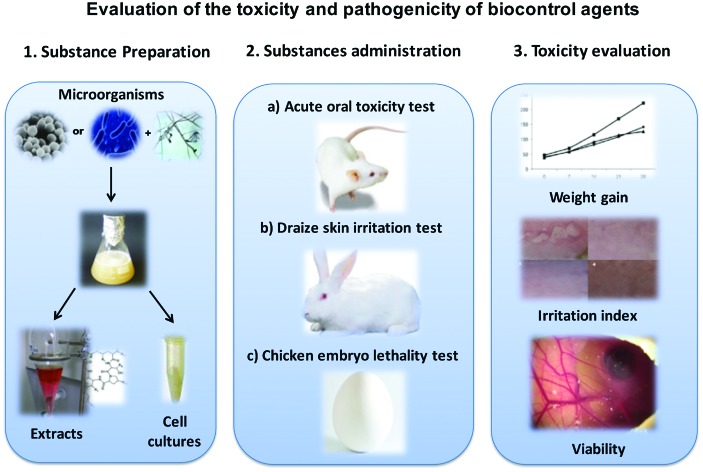
 Biological control has emerged as an alternative to the use of crop fungicides in fields and postharvest.
Biological control has emerged as an alternative to the use of crop fungicides in fields and postharvest.
Abstract
Biological control has emerged as an alternative to the use of crop fungicides in fields and postharvest. It has already been demonstrated that strains of Candida famata, Bacillus subtilis Pla10, Meyerozyma guilliermondii, Meyerozyma caribbica and Debaryomyces hansenii are effective in controlling fungal diseases in tropical fruits. However, in order to develop applications on a field-scale, it is necessary to show that these biocontrol agents are innocuous to humans. In this study, three common toxicity studies were carried out to measure the safety of their use in food products: acute oral toxicity in adult Wistar rats, chicken embryo lethality and skin irritation studies in rabbits using concentrations of 1 and 10 mg of microbial extracts and the administration of 3 and 6 × 108 cells per mL of live cells for each one of the tested strains used for each model. The rats showed no toxic symptoms and none died during testing. The extracts and strain cells under study did not produce a life-cycle interruption in chicken embryos. For the skin irritation studies in rabbits, the substance being studied produced no skin alteration in the animals. With these results it was concluded that the lyophilized extracts in concentrations of 1 and 10 mg, as well as the cells of the studied strains in concentrations of 3 and 6 × 108 cells per mL, were safe in the studied models. Therefore, their use in controlling postharvest diseases in tropical fruits is possible. Their efficiency in controlling plagues in fields and their possible effects on humans, however, require further study.
1. Introduction
Mexico is one of the most important fruit exporters,1 nonetheless, postharvest losses of up to 40% have been reported at the national level, with an estimated loss of 932 692.79 dollars in tropical fruit caused primarily by phytopathogenic microorganisms (fungi and bacteria).2 Other sources report postharvest losses representing up to 25% of total production in industrialized nations and more than 50% in developing nations.3 These losses generate a major economic impact due to the costs of production, harvest, transport and storage.4 Traditionally, damage caused by microorganisms has been controlled by synthetic pesticides. However, due to the acute and chronic effects on human health,5–7 as well as on animals, fauna, pollinators, natural enemies, terrestrial and aquatic invertebrates,8,9 the reduction of their use has gained global importance. This has led to the search for other alternatives, namely green technologies for the control of pathogens as in the case of biological control.10,11 Biological control is an emerging technology that consists of using microorganisms such as yeasts and bacteria that present antagonistic effects towards other microorganisms to control diseases caused by phytopathogens. Biological control, therefore, represents an alternative to the use of harmful chemical substances by being highly compatible with the environment and human health.4,10,12,13 Bacteria that show antagonistic properties are under widespread study, such as the genus Bacillus, a species that includes various strains with biocontrol activity: Bacillus subtilis, Bacillus amyloliquefaciens, Bacillus licheniformis, and Bacillus pumilus, among others.14–17 These microorganisms have the capacity to form spores, these act as microparasites competing for nutrients and are able to secrete metabolites such as enzymes and lipopeptides; Bacillus sp. UM96 inhibits the growth of phytopathogens such as Botrytis cinerea through the production of chitinase.18,19 In particular, the lipopeptides produced by Bacillus spp., such as fengycins, surfactin and iturins (iturin A), bacillomycin D and micosubtilin have shown their effectiveness in suppressing the growth of phytopathogens.20–30 At the same time, the utilization of certain species of yeasts has been widely studied.4,10,31–34 Some yeasts have been reported to be effective for controlling pathogens postharvest in citrus fruit:35Debaryomyces hansenii, Cryptococcus laurentii, Meyerozyma guilliermondii and Meyerozyma caribbica in mango,36,37 Rhodotorula mucilaginosa and Candida famata in papaya,38Candida magnus and Candida sake in grape,39Rhodotorula glutinis in apple,40 and Rhodosporidium paludigenum in pear,41 among others. Yeasts inhibit phytopathogenic fungi through different action mechanisms such as the production of lytic enzymes,37,38,42 parasitism,36 induced resistance,43 competition for nutrients and/or space,33,36,37,43 and the formation of microbial biofilms.37,44,45 It is important to understand the action mechanisms of the antagonist microorganisms and their metabolites for their best use and selection of new effective antagonists.4,10,33,46 Relevant literature reports the effective use of these biocontrol agents in agriculture during harvest and postharvest, however, the introduction of these agents is subject to approval by regulatory authorities such as the FDA (Food and Drug Administration); to this end it is necessary to evaluate the toxic effects and thereby ensure the safety of such products.47 Since the 1960s, toxicity studies have been developed as a requisite for all new products that reach the market, and the said studies are conducted in animals following well-established protocols laid out by the Organization for Economic Cooperation and Development (OECD). In Mexico, the biocontrol agents’ liberation and introduction is regulated by the NOM-070-FITO-1995.48 As studies and research on yeast and bacterial biocontrol agents have moved forward, there have also been reports, although scarce, about their toxicity as well as the toxicity of purified metabolites. The first research done on the toxicity of lipopeptides was conducted by Walton et al. (1949),49 in which Micosubtilin isolated from B. subtilis is tolerated by rats up to concentrations below 20 mg kg–1via subcutaneous injections. Korzybski et al. (1978)50 demonstrated that Bacillomycin isolated from B. subtilis caused toxic effects in rats in concentrations up to 75 mg kg–1 when administered peritoneally. In more recent studies, it has been shown that the lipopeptides from B. subtilis administered to rats orally caused no toxicity.51,52 Furthermore, studies on the toxicity of Bacillus extract—which contained lipopeptides—exist, and report doses of up to 475 mg kg–1 not being toxic in rats.53 Additionally, purified surfactin C administered to rats orally, did not generate toxic effects, using up to 2500 mg kg–1 for 14 days 52 and in another study, a daily dose of 500 mg kg–1 of purified surfactin C did not cause genotoxicity or teratogenic effects in fetuses.54 In the particular case of purified Iturin from Bacillus amyloliquefaciens IUB158-03 extracts, Kim et al. (2009)52 reported doses of up to 5000 mg kg–1 in rats causing no toxic effects. Additionally, Rodríguez et al. (2003)55 assessed the dermal irritation of a commercial product that contains live bacteria from Bacillus sphaericus in rabbits, and found no irritation. Also, in another study with B. thuringiensis, Mancebo et al. (2011)56 showed the absence of toxicity and pathogenicity measured in rats when administered orally, intranasally and intravenously, and no deaths or signs of toxicity were found. The ecotoxicity of those lipopeptides has also been studied, in this way. Deravel et al. (2014)57 showed that pure lipopeptide compounds such as micosubtilin and surfactin in relatively high concentrations of 125 mg L–1 and 25 mg L–1, respectively, did not affect the development of lettuce plants. Even when there are no complete studies evaluating the toxicity of subproducts such as lipopeptides, it is necessary that every compound and biocontrol strain that is purported to be used on fields or postharvest on farm products, is accounted with formal toxicological studies that prove their safety in application and consumption. There are also no studies on the toxicity of hydrolytic enzymes produced by biocontrol yeasts. The objective of this study, therefore, is to demonstrate the low or non-existing toxicity of the extracts and cellular suspensions of five yeast cultures Meyerozyma guilliermondii L6D, Meyerozyma caribbica L6A2, Cryptococcus laurentii L5D, Candida famata, Debaryomyces hansenii and the bacterium Bacillus subtilis PLA10, through three different toxicity studies in different animal models, such as acute oral toxicity in a murine model, a chicken embryo model and a dermal irritation test in rabbits, in order to be in agreement with the NOM-070-FITO-1995.48
2. Materials and methods
2.1. Microorganisms, media and culture conditions
The yeasts used in this study, M. guilliermondii L6D, M. caribbica L6A2,36C. laurentii L5D,37C. famata,38D. hansenii35,58 and the bacterium B. subtilis,28 phytopathogenic fungi C. gloeosporioides and P. digitatum were isolated from the surface of the Ataulfo mango and Persian lime, respectively. All of the previously mentioned strains form part of the strain repository of the Instituto Tecnológico de Tepic and are preserved in 40% glycerol at –80 °C. The Staphylococcus aureus isolated from infection in the urinary tract was provided by the strain repository of the Universidad de las Américas Puebla and strains of Vibrio cholerae were donated by the Microbiology Laboratory of the Benemérita Universidad Autónoma de Puebla and were used as positive controls for pathogenicity. Both strains were preserved in a nutrient broth with 20% glycerol at –80 °C. For their certification, S. aureus was grown in Mannitol Salt agar (Bioxon) and the strain of V. cholerae was grown in agar TCBS (Bioxon) at 35 °C. Additionally, the biochemical tests miniAPI ID32 STAPH 32500 and ID32 GN 32100 (BioMérieux®) were used for their identification. For the growth of the yeasts, a potato dextrose broth PDB (Bioxon) was used, inoculating every cryopreserved strain separately, and they were incubated at 28 °C and 120 rpm for 24 h. In the particular case of D. hansenii, a YPD broth was used with incubation at 25 °C and 80 rpm for 24 h. For B. subtilis Pla10 a Luria Bertani broth was used with incubation at 30 °C and 120 rpm for 48 h. For the growth of the pathogenic strains S. aureus and V. cholerae, a nutrient broth was used with incubation at 35 °C and 150 rpm for 12 h to be in the log phase. All of the cultures were adjusted to 3 × 108 and 6 × 108 cells per mL, using the McFarland turbidity standards and a spectrophotometer (Spectronic 20, Bausch & Lomb) at 600 nm corresponding to a DO of 0.145 and 0.275, respectively. These concentrations were assessed in the acute oral toxicity test, DRAIZE skin irritation test (cited in the Official Mexican Standard NOM-039-SSA-a-1993 59) and the chicken embryo lethality test.60
2.1.1. Preparation of hydrolytic yeast enzyme extracts and lipopeptides from Bacillus subtilis Pla10
For stimulation of hydrolytic yeast enzyme secretion, the method described by Bautista-Rosales et al.37 was used. First, C. gloeosporioides and P. digitatum were cultivated separately in 50 mL of PDB. Each culture was maintained under stirred conditions (110 rpm) at 25 °C for 72 h until the appearance of mycelium. Each strain culture was sterilized at 121 °C for 20 min and then centrifuged at 1400g for 10 min. Afterwards, each one of the yeasts M. guilliermondii L6D, M. caribbica L6A2, C. laurentii L5D, and C. famata was cultivated for 72 h at 28 °C (500 mL, PBD) in the presence of 10% v/v sterile mycelium of C. gloeosporioides. Only D. hansenii grew with sterile mycelium in the presence of P. digitatum. The cultures were centrifuged at 1400g for 10 min and filtered through a nitrocellulose membrane with 0.20 μm pores. These extracts presented β-1,3-glucanase, N-acetyl-β-d-glucosaminidase (Nagase) and chitinase activities.35–38 The supernatant fluid was precipitated with ethanol (10 : 1 v/v) for 3 h at –20 °C and was centrifuged (5 min, 11 400g at 4 °C). The precipitate was recovered and lyophilized. In the case of B. subtilis, an inoculum of 20 μl from a recent culture of 250 mL of Luria Bertani broth, was incubated in a water bath at 30 °C and stirred at 120 rpm for 18 to 22 h. The culture was centrifuged at 10 000g for 10 min. The supernatant fluid was filtered in a 0.20 μm nitrocellulose membrane. The clarified supernatant was extracted two times with butan-1-ol (1/10 of the broth's volume). The layer of butan-1-ol was removed and frozen at –70 °C for its subsequent lyophilization. The butanolic fraction contained the lipopeptides iturin and surfactin.28 All lyophilized samples were re-suspended in a PBS buffer solution (pH 7.0, 1.0 M) at a concentration of 1 mg L–1 and 10 mg mL–1 for their subsequent use in the animal and chicken embryo models.
2.2. Animals
In the acute oral toxicity test, 100 Wistar Hannover female rats were used, with a body weight between 150 and 200 g, and 7–8 weeks old. The animals were maintained in temperature-controlled rooms at 22 ± 2 °C and dark–light cycles of 12 : 12 h, housing four animals per cage in polypropylene compartments. The animals’ feeding consisted of a standard CMO-1000 diet for rats and water ad libitum. For the skin irritability test, 24 male New Zealand rabbits were used, their weights ranged between 2 and 2.5 kg and they were placed in stainless steel cages. 24 h prior to experimentation, an area of the skin approximately 10 × 10 cm in size was shaven on the animals’ backs. 100 SPF (Specific Pathogenic Free) chicken embryos at 11 days of embryogenesis were used for the study. Applicable standards and procedures for the handling of animals established by the vivarium as well as those established internationally were taken into account.59,61–63 All of the studied animals were supplied by the Claude Bernard vivarium of Benemérita Universidad Autónoma de Puebla.
2.3. Acute oral toxicity test
The acute oral toxicity test was conducted according to the methods in OECD Test Guideline 401. 96 rats were used (4 rats per extract and 4 for each cell culture). The animals were subjected to an acclimatization period of 3 days. On the start day of the test, the experimental groups were each formed with 4 animals. The positive control group was administered V. cholerae and the negative control group was administered sterile PBS. Each of the animal in the experimental groups was administered two concentrations (1 and 10 mg mL–1) of lyophilized extracts and two 3 × 108 and 6 × 108 cells per mL cellular suspensions. The body weight of each animal was registered twice a week beginning on the first day of the study for a period of 14 days. During this period, food and water consumption were also registered. The daily observations of the animals included: changes in the skin and hair, eyes, mucous membranes, occurrence of secretions and autonomic activity such as tearing, piloerection, pupil size, respiratory pattern, changes in gait, reaction to handling as well as the presence of clonic or tonic movements, stereotypes (e.g. excessive grooming, repetitive circling) or bizarre behavior (e.g. self-mutilation, walking backwards). At the end of the study, the average gain in weight in grams per day as well as the average of solids and liquids consumed per day was obtained in grams or milliliters. At the end of this study, one animal per group was selected and a macroscopic necropsy was performed, including examination of the outer surface of the body, all holes and cranial, thoracic and abdominal cavities. The morphological characteristics of the heart, kidney, spleen and liver were also assessed.
2.4. Draize skin irritation test
The Draize skin test (described in 1944)64 cited in the Official Mexican Standard: NOM-039-SSA1-199358 was used. The extracts and cell cultures were administered on the first day of the study. For each concentration, two rabbits were used applying a single solution directly on the back with the help of a sterile cotton swab. After application, a patch of surgical gauze with 4 layers and an elastic bandage were fixed thereon in order to prevent the animal from accessing the site where the test substances were applied. The total observation period lasted 72 h and special attention was given to signs of edema and erythema at 4, 24, 48 and 72 h after removing the patches (using the value scale for skin lesions as described by Draize). The erythema values were averaged (Table 1) and added to the edema values (eqn (1)) to calculate the Dermal irritation index (DII).
| Primary dermal irritation index = x– of erythema + x– of edema. | 1 |
Table 1. Classification of dermal responses (Draize Scale NOM-039-SSA1-1993).
| Formation of erythema and flaking | Grade |
| No erythema | 0 |
| Slight erythema (barely perceivable) | 1 |
| Defined erythema | 2 |
| Moderate to severe erythema | 3 |
| Grave erythema (redness) and formation of pressure sores (minor and deep lesions) | 4 |
| Formation of edema | Grade |
| No edema | 0 |
| Slight edema (barely perceivable) | 1 |
| Minor edema (presence of borders with defined concrete elevations) | 2 |
| Moderate edema (affecting an area of 1 mm) | 3 |
| Severe edema (affecting an area greater than 1 mm) | 4 |
Based on this index for primary dermal irritation, values between 0 and 5 are considered to be within the acceptance criteria for the safe use of these extracts and cells on humans. When values range from 6 to 8, the product cannot be utilized on human skin due to it being considered as an irritant.
2.5. Viability tests in chicken embryos
The preferred inoculation route for this study is the chorioallantoic membrane (MCA) utilized according to the technique described by Hitchner (1970),65 with embryos at 11 days of embryogenesis; for the inoculation, an artificial air chamber was formed, to achieve this, two holes were made; one on the thickest pole of the egg that possesses a natural air chamber and the other on one of the sides of the egg opposite to the embryo position as observed through a candling lamp. A syringe with a needle was placed at the larger pole and a bit of air was extracted favoring the formation of the artificial chamber through separation of the MCA from the shell on the inner side. It is in this artificial chamber that the extracts or cells of each test strain were inoculated using an insulin syringe and a candling lamp as a guide so as to not perforate the MCA. Any embryo whose membrane was perforated was discarded. After completing the inoculation, the holes were covered with sterile tape. Afterwards, the embryos were incubated at 35 °C and their viability examined until completing 19 days of embryogenesis using a candling lamp at all times to observe and ensure that the movement of the embryo and blood stream were in good condition. A dead embryo loses the circulatory function and stops moving. For this experiment, four embryos were given doses of lyophilized extracts as well as the cultures of the strains in question, using a total of 96 embryos. An additional four embryos were used as a positive control and four embryos were used for the negative control.
All experimentation, transportation and care of the animals were performed in compliance with the relevant laws and institutional guidelines according to the Mexican norms NOM-062-ZOO-1999 (policy for specific techniques for production, care and uses of laboratory animals),66 NOM-003-ZOO-1994 (policy criteria for laboratory operation for animal test approved in zoo sanitary matter),67 NOM-051-ZOO-1995 (policy for humanity in animal treatment and movement),68 NOM-033-ZOO-1995 (policy for humanity animal treatment for domestic and wild animals sacrifice),69 NOM-087-ECOL-1995 (requirements for all processes related to the collection, storage and disposal of all biologicals generated during animal testing).70 All these norms are a requirement and establishment in the Bioterio Claude Bernard vivarium of Benemérita Universidad Autónoma de Puebla. At the same time this place has its own Bioethical committee to analyze each research protocol before any animal can be used.
3. Results and discussion
3.1. Acute oral toxicity tests
Acute oral toxicity tests imply the determination of a dosage that causes the death of 50% of the treated animals. In this type of test, if the substance being tested is toxic, the death of animals after testing can span from 14 days to several months depending on the case. If only toxic effects appear, these will show in the general characteristics of the animal: like a decrease in food and water consumption and therefore weight reduction. The animal's behavior can also be affected, as well as its hair, mucous membranes, eyes, etc.62 Many studies have been conducted on the correlation between animal and human toxicity, coming to the conclusion that no animal responds to toxins exactly the same way as a human, however, most of the effects that various lab animals experience with different substances manifest themselves in humans and vice versa. Therefore, it is necessary to evaluate different models to avoid false negatives.71
3.1.1. Body weight in Wistar rats
The animals (rats) that received the extracts or the live cells orally showed no decrease in body weight during the study, in fact, they were able to gain weight, which varied between 1.72 ± 0.07 and 1.94 ± 0.13 g per day and was similar to the control group that was only administered a PBS buffer solution. This is in contrast to the control groups that received the V. cholerae strain, where the rats died the third day (Table 2).
Table 2. Average values and standard deviation in weight gain expressed in grams per day in the Wistar rat groups treated with lyophilized extracts and live cells of biocontrol agents.
| Strain/treatment | C. laurentii L5D g per dayX (SE) | M. guilliermondii L6D g per day (SE) | M. caribbica L6A2 g per day (SE) | C. famata g per day (SE) | D. hansenii g per day (SE) | B. subtilis PLA10 g per day (SE) |
| 1 mg | 1.79 ± 0.08 | 1.79 ± 0.07 | 1.84 ± 0.13 | 1.74 ± 0.03 | 1.84 ± 0.13 | 1.94 ± 0.13 |
| 10 mg | 1.79 ± 0.14 | 1.81 ± 0.17 | 1.81 ± 0.17 | 1.91 ± 0.39 | 1.72 ± 0.07 | 1.88 ± 0.17 |
| 3 × 108 cells per ml | 1.86 ± 0.11 | 1.74 ± 0.03 | 1.77 ± 0.06 | 1.79 ± 0.06 | 1.80 ± 0.15 | 1.88 ± 0.15 |
| 6 × 108 cells per ml | 1.88 ± 0.15 | 1.74 ± 0.03 | 1.72 ± 0.07 | 1.80 ± 0.18 | 1.91 ± 0.39 | 1.87 ± 0.17 |
| Control (+): died on the third day | ||||||
| Control (–): X = 1.77; DE: 0.07 | ||||||
3.1.2. Ingestion of food and liquids in Wistar rats
The ingestion of food and liquids during the test phase, in which each subject of the experimental groups had similar values to the subjects in the control group can be observed in Table 3. The inoculated rats with V. cholerae stopped consuming food and water and died on the third day after the experiment was started. The other animals had apparently normal behavior after 14 days, demonstrating the safety of not only the metabolites produced by the studied strains, but also the direct inoculation of the live cells of each test strain. The results in weight gain and food and liquid ingestion, indicate that the metabolites of each of the studied strains, extracts or the cells themselves, did not contain any substances with any activity that would diminish the absorption of nutrients or block the digestion of liquids and/or food in these animals. These metabolites or their cells also didn't show any inflammatory or enterotoxigenic effect that could have induced gastroenteritis such as diarrhea. Even in the macroscopic analysis of the autopsies, no apparent change could be observed by plain sight. The possibility of an enterotoxigenic substance exists, however, its concentration is either so low that no evident effect is present or that these metabolites, if they have any activity in the gastrointestinal tract, could be sensitive to the stomach's pH level and therefore become inactive when passing through this section of the animal; this last hypothesis is left for future investigation. With regard to the live cells of each strain, three possibilities exist: (a) that they are inactivated by the stomach's pH and do not reach the intestines in a viable manner, in which they have the opportunity to adhere to the intestinal epithelium and produce enterotoxigenic substances; (b) that these strains do not contain adhesins that favor adhesion and colonization and therefore cannot affect the normal functions of the gastrointestinal tract or, (c) even though they can survive the acidic pH of the stomach, adhere to and colonize the rats’ gastrointestinal tract, these are completely safe and their presence does not negatively affect these animals. This stands in contrast to V. cholerae that adheres to the intestinal epithelium upon entry, colonizes it and favors the production of choleric toxins that, as is well known, foment the hypersecretion of liquids and electrolytes, in addition to blocking nutrient absorption which ultimately cause the animal's death.72,73
Table 3. Ingestion of foods and liquids in Wistar rats.
| Strain/treatment | C. laurentii L5D | M. guilliermondii L6D | M. caribbica L6A2 | C. famata | D. hansenii | B. subtilis PLA10 | |
| 1 mg | SI (g per day) | 54 | 48 | 60 | 57 | 55 | 60 |
| LI (mL per day) | 60 | 50 | 70 | 50 | 50 | 65 | |
| 10 mg | SI (g per day) | 61 | 54 | 67 | 57 | 66 | 58 |
| LI (mL per day) | 65 | 55 | 60 | 60 | 50 | 50 | |
| 3 × 108 cells per ml | SI (g per day) | 59 | 58 | 63 | 54 | 50 | 60 |
| LI (mL per day) | 60 | 55 | 50 | 55 | 50 | 50 | |
| 6 × 108 cells per ml | SI (g per day) | 63 | 53 | 55 | 57 | 66 | 68 |
| LI (mL per day) | 65 | 55 | 60 | 60 | 50 | 50 | |
| Control (+): died on the third day | |||||||
| Control (–): IS: 62 g per day; IL: 55 ml per day | |||||||
3.1.3. Evaluation of the signs and symptoms of toxicity
The assessment of the signs and symptoms of toxicity as well as the general level of activity and reflexes showed normal parameters, and at no stage did the administering of the studied substances generate anatomic-structural changes in the experimentation animals or the general level of activity and/or reflexes in the animals of each group. Similar characteristics were found with the control group that was only administered the sterile PBS solution. In summary, no toxic symptoms were observed in the experimentation groups with the administered dosages, neither with the extracts nor with the cells of the cell cultures of the strains in question. These results indicate that the live cells as well as their metabolites had no negative effects, whether over the nervous system or over organs and tissue that could affect the animals’ normal behavior.
3.1.4. Mortality in Wistar rats
The main parameter measured in the acute oral toxicity studies is death. During the test study, and with the administering of lyophilized extracts in concentrations of 1 and 10 mg as well as live cells of the cultures of each one of the studied strains with concentrations of 3 × 108 and 6 × 108 cells per mL, death did not occur in the treated animals; contrary to what happened with the positive control group that was inoculated with V. cholerae, where animals died on the third day. Furthermore, these animals stopped consuming food and drink after 8 h of having administered the bacterial control. In the macroscopic autopsies, no evidence of pathological alterations in the organs analyzed could be found in each of the groups treated with lyophilized extracts or live cells of the studied strains, showing similar characteristics to the negative control group. Therefore, the need to carry out histopathological studies was not discarded. Reports about the toxicity of biocontrol agents and of the metabolites that exercise a biocontrol function, such as hydrolytic enzymes, are scarce. Nonetheless, hydrolytic enzymes, produced by biocontrol yeasts have the capacity to hydrolyze essential components of the cellular wall of fungi and bacteria, β-1,3-glucanase hydrolyzes β-1,3-glucan, chitinase hydrolyzes chitin and Nagase hydrolyzes N-acetyl-β-d-glucosamine,36 considering that these substrates are not from cells or human tissue, it is not possible that these cause lysis or structural damage. Fuentes-Silva (2006)74 reports that enzymes such as plant β-1,3-glucanase, have manifested the capacity to induce allergic reaction in humans, in comparison with this study, no symptoms of gastric allergies in the animals related to the administered dosages were registered. With regard to reports of lipopeptide toxicity produced by Bacillus, similar experiments have shown a close relationship with the results of our work. Sahnoun et al. (2014)53 assessed the toxicity of crude lipopeptide extracts produced by Bacillus subtilis SPB1 through the determination of LD50 in rats, and no abnormal behavior, or toxic symptoms in any of the treated animals were observed, clearly demonstrating that the extracts are not toxic. Ayed et al. (2015)75 assessed the acute oral and subchronic toxicity in rats administered a mix of lipopeptides produced by Bacillus majovensis A21. The results showed that administering 400 mg kg–1 did not cause any bodily changes in the rats as well as no toxic alterations. This represents a great advantage, since the lipopeptides from the strain B. subtilis PLA10 would be applied in the form of extracts, not purified, in such a way, that even when in this study pure compounds were assessed, the purified extracts contain smaller quantities than the amount reported, thus making the extracts safer. There are cases in which the extracts are less toxic than the pure compounds, as is the case with acetogenins of Annona muricata, where the anticancer compound is not only more potent, but also more toxic, supporting the safe use of the complete extract from this plant.76
3.2. Viability in chicken embryos
Chicken embryos are a biological model utilized in a wide range of toxicological studies due to their easy use, rapid development, early embryogenesis and sensitivity to substances and pathogenic microorganisms so that the virulence factors of a microorganism are directly reflected in the development of embryogenesis.77 These grow under controlled conditions and small variations of these result in abnormal development or even death; that characteristic makes them highly appreciated in toxicity studies since any change in their environment offers immediate answers to the toxic effects of the substances under study.78 For our experiments, the lyophilized extracts in concentrations of 1 and 10 mg as well as the live cells of the cultures of each one of the studied strains in concentrations of 3 × 108 and 6 × 108 cells per mL, inoculated in the chicken embryos, did not cause interruption of embryogenesis; these remained alive similar to those embryos inoculated with a sterile PBS solution for 19 days, even with a dosage of 6 × 108 cells per mL. The embryos inoculated with Staphylococcus aureus at a dose of 3 × 108 cells per mL did not survive more than 36 h after being inoculated. In this manner, it can be affirmed that the strains analyzed in this study lacked the capacity to invade tissue and therefore could not cause damage in embryo development.
3.3. Skin irritation tests in rabbits with extracts and live cells of biocontrol agents
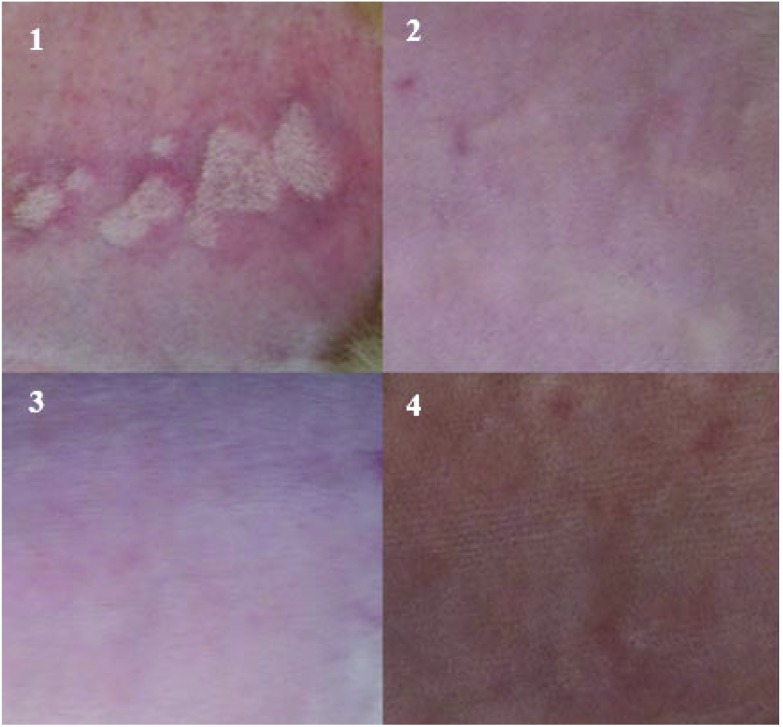
In the case of studies determining skin irritation and skin corrosion, the traditional test method is the Draize test, (1944),69 which consists of qualitative values of the degree of inflammation or redness caused on the animal's skin after applying a chemical or biosynthetic product. Skin irritation tests in rabbits are currently readily utilized as models in which the skin irritation of pharmaceuticals—that can be used on sensitive skin such as that of babies—is tested. Cosmetics that are applied to sensitive tissues of the face and that could produce some irritation of the skin or dermatitis or even hypersensitivity (allergies) are also assessed. For our experiments, the lyophilized extracts at concentrations of 1 and 10 mg, as well as the live cell cultures of each of the studied strains with a concentration of 3 × 108 and 6 × 108 cells per mL did not generate changes in any of the assessed times on the rabbit skin, measured for 72 h (Table 4) and (Table 5). In general, the areas of the skin maintained their normal base condition and showed no change. The measurements of skin irritation in the skin of experimental rabbits for each of the assessed concentrations had a value of zero, and, the value for the Primary Skin Irritation Index was also zero. Therefore, it is considered that the extracts and cells of the biocontrol agents subject to study in this investigation cause no irritation in skin. These results were compared with those of the positive control where the lowest concentration was used (S. aureus: 3 × 108 cells per mL). This strain showed irritation 24 h after its application and the substance according to the Draize scale was considered an irritant after obtaining a value of 6 on the Primary Irritation Index. In Fig. 1, the positive effect of S. aureus, the negative control (PBS) and the effect of an extract and a strain of C. laurentii that is similar to the effects observed for the other extracts and tested strains are shown. Due to the dramatic increase in the number of people affected by this pathology, the phenomenon of skin allergies has gained importance globally. Genetic predisposition, environmental conditions, and the frequency of exposure to allergens are the main characteristics observed that appear to participate in an important way to sensitization. In the case of allergies caused by hydrolytic enzymes, a few were reported to be produced by plants, a reduced group being responsible for triggering type I immediate hypersensitive reactions. A representative of this class of allergens is β-1,3-glucanase of H. brasiliensis of plant origin, related to latex allergy.79 Even when scant information about the allergenic properties of hydrolytic enzymes produced by plants is available, there are no reports of studies that relate microbial enzymes with allergies in humans. On the other hand, among the few studies on lipopeptide skin irritation, there is one conducted by Hwang et al. (2005),80 where the dermal skin irritation index in rabbits of surfactin produced by Bacillus subtilis complex BC2121 was studied. This study placed surfactin on the Draize scale as a non-irritant with a scale value of 0.125. In our study, the lyophilized extracts used contained lipopeptides such as surfactin and Iturin28 derived from B. subtilis PLA10 and did not cause inflammatory or erythematous reactions in the skin of treated rabbits, therefore the dermal irritation index acquired a value of zero, placing it according to the Draize scale as a non-irritant substance. It is rare for individuals of the general population who are exposed to these substances to become sensitized. In fact, within the general population, the prevalence of sensitization to fungal enzymes, for example, has been reported to be as low as 1% and as high as 15%.81
Table 4. Dermal irritation index of lyophilized extracts of biocontrol agents in rabbit skin according to the Draize scale.
| Strain | Evaluation (H) | DII | Classification |
| C. laurentii L5D | 4, 24, 48, and 72 | 0 | No irritant |
| M. guilliermondii L6D | 4, 24, 48, and 72 | 0 | No irritant |
| M. caribbica L6A2 | 4, 24, 48, and 72 | 0 | No irritant |
| C. famata | 4, 24, 48, and 72 | 0 | No irritant |
| D. hansenii | 4, 24, 48, and 72 | 0 | No irritant |
| B. subtilis PLA10 | 4, 24, 48, and 72 | 0 | No irritant |
| Control (+) | 4, 24, 48, and 72 | 6 | Irritant |
| Control (–) | 4, 24, 48, and 72 | 0 | No irritant |
Table 5. Primary dermal irritation index of live cells of biocontrol agents in rabbit skin according to the Draize scale.
| Strain | Evaluation (H) | DII | Classification |
| C. laurentii L5D | 4, 24, 48, and 72 | 0 | No irritant |
| M. guilliermondii L6D | 4, 24, 48, and 72 | 0 | No irritant |
| M. caribbica L6A2 | 4, 24, 48, and 72 | 0 | No irritant |
| C. famata | 4, 24, 48, and 72 | 0 | No irritant |
| D. hansenii | 4, 24, 48, and 72 | 0 | No irritant |
| B. subtilis PLA10 | 4, 24, 48, and 72 | 0 | No irritant |
| Control (+) | 4, 24, 48, and 72 | 3 | Irritant |
| Control (–) | 4, 24, 48, and 72 | 0 | No irritant |
Fig. 1. Analysis of the irritation index in rabbit skin: (1) positive control: S. aureus (irritant). (2) Negative control: sterile PBS solution (non-irritant). (3) Lyophilized extract of C. laurentii L5D (10 mg mL–1: non-irritant). (4) Culture of C. laurentii (6 × 108 cells per mL: non-irritant).
4. Conclusions
According to the results found in this study, we can conclude that the different administered doses of lyophilized extracts (1 and 10 mg per mL) and live cells of M. guilliermondii L6D, M. caribbica L6A2, C. laurentii L5D, C. famata, D. hansenii, and B. subtilis, did not show any toxicity after the administration of high doses of more than 600 million cells. This number is more than a 100 times higher than the amount of cells that a fruit treated with these microorganisms could have or 100 times more than the metabolites that could be applied to them for phytopathogenic biocontrol. This is supported by the lack of deaths in both models (rat and chicken embryo), toxic symptoms, or any type of change in the rest of the studied parameters, such as body weight, and microscopic analyses of organs and tissues in the studied rats. The same products in the studied concentrations had no effect on rabbit skin. These types of products, due to their low toxicity and high biodegradability in the environment, low toxicity to plants and animals, are considered green products that will reduce the use of synthetic chemical products, providing a more sustainable form of agriculture with less toxic effects to humans and the environment.
Acknowledgments
The authors thank CONACYT for the scholarship granted to Mrs Ocampo Suárez Iris Betsabee.
References
- Maya-Ambía C. J., Sakamoto K., Camacho L. A. R. México y la Cuenca del Pacífico. 2011;14(42):67–96. [Google Scholar]
- (SAGARPA, 2015)
- Nunes C. A. Eur. J. Plant Pathol. 2012;133(1):181–196. [Google Scholar]
- Sharma R. R., Singh D., Singh R. Biol. Control. 2009;50(3):205–221. [Google Scholar]
- Kester J. E., Endocrine-Disrupting, Clinical Environmental Health and Toxic Exposures, 2001, p. 362. [Google Scholar]
- Soderlund D. M., Clark J. M., Sheets L. P., Mullin L. S., Piccirillo V. J., Sargent D., Weiner M. L. Toxicology. 2002;171(1):3–59. doi: 10.1016/s0300-483x(01)00569-8. [DOI] [PubMed] [Google Scholar]
- Ferrer A. Ann. Sis., San Navarra. 2003;26:155–171. [PubMed] [Google Scholar]
- Weiss B., Amler S., Amler R. W. Pesticides Pediatrics. 2004;113:1030–1036. [PubMed] [Google Scholar]
- Watts M. and Williamson S., Replacing Chemicals with Biology: Phasing out highly hazardous pesticides with agroecology, Copyright © Pesticide Action Network Asia and the Pacific, 2015. All rights reserved ISBN 978-983-9381-70-2. [Google Scholar]
- Droby S., Wisniewski M., Macarisin D., Wilson C. Postharvest Biol. Technol. 2009;52(2):137–145. [Google Scholar]
- Jamalizadeh M., Etebarian H. R., Aminian H., Alizadeh A. EPPO Bull. 2011;41(1):65–71. [Google Scholar]
- Zhimo V. Y., Bhutia D. D., Saha J., Panja B. World Appl. Sci. J. 2014;31(5):785–793. [Google Scholar]
- Liu F., Zhan R. L., He Y. B., Zhao Y. L., Yang S. J., Chang J. M. J. Fruit Sci. 2011;28(4):651–656. [Google Scholar]
- Jacobsen B. J., Zidack N. K., Larson B. J. Phytopathology. 2004;94:1272–1275. doi: 10.1094/PHYTO.2004.94.11.1272. [DOI] [PubMed] [Google Scholar]
- Ongena M., Jacques P. Trends Microbiol. 2007;16:115–125. doi: 10.1016/j.tim.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Jiang C. H., Wu F., Yu Z. Y., Xie P., Ke H. J., Li H. W., Guo J. H. Microbiol. Res. 2015;170:95–104. doi: 10.1016/j.micres.2014.08.009. [DOI] [PubMed] [Google Scholar]
- Pane C., Zaccardelli M. Biol. Control. 2015;84:11–18. [Google Scholar]
- Martinez-Absalon S. C., Orozco-Mosqueda M. D. C., Martínez-Pacheco M. M., Farias-Rodriguez R., Govindappa M., Santoyo G. Genet. Mol. Res. 2012:1–9. doi: 10.4238/2012.July.10.15. [DOI] [PubMed] [Google Scholar]
- Martínez-Absalón S., Rojas-Solís D., Hernández-León R., Prieto-Barajas C., Orozco-Mosqueda M. D. C., Peña-Cabriales J. J., Santoyo G. Biocontrol Sci. Technnol. 2014;24(12):1349–1362. [Google Scholar]
- Volpon L., Besson F., Lancelin J. M. Eur. J. Biochem. 1999;264(1):200–210. doi: 10.1046/j.1432-1327.1999.00605.x. [DOI] [PubMed] [Google Scholar]
- Bongers R. S., Veening J. W., Van Wieringen M., Kuipers O. P., Kleerebezem M. Appl. Environ. Microbiol. 2005;71(12):8818–8824. doi: 10.1128/AEM.71.12.8818-8824.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X. H., Koumoutsi A., Scholz R., Schneider K., Vater J., Süssmuth R., Piel J., Borriss R. J. Biotechnol. J. 2009;140:27–37. doi: 10.1016/j.jbiotec.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Leclère V., Béchet M., Adam A., Guez J. S., Wathelet B., Ongena M., Thonart P., Gancel F., Chollet-Imbert M., Jacques P. Appl. Environ. Microbiol. 2005;71:4577–4584. doi: 10.1128/AEM.71.8.4577-4584.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongena M., Jaques P., Touré Y., Destain J., Jabrane A., Thonart P. Appl. Microbiol. Biotechnol. 2005;69:29–38. doi: 10.1007/s00253-005-1940-3. [DOI] [PubMed] [Google Scholar]
- León M., Yaryura P. M., Montecchia M. S., Hernández A. I., Correa O. S., Pucheu N. L., Kerber N. L., García A. F. Int. J. Microbiol. 2009;57:20–49. doi: 10.1155/2009/572049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawoy H., Bettiol W., Fickers P. and Ongena M., Bacillus-based biological control of plant diseases, in Pesticides in the Modern World Pesticides Use and Management, ed. M. Stoytcheva, InTech, 2011, pp. 273–302. [Google Scholar]
- Lee H. J., Kim H. Y. J. Microbiol. Biotechnol. 2011;21(3):229–235. [PubMed] [Google Scholar]
- Ragazzo-Sánchez J. A., Robles-Cabrera A., Lomelí-González L., Luna-Solano G., Calderón-Santoyo M. Rev. Chapingo Ser. Hortic. 2011;17(SPE. 1):5–11. [Google Scholar]
- Pane C., Zaccardelli M. Biol. Control. 2015;84:11–18. [Google Scholar]
- Khedher S. B., Boukedi H., Kilani-Feki O., Chaib I., Laarif A., Abdelkefi-Mesrati L., Tounsi S. J. Invertebr. Pathol. 2015;132:42–47. doi: 10.1016/j.jip.2015.08.010. [DOI] [PubMed] [Google Scholar]
- Spadaro D., Vola R., Piano S., Gullino M. L. Postharvest Biol. Technol. 2002;24:123–134. [Google Scholar]
- Spadaro D., Gullino M. L. Int. J. Food Microbiol. 2004;91(2):185–194. doi: 10.1016/S0168-1605(03)00380-5. [DOI] [PubMed] [Google Scholar]
- Janisiewicz W. J., Korsten L. Annu. Rev. Phytopathol. 2002;40(1):411–441. doi: 10.1146/annurev.phyto.40.120401.130158. [DOI] [PubMed] [Google Scholar]
- Liu J., Sui Y., Wisniewski M., Droby S., Liu Y. Int. J. Food Microbiol. 2013;167(2):153–160. doi: 10.1016/j.ijfoodmicro.2013.09.004. [DOI] [PubMed] [Google Scholar]
- Hernández-Montiel L. G., Larralde-Corona C. P., Vero S., López-Aburto M. G., Ochoa J. L., Ascencio-Valle F. CyTA–J. Food. 2010;8(1):49–56. [Google Scholar]
- Bautista-Rosales P. U., Calderon-Santoyo M., Servín-Villegas R., Ochoa-Álvarez N. A., Ragazzo-Sánchez J. A. Biol. Control. 2013;65(3):293–301. [Google Scholar]
- Bautista-Rosales P. U., Calderón-Santoyo M., Servín-Villegas R., Ochoa-Álvarez N. A., Vázquez-Juárez R., Ragazzo-Sánchez J. A. Crop Prot. 2014;65:194–201. [Google Scholar]
- Magallon-Andalon C. G., Determinación de los mecanismos de acción en el control de Colletotrichum gloeosporioides en papaya (Carica papaya L.), Maestría en Ciencias en alimentos, Inst. Tecnol. de Tepic, 2008. [Google Scholar]
- Pantelides I. S., Christou O., Tsolakidou M. D., Tsaltas D., Ioannou N. Biol. Control. 2015;88:46–53. [Google Scholar]
- Zhang H., Wang L., Ma L., Dong Y., Jiang S., Xu B., Zheng X. Bio Control. 2009;48(1):79–83. [Google Scholar]
- Yu C., Zhou T., Sheng K., Zeng L., Ye C., Yu T., Zheng X. Int. J. Food Microbiol. 2013;164(2):155–160. doi: 10.1016/j.ijfoodmicro.2013.04.012. [DOI] [PubMed] [Google Scholar]
- Yehuda H., Droby S., Bar-Shimon M., Wisniewski M., Goldway M. Yeast. 2003;20(9):771–780. doi: 10.1002/yea.1006. [DOI] [PubMed] [Google Scholar]
- Droby S., Vinokur V., Weiss B., Cohen L., Daus A., Goldschmidt E. E., Porat R. Phytopathology. 2002;92(4):393–399. doi: 10.1094/PHYTO.2002.92.4.393. [DOI] [PubMed] [Google Scholar]
- Chi M., Li G., Liu Y., Liu G., Li M., Zhang X., Sun Z., Sui Y., Liu J. Biol. Control. 2015;90:113–119. [Google Scholar]
- Spadaro D., Droby S. Trends Food Sci. Technol. 2016;47:39–49. [Google Scholar]
- D'aes J., De Maeyer K., Pauwelyn E., Höfte M. Environ. Microbiol. Rep. 2009;2:359–372. doi: 10.1111/j.1758-2229.2009.00104.x. [DOI] [PubMed] [Google Scholar]
- Skrobek A., Boss D., Défago G., Butt T. M., Maurhofer M. Toxicol. Lett. 2006;161(1):43–52. doi: 10.1016/j.toxlet.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Norma Oficial Mexicana NOM-070-FITO-1995, Requisitos y Especificaciones Fitosanitarios para la Importación, Introducción, Movilización y Liberación de Agentes de Control Biológico (http://www.economia-noms.gob.mx/noms/consultaXNormaAction.do.
- Walton R. B., Woodruff H. B. J. Clin. Invest. 1949;28(5 Pt 1):924. doi: 10.1172/JCI102180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzybski T., Kowszyk-Gindifer Z. and Kurylowicz W., Antibiotics isolated from the genus Bacillus (Bacillaceae), in Antibiotics - Origin, Nature and Properties, American Society of Microbiology, Washington, DC, 1978, vol. III, pp. 1529–1661. [Google Scholar]
- Park B. K., Lim J. H., Hwang Y. H., Kim M. S., Song I. B., Lee H. G., Park S. C. Toxicol. Res. 2006;22(4):453–458. [Google Scholar]
- Kim H. K., Lee T. S. Life Sci. 2009;19(11):1672–1678. [Google Scholar]
- Sahnoun R., Mnif I., Fetoui H., Gdoura R., Chaabouni K., Makni-Ayadi F., Ghribi D. Int. J. Pept. Res. Ther. 2014;20(3):333–340. [Google Scholar]
- Hwang Y. H., Park B. K., Lim J. H., Kim M. S., Song I. B., Park S. C., Yun H. I. J. Health Sci. 2008;54(1):101–106. [Google Scholar]
- Rodríguez A. M., Navarro B. G., Riera L., Lugo S., Torres Y. G., Arteaga M. E., Fuentes D. J. Toxicol. 2003;20(3):210–215. [Google Scholar]
- Mancebo A., Molier T., González B., Lugo S., Riera L., Arteaga M. E., González C. Regul. Toxicol. Pharmacol. 2011;59(1):184–190. doi: 10.1016/j.yrtph.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Deravel J., Lemière S., Coutte F., Krier F., Van Hese N., Béchet M., Jacques P. Appl. Microbiol. Biotechnol. 2014;98(14):6255–626l. doi: 10.1007/s00253-014-5663-1. [DOI] [PubMed] [Google Scholar]
- González-Estrada R., Calderón-Santoyo M., Carvajal-Millan E., Valle F. D. A. J., Ragazzo-Sánchez J. A., Brown-Bojorquez F., Rascón-Chu A. Molecules. 2015;20(6):11373–11386. doi: 10.3390/molecules200611373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norma Oficial Mexicana NOM-039-SSA-1-1993, Bienes y Servicios. Productos de perfumería y belleza. Determinación de los índices de irritación ocular, primaria dérmica y sensibilización (http://www.salud.gob.mx/unidades/cdi/nom/039ssa13.html.
- Jacobsen I. D., Große K., Slesiona S., Hube B., Berndt A., Brock M. Infect. Immun. 2010;78(7):2995–3006. doi: 10.1128/IAI.00268-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norma Oficial Mexicana NOM-062-ZOO-1999, Especificaciones Tecnicas para la Producción, Cuidado y Uso de los animals de Laboratorio. NOM-062-ZOO-1999
- OECD, Guidelines for Testing of Chemicals No 401 Acute Oral Toxicity, Principles and Methos of Toxicology, Ginebra, 1996, https://ntp.niehs.nih.gov/iccvam/docs/acutetox_docs/udpproc/udpfin01/append/appi.pdf; 10/Febrero/2016. [Google Scholar]
- OECD, Guidelines for the Testing of Chemicals No 402: Acute Dermal Toxicity. Fixed Dose Procedure, Organisation for Economic Co-operation and Development, Paris, 1987, http://www.oecd.org/chemicalsafety/risk-assessment/1948333.pdf; 10/Febrero/2016. [Google Scholar]
- Draize J. H., Woodward G., Calvery H. O. J. Pharmacol. Exp. Ther. 1944;82:377–390. [Google Scholar]
- Hitchner S. B. Poult. Sci. 1970;49(2):511–516. doi: 10.3382/ps.0490511. [DOI] [PubMed] [Google Scholar]
- Norma Oficial Mexicana NOM-062-ZOO-1999, Especificaciones técnicas para la producción, cuidado y uso de los animales de laboratorio (http://www.economia-noms.gob.mx/noms/consultaXNormaAction.do.
- Norma Oficial Mexicana NOM-003-ZOO-1994, Criterios para la operación de laboratorios de pruebas aprobados en materia zoosanitaria (http://www.economia-noms.gob.mx/noms/consultaXNormaAction.do.
- Norma Oficial Mexicana NOM-051-ZOO-1995, Trato humanitario en la movilización de animales (http://www.economia-noms.gob.mx/noms/consultaXNormaAction.do.
- Norma Oficial Mexicana NOM-033-ZOO-1995, Sacrificio humanitario de los animales domésticos y silvestres (http://www.dof.gob.mx/nota_detalle.php?codigo=5376424&fecha=18/12/2014.
- Norma Oficial Mexicana NOM-087-ECOL-1995, Requisitos para la separación, envasado, almacenamiento, recolección, transporte, tratamiento y disposición final de los residuos peligrosos biológico-infecciosos que se generan en establecimientos que presten atención médica (http://www.dof.gob.mx/nota_detalle.php?codigo=4884397&fecha=07/11/1995.
- Olson H., Betton G., Robinson D., Thomas K., Monro A., Kolaja G., Dorato M. Regul. Toxicol. Pharmacol. 2000;32(1):56–67. doi: 10.1006/rtph.2000.1399. [DOI] [PubMed] [Google Scholar]
- Vázquez E. G., Torres A. H., Martínez J. H., Gómez J. G. Medicine. 2014;11(56):3317–3321. doi: 10.1016/S0304-5412(14)70779-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheshwari M., Nelapati K., Kiranmayi B. Vet. World. 2011;4(9):423–428. [Google Scholar]
- Fuentes-Silva D., Rodríguez-Romero A. Alergia Inmunol. Pediatr. 2006;15:35–42. [Google Scholar]
- Ayed H. B., Nasri R., Jemil N., Amor I. B., Gargouri J., Hmidet N., Nasri M. Chem.–Biol. Interact. 2015;236:1–6. doi: 10.1016/j.cbi.2015.04.018. [DOI] [PubMed] [Google Scholar]
- Yang C., Gundala S. R., Mukkavilli R., Vangala S., Reid M. D., Aneja R. Carcinogenesis. 2015:046. doi: 10.1093/carcin/bgv046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequera D., Ercolino J. M., Álvarez M. Retel. 2010;28:37–48. [Google Scholar]
- Giannaccini M., Cuschieri A., Dente L., Raffa V. Nanomedicine. 2014;10(4):703–719. doi: 10.1016/j.nano.2013.09.010. [DOI] [PubMed] [Google Scholar]
- López C. A. S. R., Romero A. R. Alergia, Asma e Inmunología Pediátrica. 2002;11(3):92–100. [Google Scholar]
- Hwang M. H., Yun H. I., Lim J. H., Kim K. S., Rhee M. H., Kim N. W., Park S. C. Toxicol. Res. 2005;21(1):39–43. [Google Scholar]
- Green B. J., Beezhold D. H. J. Allergy. 2011 doi: 10.1155/2011/682574. [DOI] [PMC free article] [PubMed] [Google Scholar]