 Scope: Particle-induced lung injury is a kind of comprehensive pulmonary disease with not only inflammation but also fibrosis.
Scope: Particle-induced lung injury is a kind of comprehensive pulmonary disease with not only inflammation but also fibrosis.
Abstract
Scope: Particle-induced lung injury is a kind of comprehensive pulmonary disease with not only inflammation but also fibrosis. Bixin is a natural compound that is widely used as a food additive. Our previous studies demonstrated that bixin could alleviate inflammation in ventilation-induced acute lung injury as well as UV-exposure caused skin damage. But whether it could depress silica-induced long-term comprehensive lung injury and the mechanism of bixin in this protection have not yet been studied. Methods: A murine SiO2-induced long-term comprehensive lung injury model was established through silica intratracheal instillation. To elucidate the effects and mechanisms of bixin in silica-induced pulmonary inflammation and fibrosis, we treated mice with bixin following silica instillation. Results: Bixin treatment attenuated the accumulation of inflammatory cells which significantly ameliorated pathological inflammation and fibrotic development in the lungs. In addition, intraperitoneal (i.p.) injection of bixin in mice led to the upregulation of the NRF2 response in the lungs. Since alveolar macrophage activation plays a vital role in the initiation and progression of this injury, the mechanism was further studied in the THP-1 macrophage cells. Bixin activated NRF2 signals via blocking KEAP1 mediated ubiquitylation and degradation of NRF2. Conclusions: Our work has brought insights into exploring anti-particle-induced lung injury activities in the daily consumption of natural products. In addition, our study also inspires the discovery of new beneficial effects of bixin and its application in the treatment of other inflammatory diseases.
Introduction
Particle-induced lung injury is a comprehensive pulmonary disease with progressive inflammation and fibrosis, and SiO2 exposure caused lung injury is the major one, which ultimately leads to diffuse pulmonary fibrosis, lung structure distortion, and respiratory failure.1,2 The early stages may be asymptomatic, but the advanced stages of the disease can result in airflow limitation, hypoxia, pulmonary hypertension, respiratory or heart failure, and premature death.3 During its development, the pulmonary injury develops progressively, even without further exposure to silica particles. Unfortunately, there are currently no curative treatments for this injury, which makes lung transplantation the only remaining therapy.4 However, the high cost, limited donor supply, and surgical invasiveness of lung transplantation continue to be challenges. Therefore, the development of an effective alternative treatment is urgently needed.
SiO2-induced lung injury is characterized by the deposition of inhaled silica particles, prolonged cycles of massive inflammation, epithelial hyperplasia and the formation of dense nodules with a central hyalinized whorled collagen in the lungs.5,6 Previous studies have shown that alveolar macrophage activation plays a vital role in the initiation and progression of this injury.7,8 Activated alveolar macrophages secrete a series of pre-inflammatory cytokines, including IL6, IL8 and TNFα, through the p38/MAPK signaling pathway and NF-κB nuclear translocation, which stimulate the neighboring fibroblasts and macrophages.9 This process causes fibroblast proliferation and eventually leads to pulmonary fibrosis.10,11 In addition, phagocytosing silica particles by lung macrophages leads to the generation of reactive oxygen species (ROS) which further exacerbates this lung injury. Endogenous anti-inflammatory and anti-oxidative reactions can also be activated in response to the inflammation and redox imbalance triggered by particle exposure.
The cellular protective response against oxidative stress is mainly orchestrated by the transcription factor NRF2 (nuclear factor-E2-related factor 2). It regulates the expression of numerous antioxidant, anti-inflammatory, and pro-survival genes with antioxidant response elements (ARE) in their promoters.12,13 In general, NRF2 is ubiquitously expressed at low levels. However, its expression can be quickly up-regulated in response to various cellular stresses, including stress caused by exogenous particle exposure.14 In addition, the pre-activation of the NRF2/ARE signaling pathway facilitates an adaptive response that protects against various types of stresses encountered subsequently. Employing natural compounds to activate the NRF2 signaling pathway has been proved to be an effective chemo-preventive strategy, as demonstrated in various preclinical studies.15–18 In fact, many natural compounds used in traditional medicines for their antioxidant and anti-inflammatory properties have been shown to be NRF2 inducers that elicit a therapeutic effect through the NRF2/ARE signaling pathway.15–17 Collectively, NFR2 plays a key role in cellular defense against pathological oxidative stress and inflammation.19,20 It has been previously reported that NRF2 activation in response to chemo-preventive agents or oxidative stress is regulated by KEAP1, a substrate adaptor protein of an E3-ubiquitin ligase which binds to and negatively regulates NRF2.21,22 Specifically, the critical cysteine residues in KEAP1 can be oxidized by ROS or modified by electrophilic compounds, which releases it from NRF2 and prevents NRF2 from degradation.23,24
Bixin is a carotenoid extracted from Bixa orellana seeds and is used as a colorant for food, cosmetics and textiles approved by the FDA.25 Traditionally, in Mexico and South America, bixin was used in the treatment of infections and inflammatory diseases of the skin, prostate, and gastrointestinal tract, as well as chest pain.26 Our laboratory has previously demonstrated that bixin is able to quench the ROS generated during ventilation-induced acute oxidative lung injury.25 As a result of its antioxidant properties, bixin successfully prevented oxidative DNA damage and lipid peroxidation.25,27 Additionally, bixin also attenuated cisplatin-induced clastogenicity and carbon tetrachloride mediated hepatotoxicity.28 Bixin has been proved to be safe in daily consumption and there is no epidemiological evidence showing carcinogenicity or acute toxicity associated with the ingestion of or the occupational exposure to bixin, except for rare reports of allergies caused by bixin ingestion.29,30
To date, multiple natural products have been used for the treatment of SiO2-induced lung injury, but their mechanisms are still unclear.31,32 Applications of monomeric compounds derived from natural products to particle-induced pulmonary disease treatment are rarely reported. The potential protection effects of bixin on the development of this damage have not yet been studied. In this study, we established a murine model with particle exposure through silica intratracheal instillation. We showed that bixin significantly alleviates silica-induced pulmonary inflammation and fibrosis in mice. We also proved that bixin exhibits its cytoprotective effects via activating the NRF2 signaling pathway in both in vivo and in vitro studies.
This study discovered the antioxidant mechanisms of bixin in the treatment of particle-induced lung injury. Our work has brought insights into exploring anti-particle-related pulmonary disease activities among other natural products to contribute to particle-related pulmonary disease medical therapies. Furthermore, our study also inspires the discovery of new beneficial effects of bixin and its application in the treatment of other indications.
Materials and methods
Chemicals, antibodies and cell culture
Bixin was purchased from Spectrum (New Brunswick, NJ), tBHQ and silica (SiO2) were purchased from Sigma (St Louis, MO), and sulforaphane (SF) was purchased from Santa Cruz (Santa Cruz, CA). MG132 was purchased from Amquar (AMQUAR Bio., Colorado, USA). Primary antibodies against NRF2, KEAP1, GCLM, AKR1C1, P65, p-P65, LC3, and GAPDH were purchased from Santa Cruz. Hemagglutinin (HA) epitope antibody was purchased from Covance (Branford, CT). Ubiquitin antibody was purchased from Sigma. 8-Oxo-dG antibody was purchased from Trevigen (Gaithersburg, MD). Horseradish peroxidase (HRP)-conjugated secondary antibodies were purchased from Immunoway (Plano, TX). Human THP-1 acute monocytic leukemia cells were purchased from ATCC (Manassas, VA). THP-1 cells were cultured in RPMI 1640 medium supplemented with 10% FBS (Hyclone, Logan, UT), 0.1% gentamycin (Invitrogen, Carlsbad, CA) and 5 ng ml–1 phorbol-12-myristate-13-acetate (PMA) (Sigma). The cells were maintained at 37 °C in a humidified incubator containing 5% CO2.
siRNA silencing and cDNA transfection
The transfection of cDNA was performed using lipofectamine 2000 (Invitrogen). Hiperfect was used for small interfering RNA (siRNA) silencing. Control siRNA #1027281 and NRF2 siRNA #SI03187289 were purchased from Qiagene. The transfections of cDNA and siRNA were performed according to the manufacturer's instructions.
Cell viability assay
The potential cytotoxicity of bixin and its cyto-protective effects in THP-1 cells were measured by the functional impairment of the mitochondria using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Sigma). Briefly, approximately 2 × 104 THP-1 cells per well were seeded in a 96-well plate followed by 24 h of incubation. The cells were then treated with multiple doses of bixin for 48 h and were subjected to cell viability assay. On the other hand, THP-1 cells were transfected with either control-siRNA (Ctrl siRNA) or NRF2-siRNA (NRF2 siRNA) for 24 h. Approximately 2 × 104 transfected cells per well were seeded in a 96-well plate and pretreated with DMSO or bixin (40 μM) for 4 h. Cells were then exposed to multiple concentrations of SiO2 (0–2000 μg ml–1) for 48 h. For cell viability measurement, 20 μL of 2 mg mL–1 MTT was directly added to the cells followed by incubation at 37 °C for 2 h. 100 μl of isopropanol/HCl was added into each well after MTT incubation. The plate was then put on a shaker at room temperature for 15 min. The absorbance at 570 nm was measured using a Synergy 2 multi-mode microplate reader (Biotek, Seattle, USA). All samples were run in triplicates for each experiment and the data represent the means of three independent experiments.
ROS detection
THP-1 cells were transfected with either control-siRNA (Ctrl siRNA) or NRF2-siRNA (NRF2 siRNA) for 24 h. Approximately 1.5 × 106 transfected cells per well were seeded in a 6-well plate. The cells were pretreated with DMSO or bixin (40 μM) for 4 h prior to SiO2 exposure (0–100 μg ml–1) for 24 h. The cells were then washed with PBS followed by incubation with fresh medium containing 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) (Sigma, St Louis, MO, 10 μg ml–1 final concentration) for 30 min at 37 °C. The cells were then rinsed with PBS twice and trypsinized and resuspended in PBS to approximately 106 cells per ml. Fluorescence was measured using flow cytometry with excitation at 488 nm and emission at 515–545 nm. The procedures following H2DCFDA incubation were performed in the dark.
Immunoblot analysis, ubiquitination assay and protein half-life
THP-1 cells were harvested in Laemmli buffer (62.5 mM Tris-HCl (pH 6.9), 3% SDS, 10% glycerol, 5% beta-mercaptoethanol, and 0.1% bromophenol blue). Cell lysates were sonicated on ice to allow full digestion followed by boiling for 10 min. The denatured lysates were electrophoresed through an SDS-polyacrylamide gel and subjected to immunoblot analysis. For endogenous ubiquitination assay, THP-1 cells were left untreated (control) or treated with bixin (40 μM) along with MG132 (10 μM) for 4 h. For exogenous ubiquitination assay, THP-1 cells were first co-transfected with the expression vectors of HA-tagged ubiquitin and NRF2 for 24 h. The cells were then left untreated or treated with SF (5 μM), tBHQ (40 μM) or bixin (40 μM) along with MG132 (10 μM) for 4 h. At the completion of the treatments, the cells were harvested in a digesting buffer containing 2% SDS, 150 mM NaCl, 10 mM Tris-HCl (pH 8.0), and 1 mM DTT and were immediately boiled. For immunoprecipitation, cell lysates were incubated with 1 μg of NRF2 antibody along with protein A agarose beads (Invitrogen) at 4 °C overnight. The immunoprecipitated complexes were then washed with a buffer containing 0.5 M LiCl/TBS and 1 mM DTT followed by two additional washes with a buffer containing 1% Triton-X-100/TBS and 1 mM DTT. The immunoprecipitated protein was then eluted in Laemmli buffer followed by boiling for 10 min. The samples were resolved by SDS-PAGE and analyzed by immunoblotting with the antibodies against ubiquitin (endogenous ubiquitination assay) and the HA epitope (exogenous ubiquitination assay). To measure the half-life of NRF2, THP-1 cells were either left untreated or treated with 40 μM bixin for 4 hours. 50 μM cycloheximide was then added to block protein synthesis. Total cell lysates were collected at different time points and were subjected to immunoblot analysis with an anti-NRF2 antibody. The relative intensities of the immunoblot bands were quantified using the Syngene gel documentation system and GeneTools software from Syngene (Frederick, MD).
mRNA extraction and real-time RT-PCR
Total RNA was extracted from THP-1 cells and mice lung tissues using TRIzol reagent from CWBIO (Beijing, China). Equal amounts of mRNA were used to generate cDNA using a HiFiScript cDNA synthesis kit following the manufacturer's instructions (CWBIO, Beijing, China). The RT-PCR procedures and primer sequences of NRF2, KEAP1, GCLM, AKR1C1 and GAPDH were described previously.25,27,33 ABI 7500 (Applied Biosystems) was used to evaluate mRNA expression. The quantification of the cDNA expression of mouse Nrf2, Keap1, Gclm, Akr1c1, Il6 and Tnfα in the lung tissue samples was performed using an UltraSYBR Mixture (Low ROX) qPCR kit (CWBIO, Beijing, China). The primers were designed with Primer 3 (; http://www-genome.wi.mit.edu/genome_software/other; /primer3.html) and were synthesized by Genewiz. The sequences of the primers are listed as follows:
Nrf2: forward (CTCAGCATGATGGACTTGGA);
reverse (TCTTGCCTCCAAAGGATGTC);
Keap1: forward (GATCGGCTGCACTGAACTG);
reverse (GGCAGTGTGACAGGTTGAAG);
Akr1c1: forward (GGAGGCCATGGAGAAGTGTA);
reverse (GCACACAGGCTTGTACCTGA);
Gclm: forward (TCCCATGCAGTGGAGAAGAT);
reverse (AGCTGTGCAACTCCAAGGAC);
β-actin: forward (AAGGCCAACCGTGAAAAGAT);
reverse (GTGGTACGACCAGAGGCATAC).
The real-time PCR conditions used were: initial denaturation (95 °C, 10 min), 40 cycles of amplification (95 °C, 10 s; 60 °C, 30 s; 72 °C, 32 s), melting curve (95 °C, 15 s; 60 °C, 1 min; 95 °C, 15 s), and cooling cycle (60 °C, 15 s). The mean crossing point (Cp) values and standard deviations (SD) were determined. The Cp values were normalized to the respective Cp values of the mouse β-actin reference gene. The data are presented as a fold change in gene expression compared to the control group.
Animals and treatments
Male C57BL/6 mice (7 weeks old) were purchased from SLAC Laboratory Animal Co. Ltd (Shanghai, China) and were maintained in 12 h light/dark cycle, climate-controlled and pathogen-free rooms. All mice were fed with standard mouse chow, and permitted food and water consumption ad libitum. Mice handling in this study followed the Guide for the Care and Use of Laboratory Animals and the study protocols were approved by the Soochow University Institutional Animal Care and Use Committee (permission number: SYXK 2014-0030). Eight-week-old mice were randomly allocated into four experimental groups (n = 18 per group): (i) control (tea oil); (ii) bixin (200 mg kg–1, dissolved in tea oil); (iii) SiO2; and (iv) bixin + SiO2. The mice were first intratracheally instilled with SiO2 (3 mg in 50 μl sterile saline). Bixin was administered by intraperitoneal (i.p.) injection every three days (starting from 72 h before SiO2 intratracheal instillation) until the end of the experiment. The mice were weighed once a week during the experiments before they were euthanized at day 7, day 28, and day 56 following SiO2 instillation (n = 6 per group).
Silica preparation
The content of the free SiO2 dust was more than 99%, and the particle size of 80% of the SiO2 dust was between 1 and 5 μm. Silica was ground in saline for 3 h, and boiled in 1 N HCl. After washing, the particles were resuspended in sterile saline. The suspensions were subjected to sonication for 10 min before use.
Bronchoalveolar lavage (BAL) and lung tissue collection
After the completion of the treatments, the mice were euthanized and BAL fluids were obtained by lavaging the lungs with 1 mL HBSS (Invitrogen) through a tracheal cannula.34 The BAL fluid was centrifuged at 500g for 20 min at 4 °C to collect the cells. The cell pellets were then resuspended in PBS and the total cell counts were determined using a TC20 automated cell counter (BioRad). The rest of the BAL cells were subjected to cytospin (Cytospin 4, Thermo Fisher Scientific) preparation as previously reported and slides were stained with a Shandon Kwik-Diff kit (Thermo Fisher Scientific).25 Macrophages, neutrophils and lymphocytes were identified based on standard morphological criteria and in total 200 cells were examined per sample. The mean cell counts ± SD were obtained from 6 mice from each group. The BAL fluid supernatants were centrifuged again at 15 000g for 10 min at 4 °C and were stored at –80 °C for protein analysis. Lung tissues from each experimental group were harvested and divided into two parts: one part was frozen in liquid nitrogen for total RNA extraction and protein analysis and the other part was fixed in 10% buffered formalin and embedded in paraffin for histological and immunochemical analysis.
H&E (hematoxylin and eosin) and IHC (immunohistochemical) staining
Lung tissue sections (4 μm) were baked and deparaffinized. H&E staining was performed for pathology analysis. IHC analysis was performed as previously described.18 Briefly, antigen retrieval was performed by boiling the slides with a retrieval solution (citric acid monohydrate 2.1 g L–1 in H2O, pH = 6.0) three times (5 min each time). The tissue sections were then exposed to 3.5 M HCl for 15 min at room temperature and washed with PBS three times for 5 min each time. Subsequently, the tissue sections were treated with 0.3% peroxidase to quench endogenous peroxidase activity. The tissue sections were blocked for 30 min with 5% normal goat serum followed by 2 h of incubation with NRF2 antibody at 1 : 100 dilution at room temperature. Staining was performed using an EnVision+System-HRP kit (Dako) following the manufacturer's instructions.
IHC analysis for 8-hydroxy-2′-deoxyguanosine (8-oxo-dG)
Tissue sections were baked and deparaffinized. IHC analysis for 8-oxo-dG was performed as previously described.25 Briefly, lung tissue sections were incubated with proteinase K (800 U ml–1, in PBS) for 20 min at 37 °C, followed by washing with PBS 3 times (5 min each time). The sections were then treated with RNase A (10 mg ml–1 in a buffer containing 150 mM NaCl and 15 mM sodium citrate) for 1 h at 37 °C and washed with PBS 3 times (5 min each time). After the DNA in the tissue sections was denatured with 2 N HCl for 5 min at room temperature, the sections were neutralized with 1 M Tris-base for 5 min. Subsequently, the sections were blocked with 5% normal goat serum for 30 min followed by 2 h of incubation with 8-oxo-dG antibody at 1 : 5000 dilution at room temperature. Staining was performed using an EnVision+System-HRP kit (Dako) according to the manufacturer's instructions.
In situ TUNEL assay
Apoptotic cell death in lung tissues was measured using an in situ cell death detection kit (Roche) following the manufacturer's instructions. Briefly, the tissue sections were pretreated with proteinase K (800 U ml–1) in 10 mM Tris/HCl (pH = 7.8) at 37 °C for 30 min. The sections were then washed 3 times with PBS followed by incubation with a TUNEL reaction mixture for 1 h at 37 °C in the dark. The tissue sections were then stained with Hoechst stain, and were analyzed using a fluorescence microscope (LEICA DM 2500; excitation wavelength: 450–500 nm; detection wavelength: 515–565 nm; Hoechst stain was visualized under UV light).
Statistics
The results are presented as fold changes to the control groups. The data are shown as mean ± SD of three independent experiments performed in duplicate (real-time RT-PCR) or triplicate (MTT and ROS detection). Statistical tests were performed using SPSS 20.0. Unpaired Student's t-test was applied to compare the means of two groups. One-way ANOVA with Bonferroni's correction was applied to compare the means of three or more groups. A value of p < 0.05 was considered to be significant.
Results
Bixin protects against SiO2-induced lung tissue damage
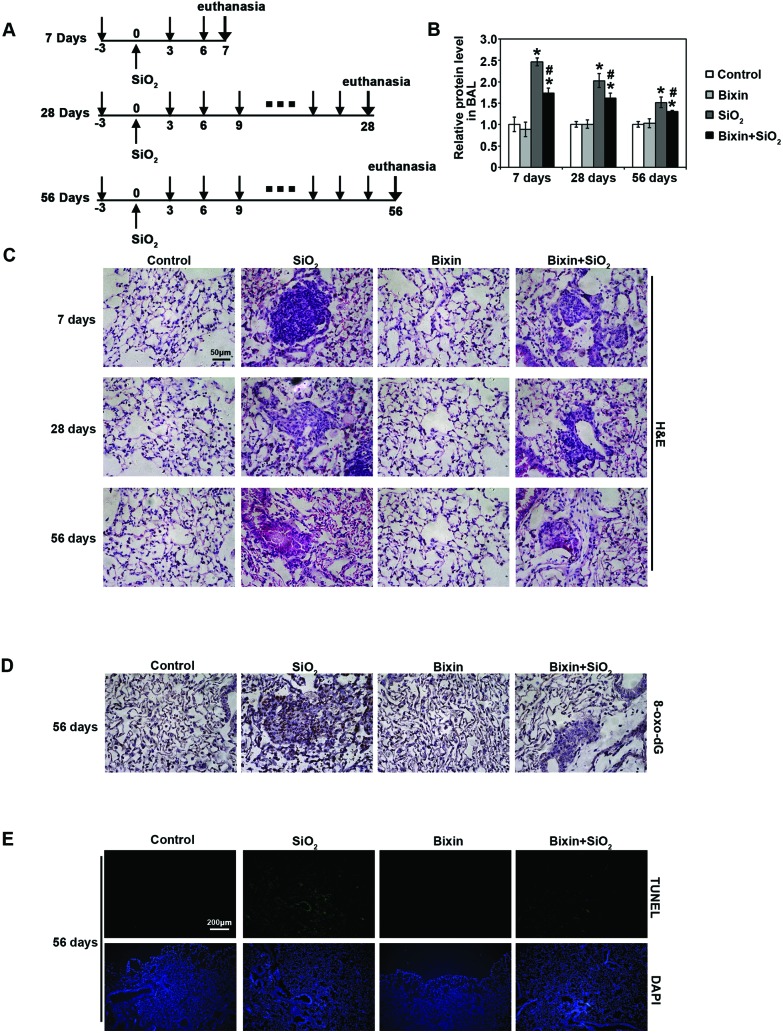
To investigate the potential protective effects of bixin on the development of lung injury caused by SiO2 exposure, mice were instilled with SiO2 (3 mg in 50 μl sterile saline) intratracheally on day 0. Bixin (200 mg kg–1) or control vehicle (tea oil) were given three days before SiO2 instillation and every three days after the exposure until they were euthanized on day 7, day 28 and day 56 (Fig. 1A). There were no significant differences in the body weight and lung weight among each experimental group (S1†). First, to evaluate the general lung tracheal epithelium injuries induced by SiO2, the total BAL protein amount from each experimental group of mice at indicated time points was measured (Fig. 1B). The total protein level in the BAL fluid increased by ∼2.5-fold as compared to the control group after 7 days of SiO2 exposure. With longer exposure durations, the protein levels decreased, but they remained significantly higher than that in the control group. In addition, the elevated BAL protein levels induced by SiO2 exposure were substantially reduced with bixin co-treatment at all time points. Bixin alone had no effects on the basal BAL protein levels. These data suggest protective effects of bixin on SiO2 instillation mediated alveolar leakage.
Fig. 1. Bixin protects against SiO2-induced tissue damage. (A) Three different time points of the experiments performed: 7 days, 28 days and 56 days. Treatment regimens: the respective groups of mice received i.p. injection of carrier control (tea oil) or bixin (200 mg kg–1) three days before the SiO2 exposure and every three days after. SiO2 was intratracheally instilled at day 0. The mice were harvested at 7 days, 28 days and 56 days. (B) Total BAL protein amount from the indicated treatment groups at different time points was measured. The data are presented as fold changes compared to the control group. The results are expressed as mean ± SD (*p < 0.05, control vs. treatment groups; #p < 0.05, SiO2vs. bixin + SiO2). (C) H&E staining of lung tissue sections from the respective groups of mice at different time points was performed. (D) IHC of 8-oxo-dG and (E) in situ TUNEL analysis (visualizing apoptotic cells) of lung tissue sections after SiO2 intratracheal instillation for 56 days were performed [n = 6, the representative images of the lung tissue from each group are shown (scale bar: 50 μm)].
Next, to investigate the effects of SiO2-induced lung injury and the protective effects of bixin during the disease development, the lung tissues collected at each time point were subjected to pathology analysis (Fig. 1C). H&E staining of the lung tissues revealed that SiO2 exposure caused the infiltration of inflammatory cells and alveolar septal thickening starting at day 7 post-instillation, as compared to the control group. In addition, irregular cellular nodules were observed at day 7 with SiO2 exposure. Bixin injection alone did not affect the lung morphology; however, it dramatically attenuated the pulmonary pathological changes caused by SiO2 instillation. This inflammatory response decreased at day 28 post-instillation, and clear fibrosis began to form across the cellular nodules, which indicated that the cellular nodules were transformed into cellular fibrotic nodules. In addition, the size of the cellular nodules also decreased. At day 56, the inflammation of the lungs with SiO2 exposure was evidently resolved, and the majority of the cellular fibrotic nodules became mostly fibrotic (fibrotic cellular nodules). In contrast, bixin treatment dramatically decreased the size of nodules starting at day 7. In addition, the fibrotic cellular nodules were dramatically shrunk, and the fibrotic level of the nodules was also significantly decreased at day 56 post-exposure. Collectively, these results indicated that SiO2 instillation caused a pronounced lung inflammation, which progressed from initial irregular cellular nodules to fibrotic nodules. However, bixin significantly attenuated the pulmonary inflammation and the subsequent lung fibrosis caused by SiO2 exposure.
SiO2 mediated oxidative DNA damage during the development of lung injury was also determined by 8-oxo-dG staining. As shown in Fig. 1D, SiO2 instillation markedly enhanced oxidative DNA damage in the lung tissue after 56 days of exposure, which was significantly suppressed with bixin cotreatment. Bixin treatment alone did not have any effects, indicating that there was no pro-oxidant effects at the dose of bixin used (Fig. 1D). Consistent with this observation, TUNEL analysis also showed that the apoptotic cell death caused by SiO2 exposure was dramatically reduced by bixin cotreatment (Fig. 1E). In summary, these observations suggest that bixin exhibits protective effects on SiO2-mediated pulmonary injury via reducing inflammation, oxidative DNA damage and cell apoptosis.
Bixin attenuates SiO2-induced injury by decreasing P65 phosphorylation in the lung tissues
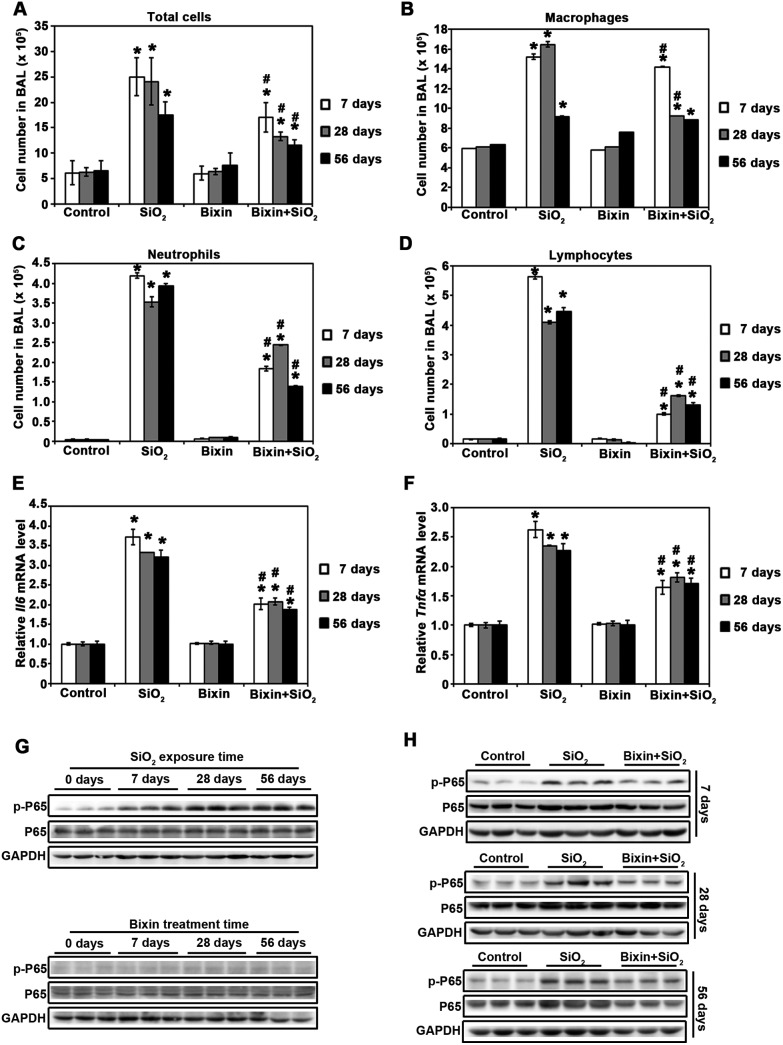
In addition to the pathological changes, silica particle inhalation also induces lung inflammation, which is characterized by the accumulation of inflammatory cells and secretion of pro-inflammatory cytokines. We first quantified the total and distinct inflammatory cell types in BAL to further our understanding of the pathological observations. SiO2 instillation induced dramatic inflammatory cell infiltration as assessed by the increase in the total cell numbers as well as the numbers of macrophages, neutrophils, or lymphocytes on day 7, 28, and 56 (Fig. 2A–D). Bixin treatment alone did not have any effect on cell infiltration; however, it significantly reduced inflammatory cell infiltration (total, macrophages, neutrophils, and lymphocytes) mediated by SiO2 exposure at all of the time points. We then measured the mRNA levels of the pro-inflammatory cytokines (Il6 and Tnfα) which are surrogate markers for the activation of the NF-κB signaling pathway using real-time RT-PCR. SiO2 exposure greatly induced the mRNA expressions of Il6 and Tnfα, which were both blocked by bixin treatment at all of the time points (Fig. 2E and F). We then investigated the activation of the NF-κB signaling pathway through measuring the phosphorylation of its P65 subunit. SiO2 exposure dramatically induced the phosphorylated (active) form of P65 (p-P65), while the total P65 protein level remained unchanged (Fig. 2G). In contrast, bixin treatment successfully blocked the phosphorylation of p-P65 mediated by SiO2 without reducing the total protein level (Fig. 2H). Collectively, our data suggest that bixin reduces SiO2 mediated pulmonary inflammation through blocking P65 phosphorylation in the lung tissues.
Fig. 2. Bixin attenuates SiO2-induced inflammation by decreasing P65 phosphorylation in the lung tissues. (A) Total cell numbers in BAL were measured in the indicated treatment groups. Cell differential analysis was performed on the BAL cells for each mouse sample. At least 200 cells were counted per sample. The numbers of (B) macrophages, (C) neutrophils and (D) lymphocytes were counted (n = 6). The mRNA levels of (E) Il6 and (F) Tnfα were measured by RT-PCR analysis. The results are expressed as mean ± SD (*p < 0.05, control vs. treatment groups; #p < 0.05, SiO2vs. bixin + SiO2). Lung tissue lysates from the indicated groups of mice (n = 3) were subjected to immunoblot analysis with the NF-κB signaling pathway (P65, p-P65) antibodies.
Bixin induces the NRF2 signaling pathway in the lung tissues
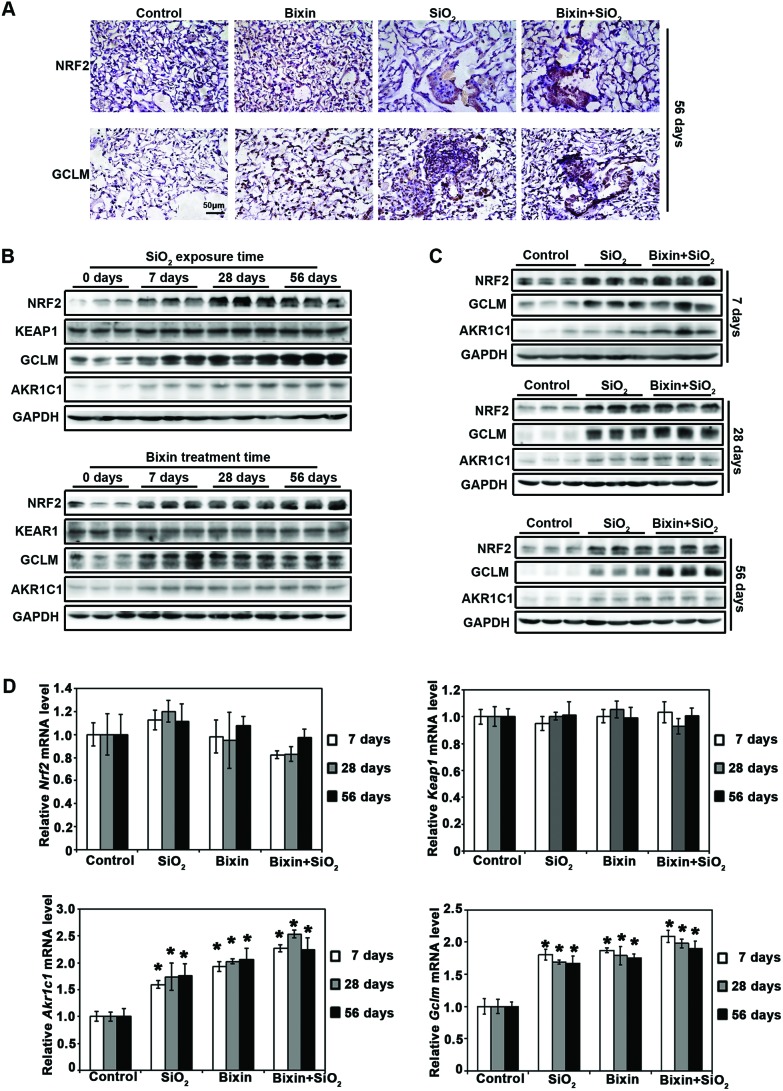
To explore the mechanism of bixin's protective effects on SiO2-induced lung injury, the activities of the NRF2 signaling pathway were determined in each experimental group. Consistent with our previous discovery of bixin's antioxidant activities,25 we here showed that bixin treatment markedly increased the protein expression of NRF2 and its target GCLM, as assessed by IHC staining of the lung tissues. Particle exposure has been previously reported to induce the NRF2 signaling pathway, and as expected, elevated NRF2 expression was observed with SiO2 exposure.18,35 In addition, an increased NRF2 expression was also detected in the combination exposure to bixin and SiO2 (Fig. 3A). This observation was confirmed by immunoblot analyses of the total protein extracted from the lung tissue collected at all time points, where we showed that the protein expression of NRF2 and its targets (GCLM and AKR1C1) was induced by bixin and SiO2 exposure (either alone or in combination), and there is no effect on KEAP1 expression (Fig. 3B and C). Furthermore, the mRNA levels of Nrf2, Keap1, Akr1c1, and Gclm were also assessed (Fig. 3D). The Nrf2 mRNA level was not affected by the treatments with bixin or SiO2, which is consistent with our previous reports.25,27 In addition, bixin had no effects on the mRNA levels of Keap1 as we expected. However, the mRNA expressions of Akr1c1 and Gclm were significantly induced by the exposure to bixin and SiO2 (either alone or in combination) in the lung tissues. These data suggest that NRF2 is an important regulator in the inflammatory response, and that the protective effects of bixin on SiO2-induced lung injury may be conducted through the NRF2 signaling pathway.
Fig. 3. Bixin induces the NRF2 signaling pathway in the lung tissues. (A) IHC staining of NRF2 and its target GCLM in the lung tissue sections from the indicated treatment groups after 56 days of SiO2 exposure was performed [n = 6, a representative image of the lung tissue for each group is shown (scale bar: 50 μm)]. (B) Lung tissue lysates from SiO2 and bixin groups were subjected to immunoblot detection of the NRF2 pathway antibodies at the indicated time points. (C) Lung tissue lysates from the control, SiO2 and bixin + SiO2 treatment groups were subjected to immunoblot analyses with the NRF2 pathway antibodies at the indicated time points. (D) The mRNA levels of Nrf2, Keap1, Akr1c1, and Gclm were measured by RT-PCR analysis. The results are expressed as mean ± SD (*p < 0.05, control vs. treatment groups).
Bixin upregulates the NRF2 signaling pathway in THP-1 cells
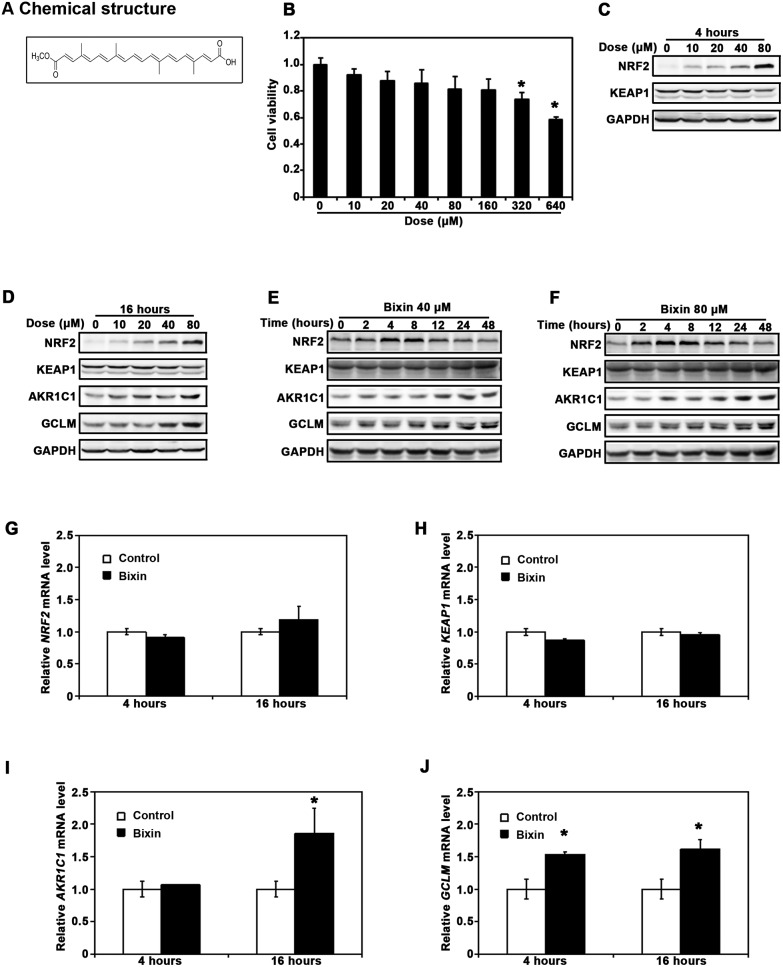
The process of SiO2-induced lung injury is initiated by macrophage activation in the lung tissues. Previous studies have shown that silica particle exposure can activate lung macrophages, followed by the activation of the NF-κB signaling pathway and induction of the apoptosis associated signaling pathways.7,36 To gain insights into the mechanism of bixin's protective effects on SiO2-mediated lung injury, the differentiated THP-1 cells were utilized for our in vivo study. We hypothesize that bixin can block the SiO2 mediated activation of the NF-κB signaling pathway, which plays critical roles in macrophage infiltration and fibrotic formation in lung injury. Based on the chemical structure of bixin and the previous data in our lab (Fig. 4A), we needed to first determine if bixin has any cytotoxicity before investigating its capability for NRF2 induction in THP-1 cells. The cells were treated with bixin (0–640 μM) for 48 h followed by cell viability measurement using the MTT assay. The result showed that cell viabilities were not significantly affected by bixin administration at dosage levels below 160 μM (Fig. 4B). We then tested the effects of bixin treatment on regulating the NRF2 signaling pathway. As shown in Fig. 4C and D, bixin treatments markedly up-regulated the protein levels of NRF2 (4 h) as well as its downstream targets (AKR1C1 and GCLM) (16 h) in a dose-dependent manner. Meanwhile, no changes were observed in KEAP1 expression (Fig. 4C). We then picked 40 μM and 80 μM as treatment dosages in the subsequent time course studies, as bixin at these two dosages can visibly induce NRF2 and its downstream targets without affecting the cell viability. As shown in Fig. 4D and E, the increase in the NRF2 protein levels started as early as 2 h after bixin treatment. This increase peaked during 4 to 8 h post-treatment followed by returning to its basal levels by 24 h (Fig. 4D and E). In addition, an increase in the AKR1C1 and GCLM protein levels occurred at 8 h post-bixin treatment and peaked at 48 h. No change was observed in the KEAP1 protein levels. On the other hand, bixin treatments at 40 μM for either 4 or 16 h did not affect the mRNA levels of NRF2 or KEAP1 (Fig. 4F and G), as expected of a ‘classical’ NRF2 inducer.37 In contrast, bixin significantly increased the mRNA levels of both AKR1C1 and GCLM at 16 h after the treatment, where an increase in the level of GCLM occurred as early as 4 h post-treatment (Fig. 4H and I).
Fig. 4. Bixin upregulates the NRF2 signaling pathway in THP-1 cells. (A) Bixin chemical structure. (B) Cell viability was measured in THP-1 cells treated with the indicated doses of bixin for 48 h. THP-1 cells were treated with the indicated doses of bixin for (C) 4 h and (D) 16 h. Cell lysates were subjected to immunoblot analyses with the NRF2 signaling pathway antibodies. THP-1 cells were treated with (E) bixin (40 μM) and (F) bixin (80 μM) for the indicated time points, and the cell lysates were subjected to immunoblot analyses. THP-1 cells were either left untreated (control) or treated with bixin (40 μM) for 4 h and 16 h, and mRNA was extracted. The relative mRNA levels of (G) NRF2, (H) KEAP1, (I) AKR1C1 and (J) GCLM were then determined by quantitative real-time RT-PCR. The data are expressed as mean ± SD (*p < 0.05, control vs. bixin treatment).
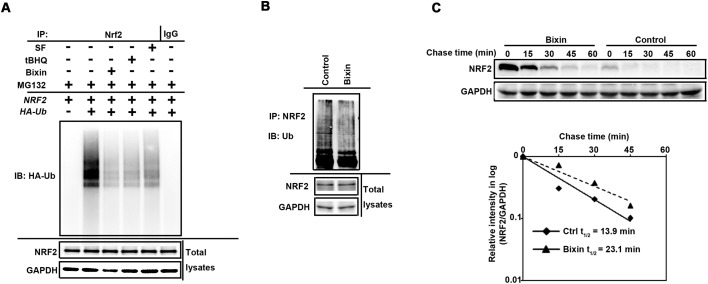
Bixin activates the NRF2 signaling pathway by decreasing NRF2 ubiquitination and increasing NRF2 protein stability
With the evidence showing that bixin is a stronger NRF2 inducer, we then investigated the mechanism of bixin's regulation in the NRF2 signaling pathway. Previous studies have demonstrated that the canonical NRF2 inducers, such as sulforaphane (SF) and Tanshinone-I (T-I), cause NRF2 activation by inhibiting its KEAP1-mediated ubiquitination.15,17,18 According to bixin's chemical structure, we postulate that bixin activates NRF2 in a similar mechanism. Therefore, cell-based ubiquitination assays were performed. For the exogenous ubiquitination assay, THP-1 cells were co-transfected with the expression vectors of HA-tagged ubiquitin and NRF2. The transfected cells were left untreated or treated with SF (5 μM), tBHQ (40 μM) or bixin (40 μM) along with MG132 (10 μM) for 4 h. For the endogenous ubiquitination assay, THP-1 cells were left untreated (control) or treated with bixin (40 μM) along with MG132 (10 μM) for 4 h. Similar to SF and tBHQ, the widely used NRF2 inducers, bixin treatment markedly reduced the ubiquitination level of NRF2 as compared to the untreated control (Fig. 5A and B); to further support the observation that bixin activates the NRF2 signaling pathway by attenuating its ubiquitination, the half-life of the endogenous NRF2 protein was determined. THP-1 cells were either left untreated or treated with 40 μM bixin for 4 hours. 50 μM cycloheximide was added to block protein synthesis. Total cell lysates were collected at different time points and subjected to immunoblot analysis with an anti-NRF2 antibody (Fig. 5C, upper panel). Quantification of the NRF2 immunoblot bands was performed and the results were plotted and the half-life of NRF2 was calculated (Fig. 5C, lower panel). The half-life of NRF2 of the untreated cells was 13.9 min; however, bixin treatment elongated the NRF2 half-life to 23.1 min. These results indicate that bixin activates NRF2 by blocking its ubiquitination and enhancing NRF2 protein stability.
Fig. 5. Bixin activates the NRF2 signaling pathway by decreasing NRF2 ubiquitination and increasing NRF2 protein stability. (A) THP-1 cells were cotransfected with plasmids encoding the indicated proteins for 24 h. The transfected cells were treated with SF (5 μM), tBHQ (40 μM) or bixin (40 μM) along with MG132 (10 μM) for 4 h. Anti-NRF2 immunoprecipitates were analyzed by immunoblotting with anti-HA antibody for the detection of ubiquitin-conjugated NRF2. (B) THP-1 cells were treated with bixin (40 μM) along with MG132 (10 μM) for 4 h. Anti-NRF2 immunoprecipitates were analyzed by immunoblotting with ubiquitin antibody for the detection of endogenous unbiquitin-conjugated NRF2. (C) THP-1 cells were either left untreated or treated with bixin (40 μM) for 4 h. Cycloheximide (CHX, 50 μM) was added and the cells were lysed at the indicated time points. Cell lysates were subjected to immunoblot analysis using anti-NRF2 and anti-GAPDH antibodies. The intensities of the bands were quantified using the GeneTools software and plotted against time after CHX treatment to obtain the half-life values.
Bixin reduces NF-κB mediated inflammation response and prevents SiO2 caused cell damage in an NRF2-dependent manner
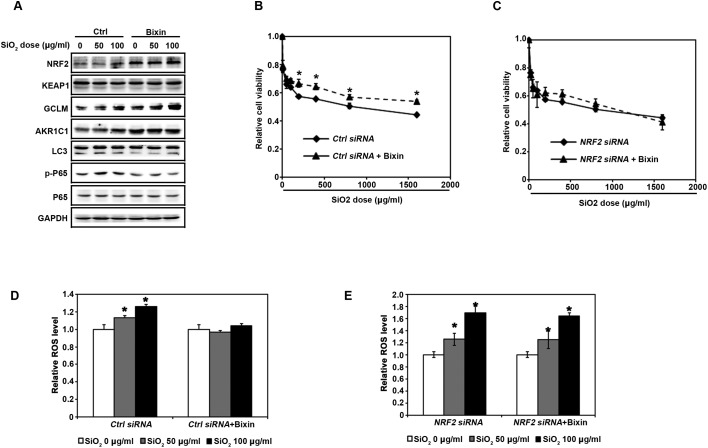
With the knowledge that bixin can activate the NRF2 signaling pathway in THP-1 cells, we then wonder if bixin has cytoprotective effects upon SiO2 exposure. We first investigated bixin's regulation of the NF-κB signaling pathway induced by SiO2 exposure. THP-1 cells were pretreated with DMSO (Ctrl) or bixin (40 μM) for 4 h followed by exposure to SiO2 (0–100 μg ml–1) for an additional 24 h. Consistent with our observations in Fig. 3, both SiO2 and bixin induced the protein expression of NRF2, GCLM, and AKR1C1. In addition, SiO2 significantly increased the phosphorylation of P65 without changing the total protein levels of P65. Bixin treatment alone did not have any effect on the protein expression levels of p-P65 and P65. However, the treatment of bixin dramatically reduced the phosphorylation of P65 induced by SiO2 exposure. Autophagy is a process that can be induced by intracellular stress. SiO2 mediated cell stress was represented by the increased protein expression of LC3 II, which is a classic autophagy indicator. However, the induced cellular stress was blocked by bixin treatment (Fig. 6A). To confirm this observation and to further explore the mechanism of bixin's protective effects, ROS production and cell viability were measured in THP-1 cells. For the cell viability study, THP-1 cells were transfected with either control-siRNA (Ctrl siRNA) or NRF2-siRNA (NRF2 siRNA) for 24 h. The cells were then pretreated with DMSO or bixin (40 μM) for 4 h before the addition of the indicated dose of SiO2 (0–2000 μg ml–1). Cell viability was measured at 48 h after exposure to SiO2. Bixin pretreatment markedly improved cell viability in response to SiO2 exposure (Fig. 6B). However, this protective effect is gone when the NRF2 protein expression was silenced by NRF2-siRNA (Fig. 6C). The ROS activation was blocked by bixin co-treatment. Bixin treatment alone did not affect ROS production, indicating that the dosages of bixin used did not trigger any intracellular oxidative stress (Fig. 6D). However, bixin failed to suppress ROS production in THP-1 cells in the presence of NRF2-siRNA, indicating that bixin is able to maintain the cellular redox balance upon SiO2 challenge and, therefore, protects the cells against SiO2-induced cell death.
Fig. 6. Bixin decreases the NF-κB inflammation response and protects against the cell damage induced by the SiO2 administration in an NRF2-dependent manner. (A) THP-1 cells were pretreated with DMSO (Ctrl) or bixin (40 μM) for 4 h prior to the treatment with SiO2 (0–100 μg ml–1) for an additional 24 h. Cell lysates were subjected to immunoblot analysis with the indicated antibodies. THP-1 cells were transfected with either (B) control-siRNA (Ctrl siRNA) or (C) NRF2-siRNA (NRF2 siRNA) for 24 h. The cells were then pretreated with DMSO or bixin (40 μM) for 4 h before the addition of the indicated dose of SiO2 (0–2000 μg ml–1). Cell viability was measured at 48 h after the addition of SiO2. The data are expressed as mean ± SD (*p < 0.05, control vs. bixin treatment). Following the transfection with either (D) control-siRNA or (E) NRF2-siRNA for 24 h, THP-1 cells were pretreated with DMSO or bixin (40 μM) for 4 h prior to the treatment with SiO2 (0–100 μg ml–1) for an additional 24 h. DCF-based fluorescence was measured using flow cytometry. The data are expressed as mean ± SD (*p < 0.05, control vs. bixin treatment).
Discussion
SiO2-induced lung injury is a form of lung disease caused by the inhalation of crystalline silica dust. It is characterized by massive inflammation and scarring in the form of nodular lesions in the upper lobes of the lungs. Despite the work of many previous studies, a cure has not yet been discovered. Thus, the treatment of SiO2-induced lung injury remains an area of urgent medical need. In addition, the mechanisms of lung inflammation and fibrosis induced by silica particles still need to be explored. In recent years, the applications of natural plant extractions have become promising approaches for the therapeutic treatment of many inflammatory disorders. Bixin has been shown to possess anti-inflammatory properties and exert therapeutic effects on various inflammatory diseases. Our previous studies have shown that bixin was able to inhibit inflammatory cell infiltration in ventilation induced acute lung injury and UV exposure mediated skin damage.25,27 However, the beneficial effects of bixin on silica-induced lung inflammation and fibrosis have been poorly studied.
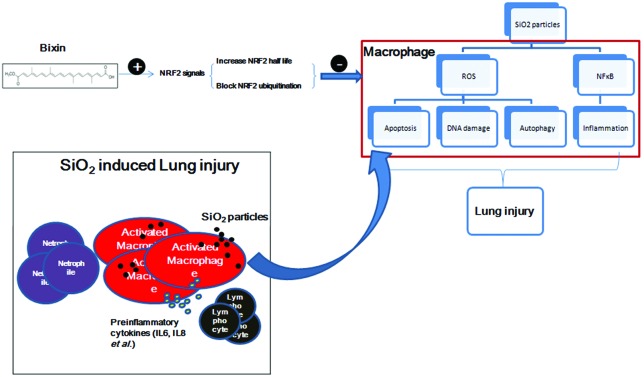
In this study, we demonstrated that bixin attenuated silica-induced lung inflammation and fibrosis through up-regulating the NRF2 signaling pathway. We first investigated the protective effects of bixin on the development of SiO2-induced lung injury in our in vivo mice model. We discovered that silica particle exposure induced the expression of NRF2, GCLM and AKR1C1, which is in agreement with the role of the NRF2 signaling pathway as a stress rescuer (Fig. 3). However, silica exposure also elicited severe inflammatory response (as measured by elevated p-P65 protein and total BAL protein levels, increased total BAL cells, neutrophils, and lymphocytes and increased secretion of inflammatory cytokines) and oxidative stress (as measured by DNA oxidative damage) (Fig. 1 and 2). Bixin induced the expression of NRF2 and its downstream targets in the lung tissues (Fig. 3). Meanwhile, bixin pretreatment also restored the normal lung morphology and alleviated silica exposure induced inflammation and oxidative stress (Fig. 3). We then proved that bixin is a canonical activator of the NRF2-orchestrated cytoprotective response in human THP-1 acute monocytic leukemia cells (Fig. 4 and 5). Bixin prevented KEAP1 mediated NRF2 ubiquitination which resulted in a prolonged half-life of NRF2. In addition, bixin treatment significantly attenuated the elevated NF-κB inflammatory response and ROS production caused by SiO2 exposure, which also contributed to the improved cell viability. However, bixin failed to reverse these cell damages when the NRF2 expression was silenced by NRF2 siRNA (Fig. 6). Collectively, we demonstrated that bixin reduces the SiO2 mediated NF-κB inflammatory response and cellular oxidative damage in an NRF2-dependent manner.
NRF2 is widely acknowledged as a major antioxidant and anti-inflammatory factor and its beneficial effects on lung inflammation have been previously reported.17,18,25 However, the role of NRF2 in SiO2-induced lung injury has not yet been well studied. Our group and others have identified that silica particle exposure up-regulates the NRF2 response in lung tissues,14,35,38,39 and that blocking the NRF2 signaling pathway leads to increased inflammation and oxidative injuries in mice. Yang et al. have previously reported that NRF2 activation significantly reduced lung inflammation and fibrosis, and therefore improved the lung structure and function in silica particles instilled mice. They have also proved that earthworm extract could induce NRF2, and protected the lung epithelial cells against SiO2-induced oxidative stress, mitochondrial apoptotic response, and epithelial–mesenchymal transition. Our observation in this study is consistent with their conclusion. Furthermore, we studied the mechanism of bixin's protective effects on lung injury caused by silica exposure.
Alveolar macrophage plays an essential role in lung injury caused by particle inhalation. They are the primary cells that phagocytize inhaled particles in the alveoli which initiates the subsequent pro-inflammatory response and tissue injury.40,41 Zhang et al. reported that silica particle exposure in THP-1 macrophages induced both NF-κB and NRF2 signaling pathways. Their data indicated that the NF-κB signaling pathway is required for the activation of NRF2 and the induction of NRF2-regulated genes in response to SiO2 particles in macrophages.38 In contrast, our results showed that bixin treatment induced the NRF2 signaling pathway while it inhibited the NF-κB/P65 signaling pathway, which led to reduced lung inflammatory injury in the mice with silica exposure (Fig. 1–3). In addition, the NF-κB/P65 signaling pathway in the SiO2 challenged THP-1 macrophage cells was also reduced with bixin treatment (Fig. 6).
The molecular crosstalk between the NRF2 and NF-κB response pathways is complicated. NF-κB regulates the gene expression of a broad range of inflammatory factors including pro-inflammatory cytokines, chemokines, and other stress response proteins.42 Cytokines such as TNFα and IL-1β or other inflammatory mediators such as cyclooxygenase-2 (COX-2) can activate NADPH oxidases causing increased production of H2O2, and/or electrophiles, which subsequently activate or induce other redox-sensitive proteins and genes.43–45 For instance, 15-deoxy-delta-12,14-prostaglandin J2 (15d-PGJ2), a product of COX-2, is a well-established NRF2 activator.46 In addition, other NF-κB-regulated proteins including NADPH oxidase 4 and iNOS that produce oxidants may also activate NRF2 through the direct oxidation of KEAP1 cysteine residues.47,48 On the contrary, it was previously reported that RAC1, a small G-protein of the Pho family, is involved in the activation of both NF-κB and NRF2 signaling pathways. Specifically, RAC1 can block RAC1-dependent NF-κB activation through inducing NRF2/EpRE signaling.49 NF-κB/P65 can also compete for co-activators that are required for NRF2 binding with the target DNA, and at the same time NF-κB/P65 can activate histone deacetylase 3 to suppress NRF2-DNA binding.50 Thus, there should be a balance between the NF-κB and NRF2 signaling pathways. Activating the NRF2 signaling pathway using molecular inducers can be a potential therapeutic method to attenuate the NF-κB signaling pathway mediated inflammatory response, and to protect against tissue injuries from toxicant exposure.
Autophagy is an evolutionarily conserved process during cellular development. During the autophagy process, specific intracellular proteins and organelles can be engulfed by autophagosomes for degradation and recycling.51 Emerging studies have suggested that ROS production plays an essential role in the activation of the autophagy process.52 It has been previously reported that silica particle exposure induces autophagy in the macrophage cells, which results in cell activation and apoptosis.53 This agrees with our observation where we found that bixin treatment reduced the elevated LC3 protein expression levels caused by SiO2 exposure (Fig. 6A). Additionally, bixin treatment also successfully restored cell viability in THP-1 cells upon SiO2 exposure (Fig. 6B–C). Consistently, our animal studies demonstrated that bixin treatment significantly suppressed cellular DNA damage and apoptotic cell death induced by SiO2 exposure (Fig. 1D–E). Taken together, these results suggest that bixin reduces SiO2-induced pulmonary injury through suppressing inflammation, oxidative DNA damage and cell apoptosis.
In summary, this present study demonstrates that bixin blocks the NF-κB mediated inflammatory response and prevents SiO2-induced cell damage in an NRF2-dependent manner. Bixin is currently one of the most commonly consumed food colorants worldwide distinguished by a long record of dietary and ethnopharmacological use.29,54 In prior studies, bixin has demonstrated antigenotoxic and antioxidant cytoprotective activities. The systemic availability of oral bixin and its demethylated metabolite norbixin has been documented in rodent studies and healthy human subjects.55–58 More importantly, the acceptable daily intake (ADI) of bixin over a lifetime does not seem to project any appreciable health risk, which makes bixin surpass other carotenoids to be approved as a food additive.59 We have demonstrated that bixin exhibits remarkable pulmonary protection effects during the development of SiO2-induced lung injury. Together with the reported safety data and systemic availability analysis of bixin, we suggest that bixin based diets can be applied as a novel strategy for the treatment of SiO2-induced lung injury.
Funding information
The work was supported by the following grants: Chinese National Nature Science Foundation (Grant ref: 81703205, 81473008, 81673203); Youth Foundation of Jiangsu Province (Grant ref: BK20160333); China Postdoctoral Science Foundation (Grant ref: 2016M600440, 2017T100402); Natural Science Research in Colleges and Universities of Jiangsu Province (Grant ref: 16KJB330008, 16KJB330009); and Postdoctoral Science Foundation of Jiangsu Province (Grant ref: 1601079C).
Conflicts of interest
There are no conflicts of interest to declare.
Supplementary Material
Acknowledgments
We thank Sherly Huang for editing this manuscript.
Footnotes
†Electronic supplementary information (ESI) available: S1 – The relative changes of body weight (A) and the relative changes of lung weight of the mice with the indicated treatments (B). See DOI: 10.1039/c7tx00304h
References
- Steenland K., Ward E. CA-Cancer J. Clin. 2014;64:63–69. doi: 10.3322/caac.21214. [DOI] [PubMed] [Google Scholar]
- Greenberg M. I., Waksman J., Curtis J. DM, Dis.-Mon. 2007;53:394–416. doi: 10.1016/j.disamonth.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Laney A. S., Petsonk E. L., Attfield M. D. Occup. Environ. Med. 2010;67:652–656. doi: 10.1136/oem.2009.047126. [DOI] [PubMed] [Google Scholar]
- Leung C. C., Yu I. T., Chen W. Lancet. 2012;379:2008–2018. doi: 10.1016/S0140-6736(12)60235-9. [DOI] [PubMed] [Google Scholar]
- Perkins T. N., Dentener M. A., Stassen F. R., Rohde G. G., Mossman B. T., Wouters E. F., Reynaert N. L. Toxicol. Appl. Pharmacol. 2016;301:61–70. doi: 10.1016/j.taap.2016.04.003. [DOI] [PubMed] [Google Scholar]
- Castranova V., Vallyathan V. Environ. Health Perspect. 2000;108(Suppl 4):675–684. doi: 10.1289/ehp.00108s4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao S. Q., Rojanasakul L. W., Chen Z. Y., Xu Y. J., Bai Y. P., Chen G., Zhang X. Y., Zhang C. M., Yu Y. Q., Shen F. H., Yuan J. X., Chen J., He Q. C. Apoptosis. 2011;16:1195–1204. doi: 10.1007/s10495-011-0647-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misson P., van den Brule S., Barbarin V., Lison D., Huaux F. J. Leukocyte Biol. 2004;76:926–932. doi: 10.1189/jlb.0104019. [DOI] [PubMed] [Google Scholar]
- Peng H. B., Wang R. X., Deng H. J., Wang Y. H., Tang J. D., Cao F. Y., Wang J. H. Mol. Med. Rep. 2017;15:3121–3128. doi: 10.3892/mmr.2017.6402. [DOI] [PubMed] [Google Scholar]
- Orfila C., Lepert J. C., Gossart S., Frisach M. F., Cambon C., Pipy B. Histochem. J. 1998;30:857–867. doi: 10.1023/a:1003485312164. [DOI] [PubMed] [Google Scholar]
- Mohr C., Gemsa D., Graebner C., Hemenway D. R., Leslie K. O., Absher P. M., Davis G. S. Am. J. Respir. Cell Mol. Biol. 1991;5:395–402. doi: 10.1165/ajrcmb/5.4.395. [DOI] [PubMed] [Google Scholar]
- Jaramillo M. C., Zhang D. D. Genes Dev. 2013;27:2179–2191. doi: 10.1101/gad.225680.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensler T. W., Wakabayashi N., Biswal S. Annu. Rev. Pharmacol. Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- Yang J., Wang T., Li Y., Yao W., Ji X., Wu Q., Han L., Han R., Yan W., Yuan J., Ni C. Lab. Invest. 2016;96:1279–1300. doi: 10.1038/labinvest.2016.101. [DOI] [PubMed] [Google Scholar]
- Tao S., Justiniano R., Zhang D. D., Wondrak G. T. Redox Biol. 2013;1:532–541. doi: 10.1016/j.redox.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long M., Tao S., Rojo de la Vega M., Jiang T., Wen Q., Park S. L., Zhang D. D., Wondrak G. T. Cancer Prev. Res. 2015;8:444–454. doi: 10.1158/1940-6207.CAPR-14-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Tao S., Lian F., Chau B. T., Chen J., Sun G., Fang D., Lantz R. C., Zhang D. D. Toxicol. Appl. Pharmacol. 2012;265:292–299. doi: 10.1016/j.taap.2012.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao S., Zheng Y., Lau A., Jaramillo M. C., Chau B. T., Lantz R. C., Wong P. K., Wondrak G. T., Zhang D. D. Antioxid. Redox Signaling. 2013;19:1647–1661. doi: 10.1089/ars.2012.5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T., Tian F., Zheng H., Whitman S. A., Lin Y., Zhang Z., Zhang N., Zhang D. D. Kidney Int. 2014;85:333–343. doi: 10.1038/ki.2013.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H. Y., Reddy S. P., Yamamoto M., Kleeberger S. R. FASEB J. 2004;18:1258–1260. doi: 10.1096/fj.03-1127fje. [DOI] [PubMed] [Google Scholar]
- Kobayashi A., Kang M. I., Okawa H., Ohtsuji M., Zenke Y., Chiba T., Igarashi K., Yamamoto M. Mol. Cell. Biol. 2004;24:7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D. D., Lo S. C., Cross J. V., Templeton D. J., Hannink M. Mol. Cell. Biol. 2004;24:10941–10953. doi: 10.1128/MCB.24.24.10941-10953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D. D., Hannink M. Mol. Cell. Biol. 2003;23:8137–8151. doi: 10.1128/MCB.23.22.8137-8151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkova-Kostova A. T., Holtzclaw W. D., Cole R. N., Itoh K., Wakabayashi N., Katoh Y., Yamamoto M., Talalay P. Proc. Natl. Acad. Sci. U. S. A. 2002;99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao S., Rojo de la Vega M., Quijada H., Wondrak G. T., Wang T., Garcia J. G., Zhang D. D. Sci. Rep. 2016;6:18760. doi: 10.1038/srep18760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Kong X. Q. Biomed. Pharmacother. 2017;89:991–1004. doi: 10.1016/j.biopha.2017.02.052. [DOI] [PubMed] [Google Scholar]
- Tao S., Park S. L., Rojo de la Vega M., Zhang D. D., Wondrak G. T. Free Radical Biol. Med. 2015;89:690–700. doi: 10.1016/j.freeradbiomed.2015.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira P. R., Maioli M. A., Medeiros H. C., Guelfi M., Pereira F. T., Mingatto F. E. Biol. Res. 2014;47:49. doi: 10.1186/0717-6287-47-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohs S. J. Phytother. Res. 2014;28:956–960. doi: 10.1002/ptr.5088. [DOI] [PubMed] [Google Scholar]
- Auttachoat W., Germolec D. R., Smith M. J., White Jr. K. L., Guo T. L. Food Chem. Toxicol. 2011;49:2638–2644. doi: 10.1016/j.fct.2011.07.009. [DOI] [PubMed] [Google Scholar]
- Zhang H. N., Xin H. T., Zhang W. D., Jin C. J., Huang S. Y., Zhang Y. Zhonghua Yufang Yixue Zazhi. 2007;41:290–294. [PubMed] [Google Scholar]
- He L. Z., Huang Z. H., Wang H. R., Tu D. Y., Mao Z. F. J. Pharm. Pharmacol. 1998;50:351–354. doi: 10.1111/j.2042-7158.1998.tb06872.x. [DOI] [PubMed] [Google Scholar]
- Tao S., Wang S., Moghaddam S. J., Ooi A., Chapman E., Wong P. K., Zhang D. D. Cancer Res. 2014;74:7430–7441. doi: 10.1158/0008-5472.CAN-14-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Vinasco L., Quijada H., Sammani S., Siegler J., Letsiou E., Deaton R., Saadat L., Zaidi R. S., Messana J., Gann P. H., Machado R. F., Ma W., Camp S. M., Wang T., Garcia J. G. Am. J. Respir. Cell Mol. Biol. 2014;51:223–228. doi: 10.1165/rcmb.2012-0519OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C., Xia Y., Niu P., Jiang L., Duan J., Yu Y., Zhou X., Li Y., Sun Z. Int. J. Nanomed. 2015;10:1463–1477. doi: 10.2147/IJN.S76114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B. L. Zhonghua Yufang Yixue Zazhi. 1993;27:10–12. [Google Scholar]
- Wang X. J., Sun Z., Chen W., Li Y., Villeneuve N. F., Zhang D. D. Toxicol. Appl. Pharmacol. 2008;230:383–389. doi: 10.1016/j.taap.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Zhou L., Yuen J., Birkner N., Leppert V., O'Day P. A., Forman H. J. Free Radical Biol. Med. 2017;108:311–319. doi: 10.1016/j.freeradbiomed.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C., Zhao X., Sun D., Zhang L., Fang W., Zhu T., Wang Q., Liu B., Wei S., Chen G., Xu Z., Gao X. Sci. Rep. 2016;6:21133. doi: 10.1038/srep21133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdorster G., Oberdorster E., Oberdorster J. Environ. Health Perspect. 2005;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A., Veronesi B., Calderon-Garciduenas L., Gehr P., Chen L. C., Geiser M., Reed W., Rothen-Rutishauser B., Schurch S., Schulz H. Part. Fibre Toxicol. 2006;3:13. doi: 10.1186/1743-8977-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahl H. L. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- Chen X., Andresen B. T., Hill M., Zhang J., Booth F., Zhang C. Curr. Hypertens. Rev. 2008;4:245–255. doi: 10.2174/157340208786241336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M., Miyazaki T., Nagaya T., Murata Y., Ida N., Maeda K., Seo H. J. Rheumatol. 1996;23:432–438. [PubMed] [Google Scholar]
- Rahman A., Kefer J., Bando M., Niles W. D., Malik A. B. Am. J. Physiol. 1998;275:L533–L544. doi: 10.1152/ajplung.1998.275.3.L533. [DOI] [PubMed] [Google Scholar]
- Itoh K., Mochizuki M., Ishii Y., Ishii T., Shibata T., Kawamoto Y., Kelly V., Sekizawa K., Uchida K., Yamamoto M. Mol. Cell. Biol. 2004;24:36–45. doi: 10.1128/MCB.24.1.36-45.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manea A., Tanase L. I., Raicu M., Simionescu M. Biochem. Biophys. Res. Commun. 2010;396:901–907. doi: 10.1016/j.bbrc.2010.05.019. [DOI] [PubMed] [Google Scholar]
- Koga T., Zhang W. Y., Gotoh T., Oyadomari S., Tanihara H., Mori M. Exp. Eye Res. 2003;76:15–21. doi: 10.1016/s0014-4835(02)00274-9. [DOI] [PubMed] [Google Scholar]
- Cuadrado A., Martin-Moldes Z., Ye J., Lastres-Becker I. J. Biol. Chem. 2014;289:15244–15258. doi: 10.1074/jbc.M113.540633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G. H., Qu J., Shen X. Biochim. Biophys. Acta. 2008;1783:713–727. doi: 10.1016/j.bbamcr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Mizushima N. Genes Dev. 2007;21:2861–2873. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- Scherz-Shouval R., Elazar Z. Trends Biochem. Sci. 2011;36:30–38. doi: 10.1016/j.tibs.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Liu H., Cheng Y., Yang J., Wang W., Fang S., Zhang W., Han B., Zhou Z., Yao H., Chao J., Liao H. Cell Death Dis. 2017;8:e2657. doi: 10.1038/cddis.2017.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulbricht C., Windsor R. C., Brigham A., Bryan J. K., Conquer J., Costa D., Giese N., Guilford J., Higdon E. R., Holmes K., Isaac R., Jingst S., Kats J., Peery L., Rusie E., Savinainen A., Schoen T., Stock T., Tanguay-Colucci S., Weissner W. J. Diet. Suppl. 2012;9:57–77. doi: 10.3109/19390211.2012.653530. [DOI] [PubMed] [Google Scholar]
- Levy L. W., Regalado E., Navarrete S., Watkins R. H. Analyst. 1997;122:977–980. doi: 10.1039/a701304c. [DOI] [PubMed] [Google Scholar]
- Junior A. C., Asad L. M., Oliveira E. B., Kovary K., Asad N. R., Felzenszwalb I. GMR, Genet. Mol. Res. 2005;4:94–99. [PubMed] [Google Scholar]
- Somacal S., Figueiredo C. G., Quatrin A., Ruviaro A. R., Conte L., Augusti P. R., Roehrs M., Denardin I. T., Kasten J., da Veiga M. L., Duarte M. M., Emanuelli T. Mol. Cell. Biochem. 2015;403:243–253. doi: 10.1007/s11010-015-2354-x. [DOI] [PubMed] [Google Scholar]
- Roehrs M., Figueiredo C. G., Zanchi M. M., Bochi G. V., Moresco R. N., Quatrin A., Somacal S., Conte L., Emanuelli T. Int. J. Endocrinol. 2014;2014:839095. doi: 10.1155/2014/839095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O. World Health, Evaluation of certain food additives and contaminants. Eightieth report of the Joint FAO/WHO Expert Committee on Food Additives, World Health Organization technical report series, 2016, pp. 1–114, back cover. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.