Abstract
Cadmium (Cd) is a common environmental pollutant. Its effects on human health have attracted great attention. The kidney is the organ that is the most affected by Cd exposure. Thus, it is highly desirable to develop a reliable model to evaluate Cd-induced nephrotoxicity in vitro. We present a kidney-on-a-chip with three compartmentalized culture chambers to examine Cd-induced nephrotoxicity. The culture and collection channels represent the capillary and the glomerular capsule sides of the glomerular filtration barrier, respectively. Isolated primary rat glomerular endothelial cells (GECs) were cultured on the side surface of the middle gel channel. The integrated GEC layer demonstrated the selective permeability of the renal barrier. Therefore, it was further utilized to study the nephrotoxicity induced by Cd exposure at different concentrations. Cd induced significant cytotoxicity and disrupted the expression of tight junction protein ZO-1 in a dose-dependent manner. Moreover, Cd exposure increased the permeability of the endothelial layer to large molecules, immunoglobulin G and albumin. These results facilitate the understanding of the underlying mechanism of kidney dysfunction and glomerular disease. This is the first study on Cd-induced nephrotoxicity using primary GECs in a microfluidic device. The kidney-on-a-chip device enables direct visualization and quantitative analysis of GEC responses to Cd in real time. It may provide a micro-scale platform based on the human system for nephrotoxicity testing under varying environmental exposure.
1. Introduction
The toxic metal cadmium (Cd) poses a significant health risk to humans and is thus a major public health concern. Cd is ubiquitous in the environment. The main contamination sources are cigarette smoke, welding, contaminated food, beverages and ambient air.1–3 The accumulation of Cd in the environment has caused various health concerns and chronic illnesses in humans, such as kidney diseases, diabetes, neurological diseases, bone diseases and cardiovascular diseases.4–9 Although Cd can accumulate in various organs and tissues, the most extensive accumulation occurs in the kidney.10 Toxic effects on the functions of the glomerular and proximal tubular systems of the kidney are recognized as characteristic responses to Cd exposure.11–13
The vascular endothelium is highly sensitive to toxic Cd exposure. After exposure, Cd is absorbed into the bloodstream-vascular system in the kidney. The renal endothelium is the principal regulator of blood filtration and is essential to the maintenance of homeostasis in the body. Thus, the kidney is greatly vulnerable to Cd exposure. Most studies on Cd-induced nephrotoxicity have depended on in vivo animal and in vitro cell-based models.14–18 Animal models have contributed to studies on Cd-induced nephrotoxicity under physiological conditions, but they limit the full understanding of the mechanism underlying normal physiological or pathological processes in the human body. Moreover, it is difficult to perform quantitative studies via high throughput assays using animal models. In contrast, in vitro cell-based assays based on two-dimensional cultures of renal cell lines, such as renal tubular cells, are popular for their simplicity.19–22 However, these simplified models lack the key representative characteristics of the glomerular filtration barrier that are crucial to maintaining renal function. The glomerular filtration barrier is a dynamic selective barrier between the circulatory system and the urinary system, which is impermeable to large protein molecules (e.g., albumin), but allows the passage of small molecules and water.23 The barrier is mainly composed of glomerular endothelial cells (GECs), fused basement membranes and podocytes. As the glomerular vascular endothelium is an important target of Cd exposure in vivo, reproducing the physiological characteristics and the functional responses of the glomerular endothelial barrier in vitro is valuable to the study of Cd-induced renal toxicity in a physiologically relevant manner.
Recent advances in engineering technology have made it possible to mimic the in vivo environment of cells and tissues. Near-physiological conditions can be created in microfluidic devices based on the flexible design of complex and well-controlled miniature devices.24,25 Many microfluidic devices have used living cells to mimic the micro-architecture of living organs in vivo, including the establishment of the renal tubular system.26–31 By creating in vivo-like extracellular environments, micro-scale kidney models have been found to sufficiently enhance cell polarization, rearrange the cytoskeleton and increase cell junctions. Although significant progress has been made in building functional kidney models for renal toxicity testing, most of these studies have focused on the renal tubular system. No attempts have been made to establish the glomerular endothelial barrier on a microdevice. Also, such technology has not been able to create a glomerular filtration barrier model that incorporates multiple physiological parameters for toxicity analysis.
Here, we present a novel compartmentalized microfluidic device lined with primary rat GECs to investigate the nephrotoxicity induced by Cd exposure in vitro. Cell viability, lactate dehydrogenase (LDH) leakage and expression of tight junction protein in GECs were examined under Cd exposure. The permeability of the GEC layer on a chip was also compared after exposure to Cd at different concentrations. All of the results demonstrated abnormal cell morphology, reduced cell viability, disrupted cell–cell junctions and enhanced permeability in the GECs in the presence of Cd, which aligns with Cd-induced responses in vivo. This kidney model facilitates the evaluation of nephrotoxicity testing in a physiologically relevant manner. It may provide a simple and efficient platform for toxicology research and drug testing in vitro as an alternative to animal models.
2. Materials and methods
2.1. Materials
SU-8 3035 negative photoresist was purchased from MicroChem Corp. A polydimethylsiloxane (PDMS) pre-polymer and curing agent were purchased from Dow Corning Corp. to fabricate the microfluidic devices. Endothelial cell medium (Gibco), fetal bovine serum (FBS, Gibco), trypsin/EDTA (Gibco), rat tail type I collagen (BD), live/dead kit (BD), cell counting kit-8 (CCK-8, Dojindo), ZO-1 (Abcam), CD31 (Cell Signaling Technology), DAPI (Sigma), Alexa 594 and 488 conjugated goat secondary antibodies (Beyotime Company), sodium fluorescein (NaFl), fluorescein IgG, albumin assay kit, LDH assay kit and cadmium acetate were purchased from Casmart Mall (Beijing, China) for cell-related experiments. All of the chemical reagents used in this experiment were analytical reagent grade.
2.2. Design and fabrication of the microfluidic device
The microfluidic chip was fabricated using soft lithography and micromolding. The masks were designed using AutoCAD (Autodesk) and printed on the plastic film at 4000 dpi resolution. First, to prepare the template, the SU-8 photoresist was spin-coated onto clean glass wafers and then selectively cured under an ultraviolet light source by using two masks continuously. Next, the microdevice was fabricated by replicate molding the master with PDMS at a 10 : 1 base-to-curing agent weight ratio. Finally, the microdevice was firmly sealed with the glass. The microfluidic device consisted of two higher channels separated from a lower channel with collagen. The higher channels were 300 μm in height and the lower channel was 100 μm in height.
2.3. Isolation and identification of glomerular endothelial cells
Primary glomerular micro-tissues were isolated from rat kidneys according to a previously described protocol.32 The isolated glomerular micro-tissues were cultured on a collagen I-coated Petri dish in endothelial cell medium supplemented with 10% FBS, 100 U mL–1 of penicillin and 100 U mL–1 of streptomycin with 5% CO2 at 37 °C. The cells spread around the glomerular tissues after being cultured for 3 days under static conditions. As the glomerular micro-tissues contained podocytes and mesangial cells, we used differential digestion to purify the endothelial cells. As GECs are more easily digested than podocytes and mesangial cells, the endothelial cells were digested using trypsin for 2 to 3 min after culturing for 5 to 7 days. The digested endothelial cells were transferred to a new Petri dish to expand. The GECs were identified with immunofluorescence testing using the CD31 antibody, an endothelial cell marker.
2.4. Culturing glomerular endothelial cells on a chip
Primary GECs isolated from rat glomeruli were cultured on the concave surface of the collagen channel between the cell culture and collection channels, mimicking glomerular capillaries. Natural-type collagen I was fused to the middle gel channel for three-dimensional (3D) cell culturing.33,34 Each channel on the microdevice had one flow inlet and one outlet, facilitating the injection of different reagents and cells. After fabricating the PDMS device, the collagen solution was compounded at a final concentration of 6 mg mL–1 according to an alternative gelation procedure at 4 °C, aseptically pumped into the collagen channel and allowed to gel at 37 °C for 30 min. After the microchip was prepared, the glomerular micro-tissues were mechanically pipetted from the Petri dishes. The glomeruli were centrifuged and re-suspended in the cell culture medium at a density of 1 × 104 cells per mL. The glomeruli were then injected into the cell culture channel. The microchip was positioned on the side to enable the cells to adhere to the concave surface of the collagen-coated micro-channel. After being incubated in a humidified incubator at 37 °C for 72 h, the GECs cultured on the surface of the collagen gel formed an endothelial cell barrier in vitro. The microchip was put in a Petri dish and placed in the cell culture incubator with 5% CO2 at 37 °C.
2.5. Permeability testing of the glomerular endothelial cell layer
To evaluate the barrier permeability of the GEC layer, we monitored the diffusion of different sized molecules, such as the fluorescein tracer NaFl (MW = 376 Da, 250 μM), FITC-IgG (MW = 150 kDa, 250 μM) and albumin (MW = 70 kDa, 40 mg mL–1) into the collection channel across the barrier over 60 min. NaFl and IgG were introduced into the cell channel. Time-lapse images of NaFl and IgG were collected at 0 min, 15 min, 30 min and 60 min. The diffusion of NaFl and IgG across the barrier was measured by the fluorescence intensity. The permeability of the barrier for large molecule albumin was assessed using the albumin assay kit.
2.6. Cd-Induced toxicity assessment in glomerular endothelial cells
To assess Cd toxicity, the cells were exposed to a series of cadmium acetate concentrations (2 μM to 16 μM) for 48 h. LDH release from cells was measured using the LDH detection kit and a microplate reader. To measure apoptosis, the cells were stained using the live/dead Kit. The live cells were stained green and the dead cells were stained red. Intracellular fluorescence was measured using fluorescence microscopy. Quantitative cell vitality data were tested using a CCK-8 assay. 5 μL of the CCK-8 reagent and 45 μL of the cell culture medium were added to the cell culture channel. The solution was collected in a 96-well plate and the absorbance at 450 nm was measured by a microplate reader after incubation for 2 h.
2.7. Immunofluorescence assay
All of the cell samples were quickly rinsed with PBS and fixed in 4% paraformaldehyde for 15 min. The fixed cells were permeabilized in 0.2% Triton-X100 before being incubated with a primary antibody, ZO-1 (diluted 1 : 300) or CD31 (diluted 1 : 300), overnight at 4 °C. The samples were then incubated with Alexa 594 or 488 conjugated goat secondary antibodies (diluted 1 : 100) for 1 h at room temperature. After washing with PBS, the nuclei were counterstained with DAPI for 5 min. All of the images were photographed using a Leica fluorescence microscope and a confocal microscope. The image analysis was performed using Image J.
2.8. Statistics and analysis
All of the data were presented as means ± standard deviation and all of the experiments were performed at least in triplicate. Comparisons of the two groups were performed using Student's t-test and p < 0.01 was considered to be statistically significant.
3. Results
3.1. Design and fabrication of a microfluidic device for renal toxicity assays
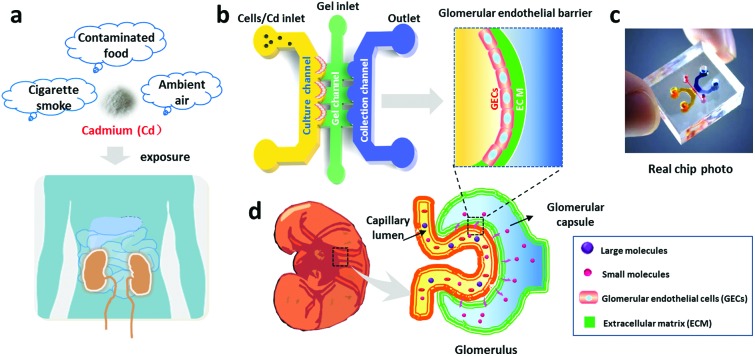
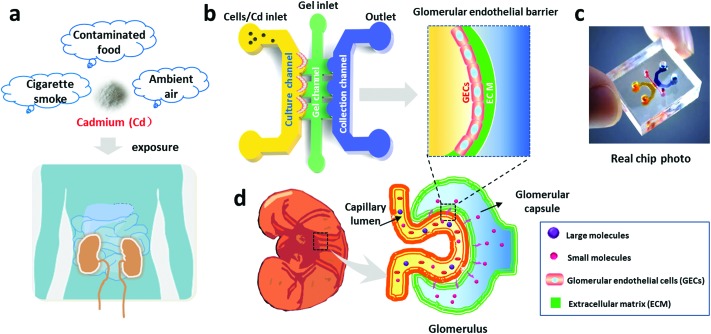
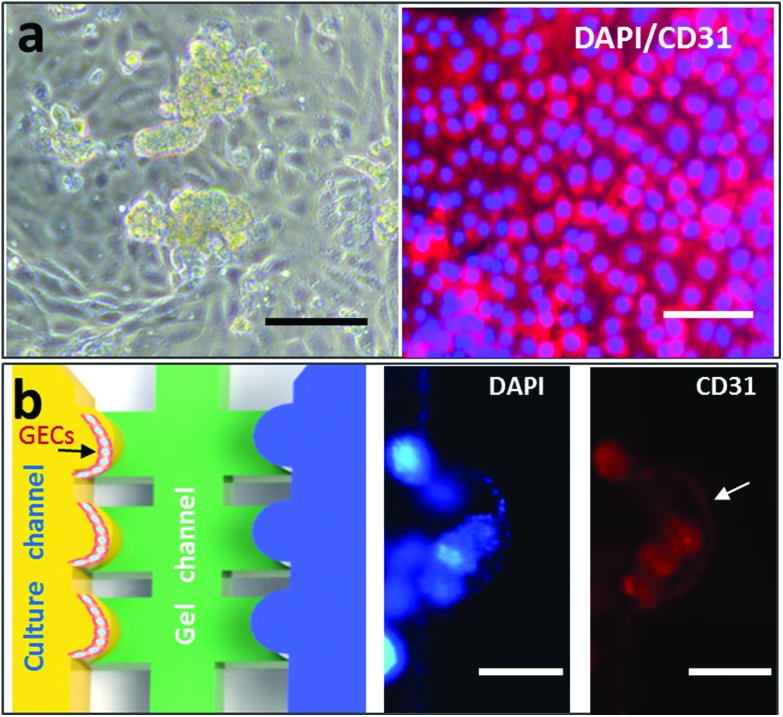
GECs are susceptible to toxic substances, such as Cd (Fig. 1). We designed and fabricated a PDMS microfluidic device containing three channels, a cell culture channel, a gel channel and a collection channel (Fig. 1b and c), to study Cd-induced nephrotoxicity. The middle channel was perfused with collagen I to support cell adhesion and growth on the 3D matrix. The culture channel was loaded with GECs to mimic the capillary side of the glomerular filtration barrier. The collection channel was used to represent the glomerular capsule side and collect the substances filtered from the culture channel across the matrix. Before the assay, the GECs isolated from rat renal cortexes were identified via an immunofluorescence assay with a specific CD31 antibody. As shown in Fig. 2a, the cells strongly expressed the CD31 protein on the cellular membrane and displayed cobblestone-like endothelial cell morphology.
Fig. 1. Schematic illustration of glomerular endothelial barrier formation on a chip. (a) Illustration of Cd exposure in daily life. (b) Schematic design of the microfluidic chip for renal toxicity testing. The microfluidic device consists of a cell culture channel, a gel channel and a collection channel, in which the GECs are cultured on the gel-coated surface to form the glomerular endothelial barrier. (c) Photo of the real microfluidic device. (d) Illustration of glomerular structure.
Fig. 2. Characterization of the glomerular endothelial cell layer cultured on the microdevice. (a) Identification of the endothelial cells purified from the glomerular micro-tissues using specific marker CD31 (red) immunostaining. Scale bar: 50 μm. (b) Identification of the formed glomerular endothelial cell layer on a chip with the expression of cell-specific marker CD31 (red). Scale bar: 200 μm.
The GECs were loaded into the culture channel after collagen infusion into the middle gel channel. Then, the cells were seeded onto the collagen surface forming an intact cell layer that was reminiscent of the endothelial barrier in vivo (Fig. 2b). The design of the kidney chip with compartmentalized regions allowed various constituents to transit from the cell culture channel to the collecting channel, assisting the study of Cd-exposed GECs in a dynamic and real-time manner.
3.2. Characterizing the integrity of the glomerular endothelial barrier
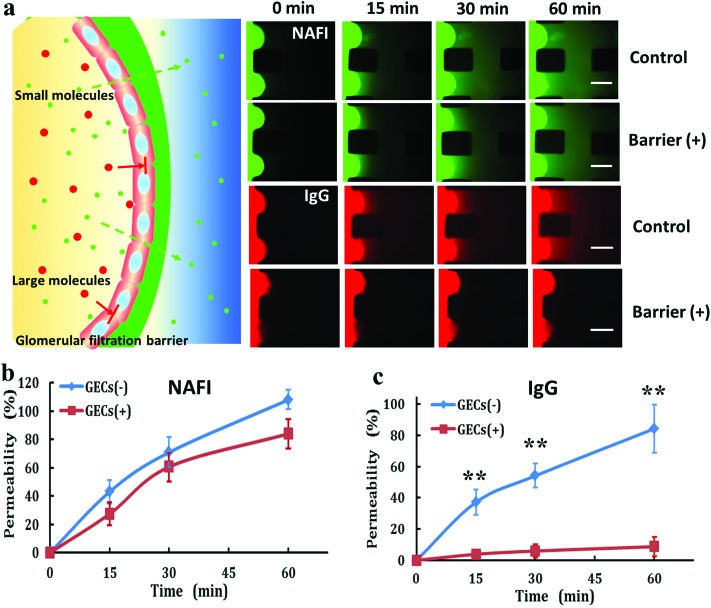
In vivo, GECs are major cellular components of the glomerular filtration barrier in the kidney. To evaluate the function of the endothelial barrier in the microfluidic device, we performed a permeability assay using fluorescent tracers, including a small molecule (NaFl) and a large protein molecule (IgG) (Fig. 3). The diffusion of both fluorescent tracers was monitored for 60 min. It was further quantified based on the fluorescence intensity. As shown in Fig. 3b, there was no significant difference between the two groups, showing that the small molecule NaFl diffused as freely through the glomerular endothelial barrier as in the control group without an endothelial layer. In contrast, compared with the control, the fluorescence-labeled IgG was unable to permeate the renal barrier (Fig. 3c). These findings indicate the integrity of the glomerular endothelial barrier on the designed kidney chip and the selectivity of renal filtration. These findings are highly relevant, as increased urinary albumin filtration can be an early sign of glomerular dysfunction and chemically induced nephrotoxicity.
Fig. 3. Evaluation of glomerular endothelial barrier permeability using fluorescein tracers with different molecular weights. (a) Time-lapse images of a permeable fluorescein tracer crossing the endothelial barrier using NaFl (green) and IgG (red) at 250 μM. Scale bar: 200 μm. Permeability of NaFl (b) and IgG (c) across the glomerular endothelial barrier over 60 min. Data are presented as mean ± standard deviation. n = 3, **p < 0.01.
3.3. Evaluation of Cd-induced cytotoxicity in glomerular endothelial cells
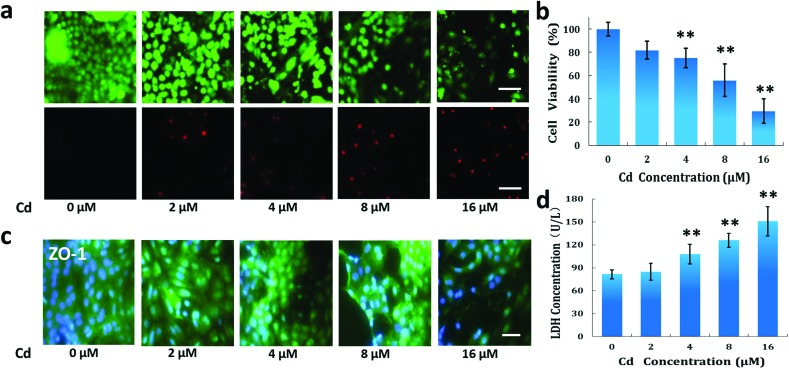
GECs are vulnerable to exotic toxicants, resulting in filtration barrier dysfunction and nephrotoxicity. To assess the effects of Cd on GECs, we tested cell viability, LDH leakage and tight junction protein (ZO-1) expression after GEC exposure to Cd at different concentrations (2 μM to 16 μM). We evaluated the responses of the cells to Cd at these concentrations.18 As shown in Fig. 4a, dead cells increased gradually as Cd concentration increased. Cell viability declined in a dose-dependent manner after treatment with 4 μM to 16 μM of Cd (Fig. 4b). Cell viability reduced to 29.4% when exposed to 16 μM of Cd. Furthermore, LDH leakage, a classic marker of endothelial cell injury, was examined. We found that Cd exposure increased LDH leakage under similar conditions, indicating the significant biochemical changes in GECs after exposure to Cd. However, we did not find cell viability to decrease or LDH leakage to increase under treatment with 2 μM of Cd. This is consistent with previous reports that low-dose Cd exposure may be less acutely toxic.35
Fig. 4. Effects of Cd exposure on the cell viability and LDH leakage from GECs. The cells were treated with Cd for 48 h. (a) Images of GEC cell viability after exposure to Cd using the live/dead assay kit. The cells were treated with Cd at different concentrations (2 μM to 16 μM). The live cells were stained green and the dead cells were stained red. (b) Quantitative cell viability of GECs using the CCK-8 kit. (c) Expression of tight junction protein ZO-1 in GECs after exposure to Cd. ZO-1 (green), DAPI (blue). (d) LDH leakage assay in GECs. Scale bar: 20 μm. Data are presented as mean ± standard deviation. n = 3, **p < 0.01.
The expression of tight junction proteins is critical to maintaining the glomerular filtration function. We further examined the effect of Cd on the expression of ZO-1 in endothelial cells using an immunofluorescence assay. Cd exposure clearly reduced the expression of ZO-1 in GECs compared with the control group (Fig. 4c). This effect was exhibited in a dose-dependent manner. This suggests that Cd may disrupt barrier integrity by reducing the expression of tight junction proteins.
3.4. Change in glomerular endothelial barrier permeability after Cd exposure
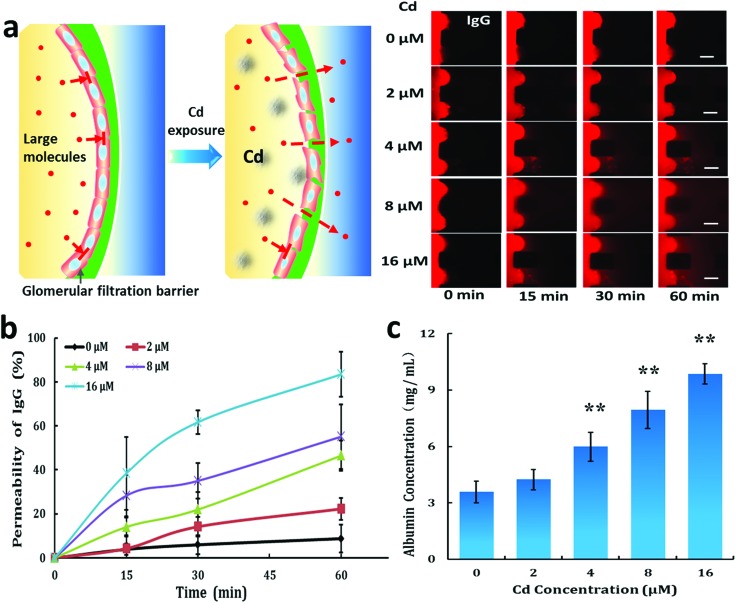
Due to the reduction of tight junction proteins in GECs, we assumed that GECs would shrink, leading to the filtration of large molecules through the glomerular endothelial layer. Thus, we studied the effects of Cd on the permeability of the glomerular endothelial barrier for large molecules using the microfluidic device (Fig. 5a). Fluorescence-labeled IgG diffused through the GEC layer after exposure to Cd in a dose-dependent manner (Fig. 5b). We further tested the permeability of this barrier to albumin, which is the major protein component of blood (Fig. 5c). The permeability of the barrier to albumin increased after exposure to Cd at different concentrations (4 μM to 16 μM). The on-chip assay exhibited GECs’ toxic responses to Cd and offered a powerful alternative to conventional animal and cell-based methods of toxicity testing.
Fig. 5. Effect of Cd on the permeability of the glomerular endothelial barrier on a chip. (a) Time-lapse images of the permeable fluorescein tracer IgG crossing the barrier after Cd exposure at different concentrations over time. The cells were treated with Cd for 48 h. Scale bar: 200 μm. (b) Endothelial barrier permeability to IgG over time. (c) Barrier permeability to albumin after 60 min under Cd exposure at different concentrations. Data are presented as mean ± standard deviation. n = 3, **p < 0.01.
4. Discussion
We describe a functional microdevice that mimics the in vivo conditions of glomerular endothelium when exposed to Cd. We characterize the effect of Cd exposure on the cytotoxicity and permeability of the glomerular endothelial barrier. The results indicate kidney-specific barrier functionalities and more in vivo-like nephrotoxic responses to Cd. In vivo, the regulation of endothelial cells for vascular permeability mainly depends on intercellular connections. Many studies have found that Cd has a destructive effect on cell connections.10,36,37 Consistent with these nephrotoxic responses to Cd, cell viability, LDH leakage and GEC layer permeability were greatly altered when the cells were exposed to Cd on a chip. Specifically, our data suggest that disrupted intercellular junctions in the endothelium may injure the GEC layer filtration function.
Previous toxicity studies on Cd have focused on kidney and bone damage, and epidemiological evidence has linked Cd exposure to adverse effects. Some studies have demonstrated that Cd exposure induces significant renal cell apoptosis, indicating impairment of cell function.38,39 These studies have primarily been focused on proximal tubular cells rather than glomerular cells. Our cytotoxicity data provide direct evidence of dose-dependent Cd-induced nephrotoxicity. Primary rat GECs were chosen to construct the glomerular barrier, as primary cells possess original tissue characteristics and functions. In contrast to previous studies,40,41 a lower concentration of Cd at 4 μM was found to affect the viability of GECs. This could have been caused by distinct cell resources.42–44 It is well known that the most extensive accumulation of Cd occurs in the kidney10 and GECs are more sensitive to Cd stimuli. This implies the potential of GECs to be target cells for nephrotoxicity testing during Cd exposure.
Collagen I was used as a natural extracellular matrix to support GEC adhesion and growth. Although the glomerular basement membrane is mainly composed of laminin, nidogens and collagen,45 collagen I is also widely used to culture renal cells and other endothelial cells in vitro.46–48 The primary GECs maintained cell activity and cell–cell contact on the collagen I matrix. Moreover, the endothelial cells formed an integrated barrier on the collagen substrate that was impermeable to large molecules, indicating the feasibility of collagen I in GEC growth and function in the established chip model. Li et al. found that low-dose Cd (1 μM) induced hyperpermeability in human GECs.16 However, we did not find significant changes in barrier permeability or cytotoxicity in GECs after exposure to 2 μM of Cd. This inconsistency may be explained by the different GEC species. In addition, we used a collagen substrate as a natural extracellular matrix to support GEC adhesion and growth on the chip. The 3D matrix could have helped maintain cell layer integrity,49–51 thereby leading to differential GEC layer permeability.
The glomerulus plays a central role in regulating the filtration of molecules across the renal barrier. As a semipermeable membrane, small molecules and water are allowed to pass through the barrier. Conventional kidney systems have limited ability to evaluate glomerular function, making them unsuitable for nephropathy studies on exposure to various environmental toxins. Our kidney-on-a-chip model facilitates the assessment of Cd-induced renal toxicity in a physiologically relevant manner. As the representative function of the barrier in vivo, its permeability to different molecules was characterized and quantified. Cd exposure was found to increase the barrier's permeability to large molecules, such as IgG and albumin. The data support the appearance of pathological changes that present in glomerular diseases in vivo under Cd exposure, such as proteinuria.
Our model enables the direct visualization and quantitative analysis of the biological processes of the glomerular endothelial barrier in ways that have not been achieved in traditional cell culture or animal models. The proposed kidney-on-a-chip model may provide a useful and cost-effective platform for the study of renal pharmacology, nephrotoxicity and glomerular disease under various pathological conditions and environmental exposures.
Conflict of interest
There is no conflict of interest to declare.
Acknowledgments
This research was supported by the National Nature Science Foundation of China (No. 91543121, 81573394, 81273483, 21607151 and 31671038), the International Science & Technology Cooperation Program of China (2015DFA00740), the National Scientific Instrument Development Project (Chinese Academy of Sciences) and the Key Laboratory of Separation Science for Analytical Chemistry (Dalian Institute of Chemical Physics, Chinese Academy of Sciences).
References
- Chargui A., Zekri S., Jacquillet G., Rubera I., Ilie M., Belaid A., Duranton C., Tauc M., Hofman P., Poujeol P., El May M. V., Mograbi B. Toxicol. Sci. 2011;121:31–42. doi: 10.1093/toxsci/kfr031. [DOI] [PubMed] [Google Scholar]
- Jarup L., Akesson A. Toxicol. Appl. Pharmacol. 2009;238:201–208. doi: 10.1016/j.taap.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Tripathi S., Srivastav A. K. Tissue Cell. 2011;43:131–136. doi: 10.1016/j.tice.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Schwartz G. G. Diabetes Care. 2003;26:468–470. doi: 10.2337/diacare.26.2.468. [DOI] [PubMed] [Google Scholar]
- Waalkes M. Mutat. Res., Fundam. Mol. Mech. Mutagen. 2003;533:107–120. doi: 10.1016/j.mrfmmm.2003.07.011. [DOI] [PubMed] [Google Scholar]
- Navas-acien A., Tellez-plaza M., Guallar E., Muntner P., Silbergeld E., Jaar B., Weaver V. Am. J. Epidemiol. 2009;170:1156–1164. doi: 10.1093/aje/kwp248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerberg B., Bergstrom G., Boren J., Barregard L. J. Intern. Med. 2012;272:601–610. doi: 10.1111/j.1365-2796.2012.02578.x. [DOI] [PubMed] [Google Scholar]
- Mates J. M., Segura J. A., Alonso F. J., Marquez J. Free Radical Biol. Med. 2010;49:1328–1341. doi: 10.1016/j.freeradbiomed.2010.07.028. [DOI] [PubMed] [Google Scholar]
- Peters J. L., Perlstein T. S., Perry M. J., Mcneely E., Weuve J. Environ. Res. 2010;110:199–206. doi: 10.1016/j.envres.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Dong F., Xu D., Du L., Yan S., Hu H., Lobe C. G., Yi F., Kapron C. M., Liu J. J. Appl. Toxicol. 2016;36:257–265. doi: 10.1002/jat.3168. [DOI] [PubMed] [Google Scholar]
- Bernhoft R. A. Sci. World J. 2013;2013:394652. doi: 10.1155/2013/394652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A. R., Degheselle O., Smeets K., Van Kerkhove E., Cuypers A. Int. J. Mol. Sci. 2013;14:6116–6143. doi: 10.3390/ijms14036116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba T., Kobayashi E., Suwazono Y., Uetani M., Oishi M., Nakagawa H., Nogawa K. Toxicol. Lett. 2005;159:192–201. doi: 10.1016/j.toxlet.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Baiomy A. A., Mansour A. A. Biol. Trace Elem. Res. 2016;170:320–329. doi: 10.1007/s12011-015-0491-4. [DOI] [PubMed] [Google Scholar]
- Su Y., Peng F., Jiang Z., Zhong Y., Lu Y., Jiang X., Huang Q., Fan C., Lee S. T., He Y. Biomaterials. 2011;32:5855–5862. doi: 10.1016/j.biomaterials.2011.04.063. [DOI] [PubMed] [Google Scholar]
- Ye Y., Li Z., Xing D. Plant, Cell Environ. 2013;36:1–15. doi: 10.1111/j.1365-3040.2012.02543.x. [DOI] [PubMed] [Google Scholar]
- Milek M., Marcinčáková D., Csank T., Kšonžeková P., Falis M., Legáth J., Pistl J. Acta Vet. Brno. 2015;84:351–356. [Google Scholar]
- L'Azou B., Passagne I., Mounicou S., Tréguer-delapierre M., Puljalté I., Szpunar J., Lobinski R., Ohayon-courtès C. Toxicol. Res. 2014;3:32–41. [Google Scholar]
- Gunness P., Aleksa K., Kosuge K., Ito S., Koren G. Can. J. Physiol. Pharmacol. 2010;88:448–455. doi: 10.1139/y10-023. [DOI] [PubMed] [Google Scholar]
- Ryan M. J., Johnson G., Kirk J., Fuerstenberg S. M., Zager R. A., Torok-storb B. Kidney Int. 1994;45:48–57. doi: 10.1038/ki.1994.6. [DOI] [PubMed] [Google Scholar]
- Adler M., Ramm S., Hafner M., Muhlich J. L., Gottwald E. M., Weber E., Jaklic A., Ajay A. K., Svoboda D., Auerbach S., Kelly E. J., Himmelfarb J., Vaidya V. S. J. Am. Soc. Nephrol. 2016;27:1015–1028. doi: 10.1681/ASN.2015010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiong H. Y., Huang P., Xiong S., Li Y., Vathsala A., Zink D. Mol. Pharm. 2014;11:1933–1948. doi: 10.1021/mp400720w. [DOI] [PubMed] [Google Scholar]
- Anil Kumar P., Welsh G. I., Saleem M. A., Menon R. K. Front. Endocrinol. 2014;5:151. doi: 10.3389/fendo.2014.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. J., Valadez A. V., Zuo P., Nie Z. Bioanalysis. 2012;4:1509–1525. doi: 10.4155/bio.12.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang Q., Ren Y., Wu Y., Guo Y., Zhao X., Tao Y., Liu J., Zhao H., Lei L., Jiang H. RSC Adv. 2016;6:27183–27190. [Google Scholar]
- Huang H. C., Chang Y. J., Chen W. C., Harn H. I., Tang M. J., Wu C. C. Tissue Eng., Part A. 2013;19:2024–2034. doi: 10.1089/ten.tea.2012.0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang K. J., Mehr A. P., Hamilton G. A., Mcpartlin L. A., Chung S., Suh K. Y., Ingber D. E. Integr. Biol. 2013;5:1119–1129. doi: 10.1039/c3ib40049b. [DOI] [PubMed] [Google Scholar]
- Choucha Snouber L., Jacques S., Monge M., Legallais C., Leclerc E. Genomics. 2012;100:27–34. doi: 10.1016/j.ygeno.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Wilmer M. J., Ng C. P., Lanz H. L., Vulto P., Suter-dick L., Masereeuw R. Trends Biotechnol. 2016;34:156–170. doi: 10.1016/j.tibtech.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Frohlich E. M., Zhang X., Charest J. L. Integr. Biol. 2012;4:75–83. doi: 10.1039/c1ib00096a. [DOI] [PubMed] [Google Scholar]
- Maschmeyer I., Lorenz A. K., Schimek K., Hasenberg T., Ramme A. P., Hubner J., Lindner M., Drewell C., Bauer S., Thomas A., Sambo N. S., Sonntag F., Lauster R., Marx U. Lab Chip. 2015;15:2688–2699. doi: 10.1039/c5lc00392j. [DOI] [PubMed] [Google Scholar]
- Shankland S. J., Pippin J. W., Reiser J., Mundel P. Kidney Int. 2007;72:26–36. doi: 10.1038/sj.ki.5002291. [DOI] [PubMed] [Google Scholar]
- Jabaji Z., Brinkley G. J., Khalil H. A., Sears C. M., Lei N. Y., Lewis M., Stelzner M., Martin M. G., Dunn J. C. PLoS One. 2014;9:e107814. doi: 10.1371/journal.pone.0107814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross V. L., Zheng Y., Won Choi N., Verbridge S. S., Sutermaster B. A., Bonassar L. J., Fischbach C., Stroock A. D. Biomaterials. 2010;31:8596–8607. doi: 10.1016/j.biomaterials.2010.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ba Q., Li M., Chen P., Huang C., Duan X., Lu L., Li J., Chu R., Xie D., Song H., Wu Y., Ying H., Jia X., Wang H. Environ. Health Perspect. 2017;125:437–446. doi: 10.1289/EHP360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu E. R., Mruk D. D., Porto C. S., Cheng C. Y. Toxicol. Appl. Pharmacol. 2009;238:240–249. doi: 10.1016/j.taap.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leussink B. T., Litvinov S. V., de Heer E., Slikkerveer A., van der Voet G. B., Bruijn J. A., de Wolff F. A. Toxicol. Appl. Pharmacol. 2001;175:54–59. doi: 10.1006/taap.2001.9228. [DOI] [PubMed] [Google Scholar]
- Liu L., Yang B., Cheng Y., Lin H. Biol. Trace Elem. Res. 2015;167:308–319. doi: 10.1007/s12011-015-0314-7. [DOI] [PubMed] [Google Scholar]
- Yuan G., Dai S., Yin Z., Lu H., Jia R., Xu J., Song X., Li L., Shu Y., Zhao X., Chen Z., Fan Q., Liang X., He C., Yin L., Lv C., Lei Q., Wang L., Mi Y., Yu X., Zhang M. Int. J. Clin. Exp. Pathol. 2014;7:2905–2914. [PMC free article] [PubMed] [Google Scholar]
- Dong F., Guo F., Li L., Guo L., Hou Y., Hao E., Yan S., Allen T. D., Liu J. Biochem. Biophys. Res. Commun. 2014;450:447–452. doi: 10.1016/j.bbrc.2014.05.140. [DOI] [PubMed] [Google Scholar]
- Prozialeck W. C., Edwards J. R., Woods J. M. Life Sci. 2006;79:1493–1506. doi: 10.1016/j.lfs.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Wang J., Hao M., Liu C., Liu R. RSC Adv. 2015;5:31798–31806. [Google Scholar]
- Wang L., Wang H., Li J., Chen D., Liu Z. Arch. Environ. Contam. Toxicol. 2011;61:500–511. doi: 10.1007/s00244-011-9644-4. [DOI] [PubMed] [Google Scholar]
- Yuan Y., Jiang C.Y., Hu F.F., Wang Q.W., Zhang K.B., Wang Y., Gu J.H., Liu X.Z., Bian J.C., Liu Z. J. Trace Elem. Med. Biol. 2015;29:275–283. doi: 10.1016/j.jtemb.2014.06.006. [DOI] [PubMed] [Google Scholar]
- Miner J. H. Pediatr. Nephrol. 2011;26:1413–1417. doi: 10.1007/s00467-011-1785-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen C. L., Li Y. J., Wu H. H., Weng C. H., Lee C. C., Chen Y. C., Chang M. Y., Yen T. H., Hsu H. H., Hung C. C., Yang C. W., Tian Y. C. Biomed. J. 2016;39:39–49. doi: 10.1016/j.bj.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs S., Jiang X., Schmidt H., Dohle E., Ghanaati S., Orth C., Hofmann A., Motta A., Migliaresi C., Kirkpatrick C. J. Biomaterials. 2009;30:1329–1338. doi: 10.1016/j.biomaterials.2008.11.028. [DOI] [PubMed] [Google Scholar]
- Allen P., Melero-martin J., Bischoff J. J. Tissue Eng. Regener. Med. 2011;5:e74–e86. doi: 10.1002/term.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason B. N., Starchenko A., Williams R. M., Bonassar L. J., Reinhart-king C. A. Acta Biomater. 2013;9:4635–4644. doi: 10.1016/j.actbio.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. B., Polio S., Lee W., Dai G., Menon L., Carroll R. S., Yoo S. S. Exp. Neurol. 2010;223:645–652. doi: 10.1016/j.expneurol.2010.02.014. [DOI] [PubMed] [Google Scholar]
- Davis G. E., Senger D. R. Circ. Res. 2005;97:1093–1107. doi: 10.1161/01.RES.0000191547.64391.e3. [DOI] [PubMed] [Google Scholar]