Abstract
Type 2 diabetes (T2DM) is associated with structural cortical and subcortical alterations, although it is insufficiently clear if these alterations are driven by obesity or by diabetes and its associated complications. We used FreeSurfer5.3 and FSL-FIRST to determine cortical thickness, volume and surface area, and subcortical gray matter volume in a group of 16 normoglycemic obese subjects and 28 obese T2DM patients without clinically manifest micro- and marcoangiopathy, and compared them to 31 lean normoglycemic controls. Forward regression analysis was used to determine demographic and clinical correlates of altered (sub)cortical structure. Exploratively, vertex-wise correlations between cortical structure and fasting glucose and insulin were calculated. Compared with controls, obese T2DM patients showed lower right insula thickness and lower left lateral occipital surface area (PFWE < 0.05). Normoglycemic obese versus controls had lower thickness (PFWE < 0.05) in the right insula and inferior frontal gyrus, and higher amygdala and thalamus volume. Thalamus volume and left paracentral surface area were also higher in this group compared with obese T2DM patients. Age, sex, BMI, fasting glucose, and cholesterol were related to these (sub)cortical alterations in the whole group (all P < 0.05). Insulin were related to temporal and frontal structural deficits (all PFWE < 0.05). Parietal/occipital structural deficits may constitute early T2DM-related cerebral alterations, whereas in normoglycemic obese subjects, regions involved in emotion, appetite, satiety regulation, and inhibition were affected. Central adiposity and elevated fasting glucose may constitute risk factors.
Keywords: Obesity, Type 2 diabetes, Neuroimaging, Brain structure, Insulin, Glucose
Introduction
Type 2 diabetes mellitus (T2DM) and obesity are both worldwide health concerns, which are related to an increased risk of cardio- and cerebrovascular disease, cancer, cognitive impairment, and dementia (Reijmer et al. 2010; McCrimmon et al. 2012; Karlin et al. 2012). Cerebral damage, such as loss of (sub)cortical gray and white matter volume, and changes in brain functioning are also frequently observed in obese and T2DM groups (Widya et al. 2011; McCrimmon et al. 2012; Moran et al. 2013; Moulton et al. 2015).
In T2DM patients, a recent meta-analysis of 23 volumetric neuroimaging studies showed reduced global brain, as well as loss of hippocampus, basal ganglia, orbitofrontal, and occipital volume (Moulton et al. 2015), and a large case-control study reported loss of brain volume in T2DM patients compared to controls in areas that are commonly atrophic in Alzheimer’s disease, such as the temporal lobe, precuneus, and anterior cingulate cortex (Moran et al. 2013). Other studies have shown deficits in cortical thickness and surface area diffusely distributed throughout the brain (Brundel et al. 2010; Peng et al. 2015). It is generally accepted that micro- and macroangiopathy, the first serving as a marker for chronic hyperglycemic exposure, constitute major risk factors for these cerebral deficits, although not all studies agree (Moran et al. 2016). Almost all of the studies so far have not used the presence of micro- or macroangiopathy as an exclusion criterion for participation, and therefore the effects of T2DM in its early stages on the brain are insufficiently clear. Ideally, in the absence of clinically manifest angiopathy it should be possible to better determine the effects of obesity, blood pressure, and disturbances in glucose and insulin metabolism/production on the brain.
The effect of obesity on cortical structure is less clear-cut. Studies in the general population have found that increased BMI is related to thinning of the left lateral occipital and right ventromedial prefrontal cortex (Medic et al. 2016), and larger waist-hip-ratio and waist circumference to total brain and global gray matter volume (Debette et al. 2014). In the latter study, higher BMI was related to increased white matter hyperintensity volume. In contrast, higher visceral fat indices were found to relate to cortical thickening in both adolescents (Saute et al. 2016) and adults (Kaur et al. 2015). Studies directly comparing obese versus non-obese participants have shown increased cortical thickness (Ronan et al. 2016), and amygdala and hippocampus volume (Widya et al. 2011). Other studies, however have shown lower cortical thickness and volume diffusely distributed throughout the brain in elderly (Brooks et al. 2013; Marqués-Iturria et al. 2013). Willette and Kapogiannis (2015) reviewed 44 studies that assessed gray and white matter volume in obesity, concluding that there may be an association between obesity and frontal gray matter atrophy across all ages, as well as temporal and parietal gray matter atrophy in middle and old age (Willette & Kapogiannis, 2015). These different results could be the result of different processes. The first possible explanation is what is called the ‘obesity-paradox’, stating that midlife obesity, but not obesity in elderly, is especially harmful for the brain. A recent meta-analysis on dementia diagnosis by Kivimäky et al. demonstrated this ‘obesity paradox’. The hazard ratio for dementia per 5-points BMI increase was below 1 for those groups measured 10 and 10–20 years before diagnosis of dementia, but was 1.16 for those measured > 20 years before dementia diagnosis (Kivimäki et al. 2017). This indicates that midlife obesity was related to an increased dementia risk, but that later life obesity resulted in lower risk of dementia. A similar effect is observed in T2DM, where midlife onset T2DM is related to (more severe) cognitive dysfunction than later-life onset T2DM (Ryan et al. 2016). This ‘reverse epidemiology’, may be driven by the shorter time of late-life obesity/T2DM to exert their negative effects on the brain, compared with midlife obesity/T2DM. Alternatively, obese people are sometimes healthy, without blood pressure, lipid or glucose metabolism problems, even though they possess several risk factors for major diseases, especially regarding cardiovascular conditions (Kim et al., 2016). The differences may also be driven by various methodological differences, such as use of different variables, i.e. visceral fat or BMI, or the inclusion of obese subjects with and without glucose disorders. Due to these diverging results, it remains difficult to determine the risk factors of cerebral damage in obesity. Risk factors identified so far include BMI, hypertension, increased glucose levels, insulin resistance and cholesterol (Kullmann et al. 2016; Wennberg et al. 2016).
As it is less clear if the alterations in T2DM are driven by obesity or by diabetes and its associated complications, we aimed to perform a detailed evaluation of cortical and subcortical gray matter structural alterations in normoglycemic obese subjects, obese T2DM patients without clinically manifest micro- or macroangiopathy, and matched lean normoglycemic controls. It was hypothesized that structural alterations would be present in obesity, but more aggravated in T2DM patients. As T2DM is most prevalent during middle-age/old age, and because T2DM related comorbidities during this period are related to cognitive problems and dementia later in life (Ryan et al. 2016), patients during midlife/older age were included. Exploratively, the correlation between glucose and insulin and (sub)cortical structure was determined.
Materials and methods
Participants
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Medical Ethics Committee of the VU University Medical Center. Written informed consent was given by all participants. In the current study, data was pooled from 2 different studies performed at the VU University Medical Center. The first study assessed the acute effects of GLP-1 infusion on reward and satiety centers in the brain (NCT01281228) (van Bloemendaal et al. 2014), from which we included 14 controls, 16 obese participants and 13 obese T2DM patients. From the other study, assessing the long-term effects of GLP-1 treatment on reward and satiety centers in the brain (NCT01363609) (ten Kulve et al. 2016), 17 controls and 15 overweight/obese T2DM patients were included.
Inclusion criteria were age between 40 and 70 years, Caucasian ancestry, right-handedness, and stable body weight (i.e. <5% change during the 3 previous months). Women had to be post-menopausal, BMI had to be >30 kg/m2 for normoglycemic obese, >25 kg/m2 for overweight/obese T2DM participants and < 25 kg/m2 for controls. Controls and obese participants had to be normoglycemic (i.e. fasting plasma glucose <5.6 mmol/l and 2-h glucose <7.8 following a 75 g oral tolerance test), HbA1c of obese T2DM patients had to be between 42 and 69 mmol/mol (6.0–8.5%), with only oral (metformin/sulphonylurea derivates) treatment.
Exclusion criteria were: history of neurological, cardiovascular, renal or liver diseases, psychiatric disorders, malignancies, substance abuse, use of centrally acting agents or oral glucocorticoids, and the inability to undergo MRI scanning.
MRI
A 3 Tesla GE Signa HDxt scanner (General Electric, Milwaukee, Wisconsin, USA) was used for MRI scanning, using an 8-channel phased-array head coil. For this study, a T1-weighted fast spoiled gradient-echo (TR: 8.2 ms, TE: 3.2 ms, 1 mm slice thickness) and a T2-based Fluid Attenuating Inverse Recovery (3D-FLAIR; TR: 8000 ms, TE: 126 ms, slice thickness 1.2 mm) sequences was used. Excessive neck signal was removed by registration of a Montreal Neurological Institute standard brain (MNI-152) to each participant’s T1-MPRAGE, thereby identifying the lower border of the brain, to increase reliability of the analyses.
Whole-brain cortical structure analysis
To calculate cortical thickness, surface area, and volume the surface-based stream of Freesurfer 5.3 (http://surfer.nmr.mgh.harvard.edu) was used. A detailed description of the pipeline can be found elsewhere (Dale et al. 1999; Fischl and Dale 2000). In short, brain images were linearly registered to Talairach space to compute seed points, the bias field inhomogeneity was corrected, skull stripped, and white matter segmented using volumetric classification. Cutting planes derived from Talairach space were used to separate both hemispheres. An initial white matter surface was generated for each hemisphere from the results of the white matter segmentation. To find white and gray matter and pial surface, these initial surfaces were nudged into the direction of the gradient. To improve the estimation of the pial surface the 3D-FLAIR was added, as the contrast between the pial surface and dura is better on a 3D-FLAIR than on a T1-weighted image. The cortical surface of each hemisphere was automatically labeled by nonlinear surface-based registration of the Desikan-Killiany atlas, which resulted in 35 cortical parcellations per hemisphere (Fischl et al. 2004). The resulting white and pial surfaces were manually checked, and corrected if necessary.
Subcortical volume analysis
FMRIB’s Software Library 5.0.8 (FSL; http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/) FIRST was used for subcortical analyses (Patenaude et al. 2011). Further details on FSL-FIRST can be found elsewhere (Patenaude et al. 2011). In short, FSL-FIRST models the outer surface of the bilateral hippocampus, thalamus, amygdala, nucleus accumbens, caudate nucleus, globus pallidus and putamen by creating a vertex-based mesh for each image. Subsequently, each voxel within the images is assigned the label of the structure to which that voxel belongs, taking into account individual variations in surface shape of each structure, as well as the presence of neighboring structures. Next, in the participant’s native space, volume of each subcortical structure is calculated. All segmentations were manually checked, and corrected if necessary. To be able to perform group comparisons, all volumes were corrected for head size by multiplying the participant’s subcortical volume by its own V-scaling factor. The V-scaling factor is obtained from FSL-SIENAX (Smith et al. 2004) and calculated by affine registration of the T1-MPRAGE brain image to MNI-152 standard brain (Jenkinson et al. 2002). This value represents the factor with which the brain volume needs to be multiplied to normalize to MNI-152 standard brain.
Statistical analyses
Between-group participant characteristics were analyzed using one-way ANOVA with Bonferroni correction for continuous variables, Kruskal-Wallis test for non-normally distributed variables, and χ2-test for categorical variables.
First, to assess if obesity and T2DM had an effect on global cortical structure, whole brain cortical thickness, volume and surface area were compared between the groups, correcting for age, sex, hypertension, and estimated intracranial volume. Next, local effects on thickness, volume, and surface area were analyzed using FreeSurfer 5.3 vertex-wise general linear modeling for the main effect of group, again correcting for age, sex, hypertension and estimated intracranial volume. To allow for group comparisons, thickness, surface area, and volume data were smoothed with a 10 mm full width at half maximum Gaussian kernel and transformed into fsaverage standard space, to ensure comparability between scans. Clusters were identified using a cluster-wise threshold of P < 0.01. Correction for multiple comparisons was performed using Monte Carlo Z simulation with 10,000 iterations. The Family Wise Error (FWE) corrected P-value was set a P < 0.05, after multiplying the P-value by 2 correcting for testing two hemispheres. To identify brain regions with a shared influence of BMI/obesity in both T2DM and normoglycemic obese participants, a conjunction analysis was performed (Nichols et al. 2005). Normalized for head size bilateral subcortical volume was compared between groups using a multivariate ANCOVA model, corrected for age, sex, and hypertension, applying Bonferroni correction for multiple comparisons in SPSS 20. Correlations between (sub)cortical structure and medical and demographical variables were determined using forward linear regression modeling. To increase power these correlations were calculated in the whole group, adding group allocation as confounding factor. Correlations between thickness/surface area/volume and glucose/insulin were calculated in a vertex-wise way correcting for estimated intracranial volume and group allocation.
All analyses were preformed using IBM SPSS Statistics 20 (IBM SPSS, Chicago, IL) or FreeSurfer 5.3. A P < 0.05 was considered to be statistically significant.
Results
Participant characteristics
As is shown in Table 1, obese T2DM patients had higher HbA1c, fasting glucose levels, and hypertension rates, but a more favorable lipid profile than the other groups (P < 0.05). Compared with controls, obese T2DM patients had higher systolic and diastolic blood pressure (P < 0.05), triglycerides and fasting insulin levels were higher in obese T2DM and obesity groups as compared to controls (P < 0.05). Manual edits (mainly editing brain voxels), were performed for 8 controls, 5 normoglycemic obese, and 10 T2MD participants (P = 0.729). Estimated total intracranial volume was not statistically significantly different between the groups (P = 0.149).
Table 1.
Participant characteristics
| Controls | Obese | Type 2 diabetes | P value | |
|---|---|---|---|---|
| Age (years) | 57.08 ± 7.10 (41.03–66.92) |
58.01 ± 8.39 (40.32–68.09) |
60.44 ± 5.05 (50.86–70.24) |
0.158 |
| Sex, male/female (% male) | 16/15 (51.6) | 8/8 (50) | 15/13 (53.6) | 0.999 |
| Diabetes duration (years) | – | – | 8.15 ± 4.81 (1–20) |
– |
| BMI (kg/m2) | 22.96 ± 1.64 (20.00–25.41) |
32.58 ± 2.86a (29.28–39.35) |
32.25 ± 4.51a (26.90–42.70) |
<0.001 |
| Systolic blood pressure (mmHg) | 118.32 ± 16.17 (88.67–159.00) |
126.75 ± 12.08 (99.00–148.00) |
135.31 ± 13.11a (110.67–163.00) |
<0.001 |
| Diastolic blood pressure (mmHg) | 74.60 ± 10.51 (58.00–98.67) |
79.14 ± 7.73 (62.00–92.00) |
81.00 ± 8.84a (65.33–100.00) |
0.033 |
| Antihypertensive medication use (%) | 0 (0) | 3 (18.8) | 17 (60.7)a, b | <0.001 |
| Hypertension (%)c | 5 (16.1) | 4 (25) | 18 (64.3)a, b | <0.001 |
| HbA1c (%) | 5.61 ± 0.36 (5.00–6.00) |
5.58 ± 0.27 (5.00–6.10) |
6.99 ± 1.05a, b (5.70–9.00) |
<0.001 |
| HbA1c (mmol/mol) | 37.24 ± 1.57 (33.00–40.00) |
37.62 ± 3.03 (31.00–43.00) |
53.46 ± 10.74a, b (39.00–77.00) |
<0.001 |
| Total cholesterol (mmol/L) | 5.43 ± 0.89 (4.00–7.00) |
5.66 ± 0.89 (3.70–6.90) |
4.55 ± 1.38a, b (3.00–8.00) |
0.002 |
| HDL cholesterol (mmol/L) | 1.91 ± 0.48 (1.00–3.00) |
1.42 ± 0.43 (0.81–2.44) |
1.17 ± 0.33a (0.87–2.00) |
<0.001 |
| LDL cholesterol (mmol/L) | 3.13 ± 0.80 (2.00–5.00) |
3.45 ± 0.69 (2.10–4.60) |
2.39 ± 0.92a, b (1.00–5.00) |
<0.001 |
| Triglycerides (mmol/L) | 0.91 ± 0.41 (0.00–2.00) |
1.76 ± 1.31a (0.50–5.50) |
1.66 ± 1.01a (0.80–5.60) |
0.002 |
| Cholesterol medication use (%) | 0 (0) | 1 (6.3) | 19 (67.9)a, b | <0.001 |
| Fasting plasma glucose (mmol/L) | 4.85 ± 0.56 (4.00–5.70) |
5.27 ± 0.41 (4.40–5.90) |
8.50 ± 2.31a, b (5.70–15.00) |
<0.001 |
| Fasting insulin (pmol/L) | 37.56 ± 18.75 (16.00–109.50) |
83.62 ± 51.25a (42.40–243.40) |
90.77 ± 35.42a (40.80–157.10) |
<0.001 |
| Estimated intracranial volume (mL) | 1547 ± 127.70 (1352–1768) | 1505 ± 173.35 (1266–1874) | 1473 ± 143.67 (1272–1884) | 0.149 |
| Manual edits (%) | 8 (25.8) | 5 (31.3) | 10 (35.7) | 0.729 |
| Control pointsd | 1 | 0 | 0 | – |
| Brain editinge | 7 | 5 | 10 | – |
Data are presented as mean with standard deviation or absolute number with percentage between parentheses. The P-value represents the P-value of the overall F-test
adifferent from controls
bdifferent from obese
cHypertension was defined as a systolic blood pressure of 140 mmHg or above, a diastolic blood pressure of 90 mmHg or above, or the use of antihypertensive medication
dAdding control point in the white matter to push the white matter segmentation forward
eBrain editing consisting of removing excessive skull or changing the intensity of voxels that were wrongly labeled
Cortical gray matter structure
No significant differences were found when testing for a global effect of obesity and T2MD on mean cortical thickness, mean surface area or total grey matter volume between groups (P > 0.05; Table 2).
Table 2.
Values of whole brain indices of cortical structure and subcortical volume
| Controls | Normoglycemic obese | Type 2 diabetes | P valued | |
|---|---|---|---|---|
| Cortical thickness (mm) | ||||
| Whole brain | 2.47 ± 0.08 | 2.43 ± 0.08 | 2.43 ± 0.08 | 0.368 |
| Left hemisphere | 2.47 ± 0.08 | 2.44 ± 0.08 | 2.43 ± 0.09 | 0.313 |
| Right hemisphere | 2.46 ± 0.09 | 2.43 ± 0.08 | 2.43 ± 0.08 | 0.439 |
| Cortical surface area (mm2) | ||||
| Whole brain | 2511.09 ± 221.00 | 2435.03 ± 318.07 | 2398.78 ± 248.28 | 0.951 |
| Left hemisphere | 2507.86 ± 219.64 | 2436.05 ± 310.31 | 2397.12 ± 250.98 | 0.973 |
| Right hemisphere | 2514.31 ± 224.07 | 2434.00 ± 326.67 | 2400.44 ± 246.41 | 0.909 |
| Cortical volume (mL) | ||||
| Whole brain | 465.38 ± 43.61 | 449.97 ± 62.22 | 439.65 ± 35.27 | 0.833 |
| Left hemisphere | 233.22 ± 22.42 | 225.22 ± 30.57 | 219.98 ± 17.20 | 0.743 |
| Right hemisphere | 232.17 ± 21.52 | 224.74 ± 31.73 | 219.67 ± 18.38 | 0.911 |
| Subcortical volume (mL)e | ||||
| Bilateral amygdala | 1.86 ± 0.29 | 2.17 ± 0.25a | 2.01 ± 0.23 | 0.001 |
| Bilateral thalamus | 10.16 ± 0.72 | 10.66 ± 0.57a, c | 10.04 ± 0.63b | 0.010 |
| Bilateral caudate nucleus | 4.65 ± 0.47 | 4.61 ± 0.32 | 4.59 ± 0.36 | 0.871 |
| Bilateral putamen | 6.41 ± 0.71 | 6.45 ± 0.57 | 6.24 ± 0.58 | 0.833 |
| Bilateral pallidum | 2.35 ± 0.21 | 2.44 ± 0.15 | 2.35 ± 0.17 | 0.174 |
| Bilateral hippocampus | 5.10 ± 0.52 | 5.31 ± 0.39 | 5.17 ± 0.46 | 0.305 |
| Bilateral nucleus accumbens | 0.63 ± 0.13 | 0.61 ± 0.08 | 0.60 ± 0.12 | 0.962 |
Data are presented as mean with standard deviation. The P-value represents the overall P-value of the F-test
adifferent from controls
bdifferent from obese
cdifferent from type 2 diabetes
dAnalyses of cortical thickness, surface area and volume are corrected for age, sex, hypertension and estimated total intracranial volume. Subcortical analyses were corrected for age, sex and hypertension only
eSubcortical volume was corrected for head size by multiplying the participant’s subcortical volume by its own V-scaling factor, obtained by FSL-SIENAX
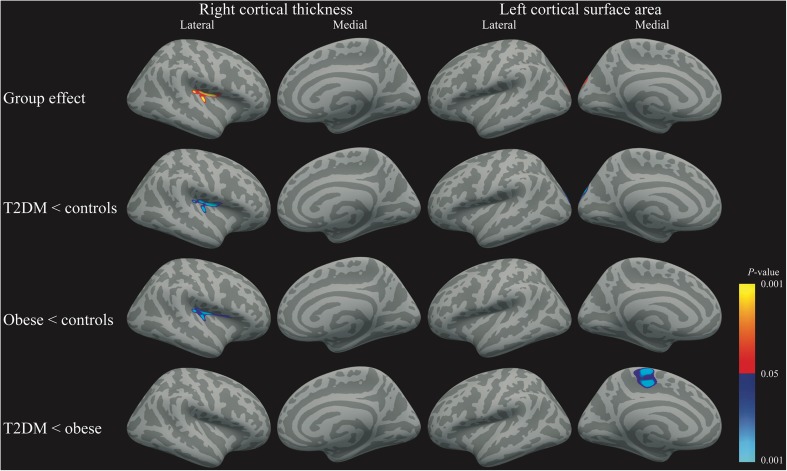
Corrected for age, sex, hypertension, and estimated intracranial volume there was an overall group-effect for cortical thickness in the right insula region extending into the transverse and superior temporal, supramarginal, and precentral regions (PFWE = 0.024; Fig. 1; Table 3). For surface area, the overall group analysis showed a borderline significant cluster comprising the left lateral occipital, superior parietal and cuneus regions (PFWE = 0.063; Fig. 1; Table 3). For cortical volume, there was no overall group-effect (all PFWE > 0.05). Given the explorative nature of this study between-group differences for right thickness and left surface area were tested. Between-group testing showed lower cortical thickness in these regions in the T2DM group versus controls (PFWE = 0.017; Fig. 1; Table 3). Thickness was also lower in these regions and the pars opercularis in the normoglycemic obese participants compared with the controls (PFWE = 0.019; Fig. 1; Table 3). There were no differences between the normoglycemic obese and obese T2DM groups (PFWE > 0.05), and the conjunction analysis showed a trend toward statistical significance for a cluster comprising the insula, extending into the transverse and superior temporal and supramarginal regions (PFWE = 0.073). A group-effect was borderline significant for left hemisphere surface area in the lateral occipital, superior parietal and cuneus regions (PFWE = 0.063; Fig. 1; Table 3). This effect was driven by lower surface area in T2DM patients relative to controls (PFWE = 0.007; Figure 1; Table 3). Interestingly, T2DM patients also showed lower surface area compared with the normoglycemic obese participants, yet in the left paracentral regions (PFWE = 0.040; Fig. 1; Table 3). There were no differences between the obese and control subjects (PFWE > 0.05). There was no overall group-effect for cortical volume, hence no further group testing was performed (PFWE > 0.05).
Fig. 1.
Clusters of lower cortical gray matter surface indices overlaid on a standard brain for right cortical thickness, left panel, and left surface area, right panel. Red-Yellow indicate the positive group effect, whereas blue-light blue indicates a negative effect
Table 3.
Information of between-group vertex-wise analyses and whole group vertex-wise correlation analyses
| Cluster size (mm2) | Peak t-value | MNI coordinates peak value (x, y, z) | Anatomical location | P-value | |
|---|---|---|---|---|---|
| Right cortical thickness | |||||
| Cluster group effect | 537.38 | 4.347 | 34.0, −11.1, 16.3 | Insula / transverse temporal / superior temporal / supramarginal / precentral | 0.024 |
| Cluster T2DM lower than controls | 571.43 | 4.720 | 34.1, −10.3, 16.1 | Insula / transverse temporal / superior temporal / supramarginal / precentral | 0.017 |
| Cluster obese lower than controls | 556.05 | 3.380 | 37.9, −34.4, 12.5 | Insula / transverse temporal / superior temporal / supramarginal / precentral / pars opercularis | 0.019 |
| Left surface area | |||||
| Cluster group effect | 678.51 | 2.892 | −12.7, −95.2, 20.0 | Lateral occipital / superior parietal / cuneus | 0.063 |
| Cluster T2DM lower than controls | 972.56 | 3.478 | −13.3, −94.9, 20.3 | Lateral occipital / superior parietal / cuneus | 0.007 |
| Cluster T2DM lower than obese | 748.11 | 4.890 | −6.9, −19.6, 67.0 | Paracentral | 0.040 |
| Whole group insulin | |||||
| Cluster 1 left area negative | 2008.94 | −4.278 | −23.6, 49.2, 9.6 | Rostral middle frontal / superior frontal | 0.0002 |
| Cluster 2 left area negative | 1373.58 | −2.697 | −64.1, −31.9, 8.5 | Superior temporal / supramarginal / banks of the superior temporal sulcus | 0.0008 |
| Cluster 1 left volume negative | 951.04 | −2.831 | −58.7, −51.3, 22.2 | Superior temporal / supramarginal / banks of the superior temporal sulcus | 0.001 |
| Cluster 2 left volume negative | 599.10 | −3.057 | −35.9, −17.2, 7.4 | Insula / transverse temporal | 0.020 |
| Cluster 1 left thickness positive | 491.43 | 4.212 | −7.3, 35.2, −22.7 | Medial orbitofrontal / lateral orbitofrontal | 0.040 |
Subcortical gray matter structure
The overall F-test including all normalized subcortical structures, corrected for age, sex, and hypertension, was statistically significant (F(2, 69) = 1.83; P = 0.04), indicating a difference in at least 1 subcortical structure between the groups. Post-hoc analysis showed that, after Bonferroni correction for multiple hypothesis testing, the obese group had significantly higher volume in the bilateral thalamus when compared to both other groups (all P < 0.03; Table 2), and in the bilateral amygdala when compared with the control group (P = 0.001; Table 2).
Correlations with cortical gray matter structure
In a forward regression model, including age, sex, BMI, hypertension, waist-hip ratio, HbA1c, total cholesterol, and fasting glucose/insulin, it was tested which variables were independently related to altered gray matter structure in the whole group. We choose to do this in the whole group, because of the lack of power in the subgroups. To avoid influence of group allocation this variable was added as confounding factor.
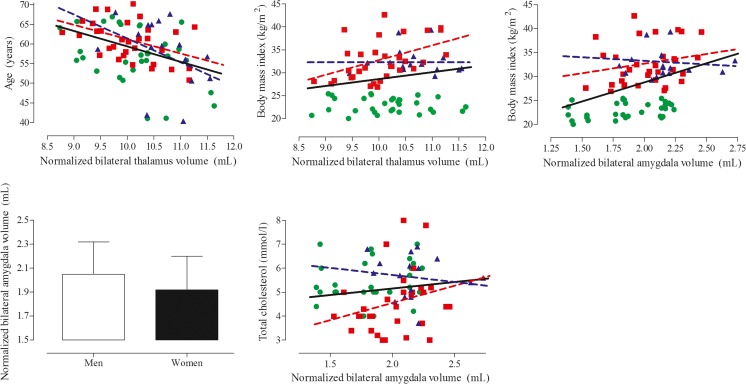
Uncorrected for group allocation there was an association between higher BMI and lower right insula thickness (cluster controls vs T2DM: β = −0.339, P = 0.001; cluster controls vs obesity: β = −0.431, P < 0.001, Fig. 2). Higher glucose (β = −0.366; P = 0.001) and being female (β = −0.325; P = 0.003) were related to lower left lateral occipital surface area. Lower left paracentral surface area was also related to being female (β = −0.238; P = 0.046). After correction for group allocation only the correlations between being female and lower left lateral occipital (β = −0.329; P = 0.003) and paracentral (β = −0.236; P = 0.049) surface area remained statistically significant.
Fig. 2.
Scatter plot of the correlations between clusters of altered cortical structure and medical and anthropometric variables. Green circles depict the healthy lean controls, blue triangles the normoglycemic obese, and red squares the obese T2DM patients. The black regression line shows the correlation for the whole group. The colored regression lines depict the correlation for either the normoglycemic obese (blue) or obese T2DM (red) participants. The correlation with sex is presented as mean with standard deviation and represents men and women irrespective or group allocation
Correlations with subcortical gray matter structure
Higher thalamus volume was related to lower age (β = −0.429; P < 0.001) and higher BMI (β = 0.215; P = 0.044; Fig. 3). Both remained statistically significantly related to thalamus volume after correction for group allocation (age: β = −0.369, P = 0.001; BMI: β = 0.444; P = 0.006). Higher amygdala volume was related to higher BMI (β = 0.495; P < 0.001), being male (β = −0.332; P = 0.002), and higher cholesterol (β = 0.294; P = 0.006; Fig. 3). Although the correlations were slightly attenuated after correction for group allocation, all remained statistically significant.
Fig. 3.
Scatter plot of the correlations between altered subcortical structures and medical and anthropometric variables. Green circles depict the healthy lean controls, blue triangles the normoglycemic obese, and red squares the obese T2DM patients. The black regression line shows the correlation for the whole group. The colored regression lines depict the correlation for either the normoglycemic obese (blue) or obese T2DM (red) participants. The correlation with sex is presented as mean with standard deviation and represents men and women irrespective or group allocation
Vertex-wise correlations with glucose and insulin
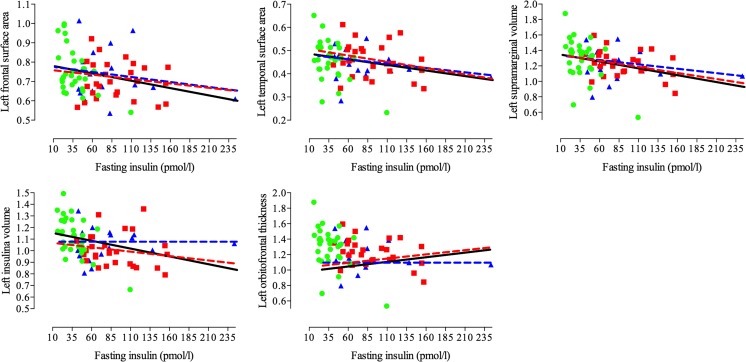
All correlations are graphically presented in Fig. 4, scatter plots are shown in Fig. 5, and statistics can be found in Table 3. To increase power, these correlations were calculated in the whole group, but were corrected for group allocation and estimated intracranial volume.
Fig. 4.
Schematic representation of the clusters where insulin, was significantly negatively related to either, surface area, thickness, or volume in all participants. Blue-light blue indicates a negative correlation, whereas red-yellow indicates a positive correlation
Fig. 5.
Scatter plot of the correlations between clusters that showed a vertex-wise correlation with insulin. Green circles depict the healthy lean controls, blue triangles the normoglycemic obese, and red squares the obese T2DM patients. The black regression line shows the correlation for the whole group. The colored regression lines depict the correlation for either the normoglycemic obese (blue) or obese T2DM (red) participants
Higher levels of insulin were related to lower surface area in the left rostral middle and superior frontal gyri (PFWE < 0.001) and in a cluster comprising the left superior temporal, supramarginal, and banks of the superior temporal sulcus regions (PFWE < 0.001). Higher insulin was also related to lower cortical volume in the left superior temporal, supramarginal, and banks of the superior temporal sulcus regions (PFWE = 0.001) and in the left insula and transverse temporal regions (PFWE = 0.020). Contrary, higher insulin was also related to higher cortical thickness in the left medial and lateral orbitofrontal gyri (PFWE = 0.040). There were no correlations between insulin and the right hemisphere or with glucose and brain structure (all PFWE > 0.05).
Discussion
In this study, cortical and subcortical gray matter structure was studied in normoglycemic obese subjects and obese T2DM patients and compared to lean normoglycemic healthy controls. Firstly, comparing obese T2DM patients without clinically manifest micro- and macroangiopathy to controls, we saw lower right insular and temporal thickness and lower left occipital and superior parietal surface area. Secondly, comparing normoglycemic obese to normoglycemic lean control subjects, right insular, temporal and inferior frontal thickness was lower. In contrast, subcortical thalamic and amygdala gray matter volume was found to be higher. Between normoglycemic obese and obese T2DM patients, thalamic volume and left paracentral surface area were found to be lower in the latter group compared with the first. The cortical alterations were mainly related to sex, whereas the subcortical alterations were related to BMI, total cholesterol, age, and being female. Vertex-wise in the whole group, higher fasting insulin was related to lower left frontal and temporal surface area, lower temporal and insular volume, but higher orbitofrontal thickness.
The cortical structural findings of lower thickness and surface area in the temporal, parietal and occipital cortex in obese T2DM patients corroborate previous studies that also found alterations in T2DM patients in similar regions (Brundel et al. 2010; Moran et al. 2013; Moulton et al. 2015; Peng et al. 2015). Many of these previous studies, however, showed results that were spatially more widespread and found in other regions than the results from our study. An important difference with the previous studies is that in the current study no T2DM patients were included who had clinically manifest micro- or macroangiopathy. It is known that both microvascular complications and macrovascular events have a strong negative effect on brain structure in T2DM (van den Berg et al. 2009), which may explain the differences in results.
In previous obesity studies, results have been mixed; with studies showing both increased and decreased cortical and subcortical structural indices. In this study, including solely normoglycemic obese subjects, we found decreased right insular cortical thickness, which extended into the temporal and inferior frontal gyri, but no increased indices of cortical gray matter structure. On the other hand, both amygdala and thalamus gray matter volume was increased in normoglycemic obese participants. A recent study also showed decreased insula and inferior frontal gyrus volume in obese patients, thus corroborating the current results (Zhang et al. 2016). Interestingly, after bariatric surgery, there was an increased volume in the obese patients in the inferior frontal gyrus (Zhang et al. 2016). Although increased thalamus volume has not been observed previously as far as we know, higher amygdala volume has been observed in obese individuals (Widya et al. 2011). Both the amygdala and the insula are part of the limbic system and as such involved in emotion regulation. Activation due to watching food pictures in these regions has been shown to be increased in this sample of normoglycemic obese and obese T2DM patients, whereas response to actual food was decreased, linking these structures to food and satiety as well (van Bloemendaal et al. 2014; ten Kulve et al. 2016). The inferior frontal gyrus, besides involved in language processing, has a major role in response inhibition (Weywadt et al. 2016). Taking these results together, it might be hypothesized that inhibition of feeding behavior is disrupted in these obese subjects. If and how structural changes in these regions affect feeding behavior, and how and if they are involved in the pathophysiology of obesity needs to be determined in further studies.
It may be hypothesized that, as obesity is a strong risk factor for T2DM, the brain changes in T2DM are aggravated in comparison with obese subjects. In this study, in the right insula, both T2DM and normoglycemic obese participants showed lower cortical thickness, and the conjunction analysis showed a trend towards overlap within this cluster, possibly indicating that the insula is an area of overlap between both groups. Regarding cortical surface area, obese T2DM patients showed lower indices than normoglycemic obese subjects in the paracentral region. Instead of showing a continuum, both groups showed specific cortical changes in distinct regions. These diverging results may be driven by the selection of our obese participants. They had to be normoglycemic as objectified by an oral glucose tolerance test, and therefore represent a special group of obese subjects that has previously been labeled as healthy obese. The absence of impaired glucose metabolism may be driving the observed differences. It was, however, not possible to test this hypothesis as obese participants with glucose metabolism disturbances were not included in this study. Alternatively, the sample of normoglycemic obese subjects in this study was modest and lower than that of the obese T2DM and lean control participants, which may have resulted in lower power to detect alterations in other brain regions.
The structural cortical gray matter alterations found were related to being female, fasting glucose, and BMI. However, after correction for group allocation only the correlation with sex remained statistically significant. Sex is commonly known for its influence on cortical structure. Although BMI and fasting glucose were not correlated with cortical structure after correction for group allocation, it hints towards the influence of both central adiposity and glucose metabolism disturbances on the brain. Many previous studies have suggested these factors have negative consequences on the brain, but future studies should determine if they have an effect on different brain regions.
The subcortical alterations were related to age, sex, BMI, and cholesterol, independent of group allocation. Previous studies showed that cholesterol and free fatty acids were related to increased white matter integrity (Haltia et al. 2007; Verstynen et al. 2013), suggesting that abnormal lipid metabolism may relate to increased indices of cortical structure. Whereas these effects may be most profound in white matter, as the primary component of myelin is cholesterol, the results of this study show it might also be connected to amygdala volume, a deep gray matter structure that is connected and adjacent to major white matter tracts. Further studies are warranted to understand the relationship between increased brain structure and lipid metabolism.
Exploratively, the correlation between cortical gray matter structure and fasting glucose and insulin levels was determined. After correction for group allocation and estimated intracranial volume, there were no correlations with glucose. On the other hand, higher insulin was related to lower frontal, temporal, and insula surface area and volume, regions that were found affected in this study or in other obesity and T2DM studies (Moran et al. 2013; Zhang et al. 2016). Interestingly, some of these regions are part of the default mode network, a network that is highly active during rest (Greicius et al. 2004), and are considered hub regions, i.e. regions with high importance within the brain because of extensive structural and functional connections (de Haan et al. 2012). It might be hypothesized that high levels of insulin in the brain have a greater negative effect on these hub regions. This should be studied in future studies.
Limitations of this study include the relatively small sample size, especially the sample size of the normoglycemic obese subjects. Although this will have limited statistical power, the current sample size yielded sufficient power to detect between-group differences. The smaller sample size also prohibited the calculation of meaningful correlations in the groups separately. Therefore, correlations were determined in the whole group. This, however, prevents us from drawing group specific conclusions. Although the well-phenotyped normoglycemic obese and complication free obese T2DM subjects constitute a strength of the current study, it is not possible to generalize the results of this study to the general obese or T2DM population. The cross-sectional nature of this study did not allow for determination of a causal relationship. Another limitation is the difference in age range between T2DM patients and the other groups. However, this difference was small (3 years) and not statistically significant. In addition, we corrected for age in the statistical analyses. It would have been interesting to compare functional connectivity or DTI data between these participants as well, however, as this is a combined study, both fMRI and DTI data are only available for a subset of participants. Also, as the parent studies did not include cognitive testing or APOE genotyping this information is not available in this study.
In conclusion, we found insula, parietal and occipital cortical deficits in obese patients with uncomplicated T2DM, which may constitute early T2DM-related brain damage. In normoglycemic obese subjects, insula and inferior frontal gyrus deficits, but increased amygdala and thalamus volume were found, indicating a distinctly differential pattern of brain alterations in normoglycemic obese and complication free obese T2DM participants. These deficits were related to sex, but also central adiposity, and altered lipid metabolism. Lastly, insulin seems to be related to poorer cortical structural integrity in several hub regions. Future studies should focus on the pathophysiology of these cortical deficits, ultimately to ameliorate or prevent the deleterious effects of obesity and T2DM on the brain.
Authors contributions
G.B. performed the neuroimaging analyses, quality checks and wrote the manuscript. R.G.IJ. participated in the design of the overall studies. J.S.t.K. took part in the design of both studies and collected part of the data of the current study. F.B. clinically rated all MRI-scans. D.J.V. participated in the design of both studies. J.L-F. provided the research infrastructure at PUC-Rio. L.v.B. took part in the design of both studies and collected part of the data. E.v.D designed the current study, supervised the neuroimaging analyses, performed the statistical analyses and wrote the manuscript. All above mentioned authors have participated in the interpretation of the results and have made critical revision to the manuscript. M.D. was principal investigator of both studies, obtained funding, and participated in the design of the studies, passed away in April 2014.
Funding
The studies of which this research is part were supported by investigator-initiated grants from Eli Lilly and Company, Bristol-Myers Squibb (BMS), and a partial grant from Novo Nordisk. E.v.D. received a personal grant from the Brazilian National Council for Scientific and Technological Development (CNPq). The sponsors had no role in the design, analyses, or reporting of the current research.
Compliance with ethical standards
Conflict of interest
The authors report no conflict of interest pertaining to this study.
Ethical approval
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Medical Ethics Committee of the VU University Medical Center.
Informed consent
Written informed consent was given by all participants.
Footnotes
Michaela Diamant died before publication of this work was completed.
References
- Brooks SJ, Benedict C, Burgos J, et al. Late-life obesity is associated with smaller global and regional gray matter volumes: a voxel-based morphometric study. Int J Obes. 2013;37:230–236. doi: 10.1038/ijo.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundel M, van den Heuvel M, de Bresser J, et al. Cerebral cortical thickness in patients with type 2 diabetes. J Neurol Sci. 2010;299:126–130. doi: 10.1016/j.jns.2010.08.048. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis: I. Segmentation and surface reconstruction. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- de Haan W, Mott K, van Straaten ECW, et al. Activity dependent degeneration explains hub vulnerability in Alzheimer’s disease. PLoS Comput Biol. 2012;8:e1002582. doi: 10.1371/journal.pcbi.1002582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debette S, Wolf C, Lambert J-C, et al. Abdominal obesity and lower gray matter volume: a Mendelian randomization study. Neurobiol Aging. 2014;35:378–386. doi: 10.1016/j.neurobiolaging.2013.07.022. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haltia LT, Viljanen A, Parkkola R, et al. Brain white matter expansion in human obesity and the recovering effect of dieting. J Clin Endocrinol Metab. 2007;92:3278–3284. doi: 10.1210/jc.2006-2495. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1006/nimg.2002.1132. [DOI] [PubMed] [Google Scholar]
- Karlin N, Dueck A, Cook C. Cancer with diabetes: prevalence, metabolic control, and survival in an academic oncology practice. Endocr Pr. 2012;18:898–905. doi: 10.4158/EP12128.OR. [DOI] [PubMed] [Google Scholar]
- Kaur S, Gonzales MM, Strasser B, Pasha E, McNeely J, Tanaka H, Haley AP. Central adiposity and cortical thickness in midlife. Psychosom Med. 2015;77:671–678. doi: 10.1097/PSY.0000000000000202. [DOI] [PubMed] [Google Scholar]
- Kim SH, Després J-P, Koh KK (2016) Obesity and cardiovascular disease: friend or foe? Eur Heart J, 37:3560–3568. 10.1093/eurheartj/ehv509 [DOI] [PubMed]
- Kivimäki M, Luukkonen R, Batty GD et al (2017) Body mass index and risk of dementia: analysis of individual-level data from 1.3 million individuals. Alzheimers Dement:1–9. 10.1016/j.jalz.2017.09.016 [DOI] [PMC free article] [PubMed]
- Kullmann S, Heni M, Hallschmid M, et al. Brain insulin resistance at the crossroads of metabolic and cognitive disorders in humans. Physiol Rev. 2016;96:1169–1209. doi: 10.1152/physrev.00032.2015. [DOI] [PubMed] [Google Scholar]
- Marqués-Iturria I, Pueyo R, Garolera M, et al. Frontal cortical thinning and subcortical volume reductions in early adulthood obesity. Psychiatry Res. 2013;214:109–115. doi: 10.1016/j.pscychresns.2013.06.004. [DOI] [PubMed] [Google Scholar]
- McCrimmon RJ, Ryan CM, Frier BM. Diabetes and cognitive dysfunction. Lancet. 2012;379:2291–2299. doi: 10.1016/S0140-6736(12)60360-2. [DOI] [PubMed] [Google Scholar]
- Medic N, Ziauddeen H, Ersche KD, et al. Increased body mass index is associated with specific regional alterations in brain structure. Int J Obes. 2016;40:1177–1182. doi: 10.1038/ijo.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran C, Phan TG, Chen J, et al. Brain atrophy in type 2 diabetes: regional distribution and influence on cognition. Diabetes Care. 2013;36:4036–4042. doi: 10.2337/dc13-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran C, Tapp RJ, Hughes AD, et al. The Association of Type 2 diabetes mellitus with cerebral gray matter volume is independent of retinal vascular architecture and retinopathy. J Diabetes Res. 2016;2016:6328953. doi: 10.1155/2016/6328953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton CD, Costafreda SG, Horton P, et al. Meta-analyses of structural regional cerebral effects in type 1 and type 2 diabetes. Brain Imaging Behav. 2015;9:651–662. doi: 10.1007/s11682-014-9348-2. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, et al. Valid conjunction inference with the minimum statistic. NeuroImage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. NeuroImage. 2011;56:907–922. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng B, Chen Z, Ma L, Dai Y. Cerebral alterations of type 2 diabetes mellitus on MRI: a pilot study. Neurosci Lett. 2015;606:100–105. doi: 10.1016/j.neulet.2015.08.030. [DOI] [PubMed] [Google Scholar]
- Reijmer YD, van den Berg E, Ruis C, et al. Cognitive dysfunction in patients with type 2 diabetes. Diabetes Metab Res Rev. 2010;26:507–519. doi: 10.1002/dmrr.1112. [DOI] [PubMed] [Google Scholar]
- Ronan L, Alexander-Bloch AF, Wagstyl K, et al. Obesity associated with increased brain age from midlife. Neurobiol Aging. 2016;47:63–70. doi: 10.1016/j.neurobiolaging.2016.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan CM, van Duinkerken E, Rosano C. Neurocognitive consequences of diabetes. Am Psychol. 2016;71:563–576. doi: 10.1037/a0040455. [DOI] [PubMed] [Google Scholar]
- Saute RL, Soder RB, Alves Filho JO et al (2016) Increased brain cortical thickness associated with visceral fat in adolescents. Pediatr Obes. 10.1111/ijpo.12190 [DOI] [PubMed]
- Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- ten Kulve JS, Veltman DJ, van Bloemendaal L, et al. Endogenous GLP1 and GLP1 analogue alter CNS responses to palatable food consumption. J Endocrinol. 2016;229:1–12. doi: 10.1530/JOE-15-0461. [DOI] [PubMed] [Google Scholar]
- van Bloemendaal L, IJzerman RG, Ten Kulve JS, et al. GLP-1 receptor activation modulates appetite- and reward-related brain areas in humans. Diabetes. 2014;63:4186–4196. doi: 10.2337/db14-0849. [DOI] [PubMed] [Google Scholar]
- van den Berg E, Kloppenborg RP, Kessels RPC, et al. Type 2 diabetes mellitus, hypertension, dyslipidemia and obesity: a systematic comparison of their impact on cognition. Biochim Biophys Acta. 2009;1792:470–481. doi: 10.1016/j.bbadis.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Verstynen TD, Weinstein A, Erickson KI, et al. Competing physiological pathways link individual differences in weight and abdominal adiposity to white matter microstructure. NeuroImage. 2013;79:129–137. doi: 10.1016/j.neuroimage.2013.04.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennberg AMV, Spira AP, Pettigrew C, et al. Blood glucose levels and cortical thinning in cognitively normal, middle-aged adults. J Neurol Sci. 2016;365:89–95. doi: 10.1016/j.jns.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weywadt CR, Kiehl KA, Claus ED (2016) Neural correlates of response inhibition in current and former smokers. Behav Brain Res. 10.1016/j.bbr.2016.11.030 [DOI] [PubMed]
- Widya RL, de Roos A, Trompet S, et al. Increased amygdalar and hippocampal volumes in elderly obese individuals with or at risk of cardiovascular disease. Am J Clin Nutr. 2011;93:1190–1195. doi: 10.3945/ajcn.110.006304. [DOI] [PubMed] [Google Scholar]
- Willette AA, Kapogiannis D (2015) Does the brain shrink as the waist expands? Ageing Res Rev 20:86–97. 10.1016/j.arr.2014.03.007 [DOI] [PMC free article] [PubMed]
- Zhang Y, Ji G, Xu M, et al. Recovery of brain structural abnormalities in morbidly obese patients after bariatric surgery. Int J Obes. 2016;40:1558–1565. doi: 10.1038/ijo.2016.98. [DOI] [PubMed] [Google Scholar]