Abstract
Introduction:
Anti-neuronal antibodies (ANA) are found in paraneoplastic neurological syndrome and autoimmune encephalitis patients. Our aim was to analyze prognostic factors related with ANA seropositivity.
Methods:
Twenty-seven consecutive ANA seropositive patients were included in the study. ANA were detected by immunofluorescent staining, immunoblot and cell-based assay methods. All patients were followed with a standard treatment protocol. Clinical syndromes, tumor types, modified Rankin scores, cranial MRI and oligoclonal band (OCB) findings were recorded. Cases were divided into subgroups due to clinical-laboratory features and ANA types. Prevalence of good prognosis, response to treatment and survival were compared among these subgroups.
Results:
Patients showed antibodies to N-methyl-D-aspartate receptor (NMDAR) (6 cases), Hu (6 cases), Ma2 (5 cases), glutamic acid decarboxylase (GAD) (3 cases), Yo (3 cases), amphiphysin (1 case), gamma-amino butyric acid B receptor (GABABR) (1 case), Ri (1 case) and Zic4 (1 case). Associated neurological syndromes were limbic encephalitis (8 cases), subacute cerebellar degeneration (7 cases), brainstem encephalitis (5 cases), subacute sensory neuronopathy (4 cases), stiff-person syndrome (2 cases) and opsoclonus-myoclonus (1 case). A tumor (ductal breast, small cell lung cancer) was detected in six cases at first admission. Six patients died in an average follow-up time of 1.0±1.5 years. Detection of antibodies to extracellular or synaptic target antigens, but not presence of tumor, cranial MRI lesions or OCB, was associated with good prognosis and response to treatment.
Conclusion:
NMDAR, Hu and Ma2-antibodies were the most prevalent ANA in this first antibody screening study in a Turkish cohort. Antibody type was determined to be the foremost prognostic factor in ANA seropositive cases.
Keywords: Paraneoplastic, antibody, survival, prognosis, cancer
INTRODUCTION
Paraneoplastic neurological syndrome (PNS) is a rare neurological condition and comprises a group of syndromes that can affect any part of the nervous system. Although PNS occurs in cancer patients, majority of the patients do not exhibit a detectable tumor at the time of admission. Therefore, diagnosis is established with demonstration of a classical PNS (e.g. limbic encephalitis, subacute cerebellar degeneration, and subacute sensory neuronopathy), and detection of well-characterized cancer-related anti-neuronal antibodies (ANA) (e.g. Hu, Yo, and Ma2 antibodies) (1–3). ANA are also detected in non-paraneoplastic autoimmune encephalitis patients, and constitute one of the hallmark findings of diagnostic criteria (4). Diagnosis of PNS and autoimmune encephalitis requires elimination of other acute encephalopathy-associated pathogenic mechanisms such as metastasis, opportunistic infections, vascular events, and side effects of cancer treatment (1–4). The significance of ANA in diagnosis of paraneoplastic and autoimmune neurological syndromes is well recognized, and presence of ANA directed against cell surface antigens has been associated with good response to immunosuppressive treatment and favorable diagnosis (3). However, there are only a few publications on the influence of ANA and other diagnostic laboratory methods on the long-term prognosis, and survival of PNS and autoimmune encephalitis cases. In this study, we aimed to define frequently encountered ANA types, and associated neurological syndromes in the Turkish population, and analyze the impact of laboratory findings obtained at admission to the long-term prognosis of ANA seropositive patients.
METHODS
Patients
Twenty-seven consecutive cases with ANA were included in the study. All patients fulfilled the diagnostic criteria for PNS (1), or autoimmune encephalitis (4). Since underlying tumors are often not detected during the initial presentation of neurological symptoms, presence of cancer is not necessary for PNS diagnosis. PNS criteria require presence of cancer, and/or well-characterized (Hu, Yo, Ri, Ma2, CV2 and amphiphysin) ANA in cases with classical PNS (e.g. limbic encephalitis, subacute cerebellar degeneration, subacute sensory neuronopathy and opsoclonus-myoclonus syndrome). In non-classical PNS (e.g. brainstem encephalitis, or stiff-person syndrome), detection of a well-characterized ANA is sufficient for the diagnosis. In non-classical PNS cases without a well-characterized ANA (e.g. Zic4), detection of an underlying tumor is also required for definite diagnosis (1). All patients underwent a detailed neurological and systemic examination. The worst (maximum) and the last modified Rankin score (mRS) values, cranial MRI, and cerebrospinal fluid (CSF) oligoclonal band (OCB) findings were recorded. Only laboratory findings obtained during the initial admission of the patient were used in statistical analyses conducted to delineate prognostic values of laboratory methods. Cranial MRI was not performed in two patients with subacute sensory neuronopathy due to the absence of central nervous system symptoms. A detailed laboratory and imaging protocol was performed for elimination of non-paraneoplastic etiologies. A standard immunotherapy protocol was applied to all cases. Immunotherapy was initiated in conjunction with tumor resection in cases with a detectable tumor. High-dose methylprednisolone (5 days x 1 g) and intravenous immunoglobulin (IVIg) (0.4 g/kg x 5 days) were administered as first-line treatment. This treatment continued with monthly booster doses for 6 months when the mRS value declined. Second-line treatment [cyclophosphamide (750 mg/m2)] was started in patients, whose mRS values did not change within three months of treatment initiation. Patients with a final mRS of 4–6 were considered to have poor prognosis, whereas those with a final mRS of 1–3 were considered to have good prognosis. Patients with no difference between the maximal and final mRS values were considered treatment-resistant.
ANA detection
Serum samples obtained from all cases were stored at-80°C in freezer. Antibodies to N-methyl-D-aspartate receptor (NMDAR), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR), leucine rich glioma inactivated 1 (LGI1), contactin associated protein 2 (CASPR2), gamma-amino butyric acid B receptor (GABABR), glutamic acid decarboxylase (GAD), Hu, Yo, CV2, Ma2, Ri, amphiphysin, Zic4, Sox1, titin, recoverin, and Tr/delta/notch-like epidermal growth factor-related receptor (DNER) were tested in sera. NMDAR, AMPAR, LGI1, CASPR2 and GABABR antibodies were tested with human embryonal kidney-293 (HEK293) cells transfected with plasmids encoding the relevant subunits of the target ion channel autoantigens using an immunofluorescence staining-based method (Euroimmun, Luebeck, Germany). GAD antibodies were measured by ELISA according to the instructions of the manufacturer (Euroimmun). For detection of Hu, Yo, CV2, Ma2, Ri, amphiphysin, Zic4, Sox1, titin, recoverin, and Tr/DNER antibodies, a kit based on immunofluorescence staining (performed on cerebrum, cerebellum, pancreas, intestine, and sural nerve sections) and immunoblotting (using recombinant proteins of the target antigens) was used (Euroimmun). Only sera, which gave positive results with both immunofluorescence and immunoblotting methods, were accepted as seropositive.
Antibodies were classified as intracellular (Hu, CV2, Ma2, Yo, Ri, amphiphysin, GAD, titin, Zic4, Sox1, recoverin), extracellular (NMDAR, AMPAR, LGI1, CASPR2, GABABR, Tr/DNER), synaptic (NMDAR, AMPAR, LGI1, CASPR2, GABABR, Tr/DNER, GAD, amphiphysin), and non-synaptic (Hu, CV2, Ma2, Yo, Ri, titin, Zic4, Sox1, recoverin) according to the cellular localization of target antigens.
Statistical Analysis
Gender, cancer, intracellular-extracellular antibody, synaptic-non-synaptic antibody, CSF OCB and cranial MRI positivity frequencies of patient subgroups were compared with Fisher’s exact test. Kaplan-Meier analysis was used to compare the cumulative survival rates of patients with and without synaptic antibodies; p<0.05 was considered as statistically significant.
RESULTS
Clinical features and ANA findings
Clinical and demographic features of 27 ANA seropositive patients are listed in Table 1. Most frequently encountered neurological syndromes were limbic encephalitis (8 cases), subacute cerebellar degeneration (7 cases), brainstem encephalitis (5 cases), and subacute sensory neuronopathy (4 cases). Stiff-person syndrome (2 cases) and opsoclonus myoclonus syndrome (1 case) were also observed. Follow-up durations ranged between 1–8 years (average ± standard deviation, 3.4±2.4 years). Three patients died within the first few months of admission.
Table 1.
Clinical and demographic features of ANA seropositive patients
| Age | Gender | Follow-up (years) | ANA | Syndrome | Cancer | Maximum mRS | Final mRS | CSF OCB | Cranial MRI* |
|---|---|---|---|---|---|---|---|---|---|
| 53 | M | 1 | Ma2 | BE | - | 6 | 6 | + | BL MTL |
| 63 | M | 0 | Hu | LE | - | 6 | 6 | + | Pons, thalamus |
| 2 | M | 3 | Hu | OMS | - | 5 | 1 | - | Normal |
| 36 | F | 7 | Hu | SSN | Breast | 4 | 4 | + | Basal frontal |
| 40 | F | 7 | Zic4 | SSN | Breast | 3 | 2 | + | - |
| 53 | F | 2 | Hu | SSN | - | 4 | 4 | + | - |
| 42 | F | 4 | Hu | SSN | SCLC | 6 | 6 | + | Normal |
| 69 | M | 6 | Hu | SCD | SCLC | 3 | 3 | - | Cerebellum |
| 69 | M | 4 | Ma2 | SCD | - | 4 | 4 | - | Normal |
| 69 | M | 4 | Ma2 | SCD | - | 5 | 5 | - | Normal |
| 66 | F | 1 | Ri | BE | Breast | 6 | 6 | + | Normal |
| 61 | M | 1 | Ma2 | SCD | - | 4 | 4 | + | Normal |
| 51 | F | 1 | Yo | SCD | - | 4 | 4 | + | Normal |
| 10 | M | 4 | Ma2 | BE | - | 2 | 0 | - | Normal |
| 46 | F | 4 | Yo | SCD | - | 5 | 5 | - | Normal |
| 53 | F | 7 | Yo | SCD | Breast | 4 | 4 | - | Normal |
| 21 | F | 8 | GAD | BE | - | 4 | 1 | + | Pons, thalamus |
| 19 | F | 2 | GAD | SPS | - | 4 | 4 | + | Normal |
| 60 | F | 3 | GAD | LE | - | 5 | 2 | + | Normal |
| 64 | M | 7 | Amphiphysin | SPS | - | 4 | 4 | - | Normal |
| 58 | M | 2 | NMDAR | LE | - | 4 | 1 | - | Normal |
| 45 | F | 3 | NMDAR | BE | - | 1 | 0 | - | Pons |
| 76 | M | 0 | NMDAR | LE | - | 6 | 6 | - | Normal |
| 42 | F | 3 | NMDAR | LE | - | 4 | 1 | + | Pons, thalamus |
| 22 | F | 2 | NMDAR | LE | - | 5 | 1 | - | BL MTL |
| 56 | M | 0 | GABABR | LE | - | 6 | 6 | - | Normal |
| 59 | M | 6 | NMDAR | LE | - | 3 | 1 | - | Normal |
M, male; F, female; BE, brainstem encephalitis; LE, limbic encephalitis; OMS, opsoclonus myoclonus syndrome; SSN, subacute sensory neuronopathy; SCD, subacute cerebellar degeneration; SPS, stiff-person syndrome; SCLC, small cell lung cancer; CSF OCB, cerebrospinal fluid-exclusive oligoclonal bands (pattern 2 or 3); BL MTS, bilateral medial temporal lobes.
Note that 2 patients did not undergo cranial MRI examination.
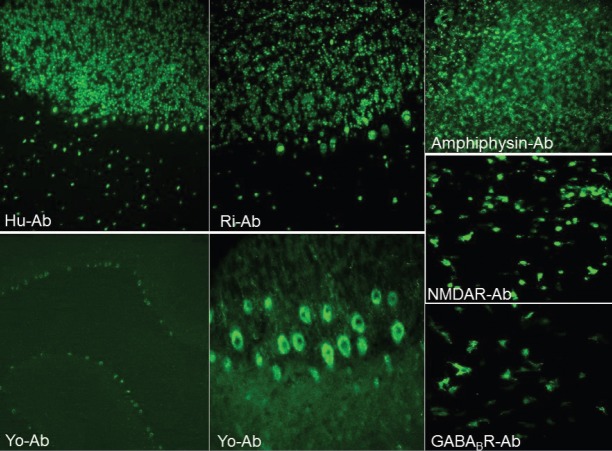
NMDAR (6 cases) and Hu (6 cases) antibodies were the most frequently detected antibodies followed by Ma2 (5 cases), GAD (3 cases), Yo (3 cases), amphiphysin (1 case), Ri (1 case), Zic4 (1 case), and GABABR (1 case) antibodies (Figure 1). CV2, titin, recoverin, Sox1, Tr/DNER, AMPAR, LGI1, and CASPR2 antibodies were not found in any of the patients. Antibodies directed against intracellular and extracellular antigens were found in 20 and 7 patients, respectively. In 11 patients, antibodies were directed to synaptic antigens, whereas 16 patients had antibodies to non-synaptic antigens.
Figure 1.
Representative images of different anti-neuronal antibody binding patterns obtained by immunofluorescence staining (original magnification x4 for the lower left panel and x20 for other panels).
During the first admission, only 6 patients (22.2%) were found to have cancer with imaging and pathology investigations. These patients had ductal breast (4 cases), or small cell lung cancer (2 cases). Ductal breast cancer patients displayed Hu, Yo, Ri and Zic4 antibodies and subacute sensory neuronopathy (2 cases), subacute cerebellar degeneration (1 case), and brainstem encephalitis (1 case) syndromes. Both lung cancer patients had Hu antibodies and subacute sensory neuronopathy, and subacute cerebellar degeneration syndromes. Overall, the diagnosis was PNS and autoimmune encephalitis in 17, and 10 ANA seropositive patients, respectively.
Thirteen patients showed CSF-exclusive OCB, CSF lymphocytosis, increased protein concentration, and increased IgG index. Eight patients had hyperintense lesions on T2-and FLAIR-weighted cranial MRI. Lesions were located in brainstem (±thalamus) (4 cases), bilateral medial temporal lobes (2 cases), basal frontal lobe (1 case), and cerebellum (1 case). None of the lesions were contrast-enhancing.
In the evaluation based on mRS, 16 patients had poor prognosis (final mRS 4–6), and 17 patients were treatment-resistant. Out of 11 patients with good prognosis (final mRS 1–3), 6 had maximum mRS of 4 or 5, and 5 had a maximum mRS of 1–3. Six patients died in an average follow-up time of 1.0±1.5 years due to complications of cancer or severe neurological involvement. Only two of these patients had cancer (1 breast, and 1 lung cancer) during admission. Serum ANA were directed to Hu in 2 patients, and Ri, Ma2, GABABR, and NMDAR in each one of the other 4 patients. Clinical syndromes were limbic encephalitis (3 cases), brainstem encephalitis (2 cases), and subacute sensory neuronopathy (1 case).
Comparison of PNS patients with different prognostic outcomes
ANA seropositive patients with a poor prognosis showed trends towards exhibiting antibodies directed against intracellular (p=0.071), and non-synaptic (p=0.054) antigens. However, these comparisons did not attain statistical significance (Table 2). Moreover, treatment-responsive patients had significantly higher prevalence of extracellular (p=0.043) and synaptic (p=0.024) antibodies (Table 3). By contrast, patients who died during their follow-up showed intracellular (p=0.633) and non-synaptic (p=0.527) antibody prevalence comparable to those who were alive at their final visit (Table 3). There were no differences between different prognostic outcome groups by means of gender, presence of detectable cancer on admission, CSF-OCB positivity, and detection of lesions on the initial cranial MRI (Table 2–4).
Table 2.
Comparison of demographic and clinical characteristics of ANA seropositive cases with good and poor prognosis*
| Poor Prognosis (16 cases) | Good Prognosis (11 cases) | p | |
|---|---|---|---|
| Gender | 8 Male | 5 Male | 0.564 |
| 8 Female | 6 Female | ||
| Presence of cancer | 12 Cancer- | 9 Cancer- | 0.528 |
| 4 Cancer + | 2 Cancer + | ||
| Anti-neuronal antibody (intracellular vs extracellular) | 14 Intracellular | 6 Intracellular | 0.071 |
| 2 Extracellular | 5 Extracellular | ||
| Anti-neuronal antibody (synaptic vs non-synaptic) | 4 Synaptic | 7 Synaptic | 0.054 |
| 12 Non-synaptic | 4 Non-synaptic | ||
| Oligoclonal band positivity | 9 Positive | 4 Positive | 0.267 |
| 7 Negative | 7 Negative | ||
| Cranial MR positivity** | 3 Pathologic | 5 Pathologic | 0.128 |
| 12 Normal | 5 Normal |
Patients with a final mRS score of 4–6 were considered to have poor prognosis, patients with a final mRS score of 1–3 were considered to have a good prognosis
Cranial MR examination was not performed in 2 cases.
Table 3.
Comparison of the demographic and clinical characteristics of treatment responsive and non-responsive* ANA seropositive cases
| Responsive (17 cases) | Non-Responsive (10 cases) | p** | |
|---|---|---|---|
| Gender | 9 Male | 4 Male | 0.695 |
| 8 Female | 6 Female | ||
| Presence of cancer | 12 Cancer- | 9 Cancer- | 0.251 |
| 5 Cancer+ | 1 Cancer+ | ||
| Anti-neuronal antibody (intracellular vs extracellular) | 15 Intracellular | 5 Intracellular | 0.043 |
| 2 Extracellular | 5 Extracellular | ||
| Anti-neuronal antibody (synaptic vs non-synaptic) | 4 Synaptic | 7 Synaptic | 0.024 |
| 13 Non-synaptic | 3 Non-synaptic | ||
| Oligoclonal band positivity | 9 Positive | 4 Positive | 0.402 |
| 8 Negative | 6 Negative | ||
| Cranial MR positivity*** | 4 Pathologic | 4 Pathologic | 0.287 |
| 12 Normal | 5 Normal |
Response to treatment was defined as a decrease of at least one point in the first mRS.,
Statistically significant p values are written in bold font.
Cranial MR examination was not performed in 2 cases.
Table 4.
Comparison of the demographic and clinical characteristics of ANA seropositive cases with (mRS=6) or without (mRS<6) death during follow-up
| Dead (6 cases) | Alive (21 cases) | p | |
|---|---|---|---|
| Gender | 4 Male | 9 Male | 0.384 |
| 2 Female | 12 Female | ||
| Presence of cancer | 4 Cancer- | 17 Cancer- | 0.586 |
| 2 Cancer+ | 4 Cancer+ | ||
| Anti-neuronal antibody (intracellular vs extracellular) | 4 Intracellular | 16 Intracellular | 0.633 |
| 2 Extracellular | 5 Extracellular | ||
| Anti-neuronal antibody (synaptic vs non-synaptic) | 2 Synaptic | 9 Synaptic | 0.527 |
| 4 Non-synaptic | 12 Non-synaptic | ||
| Oligoclonal band positivity | 4 Positive | 9 Positive | 0.286 |
| 2 Negative | 12 Negative | ||
| Cranial MR positivity* | 2 Pathologic | 6 Pathologic | 0.525 |
| 3 Normal | 14 Normal |
Brain MR examination was not performed in 2 cases
Survival Analysis
Since significant prognostic differences could only be found among patients with different antibody types, the impact of antibody types on survival was further investigated by Kaplan-Meier analysis. Kaplan-Meier curves were constructed by plotting cumulative survival rates (vertical axis) against follow-up years (horizontal axis). No significant difference could be found between survival rates of patients with or without extracellular antibodies (p=0.908), and patients with and without synaptic antibodies (p=0.343) (Figure 2).
Figure 2.
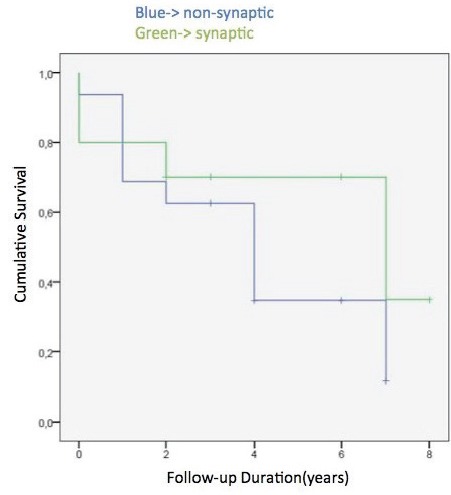
Comparison of Kaplan-Meier survival curves of patients with and without synaptic anti-neuronal antibodies.
DISCUSSION
In this study, an extensive antibody screening was performed for the first time in a Turkish cohort of PNS and autoimmune encephalitis. In our ANA seropositive cohort, most prevalent syndromes were limbic encephalitis, subacute cerebellar degeneration, and brainstem encephalitis, and most frequently detected antibodies were NMDAR, Hu, GAD, Yo, and Ma2. Tumors of the lung and breast tissues were detected in 6 cases by whole-body imaging, and pathological examination. With these characteristics, our patients’ findings were similar to those of previously reported ANA positive cohorts (5–13). Some of the antibodies examined in our study (Sox1, titin, recoverin and Tr-DNER) were not detected in any of the cases. These antibodies should probably not be examined during routine screening for PNS and autoimmune encephalitis patients, but should be investigated in cases with specific syndromes associated with these antibodies.
The most prominent prognostic factor in our study was the type of antibody (presence or absence of extracellular-synaptic antibodies). By contrast, detection of cancer, brain lesions or CSF-exclusive OCB in the first visit was not related with survival and prognosis. It is especially interesting that presence of cancer is not prognostic, implicating the significance of neurological disability in long-term outcome of PNS and autoimmune encephalitis patients.
Prevalence of cancer at first admission (6/27; 22% in all ANA positive patients) in our cohort might be considered to be somewhat lower than expected. This is probably due to the high number of patients with extracellular and GAD antibodies, which are known to be associated with a cancer prevalence of 0–50% at the initial admission. By contrast, among patients with non-synaptic intracellular antibodies (Hu, Yo, Ri, Ma2, CV2 and Zic4-antibodies), cancer prevalence at first admission was higher (6/16; 37.5%). An underlying tumor is detected in approximately 2/3 of cases with intracellular antibodies at first admission, and subsequently almost all of these patients develop a detectable tumor within 5 years. Thus, the cancer prevalence of our patients with extracellular and intracellular antibodies are congruent with the literature (13–19).
Another intriguing feature of our study was the low mortality rate (6/27; 22%). Also, although the death rate of patients with intracellular antibodies is known to be quite high (20), only 4 out of 16 patients with well-characterized paraneoplastic antibodies (Hu, Yo, Ri, Ma2, CV2 and amphiphysin) had died during their follow-up. A potential reason for the low cancer and mortality rates in our study might be the relatively short follow-up duration. Additionally, survival rates of our patients might have been increased by early diagnosis and treatment as a result of the fact that all antibody detection and treatment decisions were given by a single team working in the same facility. Thus mortality of PNS may be reduced with rapid antibody detection, early treatment, and a standard immunotherapy protocol.
Another notable finding was that positivity for extracellular and synaptic antibodies predicted good prognosis and immunotherapy responsiveness but not mortality. Extracellular antibodies bind target antigens expressed by the membrane, and cause pathogenic effects. Removal of these antibodies from the circulation by immunotherapy affects the clinical course positively, and thus detection of extracellular antibodies has a favorable effect on prognosis (21). Nevertheless, 2 of the patients who were lost during follow-up despite appropriate and early treatment had extracellular/synaptic antibodies (NMDAR and GABABR), which have been associated with a favorable outcome (3, 10, 13, 17), suggesting that prognosis is not necessarily favorable in extracellular ANA positive patients. Both NMDAR and GABABR-antibody positive patients were lost within the first months of admission due to respiratory problems indicating significance of autonomic involvement in survival of patients.
An important poor prognostic indicator is hospitalization of ANA positive neurological patients in an intensive care unit during the clinical course (13). Since this study was conducted in a tertiary referral hospital, severe patients requiring an intensive care unit were not included. This may have caused our cohort to have a better-than-expected prognosis. Immunotherapy methods are being standardized but palliative treatment methods (e.g. anti-epileptic, and anti-pyretic medications) is administered due to specific requirements of individual patients. Therefore, these non-standard treatments may constitute a confounding variable inducing an unpredictable effect on survival. Another limitation of our study is the lack of CSF antibody measurements. Conceivably some patients may only have ANA in the CSF, and thus serum-based measurements may give false negative results in these patients (17–19). This may have resulted in elimination of ANA-positive patients ultimately affecting prognostic outcome measures.
In conclusion, antibody screening panel has both diagnostic and prognostic value in the management of PNS and autoimmune encephalitis patients. Early detection of the ANA leads to early initiation of cancer and neurologic syndrome treatment, and may improve survival and prognosis of patients even with cancer. Laboratory methods other than antibody screening have limited effects on survival and prognosis. Apparently, prediction of mortality requires additional biomarkers to be developed.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of İstanbul University.
Informed Consent: As the study was planned retrospectively informed consent was not needed to be taken.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – E.T., M.K.; Design – E.T., Ç.A., Ş.Y.Ç., T.G.; Supervision – E.T., M.K., G.A.D.; Resources– E.T., Ç.A., C.U., S.İ.; Materials – E.T., T.G., G.A.D.; Data Collection and/or Processing – Ç.A, C.U., S.İ., M.K., G.A.D.; Analysis and/or Interpretation – Ç.A., C.U., S.İ., E.T., M.K.; Literature Search – Ç.A., E.T.; Writing Manuscript – E.T., Ç.A., M.K.; Critical Review – E.T., M.K., T.G.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: This work was supported by the Research Fund of the University of İstanbul. Project number: 23979 and 27301.
REFERENCES
- 1.Graus F, Delattre JY, Antoine JC, Dalmau J, Giometto B, Grisold W, Honnorat J, Smitt PS, Vedeler Ch, Verschuuren JJ, Vincent A, Voltz R. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry. 2004;75:1135–1140. doi: 10.1136/jnnp.2003.034447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Honnorat J, Antoine JC. Paraneoplastic neurological syndromes. Orphanet J Rare Dis. 2007;2:22. doi: 10.1186/1750-1172-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tüzün E, Dalmau J. Limbic encephalitis and variants: classification, diagnosis and treatment. Neurologist. 2007;13:261–271. doi: 10.1097/NRL.0b013e31813e34a5. [DOI] [PubMed] [Google Scholar]
- 4.Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, Cortese I, Dale RC, Gelfand JM, Geschwind M, Glaser CA, Honnorat J, Höftberger R, Iizuka T, Irani SR, Lancaster E, Leypoldt F, Prüss H, Rae-Grant A, Reindl M, Rosenfeld MR, Rostásy K, Saiz A, Venkatesan A, Vincent A, Wandinger KP, Waters P, Dalmau J. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15:391–404. doi: 10.1016/S1474-4422(15)00401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelley BJ, Boeve BF, Josephs KA. Young-onset dementia: demographic and etiologic characteristics of 235 patients. Arch Neurol. 2008;65:1502–1508. doi: 10.1001/archneur.65.11.1502. [DOI] [PubMed] [Google Scholar]
- 6.Chitravas N, Jung RS, Kofskey DM, Blevins JE, Gambetti P, Leigh RJ, Cohen ML. Treatable neurological disorders misdiagnosed as Creutzfeldt-Jakob disease. Ann Neurol. 2011;70:437–444. doi: 10.1002/ana.22454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKeon A. Immunotherapeutics for autoimmune encephalopathies and dementias. Curr Treat Options Neurol. 2013;15:723–737. doi: 10.1007/s11940-013-0251-8. [DOI] [PubMed] [Google Scholar]
- 8.Flanagan EP, McKeon A, Lennon VA, Boeve BF, Trenerry MR, Tan KM, Drubach DA, Josephs KA, Britton JW, Mandrekar JN, Lowe V, Parisi JE, Pittock SJ. Autoimmune dementia: clinical course and predictors of immunotherapy response. Mayo Clin Proc. 2010;85:881–897. doi: 10.4065/mcp.2010.0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vincent A, Buckley C, Schott JM, Baker I, Dewar BK, Detert N, Clover L, Parkinson A, Bien CG, Omer S, Lang B, Rossor MN, Palace J. Potassium channel antibody-associated encephalopathy: a potentially immunotherapy-responsive form of limbic encephalitis. Brain. 2004;127:701–712. doi: 10.1093/brain/awh077. [DOI] [PubMed] [Google Scholar]
- 10.Titulaer MJ, McCracken L, Gabilondo I, Armangué T, Glaser C, Iizuka T, Honig LS, Benseler SM, Kawachi I, Martinez-Hernandez E, Aguilar E, Arribas NG, Florance NR, Torrents A, Saiz A, Rosenfeld MR, Gordon RB, Graus F, Dalmau J. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12:157–165. doi: 10.1016/S1474-4422(12)70310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vitaliani R, Mason W, Ances B, Zwerdling T, Jiang Z, Dalmau J. Paraneoplastic encephalitis, psychiatric symptoms, and hypoventilation in ovarian teratoma. Ann Neurol. 2005;58:594–604. doi: 10.1002/ana.20614. https://doi.org/10.1002/ana.20614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKeon A. Paraneoplastic and other autoimmune disorders of the central nervous system. Neurohospitalist. 2013;3:53–64. doi: 10.1177/1941874412453339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mittal MK, Rabinstein AA, Hocker SE, Pittock SJ, Wijdicks EF, McKeon A. Autoimmune encephalitis in the ICU. Analysis of phenotypes, serologic findings, and outcomes. Neurocrit Care. 2016;24:240–250. doi: 10.1007/s12028-015-0196-8. [DOI] [PubMed] [Google Scholar]
- 14.Ramanathan S, Mohammad SS, Brilot F, Dale RC. Autoimmune encephalitis: recent updates and emerging challenges. J Clin Neurosci. 2014;21:722–730. doi: 10.1016/j.jocn.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Graus F, Saiz A, Lai M, Bruna J, López F, Sabater L, Blanco Y, Rey MJ, Ribalta T, Dalmau J. Neuronal surface antigen antibodies in limbic encephalitis: clinical-immunologic associations. Neurology. 2008;71:930–936. doi: 10.1212/01.wnl.0000325917.48466.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finke C, Prüss H, Heine J, Reuter S, Kopp UA, Wegner F, Then Bergh F, Koch S, Jansen O, Münte T, Deuschl G, Ruprecht K, Stöcker W, Wandinger KP, Paul F, Bartsch T. Evaluation of Cognitive Deficits and Structural Hippocampal Damage in Encephalitis with Leucine-Rich, Glioma-Inactivated 1 Antibodies. JAMA Neurol. 2017;74:50–59. doi: 10.1001/jamaneurol.2016.4226. [DOI] [PubMed] [Google Scholar]
- 17.Chi X, Wang W, Huang C, Wu M, Zhang L, Li J, Zhou D. Risk factors for mortality in patients with anti-NMDA receptor encephalitis. Acta Neurol Scand. 2017;136:298–304. doi: 10.1111/ane.12723. [DOI] [PubMed] [Google Scholar]
- 18.Suthar R, Saini AG, Sankhyan N, Sahu JK, Singhi P. Childhood anti-NMDA Receptor Encephalitis. Indian J Pediatr. 2016;83:628–633. doi: 10.1007/s12098-015-1988-8. [DOI] [PubMed] [Google Scholar]
- 19.Lim JA, Lee ST, Jung KH, Kim S, Shin JW, Moon J, Byun JI, Kim TJ, Shin YW, Lee KJ, Kim YS, Park KI, Lee SK, Chu K. Anti-N-methyl-d-aspartate receptor encephalitis in Korea: clinical features, treatment, and outcome. J Clin Neurol. 2014;10:157–161. doi: 10.3988/jcn.2014.10.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bentea G, Sculier C, Grigoriu B, Meert AP, Durieux V, Berghmans T, Sculier JP. Autoimmune paraneoplastic syndromes associated to lung cancer: A systematic review of the literature: Part 3: Neurological paraneoplastic syndromes, involving the central nervous system. Lung Cancer. 2017;106:83–92. doi: 10.1016/j.lungcan.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 21.McKeon A, Martinez-Hernandez E, Lancaster E, Matsumoto JY, Harvey RJ, McEvoy KM, Pittock SJ, Lennon VA, Dalmau J. Glycine receptor autoimmune spectrum with stiff-man syndrome phenotype. JAMA Neurol. 2013;70:44–50. doi: 10.1001/jamaneurol.2013.574. [DOI] [PMC free article] [PubMed] [Google Scholar]