We evaluate the effects of Eugenia uniflora essential oil on mitochondrial bioenergetics in Drosophila melanogaster.
We evaluate the effects of Eugenia uniflora essential oil on mitochondrial bioenergetics in Drosophila melanogaster.
Abstract
Eugenia uniflora L. (Myrtaceae family) has demonstrated several properties of human interest, including insecticide potential, due to its pro-oxidant properties. These properties likely result from the effects on its mitochondria, but the mechanism of this action is unclear. The aim of this work was to evaluate the mitochondrial bioenergetics function in Drosophila melanogaster exposed to E. uniflora leaf essential oil. For this, we used a high-resolution respirometry (HRR) protocol. We found that E. uniflora promoted a collapse of the mitochondrial transmembrane potential (ΔΨm). In addition the essential oil was able to promote the disruption of respiration coupled to oxidative phosphorylation (OXPHOS) and inhibit the respiratory electron transfer system (ETS) established with an uncoupler. In addition, exposure led to decreases of respiratory control ratio (RCR), bioenergetics capacity and OXPHOS coupling efficiency, and induced changes in the substrate control ratio. Altogether, our results suggested that E. uniflora impairs the mitochondrial function/viability and promotes the uncoupling of OXPHOS, which appears to play an important role in the cellular bioenergetics failure induced by essential oil in D. melanogaster.
1. Introduction
Effective biological control of insects is important because of the role of the insect population in disease transmission and agriculture. From a health perspective, insects, including flies and mosquitoes, are vectors for several infectious diseases and controlling their population can be an effective method to limit disease outbreak.1 From an agricultural perspective, overabundant insect population can be of agricultural pests, attacking crops and reducing productivity leading to economic loss.2,3 Because of these negative impacts, chemical insecticides are widely used to control the insect population, but insecticides can have a broad range of adverse effects both on the environment and humans exposed to these chemicals.3 The need to limit these negative consequences is driving the search for alternative insecticidal strategies that have minimal side effects on non-target organisms with special interest in natural compounds that result from secondary metabolism in plants.4,5
One such plant is the Brazilian cherry, Eugenia uniflora, from the Myrtaceae family. The compounds from this plant have several potential medicinal properties and applications of possible commercial value,6 including anti-inflammatory, anti-hypertensive and antioxidant properties.7–9 In addition, the plant's toxic properties, including antimicrobial activity, anti-fungal and antiviral properties, have been explored as possible control agents for infectious conditions.9–12 At least in part, the toxic effects of E. uniflora are related to its pro-oxidant effect, acting to increase the reactive oxygen species (ROS) generation that have promising insecticidal potential.13 The pro-oxidant properties found in E. uniflora leaves’ essential oil derive from secondary metabolites, primarily monoterpenes, sesquiterpenes, and phenylpropanoids,7,8,13 which may trigger the impairment of the redox homeostasis. On the organism scale, exposure to E. uniflora significantly increases mortality and impairs locomotor capacity (e.g., geotaxis behavior), in Drosophila melanogaster, the fruit fly model system.13
Mitochondria are the powerhouses of cells and mitochondrial function is associated with a range of metabolic processes and conditions, including oxidative stress.14–16 Mitochondria generate most cellular energy through the electron transport system (ETS) coupled with oxidative phosphorylation (OXPHOS), a combination of electron flow and proton-motive force driving mitochondrial ATP synthesis.17 Compounds, with fumigant-like insecticidal activity, are able to induce mitochondrial dysfunction leading to decreases in ATP levels.18 Even normal mitochondrial function, however, is accompanied by unavoidable proton and electron leaks within mitochondria which generate the superoxide radical that, as an ROS, has a series of deleterious effects.19,20 Furthermore, damage to this organelle, for example by plant metabolites, can play both a direct and indirect role in the ROS generation, and can induce a drastic impairment of cellular bioenergetics metabolism.21,22 In this context, plant metabolites can impair redox homeostasis, leading to the oxidation of mitochondrial protein thiol groups and triggering mitochondrial transition permeability that have been associated with the enhancement of ROS generation and dissipation of the mitochondrial transmembrane potential.23
The fruit-fly, D. melanogaster, is an outstanding model system to investigate the toxicological mechanism of drugs and natural compounds, featuring low cost, easy manipulation, a broad range of resources and established protocols, and metabolic similarity to mammals.24–26 In the fly, and all aerobic organisms, maintenance of cellular homeostasis is closely related to mitochondrial function,20 and mitochondrial compartments are often the functional targets of insecticidal compounds. In a previous study, our group has demonstrated that the essential oil from E. uniflora has insecticidal properties.13 In the current work, we demonstrate the effects of this oil on mitochondrial bioenergetics from D. melanogaster by High-Resolution Respirometry (HRR) and explore the potential modes of action related to the insecticidal effect of E. uniflora leaves’ essential oil.
2. Materials and methods
2.1. Chemical reagents
All chemicals were purchased from Sigma–Aldrich (São Paulo, SP, Brazil). Other materials, including fly food ingredients, were obtained from standard commercial suppliers.
2.2. Eugenia uniflora
2.2.1. Plant material and essential oil
The botanical material from Eugenia uniflora L. was collected in June 2013 at 9:00 ± 00:30 h at the Botanical Garden of Crato, CE, Brazil (coordinates: 07°14′28.0′′S and 39°24′56.7′′W). The referred time of collection was chosen because it yielded a higher amount of oil compared to other collection times. After species identification one voucher specimen was deposited in the Herbarium Dárdano de Andrade Lima, Universidade Regional do Cariri-URCA under number #3106.
Leaves of E. uniflora L. were collected and perforated into pieces of about 1 cm2. Then, the plant material was immersed in a 5 liter glass flask filled with distilled water, and subjected to extraction with the Clevenger apparatus by hydro-distillation, as previously described,27 obtaining a yield of 0.136%. Such distillations were made in quadruplicate following the addition of anhydrous sodium sulfate. Then the essential oil was cotton-filtered using a Pasteur pipette and stored in amber glass vials at –20 °C.
2.2.2. Gas chromatography (GC-FID)
Gas chromatography (GC) analysis was performed using an Agilent Technologies 6890N GC-FID system, equipped with a DB-5 capillary column (30 m × 0.32 mm; 0.50 mm) and connected to an FID detector. The thermal programmer was 60 °C (1 min) to 180 °C at 3 °C min–1; injector temperature 220 °C; detector temperature 220 °C; split ratio 1 : 10; carrier gas helium; flow rate 1.0 mL min–1. The volume injected was 1 μL diluted in chloroform (1 : 5). Two replicates of samples were processed in the same way. Component relative concentrations were calculated based on GC peak areas without using correction factors.13
2.2.3. Gas chromatography-mass spectrometry (GC-MS)
GC-MS analyses were performed on an Agilent Technologies AutoSystem XL GC-MS system operating in the EI mode at 70 eV, equipped with a split/splitless injector (220 °C). The transfer line temperature was 220 °C. Helium was used as the carrier gas (1.0 mL min–1) and the capillary columns used were an HP 5MS (30 m × 0.35 mm; film thickness 0.50 mm) and an HP Innowax (30 m × 0.32 mm i.d., film thickness 0.50 mm). The temperature programmer was the same as that used for the GC analyses. The injected volume was 1 μL of the essential oil diluted in chloroform (1 : 10).
2.2.4. Identification of the components
Constituents were identified based on the retention index (RI), determined with reference to the homologous series of n-alkanes, C7–C30, under identical experimental conditions, comparing with the mass spectra library search (NIST and Wiley), and mass spectra previously demonstrated in the literature.28 The relative amounts of individual components were calculated based on the CG peak area (FID response).
2.3. Drosophila stock and culture
The Harwich strain Drosophila melanogaster was obtained from the National Species Stock Center, Bowling Green, OH, USA. Flies were maintained at 25 ± 1 °C, under a 12:12 dark–light photoperiod and 60–70% relative humidity on a basic cornmeal diet composed of cereal flour, corn flour and water, supplemented with dried yeast and with Nipagin added as an antifungal agent, as previously described.29 All experimental treatments were conducted on two to five day old flies.
2.4. Essential oil exposure and D. melanogaster assays
Fruit flies were exposed to different concentrations of essential oil by a fumigation protocol, as previously described.13,30 In brief, fifty adult flies (males and females) were placed in 330 cm3 flasks, containing standard medium at the bottom. A counter-lid of polyethylene terephthalate (PET) was introduced on the screw cap of the flask, to which a filter paper was fixed at the inner side of the cap for application of the different doses of essential oil. By doing this, the flies feed and hydrate on standard medium at the bottom of flasks and essential oil is allowed to volatilize from the top in order to reach the fly's respiratory system. Each flask received one of the following treatments: control and 3, 15 and 30 μg mL–1 of essential oil. All the different essential oil concentrations were exposed at 3 hour or 12 hours. Previous research showed that these essential oil concentrations and exposure times have minimal effects on flies’ survival.13
2.5. Survival assays
Survival assays represent a measure of fly mortality after E. uniflora leaves’ essential oil exposure. Sixty flies per treatment were replicated a further two times. Flies were placed in 330 cm3 flasks containing standard medium and maintained in incubators at a controlled temperature (25 ± 1 °C). Mortality was recorded until 72 h.
2.6. Isolation of mitochondria
Mitochondria were isolated according to previous work with minor modifications.31 Around 50 flies were immobilized by chilling on ice and then decanted into a chilled mortar; 2 mL of ice-cold isolation medium containing 250 mM sucrose, 0.1% free fat acid bovine serum albumin, 2 mM EGTA and 5 mM Tris-HCl (pH 7.4) was added and the flies were pressed gently with a pestle. The resulting homogenate was filtered to remove the tissue particles with a nylon filter membrane (10 μm pore size) and centrifuged at 200g for 3 min. The supernatant was collected and a further centrifugation at 9000g for 10 min was performed. Next, the mitochondrial pellet was carefully resuspended in 2 mL of ice-cold albumin free isolation medium, followed by centrifugation at 9000g for 10 min. This final pellet was resuspended in 100 μl of albumin free isolation medium; this resuspension contained the isolated mitochondria and was used in the respiration assay described below. All the above procedures were performed at 4 °C. Protein concentration was determined using a Bradford assay using bovine serum albumin standards.32
2.7. High-resolution respirometry (HRR)
An Oxygraph-2k (O2k, Oroboros Instruments, Innsbruck, Austria) was used to determine mitochondrial bioenergetics. Isolated mitochondria from D. melanogaster (0.05 mg mL–1) were transferred to 2 mL of MiR05 solution (110 mM sucrose, 60 mM K-lactobionate, containing 0.5 mM EGTA, 3 mM MgCl2, 20 mM taurine, 10 mM KH2PO4, 20 mM HEPES and 0.1% essentially fatty acid-free BSA, pH 7.1).33 All experiments were performed at 24 °C using DatLab 4.0 software (Oroboros Inc., Austria), with continuous stirring at 750 rpm.
2.7.1. Mitochondrial respiration assays
Using titration, we assayed the abilities of a series of substrates and inhibitors to influence mitochondrial function as reflected in the difference in respiration states. Mitochondrial bioenergetics in D. melanogaster mitochondria was carried out according to the literature.34,35 l-Proline + pyruvate + malate and succinate were used as oxidizable substrates in all experiments. After signal stabilization, the basal respiration supported by endogenous substrates, the complex I (CI)-mediated leak (LEAK) respiration, was determined using 5 mM pyruvate, 5 mM l-proline and 1 mM malate. CI-mediated OXPHOS (OXPHOS) was determined using ADP (2.5 mM). The functional integrity of the outer mitochondrial membrane (CIcOXPHOS) was determined by addition of exogenous cytochrome c (cyt c) from equine heart (10 μM). Outer mitochondrial membrane disruption is associated with cyt c release; so, the respiration stimulated after the cyt c addition is proportional to membrane damage.35 Respiratory control ratios (RCR = CIOXPHOS/CILEAK) and the increase of oxygen flux after injection of cyt c (CIcOXPHOS/CIOXPHOS) were used as quality control for isolated mitochondria. The convergent electron flow during the maximal OXPHOS respiration (CIc + CIIOXPHOS) was determined with substrates of CIc and CII (10 mM succinate). The ETS respiration represents the noncoupled respiration using FCCP (optimum concentration reached between 0.5 and 1.5 μM); CIc + CII-mediated ETS respiration (CIc + CIIETS) was determined using FCCP (optimum concentration reached between 0.5 and 1.5 μM). CII-mediated ETS respiration (CIIETS) was determined with 0.5 μM rotenone. The addition of 2.5 μM antimycin A inhibited complex III, resulting in nonmitochondrial respiration, the residual oxygen consumption (Rox) with small contributions from electron leak in the uncoupled state.
2.7.2. Mitochondrial membrane potential (ΔΨm) assays
The mitochondrial membrane potential, ΔΨm, was determined using safranine dye following previously published protocols.36,37 Briefly, the O2k-Fluorescence LED2 module was equipped with filter sets for safranine O (excitation at 495 nm and emission at 587 nm). Safranine dissolved in distilled water was titrated up to a final concentration of 2.0 μM. Isolated mitochondria were then added to chambers in the presence of oxidizable substrates for complex I (l-proline + pyruvate + malate).
2.8. Statistics
Statistical analysis was performed using GraphPad (version 5.0 for Macintosh OS X, GraphPad Software, San Diego, CA). Significance was assessed by one-way analysis of variance (ANOVA), followed by Bonferroni's test for post hoc comparison. Values of p ≤ 0.05 were considered statistically significant.
3. Results
3.1. Chemical compounds in the E. uniflora essential oil
Phytochemical analyses of essential oil showed that the main compounds found were curzerene (39.27%), γ-elemene (12.68%), trans-β-elemenone (8.79%) and atractylone (5.48%) (Table 1).
Table 1. Composition of Eugenia uniflora leaves’ essential oil.
| Compounds | RI a | RI b | Oil |
| % | |||
| β-Pinene | 982 | 980 | 0.95 |
| β-Myrcene | 991 | 991 | 0.13 |
| Limonene | 1034 | 1031 | 0.78 |
| γ-Terpinene | 1059 | 1062 | 2.04 |
| Linalool | 1097 | 1098 | 0.05 |
| δ-Elemene | 1338 | 1338 | 0.86 |
| α-Cubebene | 1345 | 1345 | 0.10 |
| β-Caryophyllene | 1419 | 1418 | 2.39 |
| γ-Elemene | 1436 | 1433 | 12.68 |
| Aromadendrene | 1438 | 1439 | 0.15 |
| α-Humelene | 1456 | 1454 | 1.29 |
| γ-Muurolene | 1475 | 1477 | 2.73 |
| Germacreno D | 1479 | 1480 | 2.95 |
| β-Selilene | 1489 | 1485 | 0.64 |
| Curzerene | 1497 | 1496 | 39.27 |
| γ-Cadinene | 1508 | 1508 | 0.21 |
| α-Cadidene | 1535 | 1538 | 0.09 |
| Germacrene B | 1558 | 1556 | 3.72 |
| Spathulenol | 1578 | 1576 | 1.06 |
| Viridiflorol | 1591 | 1590 | 2.65 |
| trans-β-Elemenone | 1603 | 1601 | 8.79 |
| Atractylone | 1653 | 1653 | 5.48 |
| Total identified (%) | 89.01 | ||
aRetention indices experimental (based on the homologous series of n-alkane C7–C30).
bRetention indices from the literature (Adams, 1995).
3.2. Effects of E. uniflora essential oil on survival
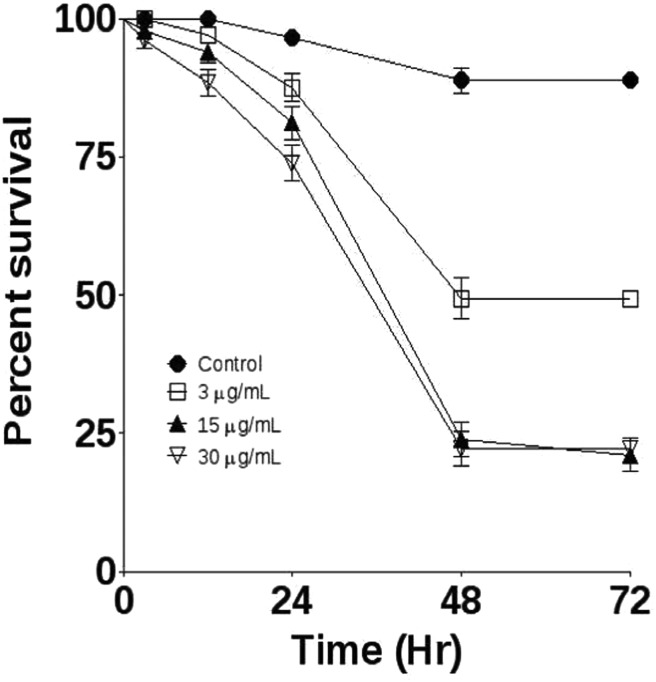
Fruit flies were monitored for 72 h to determine the effects of treatment with essential oil. All concentrations caused significant mortality. At 72 h, treatment with E. uniflora 3 μg mL–1, 15 μg mL–1 and 30 μg mL–1 presented 51, 79 and 78% mortality, respectively (Fig. 1).
Fig. 1. Effects of treatment with E. uniflora leaves’ essential oil on the survival challenge. Fruit flies were exposed through the fumigation method to 3, 15 and 30 μg mL–1. Survival was followed for 72 h, n = 180 per group.
3.2. Effects of E. uniflora essential oil on the mitochondrial membrane potential (ΔΨm)
To determine the effects of E. uniflora essential oil on mitochondrial function, we exposed flies to the oil through fumigation and measured the ΔΨm at 3 and 12 h posttreatment. Only the highest concentration, 30 μg mL–1, of E. uniflora essential oil was able to significantly reduce the ΔΨm at 3 and 12 h (Fig. 2).
Fig. 2. Determination of mitochondrial transmembrane potential (ΔΨm) in the mitochondria of D. melanogaster exposed to E. uniflora leaves’ essential oil through the fumigation protocol at 3 h and 12 h. Results are presented as means ± S.E.M, from 4 different preparations. *p ≤ 0.05 indicates statistical difference from the control group by one-way ANOVA, followed by Bonferroni's post hoc test.
3.3. Respiratory control ratios (RCRs) and cytochrome c effect
To evaluate mitochondrial functionality, the RCRs were determined as an indicator of the state of mitochondrial coupling. All oil concentrations tested were able to reduce the RCR at the 12 h time point, and all except the 3 μg mL–1 concentration were able to reduce RCR at 3 h (Table 2). To test the integrity of the mitochondrial outer membrane cyt c was added, and the rates with and without cyt c were compared. A satisfactory ratio of the membrane integrity should be less than 1.1.38 As expected, respiration rates before and after cyt c additions were similar in all preparations, attesting the integrity of mitochondria (Table 2).
Table 2. Effects of E. uniflora essential oil on the values of respiratory control ratio, uncoupling control ratio and cytochrome C effect in the mitochondria of D. melanogaster.
| RCR for complex I | Cyt C effect | |
| 3 h | ||
| Control | 11.4 ± 0.03 | 1.09 ± 0.003 |
| 3 μg mL–1 | 7.8 ± 0.03 | 1.07 ± 0.002 |
| 15 μg mL–1 | 6.4 ± 0.7* | 1.08 ± 0.006 |
| 30 μg mL–1 | 4.8 ± 1.0** | 1.07 ± 0.02 |
| 12 h | ||
| Control | 16.4 ± 3.7 | 1.09 ± 0.009 |
| 3 μg mL–1 | 9.6 ± 0.6** | 1.09 ± 0.01 |
| 15 μg mL–1 | 3.8 ± 0.4*** | 1.04 ± 0.01 |
| 30 μg mL–1 | 3.9 ± 0.1*** | 1.05 ± 0.01 |
3.4. Mitochondrial O2 flux consumption
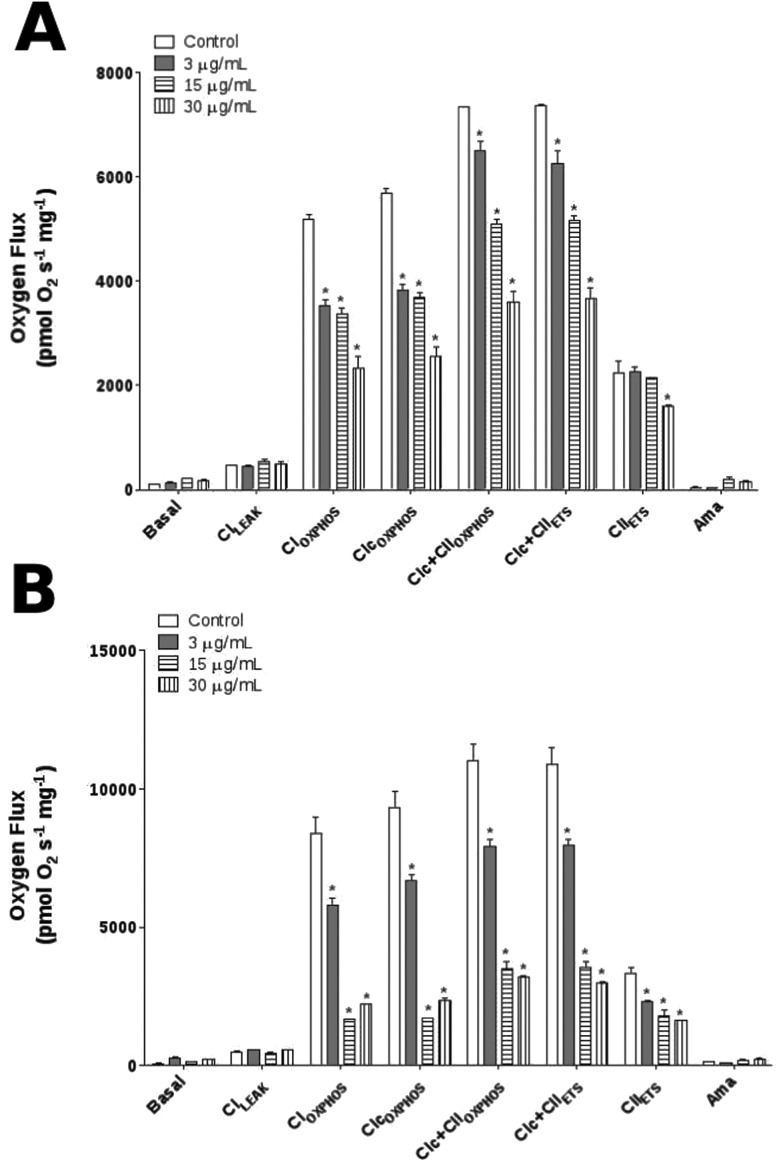
To investigate the effects of E. uniflora at the mitochondrial level, we verified the mitochondrial bioenergetics function using high-resolution respirometry (HRR) (Fig. 3). As expected, the basal and CILEAK respiration values showed no significant differences between experimental treatment and controls at 3 and 12 h. In contrast, when OXPHOS was induced by the addition of saturating ADP, all oil concentrations tested were able to decrease CIOXPHOS and CIc + CIIOXPHOS at 3 and 12 h, except for 3 μg mL–1 concentration that remained unchanged at 3 h. Furthermore, the results were similar when the respiration was uncoupled using FCCP indicating a significant decrease of ETS in the CIc + CIIETS respiration. However, 30 μg mL–1 was able to reduce the CIIETS at 3 h, while all oil concentrations promoted the reduction of CIIETS at 12 h. As expected, antimycin A (residual oxygen consumption, ROX) decreased the O2 flux consumption to the basal levels without the above-mentioned differences.
Fig. 3. O2 flux measured in the mitochondria of D. melanogaster exposed to E. uniflora leaves’ essential oil through the fumigation protocol. (A) HRR representative at 3 h and (B) HRR representative at 12 h. Mitochondrial functions are presented with the abbreviation(s) of the complex(es) involved followed by the state of respiration measured in the presence of l-proline + pyruvate + malate (CILEAK), + ADP (CIOXPHOS), + cytochrome c (CIcOXPHOS), + succinate (CIc + CIIOXPHOS), + FCCP (CIc + CIIETS), + rotenone (CIIETS), + antimycin A (Ama) used to correct for residual O2 consumption. Results are presented as means ± S.E.M, from 4 different preparations. *p ≤ 0.05 indicates statistical difference from the control group by one-way ANOVA, followed by Bonferroni's post hoc test.
3.5. Mitochondrial flux control ratio
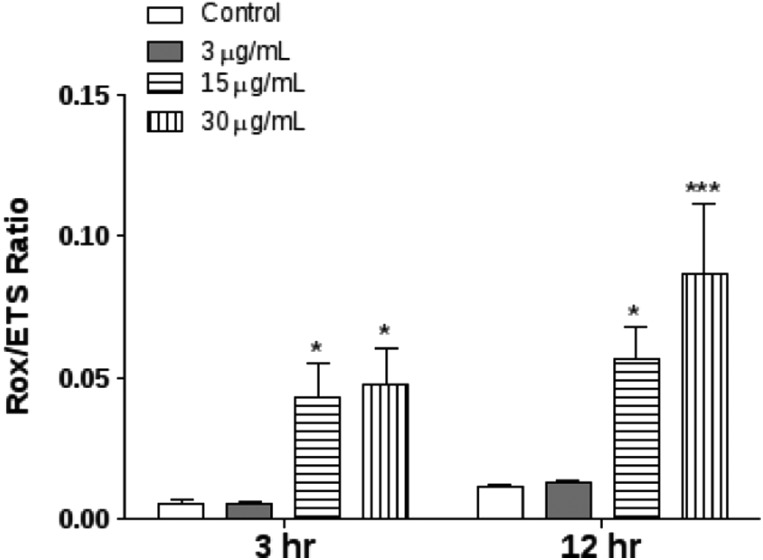
To analyze the magnitude of residual oxygen consumption relative to the maximum oxygen consumption capacity the ROX/ETS ratio was determined after E. uniflora essential oil treatment. The ROX/ETS ratio was significantly increased at 15 and 30 μg mL–1 in both 3 and 12 h post treatment (Fig. 4).
Fig. 4. Determination of the respiratory control ratio of D. melanogaster exposed to E. uniflora leaves’ essential oil through the fumigation protocol. Residual oxygen consumption relative to the maximum oxygen consumption capacity the (ROX/ETS) ratio. Results are presented as means ± S.E.M, from 4 different preparations. *p ≤ 0.05 and ***p ≤ 0.001 indicate statistical difference from the control group by one-way ANOVA, followed by Bonferroni's post hoc test.
3.6. Bioenergetics capacity and OXPHOS coupling efficiency
We quantified the mitochondrial bioenergetics capacity by subtracting the ADP-induced CIOXPHOS values from the CILEAK values (Fig. 5A). At both 3 and 12 h, all the E. uniflora essential oil concentrations reduced the flies mitochondrial bioenergetics capacity. It is noteworthy that the OXPHOS coupling efficiency was also significantly reduced by treatment with any of the oil concentrations at both 3 and 12 h (Fig. 5B).
Fig. 5. Determination of bioenergetics parameters of D. melanogaster exposed to E. uniflora leaves’ essential oil through the fumigation protocol. (A) Analysis of bioenergetics capacity; and (B) Analysis of oxidative phosphorylation coupling in D. melanogaster exposed to E. uniflora leaves’ essential oil through the fumigation protocol. Results are presented as means ± S.E.M, from 4 different preparations. *p ≤ 0.05, **p ≤ 0.01 and ***p ≤ 0.001 indicate statistical difference from the control group by one-way ANOVA, followed by Bonferroni's post hoc test.
3.7. Substrate control ratio (SCR)
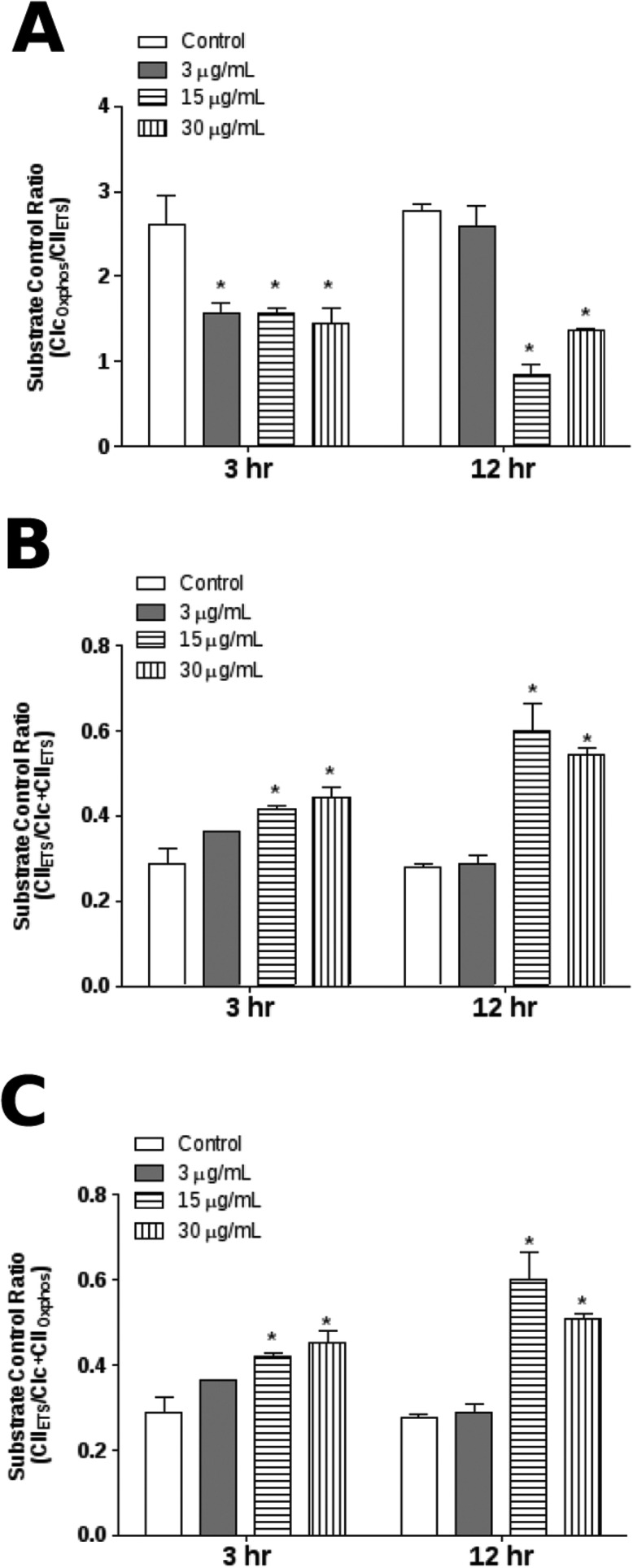
Substrate control ratios (SCR) are flux ratios normalized with respect to a common reference state of substrate supply at a fixed coupling state.33 In order to determine the effects of E. uniflora on mitochondrial respiratory control, we quantified the substrate control ratio (SCR). All concentrations resulted in a significant reduction of the CIOXPHOS/CIIETS ratio at 3 h and 12 h, except for 3 μg mL–1 concentration at 12 h (Fig. 6A). In contrast, when the contribution of CII in the convergent electron flow through the Q-junction was evaluated in mitochondrial respiratory control we noticed a significant increase of the CIIETS/CIc + CIIETS ratio and the CIIETS/CIc + CIIOXPHOS ratio induced by treatment with E. uniflora (Fig. 6B and C).
Fig. 6. Analysis of substrate control ratio (SCR) of D. melanogaster exposed to E. uniflora leaves’ essential oil through the fumigation protocol. (A) CIOXPHOS/CIIETS ratio; (B) CIIETS/CIc + CIIETS ratio; and (C) CIIETS/CI + CIIOXPHOS ratio. Results are presented as means ± S.E.M, from 4 different preparations. *p ≤ 0.05 indicates statistical difference from the control group by one-way ANOVA, followed by Bonferroni's post hoc test.
4. Discussion
Mitochondrial function is absolutely critical for the survival of aerobic organisms and mitochondrial bioenergetics function can, therefore, be the target of biological compounds to induce cellular toxicity.20,39 In a previous study, E. uniflora essential oil demonstrated a marked insecticidal effect in fruit flies, an effect that was associated with the pro-oxidant properties of the essential oil.13 In that study, flies exposed to the essential oil via fumigation had high mortality and demonstrated locomotor deficits. These changes to complex phenotypes occurred in parallel to oxidative stress and antioxidant signaling responses, including Nrf2 upregulation,13 but the mechanism through which the essential oil induces toxicity is still unclear. Considering the critical role of mitochondria in toxicological processes, in the present study we investigated whether mitochondrial function was a target of E. uniflora essential oil. This is the first study, to our knowledge, demonstrating that E. uniflora essential oil is able to induce mitochondrial dysfunction in D. melanogaster, and, given the close relationship between mitochondrial function and bioenergetics, our results suggest an important mechanism of action for insecticidal effects.
The toxicity of essential oil was determined by survival assays and the E. uniflora treatment promoted an elevated mortality of D. melanogaster (Fig. 1). In order to increase our knowledge of insecticidal properties we tested the effect of E. uniflora essential oil on parameters of mitochondrial functionality and found that only the highest concentration (30 μg mL–1) of oil promoted the disruption of ΔΨm (Fig. 2). ΔΨm is an important factor for cellular energy production because, following the chemiosmotic theory of OXPHOS, the electron flow and proton-pumping to the intermembrane space drives the generation of the proton motive force used for ATP synthesis.17,40 The collapse of ΔΨm, then, plays an important role in the impairment of physiological processes necessary for the maintenance of mitochondrial homeostasis, such as the intracellular Ca2+ balance. Loss of this balance can induce mitochondrial swelling, mitochondrial transition permeability and, consequently, bioenergetics failure due to a nonspecific increase in membrane permeability.41 Some authors have linked the collapse of ΔΨm to the lipophilic compounds present in essential oils, which are able to change the mitochondrial membrane permeability.42 Our results suggest, however, that such changes in permeability are not the only possible mechanism, since the lower concentration of oil tested here did not present similar results. Results from our earlier work linking E. uniflora exposure to a significant increase of oxidative damage13 provides the possibility of oxidative stress as a mechanism of toxicity induced by essential oil triggering mitochondrial dysfunction and reducing the capacity of cellular energy production.16
Bioenergetics failure is a process associated not only with the loss of ΔΨm but also impairment of ETS and OXPHOS capacity; so, the insecticidal properties of E. uniflora cause deregulation of systems that are critical for cellular energetics.43 Altogether, the HRR assays showed that the essential oil promoted a concentration-dependent reduction of RCR (Table 1). This result strongly suggests that exposure leads to an impairment of mitochondrial functionality through damage in the outer and inner membranes driven by pro-oxidant effects of the essential oil. Consistent with this explanation, E. uniflora essential oil has been demonstrated to induce a time-dependent increase of lipid peroxidation and ROS generation.30 In addition, the involvement of oxidative stress in the mitochondria from D. melanogaster can be evaluated by the ROX/ETS ratio (Fig. 3), as an index of the magnitude of residual oxygen consumption relative to the maximum oxygen consumption capacity. This ratio was increased after treatment with E. unifora indicating a reduction of the coupling state of mitochondria and demonstrating possible electron leakage correlated with the redox impairment. These results suggest that E. uniflora treatment causes bionergetics impairment by disrupting the outer mitochondrial membrane. Altogether, the reduction of bioenergetics capacity and OXPHOS coupling efficiency (Fig. 5A and B) demonstrate bioenergetics inefficiencies compromising the capacity to re-phosphorylate ADP in OXPHOS.
Our HRR data indicate that E. uniflora essential oil caused a significant reduction of the OXPHOS in CIOXPHOS and CIc + CIIOXPHOS (Fig. 2). This reduction might be due to, at least in part, an impairment of the mitochondrial OXPHOS support system, comprising the ADP and ATP carrier and ATP synthase, acting as a limiting factor to the mitochondrial bioenergetics function during exposure to the essential oil. It is known that some insecticides exert a significant inhibitory effect on the ATPase activity.43,44 Consistent with this, we explored the effects of E. uniflora on the electron transport system (ETS) in the presence of an uncoupler (FCCP) which represents the maximum oxygen consumption capacity and noted the disruption of ETS in CIc + CIIETS and CIIETS (Fig. 2). Changes in the electron flow induce ROS generation, presenting a synergism with the pro-oxidant effect of essential oil and triggering enhancements of mitochondrial ROS generation.42 In addition, oxidative damage reportedly changes the fluidity of the mitochondrial membrane resulting in leakage of radicals.45 Previous research demonstrated that the oxidative stress condition could trigger the release of iron from the iron-sulfur center present in complexes I and II, which may exacerbate ROS generation via Fenton-like mechanisms.46 Our SCR results indicate that E. uniflora changes the mitochondrial bioenergetics metabolism of NADH oxidation (Fig. 6). Fumigation with essential oil resulted in an increase of SCR which indicates a compensatory effect on the CII respiration in relation to the convergent pathway (CIc + CIIOXPHOS). However, the dysfunction of CII respiration is able to trigger the accentuated superoxide generation in mitochondria,47,48 since the electron flow overload toward the Q-junction could promote the electron leakage which induces ROS generation and lipid peroxidation, conditions associated with oxidative stress, triggering mitochondrial dysfunction.13 Additionally, treatment with iron and E. uniflora had a synergistic toxic effect increasing the mortality in D. melanogaster.30 In line with this, the mitochondrial dysfunction induced by E. uniflora could be related to electron leakage that occurs mainly at complex I due to the forward and reverse electron flow, and complex III due to the generation of the semiquinone radical in the Q cycle producing ROS at both sides of the inner membrane.49,50 Indeed, dysfunction in ETS, and the redox imbalance in the electron donors, enhances ROS generation leading to bioenergetic failure.14,20 In summary, our work demonstrates for the first time that essential oil from E. uniflora leaves has insecticidal properties linked to mitochondrial dysfunction.
5. Conclusion
Our findings indicate that exposure of flies to E. uniflora essential oil results in substantial impairment of mitochondrial respiration. The essential oil exposure resulted in reduction of both OXPHOS and ETS respiration through uncoupling, causing a decrease in bioenergetics capacity in isolated mitochondria, and resulting in cellular energetics crisis. The effects of the E. uniflora essential oil are likely a function of a range of compounds in the oil potentially including monoterpenes, sesquiterpenes, and phenylpropanoids. Thus, the mitochondrial impairment responsible for the observed bioenergetics dysfunction could be related to multifactorial mechanisms, including pro-oxidant properties, ROS generation, and deregulation of OXPHOS, which play a central role in the toxicity elicited by the essential oil in Drosophila and highlight its potent insecticidal activity.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
The authors acknowledge CNPq, Brazil (404603/2015-7; 456207/2014-7), FAPERGS (2380-2551/14-8), and PROPESQ-Unipampa for financial support. JLF is recipient of a CNPq research fellowship (310861/2014-4).
References
- Pavela R. Phytother. Res. 2008;22:274–278. doi: 10.1002/ptr.2300. [DOI] [PubMed] [Google Scholar]
- Pimentel D., in Integrated Pest Management: Innovation-Development Process, Springer, Netherlands, Dordrecht, 2009, pp. 83–87. [Google Scholar]
- Vincent C., Hallman G., Panneton B., Fleurat-Lessard F. Annu. Rev. Entomol. 2003;48:261–281. doi: 10.1146/annurev.ento.48.091801.112639. [DOI] [PubMed] [Google Scholar]
- Dayan F. E., Cantrell C. L., Duke S. O. Bioorg. Med. Chem. 2009;17:4022–4034. doi: 10.1016/j.bmc.2009.01.046. [DOI] [PubMed] [Google Scholar]
- Isman M. B. and Akhtar Y., in Insecticides Design Using Advanced Technologies, Springer, Berlin, Heidelberg, 2007, pp. 235–248. [Google Scholar]
- Gallucci S., Neto A. P., Porto C., Barbizan D., Costa I., Marques K., Benevides P., Figueiredo R. J. Essent. Oil Res. 2010;22:176–179. [Google Scholar]
- Oliveira A. L., Lopes R. B., Cabral F. A., Eberlin M. N. Food Chem. 2006;99:1–5. [Google Scholar]
- Consolini A. E., Sarubbio M. G. J. Ethnopharmacol. 2002;81:57–63. doi: 10.1016/s0378-8741(02)00039-9. [DOI] [PubMed] [Google Scholar]
- Victoria F. N., Lenardão E. J., Savegnago L., Perin G., Jacob R. G., Alves D., da Silva W. P., da Motta A. de S., Nascente P. da S. Food Chem. Toxicol. 2012;50:2668–2674. doi: 10.1016/j.fct.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Rodrigues K. A. da F., Amorim L. V., de Oliveira J. M. G., Dias C. N., Moraes D. F. C., Andrade E. H. de A., Maia J. G. S., Carneiro S. M. P., Carvalho F. J. Evidence-Based Complementary Altern. Med. 2013;2013:279726. doi: 10.1155/2013/279726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira M. D. L., Andrade C. A. S., Santos-Magalhães N. S., Coelho L. C. B. B., Teixeira J. A., Carneiro-da-Cunha M. G., Correia M. T. S. Lett. Appl. Microbiol. 2008;46:371–376. doi: 10.1111/j.1472-765X.2007.02319.x. [DOI] [PubMed] [Google Scholar]
- Cecílio A. B., de Faria D. B., Oliveira P. de C., Caldas S., de Oliveira D. A., Sobral M. E. G., Duarte M. G. R., Moreira C. P. de S., Silva C. G., de Almeida V. L. J. Ethnopharmacol. 2012;141:975–981. doi: 10.1016/j.jep.2012.03.031. [DOI] [PubMed] [Google Scholar]
- da Cunha F. A. B., Wallau G. L., Pinho A. I., Nunes M. E. M., Leite N. F., Tintino S. R., da Costa G. M., Athayde M. L., Boligon A. A., Coutinho H. D. M., Pereira A. B., Posser T., Franco J. L. Toxicol. Res. 2015;4:634–644. [Google Scholar]
- Le Bras M., Clément M. V., Pervaiz S., Brenner C. Histol. Histopathol. 2005;20:205–219. doi: 10.14670/HH-20.205. [DOI] [PubMed] [Google Scholar]
- Lee H. C., Wei Y. H. Adv. Exp. Med. Biol. 2012;942:311–327. doi: 10.1007/978-94-007-2869-1_14. [DOI] [PubMed] [Google Scholar]
- Carvalho N. R., da Rosa E. F., da Silva M. H., Tassi C. C., Dalla Corte C. L., Carbajo-Pescador S., Mauriz J. L., González-Gallego J., Soares F. A. PLoS One. 2013;8:e81961. doi: 10.1371/journal.pone.0081961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z., Ristow M. Antioxid. Redox Signaling. 2013;19:240–242. doi: 10.1089/ars.2013.5255. [DOI] [PubMed] [Google Scholar]
- Song C., Scharf M. E. Pest Manage. Sci. 2009;65:697–703. doi: 10.1002/ps.1747. [DOI] [PubMed] [Google Scholar]
- Scialò F., Sriram A., Fernández-Ayala D., Gubina N., Lõhmus M., Nelson G., Logan A., Cooper H. M., Navas P., Enríquez J. A., Murphy M. P., Sanz A. Cell Metab. 2016;23:725–734. doi: 10.1016/j.cmet.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L., Kepp O., Trojel-Hansen C., Kroemer G. Circ. Res. 2012;111:1198–1207. doi: 10.1161/CIRCRESAHA.112.268946. [DOI] [PubMed] [Google Scholar]
- Ott M., Gogvadze V., Orrenius S., Zhivotovsky B. Apoptosis. 2007;12:913–922. doi: 10.1007/s10495-007-0756-2. [DOI] [PubMed] [Google Scholar]
- Sastre J., Pallardó F. V., Viña J. Int. Union Biochem. Mol. Biol. Life. 2000;49:427–435. doi: 10.1080/152165400410281. [DOI] [PubMed] [Google Scholar]
- Pardo-Andreu G. L., Dorta D. J., Delgado R., Cavalheiro R. A., Santos A. C., Vercesi A. E., Curti C. Chem.-Biol. Interact. 2006;159:141–148. doi: 10.1016/j.cbi.2005.10.109. [DOI] [PubMed] [Google Scholar]
- Zhao H. W., Haddad G. G. Placenta. 2011;32(Suppl 2):S104–S108. doi: 10.1016/j.placenta.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues N. R., Batista J. E. dos S., de Souza L. R., Martins I. K., Macedo G. E., da Cruz L. C., da Costa Silva D. G., Pinho A. I., Coutinho H. D. M., Wallau G. L., Posser T., Franco J. L. Arabian J. Chem. 2015 [Google Scholar]
- Pandey U. B., Nichols C. D. Pharmacol. Rev. 2011;63:411–436. doi: 10.1124/pr.110.003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos F. J. A., Introdução a Fitoquímica Experimental, Fortaleza, 2009. [Google Scholar]
- Adams R. P., Identification of Essential Oil Components By Gas Chromatography/Mass Spectrometry, Allured Pub Corp (February 28, 2007), 4th edn, 1995. [Google Scholar]
- Paula M. T., Zemolin A. P., Vargas A. P., Golombieski R. M., Loreto E. L. S., Saidelles A. P., Picoloto R. S., Flores E. M. M., Pereira A. B., Rocha J. B. T., Merritt T. J. S., Franco J. L., Posser T. Environ. Toxicol. 2014;29:621–630. doi: 10.1002/tox.21788. [DOI] [PubMed] [Google Scholar]
- Pinho A. I., Wallau G. L., Nunes M. E. M., Leite N. F., Tintino S. R., da Cruz L. C., da Cunha F. A. B., da Costa J. G. M., Douglas Melo Coutinho H., Posser T., Franco J. L. Oxid. Med. Cell. Longevity. 2014;2014:696785. doi: 10.1155/2014/696785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa S., St-Pierre J., Partridge L., Brand M. D. Free Radical Biol. Med. 2003;35:938–948. doi: 10.1016/s0891-5849(03)00464-7. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Gnaiger E. Int. J. Biochem. Cell Biol. 2009;41:1837–1845. doi: 10.1016/j.biocel.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Pesta D., Gnaiger E. Methods Mol. Biol. 2012;810:25–58. doi: 10.1007/978-1-61779-382-0_3. [DOI] [PubMed] [Google Scholar]
- Pichaud N., Messmer M., Correa C. C., Ballard J. W. O. Mitochondrion. 2013;13:817–822. doi: 10.1016/j.mito.2013.05.008. [DOI] [PubMed] [Google Scholar]
- Akerman K. E., Wikström M. K. FEBS Lett. 1976;68:191–197. doi: 10.1016/0014-5793(76)80434-6. [DOI] [PubMed] [Google Scholar]
- Perevoshchikova I. V., Sorochkina A. I., Zorov D. B., Antonenko Y. N. Biochemistry. 2009;74:663–671. doi: 10.1134/s000629790906011x. [DOI] [PubMed] [Google Scholar]
- Kuznetsov A. V., Schneeberger S., Seiler R., Brandacher G., Mark W., Steurer W., Saks V., Usson Y., Margreiter R., Gnaiger E. Am. J. Physiol.: Heart Circ. Physiol. 2004;286:H1633–H1641. doi: 10.1152/ajpheart.00701.2003. [DOI] [PubMed] [Google Scholar]
- Smith R. A. J., Hartley R. C., Cochemé H. M., Murphy M. P. Trends Pharmacol. Sci. 2012;33:341–352. doi: 10.1016/j.tips.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Tait S. W. G., Green D. R. Nat. Publ. Gr. 2010;11:621–632. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- Brookes P. S., Yoon Y., Robotham J. L., Anders M. W., Sheu S.-S. Am. J. Physiol.: Cell Physiol. 2004;287:C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- Bakkali F., Averbeck S., Averbeck D. and Idaomar M..
- Hosamani R. Arch. Insect Biochem. Physiol. 2013;83:25–40. doi: 10.1002/arch.21094. [DOI] [PubMed] [Google Scholar]
- Subash S., Gupta C., Mishra M., Sharma A., Deepak Balaji T. G. R., Kumar R., Mishra R. K., Chowdhuri D. K. Ecotoxicol. Environ. Saf. 2010;2:1–9. doi: 10.1016/j.ecoenv.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Raza H., John A., Brown E. M., Benedict S., Kambal A. Toxicol. Appl. Pharmacol. 2008;226:161–168. doi: 10.1016/j.taap.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Thomas C., Mackey M. M., Diaz A. A., Cox D. P. Redox Rep. 2009;14:102–108. doi: 10.1179/135100009X392566. [DOI] [PubMed] [Google Scholar]
- Pfeiffer M., Kayzer E. B., Yang X., Abramson E., Kenaston M. A., Lago C. U., Lo H. H., Sedensky M. M., Lunceford A., Clarke C. F., Wu S. J., McLeod C., Finkel T., Morgan P. G., Mills E. M. J. Biol. Chem. 2011;286:37712–37720. doi: 10.1074/jbc.M111.271452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S. S., Hsiao M., Zhao H. W., Dugan L. L., Haddad G. G., Zhou D. PLoS One. 2012;7:e36801. doi: 10.1371/journal.pone.0036801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezek P., Hlavatá L. Int. J. Biochem. Cell Biol. 2005;37:2478–2503. doi: 10.1016/j.biocel.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Soares J. B. R. C., Gaviraghi A., Oliveira M. F. PLoS One. 2015;10:e0120600. doi: 10.1371/journal.pone.0120600. [DOI] [PMC free article] [PubMed] [Google Scholar]