 Diallyl trisulfide (DATS) has been verified to ameliorate hepatotoxicity induced by many drugs, but the protective actions of isoniazid (INH) and rifampicin (RFP) have not been reported.
Diallyl trisulfide (DATS) has been verified to ameliorate hepatotoxicity induced by many drugs, but the protective actions of isoniazid (INH) and rifampicin (RFP) have not been reported.
Abstract
Background & Aim: Diallyl trisulfide (DATS) has been verified to ameliorate hepatotoxicity induced by many drugs, but the protective actions of isoniazid (INH) and rifampicin (RFP) have not been reported. We attempted to elucidate the potential effects and mechanisms of DATS against INH&RFP-caused hepatotoxicity. Methods: Male Kunming mice weighing 18–22 g were divided into 6 groups. For the hepatic-protective study, DATS (10 mg per kg, 20 mg per kg, and 40 mg per kg bw, respectively) was orally administered two hours before the INH&RFP (100 mg per kg, 100 mg per kg bw, respectively) treatments. After 11 days of treatment, 10 mice in each group were taken for the carbon clearance test, while the other 10 mice were sacrificed for the collection of serum and livers for further measurements, including the levels of serum alanine aminotransferase (ALT), aspartate transaminase (AST) and total bilirubin (T.Bili), the liver index, and liver histopathological examination. Malondialdehyde (MDA), glutathione (GSH), and the level of interleukin 1-β (IL-1-β) were measured, the carbon clearance test was performed and the immunohistochemistry of F4/80 marker for activated Kupffer cells (KCs) was analyzed to investigate potential mechanisms. Results: DATS co-administration significantly inhibited the increase of liver index and elevation of serum ALT, AST and T.Bili levels induced by INH&RFP, as well as improved the hepatocellular structure. The further mechanistic studies demonstrated that DATS co-administration counteracted INH&RFP-induced oxidative stress in mice, which was illustrated by the restoration of GSH levels, and the reduction of MDA levels in the liver. Furthermore, DATS co-administration reactivated the KCs inhibited by INH&RFP, which was illustrated by the increase of carbon phagocytosis, and the restoration of the number of activated KCs and IL-1-β levels in the liver. Conclusion: DATS effectively protected the liver against INH&RFP-induced hepatotoxicity, which might be due to its antioxidant effect and enhancement of KCs’ activities.
Introduction
Tuberculosis (TB), an infectious disease induced by the infection of mycobacteria, remains a major public health problem and a leading cause of morbidity and mortality in the world. It is estimated that about 1/3 of the world's population have been infected with mycobacterium TB, and the new infections occur in about 1% of the population every year.1,2
Isoniazid (INH) and rifampicin (RFP) are the first-line drugs for the treatment of TB. Unfortunately, these drugs could cause serious adverse effects including drug induced liver injury (DILI).3 Importantly, the DILI induced by INF&RFP may lead to the termination of the TB treatment, which contributes to the emergence of drug-resistant TB strains.4 Therefore, it's an urgent task to find protective drugs against the injury and/or find alternative drugs against TB.
Though the damage mechanism remains unclear, classical studies focusing on the INH metabolism assumed that after entering the body, INH is mainly metabolized into acetyl-INH by N-acetyltransferase (NAT2) in the liver and then acetyl-INH is hydrolyzed into acetyl-hydrazine and isonicotinic acid. This pathway of metabolism is mainly oxidized by CYP2E1 accompanied by the production of many reactive hepatotoxins, such as acetyldiazene, ketone, ions and radicals.5–7 Additionally, a number of INH undergo hydrolysis into hydrazine by amidase through a secondary metabolic pathway.8 Both acetylhydrazine and hydrazine will cause oxidative stress and induce hepatotoxicity. And a few studies have shown that co-administration of RFP with INH could increase the production of hepatotoxins such as hydrazine due to its positive effect of CYP2E1 activation.9–11 Moreover, Metushi et al. have demonstrated that INH could also be oxidized into diazohydroxide. INH itself and the reactive metabolite form diazohydroxide could covalently bind to the hepatic protein in humans and mice. Both INH–protein and diazohydroxide–protein could induce an increase of INH and immunity to hepatotoxicity.12,13
Kupffer cells (KCs), resident in the liver, are the largest macrophage population in the liver. The major function of KCs is to phagocytize foreign materials, including both opsonized and non-opsonized particles as well as to synthesize and release proinflammatory cytokines, such as TNF-α. Both the macrophage inhibition and increased inflammatory reaction of KCs will induce a liver injury.14 Though more and more hepatotoxins have been verified to cause damage via KCs,15–17 it's unclear whether INH&RFP could also induce hepatotoxicity by targeting KCs.
Diallyl trisulfide (DATS) is an organic sulfide that is rich in S-allyl cysteine extracted from garlic. It has been well demonstrated that organosulfur compounds are the major component for the beneficial effects of garlic and its related products such as garlic oil,5,11 which has been reported to have a series of biological effects, including anti-oxidant, anti-tuberculosis, and anti-inflammation.12–14 Moreover, recent studies showed that DATS could alleviate various hepatotoxic effects caused by ethanol, naphthalene, carbon tetrachloride (Ccl4), arsenic etc. through attenuating oxidative stress.18–21 More interestingly, DATS was also reported to regulate the immune responses and enhance the function of macrophages.20,22,23 These all imply that DATS could be a potential drug to prevent the damage caused by INH&RFP.
To address the above question, our study was aimed to investigate the effects of DATS on INH&RFP-induced liver injury. As is known, the co-treatment of INH&RFP is a standard regimen for anti-TB therapy clinically. We use a nonlethal, short-term mouse model with co-administration of INH&RFP to certify it. The dose and time frame of INH&RFP were defined according to the human-equivalent doses for mice as well as the reference from previous studies. In those reports, the dose scopes of INH were given from 50 to 200 mg per kg bw, and the dose scopes of RFP were given from 100 to 200 mg per kg bw.24–27 To ensure the hepatic injury and no lethal dose of the co-treatment regimen in mice, a pilot study was performed prior to our formal experiment and we found that the dose of 100 mg per kg bw + 100 mg per kg bw was the best regimen for the short term model. And based on this model, we found that DATS effectively protected the liver against INH&RFP-induced hepatotoxicity, which might be due to its antioxidant effect and enhancement of KCs’ activities.
Materials and methods
Materials
DATS (purity > 97%) was purchased from Chia Tai of Jiangsu CN (Chia Tai, China); isoniazid tablets (C6H7N3O 0.1 g INH per tablet) and rifampicin capsules (C43H58N4O12 0.15 g per capsule), which were produced by Xinyi of Shanghai CN (Xinyi, China), were obtained from Qilu Hospital, and then INH and RFP in 1 : 1 ratio were dissolved in physiological saline; corn oil was purchased from the local market of Jinan CN (GB19111, Gold Embryo CORVOIL Co., Shandong, China); carbon ink was purchased from Chemical Reagent of Beijing CN (Che Rea, China); ALT kit, AST kit, and T.Bili kit were purchased from Biosino of Beijing CN (Biosino, China); GSH assay kit and MDA assay kit were purchased from Njjcbio of Nanjing CN (Njjcbio, China); mouse IL-1-β sunny ELISA was purchased from Multi Sci of Beijing CN (Multi Sci, China); rat anti-mouse-F4/80 was purchased from AbD of Oxford UK (AbD Serotec, England); a rat IgG immunohistochemistry kit was purchased from Boster of Wuhan CN (Boster, China).
Animals’ treatment
SPF male Kunming mice (18–22 g) were provided by the Animal Center of Shandong University (Jinan, China), Certificate of Laboratory Animal: SYXK (Jinan China) 20100011. 120 mice were maintained at approximately 22 °C with a 12 h light : 12 h dark cycle, and had free access to standard chow and tap water. After 5 days acclimation to the laboratory conditions, the animals were randomly divided into 6 groups (n = 20): mice in the INH&RFP + DATS groups and DATS group were treated with DATS (10 mg per kg, 20 mg per kg, 40 mg per kg, and 40 mg per kg bw, respectively) by gavage every day, while the mice in the control group and INH&RFP group received an equal volume (0.1 ml per 10 g bw) of corn oil. Two hours after the DATS administration, all the animals except those in the control group and DATS (40 mg per kg bw) group orally received INH&RFP (100 mg per kg and 100 mg per kg bw, respectively), the control group and DATS (40 mg per kg bw) group orally got an equal volume (0.2 ml per 10 g bw) of physiological saline. During the treatments, the body weight was measured at 1, 4, 8, and 11 days. After 11 days of co-administration, 10 mice of each group were anesthetized at 24 hours after the last treatment. Blood was collected by the eyeball extract method and centrifuged at 1500g for 20 minutes at 4 °C to obtain serum. The liver was stripped and weighed. A portion of the liver was fixed in paraformaldehyde (4%) for histopathology and immunohistochemistry, while the other portion of liver tissue was quickly frozen in liquid nitrogen before storing at –80 °C. The other 10 mice in each group were injected with India ink through a tail vein (i.v.) to measure the phagocytic capacities by the method of Van Etten et al.10
All animal procedures were performed according to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals which were approved by the Animal Experimentation Committee of Shandong University. All efforts were made to minimize animal suffering during experiment.
Measurement of the serum biochemical index
The levels of serum alanine aminotransferase (ALT) (rate method, YZB0694, Beijing, China), aspartate aminotransferase (AST) (rate method, YZB0693, Beijing, China), and total bilirubin (T.Bili) (diazonium salt method, YZB0121, Beijing, China) were measured by using a GLAMOUR 1600 random access clinical analyzer (Buenos Airess, Argentina) according to the protocols from the manufacturers.
Measurement of mice phagocytic capacities—the carbon clearance test
The phagocytosis was measured in vivo by the carbon clearance method as previously reported.16 In brief, carbon ink diluted with saline injections (dilution 1 : 3) was injected into mice (i.v., 0.1 ml per 10 g bw) at the 2 minute and 10 minute interval after ink injection, and then 20 μl of blood, taken from the inner canthus venous plexus, were added to 2 ml of 0.1% Na2CO3. After that, the mice were sacrificed, and the liver and spleen were stripped and weighed. The absorbance (OD) of the solutions was measured at 600 nm by using Infinite M200 PROV of TECAN (Mannedorf, Switzerland) and the phagocytic index (a) was calculated using the following formula: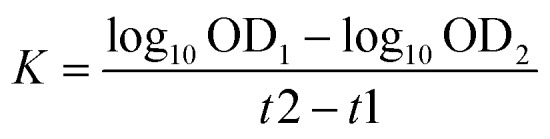
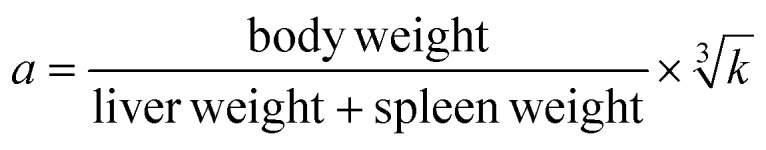
H&E staining
Liver histopathological examination was performed by hematoxylin and eosin (H&E) staining. Slices of 5 μm were prepared using a paraffin slicer (Thermo), and then deparaffinized, rehydrated, and stained with H&E and then viewed by using an Olympus AX70 microscope of Tokyo JPN (Olympus, Japan).
Immunohistochemistry of F4/80 marker for KCs
The activation of KCs was measured by immunohistochemistry detection of F4/80 which is the well characterized and extensively referenced mouse macrophage marker.17 Briefly, liver paraffin sections (5 μm) were deparaffinized, blocked using 3% H2O2 and 5% normal goat serum, and then were incubated with a rat monoclonal antibody against mouse F4/80 (1 : 200) (serotec, MCA497G, Oxford, UK) at 4 °C overnight. The following steps were performed strictly according to the procedure of a rat IgG immunohistochemistry kit (SABC method, BA1005, Wuhan, China), and then viewed by using an Olympus AX70 microscope of Tokyo JPN (Olympus, Japan).
Antioxidant status assay in the liver
Liver tissues were homogenized in ice-cold 0.8% saline (w/v = 1 : 9), and then centrifuged at 1000g for 20 min at 4 °C. The supernatant was collected and stored at –80 °C for antioxidant assay.
The levels of T-GSH, GSH and GSSH in the liver were determined by the 5,5-dithio-bis-2-nitrobenzoic acid assay using the assay kit (Micro ELISA method, A061-1, Nanjing, CN) according to the method described by the manufacturers. The reaction products, which were dependent on the amounts of T-GSH and GSSH, had an OD at 405 nm measured by using Infinite M200 PROV of TECAN (Mannedorf, Switzerland). And the amount of GSH was obtained by subtracting the two T-GSH and GSSH.
The malondialdehyde (MDA) content was measured by the accumulation of thiobarbituric acid-reactive substance and expressed for the Lipid peroxidation (LPO). Briefly, MDA reacted with 2-thiobarbituric acid (TBA) and a pink-colored product, which was developed depending on the concentration of MDA, had an OD at 532 nm measured by using Infinite M200 PROV of TECAN (Mannedorf, Switzerland). The levels of MDA (nmol per (mg pro)) were analyzed using commercial assay kits (TAB method, A003-1, Nanjing, CN) according to the manufacturer's instructions.
Quantification of IL-1β levels in the liver
The levels of IL-1β in the liver were determined using an ELISA kit (EK201B2, Multi SCI, CN) which employed the quantitative sandwich enzyme immunoassay technique. Briefly, the sample and standards were added to the ELISA plate which had been pre-coated with a monoclonal antibody specific for IL-1β, and IL-1β present was bound by the immobilized antibody. The unbound substances were washed away, and a biotin-linked monoclonal anti body specific for IL-1β was added to the wells. After a wash to remove any unbound substances, streptavidin-HRP was added. After washing, substrate solution was added and the color, which has an OD at 450 nm measured by using Infinite M200 PROV of TECAN (Mannedorf, Switzerland), developed in proportion to the amount of IL-1β.
Statistical analysis
All data were expressed as mean and standard deviation (SD). SPSS18.0 statistical software was used for statistical analysis. Data were analyzed using one-way ANOVA to compare the means among different groups and Tukey's test. For the comparisons between two experimental groups (i.e. INH&RFP group versus control group or INH&RFP + DATS groups versus INH&RFP group) the LSD test was performed by using SPSS 18.0. A p value <0.05 was considered significant.
Results
Effects of DATS alone on the liver
The DATS only administered group was examined, and the results showed that with DATS only no statistically significant differences in body weight and serum ALT, AST, T.Bili were observed compared with the control group. A normal lobular architecture with a normal cell morphology and no obvious pathological state were found in both the DATS group and control group. These all indicated that DATS is a safe drug at the studied dose to the liver (Fig. 1–3 & Tables 1 and 2).
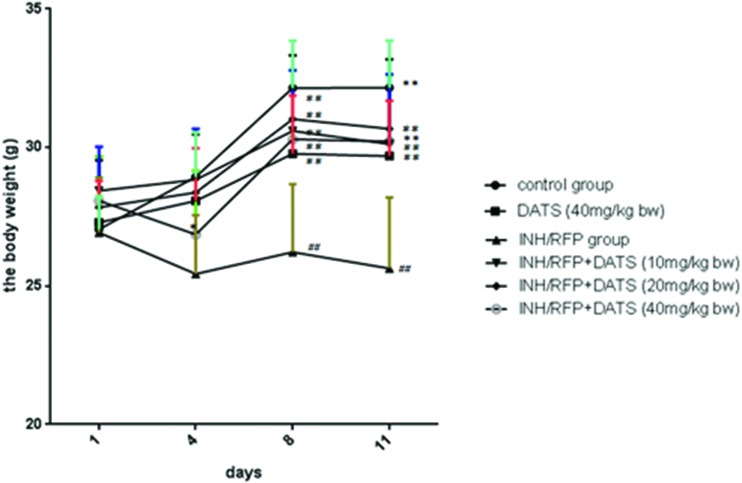
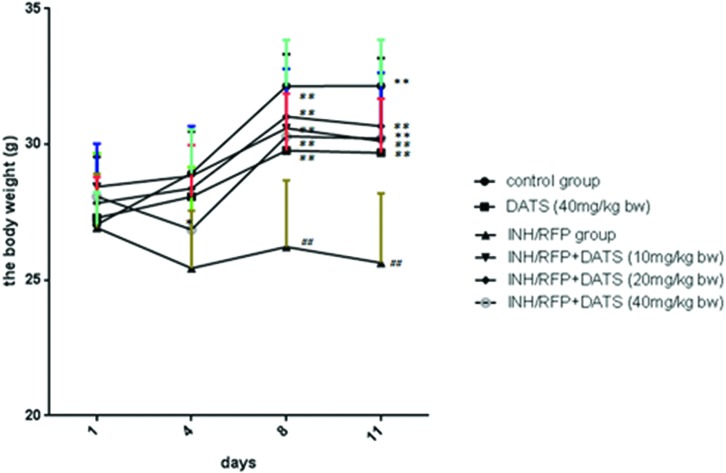
Fig. 1. The changes of body weight in six groups during the study shown as mean ± S.D. Compared with the control group, #P < 0.05, ##P < 0.01; compared with the INH&RFP group, *P < 0.05, **P < 0.01.
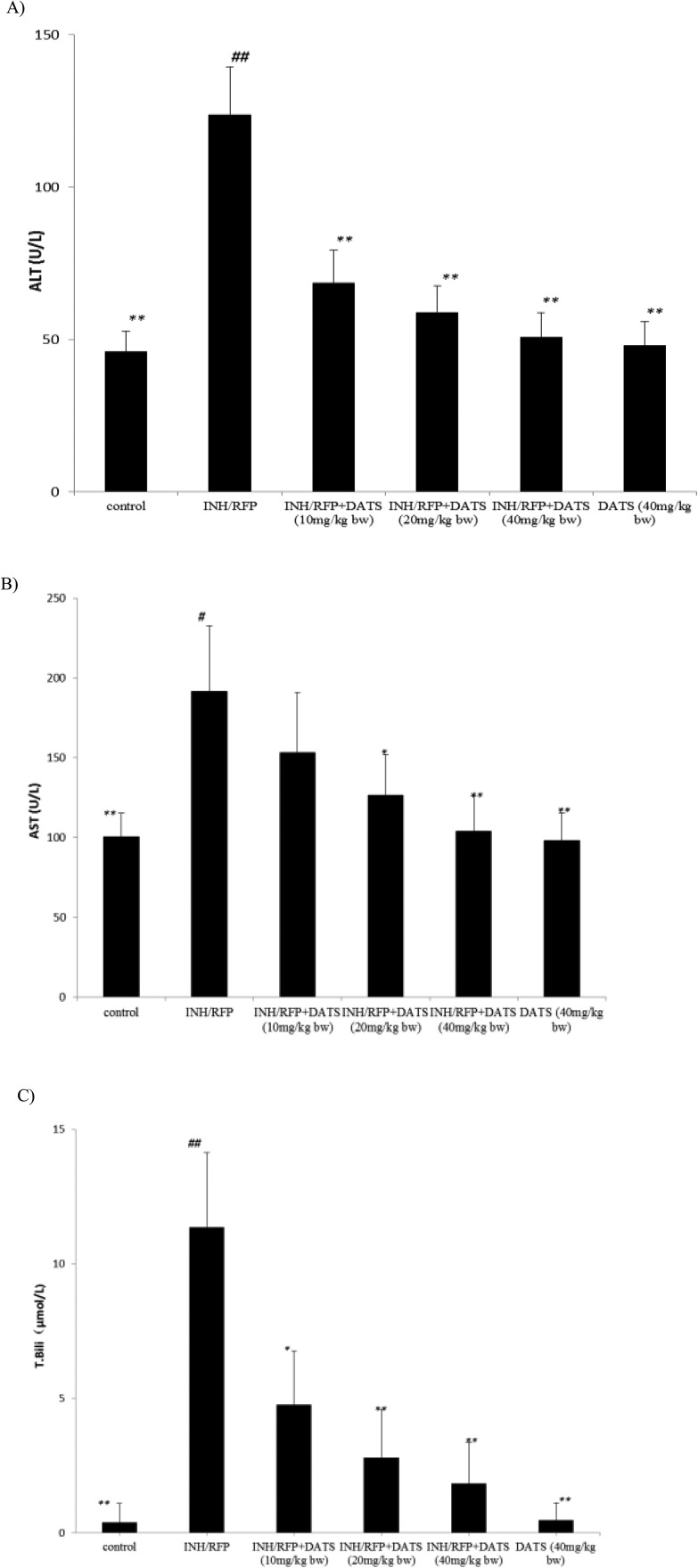
Fig. 2. (A) The levels of serum ALT (U L–1) in the six groups shown as mean ± S.D. Compared with the control group, #P < 0.05, ##P < 0.01; compared with the INH&RFP group, *P < 0.05, **P < 0.01. (B) The levels of serum AST (U L–1) in the six groups shown as mean ± S.D. Compared with the control group, #P < 0.05, ##P < 0.01; compared with the INH&RFP group, *P < 0.05, **P < 0.01. (C) The levels of serum T.Bili (μmol L–1) in the six groups shown as mean ± S.D. Compared with the control group, #P < 0.05, ##P < 0.01; compared with the INH&RFP group, *P < 0.05, **P < 0.01.
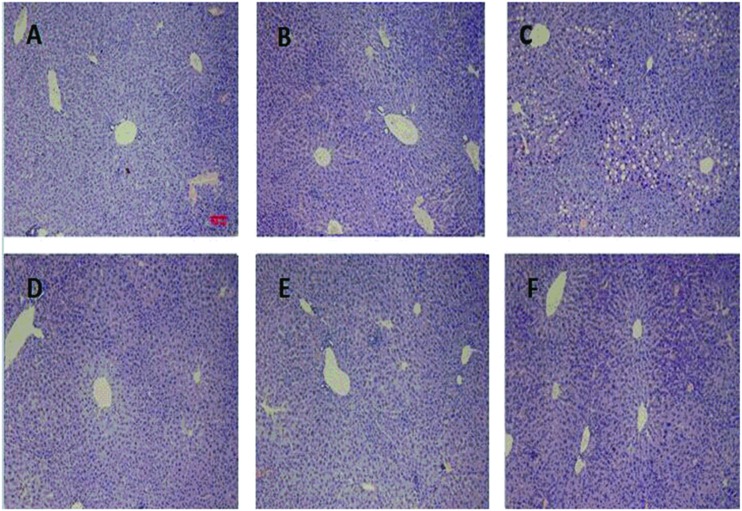
Fig. 3. The H&E staining of the livers of different groups. Pictures were originally captured at 100× magnification. The bar represents 100 μm. A was represented for the control group. B was for DATS (40 mg per kg bw). C was for the INH&RFP group. D was for INH&RFP + DATS (10 mg per kg bw). E was for INH&RFP + DATS (20 mg per kg bw). F was for INH&RFP + DATS (40 mg per kg bw).
Table 1. The comparison of the body and liver weight in the six groups (mean ± S.D.).
| Groups | Final body weight (g) | Liver weight (g) | Liver index (%) |
| Control group | 29.14 ± 2.03** | 1.23 ± 0.13** | 4.20 ± 0.35** |
| DATS (40 mg kg–1) | 27.66 ± 2.27** | 1.24 ± 0.20** | 4.45 ± 0.36** |
| INH&RFP group | 22.75 ± 3.07## | 1.82 ± 0.44## | 7.76 ± 0.7## |
| INH&RFP + DATS (10 mg per kg bw) | 27.13 ± 2.44** | 1.84 ± 0.29** | 6.79 ± 0.62**## |
| INH&RFP + DATS (20 mg per kg bw) | 27.83 ± 2.12** | 1.80 ± 0.24** | 6.41 ± 0.45**## |
| INH&RFP + DATS (40 mg per kg bw) | 27.24 ± 2.04** | 1.61 ± 0.22** | 5.86 ± 0.40**## |
Table 2. The levels of T-GSH, GSH, GSSH and MDA (mean ± S.D.).
| T-GSH, μg per (mg pro) | GSSH, μg per (mg pro) | GSH, μg per (mg pro) | MDA, nmol per (mg pro) | |
| Control group | 390.69 ± 30.72** | 50.70 ± 14.23** | 340.00 ± 29.56** | 1.56 ± 0.43** |
| DATS (40 mg per kg bw) | 405.98 ± 31.82** | 55.80 ± 13.34** | 350.18 ± 30.43** | 1.58 ± 0.47** |
| INH&RFP | 267.74 ± 57.45## | 103.01 ± 33.0## | 164.73 ± 68.62## | 4.12 ± 0.83## |
| INH&RFP + DATS (10 mg per kg bw) | 312.95 ± 47.43* | 76.02 ± 26.62* | 244.64 ± 50.16**## | 2.18 ± 0.57* |
| INH&RFP + DATS (20 mg per kg bw) | 320.37 ± 37.36** | 63.47 ± 18.67* | 249.47 ± 37.10**## | 1.81 ± 0.46** |
| INH&RFP + DATS (40 mg per kg bw) | 387.42 ± 40.55** | 61.80 ± 20.39** | 320.62 ± 43.17** | 1.69 ± 0.47** |
Effects of DATS on INH&RFP-induced hepatotoxicity
Effects of DATS and INF&RFP on the body weight, liver weight and liver index
The body weights of mice were consecutively monitored for 11 days. After INH&RFP treatment, the body weight of mice showed a negative growth in the INH&RFP group. At the end of 11 days, the final body weight of the INH&RFP group was reduced by 4% of the initial value, while that of the control group was increased by 19% (P < 0.01). By the DATS co-administration, INH&RFP + DATS groups significantly reversed the body weight depression induced by INH&RFP (5%, 10%, 5% in 10, 20, 40 mg per kg bw, respectively) (P < 0.05, P < 0.01, P < 0.01) at the end of 11 days (Fig. 1 & Table 1).
Table 1 shows the final body weight and liver weight of mice in six groups. The relative liver weight in the INH&RFP group was increased by 84.76% compared to the control group (P < 0.01). Co-administration of 10, 20 and 40 mg kg–1 DATS significantly reduced the relative liver weight compared with the INH&RFP group (P < 0.01) by 12.5%, 17.40%, 24, 28%, respectively. (Table 2)
DATS co-treatment attenuated INF&RFP-induced increase of serum ALT, AST and T.Bili levels
The levels of serum ALT, AST and T.Bili were elevated in the INH&RFP group compared to the control group (P < 0.01) (Fig. 2): ALT activity increased from 45.98 ± 6.79 to 123.5 ± 15.89 U L–1, p < 0.01; AST activity increased from 100.44 ± 14.89 to 191.85 ± 40.89 U L–1, p < 0.05; T.Bili activity increased from 0.38 ± 0.71 to 11.36 ± 2.78, which indicated the hepatic injury. DATS (10 mg kg–1, 20 mg kg–1, 40 mg kg–1, respectively) co-administration groups were ameliorated in the levels of ALT and T.Bili (P < 0.05 or P < 0.01) (Fig. 2A) (Fig. 2C), the levels of AST were also significantly refined in INH&RFP + DATS (20 and 40 mg kg–1, respectively) compared with the INH&RFP group (P < 0.01 or P < 0.05) (Fig. 2B).
DATS co-treatment improved the liver histology in INH&RFP-intoxicated mice
The control and DATS groups (40 mg per kg bw) had a normal lobular architecture with a normal cell morphology and no obvious pathological state: cells closely packed in a funicular, the cytoplasm with a red dye, and the nucleus homogeneous with a blue dye. The INH&RFP group revealed typical and obvious pathological characteristics including a loose irregular arrangement of liver cells, a large number of large, round cavity cells and cell necrosis in the portal triad region and other liver regions; nuclei were large and deep dye, with central venous blood stasis. Compared with the INH&RFP group, INH&RFP + DATS (10 mg per kg bw) had a small amount of small vacuole cells. But in the INH&RFP + DATS (20 and 40 mg per kg bw) group, the hepatic injury was obviously recovered (Fig. 3).
DATS co-treatment effectively blocked INF&RFP-induced decrease of GSH and increase of MDA
The levels of T-GSH and GSH in INH&RFP had a significant reduction of 31% (P < 0.05) and 51% (P < 0.01), respectively, compared with the control group. The contents of GSSH were higher than the control group up to 103% (P < 0.01), but this damage was well reversed by co-administration of INH&RFP and DATS (10, 20 and 40 mg per kg bw; respectively). The levels of T-GSH were increased by 17%, 20%, 45% (P < 0.05), respectively. The levels of GSH were increased by 48% (P < 0.01), 51% (P < 0.01), 95% (P < 0.01), respectively. And the levels of GSSH were declined by 26% (P < 0.05), 38% (P < 0.05), 40% (P < 0.01), respectively. (Table 2)
The levels of MDA are an index of the intensity of lipid peroxidation damage in the liver. The results showed that the INH&RFP group had a higher increase of 164% (P < 0.01) than the control group, while with the DATS co-administration, the INH&RFP + DATS (10, 20 and 40 mg per kg bw, respectively) groups showed a significant decrease in the content of MDA by 47% (P < 0.05), 56% (P < 0.01), 59% (P < 0.01) in comparison with the INH&RFP group, respectively, (Table 2).
DATS co-treatment improved the capacities of KCs induced by INH&RFP
DATS co-treatment improved the phagocytic capacities by the assay of carbon clearance
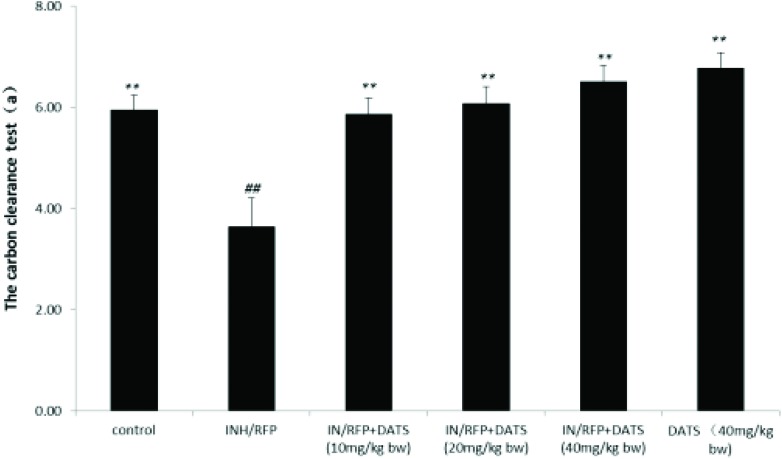
The assay of the carbon clearance test in the INH&RFP group was decreased by 39% in contrast to the control group, and the results showed that DATS (10, 20, 40 mg per kg bw, respectively) co-administration improved the carbon phagocytic capacities by 62%, 67%, 79% (P < 0.01) in contrast to the INH&RFP group (Fig. 4).
Fig. 4. The assay of the carbon clearance test (a) shown as mean ± S.D. Compared with the control group, #P < 0.05, ##P < 0.01; compared with the INH&RFP group, *P < 0.05, **P < 0.01.
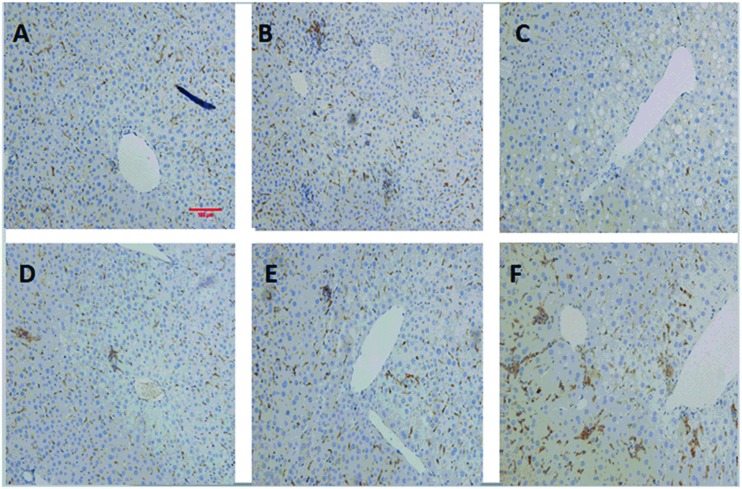
DATS co-treatment led to the activation of the Kupffer cells
The immune staining for F4/80 of KCs in the liver showed that a reduction in population of activated KCs even appeared in the INH&RFP group (Fig. 5). With co-administration of DATS, the KCs in INH&RFP + DATS (10 mg per kg bw), INH&RFP + DATS (20 mg per kg bw) and INH&RFP + DATS (40 mg per kg bw) groups were in varying degrees of activations compared to the INH&RFP group, and the activations were increased with the increasing dose of DATS (Fig. 5).
Fig. 5. The immune stain for F4/80 of KC in six groups. Pictures were originally captured at 200× magnification. The bar represents 100 μm. A was represented for the control group. B was for DATS (40 mg per kg bw). C was for the INH&RFP group. D was for INH&RFP + DATS (10 mg per kg bw). E was for INH&RFP + DATS (20 mg per kg bw). F was for INH&RFP + DATS (40 mg per kg bw).
DATS co-treatment contributed to the normal secretion of IL-1-β by Kupffer cells
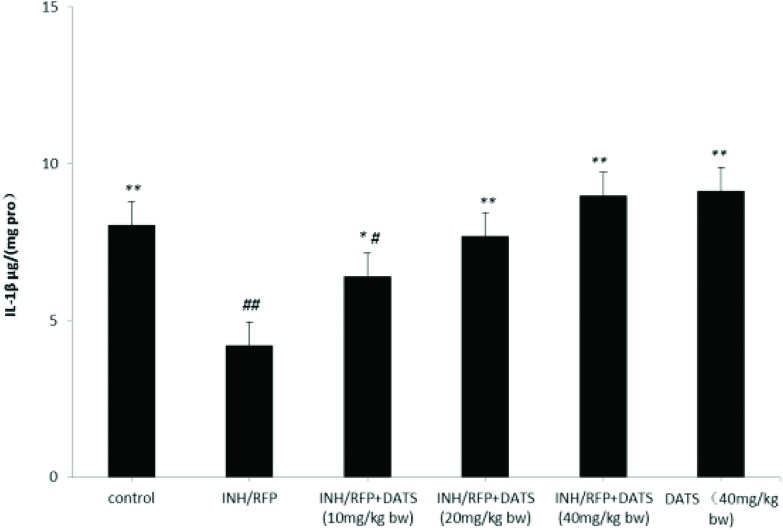
The secretions of IL-1β which were mainly secreted by KCs in the liver were decreased in the INH&RFP group in contrast to the control group (P < 0.01). By the DATS (10, 20, 40 mg per kg bw, respectively) treatments, the secretions of IL-1-β were similar to the activation of KCs and was increased by 52% (P < 0.05), 82% (P < 0.01), and 113% (P < 0.01), respectively (Fig. 6).
Fig. 6. The levels of IL-1-β (pg per (mg pro)) in the six groups shown as mean ± S.D. Compared with the control group, #P < 0.05, ##P < 0.01; compared with the INH&RFP group, *P < 0.05, **P < 0.01.
Discussion
DATS has been shown to be an effective drug against many drugs and hepatotoxins inducing liver injuries, such as ethanol, naphthalene, and carbon tetrachloride (CCl4),18–21,28 especially due to its powerful antioxidant functions. As a compound purified from garlic, DATS has less toxicity and stronger antioxidant capability than other garlic products. The hepatoprotective ability of DATS has received much attention, and it has been verified to provide multiple benefits, e.g. anti-tuberculosis and immunoregulation.21,22,29–32 But the effects of DATS on the INH&RFP-induced liver damage have not been reported. Thus, the effect of DATS against the hepatotoxicity induced by INH&RFP is worth to be studied.
ALT and AST are the most common indexes to assess the liver injury in vitro/vivo: the serum ALT level is a marker for the hepatotoxic effects while the AST level is used to measure the liver function.33 The co-treatment of RFP can also result in an increase of serum T.Bili level.34 In this study, the results showed that DATS co-treatment significantly attenuated the INF&RFP-induced increase of the serum ALT, AST, and T.Bili levels. Besides, the histological examination showed that INF&RFP treatment led to obvious liver injury shown as irregular arrangement of liver cells, vacuolar degeneration, and necrosis, which were also significantly suppressed by DATS co-treatment. These results strongly suggested that DATS effectively abrogated INF/RFP-induced liver injury.
The mechanism of INH&RFP-induced liver injury has been widely investigated in the past few decades, and the roles of GSH have been highlighted.35,36 GSH is the largest percent of non-enzymatic antioxidant in the liver, playing an important role in the antioxidant events.37,38 As is known, oxidative stress results from an imbalance between oxidants and antioxidants in favor of the oxidants. For the hepatotoxicity of INH&RFP, except for the over-production of oxidative stress, the reduced GSH level after INH or hydrazine administration to rats indicates that the decrease of GSH might also be involved in their hepatotoxicity. In the antioxidant activities, the GSH is depleted by free radicals and other oxygen species produced by hepatotoxins, such as acetyldiazene, ketone, ions and radicals metabolized by INH&RFP, and oxidized into GSSH.10,39 In this study, we found a 31% decrease of GSH and a 103% increase of GSSH after the INH&RFP administration for 11 days. Parallelly, consistent with the previous studies, it was noticed that INH&RFP exposure also led to a significant increase of hepatic MDA levels, which is a biomarker of oxidative stress and lipid peroxidation. The results showed that MDA was increased by 164%. However, DATS co-administration significantly elevated the GSH levels by 17%, 20%, and 45%, and suppressed the increase of MDA level by 47%, 56%, and 59%, which suggested that the restoration of GSH might be a mechanism for the protective effects and the antioxidant ability might at least partially account for the protection against INH&RFP-induced liver injury.
In addition to oxidative stress, a number of studies have suggested that the immune system was involved in INH&RFP-induced hepatotoxicity.40,41 Metushi et al. demonstrated that Cb-b–/–, PD1–/– mice (which have impaired immune tolerance) and the Rag–/– mice (which lack T- and B-cells) were more vulnerable to INH-induced hepatoxicity compared with the wild type C57BL/6 mice, which suggested that INH treatment led to immunosuppression.42 Several other studies also suggested that TB treatment could result in immune impairment.43,44 In view of the KCs being the largest macrophages, and their activation being the first step in the immune response of the liver, we detected the number of activated KCs by using the immunochemistry assay of the KC markers, F4/80; the results showed that INH&RFP exposure led to a significant decrease of the KC number; and then the clearance of the carbon which was often been represented for the phagocytic activity of macrophages (KCs) and the secretion of IL-1-bβ were also decreased by 39% and 39.8% after INH&RFP treatment. The results that confirmed the inhibited activity of Kupffer cells reduced by INH&RFP were parallel with the reported impairment of the immune system by INH&RFP as we mentioned. Interestingly, DATS co-treatment led to a significant increase of the number of hepatic Kupffer cells as well as the increase of the phagocytic capacity. These results suggested that Kupffer cell depletion might be involved in INH&RFP-induced hepatoxicity, and DATS could protect against INH&RFP-induced hepatoxicity by activation of Kupffer cells.
In summary, our study demonstrated that DATS could effectively suppress the INH&RFP-induced increase of serum ALT, AST, and T.Bili levels and improve the liver morphological changes, which might be associated with the antioxidant capacity and the immunoregulatory capacity. The results of the current study suggested that DATS might be a candidate hepatoprotective drug for TB patients receiving INH&RFP.
Abbreviations
- DATS
Diallyl trisulfide
- INH
Isoniazid
- RFP
Rifampicin
- ALT
Aminotransferase
- AST
Aspartate transaminase
- T.Bili
Total bilirubin
- MDA
Malondialdehyde
- GSH
Glutathione
- IL-1-β
Interleukin 1-β
- KC
Kupffer cell
- T-GSH
Total-glutathione
- GSSH
Oxidized glutathione
- TB
Tuberculosis
- DILI
Drug induced liver injury
- TBA
2-Thiobarbituric acid
- SD
Standard deviation
- ROS
Reactive oxygen species
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
We would like to thank Dr Zeng T for giving us the first antibody of F4/80. We thank Yu LH for help with the liver H&E staining. This work was in part supported by the Yong Scholars Program of Shandong University (grant no. 2015WLJH52).
References
- Stein C. M. PLoS Pathog. 2011;7:e1001189. doi: 10.1371/journal.ppat.1001189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- W. H. O. Organization, Tuberculosis
- Gaude G. S., Chaudhury A., Hattiholi J. J.f Fam. Med. Primary Care. 2015;4:238–243. doi: 10.4103/2249-4863.154661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tostmann A., Boeree M. J., Aarnoutse R. E., de Lange W. C., van der Ven A. J., Dekhuijzen R. J. Gastroenterol. Hepatol. 2008;23:192–202. doi: 10.1111/j.1440-1746.2007.05207.x. [DOI] [PubMed] [Google Scholar]
- Sotsuka T., Sasaki Y., Hirai S., Yamagishi F., Ueno K. In Vivo. 2011;25:803–812. [PubMed] [Google Scholar]
- Attri S., Rana S. V., Vaiphei K., Sodhi C. P., Katyal R., Goel R. C., Nain C. K., Singh K. Hum. Exp. Toxicol. 2000;19:517–522. doi: 10.1191/096032700674230830. [DOI] [PubMed] [Google Scholar]
- Santos N. P., Callegari-Jacques S. M., Ribeiro Dos Santos A. K., Silva C. A., Vallinoto A. C., Fernandes D. C., de Carvalho D. C., Santos S. E., Hutz M. H. Int. J. Tuberc. Lung Dis. 2013;17:499–504. doi: 10.5588/ijtld.12.0645. [DOI] [PubMed] [Google Scholar]
- Tafazoli S., Mashregi M., O'Brien P. J. Toxicol. Appl. Pharmacol. 2008;229:94–101. doi: 10.1016/j.taap.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Jenner P. J., Ellard G. A. Tubercle. 1989;70:93–101. doi: 10.1016/0041-3879(89)90033-0. [DOI] [PubMed] [Google Scholar]
- Van Etten E. W. M., Kate M. T. T., Snijders S. V., Bakker-Woudenberg I. A. J. M. Antimicrob. Agents Chemother. 1998;42(7):1677–1681. doi: 10.1128/aac.42.7.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askgaard D. S., Wilcke T., Dossing M. Thorax. 1995;50:213–214. doi: 10.1136/thx.50.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metushi I. G., Nakagawa T., Uetrecht J. Chem. Res. Toxicol. 2012;25:2567–2576. doi: 10.1021/tx300341r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imir J. U., Metushi G. J. Immunotoxicol. 2013:46. [Google Scholar]
- Boelsterli U. A., Mechanistic Toxicology, Taylor & Francis, 11 New Fetter Lane, London EC4P 4EE, 2003. [Google Scholar]
- Liu Z. X., Govindarajan S., Kaplowitz N. Gastroenterology. 2004;127:1760–1774. doi: 10.1053/j.gastro.2004.08.053. [DOI] [PubMed] [Google Scholar]
- Chen M., Gandolfi J. Drug Metab. Rev. 1997;29:103–122. doi: 10.3109/03602539709037575. [DOI] [PubMed] [Google Scholar]
- Neuberger J., Williams R. Gut. 1989;30:515–519. doi: 10.1136/gut.30.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng T., Zhang C. L., Zhu Z. P., Yu L. H., Zhao X. L., Xie K. Q. Toxicology. 2008;252:86–91. doi: 10.1016/j.tox.2008.07.062. [DOI] [PubMed] [Google Scholar]
- Fukao T., Hosono T., Misawa S., Seki T., Ariga T. Food Chem. Toxicol. 2004;42:743–749. doi: 10.1016/j.fct.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Zhang F., Zhang Y., Wang K., Zhu X., Lin G., Zhao Z., Li S., Cai J., Cao J. Int. Immunopharmacol. 2015;29:326–333. doi: 10.1016/j.intimp.2015.10.033. [DOI] [PubMed] [Google Scholar]
- Sumedha N. C., Miltonprabu S. Hum. Exp. Toxicol. 2015;34:506–525. doi: 10.1177/0960327114543933. [DOI] [PubMed] [Google Scholar]
- Shigemi Z., Furukawa Y., Hosokawa K., Minami S., Matsuhiro J., Nakata S., Watanabe T., Kagawa H., Nakagawa K., Takeda H., Fujimuro M. Int. J. Oncol. 2016;48:293–304. doi: 10.3892/ijo.2015.3247. [DOI] [PubMed] [Google Scholar]
- Hung F. M., Shang H. S., Tang N. Y., Lin J. J., Lu K. W., Lin J. P., Ko Y. C., Yu C. C., Wang H. L., Liao J. C., Lu H. F., Chung J. G. Environ. Toxicol. 2015;30:1343–1353. doi: 10.1002/tox.22005. [DOI] [PubMed] [Google Scholar]
- Sheng L., Xue Y., He X., Zhu Y., Li H., Wu Y., Dang R., Tang M., Jiang P. Steroids. 2015;104:203–207. doi: 10.1016/j.steroids.2015.10.004. [DOI] [PubMed] [Google Scholar]
- Wali A. F., Avula B., Ali Z., Khan I. A., Mushtaq A., Rehman M. U., Akbar S., Masoodi M. H. BioMed Res. Int. 2015;2015:393462. doi: 10.1155/2015/393462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y. X., Xu X. F., Zhang Q. Z., Li C., Deng Y., Jiang P., He L. Y., Peng W. X. Toxicol. Mech. Methods. 2015;25:382–387. doi: 10.3109/15376516.2015.1033074. [DOI] [PubMed] [Google Scholar]
- Thattakudian Sheik Uduman M. S., Sundarapandian R., Muthumanikkam A., Kalimuthu G., Parameswari S. A., Vasanthi Srinivas T. R., Karunakaran G. Pak. J. Pharm. Sci. 2011;24:129–134. [PubMed] [Google Scholar]
- Chen H., Zhu W., Feng J., Li S. J. Huazhong Univ. Sci. Technol. 2012;32:657–662. doi: 10.1007/s11596-012-1013-7. [DOI] [PubMed] [Google Scholar]
- Zeng T., Zhang C. L., Zhao X. L., Xie K. Q. CRC Crit. Rev. Food Sci. Nutr. 2013;53:215–230. doi: 10.1080/10408398.2010.523148. [DOI] [PubMed] [Google Scholar]
- Lii C. K., Tsai C. W., Wu C. C. J. Agric. Food Chem. 2006;54:5191–5196. doi: 10.1021/jf052484u. [DOI] [PubMed] [Google Scholar]
- Srivastava S. K., Hu X., Xia H., Zaren H. A., Chatterjee M. L., Agarwal R., Singh S. V. Cancer Lett. 1997;118:61–67. doi: 10.1016/s0304-3835(97)00237-1. [DOI] [PubMed] [Google Scholar]
- Hannan A., Ikram Ullah M., Usman M., Hussain S., Absar M., Javed K. Pak. J. Pharm. Sci. 2011;24:81–85. [PubMed] [Google Scholar]
- Turktas H., Unsal M., Tulek N., Oruc O. Tubercle Lung Dis. 1994;75:58–60. doi: 10.1016/0962-8479(94)90104-X. [DOI] [PubMed] [Google Scholar]
- Gendrel D., Nardou M., Mouba J. F., Gahouma D., Moussavou A., Boguikouma J. B. Arch. Fr. Pediatr. 1989;46:645–648. [PubMed] [Google Scholar]
- Enriquez-Cortina C., Almonte-Becerril M., Clavijo-Cornejo D., Palestino-Dominguez M., Bello-Monroy O., Nuno N., Lopez A., Bucio L., Souza V., Hernandez-Pando R., Munoz L., Gutierrez-Ruiz M. C., Gomez-Quiroz L. E. Toxicol. Sci. 2013;135:26–36. doi: 10.1093/toxsci/kft134. [DOI] [PubMed] [Google Scholar]
- Chowdhury A., Santra A., Bhattacharjee K., Ghatak S., Saha D. R., Dhali G. K. J. Hepatol. 2006;45:117–126. doi: 10.1016/j.jhep.2006.01.027. [DOI] [PubMed] [Google Scholar]
- Aruoma O. I. Food Chem. Toxicol. 1994;32:671–683. doi: 10.1016/0278-6915(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Fang Y. Z., Yang S., Wu G. Nutrition. 2002;18:872–879. doi: 10.1016/s0899-9007(02)00916-4. [DOI] [PubMed] [Google Scholar]
- Kumar S., Kumar R., Dwivedi A., Pandey A. K. Biomed Res. Int. 2014;2014:459–452. doi: 10.1155/2014/459452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metushi I. G., Cai P., Zhu X., Nakagawa T., Uetrecht J. P. Clin. Pharmacol. Ther. 2011;89:911–914. doi: 10.1038/clpt.2010.355. [DOI] [PubMed] [Google Scholar]
- Warrington R. J., Tse K. S., Gorski B. A., Schwenk R., Sehon A. H. Clin. Exp. Immunol. 1978;32:97–104. [PMC free article] [PubMed] [Google Scholar]
- Metushi I. G., Uetrecht J. J. Immunotoxicol. 2014;11:383–392. doi: 10.3109/1547691X.2013.860644. [DOI] [PubMed] [Google Scholar]
- Sharma S. K., Balamurugan A., Saha P. K., Pandey R. M., Mehra N. K. Am. J. Respir. Crit. Care Med. 2002;166:916–919. doi: 10.1164/rccm.2108091. [DOI] [PubMed] [Google Scholar]
- Tousif S., Singh D. K., Ahmad S., Moodley P., Bhattacharyya M., Van Kaer L., Das G. J. Biol. Chem. 2014;289:30190–30195. doi: 10.1074/jbc.C114.598946. [DOI] [PMC free article] [PubMed] [Google Scholar]