
Fig. 8. Transmission electron microscopy of mice spermatozoa. Nuclei of mice sperm incubated in the presence of IVFM (a) or DMSO (d) were uniformly electron-dense and fully condensed, with an intact nuclear envelope and outer membranes. The microphotographs depict well defined mitochondria and flagellum membranes in sperm with IVFM (b, c) or DMSO (e, f).
Fig. 9. Transmission electron microscopy of endosulfan exposed mice spermatozoa. The effect of endosulfan AI (a, b, c), endosulfan Master® (d, e, f) and endosulfan Zebra Ciagro® (g, h, i) was evaluated on the ultrastructure of mice spermatozoa. Following incubation with endosulfan, totally disarranged membranes were observed, including acrosome and flagellum membranes. In addition, the presence of vacuoles could be seen on the membrane of the sperm head and the flagellum (black arrows). Mitochondrial content often appeared clear as if empty (i and inset).
Discussion
When using insecticides or herbicides, the active ingredient is frequently mixed with coadjuvants, mostly undeclared, that enhance its transport across the cell membranes, because of its physicochemical characteristics. Thus, the reported effects are many times due to the combination of the coadjuvants plus the AI. Coadjuvants can contribute to the overall toxicity and may not have the same effects caused by the AI.18 Consequently, the results obtained in the laboratory when AI is used do not always reflect the effects of the commercial formulations that are used in agriculture. In the current study, mice sperm were incubated in vitro in the presence of endosulfan AI or CFs to elucidate their mechanism of action and compare the effect of the pesticide as a pure active ingredient with that of endosulfan as part of a CF. The concentrations tested in the present study were chosen in agreement with those used in other studies as shown in the scientific literature.26,27
Reported evidence show a deleterious effect of endosulfan on the mammalian sperm function,2,17,28,29 which is consistent with the drastically diminished viability and motility observed in this study when mice sperm were incubated in vitro in the presence of endosulfan Zebra Ciagro®. Sperm motility mainly depends on two factors: the generation of energy by mitochondria and the breakage of disulphide bonds in the proteins of the sperm flagellum. The study of Cabrillana et al.30 clearly suggests that during epididymal maturation disulphide bonds are modified not only in chromatin condensation but in the tail organelles. Sperm tails are responsible for the motility of sperm, and this movement requires not only structural integrity but also a normal energy supply. The damaged structure of the sperm tails, as well as the altered mitochondrial structure, could be the main reason for the decrease in sperm motility.2
It is widely known that estrogenic substances, produced because of the endogenous metabolism of androgens from male reproductive organs, or exposure to environmental toxins, could be related to ROS production and DNA fragmentation.31 Some researchers suggest that vitamins C and E successfully revert the reduction in sperm quantity and quality that endosulfan causes through oxidative stress and sperm DNA damage.2,32 Nevertheless, the results obtained in this study suggest that endosulfan does not produce oxidative stress under the evaluated conditions.
Very little is known about the action of endosulfan on chromatin decondensation. This event represents the first step towards syngamy after the spermatozoon penetrates the oocyte during fertilization and, thus, this process was used as a possible biosensor for the toxic effects of pesticides such as endosulfan. The results obtained in the present study showed that in vitro exposure of mice sperm to endosulfan increased sperm chromatin decondensation. The hydrophobicity of the molecule could be responsible for the deleterious effect on membrane permeability, thus allowing a better entry of the decondensing agents to the cells.
An increase in the in vitro chromatin decondensation could lead to greater exposure of DNA to endosulfan-generated ROS and, therefore, make it more susceptible to a direct action of endosulfan on DNA fragmentation, which apparently is not the case under the conditions of this study. Our results showed a significant increase in the percentage of TUNEL positive cells when sperm were incubated in the presence of endosulfan Master® and Zebra Ciagro ®, suggesting that other components of the CFs might be responsible for DNA fragmentation rather than the AI. Even when the TUNEL assay has been well standardized and described, Mitchell et al.33 reported that this method is not sensitive enough to analyse DNA fragmentation because of the high compaction of sperm chromatin preventing the access of TdT enzyme to cleaved DNA. Mitchell et al.33 showed that the preincubation of the sperm with DTT significantly increased the detected levels of damaged DNA. Considering these results, to analyse the effects of endosulfan on mice sperm chromatin, the assay was performed on previously decondensed sperm. Due to this variation made to the protocol, the correlation between sperm chromatin decondensation and DNA fragmentation was analysed. No correlation was found in any of the conditions evaluated, suggesting that DNA fragmentation is not increased due to chromatin decondensation, but because of the presence of endosulfan or endosulfan-generated ROS (in this study, the oxidative status was assessed using whole sperm cells rather than the isolated nuclei) that could be acting against a more susceptible chromatin. The increase in chromatin decondensation levels could have been the result of an increased entry of the decondensing agents through the plasma membrane affected by the pesticide. Endosulfan could directly damage sperm structures by oxidative stress, leading to a decrease in sperm quantity and quality, considering the role of endosulfan on ROS production because of the NADPH oxidase as well as the NOX oxidase family and the Akt/MAPK pathway.34 To further study the in vitro effect of endosulfan on the redox state of mice sperm, GSH and TBARs’ content as well as SOD and GST activity were determined in whole sperm cells. In the present study, the results showed that endosulfan had no effect on the systems related to the maintenance of the redox status of the cells, or on the regeneration of GSH and lipid peroxidation.
To further understand the mechanism of action of endosulfan, electron microscopy of mice sperm was performed. The results showed evident changes in the structure of the plasma and acrosome membranes of the sperm incubated with endosulfan AI or the CFs, which confirm our hypothesis that decondensing agents could more easily penetrate the cell through a more permeable membrane. Endosulfan exposure produced vacuoles in the plasma and acrosome membranes of the treated spermatozoa, leading to a higher susceptibility to chromatin decondensation. The pesticide affected the membranes of the flagellum and the lamellae of the mitochondria in the middle piece, which could explain the decrease in the percentage of sperm motility.
Conclusions
Taken together, these results indicate that endosulfan impairs the sperm viability, motility, chromatin decondensation and DNA fragmentation without modifying the redox status, most likely because of the accompanying substances in CFs rather than endosulfan itself. It is likely that all the results herein observed reflect the effects of the pesticide on the sperm plasma membrane, thus allowing the entry of decondensing agents through a more permeable membrane. As a result of a more permissive chromatin, the DNA could be more susceptible to fragmentation, with deleterious consequences on fertilization and embryo formation. These results open several possible avenues to consider for a more complete study on the effect of a pesticide that, although banned from use in agriculture for some years now, still represents a hazard for animal and human health due to acknowledge existing stocks as well as its persistence in the environment.
Conflicts of interest
There are no conflicts of interest to declare.
Acknowledgments
The authors wish to thank the generous donation of Fundación Honorio Bigand and Nicolás Gilio for his helpful assistance in handling the animals.
References
- Xu Y., Wang N., Shi Z. X., Li Y. B., Zhou X. Q., Sun Z. W. Toxicol. Ind. Health. 2016;32:1550–1563. doi: 10.1177/0748233714567525. [DOI] [PubMed] [Google Scholar]
- Wang N., Qian H. Y., Zhou X. Q., Li Y. B., Sun Z. W. Ecotoxicol. Environ. Saf. 2012;82:96–103. doi: 10.1016/j.ecoenv.2012.05.014. [DOI] [PubMed] [Google Scholar]
- Maier-Bode H. Residue Rev. 1968;22:1–44. doi: 10.1007/978-1-4615-8434-6_1. [DOI] [PubMed] [Google Scholar]
- Weber J., Halsall C. J., Muir D., Teixeira C., Smal J., Solomon K., Hermanson M., Hung H., Bidleman T. Sci. Total Environ. 2010;408:2966–2984. doi: 10.1016/j.scitotenv.2009.10.077. [DOI] [PubMed] [Google Scholar]
- Meire R. O., Khairy M., Targino A. C., Galvão P. M., Torres J. P., Malm O., Lohmann R. Chemosphere. 2016;144:2175–2182. doi: 10.1016/j.chemosphere.2015.10.133. [DOI] [PubMed] [Google Scholar]
- Astoviza M. J., Cappelletti N., Bilos C., Migoya M. C., Colombo J. C. Chemosphere. 2016;144:1459–1466. doi: 10.1016/j.chemosphere.2015.10.033. [DOI] [PubMed] [Google Scholar]
- Huang T., Guo Q., Tian H., Mao X., Ding Z., Zhang G., Li J., Ma J., Gao H. Environ. Sci. Pollut. Res. Int. 2014;21:6124–6135. doi: 10.1007/s11356-014-2505-8. [DOI] [PubMed] [Google Scholar]
- Papadakis E. N., Vryzas Z., Kotopoulou A., Kintzikoglou K., Makris K. C., Papadopoulou-Mourkidou E. Ecotoxicol. Environ. Saf. 2015;116:1–9. doi: 10.1016/j.ecoenv.2015.02.033. [DOI] [PubMed] [Google Scholar]
- Sun H., Qi Y., Zhang D., Li Q. X., Wang J. Environ. Pollut. 2016;209:177–185. doi: 10.1016/j.envpol.2015.11.040. [DOI] [PubMed] [Google Scholar]
- Sinha N., Adhikari N., Saxena D. K. Environ. Toxicol. Pharmacol. 2001;10:29–32. doi: 10.1016/s1382-6689(01)00066-7. [DOI] [PubMed] [Google Scholar]
- Choudhary N., Joshi S. C. Bull. Environ. Contam. Toxicol. 2003;70:285–289. doi: 10.1007/s00128-002-0189-0. [DOI] [PubMed] [Google Scholar]
- Jaiswal A., Parihar V. K., Sudheer Kumar M., Manjula S. D., Krishnanand B. R., Shanbhag R., Unnikrishnan M. K. Mutat. Res. 2005;585:50–59. doi: 10.1016/j.mrgentox.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Rao M., Narayana K., Benjamin S., Bairy K. L. Indian J. Physiol. Pharmacol. 2005;49:331–336. [PubMed] [Google Scholar]
- Aly H. A. A., Khafagy R. M. Food Chem. Toxicol. 2014;64:1–9. doi: 10.1016/j.fct.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Wang N., Xu Y., Zhou X. Q., Wu Y. H., Li S. L., Qiao X., Li Y. B., Sun Z. W. Environ. Toxicol. 2016;31:142–153. doi: 10.1002/tox.22029. [DOI] [PubMed] [Google Scholar]
- Ren N. Q., Zhang X. D., Zhou G. H., Chen S. J. Huanjing Kexue. 2008;29:386–390. [PubMed] [Google Scholar]
- Takhshid M. A., Tavasuli A. R., Heidary Y., Keshavarz M., Kargar H. Iran. J. Med. Sci. 2012;37:173–180. [PMC free article] [PubMed] [Google Scholar]
- Da Cuña R. H., Rey Vázquez G., Dorelle L., Rodríguez E. M., Guimarães Moreira R., Lo Nostro F. L. Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol. 2016;187:74–80. doi: 10.1016/j.cbpc.2016.05.008. [DOI] [PubMed] [Google Scholar]
- Sanchez M. C., Sedo C. A., Julianelli V. L., Romanato M., Calvo L., Calvo J. C., Fontana V. A. Syst. Biol. Reprod. Med. 2013;59:82–90. doi: 10.3109/19396368.2012.756952. [DOI] [PubMed] [Google Scholar]
- Bedford J., Bent M., Calvin H. J. Reprod. Fertil. 1973;33:19–29. doi: 10.1530/jrf.0.0330019. [DOI] [PubMed] [Google Scholar]
- Rougier N., Uriondo H., Papier S., Checa M. A., Sueldo C., Alvarez Sedó C. Fertil. Steril. 2013;100:69–74. doi: 10.1016/j.fertnstert.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Arch. Biochem. Biophys. 1976;175:710–716. doi: 10.1016/0003-9861(76)90563-4. [DOI] [PubMed] [Google Scholar]
- Chaufan G., Coalova I., Ríos de Molina M.D.C. Int. J. Toxicol. 2014;33:29–38. doi: 10.1177/1091581813517906. [DOI] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Anal. Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Anderson M. E. Methods Enzymol. 1985;113:548–555. doi: 10.1016/s0076-6879(85)13073-9. [DOI] [PubMed] [Google Scholar]
- Antherieu S., Ledirac N., Luzy A. P., Lenormand P., Caron J. C., Rahmani R. J. Cell. Physiol. 2007;213:177–186. doi: 10.1002/jcp.21108. [DOI] [PubMed] [Google Scholar]
- Sinha N., Adhikari N., Narayan R., Saxena D. K. Reprod. Toxicol. 1999;13:291–294. doi: 10.1016/s0890-6238(99)00020-9. [DOI] [PubMed] [Google Scholar]
- Guo F. Z., Zhang L. S., Wei J. L., Ren L. H., Zhang J., Jing L., Yang M., Wang J., Sun Z. W., Zhou X. Q. Environ. Sci. Pollut. Res. Int. 2016;23:20506–20516. doi: 10.1007/s11356-016-7195-y. [DOI] [PubMed] [Google Scholar]
- Sebastian R., Raghavan S. C. Cell Death Dis. 2015;6:e2022. doi: 10.1038/cddis.2015.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrillana M. E., Monclus M. A., Sáez Lancellotti T. E., Boarelli P. V., Clementi M. A., Vincenti A. E., Yunes R. F., Fornés M. W. Cytoskeleton. 2011;68:491–500. doi: 10.1002/cm.20525. [DOI] [PubMed] [Google Scholar]
- Bennetts L. E., De Iuliis G. N., Nixon B., Kime M., Zelski K., McVicar C. M., Lewis S. E., Aitken R. J. Mutat. Res. 2008;641:1–11. doi: 10.1016/j.mrfmmm.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Khan P. K., Sinha S. P. Mutagenesis. 1996;11:33–36. doi: 10.1093/mutage/11.1.33. [DOI] [PubMed] [Google Scholar]
- Mitchell L. A., De Iuliis G. N., Aitken R. J. Int. J. Androl. 2010;34:2–13. doi: 10.1111/j.1365-2605.2009.01042.x. [DOI] [PubMed] [Google Scholar]
- Kim H. G., Kim Y. R., Park J. H., Khanal T., Choi J. H., Do M. T., Jin S. W., Han E. H., Chung Y. H., Jeong H. G. Arch. Toxicol. 2015;89:2039–2050. doi: 10.1007/s00204-014-1359-7. [DOI] [PubMed] [Google Scholar]