Abstract
Intracellular organelles are membranous structures, central for maintaining cellular physiology and the overall health of the cell. To maintain cellular function, intracellular organelles are required to tightly regulate their ionic homeostasis. Any imbalance in ionic concentrations can disrupt energy production (mitochondria), protein degradation (lysosomes), DNA replication (nucleus) or cellular signaling (endoplasmic reticulum). Ionic homeostasis is also important for volume regulation of intracellular organelles and is maintained by cation and anion channels as well as transporters. One of the major classes of ion channels predominantly localized to intracellular membranes is chloride intracellular channel proteins (CLICs). They are non-canonical ion channels with six homologs in mammals, existing as either soluble or integral membrane protein forms, with dual functions as enzymes and channels. In this overview, we have provided a brief introduction to CLICs, and also summarized recent information on their localization, biophysical properties, and physiological roles.
Keywords: Chloride intracellular channels (CLICs), ion channels, intracellular organelles, mitochondria, localization, physiology
Introduction
Intracellular ion channels have been increasingly implicated in cellular and organ physiology. However, their molecular identity, localization, and biophysical properties are not completely elucidated (Singh, Stefani, & Toro, 2012). Lack of information on their identities, localization and physiological roles result in an incomplete understanding of cellular signaling and their underutilization as therapeutic targets. However, recent developments in establishing molecular identities of intracellular channels (Gururaja Rao et al., 2017; Nichols, Singh, & Grange, 2013; Ponnalagu et al., 2016a; Singh et al., 2013) has thrown light on our understanding of their roles on cellular and organ physiology.
Amongst intracellular channels, Chloride Intracellular Channel proteins (CLICs) are predominantly present in intracellular organelles (Littler et al., 2010; Ponnalagu & Singh, 2017; Singh, 2010), unlike intracellular potassium channels which are also present in the plasma membrane (Nichols et al., 2013; Singh et al., 2013). CLICs lack a membrane targeting leader sequence (Ashley, 2003; Cromer, Morton, Board, & Parker, 2002; Littler et al., 2010; Singh, 2010) and the majority of the protein is found in soluble state. While CLICs were identified 30 years ago, as IAA-94 (Indanyloxyacetic acid 94) sensitive p64 proteins (D. Landry et al., 1993; D. W. Landry et al., 1989; Redhead, Edelman, Brown, Landry, & al-Awqati, 1992), their structures of membranous forms, the mechanism by which they transit between soluble and membrane-anchored forms and their specific role in cell function are still not completely understood. CLICs are conserved across the phyla from bacteria to higher mammals. There are 6 homologs in mammals (CLIC1-6), four in plants (DHAR1-4), two in C. elegans (Exc4 and Exl1), and one each in Drosophila (DmCLIC) and Bacteria (SspA). Mammalian CLICs share ~50–60% sequential homology to each other (Gururaja Rao et al., 2017). Over the last three decades a significant progress has been made in establishing biophysical properties, localization and physiological role of CLICs (Ponnalagu et al., 2016a; Singh, 2010; Ulmasov, Bruno, Woost, & Edwards, 2007) and here we summarize key findings made in the last decade. For other chloride channels the reader is directed to some of the recently published reviews (Argenzio & Moolenaar, 2016; Boedtkjer, Matchkov, Boedtkjer, & Aalkjaer, 2016; De Felice, 2016; Elborn, 2016; Jentsch, 2016; Miller, 2015; Ponnalagu et al., 2016a; Sabirov, Merzlyak, Islam, Okada, & Okada, 2016; Stauber & Jentsch, 2013).
Localization of CLICs
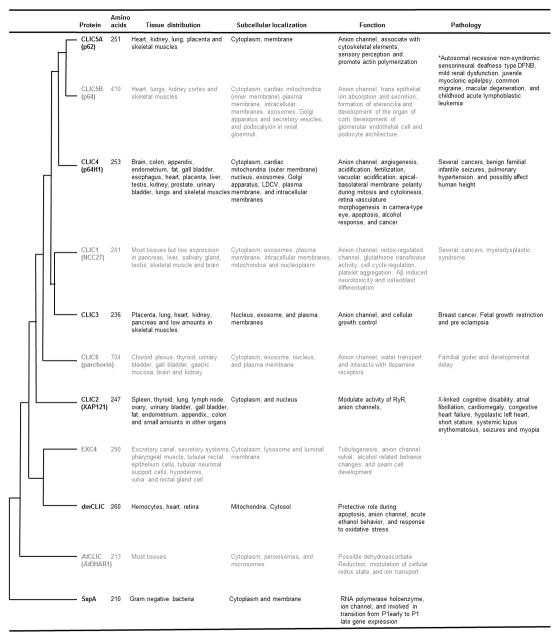
CLICs belong to the family of intracellular ion channels, that includes mitoBKCa, mitoKATP, VDAC, MCU, RyR, and IP3R (Kiselyov & Muallem, 2016; Leanza et al., 2013; O’Rourke, 2007; Singh, Stefani, et al., 2012). There are six mammalian CLIC paralogs and under physiological conditions, the majority of the CLICs are present on intracellular organelles and in the cytoplasm (Singh, 2010; Ulmasov et al., 2017). In addition to intracellular organelle membranes, CLICs can associate with the plasma membrane (Averaimo, Milton, Duchen, & Mazzanti, 2010; Milton et al., 2008; Tang et al., 2017; Warton et al., 2002) under pathological conditions or on overexpression. Information on the localization of CLICs is collectively summarized in Figure 1. Most of this information is obtained from biochemical and immnunocyto/histochemical studies of diseased as well as normal cells and tissues.
Figure 1. Summary of distribution, localization, physiological and pathological roles of family members of CLIC proteins.
There are two major branches in the phylogenetic tree of mammalian CLICs, 1) comprising of CLIC2 which is not present in mice and 2) CLIC1, 3, 4, 5, and 6 present in all mammals. CLICs present in C. elegans and Drosophila are closely associated with mammalian CLICs. Bacterial and plant paralogs were recently characterized and are placed in distal branches of the phylogenetic tree. With the exception of CLIC6 and CLIC5B, all other CLIC paralogs are ~200–300 amino acids long. Tissue distribution of proteins is also indicated and CLICs are ubiquitous in expression. CLIC proteins are present in the cytoplasm as well as other intracellular organelles such as mitochondria, nucleus, exosomes and Golgi apparatus. At functional levels, CLICs form anion channels, participate in cellular physiology, and CLIC1 is known to have enzymatic activity. Changes in expression and roles of mammalian CLICs were demonstrated in several human pathological conditions such as cancer, epilepsy and congenital heart diseases.
Nucleus
The nucleus is the key organelle defining the eukaryotic feature of cells and contains the nuclear genome and molecular components responsible for DNA replication and transcription. The nuclear envelope is important in maintaining ionic homeostasis (Bkaily et al., 2012; Matzke, Weiger, & Matzke, 2010), and is comprised of two membranes: the inner nuclear membrane (INM), and the outer nuclear membrane (ONM). The ONM is continuous with the endoplasmic reticulum (ER) and the space between the INM and ONM is contiguous with the lumen of the ER. These membranes harbor ion channels and transporters including CLICs. The nuclear localization signal (NLS) KKYR (Fig. 2) is highly conserved in mammalian CLIC 1,2,4,5 and 6. In C. elegans, Exl-1 possess a non-canonical NLS, however, Exc-4 like mammalian CLIC3 also lack an NLS (Liang, Shaulov, Savage-Dunn, Boissinot, & Hoque, 2017).
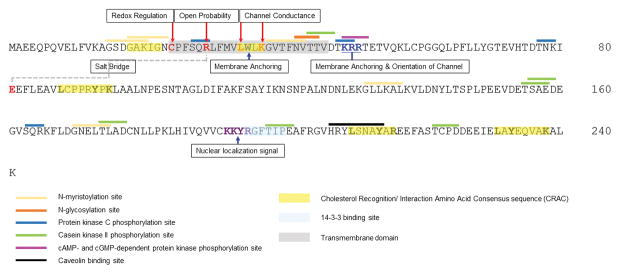
Figure 2. Sequence and key features in the secondary structure of CLIC1.
Key amino acids involved in ion channel properties and membrane anchoring are identified based on structure-function studies (Averaimo et al., 2013; Gururaja Rao et al., 2017; Hare, Goodchild, Breit, Curmi, & Brown, 2016; Liu et al., 2007; Singh & Ashley, 2006) and are highlighted with red and blue arrows within the transmembrane domain (grey box). The conserved nuclear localization signal (KKYR) is highlighted in the purple text. Grey dashed line indicates a salt bridge between R29 and E81 (Legg-E’silva et al., 2012). Key residues involved in modulation of channel activity are highlighted in red. Cholesterol Recognition/Interaction Amino Acid Consensus sequence (CRAC) are highlighted in the yellow box on the basis of specific published studies (Al Khamici et al., 2016) or predicted CRAC sequences (Fantini & Barrantes, 2013). Caveolin binding domain (black bar) (Byrne, Dart, & Rigden, 2012), PTMs (yellow, orange, pink, blue and green lines), 14-3-3 binding site (light blue box) (Johnson et al., 2010), and post-translational modification residues were identified using in silico analysis (Sigrist et al., 2010).
CLIC1 (also known as NCC27) is present in the nucleoplasm, and ONM where it forms a functional channel as shown by nuclear patch clamping (Valenzuela et al., 1997). CLIC3 and CLIC4 on overexpression are localized to the nucleus of Cercopithecus aethiops monkey kidneys (CV)-1 (Qian, Okuhara, Abe, & Rosner, 1999) and HeLa cells (Argenzio et al., 2014), respectively. This is a surprising observation for CLIC3 as it lacks the canonical NLS and perhaps possesses a non-canonical NLS yet to be characterized. In bone marrow-derived macrophages, CLIC4 was observed in the nucleus and its expression increased with LPS stimulation (Domingo-Fernandez, Coll, Kearney, Breit, & O’Neill, 2017). Hepatocellular cancer (HCC) cell lines (HepG2, Huh7, and SNU387) also showed the presence of native CLIC5 in the nucleus (Flores-Tellez, Lopez, Vasquez Garzon, & Villa-Trevino, 2015), and on ectopic expression in HeLa cells, CLIC5 localized to the nucleus (Al-Momany, Li, Alexander, & Ballermann, 2014). These findings place CLIC1 as a native nuclear membrane channel and other CLICs can translocate to nucleus either under specific conditions or on overexpression.
Mitochondria
The key organelle involved in meeting energy demands of the cell is the mitochondria. For over 100 years, mitochondria are known to undergo reversible swelling and contraction in response to changes in pH or osmotic pressure of the buffer (Lewis & Lewis, 1915). Anion channels located in mitochondrial membranes are responsible for regulating ionic homeostasis (O’Rourke, 2007; O’Rourke, Cortassa, & Aon, 2005; Ponnalagu & Singh, 2017). Studies with the Cl- channel inhibitor, IAA-94, showed that CLICs are present in mitochondrial membranes and are involved in maintaining mitochondrial functional integrity (Ponnalagu et al., 2016a). The first CLIC identified to be localized to mitochondria was CLIC4 (also known as mtCLIC4) in the inner membrane (IMM) of mouse keratinocyte mitochondria (Fernandez-Salas, Sagar, Cheng, Yuspa, & Weinberg, 1999). CLIC4 expression increased dramatically on apoptotic responses to p53 and DNA damage (Fernandez-Salas et al., 2002). In adult cardiomyocytes, CLIC4 is enriched in the outer mitochondrial membrane (OMM) (Ponnalagu et al., 2016a), and CLIC5 is present in the IMM, making it as a first inner membrane chloride channel to be identified in cardiac mitochondria (Ponnalagu et al., 2016a). In osteoblastic cells derived from mice, rats, and humans, CLIC1 was expressed in the mitochondrial membranes (Yang et al., 2009). In adult cardiac mitochondria, CLIC5 was implicated in cellular ROS generation, which was recently linked with mitochondrial stress and inflammasome activation to modulate inflammatory response (Tang et al., 2017). The only CLIC present in Drosophila is also found in the cardiac mitochondria implying conserved functional roles of CLICs in mitochondria (Ponnalagu et al., 2016a). Even though CLICs are implicated in mitochondrial functional integrity and mitochondrial-mediated cellular signaling, a complete understanding of CLICs in mitochondrial physiology and mitochondrial-mediated cellular signaling is not yet available.
Other cellular organelles
CLIC1 has been localized to phagosomes where it has been implicated in acidification and proteolysis (Jiang et al., 2012; Salao et al., 2016). In the endoplasmic reticulum (ER), CLIC1 (Ponnalagu et al., 2016b) and CLIC2 (Meng et al., 2009) were shown to be present, and CLIC2 is also known to interact with RyR1 possibly in the ER. In the Golgi complex, CLIC5 is associated with AKAP350 in HCA-7 colonic adenocarcinoma cells (Shanks et al., 2002). Most of the CLIC proteins are found in soluble forms, which make them accessible for interaction with cellular components in the cytosol. CLIC5 is one of the best-characterized cytosolic CLICs, interacting with actin and ezrin (Berryman & Bretscher, 2000; Gagnon et al., 2006) involved in maintaining the structural integrity of cells as shown in podocytes (Edwards, 2010; Pierchala, Munoz, & Tsui, 2010) and stereocilia (Salles et al., 2014). CLIC4 was also shown to be colocalized with ezrin, radixin, moesin (ERM) proteins (Tavasoli et al., 2016). The channel activity of CLIC5 and CLIC1 can be modulated by cytoskeletal filaments (Singh, Cousin, & Ashley, 2007). The extensive work relating CLIC5 to cytoskeletal filaments indicates that CLIC proteins could play an important role in cellular physiology by maintaining cytoskeletal filament integrity.
The key limitation in understanding the localization of CLIC proteins in cellular organelles is the lack of availability of specific antibodies for some of the members. In addition, some of the antibodies used are without appropriate controls to verify their quality and specificity. All the information assimilated above should be interpreted cautiously specifically for stated cell types given that CLICs can show changes in their localization according to the cell type and physiological condition.
Factors governing localization of CLICs
CLICs are known to localize to several intracellular membranes and how these dimorphic proteins unfold and refold to insert into membranes is not well-understood. However, several in silico and experimental evidence have identified regions responsible for their localization and transportation to specific cellular compartments and membranes (Fig. 2). One of the key components of bilayers presumed essential for insertion of CLICs to cellular membranes is cholesterol and CLICs possess at least five putative cholesterol interacting sites. One site is present immediately at the top of the pore-forming region, and another site is present in the transmembrane domain. Three sites are present in the C-terminus with one overlapping with the putative caveolin binding site. Cholesterol is essential for spontaneous insertion into the bilayer possibly by facilitating docking of CLICs, and formation of consistent ion channel (Goodchild, Angstmann, Breit, Curmi, & Brown, 2011; Singh & Ashley, 2007; Valenzuela et al., 2013). It is well-known that cholesterol is not present in the mitochondrial membranes or bacterial membranes under physiological conditions (Bosch, Mari, Gross, Fernandez-Checa, & Pol, 2011; Eaton, Erdos, Vreeland, & Ingram, 1981; Elustondo, Martin, & Karten, 2017). A small amount of cholesterol makes these membranes more susceptible to any physiological or pathological changes. In bacteria, the addition of cholesterol alters membrane fluidity (Eaton et al., 1981) without affecting the growth rate. In eukaryotic cells, the source of cholesterol in the majority of intracellular organelles is plasma membrane or directly by steroidogenesis. Under pathological conditions such as cancer, steatohepatitis, Alzheimer’s disease and Niemann-Pick Type C1-deficiency, there is a significant increase in the cholesterol content of mitochondrial membranes (Bosch et al., 2011).
Two important motifs were identified as being responsible for membrane anchoring in CLIC1 (Fig. 2); W at position 35 (Al Khamici, Hossain, Cornell, & Valenzuela, 2016) and KKR at 49–51(Peter, Fanucchi, & Dirr, 2014). The KKR region is involved in orienting the channel with respect to the C24 to trans side (Peter et al., 2014). In the absence of KKR, the orientation of CLIC1 was disrupted and was retained in the bilayer. CLIC4 is known to interact with dynamin 1 in a protein complex containing actin, tubulin and 14-3-3 isoforms (Suginta, Karoulias, Aitken, & Ashley, 2001), however, whether this interaction governs localization or trafficking of the channel is not deciphered. With respect to localization of CLIC5 to the plasma membrane. previous studies revealed that the last six residues of CLIC5 function to prevent expression on the plasma membrane of Panc-1 cells (Edwards, 1999). Also, it was predicted that the N-terminal half has a dominant influence over the C terminus in determining the subcellular distribution and trafficking of intact p64 (Edwards, 1999). Thus, cholesterol and motifs on CLICs are major players in determining membrane localization of CLICs.
One of the key factors in regulating protein localization and its function in response to physiological and pathological changes are posttranslational modifications (PTMs). PTMs can modulate several properties of proteins including their enzymatic activity, interaction with other cellular components and their subcellular localizations. In absence of extensive structure-function and biochemical studies, in silico prediction of PTMs has played an important role in identifying key PTMs occurring in a large number of uncharacterized proteins. As shown in Fig. 2, CLICs also possess several PTMs such as myristoylation, glycosylation and phosphorylation present either near the transmembrane domain or in the C-terminus. However, further experimental evidence should be carried out to investigate the role of these PTMs in regulating CLICs in intracellular organelles.
Biophysical properties of CLICs
CLIC proteins are known to auto insert into membranes of intracellular organelles where they form functional ion channels. Currents mediated by CLICs have been recorded from native membranes (nuclei), ectopically expressed proteins (HEK 293 cells) and planar bilayers using electrophysiological techniques. Even though CLICs are known to form ion channels, in absence of structural information, the ion conduction mechanism is not completely deciphered. Although the pattern of ionic selectivity for CLICs has emerged from studies of permeation and structure-function, they offer limited insights into the nature of the pore. As noted above, CLICs have the ability to form ion channels as well as exist as soluble proteins. To form functional ion channels, the N-terminus undergoes a structural reorganization to insert into a membrane as an oligomer (Fanucchi, Adamson, & Dirr, 2008; Peter et al., 2014). Either an individual oligomer or several of these oligomers coordinate to form a channel in intracellular membranes (Goodchild et al., 2011; Singh, 2010). Reversal potential measurements and ion substitution experiments in planar lipid bilayers indicate that CLIC channels can conduct both anions and cations (Gururaja Rao et al., 2017; Singh & Ashley, 2006, 2007). Under ectopic expression system as well as in planar bilayers, these channels indicate a preference for anions over larger cations (Averaimo et al., 2013; Tulk, Kapadia, & Edwards, 2002; Valenzuela et al., 2000).
Information on anion selectivity for CLICs is chiefly obtained from the reconstituted planar bilayer system. CLICs follow the lyotropic (mesophasic transition as a function of the concentration of ions) selectivity pattern in which permeability increases monotonically with decreasing hydration energies. For more detailed information on ion selectivity of CLICs please refer these reviews (Averaimo et al., 2013; Singh, 2010). Generally, CLICs do not discriminate between different anions, and the sort of pattern (large to small) and the relative non-selectivity of the pore suggest that these channels exhibit bias-type selectivity that is probably not associated with specific structural components such as a selectivity filter. Bias-type selectivity is characterized by patterns of ion size and little reliance on local geometry. The presence or absence of selectivity filter is yet to be verified by structure-function work, however, the impression is reinforced by the similarity of the selectivity pattern to that exhibited by CLIC1 (Averaimo et al., 2013). In a direct analysis of the channel pore vestibule of CLIC1, lysine 37 (Fig. 2) changed the channel conductance which was attributed to the removal of positively charged residues hence reducing the residence time for anions in the channel pore (Averaimo et al., 2013). In the same study arginine 29 (Fig. 2) altered the open probability without altering the selectivity (Averaimo et al., 2013) which could be attributed to the changes in the putative voltage-sensing domain of the transmembrane domain. In the predicted model, these two amino acids line the pore, where Arginine forms a ring at the top and Lysine is in the central region of the pore (Singh, 2010) and observations demonstrating changes in channel conductance and open probability support the predicted model. The Cysteine residue (position 24 in CLIC1) at the N-terminus conserved amongst the majority of CLIC proteins also changes the single-channel conductance of CLIC1 protein (Singh & Ashley, 2006) albeit removing the redox sensitivity of the protein (Fig. 2). Recently, a prokaryote homolog of CLICs, stringent starvation protein A (SspA) formed a channel in the planar bilayers(Gururaja Rao et al., 2017). The conserved amino acid in the pore region (Leucine 29, Fig. 2) of SspA resulted in increased conductance which again could be attributed to increase in flexibility of the pore region by disrupting the hydrophobic interaction with the lipid bilayer in the predicted model (Singh, 2010).
Findings to date suggest that the channel pore is a primitive structure and although CLICs are well-conserved from prokaryotes to human beings, the pore has not evolved a mechanism for the highly-selective recognition of anions or specifically Cl− ions (Averaimo et al., 2013; Gururaja Rao et al., 2017; Singh, 2010; Singh & Ashley, 2006). Smaller anions, like F−, have difficulty entering the pore due to their relatively high energy of hydration. Large molecules like SCN− actually appear to enter the pore more readily than Cl− (due to their lower ΔGhyd) but are retarded in their passage by a deeper energy well in the conduction path. The end result is a channel that is, in fact, only moderately selective, permitting ions like Br− and NO3− to transit with ease, which nevertheless is functionally adequate. It has been suggested that the relatively low abundance of these competing ions reduced the pressure for the evolution of a more selective structure (Dawson, Smith, & Mansoura, 1999), hence the pore region of CLIC is not evolved like other ion channels (Jegla, Zmasek, Batalov, & Nayak, 2009)
With just a single transmembrane domain, CLICs form functional ion channels but the number of CLIC monomers required or the number of pores per functional channel is not yet established (Ashley, 2003). A minimum of four monomers are required to form a functional channel (Singh & Ashley, 2006) and several of these functional units or monomers can assemble to form a coordinated mega-channel as reported for CLIC5 (Singh et al., 2007), Intermolecular FRET studies show that CLIC1 forms oligomers comprising 6–8 subunits upon oxidation in the presence of the membranes (Goodchild et al., 2011). All CLICs are present in the cytosol as soluble fractions; however, it is not known whether these CLICs can also interact with one other and form heteromers. Focused interaction studies on CLICs to identify their binding partners could provide beneficial insights (Singh et al., 2016; Singh, Warburton, Vondriska, & Khakh, 2009). Formation of heteromers may explain differences observed amongst native intracellular anion channels vs ectopically expressed or recombinant proteins reconstituted in planar bilayers. If CLICs can form heteromers, it will open a new direction in the CLIC field and provide new information on their physiological roles.
The channel insertion and activity of mammalian and bacterial CLICs are known to enhance at lower pH (Averaimo et al., 2013; Gururaja Rao et al., 2017; Warton et al., 2002). Open probability of CLIC-mediated currents increases at lower pH as compared to the neutral pH. Structure dynamics of CLIC1 by amide hydrogen-deuterium exchange mass spectrometry indicated that the flexibility of domain 1 of the CLIC1 increases at lower pH (5.5) (Fanucchi et al., 2008). In another study, Dirr and Colleagues also showed a presence of the salt bridge between Arginine (R) 21 and Glutamic acid (E) 81 in CLIC1 (Fig. 2) (Legg-E’silva et al., 2012). The E-R salt bridge is known to modulate open probability of several ion channels (Leng, MacGregor, Dong, Giebisch, & Hebert, 2006; Moroni & Thiel, 2006; Zhao et al., 2016). Acidic pH as in the case of K channel will break the critical salt bridge between different domains, and hence could also be directly modulating the channel activity of CLICs. As shown in Figure 2, Arginine 29 is also present in the putative phosphorylation site, hence this residue will also participate in the regulation of CLICs by phosphorylation. Further mutagenesis studies and recording channel activities could provide more information on importance of E-R salt bridge and its association with regulation of channel activity at different acidic environment
Pharmacology of CLICs
Like other Cl channels, pharmacological agents (agonists and antagonists) for CLICs are extremely limited. The first chloride intracellular channel was isolated as an IAA-94 sensitive channel (now known as CLIC5b) (D. W. Landry et al., 1989; Redhead et al., 1992). Later various members of the CLIC family in eukaryotes and prokaryotes were shown to be targets for IAA-94 including SspA, CLIC1, CLIC3, CLIC4, and CLIC5 (Gururaja Rao et al., 2017; Peretti et al., 2014; Singh, 2010). Other known Cl channel blockers such as A9C and DIDS have been tested on CLICs where only A9C but not DIDS is known to act on CLICs (Ponnalagu & Singh, 2017). Recently it was shown that IAA-94 and A9C also inhibit the enzymatic activity of CLICs (Al Khamici et al., 2015). Pharmacological inhibition of CLICs by IAA-94 is shown to reduce tumor growth as well as prevent neurodegeneration (Skaper, Facci, & Giusti, 2013). While IAA-94 is specific to CLICs at lower concentrations, it can act on other Cl channels at higher concentrations. Moreover, given how IAA94 and A9C are not specific to a particular CLIC protein and the functional versatility of various CLICs, future research is needed to focus on modified versions of IAA-94 or new molecules that are more specific to CLICs, and further to individual CLICs to take this drug into therapeutic levels. One of the biggest challenges for discovering specific molecules for CLICs is the absence of a crystal structure of the membrane form. Another limiting factor for specific pharmacological studies is the lack of specialized techniques to study intracellular organelles and ion channels associated with them. This has been circumvented to a certain extent over the last decade by usage of immuno-organelle chemistry (Singh, Lu, et al., 2012), mass spectrometry (Singh et al., 2013; Singh et al., 2009) and organellar patch clamping (Kirichok, Krapivinsky, & Clapham, 2004; Mazzanti, DeFelice, Cohn, & Malter, 1990).
Recent studies have identified the diabetic drug metformin to be targeting CLIC1 directly in human glioblastoma cells (Gritti et al., 2014). Metformin’s antiproliferative effects in cancer stem cell-enriched cultures of human glioblastomas are mimicked by metformin mediated inhibition of CLIC1. This inhibition was related to CLIC1 ion channel activity at clinically significant concentrations. This important discovery posed CLIC1 as a target in cancer therapy via metformin. This also suggests CLIC1 could be involved in metformin mediated diabetic control, mechanisms of which are still to be explored. Another exciting study has implicated Tanshinone IIA Sodium Sulfonate (STS), a traditional Chinese medicine used for treating cardiovascular diseases for its antioxidant and anti-inflammatory properties (Zhu et al., 2017) to be acting on CLICs. CLIC1 was found to be the target of STS in this study, and CLIC1 depletion inhibited the anti-oxidant and anti-inflammatory effects of STS (Zhu et al., 2017). It was also found that STS regulates the translocation of CLIC1 from cytosol to the plasma membrane as well as Cl conductance.
These studies place CLICs as attractive targets for several pathophysiological conditions including cancer, neurological, and cardiovascular conditions.
Physiological Roles of CLICs
After around 30 years of their discovery, it is still not understood how these soluble proteins unfold, insert into the membrane and then refold to form integral membrane proteins. In their soluble form, they exhibit structural similarity to GST family of proteins. Functionally, it is well established that three of the six mammalian paralogs, CLIC1, CLIC2, and CLIC4 demonstrate glutaredoxin-like glutathione-dependent oxidoreductase enzyme activity (Al Khamici et al., 2015). CLICs localizing to different intracellular organelles probably explains their significance in multiple cellular and physiological processes (summarized in Fig. 1). They are well-characterized for their role in cancer (Peretti et al., 2014; Suh, Malik, Shukla, & Yuspa, 2007) and in this section, we have provided an overview of individual CLIC proteins in other physiological and pathophysiological roles.
CLIC1 was cloned based on its enhanced expression in activated human macrophages and is highly conserved across several species (Valenzuela et al., 1997). It is predominantly involved in cell cycle regulation, cell proliferation, and differentiation (P. Wang et al., 2012). CLIC1 is highly expressed in hepatocellular carcinoma (Li et al., 2012; Song, Tang, Sun, Liu, & Wang, 2010; Wei et al., 2015), gall bladder carcinoma (J. W. Wang et al., 2009), and colorectal carcinoma (Petrova et al., 2008; P. Wang et al., 2012) and is considered as a potential prognostic biomarker for tumors. CLIC1 has a neuronal localization where it is expressed in primary or immortalized microglial cultures (Novarino et al., 2004). This results in enhanced production of proinflammatory and neurotoxic products as well as reactive oxygen species (ROS) by amyloid-β (Aβ)-stimulated microglial cells causing neurodegeneration (Averaimo et al., 2010; Milton et al., 2008; Skaper et al., 2013). Pharmacological inhibition by its inhibitor IAA-94 or small interfering RNAs (siRNA) in microglial cells prevented neurodegeneration, suggesting a therapeutic potential of CLIC1 (Paradisi et al., 2008; Skaper et al., 2013). CLIC1 null mutant mice showed lower ADP-stimulated platelet aggregation (Qiu et al., 2010) and in patients with myelodysplastic syndromes, CLIC1 expression was found to be down-regulated (Frobel et al., 2013).
CLIC1 is also shown to impact the innate arm of the immune system. It is highly expressed in macrophages and modulates the phagosomal proteolytic activity of macrophages via ROS generation (Jiang et al., 2012; Ulmasov et al., 2017). Furthermore, RAW 264.7 macrophages upon interaction with heat-inactivated Candida albicans cells, showed decreased expression of CLIC1 (Martinez-Solano, Reales-Calderon, Nombela, Molero, & Gil, 2009) and suppressed TNF-α levels suggesting the importance of CLIC1 in macrophage functions (J. S. Kim et al., 2010). Unlike macrophages, CLIC1 does not modulate ROS production by cardiac mitochondria isolated from mice suggesting tissue-specific regulation of CLIC activity (Ponnalagu et al., 2016b). Overexpression of CLIC1 in endothelial cells was also associated with increased atherosclerotic plaque development, oxidative stress, and inflammatory cytokines release (Xu et al., 2016). These findings regarding the functional role of CLIC1 in inflammation may represent a novel target for the identification of novel anti-inflammatory drugs and is worthy of additional research.
The genetic loci of CLIC2 reside in the telomeric region of Xq28 (Heiss & Poustka, 1997). It is predominantly expressed in skeletal and cardiac muscles, where it negatively regulates the activities of ryanodine receptor 1 (RyR1) and RyR2 channels respectively (Dulhunty, Gage, Curtis, Chelvanayagam, & Board, 2001; Meng et al., 2009). Therefore, CLIC2 has a significant role in regulating calcium signaling mediated by RyRs of cardiac and skeletal muscles (Takano et al., 2012). In neuronal cells, where both RyRs are present, their dysfunction leads to neurological disorders (Witham, Takano, Schwartz, & Alexov, 2011). A mutation in CLIC2 (H101Q) stimulated the activity of RyR channels and has been associated with X-linked intellectual disability (ID) in males, atrial fibrillation, cardiomegaly, congestive heart failure (CHF), seizures and cognitive impairment (Andersen, Baldwin, Ellingwood, Smith, & Lamb, 2014; Chen et al., 2016; Witham et al., 2011). The residue H101 resides in the joint loop structure of CLIC2 (Cromer et al., 2007). In silico modeling suggest that the mutation rigidifies the joint loop and inhibits the conformational change required for conversion of a soluble form of CLIC2 to membranous form (Witham et al., 2011), thus preventing the interaction of CLIC2 with RyRs. CLIC2 mRNA was also down-regulated in dilated cardiomyopathy patients (Molina-Navarro et al., 2013), further disrupting the RyR regulation and suggesting a key role of CLIC2 in maintaining cardiac physiology. Increasing evidence has shown changes in expression of CLIC2 in hypolastic left heart, short stature, systemic lupus erythematosus, intellectual and developmental disabilities (Andersen et al., 2014; Chen et al., 2016; Syahidatulamali, Wan Syamimee, Azwany, Wong, & Che Maraina, 2017). These changes in expression of CLIC2 in several pathological conditions need to be further evaluated to understand the specific role of CLIC2.
CLIC3 was discovered as an interacting partner of ERK7, a member of mitogen-activated protein kinase family (Qian et al., 1999). Similar to CLIC1, it shows nuclear localization and exhibits channel activity when expressed in cells (Qian et al., 1999). It is also required for activation of macrophages as it was required for killing of CRIg-mediated Listeria monocytogenes (LM) by the formation of anti-intracellular bacterial phagosomes (K. H. Kim et al., 2013). CLIC3 has also been implicated in pregnancy disorders such as fetal growth restriction as well as pre-eclampsia conditions (Murthi et al., 2012). CLIC3 has been localized to the syncytiotrophoblast and villous cytotrophoblast cells in human placenta from first trimester and term pregnancies. The exact function of CLIC3 or whether it exists as a channel in placental tissue is yet to be determined (Murthi et al., 2012). Microarray analysis revealed alteration of CLIC3 expression in ischemic cardiomyopathy patients (ICM) (Gronich, Kumar, Zhang, Efimov, & Soldatov, 2010) which might affect the membrane potential, intracellular pH, and cell volume.
Bovine renal protein CLIC4, also known as p64H1 was the first CLIC protein identified at the molecular level (D. Landry et al., 1993) and was termed as mtCLIC4 because of its localization in keratinocyte mitochondria (Fernandez-Salas et al., 2002; Suh et al., 2004). Recently, similar mitochondrial-specific localization (to the outer membrane) of CLIC4 was observed in isolated rat cardiomyocytes (Ponnalagu et al., 2016a). This makes CLIC4 as the second Cl channel present in the outer mitochondrial membrane after VDAC. CLIC4 was found to be associated in a complex with dynamin I, two 14-3-3 isoforms, β-actin, α-tubulin and creatine kinase and a direct interaction was observed with dynamin I and 14-3-3ζ suggesting their role in many cellular functions (Suginta et al., 2001). In addition, CLIC4 expression is required in multiple stages of angiogenesis as suppression of CLIC4 decreased cell proliferation, capillary network formation, capillary-like sprouting, and lumen formation (Bohman et al., 2005; Tung, Hobert, Berryman, & Kitajewski, 2009; Ulmasov, Bruno, Gordon, Hartnett, & Edwards, 2009). CLIC4 also influences angiogenesis by acting upstream of hypoxia-inducible factor (HIF-1α) and vascular endothelial growth factor (VEGF) signaling. Its role in angiogenesis explains its increased expression in many cancers and may represent a novel target for cancer therapeutics (Chiang, Chou, Chien, Tsai, & Chen, 2013; Peretti et al., 2014; Suh et al., 2012; Suh et al., 2007; Suh et al., 2004). CLIC4 plays a role in the acidification of intracellular vacuoles that are required for tubulogenesis (Ulmasov et al., 2009). Clic4−/− mice exhibited aberrant collateral circulation in response to ischemic injury (Chalothorn, Zhang, Smith, Edwards, & Faber, 2009; Lucitti, Tarte, & Faber, 2015).
CLIC4 was highly expressed in the lungs of patients with pulmonary arterial hypertension (PAH) in comparison to healthy patients (Wojciak-Stothard et al., 2014) and regulated the activity of the transcription factors, NF-κB and HIF-1, that are responsible for endothelial responses to inflammatory and angiogenic stimuli. Clic4−/− mice showed an attenuated development of PAH and reduced expression of HIF-1α in response to hypoxia (Wojciak-Stothard et al., 2014). Therefore, reducing CLIC4 expression in PAH patients could help in treating these patients. Consistent with its role in angiogenesis, clic4−/− mice have smaller kidneys with fewer glomeruli and less dense peritubular capillary network (Edwards, Bruno, Key, & Cheng, 2014). They suffer from albuminuria and are susceptible to acute kidney injury but there is no effect on the functional recovery or fibrosis following injury (Edwards et al., 2014). CLIC4 in association with CLIC5 can mediate ERM activation which is required for maintenance of the glomerular capillary architecture (Tavasoli et al., 2016). Inhibiting CLIC4 expression increased β-cell survival possibly caused via the increased cellular expression of Bcl-2, Bcl-xL and Bad phosphorylation (Patel, Ythier, Brozzi, Eizirik, & Thorens, 2015). Bcl-2 or Bcl-xL overexpression in β-cells increases their resistance to cytokine-induced apoptosis (Patel et al., 2015). Thus, targeting clic4 expression, its downstream signaling pathway or its interactors may provide a novel way to prevent β-cell apoptosis in the context of diabetes.
CLIC5B, an alternative transcript variant was the first member of chloride intracellular channel to be isolated from bovine kidney cortex vesicles and apical membranes of the trachea and was initially called as p64 (D. W. Landry, Akabas, Redhead, & al-Awqati, 1990; D. W. Landry et al., 1989; D. W. Landry, Reitman, Cragoe, & Al-Awqati, 1987). In avian species, an ortholog of CLIC5B known as p62 plays a role in osteoclast bone resorption and acidification of osteoclast vesicles (Edwards, 2006). There are three transcript variants of CLIC5 in mammals. An N-terminus alternative transcript variant, namely CLIC5A was initially isolated in a complex with ezrin from human placental microvilli (Berryman & Bretscher, 2000). Further, its association with F-actin suggests a role in the assembly of F-actin based structures (Berryman, Bruno, Price, & Edwards, 2004). It is also observed to interact with ERM proteins complex and playing a role in cellular architecture. Interaction of CLIC5 with ERM complex in glomeruli is necessary to maintain the architecture of endothelial as well as podocytes (Pierchala et al., 2010; Tao et al., 2015; Tavasoli et al., 2016; Wegner et al., 2010). The predominant phenotype of clic5−/− mice is inner ear dysfunction and hearing loss (Gagnon et al., 2006). In a family of Turkish origin, a homozygous nonsense mutation in CLIC5 (Cys32Ter) caused the progressive hearing impairment, vestibular and possibly mild renal dysfunction (Seco et al., 2015). Clic5−/− mice also exhibit metabolic defect as they are resistant to diet-induced obesity and compromised capacity for storing energy causing a fasting-induced gastric hemorrhaging CLIC5 is the first inner mitochondrial membrane (IMM) channel characterized to the molecular level as it was recently shown to be localized to the IMM of cardiac mitochondria (Ponnalagu et al., 2016a). In cardiac mitochondria, CLIC5 is known to modulate generation of reactive oxygen species (Ponnalagu et al., 2016a). As mitochondria are a key regulator of cell death pathways, the presence of CLIC5 in the IMM and playing a role in mitochondrial function signifies its importance in many cellular processes, which needs to be further explored.
CLIC6, also known as parchorin, is the largest member of the CLIC family with a molecular size of 120 kDa (Friedli et al., 2003). It is highly enriched in water secreting tissues such as parietal cells, choroid plexus, salivary duct and lacrimal gland, kidney, and airway epithelia (Friedli et al., 2003). In fact, it was earlier referred as parchorin because of its presence in parietal cells and choroid plexus (Debska, Kicinska, Skalska, & Szewczyk, 2001; Friedli et al., 2003). CLIC6 might be involved in gastric HCl secretion and chloride ion transport (Shin, Munson, Vagin, & Sachs, 2009). Using yeast two-hybrid system CLIC6 was identified as the interacting partners of dopamine-like receptors (D2R, D3R, and D4R) but the physiological relevance is not known (Griffon, Jeanneteau, Prieur, Diaz, & Sokoloff, 2003). CLIC6 is one of the least studied CLICs, probably due to its absence in various cells and tissues.
Conclusions
CLICs have been recognized as bona fide ion channels, as channel modulators, for their enzymatic activity, and for their roles in physiology and pathological conditions. However, a cause for concern in targeting CLIC channel protein in a single disease pathology is the ubiquitous nature of the proteins and their involvement in function and regulation of the normal physiologic state in cells of varied etiology. Though several studies have focused on identification of specific interactors of CLICs, the protein interactome (Singh et al., 2016; Singh et al., 2009) of individual CLICs is not yet identified. The major challenge for studies focusing on CLIC proteins is the absence of specific and selective pharmacological agents. The absence of crystal structure of membranous forms is another limitation for exploiting CLICs as ion channels. However, with recent key studies focusing on their structure-function, localization, and physiology, CLICs would present themselves for diagnostic, prognostic, and therapeutic applications for a multitude of human pathologies.
Acknowledgments
We thank Prof. Adam Szewczyk (Nencki Institute of Experimental Biology) and Dr. Richard H. Ashley (University of Edinburgh) for helpful discussions on CLIC literature. This work is supported by the Commonwealth Universal Research Enhancement (CURE) Program Grants to SGR and HS, American Heart Association Postdoctoral Fellowship (17POST33670360) to DP, a grant from the W. W. Smith Charitable Trust, American Heart Association National Scientist Development Grant (11SDG230059), American Heart Association Grant-in-Aid (16GRNT29430000), National Institute of Health (R01-HL133050-01) and Drexel University College of Medicine startup funds to HS.
References
- Al-Momany A, Li L, Alexander RT, Ballermann BJ. Clustered PI(4,5)P(2) accumulation and ezrin phosphorylation in response to CLIC5A. J Cell Sci. 2014;127(Pt 24):5164–5178. doi: 10.1242/jcs.147744. [DOI] [PubMed] [Google Scholar]
- Al Khamici H, Brown LJ, Hossain KR, Hudson AL, Sinclair-Burton AA, Ng JP, … Valenzuela SM. Members of the chloride intracellular ion channel protein family demonstrate glutaredoxin-like enzymatic activity. PLoS One. 2015;10(1):e115699. doi: 10.1371/journal.pone.0115699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Khamici H, Hossain KR, Cornell BA, Valenzuela SM. Investigating Sterol and Redox Regulation of the Ion Channel Activity of CLIC1 Using Tethered Bilayer Membranes. Membranes (Basel) 2016;6(4) doi: 10.3390/membranes6040051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen EF, Baldwin EE, Ellingwood S, Smith R, Lamb AN. Xq28 duplication overlapping the int22h-1/int22h-2 region and including RAB39B and CLIC2 in a family with intellectual and developmental disability. Am J Med Genet A. 2014;164A(7):1795–1801. doi: 10.1002/ajmg.a.36524. [DOI] [PubMed] [Google Scholar]
- Argenzio E, Margadant C, Leyton-Puig D, Janssen H, Jalink K, Sonnenberg A, Moolenaar WH. CLIC4 regulates cell adhesion and beta1 integrin trafficking. J Cell Sci. 2014;127(Pt 24):5189–5203. doi: 10.1242/jcs.150623. [DOI] [PubMed] [Google Scholar]
- Argenzio E, Moolenaar WH. Emerging biological roles of Cl- intracellular channel proteins. J Cell Sci. 2016;129(22):4165–4174. doi: 10.1242/jcs.189795. [DOI] [PubMed] [Google Scholar]
- Ashley RH. Challenging accepted ion channel biology: p64 and the CLIC family of putative intracellular anion channel proteins (Review) Mol Membr Biol. 2003;20(1):1–11. doi: 10.1080/0968768021000042746. [DOI] [PubMed] [Google Scholar]
- Averaimo S, Abeti R, Savalli N, Brown LJ, Curmi PM, Breit SN, Mazzanti M. Point mutations in the transmembrane region of the clic1 ion channel selectively modify its biophysical properties. PLoS One. 2013;8(9):e74523. doi: 10.1371/journal.pone.0074523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averaimo S, Milton RH, Duchen MR, Mazzanti M. Chloride intracellular channel 1 (CLIC1): Sensor and effector during oxidative stress. FEBS Lett. 2010;584(10):2076–2084. doi: 10.1016/j.febslet.2010.02.073. [DOI] [PubMed] [Google Scholar]
- Berryman M, Bretscher A. Identification of a novel member of the chloride intracellular channel gene family (CLIC5) that associates with the actin cytoskeleton of placental microvilli. Mol Biol Cell. 2000;11(5):1509–1521. doi: 10.1091/mbc.11.5.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryman M, Bruno J, Price J, Edwards JC. CLIC-5A functions as a chloride channel in vitro and associates with the cortical actin cytoskeleton in vitro and in vivo. J Biol Chem. 2004;279(33):34794–34801. doi: 10.1074/jbc.M402835200. [DOI] [PubMed] [Google Scholar]
- Bkaily G, Avedanian L, Al-Khoury J, Ahmarani L, Perreault C, Jacques D. Receptors and ionic transporters in nuclear membranes: new targets for therapeutical pharmacological interventions. Can J Physiol Pharmacol. 2012;90(8):953–965. doi: 10.1139/y2012-077. [DOI] [PubMed] [Google Scholar]
- Boedtkjer E, Matchkov VV, Boedtkjer DM, Aalkjaer C. Negative News: Cl- and HCO3- in the Vascular Wall. Physiology (Bethesda) 2016;31(5):370–383. doi: 10.1152/physiol.00001.2016. [DOI] [PubMed] [Google Scholar]
- Bohman S, Matsumoto T, Suh K, Dimberg A, Jakobsson L, Yuspa S, Claesson-Welsh L. Proteomic analysis of vascular endothelial growth factor-induced endothelial cell differentiation reveals a role for chloride intracellular channel 4 (CLIC4) in tubular morphogenesis. J Biol Chem. 2005;280(51):42397–42404. doi: 10.1074/jbc.M506724200. [DOI] [PubMed] [Google Scholar]
- Bosch M, Mari M, Gross SP, Fernandez-Checa JC, Pol A. Mitochondrial cholesterol: a connection between caveolin, metabolism, and disease. Traffic. 2011;12(11):1483–1489. doi: 10.1111/j.1600-0854.2011.01259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne DP, Dart C, Rigden DJ. Evaluating caveolin interactions: do proteins interact with the caveolin scaffolding domain through a widespread aromatic residue-rich motif? PLoS One. 2012;7(9):e44879. doi: 10.1371/journal.pone.0044879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalothorn D, Zhang H, Smith JE, Edwards JC, Faber JE. Chloride intracellular channel-4 is a determinant of native collateral formation in skeletal muscle and brain. Circ Res. 2009;105(1):89–98. doi: 10.1161/CIRCRESAHA.109.197145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CP, Chen CY, Chern SR, Wu PS, Chen YN, Chen SW, … Wang W. Molecular cytogenetic characterization of Xp22.32-->pter deletion and Xq26.3-->qter duplication in a male fetus associated with 46,Y,rec(X)dup(Xq) inv(X)(p22.3q26.3), a hypoplastic left heart, short stature, and maternal X chromosome pericentric inversion. Taiwan J Obstet Gynecol. 2016;55(5):705–711. doi: 10.1016/j.tjog.2016.05.009. [DOI] [PubMed] [Google Scholar]
- Chiang PC, Chou RH, Chien HF, Tsai T, Chen CT. Chloride intracellular channel 4 involves in the reduced invasiveness of cancer cells treated by photodynamic therapy. Lasers Surg Med. 2013;45(1):38–47. doi: 10.1002/lsm.22112. [DOI] [PubMed] [Google Scholar]
- Cromer BA, Gorman MA, Hansen G, Adams JJ, Coggan M, Littler DR, … Parker MW. Structure of the Janus protein human CLIC2. J Mol Biol. 2007;374(3):719–731. doi: 10.1016/j.jmb.2007.09.041. [DOI] [PubMed] [Google Scholar]
- Cromer BA, Morton CJ, Board PG, Parker MW. From glutathione transferase to pore in a CLIC. Eur Biophys J. 2002;31(5):356–364. doi: 10.1007/s00249-002-0219-1. [DOI] [PubMed] [Google Scholar]
- Dawson DC, Smith SS, Mansoura MK. CFTR: mechanism of anion conduction. Physiol Rev. 1999;79(1 Suppl):S47–75. doi: 10.1152/physrev.1999.79.1.S47. [DOI] [PubMed] [Google Scholar]
- De Felice LJ. Chloride requirement for monoamine transporters. Pflugers Arch. 2016;468(3):503–511. doi: 10.1007/s00424-015-1783-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debska G, Kicinska A, Skalska J, Szewczyk A. Intracellular potassium and chloride channels: an update. Acta Biochim Pol. 2001;48(1):137–144. [PubMed] [Google Scholar]
- Domingo-Fernandez R, Coll RC, Kearney J, Breit S, O’Neill LAJ. The intracellular chloride channel proteins CLIC1 and CLIC4 induce IL-1beta transcription and activate the NLRP3 inflammasome. J Biol Chem. 2017;292(29):12077–12087. doi: 10.1074/jbc.M117.797126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulhunty A, Gage P, Curtis S, Chelvanayagam G, Board P. The glutathione transferase structural family includes a nuclear chloride channel and a ryanodine receptor calcium release channel modulator. J Biol Chem. 2001;276(5):3319–3323. doi: 10.1074/jbc.M007874200. [DOI] [PubMed] [Google Scholar]
- Eaton LC, Erdos GW, Vreeland NL, Ingram LO. Failure of Escherichia coli to alter its fatty acid composition in response to cholesterol-induced changes in membrane fluidity. J Bacteriol. 1981;146(3):1151–1153. doi: 10.1128/jb.146.3.1151-1153.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JC. A novel p64-related Cl- channel: subcellular distribution and nephron segment-specific expression. Am J Physiol. 1999;276(3 Pt 2):F398–408. doi: 10.1152/ajprenal.1999.276.3.F398. [DOI] [PubMed] [Google Scholar]
- Edwards JC. The CLIC1 chloride channel is regulated by the cystic fibrosis transmembrane conductance regulator when expressed in Xenopus oocytes. J Membr Biol. 2006;213(1):39–46. doi: 10.1007/s00232-006-0059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JC. What’s a CLIC doing in the podocyte? Kidney Int. 2010;78(9):831–833. doi: 10.1038/ki.2010.238. [DOI] [PubMed] [Google Scholar]
- Edwards JC, Bruno J, Key P, Cheng YW. Absence of chloride intracellular channel 4 (CLIC4) predisposes to acute kidney injury but has minimal impact on recovery. BMC Nephrol. 2014;15:54. doi: 10.1186/1471-2369-15-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elborn JS. Cystic fibrosis. Lancet. 2016;388(10059):2519–2531. doi: 10.1016/S0140-6736(16)00576-6. [DOI] [PubMed] [Google Scholar]
- Elustondo P, Martin LA, Karten B. Mitochondrial cholesterol import. Biochim Biophys Acta. 2017;1862(1):90–101. doi: 10.1016/j.bbalip.2016.08.012. [DOI] [PubMed] [Google Scholar]
- Fantini J, Barrantes FJ. How cholesterol interacts with membrane proteins: an exploration of cholesterol-binding sites including CRAC, CARC, and tilted domains. Front Physiol. 2013;4:31. doi: 10.3389/fphys.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanucchi S, Adamson RJ, Dirr HW. Formation of an unfolding intermediate state of soluble chloride intracellular channel protein CLIC1 at acidic pH. Biochemistry. 2008;47(44):11674–11681. doi: 10.1021/bi801147r. [DOI] [PubMed] [Google Scholar]
- Fernandez-Salas E, Sagar M, Cheng C, Yuspa SH, Weinberg WC. p53 and tumor necrosis factor alpha regulate the expression of a mitochondrial chloride channel protein. J Biol Chem. 1999;274(51):36488–36497. doi: 10.1074/jbc.274.51.36488. [DOI] [PubMed] [Google Scholar]
- Fernandez-Salas E, Suh KS, Speransky VV, Bowers WL, Levy JM, Adams T, … Yuspa SH. mtCLIC/CLIC4, an organellular chloride channel protein, is increased by DNA damage and participates in the apoptotic response to p53. Mol Cell Biol. 2002;22(11):3610–3620. doi: 10.1128/MCB.22.11.3610-3620.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Tellez TN, Lopez TV, Vasquez Garzon VR, Villa-Trevino S. Co-Expression of Ezrin-CLIC5-Podocalyxin Is Associated with Migration and Invasiveness in Hepatocellular Carcinoma. PLoS One. 2015;10(7):e0131605. doi: 10.1371/journal.pone.0131605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedli M, Guipponi M, Bertrand S, Bertrand D, Neerman-Arbez M, Scott HS, … Reymond A. Identification of a novel member of the CLIC family, CLIC6, mapping to 21q22.12. Gene. 2003;320:31–40. doi: 10.1016/s0378-1119(03)00830-8. [DOI] [PubMed] [Google Scholar]
- Frobel J, Cadeddu RP, Hartwig S, Bruns I, Wilk CM, Kundgen A, … Czibere A. Platelet proteome analysis reveals integrin-dependent aggregation defects in patients with myelodysplastic syndromes. Mol Cell Proteomics. 2013;12(5):1272–1280. doi: 10.1074/mcp.M112.023168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon LH, Longo-Guess CM, Berryman M, Shin JB, Saylor KW, Yu H, … Johnson KR. The chloride intracellular channel protein CLIC5 is expressed at high levels in hair cell stereocilia and is essential for normal inner ear function. J Neurosci. 2006;26(40):10188–10198. doi: 10.1523/JNEUROSCI.2166-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodchild SC, Angstmann CN, Breit SN, Curmi PM, Brown LJ. Transmembrane extension and oligomerization of the CLIC1 chloride intracellular channel protein upon membrane interaction. Biochemistry. 2011;50(50):10887–10897. doi: 10.1021/bi2012564. [DOI] [PubMed] [Google Scholar]
- Griffon N, Jeanneteau F, Prieur F, Diaz J, Sokoloff P. CLIC6, a member of the intracellular chloride channel family, interacts with dopamine D(2)-like receptors. Brain Res Mol Brain Res. 2003;117(1):47–57. doi: 10.1016/s0169-328x(03)00283-3. [DOI] [PubMed] [Google Scholar]
- Gritti M, Wurth R, Angelini M, Barbieri F, Peretti M, Pizzi E, … Florio T. Metformin repositioning as antitumoral agent: selective antiproliferative effects in human glioblastoma stem cells, via inhibition of CLIC1-mediated ion current. Oncotarget. 2014;5(22):11252–11268. doi: 10.18632/oncotarget.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronich N, Kumar A, Zhang Y, Efimov IR, Soldatov NM. Molecular remodeling of ion channels, exchangers and pumps in atrial and ventricular myocytes in ischemic cardiomyopathy. Channels (Austin) 2010;4(2):101–107. doi: 10.4161/chan.4.2.10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gururaja Rao S, Ponnalagu D, Sukur S, Singh H, Sanghvi S, Mei Y, … Singh H. Identification and Characterization of a Bacterial Homolog of Chloride Intracellular Channel (CLIC) Protein. Sci Rep. 2017;7(1):8500. doi: 10.1038/s41598-017-08742-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare JE, Goodchild SC, Breit SN, Curmi PM, Brown LJ. Interaction of Human Chloride Intracellular Channel Protein 1 (CLIC1) with Lipid Bilayers: A Fluorescence Study. Biochemistry. 2016;55(27):3825–3833. doi: 10.1021/acs.biochem.6b00080. [DOI] [PubMed] [Google Scholar]
- Heiss NS, Poustka A. Genomic structure of a novel chloride channel gene, CLIC2, in Xq28. Genomics. 1997;45(1):224–228. doi: 10.1006/geno.1997.4922. [DOI] [PubMed] [Google Scholar]
- Jegla TJ, Zmasek CM, Batalov S, Nayak SK. Evolution of the human ion channel set. Comb Chem High Throughput Screen. 2009;12(1):2–23. doi: 10.2174/138620709787047957. [DOI] [PubMed] [Google Scholar]
- Jentsch TJ. VRACs and other ion channels and transporters in the regulation of cell volume and beyond. Nat Rev Mol Cell Biol. 2016;17(5):293–307. doi: 10.1038/nrm.2016.29. [DOI] [PubMed] [Google Scholar]
- Jiang L, Salao K, Li H, Rybicka JM, Yates RM, Luo XW, … Breit SN. Intracellular chloride channel protein CLIC1 regulates macrophage function through modulation of phagosomal acidification. J Cell Sci. 2012;125(Pt 22):5479–5488. doi: 10.1242/jcs.110072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C, Crowther S, Stafford MJ, Campbell DG, Toth R, MacKintosh C. Bioinformatic and experimental survey of 14-3-3-binding sites. Biochem J. 2010;427(1):69–78. doi: 10.1042/BJ20091834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Chang JW, Yun HS, Yang KM, Hong EH, Kim DH, … Hwang SG. Chloride intracellular channel 1 identified using proteomic analysis plays an important role in the radiosensitivity of HEp-2 cells via reactive oxygen species production. Proteomics. 2010;10(14):2589–2604. doi: 10.1002/pmic.200900523. [DOI] [PubMed] [Google Scholar]
- Kim KH, Choi BK, Song KM, Cha KW, Kim YH, Lee H, … Kwon BS. CRIg signals induce anti-intracellular bacterial phagosome activity in a chloride intracellular channel 3-dependent manner. Eur J Immunol. 2013;43(3):667–678. doi: 10.1002/eji.201242997. [DOI] [PubMed] [Google Scholar]
- Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427(6972):360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- Kiselyov K, Muallem S. ROS and intracellular ion channels. Cell Calcium. 2016;60(2):108–114. doi: 10.1016/j.ceca.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry D, Sullivan S, Nicolaides M, Redhead C, Edelman A, Field M, … Edwards J. Molecular cloning and characterization of p64, a chloride channel protein from kidney microsomes. J Biol Chem. 1993;268(20):14948–14955. [PubMed] [Google Scholar]
- Landry DW, Akabas MA, Redhead C, al-Awqati Q. Purification and reconstitution of epithelial chloride channels. Methods Enzymol. 1990;191:572–583. doi: 10.1016/0076-6879(90)91036-6. [DOI] [PubMed] [Google Scholar]
- Landry DW, Akabas MH, Redhead C, Edelman A, Cragoe EJ, Jr, Al-Awqati Q. Purification and reconstitution of chloride channels from kidney and trachea. Science. 1989;244(4911):1469–1472. doi: 10.1126/science.2472007. [DOI] [PubMed] [Google Scholar]
- Landry DW, Reitman M, Cragoe EJ, Jr, Al-Awqati Q. Epithelial chloride channel. Development of inhibitory ligands. J Gen Physiol. 1987;90(6):779–798. doi: 10.1085/jgp.90.6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leanza L, Biasutto L, Manago A, Gulbins E, Zoratti M, Szabo I. Intracellular ion channels and cancer. Front Physiol. 2013;4:227. doi: 10.3389/fphys.2013.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legg-E’silva D, Achilonu I, Fanucchi S, Stoychev S, Fernandes M, Dirr HW. Role of arginine 29 and glutamic acid 81 interactions in the conformational stability of human chloride intracellular channel 1. Biochemistry. 2012;51(40):7854–7862. doi: 10.1021/bi300874b. [DOI] [PubMed] [Google Scholar]
- Leng Q, MacGregor GG, Dong K, Giebisch G, Hebert SC. Subunit-subunit interactions are critical for proton sensitivity of ROMK: evidence in support of an intermolecular gating mechanism. Proc Natl Acad Sci U S A. 2006;103(6):1982–1987. doi: 10.1073/pnas.0510610103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MR, Lewis WH. Mitochondria (and other cytoplasmic structures) in tissue cultures. American Journal of Anatomy. 1915;17(3):339–401. doi: 10.1002/aja.1000170304. [DOI] [Google Scholar]
- Li RK, Zhang J, Zhang YH, Li ML, Wang M, Tang JW. Chloride intracellular channel 1 is an important factor in the lymphatic metastasis of hepatocarcinoma. Biomed Pharmacother. 2012;66(3):167–172. doi: 10.1016/j.biopha.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Liang J, Shaulov Y, Savage-Dunn C, Boissinot S, Hoque T. Chloride intracellular channel proteins respond to heat stress in Caenorhabditis elegans. PLoS One. 2017;12(9):e0184308. doi: 10.1371/journal.pone.0184308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littler DR, Harrop SJ, Goodchild SC, Phang JM, Mynott AV, Jiang L, … Curmi PM. The enigma of the CLIC proteins: Ion channels, redox proteins, enzymes, scaffolding proteins? FEBS Lett. 2010;584(10):2093–2101. doi: 10.1016/j.febslet.2010.01.027. [DOI] [PubMed] [Google Scholar]
- Liu W, Gnanasambandam R, Benjamin J, Kaur G, Getman PB, Siegel AJ, … Singh S. Mutations in cytochrome c oxidase subunit VIa cause neurodegeneration and motor dysfunction in Drosophila. Genetics. 2007;176(2):937–946. doi: 10.1534/genetics.107.071688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucitti JL, Tarte NJ, Faber JE. Chloride intracellular channel 4 is required for maturation of the cerebral collateral circulation. Am J Physiol Heart Circ Physiol. 2015;309(7):H1141–1150. doi: 10.1152/ajpheart.00451.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Solano L, Reales-Calderon JA, Nombela C, Molero G, Gil C. Proteomics of RAW 264.7 macrophages upon interaction with heat-inactivated Candida albicans cells unravel an anti-inflammatory response. Proteomics. 2009;9(11):2995–3010. doi: 10.1002/pmic.200800016. [DOI] [PubMed] [Google Scholar]
- Matzke AJ, Weiger TM, Matzke M. Ion channels at the nucleus: electrophysiology meets the genome. Mol Plant. 2010;3(4):642–652. doi: 10.1093/mp/ssq013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzanti M, DeFelice LJ, Cohn J, Malter H. Ion channels in the nuclear envelope. Nature. 1990;343(6260):764–767. doi: 10.1038/343764a0. [DOI] [PubMed] [Google Scholar]
- Meng X, Wang G, Viero C, Wang Q, Mi W, Su XD, … Yin CC. CLIC2-RyR1 interaction and structural characterization by cryo-electron microscopy. J Mol Biol. 2009;387(2):320–334. doi: 10.1016/j.jmb.2009.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. In the beginning: a personal reminiscence on the origin and legacy of ClC-0, the ‘Torpedo Cl(−) channel’. J Physiol. 2015;593(18):4085–4090. doi: 10.1113/jphysiol.2014.286260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton RH, Abeti R, Averaimo S, DeBiasi S, Vitellaro L, Jiang L, … Mazzanti M. CLIC1 function is required for beta-amyloid-induced generation of reactive oxygen species by microglia. J Neurosci. 2008;28(45):11488–11499. doi: 10.1523/JNEUROSCI.2431-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Navarro MM, Rosello-Lleti E, Ortega A, Tarazon E, Otero M, Martinez-Dolz L, … Rivera M. Differential gene expression of cardiac ion channels in human dilated cardiomyopathy. PLoS One. 2013;8(12):e79792. doi: 10.1371/journal.pone.0079792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroni A, Thiel G. Flip-flopping salt bridges gate an ion channel. Nat Chem Biol. 2006;2(11):572–573. doi: 10.1038/nchembio1106-572. [DOI] [PubMed] [Google Scholar]
- Murthi P, Stevenson JL, Money TT, Borg AJ, Brennecke SP, Gude NM. Placental CLIC3 is increased in fetal growth restriction and pre-eclampsia affected human pregnancies. Placenta. 2012;33(9):741–744. doi: 10.1016/j.placenta.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Nichols CG, Singh GK, Grange DK. KATP channels and cardiovascular disease: suddenly a syndrome. Circ Res. 2013;112(7):1059–1072. doi: 10.1161/CIRCRESAHA.112.300514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novarino G, Fabrizi C, Tonini R, Denti MA, Malchiodi-Albedi F, Lauro GM, … Mazzanti M. Involvement of the intracellular ion channel CLIC1 in microglia-mediated beta-amyloid-induced neurotoxicity. J Neurosci. 2004;24(23):5322–5330. doi: 10.1523/JNEUROSCI.1170-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rourke B. Mitochondrial ion channels. Annu Rev Physiol. 2007;69:19–49. doi: 10.1146/annurev.physiol.69.031905.163804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rourke B, Cortassa S, Aon MA. Mitochondrial ion channels: gatekeepers of life and death. Physiology (Bethesda) 2005;20:303–315. doi: 10.1152/physiol.00020.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradisi S, Matteucci A, Fabrizi C, Denti MA, Abeti R, Breit SN, … Mazzanti M. Blockade of chloride intracellular ion channel 1 stimulates Abeta phagocytosis. J Neurosci Res. 2008;86(11):2488–2498. doi: 10.1002/jnr.21693. [DOI] [PubMed] [Google Scholar]
- Patel D, Ythier D, Brozzi F, Eizirik DL, Thorens B. Clic4, a novel protein that sensitizes beta-cells to apoptosis. Mol Metab. 2015;4(4):253–264. doi: 10.1016/j.molmet.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretti M, Angelini M, Savalli N, Florio T, Yuspa SH, Mazzanti M. Chloride channels in cancer: Focus on chloride intracellular channel 1 and 4 (CLIC1 AND CLIC4) proteins in tumor development and as novel therapeutic targets. Biochim Biophys Acta. 2014 doi: 10.1016/j.bbamem.2014.12.012. [DOI] [PubMed] [Google Scholar]
- Peter B, Fanucchi S, Dirr HW. A conserved cationic motif enhances membrane binding and insertion of the chloride intracellular channel protein 1 transmembrane domain. Eur Biophys J. 2014;43(8–9):405–414. doi: 10.1007/s00249-014-0972-y. [DOI] [PubMed] [Google Scholar]
- Petrova DT, Asif AR, Armstrong VW, Dimova I, Toshev S, Yaramov N, … Toncheva D. Expression of chloride intracellular channel protein 1 (CLIC1) and tumor protein D52 (TPD52) as potential biomarkers for colorectal cancer. Clin Biochem. 2008;41(14–15):1224–1236. doi: 10.1016/j.clinbiochem.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Pierchala BA, Munoz MR, Tsui CC. Proteomic analysis of the slit diaphragm complex: CLIC5 is a protein critical for podocyte morphology and function. Kidney Int. 2010;78(9):868–882. doi: 10.1038/ki.2010.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnalagu D, Gururaja Rao S, Farber J, Xin W, Hussain AT, Shah K, … Singh H. Molecular identity of cardiac mitochondrial chloride intracellular channel proteins. Mitochondrion. 2016a;27:6–14. doi: 10.1016/j.mito.2016.01.001. [DOI] [PubMed] [Google Scholar]
- Ponnalagu D, Gururaja Rao S, Farber J, Xin W, Hussain AT, Shah K, … Singh H. Data supporting characterization of CLIC1, CLIC4, CLIC5 and DmCLIC antibodies and localization of CLICs in endoplasmic reticulum of cardiomyocytes. Data Brief. 2016b;7:1038–1044. doi: 10.1016/j.dib.2016.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnalagu D, Singh H. Anion Channels of Mitochondria. Handb Exp Pharmacol. 2017;240:71–101. doi: 10.1007/164_2016_39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Z, Okuhara D, Abe MK, Rosner MR. Molecular cloning and characterization of a mitogen-activated protein kinase-associated intracellular chloride channel. J Biol Chem. 1999;274(3):1621–1627. doi: 10.1074/jbc.274.3.1621. [DOI] [PubMed] [Google Scholar]
- Qiu MR, Jiang L, Matthaei KI, Schoenwaelder SM, Kuffner T, Mangin P, … Breit SN. Generation and characterization of mice with null mutation of the chloride intracellular channel 1 gene. Genesis. 2010;48(2):127–136. doi: 10.1002/dvg.20590. [DOI] [PubMed] [Google Scholar]
- Redhead CR, Edelman AE, Brown D, Landry DW, al-Awqati Q. A ubiquitous 64-kDa protein is a component of a chloride channel of plasma and intracellular membranes. Proc Natl Acad Sci U S A. 1992;89(9):3716–3720. doi: 10.1073/pnas.89.9.3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabirov RZ, Merzlyak PG, Islam MR, Okada T, Okada Y. The properties, functions, and pathophysiology of maxi-anion channels. Pflugers Arch. 2016;468(3):405–420. doi: 10.1007/s00424-015-1774-5. [DOI] [PubMed] [Google Scholar]
- Salao K, Jiang L, Li H, Tsai VW, Husaini Y, Curmi PM, … Breit SN. CLIC1 regulates dendritic cell antigen processing and presentation by modulating phagosome acidification and proteolysis. Biol Open. 2016;5(5):620–630. doi: 10.1242/bio.018119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salles FT, Andrade LR, Tanda S, Grati M, Plona KL, Gagnon LH, … Berryman MA. CLIC5 stabilizes membrane-actin filament linkages at the base of hair cell stereocilia in a molecular complex with radixin, taperin, and myosin VI. Cytoskeleton (Hoboken) 2014;71(1):61–78. doi: 10.1002/cm.21159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seco CZ, Oonk AM, Dominguez-Ruiz M, Draaisma JM, Gandia M, Oostrik J, … Schraders M. Progressive hearing loss and vestibular dysfunction caused by a homozygous nonsense mutation in CLIC5. Eur J Hum Genet. 2015;23(2):189–194. doi: 10.1038/ejhg.2014.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks RA, Larocca MC, Berryman M, Edwards JC, Urushidani T, Navarre J, Goldenring JR. AKAP350 at the Golgi apparatus. II. Association of AKAP350 with a novel chloride intracellular channel (CLIC) family member. J Biol Chem. 2002;277(43):40973–40980. doi: 10.1074/jbc.M112277200. [DOI] [PubMed] [Google Scholar]
- Shin JM, Munson K, Vagin O, Sachs G. The gastric HK-ATPase: structure, function, and inhibition. Pflugers Arch. 2009;457(3):609–622. doi: 10.1007/s00424-008-0495-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigrist CJ, Cerutti L, de Castro E, Langendijk-Genevaux PS, Bulliard V, Bairoch A, Hulo N. PROSITE, a protein domain database for functional characterization and annotation. Nucleic Acids Res. 2010;38(Database issue):D161–166. doi: 10.1093/nar/gkp885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H. Two decades with dimorphic Chloride Intracellular Channels (CLICs) FEBS Lett. 2010;584(10):2112–2121. doi: 10.1016/j.febslet.2010.03.013. [DOI] [PubMed] [Google Scholar]
- Singh H, Ashley RH. Redox regulation of CLIC1 by cysteine residues associated with the putative channel pore. Biophys J. 2006;90(5):1628–1638. doi: 10.1529/biophysj.105.072678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H, Ashley RH. CLIC4 (p64H1) and its putative transmembrane domain form poorly selective, redox-regulated ion channels. Mol Membr Biol. 2007;24(1):41–52. doi: 10.1080/09687860600927907. [DOI] [PubMed] [Google Scholar]
- Singh H, Cousin MA, Ashley RH. Functional reconstitution of mammalian ‘chloride intracellular channels’ CLIC1, CLIC4 and CLIC5 reveals differential regulation by cytoskeletal actin. FEBS J. 2007;274(24):6306–6316. doi: 10.1111/j.1742-4658.2007.06145.x. [DOI] [PubMed] [Google Scholar]
- Singh H, Li M, Hall L, Chen S, Sukur S, Lu R, … Toro L. MaxiK channel interactome reveals its interaction with GABA transporter 3 and heat shock protein 60 in the mammalian brain. Neuroscience. 2016;317:76–107. doi: 10.1016/j.neuroscience.2015.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H, Lu R, Bopassa JC, Meredith AL, Stefani E, Toro L. mitoBKCa is encoded by the Kcnma1 gene, and a splicing sequence defines its mitochondrial location. Proc Natl Acad Sci U S A. 2013;110(44):10836–10841. doi: 10.1073/pnas.1302028110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H, Lu R, Rodriguez PF, Wu Y, Bopassa JC, Stefani E, Toro L. Visualization and quantification of cardiac mitochondrial protein clusters with STED microscopy. Mitochondrion. 2012;12(2):230–236. doi: 10.1016/j.mito.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H, Stefani E, Toro L. Intracellular BK(Ca) (iBK(Ca)) channels. J Physiol. 2012;590(Pt 23):5937–5947. doi: 10.1113/jphysiol.2011.215533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H, Warburton S, Vondriska TM, Khakh BS. Proteomics to identify proteins interacting with P2X2 ligand-gated cation channels. J Vis Exp. 2009;(27) doi: 10.3791/1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaper SD, Facci L, Giusti P. Intracellular Ion Channel CLIC1: Involvement in Microglia-Mediated beta-Amyloid Peptide(1-42) Neurotoxicity. Neurochem Res. 2013 doi: 10.1007/s11064-013-1084-2. [DOI] [PubMed] [Google Scholar]
- Song MY, Tang JW, Sun MZ, Liu SQ, Wang B. Localization and expression of CLIC1 in hepatocarcinoma ascites cell lines with high or low potentials of lymphatic spread. Zhonghua Bing Li Xue Za Zhi. 2010;39(7):463–466. [PubMed] [Google Scholar]
- Stauber T, Jentsch TJ. Chloride in vesicular trafficking and function. Annu Rev Physiol. 2013;75:453–477. doi: 10.1146/annurev-physiol-030212-183702. [DOI] [PubMed] [Google Scholar]
- Suginta W, Karoulias N, Aitken A, Ashley RH. Chloride intracellular channel protein CLIC4 (p64H1) binds directly to brain dynamin I in a complex containing actin, tubulin and 14-3-3 isoforms. Biochem J. 2001;359(Pt 1):55–64. doi: 10.1042/0264-6021:3590055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh KS, Malik M, Shukla A, Ryscavage A, Wright L, Jividen K, … Yuspa SH. CLIC4 is a tumor suppressor for cutaneous squamous cell cancer. Carcinogenesis. 2012;33(5):986–995. doi: 10.1093/carcin/bgs115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh KS, Malik M, Shukla A, Yuspa SH. CLIC4, skin homeostasis and cutaneous cancer: surprising connections. Mol Carcinog. 2007;46(8):599–604. doi: 10.1002/mc.20324. [DOI] [PubMed] [Google Scholar]
- Suh KS, Mutoh M, Nagashima K, Fernandez-Salas E, Edwards LE, Hayes DD, … Yuspa SH. The organellular chloride channel protein CLIC4/mtCLIC translocates to the nucleus in response to cellular stress and accelerates apoptosis. J Biol Chem. 2004;279(6):4632–4641. doi: 10.1074/jbc.M311632200. [DOI] [PubMed] [Google Scholar]
- Syahidatulamali CS, Wan Syamimee WG, Azwany YN, Wong KK, Che Maraina CH. Association of anti-CLIC2 and anti-HMGB1 autoantibodies with higher disease activity in systemic lupus erythematosus patients. J Postgrad Med. 2017;63(4):257–261. doi: 10.4103/jpgm.JPGM_499_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano K, Liu D, Tarpey P, Gallant E, Lam A, Witham S, … Dulhunty AF. An X-linked channelopathy with cardiomegaly due to a CLIC2 mutation enhancing ryanodine receptor channel activity. Hum Mol Genet. 2012;21(20):4497–4507. doi: 10.1093/hmg/dds292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang T, Lang X, Xu C, Wang X, Gong T, Yang Y, … Zhou R. CLICs-dependent chloride efflux is an essential and proximal upstream event for NLRP3 inflammasome activation. Nat Commun. 2017;8(1):202. doi: 10.1038/s41467-017-00227-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao H, Li X, Wei K, Xie K, Ni Z, Gu L. Cyclic AMP prevents decrease of phosphorylated ezrin/radixin/moesin and chloride intracellular channel 5 expressions in injured podocytes. Clin Exp Nephrol. 2015;19(6):1000–1006. doi: 10.1007/s10157-015-1102-6. [DOI] [PubMed] [Google Scholar]
- Tavasoli M, Al-Momany A, Wang X, Li L, Edwards JC, Ballermann BJ. Both CLIC4 and CLIC5A activate ERM proteins in glomerular endothelium. Am J Physiol Renal Physiol. 2016;311(5):F945–F957. doi: 10.1152/ajprenal.00353.2016. [DOI] [PubMed] [Google Scholar]
- Tulk BM, Kapadia S, Edwards JC. CLIC1 inserts from the aqueous phase into phospholipid membranes, where it functions as an anion channel. Am J Physiol Cell Physiol. 2002;282(5):C1103–1112. doi: 10.1152/ajpcell.00402.2001. [DOI] [PubMed] [Google Scholar]
- Tung JJ, Hobert O, Berryman M, Kitajewski J. Chloride intracellular channel 4 is involved in endothelial proliferation and morphogenesis in vitro. Angiogenesis. 2009;12(3):209–220. doi: 10.1007/s10456-009-9139-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov B, Bruno J, Gordon N, Hartnett ME, Edwards JC. Chloride intracellular channel protein-4 functions in angiogenesis by supporting acidification of vacuoles along the intracellular tubulogenic pathway. Am J Pathol. 2009;174(3):1084–1096. doi: 10.2353/ajpath.2009.080625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov B, Bruno J, Oshima K, Cheng YW, Holly SP, Parise LV, … Edwards JC. CLIC1 null mice demonstrate a role for CLIC1 in macrophage superoxide production and tissue injury. Physiol Rep. 2017;5(5) doi: 10.14814/phy2.13169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov B, Bruno J, Woost PG, Edwards JC. Tissue and subcellular distribution of CLIC1. BMC Cell Biol. 2007;8:8. doi: 10.1186/1471-2121-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela SM, Alkhamici H, Brown LJ, Almond OC, Goodchild SC, Carne S, … Cornell BA. Regulation of the membrane insertion and conductance activity of the metamorphic chloride intracellular channel protein CLIC1 by cholesterol. PLoS One. 2013;8(2):e56948. doi: 10.1371/journal.pone.0056948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela SM, Martin DK, Por SB, Robbins JM, Warton K, Bootcov MR, … Breit SN. Molecular cloning and expression of a chloride ion channel of cell nuclei. J Biol Chem. 1997;272(19):12575–12582. doi: 10.1074/jbc.272.19.12575. [DOI] [PubMed] [Google Scholar]
- Valenzuela SM, Mazzanti M, Tonini R, Qiu MR, Warton K, Musgrove EA, … Breit SN. The nuclear chloride ion channel NCC27 is involved in regulation of the cell cycle. J Physiol. 2000;529(Pt 3):541–552. doi: 10.1111/j.1469-7793.2000.00541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JW, Peng SY, Li JT, Wang Y, Zhang ZP, Cheng Y, … Liu YB. Identification of metastasis-associated proteins involved in gallbladder carcinoma metastasis by proteomic analysis and functional exploration of chloride intracellular channel 1. Cancer Lett. 2009;281(1):71–81. doi: 10.1016/j.canlet.2009.02.020. [DOI] [PubMed] [Google Scholar]
- Wang P, Zhang C, Yu P, Tang B, Liu T, Cui H, Xu J. Regulation of colon cancer cell migration and invasion by CLIC1-mediated RVD. Mol Cell Biochem. 2012;365(1–2):313–321. doi: 10.1007/s11010-012-1271-5. [DOI] [PubMed] [Google Scholar]
- Warton K, Tonini R, Fairlie WD, Matthews JM, Valenzuela SM, Qiu MR, … Mazzanti M. Recombinant CLIC1 (NCC27) assembles in lipid bilayers via a pH-dependent two-state process to form chloride ion channels with identical characteristics to those observed in Chinese hamster ovary cells expressing CLIC1. J Biol Chem. 2002;277(29):26003–26011. doi: 10.1074/jbc.M203666200. [DOI] [PubMed] [Google Scholar]
- Wegner B, Al-Momany A, Kulak SC, Kozlowski K, Obeidat M, Jahroudi N, … Ballermann BJ. CLIC5A, a component of the ezrin-podocalyxin complex in glomeruli, is a determinant of podocyte integrity. Am J Physiol Renal Physiol. 2010;298(6):F1492–1503. doi: 10.1152/ajprenal.00030.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Li J, Xie H, Wang H, Wang J, Zhang X, … Zheng S. Chloride intracellular channel 1 participates in migration and invasion of hepatocellular carcinoma by targeting maspin. J Gastroenterol Hepatol. 2015;30(1):208–216. doi: 10.1111/jgh.12668. [DOI] [PubMed] [Google Scholar]
- Witham S, Takano K, Schwartz C, Alexov E. A missense mutation in CLIC2 associated with intellectual disability is predicted by in silico modeling to affect protein stability and dynamics. Proteins. 2011;79(8):2444–2454. doi: 10.1002/prot.23065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojciak-Stothard B, Abdul-Salam VB, Lao KH, Tsang H, Irwin DC, Lisk C, … Wilkins MR. Aberrant chloride intracellular channel 4 expression contributes to endothelial dysfunction in pulmonary arterial hypertension. Circulation. 2014;129(17):1770–1780. doi: 10.1161/CIRCULATIONAHA.113.006797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Zhu J, Hu X, Wang C, Lu D, Gong C, … Zong L. CLIC1 Inhibition Attenuates Vascular Inflammation, Oxidative Stress, and Endothelial Injury. PLoS One. 2016;11(11):e0166790. doi: 10.1371/journal.pone.0166790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JY, Jung JY, Cho SW, Choi HJ, Kim SW, Kim SY, … Shin CS. Chloride intracellular channel 1 regulates osteoblast differentiation. Bone. 2009;45(6):1175–1185. doi: 10.1016/j.bone.2009.08.012. [DOI] [PubMed] [Google Scholar]
- Zhao WS, Sun MY, Sun LF, Liu Y, Yang Y, Huang LD, … Yu Y. A Highly Conserved Salt Bridge Stabilizes the Kinked Conformation of beta2,3-Sheet Essential for Channel Function of P2X4 Receptors. J Biol Chem. 2016;291(15):7990–8003. doi: 10.1074/jbc.M115.711127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Xu Y, Ren G, Hu X, Wang C, Yang Z, … Lu D. Tanshinone IIA Sodium sulfonate regulates antioxidant system, inflammation, and endothelial dysfunction in atherosclerosis by downregulation of CLIC1. Eur J Pharmacol. 2017;815:427–436. doi: 10.1016/j.ejphar.2017.09.047. [DOI] [PubMed] [Google Scholar]