 The herbicide paraquate (1,1′-dimethyl-4,4′-bipyridinium dichloride) induces an inflammatory response in human macrophages which cannot be attenuated by cortisol.
The herbicide paraquate (1,1′-dimethyl-4,4′-bipyridinium dichloride) induces an inflammatory response in human macrophages which cannot be attenuated by cortisol.
Abstract
The herbicide paraquat (1,1′-dimethyl-4,4′-bipyridinium dichloride) has been banned in Europe since 2007 due to its high toxicity in humans. However, it is still widely used in Middle/South America and in Asia where it is annually associated with a high incidence of unintentional and intentional poisoning. Human macrophage-like cell lines were used to shed more light on the inflammatory response elicited by paraquat. Paraquat (3–1000 μM) reduced cell viability in a dose- and time-dependent manner. Exposure to 50 or 200 μM paraquat for 24 h elevated the release of interleukin 8 and gene expression of tumor necrosis factor-α. Expression of the 11β-hydroxysteroid dehydrogenase 1 gene tended to increase, while cellular glutathione concentrations decreased. The anti-inflammatory effect of cortisol was significantly disrupted. The paraquat-induced cortisol resistance could not be prevented by N-acetyl-l-cysteine. However, a polyphenolic extract of grape seeds consisting of monomeric and oligomeric flavan-3-ols (MOF) reduced paraquat-induced inflammation in the presence of cortisol to baseline. In conclusion, the results suggest that an impaired cortisol response may contribute to paraquat-mediated inflammation. Agents with pleiotropic cellular and subcellular effects on redox regulation and inflammation, such as plant-derived polyphenols, may be an effective add-on to the therapy of paraquat intoxications with glucocorticoids.
1. Introduction
Since 2007 the herbicide paraquat (1,1′-dimethyl-4,4′-bipyridinium dichloride) has been banned in the European Union. Only licensed technicians are allowed to use it in the United States of America, and since 2012 many of its formulations are being phased out in China. It is, however, still widely used in other parts of the world. Brazilian farmers emerge as a farming superpower worldwide and represent the biggest market for pesticides and herbicides such as paraquat.1 Between 2007 and 2013 the numbers of recorded pesticide intoxications doubled. In 2013, the Brazilian health ministry reported around 4500 human intoxications of which 206 cases ended fatally.1 Next to the unintentional poisoning of farm workers, approximately one third of all suicides are committed with pesticides which accounts for more than 300 000 annual deaths world-wide.2 Intentional self-poisoning with paraquat may vary among regions,2 but its high case fatality fairly exceeding 50%3,4 makes paraquat responsible for thousands of deaths around the globe per year. Moreover, it is likely that the estimated number of unreported cases in less developed countries is large.
Effective treatment of paraquat poisoning is lacking although nowadays paraquat is known as a classical example of a pulmonary redox-active toxicant. After being taken up by cells, e.g. pneumocytes, paraquat (as a bipyridyl di-cation) easily accepts an electron (generated from reducing enzyme systems such as NADH-ubiquinone oxidoreductase, NADPH-cytochrome P450 reductases, xanthine oxidase, nitric oxide synthase etc.) thereby becoming reduced to the monocation paraquat radical.5–7 This paraquat radical shuttles the electron to oxygen which generates superoxide anion radicals and regenerates the original paraquat di-cation. Next to this redox cycling mechanism, a large part of reactive oxygen species (ROS) produced by paraquat was shown to be a consequence of its interaction with the mitochondrial respiration, in particular an inhibition of the electron transport chain and uncoupling of oxidative phosphorylation.8,9 Finally, it has become evident that paraquat also activates NADPH oxidase which results in the production of cytotoxic ROS.10,11 The generation of oxidative stress in cells is not only responsible for the herbicide action of paraquat but also for its lung toxicity.12 Cellular oxidative stress activates the inflammatory transcription factor nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) leading to the transcription of inflammatory mediators like cytokines, chemokines and inflammatory enzymes.13 This explains why the toxicity is characterized by the manifestation of inflammation which eventually leads to irreversible fibrotic organ damage. Surprisingly, even a powerful class of anti-inflammatory drugs i.e. glucocorticoids is hardly efficient in managing paraquat poisoning.14 Such a state of glucocorticoid resistance is also known from other degenerative diseases associated with chronic inflammation.15–18 Cellular oxidative stress has been suggested as one of the mechanisms leading to the diminished anti-inflammatory effect of glucocorticoids.19 Therefore, we hypothesized that the redox-cycling compound paraquat may impair the anti-inflammatory glucocorticoid response in innate immune cells. Since alveolar macrophages play a critical role in the onset and propagation of pulmonary inflammation and are prone to become insensitive to glucocorticoids,19 we used human monocytes which were differentiated into macrophage-like cells. To investigate how far antioxidant compounds can ameliorate paraquat-induced cortisol resistance we included in our experiments N-acetylcysteine (NAC) and a well-standardized and analytically characterized blend of grape-seed derived monomeric and oligomeric flavanols (MOF). NAC has been recently proposed as an experimental therapeutic agent for paraquat intoxication.14 The grape-seed derived MOF have been shown to exert significant redox modulating and anti-inflammatory effects both, in in vitro and in humans20,21 and are available as a registered drug (Endotélon®) in France and as a food supplement in other countries of the world.
Our data propose that a state of glucocorticoid resistance in cells of the innate immune system may contribute to the fulminant pulmonary inflammation seen in paraquat intoxicated patients.22 This knowledge may have practical implications for the development of treatment strategies that enable to combat paraquat intoxications more effectively in the future than it can be currently achieved.
2. Methods
2.1. Chemicals
Cortisol, paraquat, phorbol 12-myristate 13-acetate (PMA) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma (St Louis, MO, USA). N-Acetyl-l-cysteine (NAC) was purchased from Merck (Darmstadt, Germany). The standardized and analytically well-characterized MOF (Masquelier's® Original OPCs) were derived from the seeds of grapes (Vitis vinifera L.) and provided by International Nutrition Company (INC), Loosdrecht, The Netherlands. The flavanolic composition is given in Table 1.
Table 1. Mean quantities ± standard deviation (SD) of grape seed-derived monomeric and oligomeric flavan-3-ols (MOF).
| Compound | Mean quantity ± SD (% (w/w)) |
| Total monomers | 25.6 ± 2.2 |
| (+)-Catechin | 10.9 ± 1.6 |
| (–)-Epicatechin | 12.2 ± 1.6 |
| (–)-Epicatechin-3-O-gallate | 2.5 ± 1.6 |
| Total dimers | 27.5 ± 1.6 |
| Procyanidin B1 | 7.7 ± 1.6 |
| (–)-Epicatechin-(4β → 8)-(+)-catechin | |
| Procyanidin B2 | 8.3 ± 1.6 |
| (–)-Epicatechin-(4β → 8)-(–)-epicatechin | |
| Procyanidin B3 | 2.8 ± 1.6 |
| (+)-Catechin-(4α → 8)-(+)-catechin | |
| Procyanidin B4 | 1.6 ± 1.6 |
| (+)-Catechin-(4α → 8)-(–)-epicatechin | |
| Procyanidin B2-gallate | 7.1 ± 1.6 |
| Total tri-, tetra- and pentameric proanthocyanidins | 46.9 ± 1.6 |
Roswell Park Memorial Institute 1640 (RPMI 1640) medium, fetal calf serum (FCS) and phosphate buffered saline (PBS) were obtained from Gibco (Life Technologies, Carlsbad, CA, USA).
2.2. Cell culture and exposure conditions
Human monocytes (U937 cell line, ATCC CRL-1593, LGC Standards GmbH, Wesel, Germany) were cultured in RPMI 1640 cell culture medium supplemented with 10% (v/v) FCS under a humidified atmosphere with 5% CO2 at 37 °C. Before exposures, cells were differentiated to macrophage-like cells using 50 ng ml–1 PMA for 4 h.23 4 × 105 cells per well were seeded in 24-well plates and 1 × 106 cells per well in 12-well plates, respectively and allowed to differentiate for 48 h. Prior to treatment, serum concentration on the cells was reduced to 1% (v/v) FCS overnight. Subsequently, the cells were washed with PBS and exposed to either cell culture medium (control cells) or different concentrations of paraquat (3–1000 μM) in the presence and absence of 5 mM N-acetyl-l-cysteine (NAC) at 37 °C and 5% CO2 for 24 h. The cells were harvested to determine cell viability, gene expression and cellular glutathione (GSH) concentrations. Moreover, after removing the cell supernatant, the cells were washed with PBS and incubated with the medium (control) or 100 mM cortisol. After 18 h incubation at 37 °C and 5% CO2 interleukin (IL)-8 concentrations were measured in the cell culture supernatant.
In order to corroborate our findings in a second human monocytic cell line, THP-1 cells (ATCC TIB-202) were cultured in RPMI 1640 medium supplemented with 10% (v/v) FCS, 1 mM sodium pyruvate, 0.225% glucose and 50 nM 2-mercaptoethanol under a humidified atmosphere of 5% CO2 at 37 °C. Before exposures, 2 × 105 cells per well seeded in 48-well plates were differentiated into macrophage-like cells using 200 nM PMA for 72 h. All exposures were performed in RPMI 1640 medium without any supplementation. Previous research in a human macrophage reporter gene assay revealed that 5 μg ml–1 MOF significantly inhibits NF-κB-mediated gene transcription.20 Moreover, oral supplementation of healthy volunteers with MOF (200 mg d–1 for 8 weeks) exerted redox modulatory effects in human blood cells.21 To investigate whether MOF are able to mitigate paraquat-induced cortisol resistance, macrophage-like cells were pre-exposed to either the medium (control cells) or 5 μg ml–1 MOF for 30 min. Subsequently, the cells were exposed to either the medium (control cells) or paraquat (200 μM) in the presence and absence of 5 μg ml–1 MOF at 37 °C and 5% CO2 for 4 h. Furthermore, after removing the cell supernatant, the cells were washed with PBS and incubated with the medium (control) or 100 nM cortisol. After 18 h incubation at 37 °C and 5% CO2 interleukin (IL)-8 concentrations were measured in the cell culture supernatant. All experiments were performed in duplicate or triplicate and repeated at least 3 times.
2.3. Cell viability
After 24, 48, 72 and 96 h exposure of cells to paraquat concentrations ranging from 3–1000 μM, cellular viability was colorimetrically assessed by means of the MTT assay. Briefly, the cells were incubated with a 0.5 mg ml–1 MTT solution at 37 °C and 5% CO2 for 60 min followed by solvation of the precipitated tetrazolium salt in dimethyl sulfoxide (DMSO). The absorbance was measured at λ = 540 nm using a Spectramax plate reader (Molecular Devices, Sunnyvale, CA, USA).
2.4. RNA isolation and quantitative real-time polymerase chain reaction (qRT-PCR)
After 24 h of cell exposures the cells were lysed using 300 μl QIAzol lysis reagent (Qiagen, Venlo, Netherlands). RNA was isolated with chloroform by phase separation, precipitated with isopropanol and resuspended in RNase/DNase free water. The RNA purity and quantity was assessed using the NanoDrop system (Thermo Scientific, Rockford, IL, USA). cDNA synthesis was performed with 500 ng RNA using the iScript cDNA Synthesis kit (Bio-Rad, Hercules, CA, USA) according to the manufacturer's protocol. qRT-PCR reaction mixtures (20 μl total volume) consisted of 10 μl SensiMix SYBR & Fluorescence kit (Bio-Rad, Hercules, CA, USA), 5 μl cDNA and 150 nM of each primer (Eurofins MWG Operon, Ebersberg, Germany). The following primer sequences were used: β-actin, forward: 5′-CCTGGCACCCAGCACAAT-3′, reverse: 5′-GCCGATCCACACGGAGTACT-3′; tumor necrosis factor α (TNFA), forward: 5′-TCAATCGGCCCGACTATCTC-3′, reverse: 5′-CAGGGCAATGATCCCAAAGT-3′; 11β-hydroxysteroid dehydrogenase 1 (HSD11B1), forward: 5′-AGGAAAGCTCATGGGAGGACTAG-3′, reverse 5′-ATGGTGAATATCCTCATGAAAAAGATTC-3′. The cycling conditions were initial denaturation at 95 °C for 10 min, followed by 40 alternating cycles at 95 °C and 60 °C for 15 s and 45 s respectively, using the iCycler system (Bio-Rad, Hercules, CA, USA). The specificity of the reactions was controlled by means of melting curves. The 2–ΔΔct method was applied with β-actin as a housekeeping gene and calculating fold changes relative to unexposed cells.
2.5. Analysis of total cellular GSH concentrations
After 24 h of exposures the cells were lysed with 600 μl 100 mM potassium phosphate buffer containing 10 mM EDTA (pH = 7.5) and 0.1% (v/v) Triton X-100. After 30 min incubation on ice, cell debris was removed by centrifugation at 3000g for 10 min. Sulfosalicylic acid (SSA) was added in a final concentration of 0.6% (m/V). By means of an enzymatic recycling method total cellular GSH concentrations were determined as previously described.24 The cellular protein content was quantified in the lysates via the bicinchoninic acid assay (BCA protein assay kit; Bio-Rad, Hercules, CA, USA).
2.6. Quantification of IL-8 concentrations in cell culture medium
IL-8 concentrations in the cell supernatant were measured using a commercially available ELISA kit (Sanquin, Amsterdam, Netherlands) in accordance with the manufacturer's protocol.
2.7. Statistics
Data are reported as mean ± standard deviation (SD). Outcome parameters were statistically compared by using Kruskal–Wallis H tests followed by either Dunn's test or Mann–Whitney U tests (taking into account Bonferroni corrected levels of significance for multiple comparisons) to post hoc locate statistically significant differences between the 2 test conditions. Unless stated otherwise, a two-tailed P-value < 0.05 was considered statistically different. All analyses were performed with GraphPad Prism 5.1 (GraphPad Software Inc., San Diego, CA, USA).
3. Results
3.1. Time- and concentration-dependent effects of paraquat on cell viability
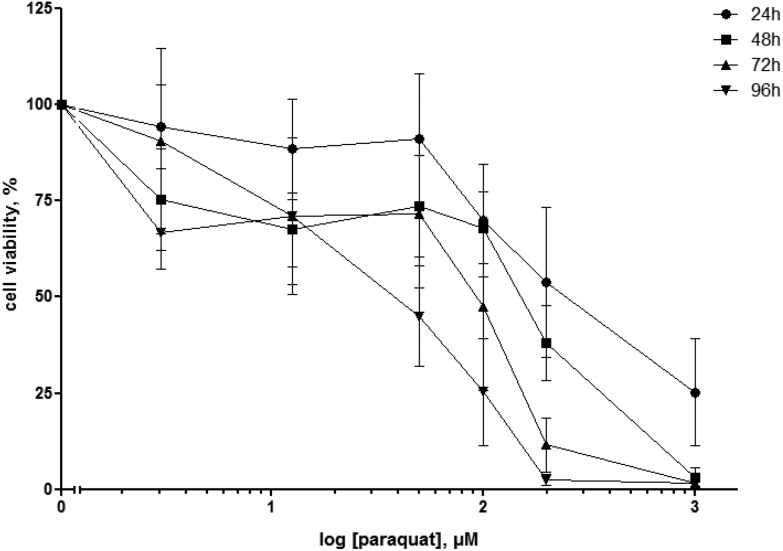
Cell viability was assessed by the MTT assay after 24 h, 48 h, 72 and 96 h exposure of human macrophage-like cells to paraquat concentrations ranging from 3 to 1000 μM. For each exposure time, cell viability decreased with increasing paraquat concentrations (Fig. 1). In line, extended exposure times led to more cell death for most test concentrations. Whereas a 24 h exposure of paraquat concentrations of 50 μM and below did not considerably affect the viability of macrophage-like cells, a reduction of 44.0 ± 13.8% in viable cells was observed for a concentration of 200 μM paraquat (Fig. 1). Based on these data, we decided to select for the subsequent experiments paraquat concentrations of 50 and 200 μM and expose the cells for 24 h, in order to investigate the early effects of both, a sub-cytotoxic and a mildly cytotoxic paraquat concentration on the anti-inflammatory action of cortisol.
Fig. 1. Cell viability measured by the MTT assay of U937 human macrophage-like cells exposed to increasing concentrations (3; 12.5; 50; 100; 200; 1000 μM) paraquat for 24, 48, 72 and 96 h. Data are expressed as percentage viability of unexposed (control) cells and depicted as mean ± standard deviation (N = 3–4).
3.2. Inflammatory effect of paraquat
To determine the inflammatory activity of paraquat, macrophage-like cells were exposed to 50 and 200 μM for 24 h. The supernatant was collected and used to quantify the release of the pro-inflammatory cytokine IL-8. The cells were used to measure the level of gene expression of the pro-inflammatory cytokine TNFA and the enzyme HSD11B1 that catalyzes the intracellular conversion of cortisone into bioactive cortisol.
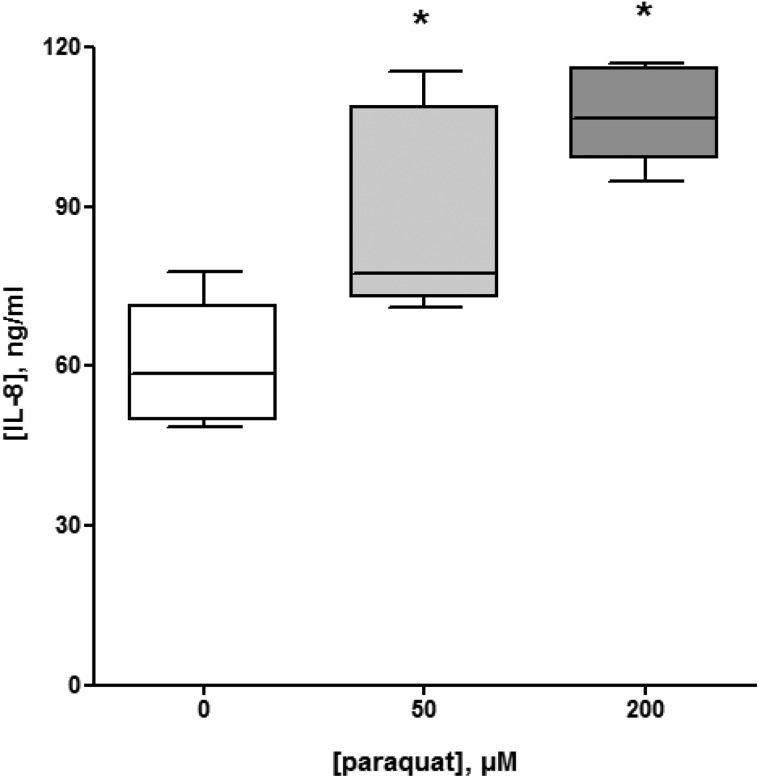
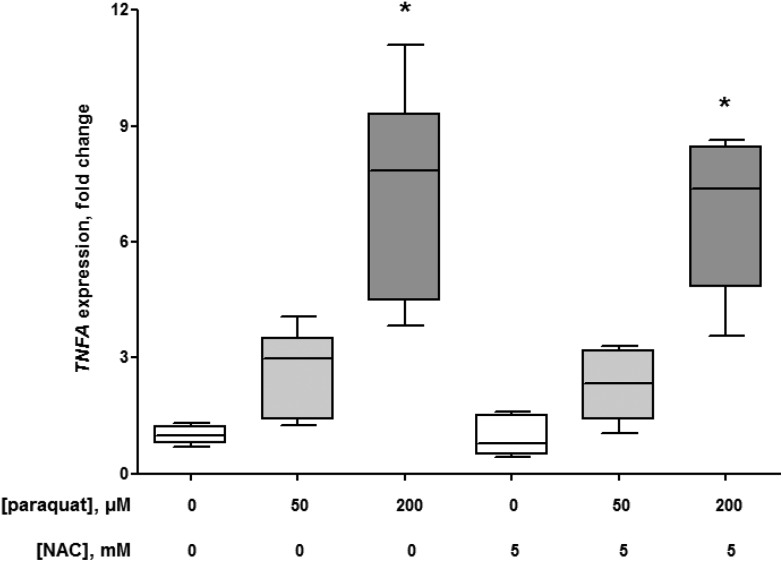
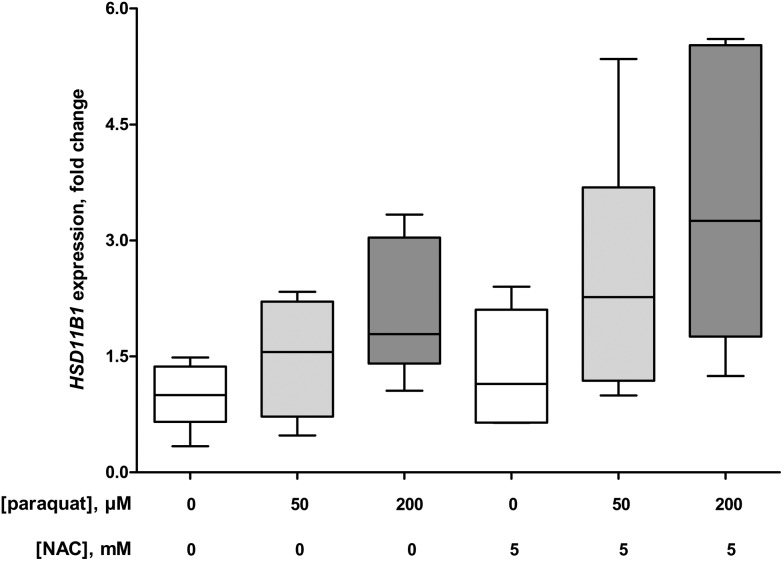
The 24 h exposure of macrophage-like cells to 50 and 200 μM paraquat elevated the release of IL-8 to 143 ± 6% (P = 0.015) and 179 ± 17% (P = 0.002), respectively compared to the control conditions without paraquat (Fig. 2). In addition, the pro-inflammatory effect of paraquat was reflected in the 2.7 ± 1.0 fold (50 μM paraquat, P = 0.004 compared to the control without paraquat) and 7.3 ± 2.2 fold (200 μM paraquat, P = 0.002 compared to the control without paraquat) increase in TNFA expression (Fig. 3). At the same time, macrophage-like cells showed a 1.5 ± 0.6 fold (50 μM paraquat; P = 0.394 compared to the control without paraquat) and 2.1 ± 0.8 fold (200 μM paraquat; P = 0.015 compared to the control without paraquat) elevation of HSD11B1 expression (Fig. 4). The presence of 5 mM of the antioxidant NAC did not significantly change the paraquat-mediated upregulation of the expression of TNFA (P = 0.589 (50 μM paraquat) and P = 0.699 (200 μM paraquat)) and HSD11B1 (P = 0.240 (50 μM paraquat) and P = 0.065 (200 μM paraquat)) compared to the exposure conditions without NAC (Fig. 3 and 4).
Fig. 2. Interleukin (IL-)8 production (ng ml–1) of U937 human macrophage-like cells in the absence (control, N = 3) and presence of 50 (N = 3) and 200 μM paraquat (N = 3) after 24 h. The box and whisker plots give the median (line in box), 25th and 75th percentiles (outer lines of box) and the minimal and maximal values (whiskers). The effect of paraquat on IL-8 levels was statistically assessed by Mann–Whitney U tests. To correct for multiple comparisons P < 0.025 was considered statistically significant. *P = 0.015 (50 μM paraquat) and P = 0.002 (200 μM paraquat), respectively compared with control cells.
Fig. 3. Expression of tumor necrosis factor (TNF)-α gene (TNFA) in U937 human macrophage-like cells in the absence (control, N = 3) and presence of 50 (N = 3) and 200 μM (N = 3) paraquat with and without 5 mM N-acetyl-l-cysteine (NAC) after 24 h. The box and whisker plots give the median (line in box), 25th and 75th percentiles (outer lines of box) and the minimal and maximal values (whiskers). The effects of paraquat in the absence or presence of NAC on TNFA expression were statistically assessed by Kruskal–Wallis H-tests and either Dunn's or Mann–Whitney U post hoc tests. To correct for multiple comparisons P < 0.007 was considered statistically significant. *P = 0.004 (50 μM paraquat), P = 0.002 (200 μM paraquat) and P = 0.002 (200 μM paraquat and 5 mM NAC), respectively compared with control cells.
Fig. 4. Expression of 11β-hydroxysteroid dehydrogenase (11β-HSD) 1 gene (HSD11B1) in U937 human macrophage-like cells in the absence (control, N = 3) and presence of 50 (N = 3) and 200 μM (N = 3) paraquat with and without 5 mM N-acetyl-l-cysteine (NAC) after 24 h. The box and whisker plots give the median (line in box), 25th and 75th percentiles (outer lines of box) and the minimal and maximal values (whiskers). The effects of paraquat in the absence or presence of NAC on HSD11B1 expression were statistically assessed by Kruskal–Wallis H-tests and either Dunn's or Mann–Whitney U post hoc tests. To correct for multiple comparisons P < 0.01 was considered statistically significant.
3.3. Effect of paraquat on cellular GSH concentration
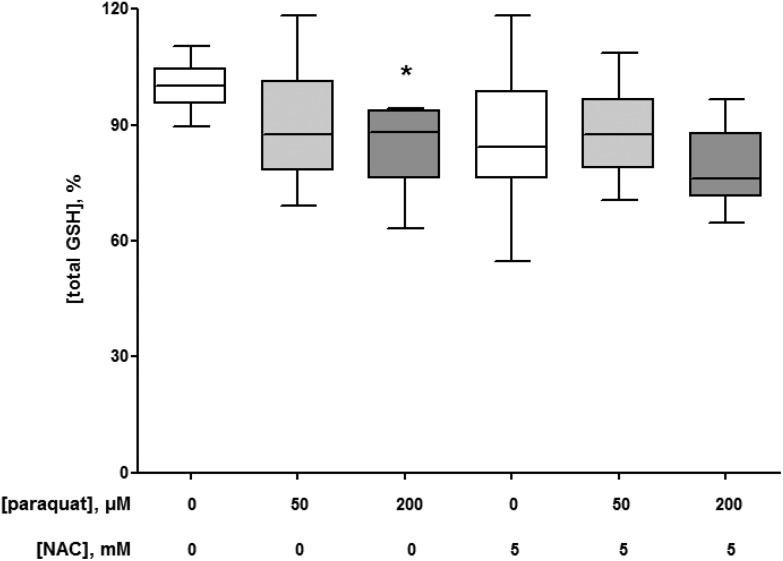
As a redox cycling compound, paraquat is known to reduce cellular GSH content.25–27 Therefore, the effects of the selected paraquat exposure conditions were assessed on the total GSH concentration in macrophage-like cells. The 24 h exposure to 50 and 200 μM paraquat decreased the cellular GSH levels. However, this decrease was statistically significant for only the higher paraquat concentration (P = 0.0002 compared to the control without paraquat, Fig. 5). The presence of 5 mM NAC did not affect the paraquat-induced alterations in the cellular GSH concentrations of the macrophage-like cells (Fig. 5).
Fig. 5. Intracellular total glutathione (GSH) concentrations of U937 human macrophage-like cells in the absence (control, N = 5) and presence of 50 (N = 5) and 200 μM (N = 5) paraquat with and without 5 mM N-acetyl-l-cysteine (NAC) after 24 h. The box and whisker plots give the median (line in box), 25th and 75th percentiles (outer lines of box) and the minimal and maximal values (whiskers). The effects of paraquat in the absence or presence of NAC on GSH concentrations were statistically assessed by Kruskal–Wallis H-tests and either Dunn's or Mann–Whitney U post hoc tests. To correct for multiple comparisons P < 0.01 was considered statistically significant. *P = 0.0002 (200 μM paraquat) compared with control cells.
3.4. Effect of paraquat on the anti-inflammatory effects of cortisol
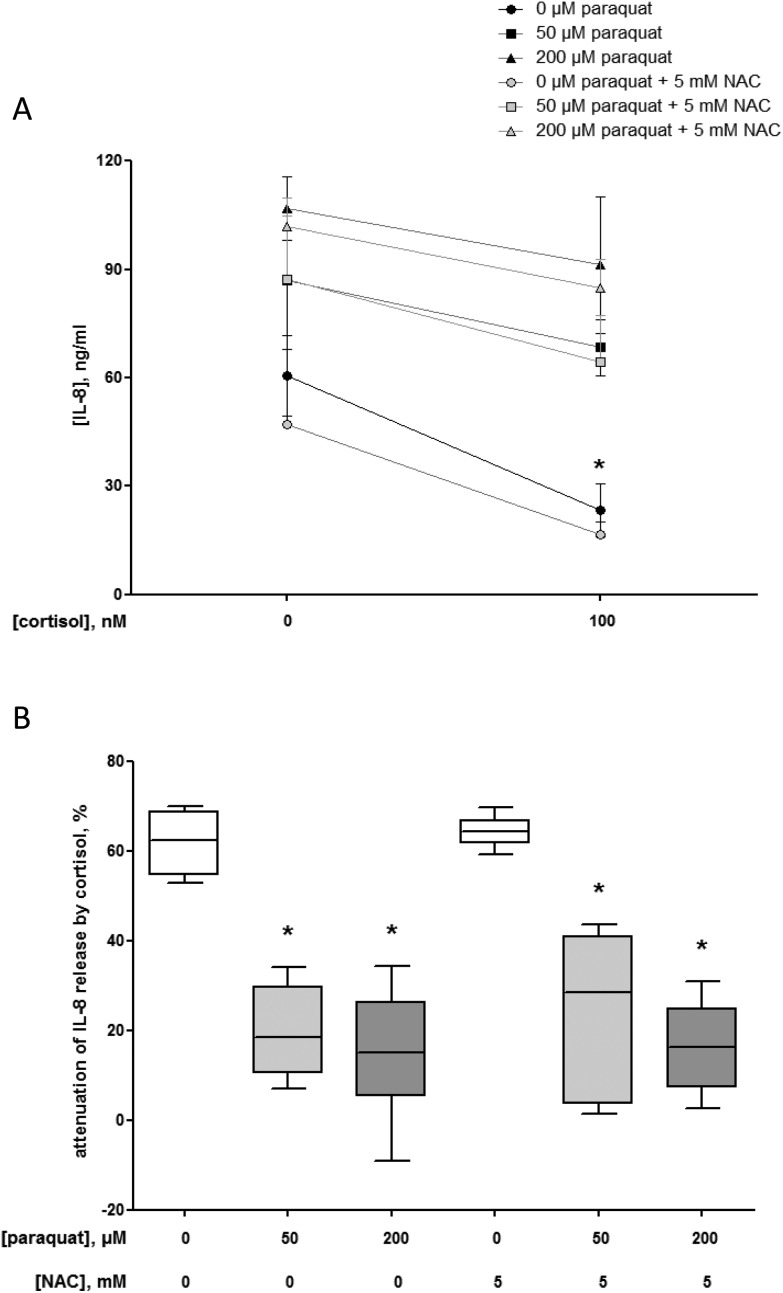
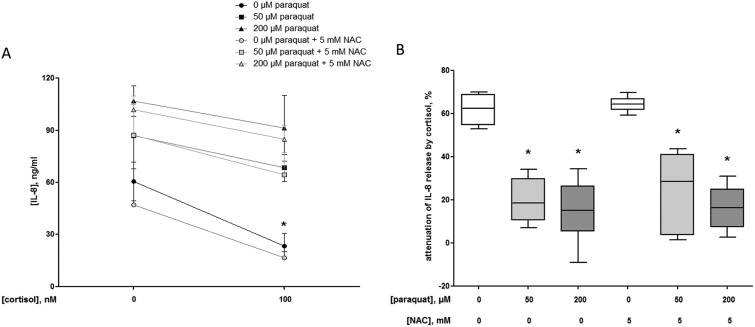
The anti-inflammatory efficacy of cortisol is shown in the significant attenuation of the IL-8 release from 60.5 ± 9.2 ng ml–1 (control without cortisol and paraquat, i.e. baseline) to 23.4 ± 6.2 ng ml–1 (P = 0.002) when macrophage-like cells were incubated with 100 nM cortisol for 18 h (Fig. 6A). This effect equals a reduction of 62 ± 5% (Fig. 6B).
Fig. 6. Interleukin (IL-)8 production (ng ml–1) in the presence or absence of 100 nM cortisol for 18 h by U937 human macrophage-like cells pre-exposed to 0 (control, N = 3), 50 (N = 3) or 200 μM (N = 3) paraquat with and without 5 mM N-acetyl-l-cysteine (NAC) for 24 h (A). The data are given as mean ± standard deviation. The effects of paraquat pre-exposure with and without NAC on the IL-8 release in the absence or presence of cortisol were statistically assessed by Kruskal–Wallis H-tests and either Dunn's or Mann–Whitney U post hoc tests. To correct for multiple comparisons P < 0.01 was considered statistically significant. *P = 0.002 (100 nM cortisol) compared with control cells. Percentage attenuation of interleukin (IL-)8 release by 100 nM cortisol (18 h) in U937 human macrophage-like cells pre-exposed to 0 (control, N = 3), 50 (N = 3) or 200 μM (N = 3) paraquat with and without 5 mM N-acetyl-l-cysteine (NAC) for 24 h (B). The box and whisker plots give the median (line in box), 25th and 75th percentiles (outer lines of box) and the minimal and maximal values (whiskers). The effects of paraquat with and without NAC pre-exposure on the anti-inflammatory action of cortisol were statistically assessed by Kruskal–Wallis H-tests and either Dunn's or Mann–Whitney U post hoc tests. To correct for multiple comparisons P < 0.008 was considered statistically significant. *P = 0.002 (50 μM paraquat), P = 0.002 (200 μM paraquat), P = 0.002 (50 μM paraquat and 5 mM NAC) and P = 0.002 (200 μM paraquat and 5 mM NAC), respectively compared with control cells.
However, due to pre-exposure of the cells to 50 or 200 μM paraquat for 24 h, the cortisol-mediated attenuation of the IL-8 release after 18 h was compromised to 20 ± 8% (P = 0.002) and 15 ± 12% (P = 0.002), respectively compared to the condition without paraquat (Fig. 6B). The decline in the anti-inflammatory effects of cortisol could not be prevented by the presence of 5 mM NAC during the pre-exposure to paraquat (Fig. 6A and B).
3.5. Effects of grape seed-derived MOF on the paraquat-induced damage of cortisol's anti-inflammatory effects
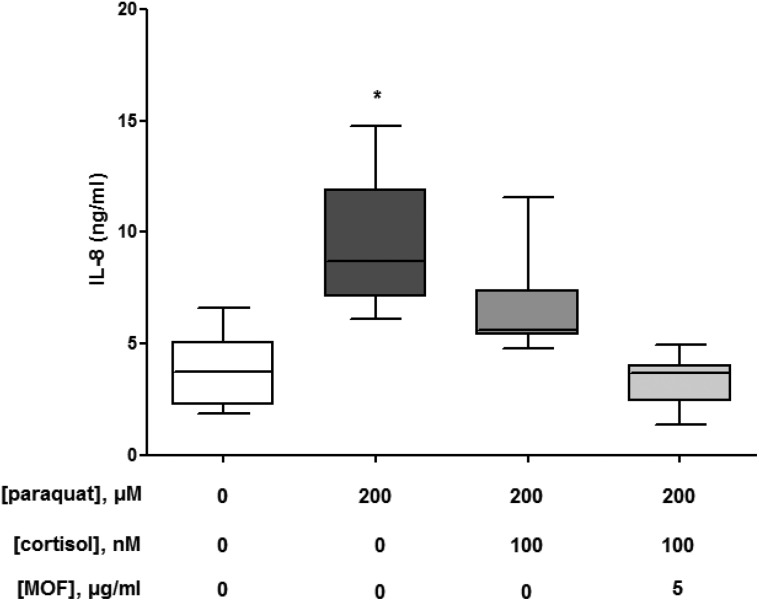
Since various flavonoids have been identified to prevent hydrogen peroxide-induced cortisol resistance,28 we assessed the ability of grape seed-derived MOF to prevent paraquat-induced inflammation. As shown in Fig. 7, the paraquat-induced release of IL-8 could only partly be attenuated by adding 100 nM cortisol to the culture medium of macrophage-like THP-1 cells. However, when the cells were incubated with 5 μg ml–1 MOF during paraquat exposure, IL-8 release was restored to the baseline level in the presence of cortisol.
Fig. 7. Percentage attenuation of interleukin (IL-)8 release by 100 nM cortisol (18 h) in THP-1 human macrophage-like cells pre-exposed to 0 (control, N = 3) or 200 μM (N = 3) paraquat with and without 5 μg ml–1 monomeric and oligomeric flavan-3-ols (MOF) from grape seeds for 6 h. The box and whisker plots give the median (line in box), 25th and 75th percentiles (outer lines of box) and the minimal and maximal values (whiskers). The effects of paraquat with and without MOF exposure on the anti-inflammatory action of cortisol were statistically assessed by Kruskal–Wallis H-tests and either Dunn's or Mann–Whitney U post hoc tests. To correct for multiple comparisons P < 0.02 was considered statistically significant. *P = 0.0006 (200 μM paraquat) compared with control cells.
4. Discussion
Paraquat poisoning is still a major problem in rural environments of developing countries such as Brazil. The herbicide is widely used without proper knowledge on safety precautions. The redox cycling properties of paraquat have been identified as a crucial cytotoxicity-mediating mechanism.12 In this process, free electrons shuttle via paraquat to oxygen. In this way superoxide anion radicals are formed which may further lead to other ROS and may result in cellular damage. Exposure to paraquat frequently takes place via inhalation and may result in severe lung injury.
Redox damage is intrinsically related to inflammation through activation of the NF-κB pathway. Reactive oxygen species activate the inhibitor of kappa B (IκB)-kinase (IKK) which leads to the stimulation of the transcription factor NF-κB and subsequent production of pro-inflammatory cytokines.29,30 This causes macrophage/neutrophil recruitment and activation eliciting an increased inflammatory response.31 Redox cycling compounds like paraquat are thought to inflict damage not only via the generation of ROS, but also directly via the activation of inflammation.32–34
Recommended pharmacotherapeutic interventions of paraquat poisoning are directed to either attenuate the oxidative damage with antioxidants or to inhibit the inflammatory response with immunosuppressive and anti-inflammatory drugs like glucocorticoids.14 Clinical data are scarce that demonstrate the therapeutic benefit of NAC, a precursor of the endogenous antioxidant glutathione. At the same time glucocorticoids appear to be hardly effective in the treatment of paraquat-intoxicated patients.14 Interestingly, animal studies revealed that quickly after poisoning the endogenous glucocorticoid cortisol was markedly increased and peaked after 24 h, shortly before the animals died.35,36 Altogether, these data point to an abrogated glucocorticoid response in paraquat intoxications.
Since macrophages play a central role in the development of pulmonary inflammation and the propagation to fibrotic lung injury,37 we used macrophage-like cells to further investigate the paraquat-induced inflammation. We showed that paraquat exposure resulted in a concentration- and time-dependent cytotoxicity in these macrophages. As expected prolonged exposure for more than 48 h considerably diminished cell viability already in lower paraquat concentrations i.e. below 12.5 μM (Fig. 1). Based on these data we decided to continue with 2 paraquat concentrations, 50 and 200 μM, and an exposure time of 24 h, to study acute conditions that cause no and a mild decrease in cell viability, respectively in our in vitro model. The selected test concentrations exceeded the concentrations reported in plasma (approximately 3–40 μM) from intoxicated patients, although these values may differ depending on the analytical method.38
Our data revealed that paraquat is a potent pro-inflammatory stimulant eliciting a significant increase in IL-8 production in macrophage-like cells (Fig. 2). Moreover, the TNF-α gene expression was significantly up-regulated in the presence of paraquat (Fig. 3), which confirms the earlier reported pro-inflammatory properties of this compound.32,33,39 The induction of the expression of the enzyme 11β-HSD1 (Fig. 4) is indicative of the stress response elicited by the herbicide in these inflammatory immune cells.40 The enzyme 11β-HSD1 catalyzes the intracellular formation of bioactive cortisol from cortisone thereby serving as a cellular counter response to inflammation. We first anticipated that paraquat triggers inflammation via its redox cycling property leading to an increased production of ROS in the cells. However, the antioxidant NAC did not protect against the paraquat-mediated up-regulation of the TNF-α gene expression (Fig. 4), and tended to further elevate the expression of 11β-HSD1 in the presence of paraquat (Fig. 4). The lacking effect of NAC suggests that oxidative stress may not be the primary stimulus for the inflammatory response of the macrophages exposed to paraquat. This is underlined by the observation that under our experimental conditions (concentrations and incubation times) paraquat reduced intracellular GSH concentrations only in the higher test concentration (Fig. 5). This is in contrast to many experimental studies which reported a pronounced decrease in intracellular GSH upon paraquat exposure, both in vitro and in vivo.41–46 However, in the in vitro studies the reduction in GSH appears to become manifest in paraquat concentrations ≤500 μM after exposures considerably exceeding 24 h (ref. 45) or in paraquat concentrations of ≥1 mM.44 Furthermore, it was shown that cellular uptake of NAC requires approximately 2 h before it confers protection against paraquat-induced cytotoxicity.47 Since we exposed the macrophage-like cells simultaneously with paraquat and NAC, the failing effects of NAC on inflammatory gene expression (Fig. 3 and 4) and cellular GSH concentrations (Fig. 5) could also be explained by the fact that NAC was yet insufficiently taken up by the cells.
The treatment of paraquat poisoning with anti-inflammatory glucocorticoids is clinically still the first choice albeit that glucocorticoids are not always very effective to mitigate paraquat toxicity.14 Poor anti-inflammatory efficacy of glucocorticoids, i.e. corticosteroid resistance, is a well-known phenomenon in diseases associated with chronic inflammation.15–18 It can be caused by oxidative stress which impairs the anti-inflammatory action of cortisol and dexamethasone,23,48 and also by a wide variety of other factors and molecular mechanisms.17 Our results revealed that 100 nM cortisol significantly attenuates the basal IL-8 release in macrophage-like cells (Fig. 6A and B). Paraquat stimulates the IL-8 formation in a dose-dependent manner which is not inhibited by cortisol added 24 h after paraquat exposure (Fig. 6A and B). Moreover, the anti-inflammatory efficacy of cortisol is significantly reduced in cells exposed to paraquat (Fig. 6B). This shows that cortisol is not able to mitigate the inflammation elicited by paraquat. It appears that paraquat leads to a cessation of the endogenous cortisol response which cannot be prevented by NAC. Remarkably, pretreatment with a polyphenolic preparation derived from grape seeds and consisting of a standardized composition of MOF (Table 1) is able to restore the inflammation induced by paraquat and only partly counteracted by cortisol (Fig. 7).
MOF have been shown to exert a plethora of physiological effects on the human vasculature21 which go beyond direct antioxidant effects. In vitro and clinical data revealed that MOF attenuate inflammation by cellular and subcellular mechanisms involving inhibition of NF-κB-mediated gene expression.20 Although our experiments do not elucidate whether MOF prevent the occurrence of cortisol resistance as shown recently for a variety of plant-derived compounds,28 or provide additive anti-inflammatory effects, our findings suggest that plant-derived polyphenols may be effective to enhance the anti-inflammatory action of glucocorticoids in paraquat-induced inflammation. This observation is supported by recent data from paraquat toxicity models with the flavonol quercetin and the flavanone glycoside naringin. While quercetin's redox modulatory effects prevented paraquat-induced cytotoxicity in lung epithelial cells in vitro,49 naringin exerted significant anti-inflammatory and anti-fibrotic effects in the lungs of paraquat intoxicated mice.50 The successful reversal of acute lung injury of paraquat by rapamycin in vivo proposes NF-κB as a relevant molecular drug target.51 Moreover, the effect of drugs targeting inflammatory cytokines (e.g. monoclonal antibodies or receptor fusion proteins) on paraquat-mediated inflammation is not yet fully known.
In the clinical situation, combination therapies are recommended for paraquat intoxications.14 Dexamethasone has been combined with either methylprednisolone or the immunosuppressant cyclophosphamide (and mesna) to increase the therapeutic efficiency. Although individual patients survived a severe paraquat intoxication when they received such a combinatory treatment,52,53 evidence for the efficacy of these approaches from randomized clinical trials is weak.14,54 The high case fatality, i.e. >50% of hospital admissions due to paraquat poisoning die from severe lung injury and multi-organ failure,3,4 and the wide-spread occurrence of unintentional and intentional self-poisoning2 increase the urgency to improve the current therapeutic strategies. The absence of protection of the cortisol action by NAC emphasizes the need for seeking agents with alternative mechanisms of action to attenuate the paraquat-induced inflammatory response.
5. Conclusions
The present study revealed that paraquat elicits an inflammatory response in human macrophages, i.e. cells of the body's first line of immune defense. Moreover, paraquat damages the anti-inflammatory action of the endogenous glucocorticoid cortisol which seems to be independent of the induction of oxidative stress. The findings suggest that damage of the endogenous cortisol action may contribute to the paraquat-initiated inflammation. Moreover, it remains important to further elucidate the enigmatic mechanisms of paraquat's cytotoxicity in order to improve established therapies and develop novel approaches for the management of paraquat intoxications. In this respect, agents with pleiotropic effects on redox regulatory and inflammatory pathways, such as plant-derived polyphenols, may be promising to clinically investigate in the future as add-ons in the therapy of paraquat intoxication with glucocorticoids.
Abbreviations
- 11β-HSD1
11β-Hydroxysteroid dehydrogenase 1
- FCS
Fetal calf serum
- GSH
Glutathione
- HSD11B1
Gene encoding 11β-hydroxysteroid dehydrogenase 1
- IKK
Inhibitor of kappa B (IκB)-kinase
- IL
Interleukin
- MOF
Monomeric and oligomeric flavan-3-ols
- MTT
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NAC
N-Acetyl-l-cysteine
- NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- PMA
Phorbol 12-myristate 13-acetate
- ROS
Reactive oxygen species
- SSA
Sulfosalicylic acid
- TNF-α
Tumor necrosis factor α
- TNFA
Gene encoding tumor necrosis factor α
Conflict of interest
There are no conflicts of interest to declare.
Acknowledgments
GV received a scholarship from the CAPES Foundation, Brazil, to conduct this research. CAPES Foundation was not involved in the design of the study and collection, analysis and interpretation of data and in writing the manuscript.
References
- Prada P., Why Brazil has a big appetite for risky pesticides, http://www.reuters.com/investigates/special-report/brazil-pesticides/.
- Gunnell D., Eddleston M., Phillips M., Konradsen F. BMC Public Health. 2007;7:357. doi: 10.1186/1471-2458-7-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald G. R., Barniville G., Flanagan M., Silke B., Carmody M., O'Dwyer W. F. Ir. Med. J. 1978;71:103–108. [PubMed] [Google Scholar]
- Hettiarachchi J., Kodithuwakku G. C. Int. J. Epidemiol. 1989;18:418–422. doi: 10.1093/ije/18.2.418. [DOI] [PubMed] [Google Scholar]
- Clejan L., Cederbaum A. I. Biochem. Pharmacol. 1989;38:1779–1786. doi: 10.1016/0006-2952(89)90412-7. [DOI] [PubMed] [Google Scholar]
- Franco R., Li S., Rodriguez-Rocha H., Burns M., Panayiotidis M. I. Chem.-Biol. Interact. 2010;188:289–300. doi: 10.1016/j.cbi.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Polo R. A., Rodriguez-Martin A., Moran J. M., Niso M., Soler G., Fuentes J. M. Brain Res. 2004;1011:170–176. doi: 10.1016/j.brainres.2004.02.078. [DOI] [PubMed] [Google Scholar]
- Palmeira C. M., Moreno A. J., Madeira V. M. Biochim. Biophys. Acta. 1995;1229:187–192. doi: 10.1016/0005-2728(94)00202-g. [DOI] [PubMed] [Google Scholar]
- Castello P. R., Drechsel D. A., Patel M. J. Biol. Chem. 2007;282:14186–14193. doi: 10.1074/jbc.M700827200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. L., Sun G. Y., Sun A. Y. Brain Res. 2007;1167:129–139. doi: 10.1016/j.brainres.2007.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristovao A. C., Choi D. H., Baltazar G., Beal M. F., Kim Y. S. Antioxid. Redox Signaling. 2009;11:2105–2118. doi: 10.1089/ars.2009.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bus J. S., Gibson J. E. Environ. Health Perspect. 1984;55:37–46. doi: 10.1289/ehp.845537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloire G., Legrand-Poels S., Piette J. Biochem. Pharmacol. 2006;72:1493–1505. doi: 10.1016/j.bcp.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Gawarammana I. B., Buckley N. A. Br. J. Clin. Pharmacol. 2011;72:745–757. doi: 10.1111/j.1365-2125.2011.04026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell R. J., Kelleher D. J. Endocrinol. 2003;178:339–346. doi: 10.1677/joe.0.1780339. [DOI] [PubMed] [Google Scholar]
- Chikanza I. C., Kozaci D. L. Rheumatology. 2004;43:1337–1345. doi: 10.1093/rheumatology/keh333. [DOI] [PubMed] [Google Scholar]
- Barnes P. J., Adcock I. M. Lancet. 2009;373:1905–1917. doi: 10.1016/S0140-6736(09)60326-3. [DOI] [PubMed] [Google Scholar]
- Barnes P. J. J. Allergy Clin. Immunol. 2015;136:531–545. doi: 10.1016/j.jaci.2015.05.052. [DOI] [PubMed] [Google Scholar]
- Barnes P. J., Ito K., Adcock I. M. Lancet. 2004;363:731–733. doi: 10.1016/S0140-6736(04)15650-X. [DOI] [PubMed] [Google Scholar]
- Milenkovic D., Vanden Berghe W., Boby C., Leroux C., Declerck K., Szarc vel Szic K., Heyninck K., Laukens K., Bizet M., Defrance M., Dedeurwaerder S., Calonne E., Fuks F., Haegeman G., Haenen G. R., Bast A., Weseler A. R. PLoS One. 2014;9:e95527. doi: 10.1371/journal.pone.0095527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weseler A. R., Ruijters E. J., Drittij-Reijnders M. J., Reesink K. D., Haenen G. R., Bast A. PLoS One. 2011;6:e28460. doi: 10.1371/journal.pone.0028460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearden L. C., Fairshter R. D., McRae D. M., Smith W. R., Glauser F. L., Wilson A. F. Am. J. Pathol. 1978;93:667–680. [PMC free article] [PubMed] [Google Scholar]
- Ruijters E. J., Haenen G. R., Weseler A. R., Bast A. Pharmacol. Res. 2014;79:28–33. doi: 10.1016/j.phrs.2013.11.004. [DOI] [PubMed] [Google Scholar]
- Rahman I., Kode A., Biswas S. K. Nat. Protoc. 2006;1:3159–3165. doi: 10.1038/nprot.2006.378. [DOI] [PubMed] [Google Scholar]
- Yang W., Tiffany-Castiglioni E. J. Toxicol. Environ. Health, Part A. 2005;68:1939–1961. doi: 10.1080/15287390500226987. [DOI] [PubMed] [Google Scholar]
- Hoffer E., Baum Y., Tabak A., Taitelman U. Toxicol. Lett. 1996;84:7–12. doi: 10.1016/0378-4274(95)03446-3. [DOI] [PubMed] [Google Scholar]
- Palmeira C. M., Moreno A. J., Madeira V. M. Arch. Toxicol. 1994;68:24–31. doi: 10.1007/s002040050025. [DOI] [PubMed] [Google Scholar]
- Ruijters E. J., Haenen G. R., Willemsen M., Weseler A. R., Bast A. Int. J. Mol. Sci. 2016;17:239. doi: 10.3390/ijms17020239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreck R., Rieber P., Baeuerle P. A. EMBO J. 1991;10:2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreck R., Albermann K., Baeuerle P. A. Free Radical Res. Commun. 1992;17:221–237. doi: 10.3109/10715769209079515. [DOI] [PubMed] [Google Scholar]
- Nathan C. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- Bianchi M., Fantuzzi G., Bertini R., Perin L., Salmona M., Ghezzi P. Cytokine. 1993;5:525–530. doi: 10.1016/1043-4666(93)90045-7. [DOI] [PubMed] [Google Scholar]
- Ishida Y., Takayasu T., Kimura A., Hayashi T., Kakimoto N., Miyashita T., Kondo T. Leg. Med. 2006;8:102–109. doi: 10.1016/j.legalmed.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Toygar M., Aydin I., Agilli M., Aydin F. N., Oztosun M., Gul H., Macit E., Karslioglu Y., Topal T., Uysal B., Honca M. Hum. Exp. Toxicol. 2015;34:198–204. doi: 10.1177/0960327114533808. [DOI] [PubMed] [Google Scholar]
- Giri S. N., Curry D. L., Stabenfeldt G., Spangler W. L., Chandler D. B., Schiedt M. J. Environ. Res. 1983;30:80–88. doi: 10.1016/0013-9351(83)90169-x. [DOI] [PubMed] [Google Scholar]
- Rose M. S., Crabtree H. C., Fletcher K., Wyatt I. Biochem. J. 1974;138:437–443. doi: 10.1042/bj1380437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thannickal V. J., Toews G. B., White E. S., Lynch, 3rd J. P., Martinez F. J. Annu. Rev. Med. 2004;55:395–417. doi: 10.1146/annurev.med.55.091902.103810. [DOI] [PubMed] [Google Scholar]
- Dinis-Oliveira R. J., Duarte J. A., Sánchez-Navarro A., Remião F., Bastos M. L., Carvalho F. Crit. Rev. Toxicol. 2008;38:13–71. doi: 10.1080/10408440701669959. [DOI] [PubMed] [Google Scholar]
- He X., Wang L., Szklarz G., Bi Y., Ma Q. J. Pharmacol. Exp. Ther. 2012;342:81–90. doi: 10.1124/jpet.112.194142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman K. E., Coutinho A. E., Gray M., Gilmour J. S., Savill J. S., Seckl J. R. Mol. Cell. Endocrinol. 2009;301:123–131. doi: 10.1016/j.mce.2008.09.031. [DOI] [PubMed] [Google Scholar]
- Peter B., Wartena M., Kampinga H. H., Konings A. W. Biochem. Pharmacol. 1992;43:705–715. doi: 10.1016/0006-2952(92)90234-a. [DOI] [PubMed] [Google Scholar]
- Yamamoto H. Toxicology. 1993;79:37–43. doi: 10.1016/0300-483x(93)90204-6. [DOI] [PubMed] [Google Scholar]
- Nakagawa I., Suzuki M., Imura N., Naganuma A. J. Toxicol. Sci. 1995;20:557–564. doi: 10.2131/jts.20.5_557. [DOI] [PubMed] [Google Scholar]
- Hoffer E., Baum Y., Tabak A., Taitelman U. Toxicol. Lett. 1996;84:7–12. doi: 10.1016/0378-4274(95)03446-3. [DOI] [PubMed] [Google Scholar]
- Tsukamoto M., Tampo Y., Sawada M., Yonaha M. Toxicol. Appl. Pharmacol. 2002;178:82–92. doi: 10.1006/taap.2001.9325. [DOI] [PubMed] [Google Scholar]
- Djukic M. M., Jovanovic M. D., Ninkovic M., Stevanovic I., Ilic K., Curcic M., Vekic J. Chem.-Biol. Interact. 2012;199:74–86. doi: 10.1016/j.cbi.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Mitsopoulos P., Suntres Z. E. J. Toxicol. 2011;2011:808967. doi: 10.1155/2011/808967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruijters E. J. B., Haenen G. R. M. M., Weseler A. R., Bast A. PharmaNutrition. 2014;2:47–52. [Google Scholar]
- Zerin T., Kim Y. S., Hong S. Y., Song H. Y. J. Appl. Toxicol. 2013;33:1460–1467. doi: 10.1002/jat.2812. [DOI] [PubMed] [Google Scholar]
- Chen Y., Nie Y. C., Luo Y. L., Lin F., Zheng Y. F., Cheng G. H., Wu H., Zhang K. J., Su W. W., Shen J. G., Li P. B. Food Chem. Toxicol. 2013;58:133–140. doi: 10.1016/j.fct.2013.04.024. [DOI] [PubMed] [Google Scholar]
- Chen D., Ma T., Liu X. W., Yang C., Liu Z. Int. J. Clin. Exp. Pathol. 2015;8:4627–4638. [PMC free article] [PubMed] [Google Scholar]
- Chen G. H., Lin J. L., Huang Y. K. Crit. Care Med. 2002;30:2584–2587. doi: 10.1097/00003246-200211000-00030. [DOI] [PubMed] [Google Scholar]
- Comchai S. J. Toxicol. Clin. Toxicol. 2003;41:520–521. [Google Scholar]
- Li L. R., Sydenham E., Chaudhary B., Beecher D., You C. Cochrane Database Syst. Rev. 2014;8:CD008084. doi: 10.1002/14651858.CD008084.pub4. [DOI] [PubMed] [Google Scholar]