Abstract
Background
The aim of this study was to investigate the long intergenic non-coding RNA (lincRNA) of the NED25 gene, and the microRNA (miR)-125b, STAT3, nitric oxide (NO), and procalcitonin (PCT) pathway in sepsis.
Material/Methods
Seventy-five age-matched and sex-matched patients were divided into three groups: 25 patients with sepsis only; 25 patients with septic shock; and 25 healthy control subjects. Computational analysis and a luciferase assay confirmed that the STAT3 and PCT genes were target genes of miR-125b, whereas the lincRNA of the NED25 gene was validated as an endogenous lincRNA competing with miR-125b for binding to STAT3 and PCT. Real-time polymerase chain reaction (PCR) and Western blot measured the expression of miR-125b, STAT3, and PCT in peripheral blood monocytes (PBM) transfected with miR-125b mimics, miR-125b inhibitors, or small interfering (siRNA).
Results
The expression of miR-125b, the PCT position ratio, the expression of PCT mRNA and protein were increased when compared with healthy individuals. When compared with the siRNA negative control, miR-125b and the lincRNA of the NED25 gene mimics, as well as STAT3 siRNA significantly downregulated the mRNA and protein expression of STAT3 and PCT; mRNA and protein expression of STAT3 and PCT in cells transfected with miR-125b inhibitors were significantly increased. Intracellular nitric oxide (NO) production was upregulated by miR-125b inhibitors and downregulated by miR-125b mimics or siRNA.
Conclusions
Downregulation of the lincRNA of the NED25 gene was associated with sepsis in patients by modulating the signaling pathways downstream of miR-125b/STAT3/PCT/NO signaling pathway.
MeSH Keywords: MicroRNAs, Nitric Oxide, Prognosis, Sepsis, STAT3 Transcription Factor
Background
Worldwide, sepsis is associated with a high risk of patient mortality in all age groups [1]. The estimated incidence of childhood pneumonia is 0.29, and 0.05 per child-year in developing and developed countries, respectively and pediatric sepsis has become the most common cause of death in children five years-of-age and younger [2]. Worldwide, there are approximately 0.156 billion new cases of pneumonia occurring annually, with 0.151 billion of these cases occurring in developing countries, where they are associated with malnutrition and lack of immunization, poor sanitation, indoor air pollution, contaminated water, overpopulation, and low birth weight, all of which can facilitate the invasion of pathogens into the body [2].
As a potential marker for risk stratification, procalcitonin (PCT) is a 116-amino acid prohormone for calcitonin, a regulator of calcium metabolism [3]. Although calcitonin is produced by the C-cells in the thyroid gland, it can also be increased during bacteria-induced activation of cytokines and lipopolysaccharides (LPS), many extra thyroid tissues can synthesize PCT, which may be regarded as a biomarker for sepsis and systemic bacterial infection [4]. Previous studies have shown a time-dependent change in the expression profile of certain biomarkers, such as C-reactive protein (CRP) and PCT, which may serve as potential prognostic indicators for patients with respiratory infections or sepsis [5]. PCT has been shown to be a helpful marker to monitor critically ill patients with sepsis and septic shock [6]. Recently, it has been shown that the production of nitric oxide (NO) and the expression of the nitric oxide synthase gene (NOS) were both promoted by PCT, which could explain the relationship between high levels of PCT and poor patient outcome in septic shock and multiple organ failure [7]. For example, following lipopolysaccharide (LPS) stimulation, the activation of the STAT3 gene and increased protein levels of Stat3 have been shown to lead to increased expression of PCT, with the expression levels of PCT occurring via the modulation of phospho-Stat3 [8].
The majority of non-coding RNAs (ncRNAs), including long non-coding RNAs (lncRNAs) and small non-coding RNAs (sncRNAs), are primarily expressed in the mammalian central nervous system (CNS) and contribute to neuronal survival as well as cell maturation and differentiation of stem cells [9].
Although lncRNAs and sncRNAs demonstrate significant differences, they can mimic each other in terms of their structures. Also, sncRNAs are located in the long intergenic non-coding RNA (lincRNA) genes, and the functionality, activity, and bioavailability of sncRNAs, including microRNAs (miRNAs), are controlled by lncRNAs [10]. For example, the microRNA (miR)-125b, a miRNA belonging to the ortholog of the heterochromic lin-4, is mainly expressed in the brain and is upregulated during neurogenesis [11]. The lincRNA of the NED25 gene has been considered to be a novel intergenic and neuronal-induced lncRNA that harbors miR-125b-1 in its intron [12,13].
Previously published studies have shown that different expression profiles of the lincRNA of the NED25 gene have been found in monocytes collected from peripheral blood samples [12,14]. After searching the online miRNA databases, STAT3 has been found to be targeted by miR-125b, which could be competitively inhibited by the lincRNA of the NED25 gene. Also, STAT3 expression has been reported to be able to alter the expression of PCT, a donor of NO in vivo.
The aim of this study was to investigate lincRNA of the NED25 gene, and the miR-125b, STAT3, NO, and the PCT pathway in patients with sepsis and septic shock by examination of peripheral blood monocytes (PBMs), and to determine whether there was an association with patient prognosis.
Material and Methods
Ethical approval and patients studied
This study was approved by the local Human Research Ethics Committee. The research protocol conformed with the latest edition of the Declaration of Helsinki. Written informed consent was obtained from all subjects, or their first-degree relatives, before the initiation of this study.
The participants in this study included 75 age-matched and sex-matched individuals and were divided into three groups: 25 patients with sepsis only; 25 patients with septic shock; and 25 healthy control subjects. All subjects were enrolled from the same single institution. The main diagnostic criterion used for inclusion in the study was the presence of systemic bacterial infection.
Isolation of peripheral blood monocytes (PBMs)
Flow cytometry was used to isolate peripheral blood monocytes (PBMs) from the peripheral blood samples collected from all 75 subjects and was performed according to the manufacturer’s instructions (BD Biosciences, New Jersey, USA). Dulbecco’s Modified Eagle’s Medium (DMEM) (Life Technologies, Gaithersburg, MD, USA) containing streptomycin (100 mg/ml), penicillin G (100 U/ml) and 10% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA, USA) were used to culture the PBMs at 37°C in an atmosphere of 5% CO2 and 95% air.
MicroRNA (miR)-125b and long intergenic non-coding RNA (lincRNA) of the NED25 gene isolation and real-time polymerase chain reaction (PCR)
Trizol reagent (Invitrogen, CA, USA) was used to extract the total RNA from monocytes and tissue samples in accordance with the supplier’s recommendation. An ultraviolet spectrophotometer (Beckman, Fullerton, CA, USA) was used to measure the purity and concentration of RNA at A260/280 and A260, respectively. Agarose gels and ethidium bromide staining were used to evaluate RNA integrity based. A high-capacity cDNA reverse transcription kit (Takara, Kyoto, Japan) was used to reverse transcribe the RNA into its complementary cDNA. A TP800 Fast RT-PCR system (Applied Biosystems, Foster City, CA, USA) was used to carry out the quantitative RT-PCR reaction with SYBR Green PCR Master Mix, diluted cDNA templates, and specific primers. The data analysis software (Takara, Kyoto, Japan) used was the Thermal Cycler DICE Real-Time System analysis software (Takara, Kyoto, Japan) and the 2-ΔΔCt method was used to quantify the relative expression of STAT3 mRNA. β-actin mRNA was used as the internal control for quantitative RT-PCR after normalization. All experiments were performed in triplicate.
Cell culture and cell transfection
Dulbecco’s Modified Eagle’s Medium (DMEM) (Life Technologies, Gaithersburg, MD, USA) containing streptomycin (100 mg/ml), penicillin G (100 U/ml), and 10% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA, USA) was used to culture the monocytes at 37°C in an atmosphere of 5% CO2 and 95% air. The PBMs were activated by the treatment of 1 ug/ml lipopolysaccharide (LPS). To analyze the functional role of the long intergenic non-coding RNA (lincRNA) of the NED25 gene and the microRNA (miR)-125b in regulating the biological activity of the cells, the lincRNA of the NED25 gene mimics or inhibitors (Genecopoeia, China) were transfected into the cells using Lipofectamine 2000 (Invitrogen, CA, USA). Each experiment was performed in triplicate.
Luciferase assay following Lipofectamine transfection
The full fragment of the lincRNA of the NED25 gene sequence containing the putative binding site for the lincRNA of the NED25 gene was inserted into a pmirGLO Dual-Luciferase miRNA Target Expression Vector (#E1330) (Promega, Madison, WI, USA) downstream of the firefly luciferase reporter gene. Subsequently, the seed region in the target site of the lincRNA of the NED25 gene was mutated by site-directed mutagenesis. DNA sequencing was performed to confirm the correct sequence of the amplified product. Before the transfection, the PBMs were incubated in a 48-well plate. After the PBMs reached the 70–80% confluence, mutant or wild-type pmirGLO-lincRNA of the NED25 gene vectors were transiently co-transfected with the lincRNA of the NED25 gene mimics or a negative control (NC) (GenePharma, Shanghai, China) into the cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) and following the manufacturer’s protocol. A Renilla luciferase reporter (pRL-TK) vector was simultaneously transfected into the monocytes as the internal control. The monocytes were harvested 24 hours post-transfection, and their luciferase activity was measured using either using an Anthos Lucy3 luminometer (Anthos Mikrosysteme, GmbH) or a SpectraMax L microplate luminometer (Molecular Devices, Sunnyvale, CA, USA). Each experiment was performed in triplicate.
Luciferase assay following electrophoresis transfection
The lincRNA of the NED25 gene DNA sequence was amplified and inserted into a pcDNA3 vector, and the 3′UTR of STAT3 containing the putative binding site for miR-125b was amplified and inserted into a pmirGLO vector (#E1330) (Promega, Madison, WI, USA) downstream of the firefly luciferase reporter gene. Subsequently, the seed region for the target site of the lincRNA of the NED25 gene was mutated by site-directed mutagenesis. DNA sequencing was performed to confirm the correct sequence of the amplified products. Before the transfection, the monocytes were incubated in a 48-well plate. After the monocytes reached 70–80% confluence, mutant or wild-type pmirGLO-PCT vectors were transiently co-transfected with pcDNA3-lincRNA of the NED25 gene, miR-125b mimics, or a negative control (NC) (GenePharma, Shanghai, China) into the cells using electrophoresis and following the manufacturer’s protocol. A Renilla luciferase reporter (pRL-TK) vector was simultaneously transfected into the monocytes as the internal control. The monocytes were harvested 24 hours post-transfection and their luciferase activity was measured either using either an Anthos Lucy3 luminometer (Anthos Mikrosysteme, GmbH) or a SpectraMax L Microplate luminometer (Molecular Devices, Sunnyvale, CA, USA). Each experiment was performed in triplicate.
Western blot assay
At 48 hours after transfection, the cells were washed twice with an ice-cold PBS and were lysed in a radioimmunoprecipitation assay buffer (Keygen, Nanjing, China) containing 1 mM phenylmethanesulfonyl fluoride (Keygen, Nanjing, China). The lysates were transferred into an EP tube and centrifuged at 4°C and 12,000×g for 15 min. A bicinchoninic acid (BCA) protein assay kit (TaKaRa, Japan) was used to measure the protein concentrations, and the total protein was separated by 10% SDS-PAGE (Bio-Rad Laboratories, Hertfordshire, UK) and transferred onto a polyvinylidene difluoride (PVDF) membrane (Immobilon-P) (Millipore, Billerica, MA, USA). The PVDF membrane was then blocked in 5% dried skimmed milk powder for three hours at room temperature, followed by incubation at 4°C with primary anti-PCT antibody (1: 3000 dilution) (Millipore, Bedford, MA, USA), primary anti-STAT3 antibody (1: 1000 dilution) (Millipore, Bedford, MA, USA) and primary anti-β-actin antibody (1: 10,000 dilution) (AB clonal, Cambridge, MA, USA) for 12 hours. The membrane was washed thrice in Tris-buffered saline (TBS) containing 1% Tween20, and then incubated with horseradish peroxidase (HRP)-labeled secondary antibody (1: 10,000 dilution) (Bioworld, Dublin, OH, USA) for 60 min at room temperature. An enhanced chemiluminescence instrument (Tanon, Shanghai, China) was used to visualize the specific bands of the antibodies. Each experiment was performed in triplicate.
Enzyme-linked immunoassay (ELISA)
A 50 mM carbonate-bicarbonate buffer containing the anti-PCT antibody (1: 500 dilution) (Cell Signaling Technology, Boston, USA) was used to coat a 96-well plate at 4°C for 12 hours, followed by treating the plate for another 60 min using PBS containing 4% bovine serum albumin (BSA). Subsequently, the plate was washed with PBS containing 0.1% Tween-20, and 50 μl of a mixture containing purified PCT antigen and clinical samples were distributed among each of 96 wells. The plate was then incubated for 120 min at 37°C and washed. Then, 100 μl of PBS containing 1% BSA and 1: 1500 dilution of the anti-PCT antibody was added to each well, and the plate was incubated for 60 min at 37°C. The plates were washed with 1% BSA (LI-COR Inc., Lincoln, NE, USA) and incubated with secondary antibodies conjugated to horseradish peroxidase (HRP) (1: 3000 dilution) (Cell Signaling Technology, Boston, USA). In the presence of O-phenylenediamine, the HRP activity in each well of the plate was measured at 492 nm absorbance using a microplate reader and H2O2 served as the substrate for the enzyme. Each experiment was performed in triplicate.
Statistical analysis
All results were shown as the mean ± standard error of the mean (SEM). Independent samples t-tests were performed to compare the results between two treatment groups. ANOVA (one-way analysis of variance) was performed to compare the results between three or more groups. SPSS 17.0 software (SPSS, Chicago, IL, USA) was used perform the statistical analysis. A P-value <0.05 were considered to be statistically significant.
Results
Patients studied
The study included 75 age and sex-matched patients: 25 patients with sepsis, without septic shock (mean age: 26.37±4.53 years; 14 men and 11 women); 25 patients with septic shock (mean age: 25.44±4.98 years; 13 men and 12 women); and 25 healthy control subjects (mean age: 26.78±5.12 years; 13 men and 12 women). All subjects were gender and age-matched and all patients provided serum samples and peripheral blood monocytes (PBMs) for the study.
The STAT3 gene as a target gene of microRNA (miR)-125b
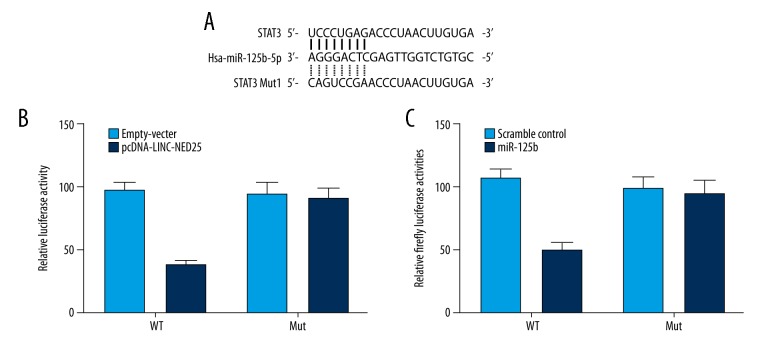
Based on a computational analysis (www.mirdb.org), STAT3 was identified as a virtual target of microRNA (miR)-125b, and the potential binding sites were located in the 3′UTR of STAT3 (Figure 1A). A site-directed mutagenesis kit was used to construct a vector containing the mutant 3′UTR of STAT3. Since it had previously been reported that the long intergenic non-coding RNA (lincRNA) of the NED25 gene acts as a host gene of miR-125b, a luciferase assay was conducted to study the regulatory relationship between the lincRNA of the NED25 gene and STAT3 (Figure 1B) and miR-125b (Figure 1C), which substantially reduced the luciferase activity of wild-type STAT3 3′UTR but did not affect the luciferase activity of mutant STAT3 3′UTR, indicating that STAT3 was a virtual target gene of miR-125b, whereas lincRNA of the NED25 gene was a host gene of miR-125b.
Figure 1.
Based on a computational analysis, STAT3 is identified as a virtual target of microRNA (miR)-125b, and the potential binding sites were located in the 3′UTR of STAT3. (A) STAT3 was identified as the candidate target gene of microRNA (miR)-125b with the ‘seed sequence’ in the 3′UTR of STAT3. (B) Long intergenic non-coding RNA (lincRNA) of the NED25 gene inhibited luciferase activity of wild-type STAT3 3′UTR. (C) miR-125b inhibited luciferase activity of wild-type STAT3 3′UTR.
Levels of the lincRNA of the NED25 gene, miR-125b, STAT3 and procalcitonin (PCT) measured in the three study groups
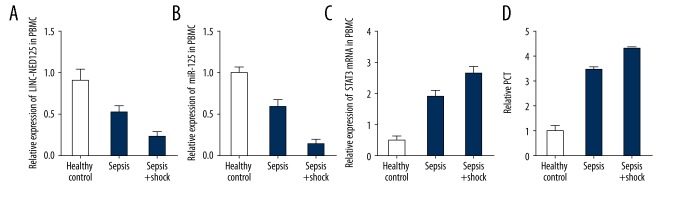
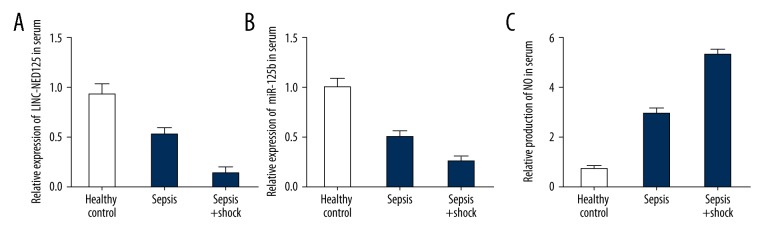
PBMs and serum were collected from each subject in the three study groups. The mRNA level of the lincRNA of the NED25 gene, miR-125b, STAT3, and PCT in the monocytes and serum was determined using real-time polymerase chain reaction (PCR). As shown in Figures 2 and 3, the PBMs (Figure 2) and serum (Figure 3) collected from the patients with sepsis (who did not have septic shock) were associated with a lower level of the lincRNA of the NED25 gene (A) and miR-125b (B) compared with those collected from healthy participants. The PBMs (Figure 2) and serum (Figure 3) collected from the patients with septic shock showed an even lower level of the lincRNA of the NED25 gene (Figures 2A, 3A) and miR-125b (Figures 2B, 3B). However, PBMs (Figure 2) and serum (Figure 3) collected from patients with sepsis were associated with a higher levels of STAT3 (Figures 2C, 3C) and PCT (Figures 2D, 3D) compared with those collected from healthy participants. The PBM (Figure 2) and serum (Figure 3) collected from patients with septic shock showed an even lower higher level of expression of STAT3 (Figures 2C, 3C) and PCT (Figures 2D, 3D).
Figure 2.
Real-time polymerase chain reaction (PCR) detected lincRNA of the NED25 gene, microRNA (miR)-125b, STAT3 and PCT expression levels in peripheral blood monocytes (PBM) from 25 patients with sepsis, 25 patients with sepsis and shock, and 25 healthy control subjects. (A) Expression levels of the lincRNA of the NED25 gene were lower in the sepsis group compared with the healthy controls and were even lower in the patient group with sepsis and shock. (B) Expression levels of the microRNA (miR)-125b were lower in the sepsis group compared with the healthy controls and were even lower in the patient group with sepsis and shock. (C) Expression levels of STAT3 were increased in the sepsis group compared with the healthy controls and were even greater in the patient group with sepsis and shock. (D) Expression levels of PCT were increased in the sepsis group compared with the healthy controls and were even greater in the patient group with sepsis and shock.
Figure 3.
Real-time polymerase chain reaction (PCR) detected lincRNA of the NED25 gene, microRNA (miR)-125b, STAT3 and PCT expression levels in serum samples from 25 patients with sepsis, 25 patients with sepsis and shock, and 25 healthy control subjects. (A) Expression levels of the lincRNA of the NED25 gene were lower in the sepsis group compared with the healthy controls and were even lower in the patient group with sepsis and shock. (B) Expression levels of the microRNA (miR)-125b were lower in the sepsis group compared with the healthy controls and were even lower in the patient group with sepsis and shock. (C) Expression levels of STAT3 were increased in the sepsis group compared with the healthy controls and were even greater in the patient group with sepsis and shock. (D) Expression levels of PCT were increased in the sepsis group compared with the healthy controls and were even greater in the patient group with sepsis and shock.
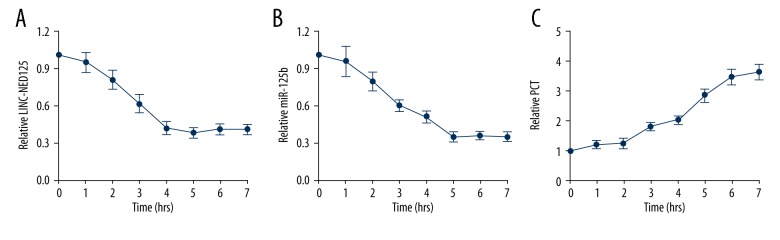
LPS treatment of PBM promoted PCT expression while suppressing the expression of miR-125b
The expression of the lincRNA of the NED25 gene, miR-125b and PCT were increased in response to LPS and bacterial infection. As shown in Figure 4, the mRNA level of the lincRNA of the NED25 gene (Figure 4A) and miR-125b (Figure 4B) decreased gradually during the initial five hours of LPS treatment and then remained stable, whereas the mRNA level of PCT (Figure 4C) was gradually upregulated and then continued following LPS treatment.
Figure 4.
Real-time polymerase chain reaction (PCR) analyzed every hour detects the effects of lipopolysaccharide (LPS) treatment of peripheral blood monocytes (PBM) on expression of the lincRNA of the NED25 gene, miR-125b, and PCT. (A) The levels of lincRNA of the NED25 gene is significantly decreased following lipopolysaccharide (LPS) treatment. (B) The level of the microRNA (miR)-125b decreases gradually during the initial 5 hours following LPS treatment.(C) PCT expression increased gradually following LPS treatment.
Activation of STAT3 increased the expression levels of PCT
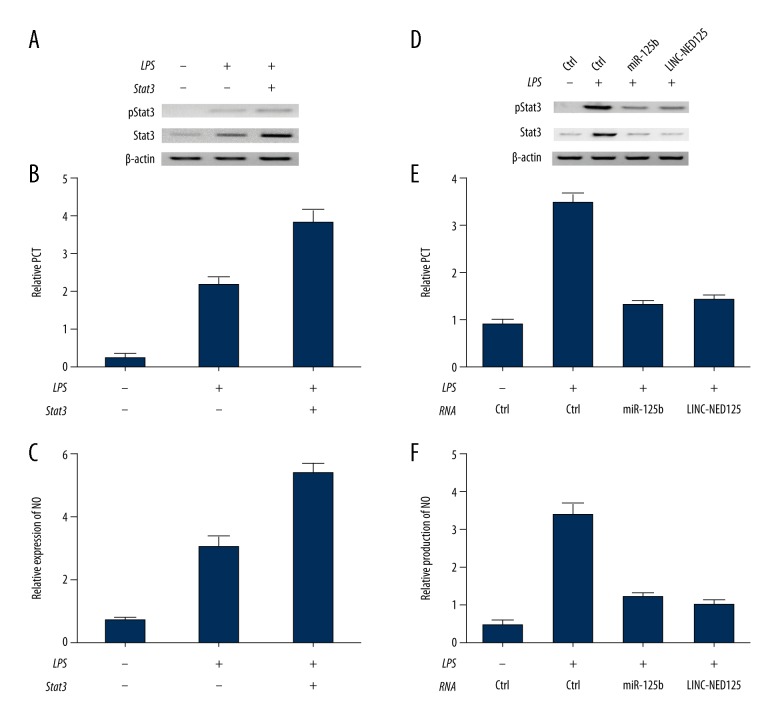
Compared with the control cells that had not undergone LPS treatment, the amount of total STAT3 and phosphorylated STAT3 rose significantly in cells treated with LPS (Figure 5A). Also, the over-expression of STAT3 and the treatment with LPS enhanced both PCT expression (Figure 5B) and STAT3 production (Figure 5C). When compared with the normal controls, the amount of total STAT3 and phosphorylated STAT3 increased significantly after the cells were treated with the lincRNA of the NED25 gene and miR-125b (Figure 5D), whereas the level of phosphorylated STAT3 was reduced after knockdown of the expression of total STAT3 with siRNAs (Figure 5D). The over-expression of the lincRNA of the NED25 gene and miR-125b also decreased the production of PCT (Figure 5E) and nitric oxide (NO) (Figure 5F), suggesting that the level of PCT was simultaneously reduced during the inhibition of STAT3 phosphorylation.
Figure 5.
Total STAT3 and phosphorylated STAT3 following lipopolysaccharide (LPS) treatment of peripheral blood monocytes (PBM) from 25 patients with sepsis, 25 patients with sepsis and shock, and 25 healthy control subjects. (A) Ectopic expression of STAT3 upregulated phosphorylated Stat3 levels following lipopolysaccharide (LPS) treatment. (B) Ectopic expression of STAT3 enhanced PCT expression following LPS treatment. (C) Ectopic expression of STAT3 upregulated STAT3 expression following LPS treatment. (D) Knockdown of STAT3 by treatment with miR-125b and the lincRNA of the NED25 gene reduced the levels of phosphorylated Stat3. (E) Downregulation of Stat3 by treatment with miR-125b and the lincRNA of the NED25 gene significantly reduced PCT expression. (F) Loss of Stat3 by treatment with miR-125b and the lincRNA of the NED25 gene significantly reduced nitric oxide (NO) levels.
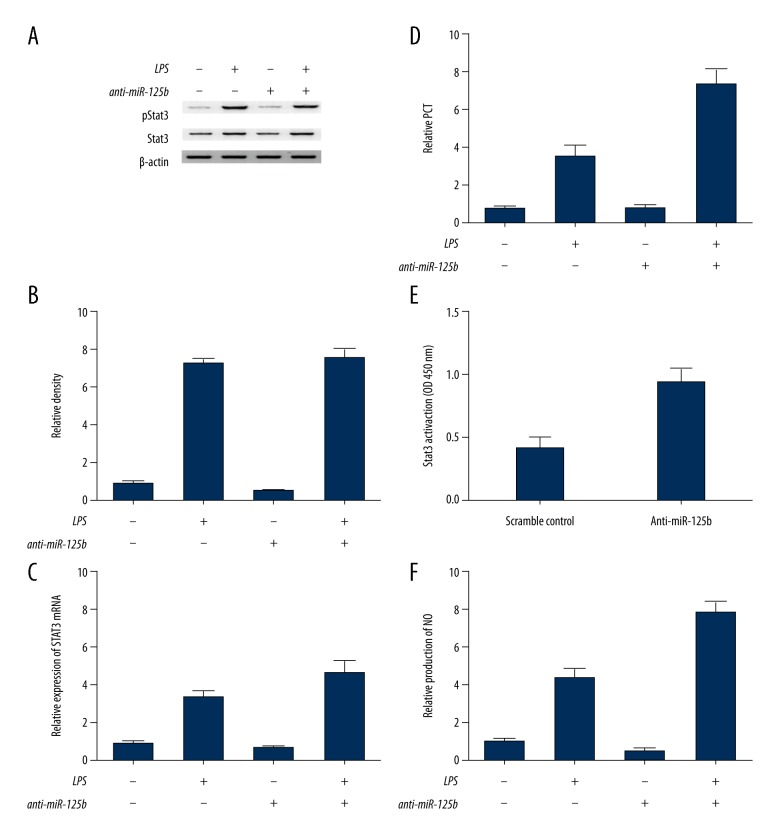
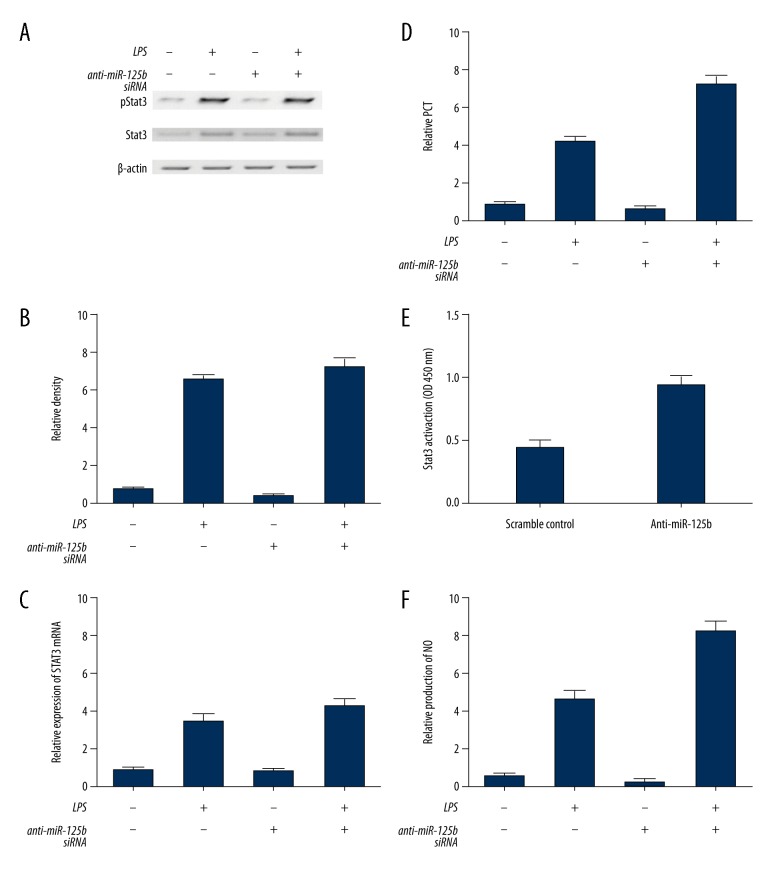
miR-125b and long intergenic non-coding RNA (lincRNA) of the NED25 gene mediated the production of PCT by directly targeting STAT3
Further investigations were carried out to clarify the underlying mechanism involved in the regulation of PCT by miR-125b and the long intergenic non-coding RNA (lincRNA) of the NED25 gene. The suppression of miR-125b increased the total level of STAT3 (Figure 6C), and significantly increased the mean level of pSTAT3 (Figure 6A). The mRNA level of PCT (Figure 6B, 6D) and total STAT3 (Figure 6C) increased significantly when compared with that in control cells. A DNA-binding assay was carried out to determine whether the transcriptional activity of STAT3 was influenced by miR-125b. The results showed that the DNA-binding activity of STAT3 in the anti-miR-125b treatment group was enhanced by LPS stimulation when compared with that in control cells (Figure 6E). Also, the suppression of miR-125b also increased the production of NO (Figure 6F), whereas the inhibition of miR-125b expression via the lincRNA of the NED25 gene small interfering (siRNA) treatment increased the level of STAT3 and elevated the mean level of pSTAT3 (Figure 7A), whereas the level of PCT (Figure 7B, 7D) and total STAT3 (Figure 7C) was significantly increased compared with the control cells. Also, following LPS stimulation and lincRNA of the NED25 gene siRNA treatment, the DNA-binding activity of STAT3 following LPS stimulation was substantially enhanced following the treatment by the lincRNA of the NED25 gene siRNA when compared with the control cells (Figure 6E). Finally, the inhibition of miR-125b significantly increased the level of NO compared with that found in the controls (Figure 6F), indicating that miR-125b and the lincRNA of the NED25 gene upregulated the production of NO and PCT by directly targeting STAT3.
Figure 6.
Suppression of microRNA (miR)-125b increased the levels of STAT3, pSTAT3, and the mRNA level of PCT and total STAT3 increased significantly when compared with control cells. (A) Loss of microRNA (miR)-125b had no influence on STAT3 activation. (B) Suppression of miR-125b upregulated phosphorylated Stat3 levels. (C) Inhibition of miR-125b increased Stat3 production. (D) Suppression of miR-125b increased PCT production. (E) miR-125b inhibited the DNA-binding activity of Stat3. (F) miR-125b reduced the production of nitric oxide (NO).
Figure 7.
The effects of inhibition of microRNA (miR)-125b expression using the long intergenic non-coding RNA (lincRNA) of the NED25 gene by treatment with the small interfering (siRNA) of peripheral blood monocytes (PBM) from 25 patients with sepsis, 25 patients with sepsis and shock, and 25 healthy control subjects. (A) Inhibition of the long intergenic non-coding RNA (lincRNA) of the NED25 gene with siRNA goes not affect STAT3 activation. (B) Inhibition of the lincRNA of the NED25 gene with siRNA increased the levels of phosphorylated STAT3 levels. (C) Inhibition of the lincRNA of the NED25 gene with siRNA increased STAT3 levels. (D) Inhibition of the lincRNA of the NED25 gene with siRNA increased procalcitonin (PCT) levels. (E) Inhibition of the lincRNA of the NED25 gene with siRNA inhibited the DNA-binding activity of STAT3. (F) Inhibition of the lincRNA of the NED25 gene with siRNA reduced nitric oxide (NO) production.
Discussion
This study has shown the functional and molecular characteristics of the long intergenic non-coding RNA (lincRNA) of the NED25 gene and has demonstrated its role in the control of cell metabolism. Previous in vitro studies have shown that the ectopic expression of the lincRNA of the NED25 gene in neuroblastoma cells could lead to a 50% reduction in cell viability, while neuronal differentiation of medulloblastoma and neuroblastoma cells were associated with upregulation of the expression of lincRNA of the NED25 gene [14]. In growing cells, the ectopic expression of the lincRNA of the NED25 gene increased the expression of the anti-apoptotic gene BCL-2 and resulted in a reduced Bax/Bcl-2 ratio, a characteristic indicator for apoptosis-resistant cells [15].
As a novel, intergenic and cytoplasmic lncRNA with neuronal-induced functions, lincRNA of the NED25 gene has been considered as the host gene for miR-125b-1, a microRNA that plays a negative regulatory role in the proliferation of human neuroblastoma cells [12]. Some in vitro studies have demonstrated the coordinated induction of these two overlapping non-coding RNAs during neuronal differentiation, and various mechanisms have been proposed for the regulation of their expression [12]. Although the production of miR-125b-1 is controlled by transcriptional regulation, the expression of lincRNA of the NED25 gene is regulated by modulating its stability at the post-transcriptional level [12,16].
miR-125 is a critical player in the immune system, and is associated with the regulation of immune responses to endotoxin shock and can inhibit the activation of Raw 264.7 macrophages in lipopolysaccharide (LPS)-stimulated mice [17]. Also, miR-125b has been shown to mediate the expression of IFN-alpha-dependent Blimp-1 in monocyte-derived dendritic cells [11]. Furthermore, the production of TNF-α in CD14-positive neonatal monocytes is regulated by miR-125b [18]. miR-125b was also reported to affect the transcriptional activity of STAT3 in myelopoiesis [17]. In this study, through an online search of a miRNA database (www.mirdb.org), STAT3 was identified as a candidate target gene of miR-125b and with the complementary binding sites of miR-125b/STAT3 was located in the 3′UTR of STAT3. Subsequently, luciferase constructs containing a wild-type or a mutant STAT3 3′UTR were generated, and a luciferase assay was performed to demonstrate that the luciferase activity of wild-type STAT3 3′UTR was inhibited by the presence of miR-125b and the lincRNA of the NED25 gene, whereas the luciferase activity of mutant STAT3 3′UTR was not affected.
In the PBMs from the study participants, the mRNA levels of STAT3 remained stable after LPS stimulation, but the total quantity of STAT3 increased with decreasing levels of miR-125b. Compared with the non-stimulated control, the quantity of activated STAT3 in the PBMs over-expressing miR-125b increased following LPS treatment. In this study, the mRNA levels of the lincRNA of the NED25 gene, miR-125b, and the procalcitonin gene (PCT) following LPS treatment were further investigated, and the results showed that the PBMs treated with LPS showed an increased level of PCT and a lower level of miR-125b. Also, LPS treatment increased the amount of total STAT3 and phosphorylated STAT3, which in turn promoted the expression of PCT. However, it has previously been reported that the lincRNA of the NED25 gene and miR-125b enhanced the production of total STAT3 and phosphorylated STAT3 while decreasing the expression of PCT [19].
In 1974, Roos et al. showed, for the first time, that PCT was the precursor of the hormone calcitonin (CT) [20]. Previously, in 1963, Hirsch et al. demonstrated that the calcitonin isolated from pulmonary endocrine cells and thyroid C-cells played a metabolic role in calcium homeostasis [21]. In 1984, LeMoullec et al. found that the amino acid sequence of PCT was comprised of 116 amino acids [22], including the 21-amino acid calcitonin carboxypeptide-1 (CCP1) located at the carboxyl terminus, the 57-amino acid NProCT located at the amino terminus, and the centrally located 33-amino acid immature non-amidated calcitonin. In a later study in 1997, Snider et al. showed the presence of all these component peptides, including ProCT, at very low serum concentrations in the normal circulation, and it was proposed that these peptides were produced by the neuroendocrine cells in the lungs and thyroid gland [23].
Depending on the prognosis and severity of sepsis, the level of PCT gradually increases during systemic inflammation [24]. Therefore, consecutive measurements of PCT in the intensive care unit (ICU) might help plan the duration of antimicrobial use as early as possible [25]. Using a 0.5 ng/ml threshold of PCT or a minimum reduction of 80% from its peak value, some studies have been unable to predict with accuracy the treatment response in patients with septic shock [26]. In one study involving 246 cases of patients suffering from septic shock, sepsis, or severe sepsis secondary to peritonitis, a correlation between the level of PCT and patient survival was demonstrated [27]. However, in another study of secondary peritonitis, a PCT level of 16 ng/ml was used to distinguish between potential survivors and non-survivors, but the predictive rate was only 30% [28]. There are also clinical studies that have supported the use of PCT as a biomarker for monitoring respiratory infections and sepsis [29]. However, interventional studies have failed to demonstrate the benefit of PCT-guided therapy prediction regarding survival in sepsis patients [30]. A European study evaluated the prognostic potential of PCT patients with sepsis [31]. A Finnish study showed that the serum levels of PCT were greater in more severe cases of advanced sepsis, whereas a substantial decrease in PCT concentration was a more useful predictor for survival when compared with the prognostic value of absolute values for PCT [29]. In a Danish study that enrolled 472 patients, the increase in PCT, or a high maximal level of PCT lasting for more than one day, was shown to be an early independent predictor for 90-day all-cause mortality in sepsis patients [24].
In the present study, the PBMs and serum samples from 25 patients with sepsis also suffering from septic shock, 25 patients suffering from sepsis only, and 25 healthy subjects without any health problems were investigated. Real-time polymerase chain reaction (PCR) was performed to determine the level of the lincRNA of the NED25 gene, miR-125b, STAT3, and PCT in PBMs and serum samples. The findings showed that the levels of lincRNA of the NED25 gene and miR-125b were the lowest in the septic shock group and the highest in the healthy control group. Also, the highest level of STAT3 and PCT were highest in the septic shock group and lowest in the healthy control group. In general, LPS or other toxins secreted from bacteria can directly trigger the release of PCT during inflammation [32]. However, cellular responses to inflammatory cytokines can trigger the release of PCT directly or indirectly [33]. Previous studies have demonstrated that miR-125b in human monocytes regulates the expression of PCT by mediating the transcriptional activity of STAT3 [12].
This study had several limitations. The sample size was relatively small, and further studies with a larger study population are recommended. Also, this study was performed in a single center. The study focused on the lincRNA of the NED25 gene, miR-125b, STAT3, PCT pathway, but in practice, the expression of a particular gene could be regulated by multiple miRNAs or lncRNAs, or lincRNAs, which form a network of interactions involved in the pathogenesis of the disease. Therefore, further studies are recommended to include larger study populations, in multiple centers, and to investigate multiple pathways in the pathogenesis and prognosis of patients with sepsis.
Conclusions
This study demonstrated that the deregulation of the long intergenic non-coding RNA (lincRNA) of the NED25 gene was involved in the prognosis of patients with sepsis by modulating the signaling pathways downstream of the microRNA (miR)-125b, STAT3, procalcitonin (PCT), and nitric oxide (NO) pathway. Also, the expression level of the lincRNA of the NED25 gene might have future potential as a novel prognostic biomarker for patients with bacterial sepsis and septic shock.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Bahl R, Martines J, Ali N, et al. Research priorities to reduce global mortality from newborn infections by 2015. Pediatr Infect Dis J. 2009;28:S43–48. doi: 10.1097/INF.0b013e31819588d7. [DOI] [PubMed] [Google Scholar]
- 2.Rudan I, Boschi-Pinto C, Biloglav Z, et al. Epidemiology and etiology of childhood pneumonia. Bull World Health Organ. 2008;86:408–16. doi: 10.2471/BLT.07.048769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muller B, Becker KL, Schachinger H, et al. Calcitonin precursors are reliable markers of sepsis in a medical intensive care unit. Crit Care Med. 2000;28:977–83. doi: 10.1097/00003246-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Linscheid P, Seboek D, Schaer DJ, et al. Expression and secretion of procalcitonin and calcitonin gene-related peptide by adherent monocytes and by macrophage-activated adipocytes. Crit Care Med. 2004;32:1715–21. doi: 10.1097/01.ccm.0000134404.63292.71. [DOI] [PubMed] [Google Scholar]
- 5.Theodorou VP, Papaioannou VE, Tripsianis GA, et al. Procalcitonin and procalcitonin kinetics for diagnosis and prognosis of intravascular catheter-related bloodstream infections in selected critically ill patients: A prospective observational study. BMC Infect Dis. 2012;12:247. doi: 10.1186/1471-2334-12-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ugarte H, Silva E, Mercan D, et al. Procalcitonin used as a marker of infection in the intensive care unit. Crit Care Med. 1999;27:498–504. doi: 10.1097/00003246-199903000-00024. [DOI] [PubMed] [Google Scholar]
- 7.Hoffmann G, Czechowski M, Schloesser M, Schobersberger W. Procalcitonin amplifies inducible nitric oxide synthase gene expression and nitric oxide production in vascular smooth muscle cells. Crit Care Med. 2002;30:2091–95. doi: 10.1097/00003246-200209000-00023. [DOI] [PubMed] [Google Scholar]
- 8.Becker KL, Snider R, Nylen ES. Procalcitonin in sepsis and systemic inflammation: A harmful biomarker and a therapeutic target. Br J Pharmacol. 2010;159:253–64. doi: 10.1111/j.1476-5381.2009.00433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao X, Yeo G, Muotri AR, et al. Noncoding RNAs in the mammalian central nervous system. Annu Rev Neurosci. 2006;29:77–103. doi: 10.1146/annurev.neuro.29.051605.112839. [DOI] [PubMed] [Google Scholar]
- 10.Fatica A, Bozzoni I. Long non-coding RNAs: New players in cell differentiation and development. Nat Rev Genet. 2014;15:7–21. doi: 10.1038/nrg3606. [DOI] [PubMed] [Google Scholar]
- 11.Parlato S, Bruni R, Fragapane P, et al. IFN-alpha regulates Blimp-1 expression via miR-23a and miR-125b in both monocytes-derived DC and pDC. PLoS One. 2013;8:e72833. doi: 10.1371/journal.pone.0072833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang F, Fan X, Bai Y, et al. miR-125b regulates procalcitonin production in monocytes by targeting Stat3. Microbes Infect. 2016;18:102–8. doi: 10.1016/j.micinf.2015.09.027. [DOI] [PubMed] [Google Scholar]
- 13.Wang Z, Ren J, Wang G, et al. Association between diabetes mellitus and outcomes of patients with sepsis: A meta-analysis. Med Sci Monit. 2017;23:3546–55. doi: 10.12659/MSM.903144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bevilacqua V, Gioia U, Di Carlo V, et al. Identification of linc-NeD125, a novel long non coding RNA that hosts miR-125b-1 and negatively controls proliferation of human neuroblastoma cells. RNA Biol. 2015;12:1323–37. doi: 10.1080/15476286.2015.1096488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adams JM, Cory S. The Bcl-2 protein family: Arbiters of cell survival. Science. 1998;281:1322–26. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 16.Shi X, Zhang Y, Wang H, Zeng S. Effect of triggering receptor expressed on myeloid cells 1 (TREM-1) blockade in rats with cecal ligation and puncture (CLP)-induced sepsis. Med Sci Monit. 2017;23:5049–55. doi: 10.12659/MSM.904386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Surdziel E, Cabanski M, Dallmann I, et al. Enforced expression of miR-125b affects myelopoiesis by targeting multiple signaling pathways. Blood. 2011;117:4338–48. doi: 10.1182/blood-2010-06-289058. [DOI] [PubMed] [Google Scholar]
- 18.Huang HC, Yu HR, Huang LT, et al. miRNA-125b regulates TNF-alpha production in CD14+ neonatal monocytes via post-transcriptional regulation. J Leukoc Biol. 2012;92:171–82. doi: 10.1189/jlb.1211593. [DOI] [PubMed] [Google Scholar]
- 19.Hua S, Liu X, Lv S, Wang Z. Protective effects of Cucurbitacin B on acute lung injury induced by sepsis in rats. Med Sci Monit. 2017;23:1355–62. doi: 10.12659/MSM.900523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hernandez-Porras BC, Plancarte-Sanchez R, Alarcon-Barrios S, Samano-Garcia M. [Complex regional pain syndrome: A review]. Cir Cir. 2017;85(4):366–74. doi: 10.1016/j.circir.2016.11.004. [in Spanish] [DOI] [PubMed] [Google Scholar]
- 21.Hirsch PF, Gauthier GF, Munson PL. Thyroid hypocalcemic principle and recurrent laryngeal nerve injury as factors affecting the response to parathyroidectomy in rats. Endocrinology. 1963;73:244–52. doi: 10.1210/endo-73-2-244. [DOI] [PubMed] [Google Scholar]
- 22.Le Moullec JM, Jullienne A, Chenais J, et al. The complete sequence of human preprocalcitonin. FEBS Lett. 1984;167:93–97. doi: 10.1016/0014-5793(84)80839-x. [DOI] [PubMed] [Google Scholar]
- 23.Snider RH, Jr, Nylen ES, Becker KL. Procalcitonin and its component peptides in systemic inflammation: immunochemical characterization. J Investig Med. 1997;45:552–60. [PubMed] [Google Scholar]
- 24.Jensen JU, Heslet L, Jensen TH, et al. Procalcitonin increase in early identification of critically ill patients at high risk of mortality. Crit Care Med. 2006;34:2596–602. doi: 10.1097/01.CCM.0000239116.01855.61. [DOI] [PubMed] [Google Scholar]
- 25.Nobre V, Harbarth S, Graf JD, et al. Use of procalcitonin to shorten antibiotic treatment duration in septic patients: A randomized trial. Am J Respir Crit Care Med. 2008;177:498–505. doi: 10.1164/rccm.200708-1238OC. [DOI] [PubMed] [Google Scholar]
- 26.Jung B, Molinari N, Nasri M, et al. Procalcitonin biomarker kinetics fails to predict treatment response in perioperative abdominal infection with septic shock. Crit Care. 2013;17:R255. doi: 10.1186/cc13082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reith HB, Mittelkotter U, Wagner R, Thiede A. Procalcitonin (PCT) in patients with abdominal sepsis. Intensive Care Med. 2000;26(Suppl 2):S165–69. doi: 10.1007/BF02900731. [DOI] [PubMed] [Google Scholar]
- 28.Rau BM, Frigerio I, Buchler MW, et al. Evaluation of procalcitonin for predicting septic multiorgan failure and overall prognosis in secondary peritonitis: A prospective, international multicenter study. Arch Surg. 2007;142:134–42. doi: 10.1001/archsurg.142.2.134. [DOI] [PubMed] [Google Scholar]
- 29.Karlsson S, Heikkinen M, Pettila V, et al. Predictive value of procalcitonin decrease in patients with severe sepsis: A prospective observational study. Crit Care. 2010;14:R205. doi: 10.1186/cc9327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diaz-Flores RF. A letter in response to: Procalcitonin-guided interventions against infections to increase early appropriate antibiotics and improve survival in the intensive care unit: a randomized trial. Crit Care Med. 2012;40:1038. doi: 10.1097/CCM.0b013e31824138c4. author reply 1038–39. [DOI] [PubMed] [Google Scholar]
- 31.Schuetz P, Amin DN, Greenwald JL. Role of procalcitonin in managing adult patients with respiratory tract infections. Chest. 2012;141:1063–73. doi: 10.1378/chest.11-2430. [DOI] [PubMed] [Google Scholar]
- 32.Oberhoffer M, Stonans I, Russwurm S, et al. Procalcitonin expression in human peripheral blood mononuclear cells and its modulation by lipopolysaccharides and sepsis-related cytokines in vitro. J Lab Clin Med. 1999;134:49–55. doi: 10.1016/s0022-2143(99)90053-7. [DOI] [PubMed] [Google Scholar]
- 33.Nijsten MW, Olinga P, The TH, et al. Procalcitonin behaves as a fast responding acute phase protein in vivo and in vitro. Crit Care Med. 2000;28:458–61. doi: 10.1097/00003246-200002000-00028. [DOI] [PubMed] [Google Scholar]