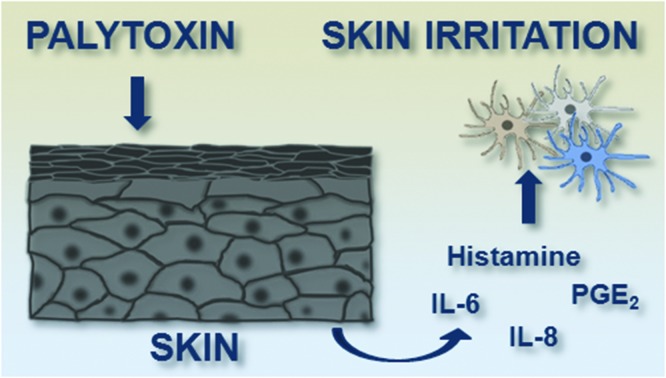
 Keratinocytes are actively involved in the recruitment of inflammatory cells in response to cutaneous contact with palytoxin.
Keratinocytes are actively involved in the recruitment of inflammatory cells in response to cutaneous contact with palytoxin.
Abstract
Palytoxin (PLTX) is one of the most harmful marine toxins known so far. Although the ingestion of contaminated seafood is the most dangerous exposure route for humans, cutaneous and inhalational exposures are far more frequent, and can cause strong inflammatory reactions. However, little is known about the inflammatory events that follow the cutaneous exposure to the toxin. In this study, we investigated (1) the effects of both short (2 h) and long (24 h) term exposures of HaCaT keratinocytes to a sub-cytotoxic PLTX concentration on pro-inflammatory mediator gene expression and release and (2) the effect of PLTX-conditioned HaCaT cell media on undifferentiated (monocytes) and differentiated (macrophages; immature dendritic cells, iDCs; mature dendritic cells, mDCs) THP-1 cells. At 10–11 M, PLTX induced interleukin (IL)-6 and IL-8 release from HaCaT keratinocytes after 24 h of continuous exposure to the toxin, as well as after 23 h in toxin-free medium preceded by 1 h exposure to PLTX. Under the same experimental conditions, release of the inflammatory mediators prostaglandin-E2 and histamine was also found after both short and long exposures to the toxin. The conditioned media collected from HaCaT cells treated with PLTX increased the migration of the differentiated and undifferentiated THP-1 cells (potency rank order: monocytes ≥ iDCs > mDCs > macrophages) but did not induce cell differentiation. These results indicate that keratinocytes can be actively involved in the recruitment of inflammatory cells in response to cutaneous contact with PLTX. The lack of a significant effect on monocyte differentiation towards mature immune cells suggests that PLTX is endowed with irritant rather than sensitizing properties.
Introduction
Palytoxin (PLTX), one of the most toxic natural non-proteinaceous compounds, has been identified in several marine organisms, including Palythoa corals (usually used as home-aquaria decorative elements), Ostreopsis dinoflagellates (frequently blooming in temperate seas in the last years) and Trichodesmium marine cyanobacteria.1–4
Human poisonings ascribed to PLTX exposure are commonly associated with three exposure routes: (i) oral exposure, after ingestion of contaminated seafood; (ii) inhalational exposure to marine aerosol during Ostreopsis blooms or vapours from Palythoa boiling during aquaria cleaning, and (iii) cutaneous exposure, after direct contact with seawater during Ostreopsis blooms or by Palythoa coral handling.4 Despite the fact that oral intake is the most harmful and even lethal exposure route, foodborne poisonings ascribed to PLTXs have been limited to tropical areas, so far, where low number of cases had been confirmed by the direct toxin detection in the leftovers. On the other hand, cutaneous and inhalational exposures to PLTXs cause the major number of poisonings, particularly in temperate areas. Several forms of toxic responses, mainly rhinorrhea, cough, respiratory distress, fever, conjunctivitis and dermatitis, have been reported during Ostreopsis blooms along both Italian and French coasts.4–7 Durando et al. estimated that erythematous dermatitis represented 5% of the recorded reactions in the 228 patients hospitalized during the Ostreopsis blooms in Genoa (Italy) during 2005–2006.6 However, this incidence could be significantly underestimated since dermatitis does not always require hospitalization and the skin lesions could be frequently ascribed to a different aetiology. Furthermore, the “French Mediterranean Coast Ostreopsis Surveillance Network” (operating along the French Mediterranean and Monaco coasts from 2006 to 2009) reported a variability of signs and symptoms in people exposed to Ostreopsis cf. ovata, skin irritation being the most common and the only one even with low Ostreopsis concentrations in seawater.7 In addition, dermatological problems have been recently ascribed to PLTX after handling Palythoa-containing home aquaria.2,5,7–10 The skin lesions suggest that the toxin induces a strong inflammatory reaction, activating pro-inflammatory signaling pathways which involve inflammatory/immune cells.11
Hence, this study was aimed to investigate a series of inflammatory events induced by PLTX at the skin level. For ethical reasons, to avoid the use of animals in compliance with the EU Directive 2010/63/EU based on the Three Rs principle, experiments were performed on human HaCaT keratinocytes as a surrogate of the skin first-line of defense12 and undifferentiated or differentiated THP-1 cells as surrogates of human inflammatory/immunity cells.
Materials and methods
Cell cultures
HaCaT cells were purchased from Cell Line Service (DKFZ, Eppelheim, Germany) and cultured in DMEM supplemented with 10% fetal bovine serum (FBS), 1.0 × 10–2 M l-glutamine, 1.0 × 10–4 g per mL penicillin and 1.0 × 10–4 g per mL streptomycin at 37 °C under a humidified 95% air/5% CO2 atmosphere. Cell passage was performed 2 days post-confluence, once a week.
THP-1 cells were purchased from ATCC (Manassas, USA) and cultured in RPMI-1640 supplemented with 10% fetal bovine serum (FBS), 1.0 × 10–2 M l-glutamine, 1.0 × 10–4 g per mL penicillin and 1.0 × 10–4 g per mL streptomycin at 37 °C under a humidified 95% air/5% CO2 atmosphere. Cell passage was performed once a week. Undifferentiated THP-1 monocytes were differentiated into macrophages by exposure to 10–7 M phorbol-12-myristate-13-acetate (PMA) for 72 h,13,14 into immature dendritic cells (iDCs) by exposure to 100 ng per mL interleukin-4 (IL-4) and a granulocyte-macrophage colony-stimulating factor (GM-CSF) for 120 h,15 and into mature dendritic cells (mDCs) with 200 ng per mL IL-4, 100 ng per mL GM-CSF, 10 ng per mL tumor necrosis factor-α (TNF-α) and 200 ng per mL ionomycin in serum-free medium for 48 h.15
Toxin exposure
PLTX from Wako Pure Chemical Industries Ltd (Osaka, Japan; lot number WKL7151, purity > 90%) was stored at –20 °C at a concentration of 10–5 M in 50% aqueous ethanol (v/v). HaCaT cells were exposed for 1, 2, 4 and 24 h to 1.0 × 10–11 M PLTX diluted in a culture medium (continuous exposure) or exposed for 1 h to 1.0 × 10–11 M PLTX followed by 1, 3 and 23 h culture in toxin-free media (recovery). Conditioned media were obtained as follows: HaCaT cells were exposed for 1 h to 1.0 × 10–11 M PLTX. Thereafter, cell media were removed, the wells were rinsed with fresh medium and the cells were cultured in a toxin-free medium for additional 3 or 23 h (1 h + 3 h and 1 h + 23 h conditioned media, respectively). Control media were collected from HaCaT keratinocytes not exposed to the toxin after 24 h in culture. PLTX sub-cytotoxic concentration (1.0 × 10–11 M) and exposure conditions were chosen on the basis of preliminary cytotoxic assays carried out on HaCaT cells.
Gene expression analysis
HaCaT cells (5 × 105) were seeded for 48 h in a 25 cm2 flask and, after treatment, were collected and washed with ice-cold PBS. Total RNA was extracted by using a High Pure RNA Isolation kit (Roche; Milan, Italy) following manufacturer's instructions and then retrotranscripted by PCR using SuperScriptII 200U (Life Technologies; Milan, Italy). To quantify the mRNA expression of specific genes, SYBR green real time qPCR assay was performed by using the LightCycler technology (Roche; Mannheim, Germany) in a 20 μl PCR mixture volume consisting of 10 μl of 2× Quantitect SYBR Green PCR Master Mix containing HotStarTaq DNA polymerase (Qiagen; Hilden, Germany), 400 nM of each oligonucleotide primer and 100 ng of retrotranscripted total RNA extracted from each sample per reaction. The amplification was performed with initial activation of HotStarTaq DNA polymerase at 95 °C for 15 min and 40 cycles in three steps: 94 °C for 10 s, 60 °C for 15 s, 72 °C for 30 s for all tested genes. Following cycling, to ensure specificity, melting curve analysis was carried out to verify the amplification of PCR products starting at 60 °C and ramping to 95 °C at 0.1 °C per second. The relative quantization was performed by using the specific standard external curves. The normalisation was performed by parallel amplification of β-actin gene expression, and data were calculated as previously described16 considering 1 as the value of gene expression of control sample not exposed to PLTX. The specific oligo pairs to amplify genes encoding pro-inflammatory cytokines (IL-6, IL-8, IL-1α and TNF-α) were chosen basing on the literature data.17–20 All oligonucleotide pairs were from Life Technologies (Milan, Italy).
Pro-inflammatory mediators release
The culture media of HaCaT cells used for gene expression analyses were collected and kept at –80° C. Inflammatory mediator release in cell media was quantified by using commercial ELISA kits according to manufacturers’ instructions, using 50 μl of cell media. In particular, interleukin (IL)-6, -8, -1α and tumor necrosis factor (TNF)-α were quantified by indirect sandwich ELISAs (Bender MedSystems GmbH; Milan, Italy), while prostaglandin (PG)-E2, leukotriene (LT)-B4 and histamine were quantified by competitive ELISAs (Oxford Biomedical Research; Milan, Italy). Absorbance (Abs) was read at 640 nm with an Automated Microplate Reader EL 311S (Bio-Tek Instruments; Winooski, VT).
Chemotaxis
Immune cell migration was evaluated in 24-well plates using Transwell® inserts with 8 μm pores. At the bottom of each well, 800 μl of HaCaT cell conditioned media (control, 1 h + 3 h, 1 h + 23 h) were added. As the positive control, 800 μl per well of THP-1 culture medium supplemented with 1.25 × 10–12 M macrophage inflammatory protein-3α (MIP-3α; Sigma-Aldrich; Milan, Italy) were added. THP-1 cells were differentiated in 25 cm2 flasks in macrophages, iDCs and mDCs as described above, collected and incubated with the fluorescent probe 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiL; Sigma-Aldrich; Milan, Italy) at the concentration of 10–4 M at 37 °C for 20 minutes. After washing, 2 × 105 cells per insert were added and allowed to migrate for 4 h at 37 °C. Fluorescence was read using a Fluorocount Microplate Fluorometer (Packard, Germany) with an excitation λ = 530 nm and an emission λ = 590 nm. After 4 hours, fluorescence was read in the wells (migrated cells) and in the inserts after two washings (migrating cells).
Cell proliferation
Undifferentiated THP-1 cells (3 × 104 cells per well) were seeded in 96-well plates and exposed to HaCaT cell conditioned media (control, 1 h + 3 h, 1 h + 23 h) for increasing time intervals up to 120 h. Twenty-two hours before the end of treatment, [3H]-thymidine (Perkin Elmer; Milan, Italy) was added at a final concentration of 2.5 μCi mL–1. Cells were then collected and filtered on 96-wells MultiScreen HTS FB 1.0/0.6 μm plates (Millipore; Milan, Italy) at a pressure of 500 mmHg. After two washes with PBS, 25 μl of the scintillation liquid (OptiPhase “Super Mix”; Perkin Elmer; Milan, Italy) were added and the sample radioactivity was determined by using a Liquid Scintillation Analyzer (Wallac 1450 Microbeta liquid scintillation counter; Perkin Elmer; Milan, Italy). Raw counts per minute (cpm) data were normalized as % proliferation compared to controls (100% proliferation).
Cell adhesion
Undifferentiated THP-1 cells were fluorometrically marked with the DiL probe as described above and seeded in 24-well plates at a density of 2 × 105 cells per well. Cells were then exposed for increasing time intervals up to 120 h to HaCaT cell conditioned media (control, 1 h + 3 h, 1 h + 23 h). After treatment, fluorescence was read before (total cells) and after two washes with PBS to measure the residual fluorescence (adherent cells) using a Fluorocount Microplate Fluorometer (Packard, Germany) with an excitation λ = 530 nm and an emission λ = 590 nm.
Phenotypic analysis
THP-1 cell differentiation was evaluated by the expression of specific differentiation markers (CD14, CD54, CD86 e HLA-DR). Cells (5 × 105 cells per flask) were seeded in 25 cm2 flasks and exposed to the HaCaT cell conditioned media (control, 1 h + 3 h, 1 h + 23 h) for increasing time intervals up to 120 h. At the end of treatment, cells were collected, washed with PBS and suspended in PBS containing 1% BSA at a final density of 1 × 107 cells per mL in which 5 μl of each of the fluorescein isothiocyanate-conjugated murine IgG antibodies (Life Technologies; Milan, Italy) targeting the considered markers were added. Cells were incubated with antibodies at 4° C for 30 minutes and subsequently fixed with 1 mL of 4% paraformaldehyde for 30 minutes. Cells were then washed with 5 mL of PBS containing 1% BSA (PBS/BSA 1%) and the pellet was suspended in 600 μl of 1% PBS/BSA. The acquisitions were performed by flow cytometry (FACScan; Becton-Dickinson; Milan, Italy) and the data, acquired as listmode files, were analyzed with the FlowJo software.
Statistical analysis
The results are presented as the mean ± SE of at least three independent experiments. Cell proliferation data are presented as % with respect to the untreated control cells (100% proliferation) and were analyzed by two-way ANOVA and Bonferroni post-test (Prism GraphPad Inc.; San Diego, CA) considering significant differences at p < 0.05. Chemotaxis data are presented as % increase with respect to the untreated control cells, using the following formula:
Chemotaxis and pro-inflammatory mediator data were analyzed by one-way ANOVA and Bonferroni post-test (Prism GraphPad Inc.; San Diego, CA) and were considered significant at p < 0.05.
Results
Effects of PLTX on inflammatory cytokine gene expression and release
The effect of PLTX on pro-inflammatory cytokines was evaluated as changes in IL-8, IL-6, IL-1α and TNF-α gene expressions and in the release of the corresponding proteins. HaCaT cells were exposed to 10–11 M PLTX for increasing time intervals (2, 4 and 24 h) or for 1 h followed by 1, 3 and 23 h culture in PLTX-free medium (recovery conditions).
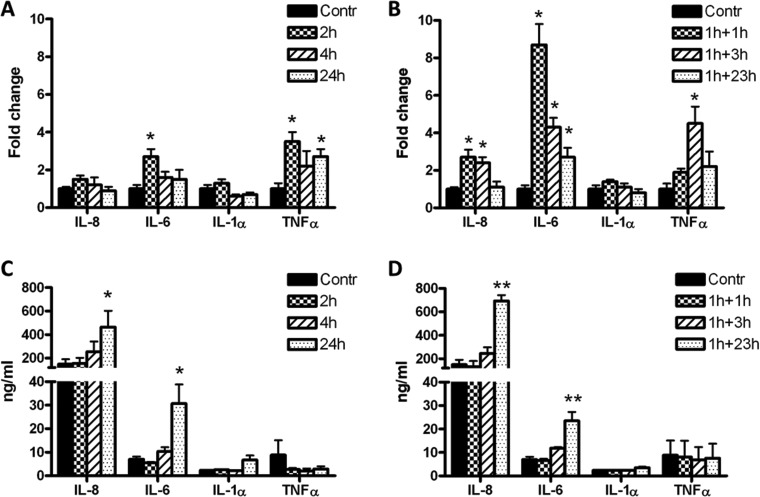
After continuous exposure to the toxin, a significant change in the expression of the genes encoding for IL-6 (2.7-fold increase) and TNF-α (3.5-fold increase) was observed at 2 h, whereas no significant variations were observed for IL-8 and IL-1α mRNA expressions (Fig. 1A). After 24 h exposure, only TNF-α expression was significantly increased (2.7-fold). Under recovery conditions, the expression of genes encoding for IL-6 and IL-8 increased 8.7- and 2.7-fold, respectively, after 2 h (1 h PLTX exposure + 1 h in toxin-free medium), whereas TNF-α gene expression increased 4.5-fold after 4 h (1 h PLTX exposure + 3 h in toxin-free medium; Fig. 1B). Again, no significant changes were found in IL-1α gene expression.
Fig. 1. Effects of PLTX on IL-8, IL-6, IL-1α and TNF-α gene expressions (A and B) and protein release in cell media (C and D). Cells were exposed to 1.0 × 10–11 M PLTX for 2, 4 and 24 h (panels A and C) or to 1 h exposure to 1.0 × 10–11 M PLTX followed by 1, 3 and 23 h culture in toxin-free medium (panels B and D). Gene expression data are expressed as the fold change with respect to the untreated sample specific mRNA considered as 1 (Kim et al. 2001)16 whereas protein release is expressed as ng mL–1 of each analyte released in cell media on the basis of the respective calibration curves and are the mean ± SE of three independent experiments performed in duplicate. Statistical differences: *, p < 0.05; **, p < 0.01 (one-way ANOVA).
The release of cytokines was evaluated under the same experimental conditions. A significant release of IL-6 and IL-8 was observed after 24 h of continuous exposure to PLTX (3.4- and 2.1-fold increase compared to untreated controls, respectively; see Fig. 1C). Similar results were obtained after 1 h exposure to the toxin followed by 23 h culture in toxin-free medium: 3.6- and 2.4-fold increase for IL-8 and IL-6 compared to the untreated controls, respectively (Fig. 1D). The IL-1α and TNF-α levels were unchanged (Fig. 1D).
Effects of PLTX on histamine and eicosanoids release
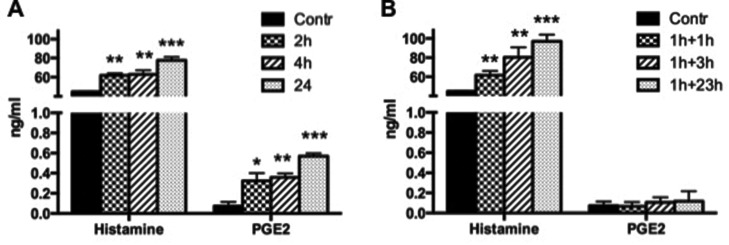
The effect of 1.0 × 10–11 M PLTX on histamine, PGE2, and LTB4 release was then investigated. Continuous exposure of HaCaT cells to PLTX for 2, 4 or 24 h induced a significant time-dependent increase of histamine and PGE2 release (Fig. 2, panel A), whereas the release of LTB4 was not affected (data not shown). Under recovery conditions (1 h exposure to the toxin followed by incubation in toxin-free medium for 1, 3 or 23 h), only histamine release increased time-dependently, with no effects on PGE2 (Fig. 2B) or LTB4 (data not shown).
Fig. 2. Effects of PLTX on histamine and PGE2 release after continuous exposure to 1.0 × 10–11 M PLTX for 2, 4 and 24 h (A) or to 1 h exposure to 1.0 × 10–11 M PLTX followed by 1, 3 and 23 h culture in toxin-free medium (B). Data are presented as ng mL–1 of each analyte released in cell media and are the mean ± SE of three independent experiments performed in duplicate. Statistical differences: *, p < 0.05; **, p < 0.01; ***, p < 0.001 (one-way ANOVA).
Effect of PLTX-conditioned media on the chemotaxis of THP-1 cells
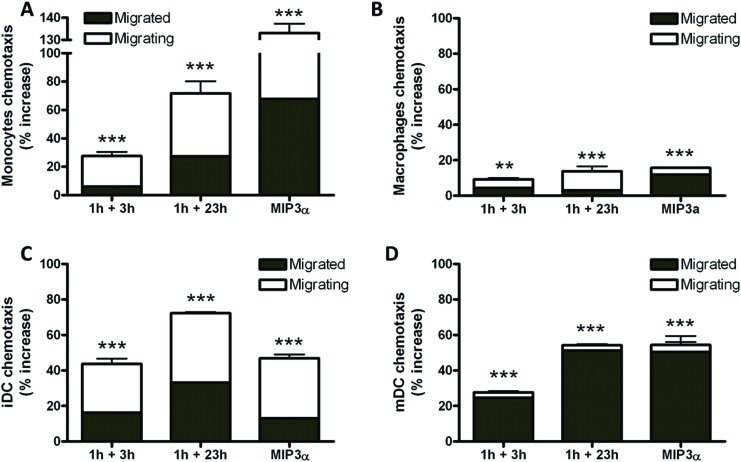
Undifferentiated (monocytes) and differentiated (macrophages, iDCs and mDCs) THP-1 cells were exposed for 4 h to conditioned media collected from HaCaT cells 3 and 23 h after a 1 h-treatment with 1.0 × 10–11 M PLTX. The effect of the treatment was evaluated considering the sum of both migrated and migrating cells. The control cells were exposed to media collected from untreated HaCaT cells.
HaCaT cell conditioned media stimulated the chemotaxis of both undifferentiated and differentiated THP-1 cells. Compared to control cells, the total migration of monocytes increased, according to the time of conditioning (28% and 72% at 3 and 23 h of recovery, respectively; Fig. 3A). The conditioned media increased the migration of macrophages (9% and 14% at 3 and 23 h of recovery, respectively; Fig. 3B), iDCs (44% and 72% at 3 and 23 h of recovery, respectively; Fig. 3C) and mDCs (28% and 54% at 3 and 23 h of recovery, respectively; Fig. 3D). The positive control MIP-3α (1.25 × 10–12 M) induced 16–133% increase of cell migration, the effect depending on the cell model.
Fig. 3. Effects of PLTX-treated HaCaT conditioned media on monocytes (A), macrophages (B), immature dendritic cells, iDC (C) and mature dendritic cells, mDC (D) migration. Cells were exposed for 4 h to HaCaT conditioned media (1 h exposure to 1.0 × 10–11 M PLTX followed by 3 and 23 h culture in toxin-free medium) and chemotaxis evaluated fluorometrically. MIP-3α (1.25 × 10–12 M) was used as the positive control. Data are presented as % increase of chemotaxis of migrated and migrating cells with respect to the untreated controls and are the mean ± SE of 3 independent experiments performed in duplicate. Statistical differences vs. untreated controls: *, p < 0.05; **, p < 0.01; ***, p < 0.001 (one-way ANOVA and Bonferroni post-test).
Effect of PLTX-conditioned media on THP-1 cell differentiation
Differentiation of THP-1 cells (monocytes) was evaluated after exposure to conditioned media obtained as described above by means of cell proliferation, cell adhesion and expression of specific surface differentiation markers.
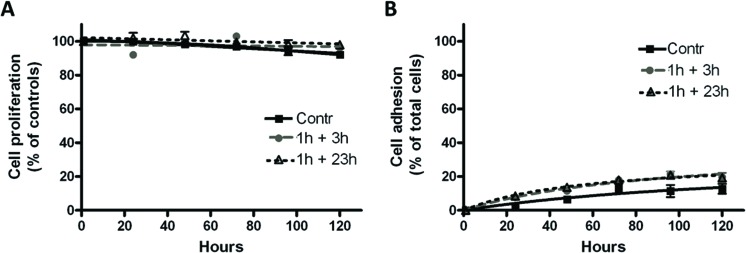
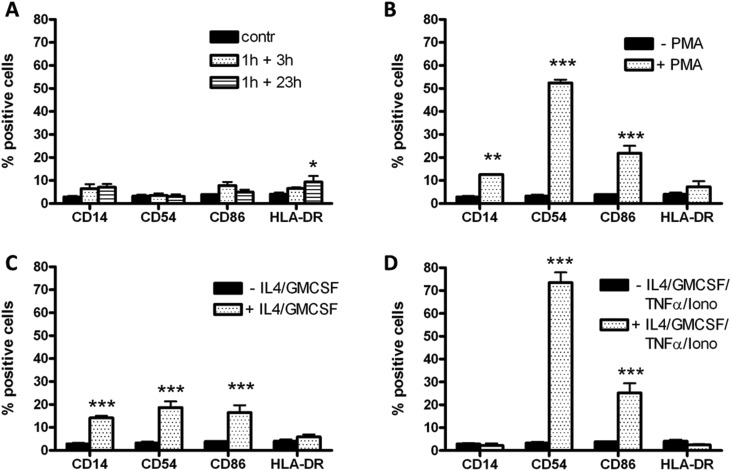
As shown in Fig. 4, the conditioned media did not significantly affect cell proliferation (panel A) or cell adhesion (panel B) up to 120 h of exposure. Similarly, the expression of the differentiation markers CD14, CD54, CD86 and HLA-DR was not changed, but with only a slight but significant increase in the expression of HLA-DR after exposure to the 1 h + 23 h-conditioned medium (Fig. 5, panel D). Positive controls are also presented in Fig. 5, showing the changes in the expression of differentiation markers after THP-1 cell differentiation into macrophages by PMA (Fig. 5A), iDCs by IL-4/GM-CSF (Fig. 5B) and mDCs by IL-4/GM-CSF/TNF-α/ionomycin (Fig. 5C).
Fig. 4. Effects of PLTX-treated HaCaT conditioned media on monocyte proliferation (A) and adhesion (B). Cells were exposed for increasing time intervals up to 120 h to HaCaT conditioned media (1 h exposure to 1.0 × 10–11 M PLTX followed by 3 and 23 h culture in toxin-free medium). The results are presented as % of proliferation with respect to untreated controls (100% proliferation, panel A) or % of adherent cells with respect to total cells (panel B) and are the mean ± SE of 6 and 3 experiments performed in triplicate, respectively.
Fig. 5. Effects of PLTX-treated HaCaT conditioned media on monocyte differentiation. (A) Cells were exposed for 120 h to HaCaT conditioned media (1 h exposure to 1.0 × 10–11 M PLTX followed by 3 and 23 h culture in toxin-free medium) and the levels of the following differentiation markers CD14, CD54, CD86 and HLA-DR evaluated by flow cytofluorometry. Panel B, C and D: positive controls. Monocytes were differentiated into (B) macrophages (10–7 M PMA), (C) iDCs (100 ng per mL IL-4 and GM-CSF) and (D) mDCs (200 ng per mL IL-4, 100 ng per mL GM-CSF, 10 ng per mL TNF-α and 200 ng per mL ionomycin). The results are expressed as % of positive cells to the markers considered and are the average of 4 experiments performed in duplicate (black bars: undifferentiated monocytes; dotted bars: differentiated cells). Statistical differences vs. undifferentiated monocytes: *, p < 0.05; **, p < 0.01; ***, p < 0.001 (one-way ANOVA and Bonferroni post-test).
Discussion
In recent decades, PLTX-producing Ostreopsis dinoflagellates have begun to populate temperate areas including the Mediterranean Sea.21 This broad distribution and the abundant algal proliferations have facilitated human exposure to PLTXs through different routes (oral, cutaneous and inhalational exposures) with adverse effects of varying severity.1,6,22–24 An increasing risk of human health concerns PLTX skin exposure, usually associated with both recreational and working activities during Ostreopsis blooms as well as with direct contact with Palythoa and Zoanthus corals, commonly used as decorative elements in home aquaria. Dermatitis ascribed to PLTX has been reported since the first toxin isolation from Palythoa corals,25 characterized by local irritation, erythema and oedema4 sometimes associated with systemic symptoms tentatively ascribed to toxin absorption.4,8,9 Considering its non-proteic nature and its relatively high molecular weight, the most probable skin reaction induced by PLTX could be irritant contact dermatitis, even though sensitizing reactions cannot be excluded.
In both irritant and sensitizing reactions, keratinocytes play a crucial role as initiators, modulators and amplifiers of cutaneous inflammation, due to their strategic positioning at the interface between the body and the environment.26 After their activation by pro-inflammatory stimuli, these epidermal cells generate mainly reactive oxygen species (ROS) and cytokines, which in turn trigger signalling pathways leading to vasodilation, increased vascular permeability and infiltration of immune/inflammatory cells into the skin.27 We have previously demonstrated that PLTX is a potent ROS inducer in HaCaT keratinocytes in vitro,28 inducing also a sustained mitochondrial dysfunction, superoxide anion production and necrotic cell death.29–31 In the present study we aimed to elucidate the sequence of selected inflammatory events that follow the contact of keratinocytes with PLTX at a concentration (1.0 × 10–11 M) that allowed the maintenance of cell viability.
We initially evaluated the induction and release of selected inflammatory mediators by HaCaT cells exposed to PLTX under two different conditions: continuous exposure for 2, 4 and 24 h or 1 h exposure of priming with the toxin, followed by a recovery period of 1, 3 and 23 h in toxin-free medium. Under both conditions, the toxin induced an early (2 h) increase in the expression of the genes encoding for IL-6, IL-8 and TNF-α, in agreement with recent evidence on the ability of PLTX-containing Ostreopsis cf. ovata extracts to increase the levels of TNF-α and IL-8 transcripts in human macrophages.11 The early increase in gene expression was associated with a significant release of IL-6 and IL-8 after 24 h, with no changes in IL-1α and TNF-α release, notwithstanding the increased transcription of TNF-α mRNA. Upregulation of TNF-α gene expression not followed by the release of the corresponding protein has been previously described in human macrophages and tentatively attributed to the translational inhibitory activity of PLTX.11
In HaCaT cells, PLTX induced the release of the inflammatory mediators PGE2 and histamine. The release started early, being significant after 2 h exposure, and in line with previous findings on the toxin ability to stimulate arachidonic acid metabolism in rat peritoneal macrophages32 and in mouse epidermal cells.33 Also histamine release started early and, in contrast to the PGE2 one, was maintained after PLTX removal, under recovery conditions. On the whole, these results suggest that not only mast cells34 but also keratinocytes are an important source of histamine that could represent a pivotal inflammatory mediator in PLTX-induced dermatitis. This conclusion is in line with previous findings demonstrating not only the presence of key enzymes (i.e. histidine decarboxylase) for histamine production in skin keratinocytes,35 but also their ability to actively produce histamine under particular pathophysiological conditions.35,36
Other marine toxins, such as tetrodotoxin, ciguatoxin 1B and okadaic acid, are able to exert pro-inflammatory effects that imply cytokine release or differentiation stimuli towards inflammatory cells. For instance, inhibition of the voltage-gated Na+-channel Nav1.7 by tetrodotoxin results in the activation of dendritic cells towards a sensitizing response.37 On the other hand, ciguatoxin 1B was shown to induce the release of cytokines from murine macrophages.38 Similarly, the protein phosphatase inhibitor okadaic acid stimulates the release of pro-inflammatory cytokines and chemokines from inflammatory cells in a MAP kinase-dependent manner39 through NF-κB activation.40–42 In this frame, it has been demonstrated that PLTX is able to activate the MAPK cascade, as a consequence of the initial overload of Na+ induced after its interaction with the Na+/K+-ATPase.43 Hence, even though PLTX and okadaic acid display very different mechanisms of action and different molecular targets, they apparently stimulate similar intracellular signal cascades that end up with the regulation of inflammatory events. Further studies are required to confirm this hypothesis.
To further characterize PLTX-induced inflammatory events, we investigated the possibility that the HaCaT cell conditioned media found to contain pro-inflammatory mediators endowed with chemotactic activity, such as IL-6,44 IL-8 45 and histamine,46 could induce migration of inflammatory/immunity cells. These cells include monocytes that, once recruited from the hematic torrent into the inflammation site, are able to differentiate into macrophages and dendritic cells (iDCs). In turn, once activated, the latter can mature into inflammatory dendritic cells (mDCs) that sustain the immune response at the site of inflammation. Four hours exposure of these inflammatory cells to keratinocytes conditioned media significantly increased the migration of undifferentiated and differentiated THP-1 cells, with the following order of potency: monocytes ≥ iDCs > mDCs > macrophages. It is thus possible to speculate that the release of IL-6, IL-8 and histamine from keratinocytes may induce migration of immune/inflammatory cells nearby the site of contact with the toxin. In particular, recruitment of monocyte-derived cells could play an important role in the modulation of skin inflammation induced by PLTX.
Finally, we investigated the effect of conditioned media from PLTX-treated HaCaT cells on monocyte differentiation, assessed as cell proliferation, cell adhesion and phenotypic analysis. Cell proliferation and adhesion did not provide significant evidence for THP-1 monocyte differentiation up to 120 h of treatment, with only a moderate (<20%) increase in cell adhesion. This result is consistent with the lack of expression of adhesion molecules, such as CD54, characteristic of cell differentiation into macrophages or mDC,47 as evidenced by the phenotypic analysis. However, from the same analysis, a slight but significant increase in HLA-DR expression was found in the cells exposed to 1 h + 23 h conditioned medium, which could result from a paracrine effect of pro-inflammatory cytokines released by keratinocytes rather than from a real antigen presentation.
Conclusions
In conclusion, this study evidenced that PLTX stimulates the release of pro-inflammatory mediators from skin HaCaT keratinocytes at a sub-cytotoxic concentration (10–11 M), representative of an expected human exposure. In fact, although a direct quantitation of PLTX during Ostreopsis blooms has never been made, its release from algal cells was indirectly estimated in cultures of O. cf. ovata. Guerrini and co-workers estimated a PLTX release in the culture medium of 6.13 × 10–6 g L–1, a concentration of about two orders of magnitude higher than that used in this study (10–11 M, equal to 2.7 × 10–8 g L–1).48 The ability of the conditioned media obtained from PLTX-treated HaCaT cells to evoke a significant migratory response in both undifferentiated (monocytes) and differentiated dendritic cells (iDCs and mDCs) suggests that keratinocytes, through the release of pro-inflammatory mediators, play an important role in mediating the chemotaxis of immune/inflammatory cells in response to PLTX exposure. Considering the lack of monocyte differentiation into dendritic cells, skin exposure to PLTX seems to induce an irritant contact dermatitis rather than a sensitizing reaction. Further studies are needed to confirm this conclusion and to define the actual role of differentiated iDCs and mDCs once recruited to the site of inflammation.
Abbreviations
- DiL
1,1′-Dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate
- GM-CSF
Granulocyte-macrophage colony-stimulating factor
- iDC
Immature dendritic cells
- mDC
Mature dendritic cells
- MIP3α
Macrophage inflammatory protein-3α
- PLTX
Palytoxin
- ROS
Reactive oxygen species
Acknowledgments
This work was supported by a grant of the University of Trieste (Università degli Studi di Trieste – Finanziamento di Ateneo per progetti di ricerca scientifica – FRA2012).
References
- Ciminiello P., Dell'Aversano C., Fattorusso E., Forino M., Tartaglione L., Grillo C., Melchiorre N. J. Am. Soc. Mass Spectrom. 2008;19:111–120. doi: 10.1016/j.jasms.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Pelin M., Brovedani V., Sosa S., Tubaro A. Mar. Drugs. 2016;14:pii:E33. doi: 10.3390/md14020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbrat A. S., Amzil Z., Pawlowiez R., Golubic S., Sibat M., Darius H. T., Chinain M., Laurent D. Mar. Drugs. 2011;9:543–560. doi: 10.3390/md9040543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubaro A., Durando P., Del Favero G., Ansaldi F., Icardi G., Deeds J. R., Sosa S. Toxicon. 2011;57:478–495. doi: 10.1016/j.toxicon.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Deeds J. D., Schwartz M. Toxicon. 2010;56:150–162. doi: 10.1016/j.toxicon.2009.05.035. [DOI] [PubMed] [Google Scholar]
- Durando P., Ansaldi F., Oreste P., Moscatelli P., Marensi L., Grillo C., Gasparini R., Icardi G. Euro Surveill. 2007;12:3212. doi: 10.2807/esw.12.23.03212-en. [DOI] [PubMed] [Google Scholar]
- Tichadou L., Glaizal M., Armengaud A., Grossel H., Lemée R., Kantin R., Lasalle J. L., Drouet G., Rambaud L., Malfait P., De Haro L. Clin. Toxicol. 2010;48:839–844. doi: 10.3109/15563650.2010.513687. [DOI] [PubMed] [Google Scholar]
- Hoffmann K., Hermanns-Clausen M., Buhl C., Buchler M. W., Schemmer P., Mebs D., Kauferstein S. Toxicon. 2008;51:1535–1537. doi: 10.1016/j.toxicon.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Nordt S. P., Wu J., Zahller S., Clark R. F., Cantrell F. L. J. Emerg. Med. 2009;40:397–399. doi: 10.1016/j.jemermed.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Wieringa A., Bertholee D., Ter Horst P., van den Brand I., Haringman J., Ciminiello P. Clin. Toxicol. 2014;52:150–151. doi: 10.3109/15563650.2013.878867. [DOI] [PubMed] [Google Scholar]
- Crinelli R., Carloni E., Giacomini E., Penna A., Dominici S., Battocchi C., Ciminiello P., Dell'Aversano C., Fattorusso E., Forino M., Tartaglione L., Magnani M. PLoS One. 2012;7:e38139. doi: 10.1371/journal.pone.0038139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs S. Skin Pharmacol. Physiol. 2009;22:103–113. doi: 10.1159/000178869. [DOI] [PubMed] [Google Scholar]
- Auwerx J. Experientia. 1991;47:22–31. doi: 10.1007/BF02041244. [DOI] [PubMed] [Google Scholar]
- Gutowska I., Baranowska-Bosiacka I., Baśkiewicz M., Milo B., Siennicka A., Marchlewicz M., Wiszniewska B., Machalinśki B., Stachowska E. Toxicol. Lett. 2010;196:74–79. doi: 10.1016/j.toxlet.2010.03.1167. [DOI] [PubMed] [Google Scholar]
- Berges C., Naujokat C., Tinapp S., Wieczorek H., Höh A., Sadeghi M., Opelz G., Volker D. Biochem. Biophys. Res. Commun. 2005;333:896–907. doi: 10.1016/j.bbrc.2005.05.171. [DOI] [PubMed] [Google Scholar]
- Kim D. W. Exp. Mol. Med. 2001;33:101–109. [PubMed] [Google Scholar]
- Lee W. J., Wu C. S., Lin H., Lee I. T., Wu C. M., Tseng J. J., Chou M. M., Sheu W. H. Int. J. Obes. 2009;33:465–472. doi: 10.1038/ijo.2009.24. [DOI] [PubMed] [Google Scholar]
- Gibellini D., De Crignis E., Ponti C., Cimatti L., Borderi M., Tschon M., Giardino R., Re M. C. J. Med. Virol. 2008;80:1507–1514. doi: 10.1002/jmv.21266. [DOI] [PubMed] [Google Scholar]
- Törmä H., Geijer S., Gester T., Alpholm K., Berne B., Lindberg M. Toxicol. In Vitro. 2006;20:472–479. doi: 10.1016/j.tiv.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Wolf K., Schulz C., Riegger G. A., Pfeifer M. Eur. Respir. J. 2002;20:369–375. doi: 10.1183/09031936.02.00303602. [DOI] [PubMed] [Google Scholar]
- Katikou P., Palytoxin and analogues: ecobiology and origin, chemistry, metabolism, and chemical analysis, in Seafood and Freshwater Toxins: Pharmacology, Physiology and Detection, ed. L. M. Botana, CRC Press, Boca Raton, 2nd edn, 2008, pp. 631–663. [Google Scholar]
- Sansoni G., Borghini B., Camici G., Casotti M., Righini P., Rustighi C. Biol. Ambient. 2003;17:17–23. [Google Scholar]
- Gallitelli M., Ungaro N., Addante L. M., Procacci V., Gentiloni N., Sabbà C. J. Am. Med. Assoc. 2005;293:2599–2600. doi: 10.1001/jama.293.21.2599-c. [DOI] [PubMed] [Google Scholar]
- Ciminiello P., Dell'Aversano C., Fattorusso E., Forino M., Magno G. S., Tartaglione L., Grillo C., Melchiorre N. Anal. Chem. 2006;78:6153–6159. doi: 10.1021/ac060250j. [DOI] [PubMed] [Google Scholar]
- Moore R. E., Helfrich P., Patterson G. M. L. Oceanus. 1982;25:54–63. [Google Scholar]
- Monteiro-Riviere N. A., Toxicology of the skin, Informa Healthcare, New York, 2010. [Google Scholar]
- Ansel J., Perry P., Brown J., Damm D., Phan T., Hart C., Luger T., Hefeneider S. J. Invest. Dermatol. 1990;94:101S–107S. doi: 10.1111/1523-1747.ep12876053. [DOI] [PubMed] [Google Scholar]
- Pelin M., Ponti C., Sosa S., Gibellini D., Florio C., Tubaro A. Toxicol. Appl. Pharmacol. 2013;266:1–8. doi: 10.1016/j.taap.2012.10.023. [DOI] [PubMed] [Google Scholar]
- Pelin M., Zanette C., De Bortoli M., Sosa S., Della Loggia R., Tubaro A., Florio C. Toxicology. 2011;282:30–38. doi: 10.1016/j.tox.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Pelin M., Boscolo S., Poli M., Sosa S., Tubaro A., Florio C. Mar. Drugs. 2013;11:584–598. doi: 10.3390/md11030584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelin M., Sosa S., Pacor S., Tubaro A., Florio C. Toxicol. Lett. 2014;229:440–450. doi: 10.1016/j.toxlet.2014.07.022. [DOI] [PubMed] [Google Scholar]
- Ohuchi K., Watanabe M., Yoshizawa K., Tsurufuji S., Fujiki H., Suganuma M., Sugimura T., Levine L. Biochim. Biophys. Acta. 1985;834:42–47. doi: 10.1016/0005-2760(85)90174-2. [DOI] [PubMed] [Google Scholar]
- Aizu E., Yamamoto S., Nakadate T., Kato R. Eur. J. Pharmacol. 1990;182:19–28. doi: 10.1016/0014-2999(90)90489-s. [DOI] [PubMed] [Google Scholar]
- Chhatwal G. S., Ahnert-Hilger G., Beress L., Habermann E. Int. Arch. Allergy Appl. Immunol. 1982;68:97–100. doi: 10.1159/000233075. [DOI] [PubMed] [Google Scholar]
- Inami Y., Andoh T., Sasaki A., Kuraishi Y. J. Pharmacol. Exp. Ther. 2013;344:459–466. doi: 10.1124/jpet.112.200063. [DOI] [PubMed] [Google Scholar]
- Gutowska-Owsiak D., Greenwald L., Watson C., Selvakumar T. A., Wang X., Ogg G. S. Br. J. Dermatol. 2014;171:771–778. doi: 10.1111/bjd.13199. [DOI] [PubMed] [Google Scholar]
- Kis-Toth K., Hajdu P., Bacskai I., Szilagyi O., Papp F., Szanto A., Posta E., Gogolak P., Panyi G., Rajnavolgyi E. J. Immunol. 2011;187:1273–1280. doi: 10.4049/jimmunol.1003345. [DOI] [PubMed] [Google Scholar]
- Matsui M., Kumar-Roine S., Darius H. T., Chinain M., Laurent D., Pauillac S. Toxicon. 2010;56:776–784. doi: 10.1016/j.toxicon.2009.05.039. [DOI] [PubMed] [Google Scholar]
- Chang J., Voorhees T. J., Liu Y., Zhao Y., Chang C. H. Proc. Natl. Acad. Sci. U. S. A. 2010;107:8340–8345. doi: 10.1073/pnas.0914703107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda Y., Kasahara T., Yamaguchi Y., Kuno K., Matsushima K., Mukaida N. J. Biol. Chem. 1997;272:15366–15372. doi: 10.1074/jbc.272.24.15366. [DOI] [PubMed] [Google Scholar]
- Tebo J. M., Hamilton T. A. Cell. Immunol. 1994;153:479–491. doi: 10.1006/cimm.1994.1044. [DOI] [PubMed] [Google Scholar]
- Feng G., Ohmori Y., Chang P. L. Carcinogenesis. 2006;27:43–52. doi: 10.1093/carcin/bgi174. [DOI] [PubMed] [Google Scholar]
- Wattenberg E. V. Toxicon. 2011;57:440–448. doi: 10.1016/j.toxicon.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clahsen T., Schaper F. J. Leukocyte Biol. 2008;84:1521–1529. doi: 10.1189/jlb.0308178. [DOI] [PubMed] [Google Scholar]
- Gouwy M., Struyf S., Noppen S., Schutyser E., Springael J. Y., Parmentier M., Proost P., Van Damme J. Mol. Pharmacol. 2008;74:485–495. doi: 10.1124/mol.108.045146. [DOI] [PubMed] [Google Scholar]
- Damaj B. B., Becerra C. B., Esber H. J., Wen Y., Maghazachi A. A. J. Immunol. 2007;179:7907–7915. doi: 10.4049/jimmunol.179.11.7907. [DOI] [PubMed] [Google Scholar]
- Hollemweguer E. J., Koppelman B., Dong J. Hotlines. 2001;6:14–21. [Google Scholar]
- Guerrini F., Pezzolesi L., Feller A., Riccardi M., Ciminiello P., Dell'Aversano C., Tartaglione L., Dello Iacovo E., Fattorusso E., Forino M., Pistocchi R. Toxicon. 2010;55:211–220. doi: 10.1016/j.toxicon.2009.07.019. [DOI] [PubMed] [Google Scholar]