Abstract
Human induced pluripotent stem cells (hiPSCs) offer great opportunities for the study of human development and disease modeling as well as for their enormous potential in future clinical cell therapies. However, most current systems to create hiPSCs often expose the cells to animal feeder layers or xenogeneic reagents. This raises safety concerns about using hiPSC-derived cells for therapeutic purposes. Here we describe a protocol to generate hiPSCs without exposing the cells to xenogeneic materials using a defined, feeder-free reprograming system. With this method, we were successfully able to reprogram not only patient-derived PBMCs but also amniocytes from the amniotic fluid of stillborn fetuses, using two independent reprogramming platforms. Importantly, hiPSCs generated in this fashion expressed pluripotent markers and had normal karyotypes. This protocol allowed us to generate and culture hiPSCs in GMP-like conditions, a necessary step for their future clinical applications.
Keywords: human induced pluripotent stem cells (hiPSCs), peripheral blood mononuclear cells (PBMCs), Amniocytes, Feeder-free reprogramming system, STEMCCA lentiviral vector, Sendai viral vector
Introduction
The first hiPSCs were derived from dermal fibroblasts by forced expression of the transcription factors Oct4, Sox2, Klf4, and c-Myc in 2007 using individual retroviral vectors(Takahashi et al., 2007). Since then, several methods have been developed to generate hiPSCs using either viral delivery systems Retro(Takahashi et al., 2007), Lentivirus(Yu et al., 2007), and Sendai virus(Fusaki et al., 2009)) or non-viral ones plasmid(Okita et al., 2011), transposons(Woltjen et al., 2009), RNA(Warren et al., 2010). These hiPSCs have been derived from various types of somatic cells such as skin fibroblasts(Takahashi et al., 2007), keratinocytes(Aasen et al., 2008), pancreatic beta cells(Stadtfeld et al., 2008a), and T lymphocytes(Seki et al., 2010; Staerk et al., 2010). hiPSCs hold enormous potential for studying diseases, drug screening, and for patient-specific cell therapies(Avior et al., 2016). However, the full potential of this hiPSC technology could be limited by using animal products for its derivation and maintenance since most of the current reprogramming methods require exposure of the cells to animal feeder layers or xenogeneic reagents. This raises safety concerns for clinical translation(Ahrlund-Richter et al., 2009; Martin et al., 2005).
Here, we describe a protocol to generate hiPSCs in GMP-like conditions. In this protocol, two different viral vector systems are employed to produce hiPSCs, namely STEMCCA vector and Sendai virus vector. STEMCCA lentiviral vector is a single floxed-excisable polycistronic lentivirus, which we originally described capable of very efficiently reprogram murine and human cells(Sommer et al., 2009; 2010). Sendai virus vector is a cytoplasmic RNA vector, which does not integrate into a host genome. By using these two reprograming systems, we were able to generate transgene-free hiPSCs.
Furthermore, we used two different somatic cell types as sources for hiPSC generation, namely PBMCs and amniocytes. These cell types have advantages as a source for reprogramming over other somatic cell types. For example, PBMCs can be collected with a minimally invasive procedure, which is relatively less traumatic and has lower risks to patients compared to skin biopsy. When it comes to amniocytes, they can be easily obtained from amniotic fluid, even in cases where the stillborn fetus is affected by a disease impairing development.
Lastly, the whole reprogramming protocol is performed in serum-free feeder-free conditions bringing us a step closer to the generation of hiPSCs that will be suitable for the clinical setting.
NOTE: All of procedures are performed in a Class II biological hazard flow hood or a laminar-floor hood.
NOTE: Sterile techniques are used to prepare all solutions and handle cells.
BASIC PROTOCOL 1 GENERATION OF hiPSCS DERIVED FROM PBMCS
This protocol is for reprogramming peripheral blood mononuclear cells (PBMCs) using a feeder-free reprogramming system to generate hiPSCs. PBMCs are cultured and expanded in vitro based on a previous published protocol(Sommer et al., 2012)that tends to produce an enriched population of erythroblasts(Yang et al., 2015). In contrast to other protocols that use T lymphocytes(Seki et al., 2010; Staerk et al., 2010), this protocol provides access to hiPSCs without pre-rearranged T cell receptor (TCR). Expanded cells can then be transduced using either the STEMCCA lentiviral system or with the Sendai viral reprogramming approach to derive hiPSCs. Upon reprogramming with the STEMCCA, the integrated reprogramming cassette can be excised by utilizing Cre recombination(Somers et al., 2010). Ultimately, this platform allows for the generation of integration-free hiPSCs in a feeder-free serum-free manner.
Materials
BD Vacutainer CPT Cell Preparation Tube with Sodium Citrate (BD Biosciences)
QBSF-60 Stem Cell Medium (Quality Biological)
PBMC Expansion Medium (see recipe)
PBMC Freezing Medium (see recipe)
Phosphate-buffered saline without CaCl2, without MgCl2 (PBS, Invitrogen)
DMEM/F12 (Invitrogen)
CytoTune-iPS 2.0 Sendai Reprogramming Kit (Invitrogen)
STEMCCA lentivirus
Polybrene (see recipe, MilliporeSigma)
hESC-qualified Matrigel (Corning)
Complete ReproTeSR (see recipe, STEMCELL Technologies)
Complete mTeSR (see recipe, STEMCELL Technologies)
Y27632/ROCK inhibitor (STEMGENT)
6-well Tissue Culture Plates
12-well Tissue Culture Plates
15-mL Conical tubes
Day -9: Isolate and Expand Peripheral Blood Mononuclear Cells (PBMCs)
-
1
Collect 4 mL of peripheral blood into a BD Vacutainer CPT Cell Preparation Tube with Sodium Citrate. After collection, keep the tube upright at room temperature (RT) until centrifugation.
-
2
Remix the blood sample right before centrifugation by gently inverting the tube 8 to 10 times. Centrifuge the tube with blood at 1,800 RCF for 30 min at RT
This step should be done within two hours of blood collection for best results.
-
3
Collect mononuclear cells within the buffy coat between the plasma layer and gel layer using 1000p pipette. Transfer them into a 15-mL conical tube.
-
4
Add PBS to bring volume to 10 mL and mix the cells gently by inverting the tube 5 times.
-
5
Centrifuge the tube at 300 RCF for 15 min at RT.
-
6
Aspirate the supernatant and Resuspend the cells in 10 mL of PBS.
-
7
Perform cell count and transfer 1 to 2 × 106 cells into a 15-mL conical tube followed by centrifugation at 300 RCF for 10 min at RT.
-
8
Aspirate the supernatant and Resuspend the cell pellet in 2 mL of PBMC Expansion Medium (EM) (see recipe). Transfer the cells in a well of a 12-well plate and incubate them in 37°C, 5% CO2 incubator for 3 days. The cells don’t need to be replenished with the media during the time. Go to Step 11.
The cells from a BD Vacutainer CPT Cell Preparation Tube with Sodium Citrate are cultured in a well of a 12-well plate. Depending on the number of tubes used, the number of wells can be scaled up according the need.
Freezing PBMCs for future use
-
9
Spin down the remaining cells at 300 RCF for 10 min at RT and freeze ~2 × 106 cells/vial in PBMC Freezing Medium (FM) (see recipe).
-
10
To begin with frozen PBMCs thaw 1 vial of cells into 10 mL of QBSF-60 Stem Cell Medium (QBSF) and centrifuge at 300 RCF for 10 min at RT. Aspirate the supernatant and Resuspend the cells using 2 mL of EM. Transfer the cells in a new well of a 12-well plate and incubate them in 37°C, 5% CO2 incubator.
Day -6 and -3: Keep expanding PBMCs
-
11
At day -6, transfer the cells into a 15-mL conical tube. Wash the well using 1 mL of QBSF to collect the adherent cells and add into the 15-mL conical tube with the cells.
-
12
Spin down the cells at 300 RCF for 10 min at RT. Aspirate the supernatant and resuspend the cells in 2 mL of fresh EM following.
-
13
Transfer the cells in a new well of a 12-well plate and incubate the cells in 37°C, 5% CO2 incubator for 3 days. The cells don’t need to be replenished with the media during the time. At day -3, repeat the process from Step 11 to 13.
Day 0: Transduce PBMCs using STEMCCA lentiviruses
-
14
Transfer the cells to a 15-mL conical tube and wash the well with 1 mL of QBSF to collect the adherent cells. Add into the 15-mL conical tube with the cells.
-
15
Centrifuge the cells at 300 RCF for 10 min at RT.
-
16
Aspirate the supernatant from the tube and resuspend the cells in 1 mL of fresh warm EM containing 5 μg/mL of polybrene and STEMCCA lentivirus (MOI=1 to 10).
Cationic polymer Polybrene helps virions diffuse to the host cell surface by neutralizing the charge repulsion. Polybrene has been proved to increase the efficiency of transduction. -
17
Mix the cells with the viruses by gently flicking the tube.
-
18
Transfer the cells to a well of a 12-well plate and spin the plate at 2,250 RPM for 90 min at RT.
-
19
After spinoculation, add an additional 1 mL of fresh EM containing 5 μg/mL of polybrene for a total of 2 mL of the medium and incubate the cells in 37°C, 5% CO2 incubator. Go to Step 29.
Day 0: Transduce PBMCs using Sendai viruses
-
20
Transfer the cells to a 15-mL conical tube and wash the well with 1 mL of QBSF to collect the adherent cells. Add into the 15-mL conical tube with the cells.
-
21
Centrifuge the cells at 300 RCF for 10 min at RT.
-
22
Meanwhile, thaw CytoTune 2.0 Sendai tubes according to the manufacturer’s instruction and keep the viruses on ice.
-
23
Aspirate the supernatant from the tube, resuspend the cells in 1 mL of fresh EM and count the cells.
-
24
Take 2 × 105 cells into a 15-mL conical tube in 1 mL of fresh warm EM containing 5 μg/mL of polybrene.
-
25Based on the cell number, calculate the volume of each virus needed to obtain appropriate MOI (KOS:hc-Myc:hKlf4=5:5:3). Add each virus into the 1 mL of polybrene-containing EM with the cells.The titer of each virus is providde by the manufacturer.
-
26
Mix the cells with the viruses by gently flicking the tube.
-
27
Transfer the cells to a well of a 12-well plate and spin the plate at 2,250 RPM for 90 min at RT.
-
28
After spinoculation, add an additional 1 mL of fresh EM containing 5 μg/mL of polybrene for a total of 2 mL of the medium and incubate the cells in 37°C, 5% CO2 incubator.
Day 1: Wash-off the reprogramming viruses and re-plate the transduced cells
-
29
To prepare a hESC-qualified Matrigel coated 6-well plate, thaw an aliquot of the Matrigel on ice and dilute it with 6.25 mL of cold DMEM/F12 in ice. Distribute the Matrigel mixture 1 mL per well and incubate the plate for an hour at RT. Aspirate the Matrigel mixture right before plating the cells without scratching the coated surface.
The hESC-qualified Matrigel coated plate should not be dried up.hESC-qualified Matrigel is aliquoted for one or two 6-well plates according to Lot-specific Dilution factor and stored at −80°C until use.Lot-specific Dilution factor is provided by the manufacturer. -
30
Collect and transfer the transduced cells into a 15-mL conical tube. Wash the well gently with 1 mL of QBSF to make sure most of cells are harvested.
-
31
Centrifuge the cells at 300 RCF for 10 min.
-
32
Remove the supernatant and Resuspend the cells in 1 mL of EM.
-
33
Perform cell count.
-
34
Plate 8.0 × 104 – 2.0 × 105 cells/well of a 6-well plate in 2 mL of EM.
Plating the cells with three different concentrations is recommended to make it easy to pick colonies.We found that when the plate was spun down at low speed (e.g., 500 RPM) to help the cells attach on the Martigel-coated wells, rather fewer cells were attached and survived compared to the cells without spinning. So the plate with the infected cells should not be centrifuged even at low speed. -
35
Incubate the cells in 37°C, 5% CO2 incubator.
Day 2: Add more PBMC Expansion medium
-
36
Add 1 mL of fresh EM into the wells without aspirating any medium from the wells. Incubate the cells in 37°C, 5% CO2 incubator.
Day 3 and 5: Add Reprogramming Medium
-
37
On day 3, add 1 mL of complete ReproTeSR (See recipe) into the wells without aspirating any medium from the wells. Incubate the cells in 37°C, 5% CO2 incubator. Repeat step on day 5.
Day 7 to 25: Feed the cells and Pick putative iPSC colonies
-
38
Aspirate the old medium in a well and feed 2 mL of complete ReproTeSR per well. Incubate the cells in 37°C, 5% CO2 incubator.
-
39
Change the medium daily with 2 mL of complete ReproTeSR per well in 6-well plates.
-
40
Monitor the cells every other day under a microscope to observe the appearance of small colonies (Figure 2).
When iPSC colonies are observed, let them grow for 3 to 4 more days unless they start merging with other colonies. The bigger colony, the easier it is picked. -
41
Once iPSC colonies become big enough (approximately 1.3 mm to 2.2 mm) to be picked without merging with other colonies, prepare a hESC-qualified Matrigel coated 12-well plate. Add 1 mL per well of complete mTeSR medium with 10uM ROCK inhibitor and keep the plate in 37°C, 5% CO2 incubator until use.
If iPSC colonies atart merging into each other, they are likely to start differenting.A iPSC colony is still small but if it starts differentiating in the middle, it should be picked before differentiated cells cover the whole colony. -
42
Manually pick putative iPSC colonies. Transfer each clone into each well in a hESC-qualified Matrigel coated 12-well plate for further expansion or analysis.
To pick only putative iPSC colonies, remove differentiated, partial reprogrammed, or not-reprogrammed cells surrounding the colony before picking.6 clones from one sample are picked and the only best 3 clones are expanded and frozen for future use.One colony is divided into 10 to 30 cell clumps and plated in the same well of a 12-well plate.The generated hiPSCs can be confirmed by immunofluorescence staining using “ES Cell Characterization Kit” from Millipore according to the manufacturer’s protocol.
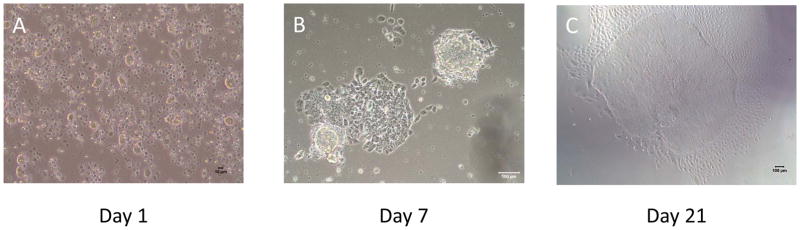
Figure 2. Representative images of morphological changes of the reprogramming PBMCs.
(A) Day 1 : STEMCCA or SeV infected PBMCs before plating onto hESC-qualified Matrigel (Scale bar = 10um)
(B) Day 7 : There is a clear change in morphology and the beginning of colony formation can be detected. (Scale bar = 100um)
(C) Typical hiPSC colonies appear on the Matrigel and they are ready to be picked. (Scale bar = 100um)
SUPPORT PROTOCOL 1 Freezing hiPSCs
Reprogrammed hiPSCs usually become stabilized within 4 passages. Stabilized and Expanded hiPSCs can be frozen and stored at −150°C for future use.
Materials
mFreSR (STEMCELL Technologies)
ReLeSR (STEMCELL Technologies)
Complete mTeSR (see recipe, STEMCELL Technologies)
Phosphate-buffered saline without CaCl2, without MgCl2 (PBS, Invitrogen)
6-well Tissue Culture Plates
Cell Scraper or Cell lifter
15-mL Conical tubes
1-mL Cryovials
Container with ice
Freezing container (e.g., Mr. Frosty or Double-layered Styrofoam box)
Thaw mFreSR at RT or at 4°C overnight. Keep it on ice after thawing.
-
Meanwhile, label cryovials with the information of the cells to be frozen (e.g., the name of the cell line, passage number, the kind of the media for culture, the date of freezing and the name of person who freeze the cells)
A cryovial should be needed per well of a 6-well plate. -
Wash the cells with PBS and aspirate it.
When the cells are around 70 – 90% confluent, they can be frozen. -
Add 1 mL of ReLeSR per well of a 6-well plate and aspirate it within a minute. Incubate the cells in 37°C, 5% CO2 incubator for 2 – 5 minutes.
The cells are covered with a thin layer of ReLeSR so the ReLeSR-treated cells are not dried up while incubating.ReLeSR can be replaced with other types of dissociation reagents (e.g., Dispase or Gentle cell dissociation reagent). In that case, the procedure should be followed the one for the reagent. -
Take the cells from the incubator and add 1 mL of mTeSR per well. Make the cells come off from the plate by gently tapping the plate or scraping the cells using a cell scraper. The cells are detached as cell aggregates from the plate.
Be careful not to make the cells as single cells.If most of the cells are not differentiated, they can be incubated only for 2 minutes followed by adding 1 mL of mTeSr and harvested by scraping. By doing this, most of the cells can be harvested and transferred into a 15-mL conical tube for the next step. ReLeSR-treated cells often tend to float on the surface of the media then, stick to the plate again while transferring. So a fraction of the cells can be lost. If the cells contain differentiated cells, incubate the cells for 5 minutes. And then, tap the plate gently 15 to 20 times followed by adding 1 mL of mTeSR. Only undifferentiated cells will be detached from the plate. -
Transfer the cell aggregates into a 15-mL conical tube.
If the cell aggregates are derived from the same cell line with the same batch, they can be combined into the same 15-mL conical tube. -
Centrifuge the cell aggregate at 200 RCF for 5 min at RT.
If the cell aggregates still float in the media, they can be centrifuged at 300 RCF. Gently aspirate the supernatant without disturbing the cell pellet.
-
Gently resuspend the cell pellet with 1 mL of cold mFreSR by pipetting. Add additional amount of mFreSR according to the number of the harvested wells (1mL per well).
If the cells are harvested from a whole 6-well plate, add 1 mL of cold mFreSR to resuspend the cell pellet and add additional 5 more mL of mFreSR. So it is 6 mL of mFreSR added in total (1mL/well).Be careful not to break-up the cell aggregate. Keep the 15-mL conical tube with the cells in mFreSR in ice. Transfer 1 mL of cell aggregates into each labeled cryovial.
Freeze the cryovials at −80°C using a freezing container for overnight, which reduce temperatures at −1°C/min. Move the frozen vials into −150°C freezer on the following day.
SUPPORT PROTOCOL 2 Thawing hiPSCs
Previously frozen hiPSCs can be thawed when needed. The thawed hiPSCs can be usually recovered within a week but may vary depending on cell lines and freezing methods used.
Materials
hESC-qualified Matrigel (Corning)
DMEM/F12 (Invitrogen)
Complete mTeSR (see recipe, STEMCELL Technologies)
Y27632/ROCK inhibitor (STEMGENT)
6-well Tissue Culture Plates
15-mL Conical tubes
Container with dry ice
-
Prepare a hESC-qualified Matrigel coated 6-well plate (see Basic protocol 1, Step 29).
One cryovial of frozen cells can be thawed into 1–3 wells of a 6-well plate. Meanwhile coating a 6-well plate with the Matrigel, warm up mTeSR at RT.
-
Add 9 mL of mTeSR in a 15-mL conical tube.
One frozen cryovial needs each 9 mL-mTeSR contained 15-mL conical tube so prepare 15-mL conical tubes with mTeSR according to the number of cryovials to be thawed. Take the frozen cryovial from −150°C freezer and keep it on dry ice until thawing.
Quickly thaw the frozen cells in a 37°C water bath by gently shaking or using a thawing instrument until only a small ice pellet left.
Remove the cryovial from the water bath or the thawing instrument. Spray 70% ethanol thoroughly all over the cryovial and wipe it to sterilize.
Transfer 1 mL of the thawed cells from the cryovial to a 15 mL-conical tube which has 9 mL of mTeSR.
-
Centrifuge the 15 mL-conical tube at 200 RCF for 5 min at RT.
If the cell aggregates still float in the media, they can be centrifuged at 300 RCF. Meanwhile, prepare mTeSR with 10 uM ROCK inhibitor (2 mL/well of a 6-well plate). Aspirate the hESC-qualified Matrigel from the 6-well plate and add 1 to 1.5 mL of mTeSR with ROCK inhibitor per well.
Gently aspirate the supernatant without disturbing the cell pellet.
-
Gently resuspend the cell pellet with 1 mL of mTeSR with ROCK inhibitor by pipetting. Plate 0.5 mL to 1 mL of the cells into a hESC-qualified Matrigel coated well or two so the total volume will be 2 mL of the cells in mTeSR/ROCK inhibitor.
The cells should be plated more confluent than routine passaging. Depending on the growth rate of cells and amount of cell aggregates, the number of wells prepared can be adjusted. Gently shake the plate to evenly distribute the cell aggregates over the well.
Incubate the cells in 37°C, 5% CO2 incubator. Do not disturb the plate until the following day.
-
Change the media every day using mTeSR and check the cells until they are ready to be passaged.
Thawed cells are usually recovered and ready to be passaged within a week but may vary depending on cell lines, freezing methods used, or the confluency of plating cells.
BASIC PROTOCOL 2 GENERATION OF hiPSCS DERIVED FROM AMNIOCYTES
In this protocol, we described the generation of hiPSCs from amniocytes using integration-free and feeder-free reprogramming system. Integrated STEMCCA lentiviral vector can be excised by using Cre recombination as described(Somers et al., 2010). Amniocytes were cultured and maintained based on a previously published method(Anchan et al., 2010) with modifications as described. It is recommended to use low-passage amniocytes that are actively proliferating.
Materials
Amniocyte medium (see recipe)
0.05% Trypsin (Invitrogen)
TrypLE Select (Invitrogen)
Ultrapure Water With 0.1% Gelatin (Millipore)
Phosphate-buffered saline without CaCl2, without MgCl2 (PBS, Invitrogen)
CytoTune-iPS 2.0 Sendai Reprogramming Kit (Invitrogen)
STEMCCA lentivirus
Polybrene (see recipe, MilliporeSigma)
hESC-qualified Matrigel (Corning)
Complete ReproTeSR (see recipe, STEMCELL Technologies)
Complete mTeSR (see recipe, STEMCELL Technologies)
Y27632/ROCK inhibitor (STEMGENT)
6-well Tissue Culture Plates
12-well Tissue Culture Plates
15-mL Conical tubes
Day-2: Prepare amniocytes for transduction
-
1
Plate amniocytes onto two wells of a 0.1% gelatin coated 6-well plate to obtain 2×105 and 5×105 cells on the day of transduction (which represents 50–80% confluency).
On day -2, 1×105 to 2.5×105 cells are usually plated to reach 50–80% confluency on the day of transduction. However, it may vary depending on cell lines so should be adjusted based on the cell growth rate.
Day 0: Transduce amniocytes using STEMCCA lentiviruses
-
2
Aspirate the old medium from one of the two wells of amniocytes and wash the well using 1 mL of PBS.
-
3
Add 1 mL of 0.05% Trypsin and incubate the cells at 37°C for 3–5 min.
-
4
Inactivate the Trypsin by adding 2 mL of amniocyte medium and transfer them into a 15-mL conical tube. Centrifuge at 300 RCF for 5 min.
-
5
Aspirate the supernatant and resuspend the cells using 1 mL of amniocyte medium.
-
6
Count the cells. These cells are only used for estimating the cell number in the other well.
-
7
Prepare 1 mL of warm amniocyte medium and thaw STEMCCA lentivirus on ice.
-
8
Add STEMCCA virus (MOI=1 to 10) and 5 μg/mL of polybrene into the 1 mL of amniocyte medium.
-
9
Aspirate the old medium in the well and add the 1 mL of amniocyte medium with the STEMCCA and polybrene into the well. Incubate the cells in 37°C, 5% CO2 incubator. Go to Step 13.
Day 0: Transduce amniocytes using Sendai viruses
-
10
From Step 6, prepare 1 mL of warm amniocyte medium and thaw CytoTune 2.0 Sendai tubes according to the manufacturer’s instruction.
-
11
Based on the cell number, calculate the volume of each virus needed to obtain the appropriate MOI (KOS:hc-Myc:hKlf4=5:5:3). Add each virus and 5 μg/mL of polybrene into 1 mL of amniocyte medium.
-
12
Aspirate the old medium in the well and add the 1 mL of amniocyte medium containing Sendai viruses and polybrene into the well. Incubate the cells in 37°C, 5% CO2 incubator. Go to Step 13.
Day 1: Wash-off the reprogramming viruses
-
13
24 hours post-infection, aspirate the medium with the viruses and add 2 mL amniocyte medium.
-
14
Incubate the cells in 37°C, 5% CO2 incubator. Culture the cells for 6 more days, changing the old medium with fresh amniocyte medium every other day.
Day 7: Plate the transduced cells onto a Matrigel-coated plate
-
15
Thaw hESC-qualified Matrigel according to the manufacturer’s protocol and coat sufficient number of 6-well plates for one hour at room temperature.
It is recommended to prepare small aliquots of hESC-qualified Matrigel ahead of time.
After an hour, aspirate the Matrigel from 6-well plates and add 2 mL per well of amniocyte medium. Keep the plates in 37°C, 5% CO2 incubator until use.
-
16
On day 7 post-infection, aspirate the old medium in the well and wash the cells with 1 mL of PBS. Then, trypsinize the transduced cells using 1 mL of TrypLE for 2 to 3 min at RT.
-
17
Add 2 mL of amniocyte medium into the well and collect the cells in a 15 mL-conical tube.
-
18
Centrifuge at 200 RCF for 5 min. Aspirate the supernatant and Resuspend the cells adding 1 mL of amniocyte medium.
-
19
Count the cells.
-
20
Take the hESC-qualified Matrigel coated 6-well plates kept in 37°C, 5% CO2 incubator. Plate 1.0 × 104 – 8.5 × 104 cells per well in a 6-well plate. Plating the cells with three different concentrations is recommended to make it easy to pick colonies.
The cells are usually plated in a well with 1.5 × 104, 5.0 × 104, and 8.0 × 104.
Day 8 to 25: Feed the cells and Pick putative iPSC colonies
-
21
Start feeding the cells with complete ReproTeSR. Change the medium daily with 2 mL per well in 6-well plates.
-
22
Monitor the cells every other day under a microscope to observe the appearance of small ESC-like colonies (Figure 4).
-
23
Once iPSC colonies become big enough (approximately 1.3 mm to 2.2 mm) to be picked without merging with other colonies, prepare a hESC-qualified Matrigel coated 12-well plate. Add 1 mL per well of complete mTeSR medium with 10uM ROCK inhibitor and keep the plate in 37°C, 5% CO2 incubator until use. (See Basic protocol 1, step 41)
Amniocyte-derived iPSC colonies appear 2–3 later than PBMC-derived iPSC colononies. -
24
Manually pick putative iPSC colonies. Transfer one clone per well into a hESC-qualified Matrigel coated 12-well plate for further expansion or analysis. (See Basic protocol 1, step 42)
Amniocyte-derived iPSC colonies are usually ready to be picked around day 21 to 25.
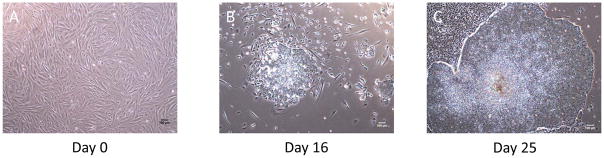
Figure 4.
Representative images of morphological changes of the reprogramming amniocytes.
(A) Day 0 : Amniocytes before infection should be around 50–80% confluency.
(B) Day 16 : Small colonies are emerged between day 13 and 17.
(C) Day 25 : hiPSC colonies are ready to be picked.
(Scale bar = 100um)
REAGENTS AND SOLUTIONS
PBMC Expansion medium
QBSF-60 Stem Cell Medium (Quality Biological) containing;
50 μL/mL Ascorbic Acid (MilliporeSigma)
50 ng/ML SCF (R&D Systems)
10 ng/mL IL-3(R&D Systems)
2 U/mL EPO(R&D Systems)
40 ng/mL IGF-1(R&D Systems)
1 μM Dexamethasone (MilliporeSigma)
1× Pen/Step (100x, Invitrogen) or 0.1 mg/mL Primocin (50mg/mL, InvivoGen)
Make the medium freshly every time. Use Pen/Strep while testing Mycoplasma contamination. After confirmed Mycoplasma negative, switch to Primocin.
PBMC Freezing medium
FBS (HyClone) containing:
10% Dimethyl Sulfoxide(DMSO, MilliporeSigma)
Store up to 2 weeks at 4°C
Amniocyte medium
Alpha-MEM (Invitrogen) containing:
10% (v/v) heat-inactivated fetal bovine serum, Characterized (FBS, HyClone)
1×, non-essential amino acids (100x, Invitrogen)
1×, GlutaMax (100x, Invitrogen)
0.1mg/mL, Primocin (50mg/mL, InvivoGen)
Store up to 2 weeks at 4°C
Complete ReproTeSR medium
Basal ReproTeSR (STEMCELL Technologies) containing:
1×, ReproTeSR Supplement (20x, STEMCELL Technologies)
1×, ReproTeSR Supplement (500x, STEMCELL Technologies)
0.1mg/mL, Primocin (50mg/mL, InvivoGen)
Store up to 2 weeks at 4°C or aliquot and store up to 6 months at −20°C
Complete mTeSR medium
Basal mTeSR (STEMCELL Technologies) containing:
1×, mTeSR Supplement (50x, STEMCELL Technologies)
0.1mg/mL, Primocin (50mg/mL, InvivoGen)
Store up to 2 weeks at 4°C
Polybrene
Dissolve Polybrene powder (MilliporeSigama) in deionized, distilled water at 5 mg/mL (1000× stock solution). Store at −20°C.
Ascorbic Acid
Dissolve L-Ascorbic Acid powder (MilliporeSigama) in deionized, distilled water at 5 mg/mL (100× stock solution). Store at −20°C.
SCF
Reconstitute lyophilized Recombinant Human SCF (R&D Systems) at 50 μg/mL in sterile PBS containing at least 0.1% bovine serum albumin. Divide into appropriately sized aliquots and store at −20°C to −80°C under sterile conditions after reconstitution.
IL-3
Reconstitute lyophilized Recombinant Human IL-3 (R&D Systems) at 10 μg/mL in sterile PBS containing at least 0.1% bovine serum albumin. Divide into appropriately sized aliquots and store at −20°C to −80°C under sterile conditions after reconstitution.
IGF-1
Reconstitute lyophilized Recombinant Human IGF-1 (R&D Systems) at 50 μg/mL in sterile PBS. Divide into appropriately sized aliquots and store at −20°C to −80°C under sterile conditions after reconstitution.
Dexamethasone
Dissolve Dexamethasone powder in 1 mL of absolute ethanol per mg product and add sterile DMEM/F12 (Invitrogen) to achieve final concentration of 50 μM (50× stock solution). Store at −20°C. Keep dexamethasone protected from light.
COMMENTARY
Background Information
In 2007, Shinya Yamanaka and his colleagues generated human induced pluripotent stem cells from somatic cells by expressing several transcription factors, such as OCT4, NANOG, SOX2, c-MYC, and KLF4(Takahashi et al., 2007). hiPSCs not only overcome many ethical concerns and potential immune rejections in future tissue transplantations, which human embryonic stem cells (hESCs) may have but also have been shown to be equivalent to hESCs(Choi et al., 2015). hiPSCs provide great opportunities to study the pathophysiology of disease and test drugs. Importantly, hiPSCs holds great promise for the ultimate personalized patient-specific treatment(Shi et al., 2016).
Initially, individual retroviruses and lentiviruses were used to derive hiPSCs. However, these viral reprogramming systems had a risk of multiple random integrations into the host genome, potentially disrupting the host genome and inducing insertional mutagenesis. Eventually, this could potentially cause disruption or aberrant activation of neighbor genes and also let to the reactivation of the reprogramming factors that can induce tumor formation(Ahrlund-Richter et al., 2009).. To avoid these risks, several reprogramming systems able to generate integration-free iPSCs have been developed using excisable lentiviruses(Somers et al., 2010), adenoviruses(Stadtfeld et al., 2008b), plasmids(Okita et al., 2011), transposons(Woltjen et al., 2009), Sendai viruses(Fusaki et al., 2009), synthetic mRNAs(Warren et al., 2010), and recombinant proteins(Kim et al., 2009).
Conventionally, hiPSCs have been generated using the support of animal feeder layers in the presence of xenogeneic reagents, which raises safety concerns for their use in clinical applications. Therefore, in order to generate hiPSCs under GMP-like conditions, reprogramming should be done to obtain integration-free colonies obtained under feeder-free and xeno-free settings. To satisfy these criteria, we use a single excisable polycistronic lentiviral STEMCCA vector or Sendai virus vector for derivation of integration-free hiPSCs. Also, a defined serum-free culture condition is used to generate and maintain hiPSCs. This reprogramming system represents a step closer for the generation of clinical-grade hiPSCs.
Critical Parameters and Troubleshooting
Regarding PBMC expansion, the expansion medium (EM) is purposed to expand erythroblasts among PBMCs. This ensures generation of hiPSCs devoid of pre-rearranged T/B cell receptors.
When plating transduced PBMCs on hESC-qualified Matrigel, the plate with the cells should not be spun down, which may prevent cell attachment and decrease cell viability.
To successfully reprogram amniocytes, we recommend using cells at lower passage (<5).
It is important to keep the number of plated cells to avoid high confluency, this can cause colonies to merged even before they become fully reprogrammed and making it very difficult to pick up single-cell-derived hiPSC clones.
Anticipated Results
Using this protocol, hiPSCs can be generated from PBMCs or Amniocytes. Both PBMC-derived and Amniocyte-derived hiPSCs have normal karyotypes. Also, they positively express typical human pluripotent cell markers such as TRA-1-81, TRA-1-60, and SSEA-4 as well as Alkaline Phosphatase. hiPSCs can be stably passaged and maintained on serum-free conditions or freeze down for later usage.
Time Considerations
PBMCs transduced both with STEMCCA lentivirus or Sendai virus start changing their morphology around day 7 post-infection. In our experience, cells reprogrammed with the Sendai system tend to be ready for picking a few days earlier (around 17–19 days post infection) than when using the STEMCCA system.
Figure 1.
Timeline for reprograming PBMCs
Figure 3.
Time course for generating hiPSCs derived from amniocytes
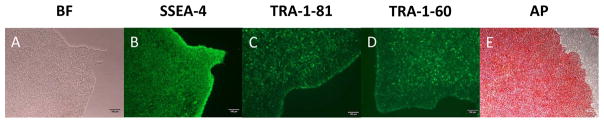
Figure 5. Characterization of hiPSCs generated from PBMCs and amniocytes.
Immunofluorescence analysis of the hiPSCs derived from PBMCs and amniocytes shows the expression of the pluripotency markers SSEA-4(B), Tra-1-81(C), and Tra-1-60 (D). The hiPSCs generated from PBMCs and amniocytes reveals positive staining with Alkaline Phosphatase. Bright field (A), Scale bar = 100um
Figure 6. Karyotyping analysis of hiPSCs.
Karyotype of the hiPSCs from both male and female samples is normal after reprogramming. (A) XX, Female (B) XY, Male
Significance Statement.
Human induced pluripotent stem cells (hiPSCs) have a great potential in regenerative medicine. Specific hiPSC-derived differentiated cells have already moved into clinical trials. To secure clinical safety, hiPSCs should be generated under Good Manufacturing Practice (GMP) conditions. However, most current protocols make use of xenogeneic reagents and/or animal feeder layers to generate hiPSCs, which increases safety concerns. In this protocol, we described how to establish and culture hiPSCs in GMP-like conditions using a few mL of peripheral blood mononuclear cells (PBMCs) as a source material for reprogramming, which is minimally invasive and an easily accessible somatic cell source. Also, this protocol allowed us to generate hiPSCs from amniocytes of stillborn fetuses with developmental incompetence, further validating the robustness of our platform and offering a unique opportunity to study and model diseases that are embryonically lethal.
Acknowledgments
The authors would like to thank the CReM for support of the main tissue culture facility. GM is supported by 1R01CA175727-01, 1R24HL123828-01 and 5R21AI126457-02.
Footnotes
Conflicts of Interest
No conflicts of interest declared.
Literature Cited
- Aasen T, Raya A, Barrero MJ, Garreta E, Consiglio A, Gonzalez F, Vassena R, Bilić J, Pekarik V, Tiscornia G, et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nature Biotechnology. 2008;26:1276–1284. doi: 10.1038/nbt.1503. [DOI] [PubMed] [Google Scholar]
- Ahrlund-Richter L, De Luca M, Marshak DR, Munsie M, Veiga A, Rao M. Isolation and Production of Cells Suitable for Human Therapy: Challenges Ahead. Cell Stem Cell. 2009;4:20–26. doi: 10.1016/j.stem.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Anchan RM, Quaas P, Gerami-Naini B, Bartake H, Griffin A, Zhou Y, Day D, Eaton JL, George LL, Naber C, et al. Amniocytes can serve a dual function as a source of iPS cells and feeder layers. Human Molecular Genetics. 2010;20:962–974. doi: 10.1093/hmg/ddq542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avior Y, Sagi I, Benvenisty N. Pluripotent stem cells in disease modelling and drug discovery. Nature Reviews Molecular Cell Biology. 2016;17:1–13. doi: 10.1038/nrm.2015.27. [DOI] [PubMed] [Google Scholar]
- Choi J, Lee S, Mallard W, Clement K, Tagliazucchi GM, Lim H, Choi IY, Ferrari F, Tsankov AM, Pop R, et al. A comparison of genetically matched cell lines reveals the equivalence of human iPSCs and ESCs. Nature Biotechnology. 2015:1–11. doi: 10.1038/nbt.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusaki N, BAN H, NISHIYAMA A, SAEKI K, Hasegawa M. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proceedings of the Japan Academy, Series B. 2009;85:348–362. doi: 10.2183/pjab.85.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Kim CH, Moon JI, Chung YG, Chang MY, Han BS, Ko S, Yang E, Cha KY, Lanza R, et al. Generation of Human Induced Pluripotent Stem Cells by Direct Delivery of Reprogramming Proteins. Cell Stem Cell. 2009;4:472–476. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin MJ, Muotri A, Gage F, Varki A. Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nature Medicine. 2005;11:228–232. doi: 10.1038/nm1181. [DOI] [PubMed] [Google Scholar]
- Okita K, Matsumura Y, Sato Y, Okada A, Morizane A, Okamoto S, Hong H, Nakagawa M, Tanabe K, Tezuka KI, et al. A more efficient method to generate integration-free human iPS cells. Nature Methods. 2011;8:409–412. doi: 10.1038/nmeth.1591. [DOI] [PubMed] [Google Scholar]
- Seki T, Yuasa S, Oda M, Egashira T, Yae K, Kusumoto D, Nakata H, Tohyama S, Hashimoto H, Kodaira M, et al. Generation of Induced Pluripotent Stem Cells from Human Terminally Differentiated Circulating T Cells. Cell Stem Cell. 2010;7:11–14. doi: 10.1016/j.stem.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Shi Y, Inoue H, Wu JC, Yamanaka S. Induced pluripotent stem cell technology: a decade of progress. Nature Reviews Drug Discovery. 2016;16:115–130. doi: 10.1038/nrd.2016.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers A, Jean JC, Sommer CA, Omari A, Ford CC, Mills JA, Ying L, Sommer AG, Jean JM, Smith BW, et al. Generation of Transgene-Free Lung Disease-Specific Human Induced Pluripotent Stem Cells Using a Single Excisable Lentiviral Stem Cell Cassette. STEM CELLS. 2010;28:1728–1740. doi: 10.1002/stem.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer AG, Rozelle SS, Sullivan S, Mills JA, Park S-M, Smith BW, Iyer AM, French DL, Kotton DN, Gadue P, et al. Generation of Human Induced Pluripotent Stem Cells from Peripheral Blood Using the STEMCCA Lentiviral Vector. Journal of Visualized Experiments. 2012:1–5. doi: 10.3791/4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer CA, Gianotti Sommer A, Longmire TA, Christodoulou C, Thomas DD, Gostissa M, Alt FW, Murphy GJ, Kotton DN, Mostoslavsky G. Excision of Reprogramming Transgenes Improves the Differentiation Potential of iPS Cells Generated with a Single Excisable Vector. STEM CELLS. 2010;28:64–74. doi: 10.1002/stem.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer CA, Stadtfeld M, Murphy GJ, Hochedlinger K, Kotton DN, Mostoslavsky G. Induced Pluripotent Stem Cell Generation Using a Single Lentiviral Stem Cell Cassette. STEM CELLS. 2009;27:543–549. doi: 10.1634/stemcells.2008-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtfeld M, Brennand K, Hochedlinger K. Reprogramming of Pancreatic β Cells into Induced Pluripotent Stem Cells. Current Biology. 2008a;18:890–894. doi: 10.1016/j.cub.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtfeld M, Nagaya M, Utikal J, Weir G, Hochedlinger K. Induced Pluripotent Stem Cells Generated Without Viral Integration. Science. 2008b;322:945–949. doi: 10.1126/science.1162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staerk J, Dawlaty MM, Gao Q, Maetzel D, Hanna J, Sommer CA, Mostoslavsky G, Jaenisch R. Reprogramming of Human Peripheral Blood Cells to Induced Pluripotent Stem Cells. Cell Stem Cell. 2010;7:20–24. doi: 10.1016/j.stem.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, Ebina W, Mandal PK, Smith ZD, Meissner A, et al. Highly Efficient Reprogramming to Pluripotency and Directed Differentiation of Human Cells with Synthetic Modified mRNA. Cell Stem Cell. 2010;7:618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woltjen K, Michael IP, Mohseni P, Desai R, Mileikovsky M, Hämäläinen R, Cowling R, Wang W, Liu P, Gertsenstein M, et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2009;458:766–770. doi: 10.1038/nature07863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Mills JA, Sullivan S, Liu Y, French DL, Gadue P. iPSC Reprogramming from Human Peripheral Blood Using Sendai Virus Mediated Gene Transfer - StemBook - NCBI Bookshelf. StemBook. 2015:1–7. [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. Induced Pluripotent Stem Cell Lines Derived from Human Somatic Cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]