 We previously reported that Asian sand dust (ASD), which contains particulate matter (PM) less than 10 μm in diameter (PM10), induced subacute inflammation in splenocytes.
We previously reported that Asian sand dust (ASD), which contains particulate matter (PM) less than 10 μm in diameter (PM10), induced subacute inflammation in splenocytes.
Abstract
We previously reported that Asian sand dust (ASD), which contains particulate matter (PM) less than 10 μm in diameter (PM10), induced subacute inflammation in splenocytes. However, it was unclear whether the PM itself or compounds attached to its surface induced the inflammation. Here we characterized the role of organic substances adsorbed onto the PM10 surface in triggering inflammation by comparing the effect on splenocyte activation of PM10 from urban areas (urPM10), which is rich in lipopolysaccharide (LPS) as compared to ASD, with that of heated PM10 (H-PM). BALB/c mice were intratracheally administered urPM10 or H-PM with or without LPS (1 ng and 10 ng) four times at 2-week intervals, and splenocytes were prepared at 24 h after the final administration to assay the immune responses. urPM10 suppressed splenocyte activation, while H-PM activated splenocytes and LPS neutralization by polymyxin B rescued urPM10-induced immunosuppression. Co-administration of LPS with H-PM clearly suppressed mitogen-induced immune responses in the spleen. Consistent with these results, H-PM induced the phosphorylation of nuclear factor κB (NF-κB) p65 and I kappa B kinase (IKK), which was inhibited by co-administration of LPS. In mice deficient in the LPS signal transducer MyD88, splenocyte activation after LPS or H-PM treatment in vivo was comparable to that in the control. Altogether, our results indicate that PM10 itself could activate NF-κB through the MyD88 pathway, which was modulated by the amount of LPS attached.
Introduction
Exposure to elevated concentrations of particulate matter (PM) is known to have adverse health impacts. Lelieveld et al.1 reported that outdoor air pollution causes 3.3 million premature deaths per year worldwide, predominantly in Asia, based on the data on the global burden of disease for 2010.2 Asian sand dust (ASD) is a PM10 (particulate matter ≤10 μm in diameter) aerosol that occurs over large areas in East Asia.3 We previously reported that ASD induces subacute inflammation in splenocytes4 and repeated ASD airway exposure induces lung inflammation.5,6 We found that lipopolysaccharide (LPS), one of the ASD-derived organic compounds, enhanced pulmonary eosinophilia in ovalbumin-sensitized mice,7 which suggested that PM-attached compounds modulate immune responses. However, it remained unclear whether the PM-attached compounds or the PM itself affects systemic inflammation, especially in splenocytes.
Pattern recognition receptors (PRRs) are key players in the innate immune response. Toll-like receptors (TLRs) belong to the PRR family and mediate pathogen-associated molecular pattern (PAMP)-induced signaling.8 LPS present in the outer membrane of Gram-negative bacteria can bind to TLR4 and activate the transcription factor nuclear factor κB (NF-κB)9 through the adaptor molecule myeloid differentiation factor 88 (MyD88). I kappa B kinase (IKK) is an upstream kinase that regulates NF-κB activation. After stimulation, the p65 component of NF-κB is phosphorylated, after which the NF-κB complex translocates to the nucleus. We previously reported that p65 is phosphorylated in splenocytes upon ASD administration in vivo,4 suggesting that activated p65 is involved in splenic inflammation; however, it is unclear whether this is a direct or indirect effect of the PM.
In this study, we administered urban PM10 (urPM10), which is different from flying PM such as ASD, in which it includes a large amount of LPS and therefore is convenient for evaluating the effects of LPS and PM on splenic inflammation. We demonstrated that urPM10 particles induced NF-κB activation in splenocytes through the MyD88 signaling pathway, which was modulated by the LPS attached to the PM surface.
Materials and methods
Preparation of the particles
The urPM10 used in the present study was collected from the atmosphere in November 2013 at Shenyang, China by using a high-volume air sampler with a Teflon filter. The size distribution peak of PM was at 3.2–4.0 μm, while the mean diameter of the ASD was 3.8 μm. The chemical composition of the ASD was 71% SiO2, 13.0% Al2O3, 5.4% Fe2O3, 2.5% CaO, 3.5% CaCo3, 3.0% MgO, and 0.6% TiO2 as described in a previous paper. The urPM10 consisted of (in μg mg–1 urPM10): Cl–, 22; NO3–, 150; SO4–, 100; NH4+, 55; K+, 8; Mg2+, 9; Ca2+, 34; Na, 10; Al, 31; Fe, 35. Heated PM (H-PM) was prepared by heating the urPM10 at 360 °C for 30 min under 80% N2 gas in an electric heater to remove toxic materials (microbiological materials, sulfate, etc.) attached to the urPM10.
Analysis of LPS
The urPM10 and H-PM were subjected to kinetic assays using Endospec ES test MK (Seikagaku Corp., Tokyo, Japan) to determine LPS activity as described previously.10 We analyzed the constituents of the PM and detected LPS (0.326 ng mg–1 PM).
Animals
Six-week-old, male BALB/c and MyD88 knockout (MyD88–/–) mice (BALB/c background) were purchased from the Oriental Bioservice, Inc. (Kyoto, Japan). The mice were acclimated for 1 week, and healthy mice were used at 7 weeks of age. The mice were fed a commercial CE-2 diet (CLEA Japan, Inc., Tokyo, Japan) and given water ad libitum, and were housed in plastic cages lined with soft-wood chips. The cages were maintained in an air-conditioned room at 25 °C with 55–70% humidity under a 12 h light/dark cycle. The study adhered to the U.S. National Institutes of Health Guidelines for the use of experimental animals. The animal care method and the experiments were approved by the Animal Care and Use Committee of the Oita University of Nursing and Health Sciences (Oita, Japan).
Intratracheal administration of particles
The urPM10 and H-PM were suspended in saline (0.9% NaCl; Otsuka Co., Kyoto, Japan) for injection. This suspension was sonicated for 5 min with a UD-201 ultrasonic disrupter by using a Micro-tip (Tomy, Tokyo, Japan) under cooling conditions. Mice were intratracheally injected with particles through a polyethylene tube under anesthesia with 4% halothane (Takeda Chemical, Osaka, Japan) as reported previously.11 The treatments (0.1 mL each of 0.9% NaCl normal saline solution) included control (0.1 ml normal saline), urPM10 (0.1 mg urPM10 with or without 20 μg polymyxin B), H-PM (0.1 mg H-PM alone), H-PM + LPS 1 (0.1 mg H-PM and 1 ng LPS), and H-PM + LPS 10 (0.1 mg H-PM and 10 ng LPS). The mice were intratracheally challenged with a mix or individual solutions of urPM10, H-PM, and LPS or the control 4 times at 2-week intervals at a dose of 0.1 mg per mouse. At 24 h after intratracheal administration (at 12 weeks of age), the mice were killed by exsanguination under deep anesthesia by intraperitoneal injection of pentobarbital, and the spleens were taken.
Reagents and antibodies
Anti-phospho (p)-IKKα and anti-p-p65 antibodies were purchased from Abcam (Cambridge, MA), and anti-IKKα and anti-p65 antibodies were purchased from Santa Cruz (Santa Cruz, CA). An anti-β-actin antibody was purchased from Sigma (St. Louis, MO). ConA was purchased from EY Laboratories (San Diego, CA). LPS was purchased from Sigma.
Spleen cell isolation and culture
Spleen cells were treated with red blood cell lysis (Tris-ammonium) buffer as described previously.12 The cells were grown in RPMI1640 medium (Nissui, Tokyo, Japan) supplemented with 10% fetal bovine serum (Thermo Scientific, South Logan, UT), l-glutamine (2 mM, Wako Pure Chemical Industries Ltd, Tokyo, Japan), and penicillin–streptomycin (Gibco, New York, NY) in a humidified incubator at 37 °C under 5% CO2 and 95% air.
Measurement of cell proliferation
ATP levels, which are correlated with the number of viable cells, were measured using a CellTiter-Glo luminescent cell viability assay system (Promega, Madison, WI) as described previously.13 In this study, mitogen (concanavalin A [ConA] or LPS)-induced relative cell viability was considered to represent the relative cell proliferation. Splenocytes were plated into 96-well plates at a concentration of 2 × 105 cells per well and then stimulated with phosphate-buffered saline (PBS), ConA (5 μg ml–1), or LPS (1 μg ml–1) for 72 h. After culturing, 20 μL of the assay reagent was added to each well, and the luminescence was measured using a luminometer (Luminescencer-JNR-II, ATTO, Tokyo, Japan).
Western blotting
Cells were lysed with RIPA lysis buffer12 to prepare whole-cell extracts. Equivalent amounts of protein (10 μg) were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to and immobilized on nitrocellulose membranes (Amersham, Buckinghamshire, UK), and probed with the appropriate primary and secondary antibodies. Immunodetection was carried out using a chemiluminescence detection system (Alpha Innotech, San Leandro, CA). Band intensities were quantified using the ImageJ software. Target protein expression was normalized to the amount of β-actin.
Enzyme-linked immunosorbent assay (ELISA)
Splenocytes were seeded into 24-well plates at a concentration of 1 × 106 cells per well and stimulated with PBS, LPS, or ConA. After incubation for 12 h, the culture supernatant was harvested to analyze the IL-2, TNF-α, and MDC concentrations. In vitro cytokine production was measured in the culture supernatants using ELISA kits (BioLegend, San Diego, CA, or Peprotech, Rocky Hill, NJ) according to the manufacturer's instructions.
Statistics
The results are expressed as the mean ± standard deviation (SD). Statistical analysis was conducted by using Student's t-test and ANOVA followed by Scheffé post-hoc tests. A p-value <0.05 was considered significant.
Results
urPM10 suppresses splenocyte activation
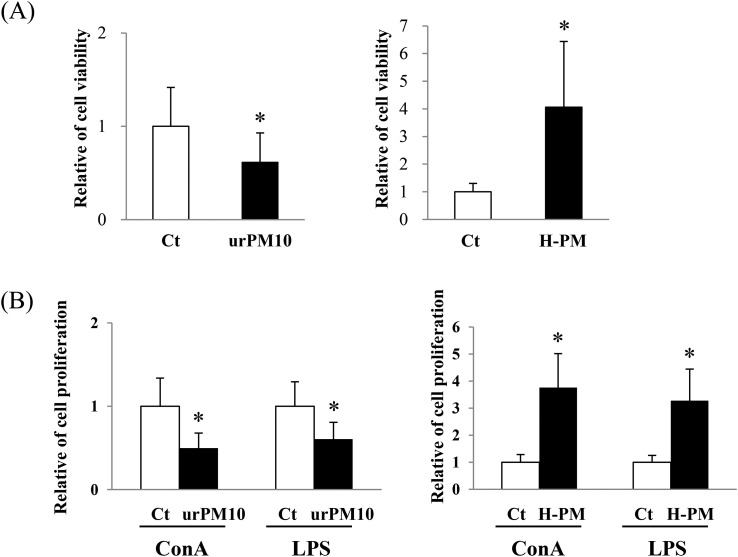
We previously demonstrated that ASD migrated to Japan enhanced splenocyte activation.4 To clarify whether urPM10 affects splenocytes, we examined the activation status of splenocytes obtained from mice treated intratracheally with urPM10. Fig. 1A shows significantly lower activity in the splenocytes of mice treated with urPM10 than in those of the control mice. However, spleen weights and cell numbers were unchanged (data not shown). These results suggested that cell viability was suppressed on a per-cell basis rather than by decreasing the total cell number. Additionally, the proliferation of splenocytes stimulated by ConA or LPS was suppressed after urPM10 administration (Fig. 1B).
Fig. 1. urPM10 suppresses splenocyte activation. Saline (Ct), urPM10, or H-PM was intratracheally administered to BALB/c mice four times at 2-week intervals, and the mice were dissected at 24 h after the last administration. Freshly isolated splenocytes (2 × 105) from saline-, urPM10-, or H-PM-treated mice were prepared and treated with PBS (A), or ConA or LPS (B). After 72 h of culture, proliferation of the splenocytes was measured. The values shown are the mean relative cell proliferation as compared to the control ± SD. *p < 0.05 vs. control (n = 7 in each group).
Substances adhering to the urPM10 surface are involved in its suppressive function
To investigate any involvement of components adsorbed onto the urPM10 surface (microbiological materials, sulfate, etc.), the urPM10 was heated to remove these substances. In contrast to urPM10, H-PM induced the activation of splenocytes as indicated by their high level of ATP and enhanced mitogen-induced immune responses (Fig. 1A and B), as we have previously reported for ASD-administrated mice.4 Additionally, mitogen-induced cytokine and chemokine production was enhanced in the H-PM-treated mice as indicated by ELISA (Fig. 2). These results suggested that the adherent substances have a key role in the suppressive effect of urPM10 on splenocyte activation.
Fig. 2. H-PM induced activation of splenocytes. Saline (Ct) or H-PM was intratracheally administered to BALB/c mice, and the mice were then dissected at 24 h after the last administration. Splenocytes (2 × 105) were treated with ConA or LPS. After 12 h of culture, supernatants were collected and cytokine levels were measured by ELISA. Production of ConA-induced IL-2, LPS-induced TNF-α, and MDC was assessed.
Polymyxin B rescues the suppressive effect of urPM10 on splenocyte activation
To clarify the involvement of microbiological materials in the suppressive effect of urPM10 on splenocytes, the concentrations of LPS in urPM10 and H-PM were measured. urPM10 had 0.326 ng LPS per mg1 PM, while no LPS was detected in H-PM. This finding indicated that urPM10 contains a substantial amount of endotoxin compared to flying PM10 (ASD), as we reported earlier.4 ASD contained 0.0614 ng LPS per mg PM.
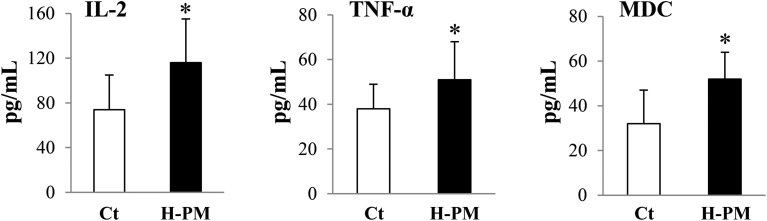
Next, we examined whether the LPS-binding antibiotic polymyxin B rescued the suppressive effect of urPM10 on splenocytes. Fig. 3A shows that polymyxin B completely reverted the suppressive effect of urPM10 on splenocytes, suggesting that the LPS attached to the particles is involved in immunosuppression.
Fig. 3. urPM10 does not induce NF-κB activation in splenocytes while cotreatment with polymyxin B does. Saline (Ct) or urPM10 in the presence of polymyxin B was intratracheally administered to BALB/c mice four times at 2-week intervals, and the mice were dissected at 24 h after the last administration. (A) Freshly isolated splenocytes (2 × 105) from saline- and urPM10-treated mice were prepared and treated with PBS, ConA, or LPS. After 72 h of culture, cell proliferation was measured. The values shown are the mean relative cell proliferation as compared to the control ± SD. *p < 0.05 vs. control. (B) Whole cell lysates of freshly isolated splenocytes were prepared and probed for NF-κB activation by western blot analysis. The levels of phospho (p)-p65 and p-IKKα are shown. β-Actin was used as a loading control. Representative blots are shown. (C) Quantification of the western blot data. The values shown are the mean of the relative density ± SD after normalization to β-actin levels. *p < 0.05 vs. control (n = 4 in each group).
urPM10 induces NF-κB activation in splenocytes only after LPS neutralization with polymyxin B
We have reported that ASD administration affects NF-κB activation in acute-phase inflammation.4 Activation of p65 and IKKα was not detected in splenocytes upon urPM10 administration (Fig. 3B and C). In contrast, p65 was strongly phosphorylated at serine 536, suggesting NF-κB activation, after simultaneous treatment with urPM10 and polymyxin B. Similarly, IKKα was slightly but significantly more phosphorylated in polymyxin B-co-administered mice than in the mice treated with urPM10 alone.
LPS inhibits H-PM-mediated NF-κB activation in vivo
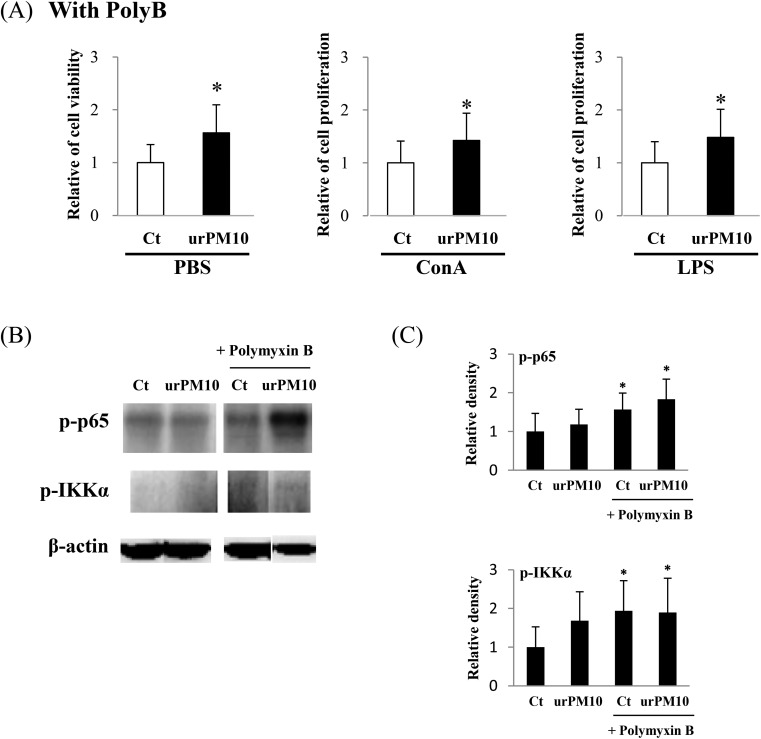
Because we observed that H-PM induced activation of splenocytes and polymyxin B inhibited the urPM10 effect, we investigated whether LPS directly affects H-PM-induced cell activation. Fig. 4A shows that H-PM-induced splenocyte activation was significantly inhibited by LPS administration in mice. Additionally, H-PM-enhanced mitogen-induced cell activities were also inhibited upon LPS administration. Consistent with these results, the activation of p65 and IKKα was significantly inhibited in the LPS-co-administered mice (Fig. 4B). IL-10 is known to be an immuno-suppressive cytokine. Fig. 4C shows that LPS administration obviously induced IL-10 production in splenocytes.
Fig. 4. LPS inhibits H-PM-mediated NF-κB activation in vivo. Saline (Ct) or H-PM in the presence or absence of LPS (1 ng per mouse [L1] or 10 ng per mouse [L10]) was intratracheally administered to BALB/c mice, and the mice were dissected at 24 h after the last administration. (A) Cell proliferation was measured after cell culture for 72 h as described in the legend of Fig. 1. *p < 0.05 vs. H-PM-administered mice (n = 7 in each group). The values shown are the mean relative cell proliferation as compared to that of the H-PM group. (B) Whole cell lysates of freshly isolated splenocytes were prepared and probed for the activation of NF-κB by performing western blot analysis. The levels of p-p65 and p-IKKα are shown. β-Actin was used as a loading control. In the right panel, quantification of the western blot data is shown. The values shown are the mean of the relative density ± SD after normalization to β-actin levels. Representative blots are shown. *p < 0.05 vs. control (n = 4 in each group). (C) Splenocytes (2 × 105) were treated with LPS and supernatants were collected at 12 h after culture. IL-10 levels were measured by ELISA. *p < 0.05 vs. control (n = 5 in each group).
H-PM-induced NF-κB activation depends on the MyD88 pathway
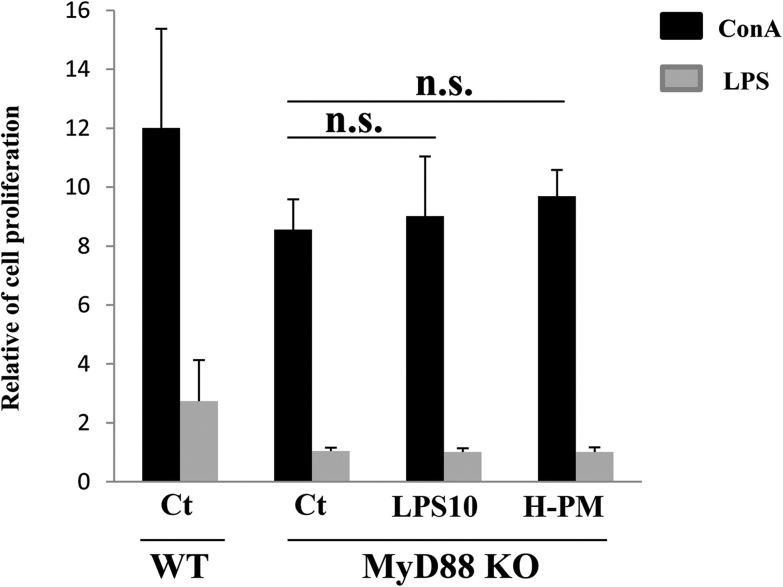
MyD88 is an essential signal transducer in the LPS signaling pathway, which is regulated by numerous pro-inflammatory events. We evaluated the effect of H-PM on LPS signaling using MyD88–/– mice. The MyD88–/– mice did not respond to LPS stimulation (Fig. 5) as compared to the wild-type mice. Splenocyte activation after in vivo LPS or H-PM treatment of MyD88–/– mice was comparable to that of the control (Fig. 5). These results suggested that H-PM-induced activation of NF-κB signaling depends on the MyD88 pathway.
Fig. 5. H-PM-induced NF-κB activation depends on the MyD88 pathway. Saline, H-PM, or LPS (10 ng per mouse) was intratracheally administered to MyD88–/– mice four times at 2-week intervals, and the mice were dissected 24 h after the last administration. Cell proliferation was measured as described in Fig. 1 (ConA stimulation, LPS stimulation for 72 h). The values shown are the mean relative cell proliferation as compared to that of PBS-stimulated cells of the control group. n.s., no statistically significant difference as compared to the control mice (n = 4 in each group). The left bar represents the data from saline-administered wild-type mice.
Discussion
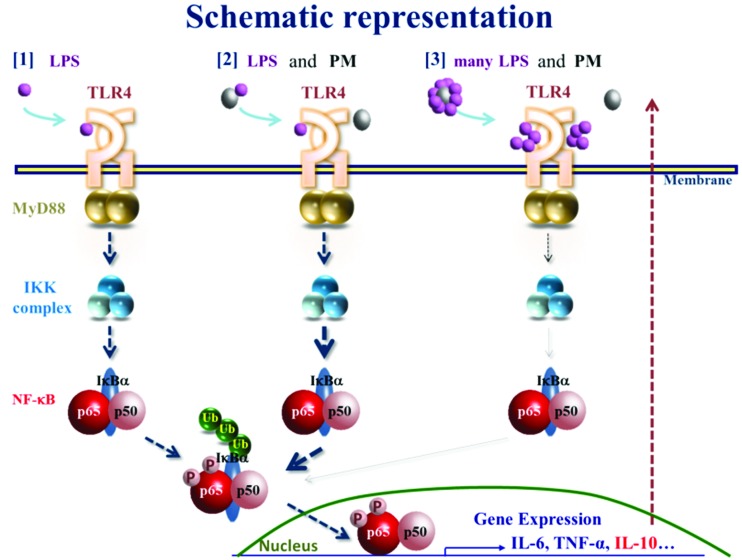
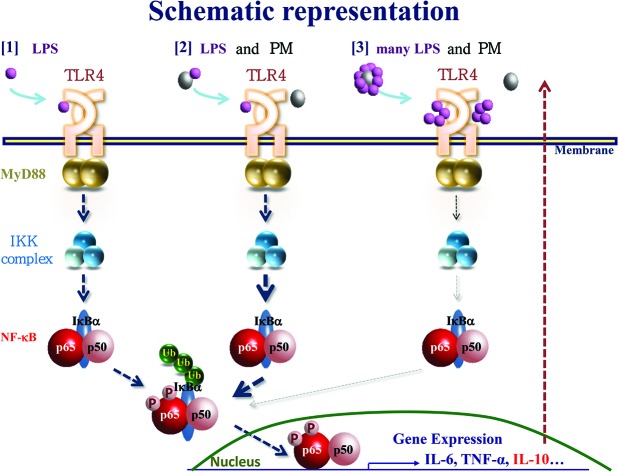
It is currently accepted that Th1 adaptive immune responses require TLR signaling.14 Particularly, respiratory infections that cause LPS signaling have been linked to asthma.15 However, the role of endotoxin exposure in asthma development in children has been debated.16 Eisenbarth et al. demonstrated that the level of exposure to LPS determines Th1- or Th2-dominant immunity.17 From these findings, we hypothesized that organic compounds attached to the PM might influence the systemic inflammation we reported previously.4 To examine this hypothesis, in this study, we used urPM10 because it contains a much higher level of LPS than flying ASD, which we used in previous studies. We clearly demonstrated that LPS-rich urPM10 suppressed immune responses in vivo, in contrast to flying ASD. The suppressive effect of urPM10 was dramatically reverted by heating of the PM or co-treatment with polymyxin B, suggesting that the high amount of LPS attached to the PM contributes to immunosuppression. This finding was confirmed upon co-administration of H-PM and LPS. Although the potential existence of protein-bound LPS on the particles might have led to underestimation of the concentration of LPS, the H-PM data corroborated our conclusion. To our knowledge, this is the first evidence that administration of PM containing different amounts of LPS induces opposite responses in a lymphoid organ. However, it remains to be clarified how many of the constituents of the PM contribute to immune responses (Fig. 6).
Fig. 6. Schematic representation of the effects of PM containing different amounts of LPS on immune responses. PM and LPS modulate NF-κB signaling. PM and LPS balance out each other's influence on immune responses.
It is well known that the TLR family has key roles in mammalian innate immunity.18 After LPS binds TLR4, the transcription factor NF-κB is activated. NF-κB modulates the expression of several proinflammatory genes. Therefore, it is a good marker for evaluating inflammatory events; however, the mechanisms underlying PM utilization of TLRs and NF-κB to elicit cellular responses were unclear. Some studies have suggested that NF-κB is activated by PM in non-immune cells. Diesel exhaust particles induce the nuclear translocation of NF-κB in human bronchial epithelium.19 Ultrafine particles promote vascular calcification by activating NF-κB signaling in human aortic endothelial cells.20 In human monocytic THP-1 cells, cobalt-alloy particles induce NF-κB activation.21 Shoenfelt et al. suggested the involvement of MyD88 in the initiation of inflammatory cytokine responses by PM2.5 in mouse macrophages.22 In the current study, we demonstrated that H-PM induced NF-κB activation in splenocytes from WT but not MyD88–/– mice. These results suggested that PM itself activates NF-κB signaling without attached components and is not directly targeted to the NF-κB protein.
Finally, we addressed the contribution of LPS to NF-κB activation in splenocytes. It is difficult to determine at which dosage LPS activates NF-κB in vivo. Morris et al. demonstrated that varying dosages of LPS caused differential activation of genes in THP-1 cells in vitro.23 A high dose of LPS (100 ng mL–1in vitro) robustly activated both pro- and anti-inflammatory genes. In contrast, a super-low dose of LPS (50 pg mL–1in vitro) preferentially caused mild activation of GSK3, resulting in the inhibition of anti-inflammatory genes. Here, we determined a mean PM-attached LPS concentration of 0.326 ng mg–1 PM and we administered 100 μL containing 32.6 pg LPS to each mouse at once. Although this is not directly comparable to the super-low dose of LPS used in the study of Morris et al., it can be regarded as a low LPS content, albeit more than 5 times higher than that in flying ASD. Further studies are needed to elucidate the involvement of other components attached to the PM in detail since we observed that urPM10 administration did not suppress the basic levels of p-p65 in splenocytes. However, we can conclude that endotoxins at the levels contained in PM can modulate the immune response, and that the range between low and high dosage is very small. The immuno-suppressive effects of LPS at very low doses are reminiscent of endotoxin tolerance.24 One mechanism of endotoxin tolerance is the polarization of macrophages from type 1 to type 2.25 Indeed, we observed high IL-10 production in the H-PM-treated mice, which was enhanced by LPS administration. Although altered polarization cannot explain all of the immuno-suppressive effects and the inactivation of NF-κB, it seems to be an adaptive response. In future, it would be interesting to examine macrophages in PM-treated mice.
In conclusion, exposure to endotoxin-rich particles generates immuno-suppressive phenomena. Exposure to environmental pollutants induces lung inflammation,10 which can be tackled by the immune system. However, chronic pollution-induced inflammation reactions cannot be overcome by an immune system that is suppressed by the action of endotoxin-rich pollutants. In turn, constitutive inflammatory events cause severe inflammation that can be linked to certain organ-specific cancers. Future studies are warranted to investigate these potential links further.
Abbreviations
- ASD
Asian sand dust
- PM
Particulate matter
- TLR
Toll-like receptor
- NF-κB
Nuclear factor κB
- ATP
Adenosine triphosphate
Conflict of interest
All the authors declare that they have no actual or potential competing financial interest.
Acknowledgments
This study was supported in part by a Grant-in-Aid for the Environment Research and Technology Development Fund (ERTDF 5-1457) from the Ministry of the Environment, Government of Japan to Y. Yoshida and T. Ichinose and by a Grant-in-Aid for Scientific Research (B) (No. 24390159) from the Japan Society for the Promotion of Science to Y. Yoshida.
References
- Lelieveld J., Evans J. S., Fnais M., Giannadaki D., Pozzer A. Nature. 2015;525:367–371. doi: 10.1038/nature15371. [DOI] [PubMed] [Google Scholar]
- Lim S. S., Vos T., Flaxman A. D., Danaei G., Shibuya K., Adair-Rohani H., Amann M. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duce R. A., Unni C. K., Ray B. J., Prospero J. M., Merrill J. T. Science. 1980;209:1522–1524. doi: 10.1126/science.209.4464.1522. [DOI] [PubMed] [Google Scholar]
- Song Y., Ichinose T., Morita K., Nakanishi T., Kanazawa T., Yoshida Y. Environ. Toxicol. 2015;30:549–558. doi: 10.1002/tox.21931. [DOI] [PubMed] [Google Scholar]
- Hiyoshi K., Ichinose T., Sadakane K., Takano H., Nishikawa M., Mori I., Yanagisawa R. Environ. Res. 2005;99:361–368. doi: 10.1016/j.envres.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Ichinose T., Yoshida S., Sadakane K., Takano H., Yanagisawa R., Inoue K., Nishikawa M. Inhalation Toxicol. 2008;20:685–694. doi: 10.1080/08958370801935133. [DOI] [PubMed] [Google Scholar]
- Ren Y., Ichinose T., He M., Song Y., Yoshida Y., Yoshida S., Nishikawa M. Allergy, Asthma, Clin. Immunol. 2014;10:30. doi: 10.1186/1710-1492-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani L. Nat. Rev. Immunol. 2004;4:1–23. doi: 10.1038/nri1255. [DOI] [PubMed] [Google Scholar]
- Wan F., Lenardo M. J. Cell Res. 2010;20:24–33. doi: 10.1038/cr.2009.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M., Ichinose T., Ren Y., Song Y., Yoshida Y., Arashidani K., Yoshida S. Inhalation Toxicol. 2015;27:287–299. doi: 10.3109/08958378.2015.1045051. [DOI] [PubMed] [Google Scholar]
- He M., Ichinose T., Liu B., Song Y., Yoshida Y., Kobayashi F., Maki T. Environ. Toxicol. 2016;31:93–105. doi: 10.1002/tox.22025. [DOI] [PubMed] [Google Scholar]
- Yoshida Y., Liu J., Sugiura T., Ishidao T., Ueno S., Yanagita H., Fueta Y. Chem.-Biol. Interact. 2009;177:137–141. doi: 10.1016/j.cbi.2008.10.053. [DOI] [PubMed] [Google Scholar]
- Ding N., Yamashita U., Matsuoka H., Sugiura T., Tsukada J., Noguchi J., Yoshida Y. Cancer. 2011;117:2735–2746. doi: 10.1002/cncr.25711. [DOI] [PubMed] [Google Scholar]
- Schnare M., Barton G. M., Holt A. C., Takeda K., Akira S., Medzhitov R. Nat. Immunol. 2001;2:947–950. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- Gern J. E. J. Allergy Clin. Immunol. 2000;105:S497–S502. doi: 10.1016/s0091-6749(00)90050-2. [DOI] [PubMed] [Google Scholar]
- Liu A. H. J. Allergy Clin. Immunol. 2002;109:379–392. doi: 10.1067/mai.2002.122157. [DOI] [PubMed] [Google Scholar]
- Eisenbarth S. C., Piggott D. A., Huleatt J. W., Visintin I., Herrick C. A., Bottomly K. J. Exp. Med. 2002;196:1645–1651. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Liao J., Chu W., Wang S., Yang T., Tao Y., Wang G. Inhalation Toxicol. 2012;24:918–927. doi: 10.3109/08958378.2012.731093. [DOI] [PubMed] [Google Scholar]
- Pourazar J., Blomberg A., Kelly F. J., Davies D. E., Wilson S. J., Holgate S. T., Sandstrom T. Part. Fibre Toxicol. 2008;5:8. doi: 10.1186/1743-8977-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Mittelstein D., Kam W., Pakbin P., Du Y., Tintut Y., Navab M. Am. J. Physiol.: Cell Physiol. 2013;304:C362–C369. doi: 10.1152/ajpcell.00322.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potnis P. A., Dutta D. K., Wood S. C. Cell. Immunol. 2013;282:53–65. doi: 10.1016/j.cellimm.2013.04.003. [DOI] [PubMed] [Google Scholar]
- Shoenfelt J., Mitkus R. J., Zeisler R., Spatz R. O., Powell J., Fenton M. J., Squibb K. A. J. Leukocyte Biol. 2009;86:303–312. doi: 10.1189/jlb.1008587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris M. C., Gilliam E. A., Button J., Li L. J. Biol. Chem. 2014;289:21584–21590. doi: 10.1074/jbc.M114.583518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porta C., Rimoldi M., Raes G., Brys L., Ghezzi P., Di Liberto D., Dieli F. Proc. Natl. Acad. Sci. U. S. A. 2009;106:14978–14983. doi: 10.1073/pnas.0809784106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S. Nat. Rev. Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]