Abstract
Mitochondrial Ca2+ is involved in heterogeneous functions, ranging from the control of metabolism and ATP production to the regulation of cell death. In addition, mitochondrial Ca2+ uptake contributes to cytosolic [Ca2+] shaping thus impinging on specific Ca2+-dependent events. Mitochondrial Ca2+ concentration is controlled by influx and efflux pathways: the former controlled by the activity of the mitochondrial Ca2+ uniporter (MCU), the latter by the Na+/Ca2+ exchanger (NCLX) and the H+/Ca2+ (mHCX) exchanger. The molecular identities of MCU and of NCLX have been recently unraveled, thus allowing genetic studies on their physiopathological relevance. After a general framework on the significance of mitochondrial Ca2+ uptake, this review discusses the structure of the MCU complex and the regulation of its activity, the importance of mitochondrial Ca2+ signaling in different physiological settings, and the consequences of MCU modulation on organ physiology.
Keywords: Mitochondria Ca2+ uptake, Animal models, Heart, Skeletal muscle, Neurons, Pancreatic β cells
Introduction
Ca2+ accumulation in energized mitochondria was first described in the early 1960s [22, 110], and since then, the contribution of Ca2+ uptake to mitochondrial bioenergetics and cellular processes has considerably evolved. The formulation of the chemiosmotic hypothesis [66], together with the measurement of sizable internally negative membrane potentials [94], led to the concept of an energetically favorable Ca2+ uptake mechanism. In the 1980s, with the identification of inositol 1,4,5-trisphosphate (InsP3) as a soluble second messenger, which triggers the release of Ca2+ from the endoplasmic reticulum (ER), and the development of accurate tools for measuring Ca2+ concentration, the attention on mitochondrial Ca2+ accumulation declined. Indeed, it appeared that the affinity of mitochondria for Ca2+ was too low to accumulate the cation, not only in resting cytosolic Ca2+ concentrations ([Ca2+]cyt) (~ 0.1 μM) but also during the transient increase (2–3 μM) generated by cell stimulation [65]. This view was drastically revised when tools allowing the selective measurement of mitochondrial Ca2+ concentration ([Ca2+]m) in living cells were developed. By targeting the Ca2+-sensitive photoprotein aequorin to mitochondria, Pozzan and coworkers demonstrated that a rapid mitochondrial Ca2+ peak, reaching values well above those of the bulk cytosol, parallels the [Ca2+]cyt rise evoked by cell stimulation [91]. Furthermore, the apparent discrepancy between the affinity of Ca2+ transporters and the high level of Ca2+ taken up by mitochondria was resolved by demonstrating that mitochondria, upon cell stimulation, are exposed to microdomains of high [Ca2+] that greatly exceed the values measured in the cytosol due to the close contacts (< 200 nm) between the mitochondria and the ER [92].
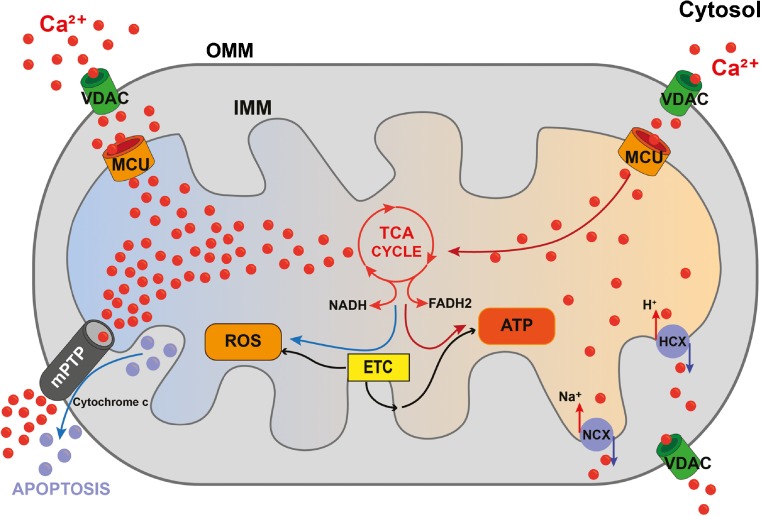
Mechanistically, to reach the mitochondrial matrix, Ca2+ needs to cross two lipid bilayers: the outer and the inner mitochondrial membranes (Fig. 1). The outer mitochondrial membrane (OMM) is permeable to ions and small proteins (MW < 10 kDa), thanks to the presence of a large conductance channel, the voltage-dependent anion channel (VDAC), whose permeability is controlled by ATP and other regulatory factors [18]. The inner mitochondrial membrane (IMM) is an ion-impermeable membrane, whose surface is significantly bigger than the one of OMM due to the presence of numerous invaginations called cristae. The huge driving force for mitochondrial Ca2+ entry is provided by the activity of the respiratory chain complexes that, by translocating H+ in the intermembrane space, leads to an electrochemical gradient (ΔμH) that in mitochondria is mainly represented by the electrical component, generating a mitochondrial membrane potential (ΔΨm) of ~ 180 mV. Accordingly, treatment with an uncoupler such as p-[trifluoromethoxyl]-phenyl-hydrazone (FCCP), that collapses the ΔΨm, abolishes mitochondrial Ca2+ uptake. [Ca2+]m is additionally regulated by Ca2+ efflux pathways through the mitochondrial Na+/Ca2+ (NCLX) and H+/Ca2+ (mHCX) exchangers [93].
Fig. 1.
Mitochondrial Ca2+ homeostasis is regulated by influx and efflux mechanism and impinges on oxidative metabolism, mROS generation, and mPTP opening. Physiologically, mitochondrial Ca2+ uptake stimulates TCA cycle and ATP production (right-hand side), while in pathological conditions, mitochondrial Ca2+ overload causes the opening of the mPTP (left-hand side). mROS play either a signaling role or behave as damaging agents depending on their concentration and on the biological context
The functional significance of mitochondrial Ca2+ accumulation started to be elucidated when it was demonstrated that mitochondrial Ca2+ regulates three key enzymes of mitochondrial metabolism: ketoglutarate dehydrogenase, isocitrate dehydrogenase, and pyruvate dehydrogenase phosphatase 1 (PDP1). The net effect on tricarboxylic acid (TCA) cycle activation is a boost in the synthesis of reduced OXPHOS substrates (NADH and FADH2), enhanced respiratory chain activity, and a subsequent increase in H+ pumping [93] (Fig. 1).
In addition, Ca2+ pulses also stimulate the adenine nucleotide transporter [63] and complex V (mitochondrial F0F1 ATP synthase) [20], harnessing the H+ gradient to upregulate ATP production. Finally, Ca2+ activates α-glycerolphosphate dehydrogenase, a component of the glycerol phosphate shuttle that supplies NAD+ for glycolysis [116]. Thus, an important role for mitochondrial Ca2+ accumulation could be inferred, i.e., a rapid upregulation of mitochondrial ATP production in stimulated cells.
The ability of the mitochondria to act as Ca2+ buffers impinges also on the pattern of the cytosolic Ca2+ signals, with different consequences depending on the arrangement of mitochondria inside cells. For example, in pancreatic acinar cells, three distinct groups of mitochondria have been identified, i.e., mitochondria located at the peripheral basal area and perigranular and perinuclear mitochondria. Each of these subsets is characterized by specific responses to cytosolic Ca2+ signals occurring in their close proximity [76].
The mitochondrial electron transport chain is the main cellular process that generates reactive oxygen species (ROS) in mammalian cells under both physiological and pathological conditions. ROS are derived from molecular oxygen by electron transfer reactions resulting in the formation of superoxide anion radical (O2−) and subsequently hydrogen peroxide (H2O2), either spontaneously or by the action of superoxide dismutases (SOD). In the presence of iron, superoxide and H2O2 can lead to the formation of highly reactive hydroxyl radicals, which can damage cellular proteins, RNA, DNA, and lipids. Interaction of ROS with nitric oxide or fatty acids can lead also to the formation of peroxynitrite or peroxyl radicals, respectively, that are also highly reactive [38]. Although mitochondrial ROS (mROS) have been previously mainly considered as by-products of oxidative metabolism, it is now clearly established that they also act as important signaling molecules controlling a plethora of cellular functions, both in physiology and in pathology [40]. Mitochondrial Ca2+ uptake, by increasing the metabolic rate, and thus O2 consumption and respiratory chain electron leakage, drives superoxide production [38]. Ca2+ may promote mROS formation both directly, by stimulating mROS generating enzymes, like glycerol phosphate and α-ketoglutarate dehydrogenase [108], and indirectly, as in the case of nitric oxide synthase (NOS) activation that, by forming NO, blocks complex IV and leads to mROS formation [30]. In addition, the mild mitochondria uncoupling effect (ΔΨm dissipation) of Ca2+ uptake contributes to mROS generation. Importantly, mROS play a crucial role in cancer progression, eliciting metabolic adaptations essential for metastasis formation and invasion [84]. It has been shown, in a triple-negative breast cancer model, that the inhibition of mitochondrial Ca2+ uptake causes a decrease in mROS production and consequently a reduction in cancer progression and metastasis formation [106].
Finally, mitochondrial Ca2+ overload triggers mitochondrial permeability transition pore (mPTP) opening (Fig. 1). mPTP is a high-conductance channel mediating mitochondrial swelling [4, 81]. Matrix Ca2+ is an essential permissive factor for mPTP opening: as [Ca2+]cyt increases beyond a certain value, mitochondrial Ca2+ overload ensues. This, together with other causal factors, most notably oxidative stress, high phosphate concentrations, and low adenine nucleotide concentrations, triggers mitochondrial “permeability transition,” i.e., the mitochondrial membrane becomes permeable to any molecule less than 1.5 kDa in size. Consequent dissipation of the ΔΨm leads to membrane depolarization, increased mROS generation, and decreased ATP production, eventually triggering apoptosis. The function of Ca2+ in apoptosis is particularly fascinating, since a small amount of cytochrome c released from mitochondria can bind to and promote Ca2+ conductance through IP3R. The increased cytosolic Ca2+ then triggers a massive exodus of cytochrome c from all mitochondria in the cell, thus activating caspases and nucleases that finalize the apoptotic process [10]. Recently, it has been proposed that mPTP forms from the F-ATP synthase through a strictly Ca2+-dependent mechanism [32–34]. However, the detailed mechanisms of mPTP activation are still debated and readers are referred to specific contributions on this topic [5, 12, 36, 37, 67].
The molecular characterization of the MCU complex
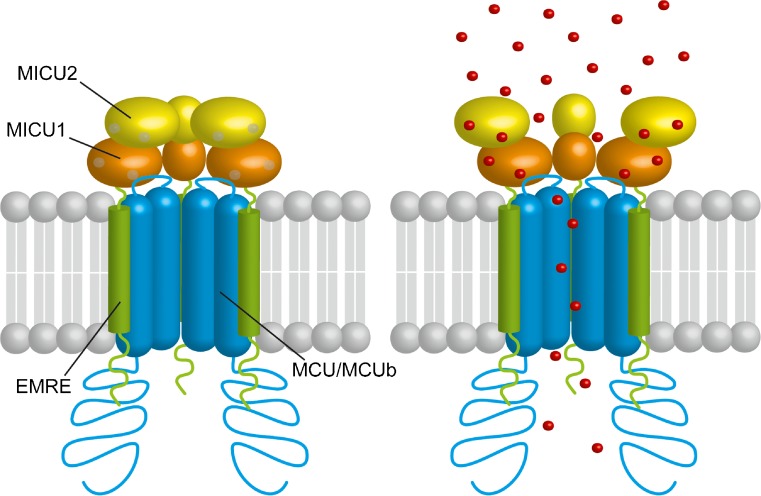
In 2011, the identification of the gene encoding the pore-forming subunit of the MCU, made by two independent groups [3, 21], marked a turning point in the field of mitochondrial physiology and paved the way for the characterization of one of the most sophisticated ion channels described so far. We will briefly describe the different components of the MCU complex distinguishing the membrane pore-forming subunits from the soluble regulatory components (Fig. 2).
Fig. 2.
The mitochondrial Ca2+ uniporter is a complex composed of pore-forming proteins (comprising the channel subunit MCU, the dominant-negative subunit of the channel MCUb, and the short transmembrane regulator EMRE) and of regulatory proteins (MICU1 and MICU2). Both MICU1 and MICU2 contain EF-hand domains facing the intermembrane space. By sensing IMS [Ca2+], MICU1 and MICU2 coordinately regulate both the threshold and the cooperativity of channel opening
The pore-forming subunits
Three proteins have been identified as components of the MCU pore-forming subunit that spans the IMM: MCU, MCUb, and EMRE (Fig. 2).
The MCU gene, originally named CCDC109a, was identified through a bioinformatics screening of the MitoCarta database, i.e., a compendium of mitochondrial proteins identified by mass spectrometry analyses on mitochondrial preparations from different mouse tissues [3, 21, 72]. The MCU gene is well conserved among metazoan and plants while is absent in yeast, that lacks the Ruthenium red-sensitive mitochondrial Ca2+ uptake, in some fungi and in protozoans [8]. It encodes a 40 kDa protein that contains two predicted transmembrane domains joined by a very short, but highly conserved, loop that faces the intermembrane space (IMS). The N- and C-domains, which represent the majority of the protein sequence, face the mitochondrial matrix [3, 61]. The MCU protein structure analysis reveals two important aspects. First, since MCU displays only two transmembrane domains, it has to undergo oligomerization to form a functional channel. This is confirmed by blue native gel separation experiments of purified mitochondria, that display a high molecular complex containing MCU with an apparent molecular weight of 450 kDa [3, 21, 87]. Second, consensus sequences of classical Ca2+-binding domains have not been identified in MCU protein sequence. This suggests that MCU is unable to regulate its own activity. In addition, the MCU loop region that faces the IMS and that connects the two transmembrane domains is too small to contain regulatory elements since it is formed by a stretch of only 11 amino acids. Nonetheless, the loop includes salient residues necessary for MCU channeling. In particular, the loop contains the “DIME” motif, characterized by negatively charged amino acids (such as D260 and E263) essential to confer selectivity to the MCU channel [3, 21]. In addition, the residue S258 is critical to confer sensitivity of MCU complex to Ru360, the most potent inhibitor of the uniporter [3].
The protein structure of the N-terminal domain of MCU was resolved by a crystallographic study [51]. This domain contains a residue (S92) that was predicted as a putative phosphorylation site for CaMKII [51]. Mutation of this residue causes a reduction in the MCU Ca2+ conductance. This finding matches with the demonstration that mitoplasts derived from hearts treated with CaMKII inhibitors display a reduced MCU current [45].
Although MCU oligomer has been predicted to be a tetramer by a molecular dynamic approach [87], NMR and cryo-EM of the Caenorhabditis elegans MCU identified a pentamer complex [71]. In both of the proposed molecular structures, the DIME motifs form the pore entrance and they are part of the channel selectivity filter [71, 87]. Whether the expression and the purification of the C. elegans MCU in a prokaryotic system, which does not express the essential complex component EMRE (see below), are sufficient to ensure the correct structure assembling is unclear. Indeed, EMRE seems required for ensuring mitochondrial Ca2+ uptake and for the assembly of the regulatory subunits MICU1 and MICU2 [98], but its role on the folding of MCU, and thus for MCU structure, is still debated and needs further clarification.
The MCUb gene, formerly known as CCDC109b, was identified through an MCU sequence homology screening [87], and the incorporation of MCUb in the MCU complex has been demonstrated also by proteomics experiments [98]. MCUb greatly impairs Ca2+ permeation through the MCU [87]. It is present only in vertebrates, while it is absent in other organisms in which MCU is present. The MCUb amino acid sequence is highly conserved among different species and shares 50% of similarity with MCU [87]. For this reason, the overall predicted domain distribution and topology are conserved between MCU and MCUb. Nonetheless, MCUb presents salient differences from MCU. Firstly, two critical and conserved amino acid substitutions in the loop region confer to MCUb a dominant-negative function. Indeed, the substitution of the MCU loop residue E256 with a noncharged residue drastically reduces the conductivity of the channel [87]. In addition, MCU and MCUb show radically different expression profiles among tissues. Consequently, some tissues, such as the heart, exhibit a high MCU:MCUb ratio, while others, such as skeletal muscle, display a lower ratio. As for the physiological relevance of the presence and the differential expression of this isoform, it might represent one of the mechanisms that underlie the different MCU currents recorded in different tissues [29].
EMRE is the last component of the MCU pore to be identified. It is a 10 kDa protein of the IMM, and it represents a metazoan innovation since it is not present in the other eukaryotic taxa where MCU and MICU1 are expressed. It is composed of a transmembrane domain, a short N-terminal domain, and a highly conserved acidic C-terminal domain [98]. EMRE is essential for MCU activity, as demonstrated by experiments in EMRE KO cells where mitochondrial Ca2+ uptake is abolished. Even though in planar lipid bilayer MCU and the regulatory subunits MICU1 and MICU2 display the ability to interact with each other without the presence of EMRE [77], this protein has been proposed to play a fundamental role in the interaction between the pore core subunits and the regulatory subunits [98]. In addition, in yeast cells, that do not present mitochondrial Ca2+ uptake, human MCU is able to assemble in a functional channel only when EMRE is present. This gave rise to the concept that EMRE is essential to assemble a functional MCU channel in metazoan organisms [48]. Furthermore, the acidic C-terminal domain has been identified as a matrix-Ca2+ sensor that governs the MCU activity. Accordingly, EMRE would be able to form a unique regulatory complex with MICU1 and MICU2 that is able to sense Ca2+ at both sides of IMM [109]. This is in opposition to another report, which proposes a structural role of EMRE and a different topology across the IMM, incompatible with the suggested matrix-Ca2+ sensor of the acidic C-terminal domain [121].
The regulatory subunits
As mentioned above, none Ca2+-sensing domains have been identified in the MCU structure, indicating that MCU is unable to regulate its own activity. Instead, it is clear that the regulation of MCU is dependent on IMS-residing proteins, namely MICU proteins. These belong to a family of proteins with common features: they are located in the mitochondria, they display EF-hand domains in their protein structure, and they interact with MCU [80] (Fig. 2). Through an integrative strategy that fuses comparative physiology, evolutionary genomics, organelle proteomics, and RNAi screenings, MICU1 was identified as a critical modulator of mitochondrial Ca2+ uptake even before the identification of MCU [80]. MICU1 is fundamental for the proper gatekeeping of the MCU channel, as demonstrated by the fact that the silencing of MICU1 causes mitochondrial Ca2+ overload [19, 59]. In addition, MICU1 acts also as cooperative activator of MCU, thus ensuring the increase in the MCU Ca2+ conductivity during cell stimulation [19].
Some years after MICU1 discovery, two MICU1 paralog genes were identified. These originate from a gene duplication event prior to vertebrate evolution. MICU2, formerly named EFHA1, displays a tissue expression pattern similar to MICU1 [83]. Instead, MICU3, formerly named EFHA2, is expressed prevalently in specific tissues, such as the nervous system and skeletal muscle [73, 83]. As MICU1, MICU2 displays two well-conserved EF-hand domains and several studies demonstrated that it is located in the IMS [19, 42]. Interestingly, MICU2 stability depends on the presence of MICU1, and the knockdown of MICU1 causes the destabilization of MICU2 protein, without affecting MICU2 mRNA levels [77, 83]. Indeed, MICU2 forms obligate heterodimers with MICU1, stabilized by a disulfide bond through two conserved cysteine residues [77], that have been hypothesized to be joined thanks to the mitochondrial oxidoreductase Mia40 [82]. The MICU1-MICU2 heterodimer is responsible for one of the most peculiar properties of mitochondrial Ca2+ uptake, i.e., the sigmoidal response to increasing [Ca2+]cyt [19, 77]. In detail, in resting conditions, the MCU complex is inhibited to prevent mitochondrial Ca2+ overload and ion vicious cycles. However, when [Ca2+]cyt increases, the MCU complex is subjected to a cooperative activation that ensures the prompt response of the mitochondria to cell challenge. Overall, the regulation of MCU complex activity by MICU1-MICU2 heterodimers is possible thanks to the ability of these proteins to sense Ca2+ concentration through their EF-hand domains [46, 77]. Nonetheless, the affinity for Ca2+ of the EF-hand domains is still controversial. As for MICU1, Kd measurements performed by isothermal titration calorimetry range from 4 to 40 μM [111, 115], while measurements of intrinsic tryptophan fluorescence record a higher affinity, with a Kd of ~ 300 nM [47]. These discrepancies, reflecting the different technical approaches, surely need further investigation. By in vivo experiments and electrophysiological studies carried out in planar lipid bilayer, our laboratory demonstrated that, at low [Ca2+]cyt, MICU2 inhibits MCU activity, thus representing the genuine gatekeeper of the channel. On the other hand, MICU1 senses high [Ca2+] and it allows the cooperative activation of MCU during cytosolic Ca2+ increases. Along these lines, MICU1 silencing causes Ca2+ overload due to the loss of the gatekeeper MICU2 and reduces the maximal activation of MCU due to the loss of cooperativity [27, 47, 77, 83, 111]. Nonetheless, the stoichiometry of MCU regulators in the complex is still completely unknown. This is a problematic issue, since it has been proposed that during Ca2+ stimulation MICU1 multimers undergo molecular rearrangement [113]. Furthermore, it has been recently proved that the ratio between MICU1 and MCU is sufficient to account for the different regulatory properties of the MCU complex in different tissues [73]. Indeed, the authors proposed that the different amounts of MCU not associated with MICU1 explain the tissue specificity of cytosolic Ca2+ transients decoding at the level of mitochondria. In particular, the low MICU1:MCU ratio measured in the heart allows mitochondrial Ca2+ uptake even for low [Ca2+]cyt transients, due to a low gating of the channel accompanied by a low cooperativity. In this way, the beat-to-beat Ca2+ transients that occur in the heart cause an integrative Ca2+ accumulation. On the other hand, the high MICU1:MCU ratio, as observed in the liver, confers high cooperative activation of the channel but strengthens the threshold of activation. Therefore, subthreshold [Ca2+] fluctuations are not sufficient to trigger mitochondrial Ca2+ uptake. Moreover, each single sustained cytosolic Ca2+ increase is effectively transmitted to the mitochondria [73].
Other mitochondrial proteins have been identified as putative modulators of MCU activity. MCUR1, formerly named CCDC90a, was identified as a modulator of MCU, since the silencing of this protein causes a decrease of mitochondrial Ca2+ uptake in HEK293T cells [58]. However, its role in the MCU complex is highly debated, because it is also important for complex IV assembly and MCUR1 silencing causes a consistent drop of mitochondrial membrane potential [78]. The other component proposed to be part of the MCU complex is SLC25A23 [39], which belongs to a family of Mg-ATP/Pi solute carriers across IMM [2]. The mutation of its EF-hand domains reduces mitochondrial Ca2+ accumulation [39], but whether this depends on a direct MCU activity regulation or whether it affects mitochondrial bioenergetics or mitochondrial Ca2+ buffering capacity is still debated.
Mitochondrial Ca2+ signaling in physiology: general framework and effects of MCU modulation
Heart
Back in 1883, the heart was the first striated muscle that was demonstrated to contract in response to Ca2+ [90]. Much of the Ca2+ needed for contraction comes from the sarcoplasmic reticulum (SR) and is released in a beat-to-beat fashion by the process named Ca2+-induced Ca2+ release [7]. Indeed, it was postulated that, in mammalian cardiac muscle, the entry of a small amount of Ca2+ through the sarcolemma during the plateau of the action potential results in a large increase of intracellular Ca2+ through the RyR2, an event considered necessary for contraction (for a review, see [28]). Specifically, the depolarization induced by the action potential opens the L-type Ca2+ channels located on the membrane and transverse tubules, resulting in the entry of a small amount of Ca2+. This induces a large increase in the dyadic space, the region bounded by the t-tubule and SR [17]. This increase makes the SR RyR2s open, thereby releasing a much larger amount of Ca2+ from the SR. The latter event causes an increase in free Ca2+ ion concentration from approximately 100 nM to 1 μM, making more Ca2+ available for binding to troponin C (TnC). The binding of Ca2+ ions to TnC initiates a cascade of events leading to force generation by the cycling of cross-bridges, i.e., the interaction between the thin and thick filaments [17]. For relaxation to occur, Ca2+ must be removed from the cytoplasm. This requires the closure of RyR2s and, concomitantly, that Ca2+ is both pumped back into the SR, by the SERCA and out of the cell, largely by the Na+/Ca2+ exchanger (NCX), with some contribution from the plasma membrane Ca2+-ATPase [17]. Importantly, physiological sympathetic stimulation of the heart through β-adrenergic receptors increases the force of contraction (inotropy) and accelerates relaxation (lusitropy) [7]. The L-type Ca2+ channel is the main route for Ca2+ entry into cardiac myocytes that not only results in contraction but, importantly, in the upregulation of ATP production that powers cardiac excitation and contraction. Indeed, maintenance of intracellular Ca2+ homeostasis is critical for the regulation of mitochondrial ATP production [112]. Importantly, most of the ATP needed for cardiac excitation and contraction is synthesized within the mitochondria via oxidative phosphorylation that, as mentioned above, is a Ca2+-dependent process [23]. The fundamental role of the mitochondria in meeting changes in energy demand, such as upon increased workload or hormonal stimulation, is demonstrated by the close apposition of the mitochondria and the major source of Ca2+ for contraction, the SR. Therefore, it was hypothesized that Ca2+ release from the SR will elevate local Ca2+ to high levels resulting in a large mitochondrial Ca2+ influx [53]. Nevertheless, direct patch clamp recordings have shown that cardiac mitochondria IMCU is substantially smaller than that of other tissues and, in particular, ~ 30 times smaller than skeletal muscle IMCU [29]. In the heart, the mitochondria occupy 37% of cellular volume. Therefore, the small IMCU might prevent excessive buffering of Ca2+ needed for contraction. Furthermore, excessive mitochondrial Ca2+ uptake, in conjunction with accumulation of ROS, has long been associated to the opening of the mPTP, leading to irreversible ΔΨ collapse, swelling of the mitochondria, with consequent loss of cytochrome c and ultimately necrotic cardiomyocyte cell death, as observed in ischemic/reperfused myocardium [24]. Therefore, reducing the amplitude of cardiac mitochondrial transients might serve as a safety mechanism.
Despite the physical proximity of the mitochondria to the SR compartment and their Ca2+-dependent role in ATP production, the ability of the mitochondria to serve as significant dynamic buffers of cytosolic Ca2+ in the heart is still debated [13]. Furthermore, highly controversial is whether the fast cytosolic Ca2+ transients in excitation-contraction coupling in beating cardiomyocytes are transmitted to the matrix compartment in a beat-to-beat fashion or in a slow integration pattern [43]. This issue has been addressed by the first study reporting the effects of MCU modulation on heart function [26]. In detail, by means of a GFP-based Ca2+ indicator targeted to the OMM of neonatal cardiomyocytes, Pozzan and coworkers demonstrated the presence of microdomains of high [Ca2+] generated at the SR/mitochondria contacts that allow the massive entrance of Ca2+ into these organelles. Indeed, a fraction of Ca2+ released during systole enters the mitochondria and is released back into the cytoplasm during diastole, resulting in a significant buffering of Ca2+ peaks. In addition, the modulation of MCU protein levels by silencing or overexpression enhances or decreases the amplitude of cytoplasmic Ca2+ oscillation, respectively, and the opposite effect takes place into the mitochondria. Furthermore, mitochondrial Ca2+ uptake in the heart mitochondria is controlled by a low MICU1:MCU ratio, as discussed above. This property has been hypothesized to ensure beat-to-beat mitochondrial Ca2+ accumulation at low frequency, while allowing an integrative matrix Ca2+ accumulation when frequency increases [73].
Despite these findings demonstrating the importance of mitochondrial Ca2+ buffering in cardiac physiology, the heart phenotype of the first model of MCU knockout mouse was surprisingly mild [41, 75]. As expected, mitochondria isolated from MCU−/− cardiomyocytes do not take up Ca2+ [75] and have lower resting Ca2+ levels compared to controls. However, basal ATP levels are unchanged, demonstrating a preserved basal mitochondrial energetics [41]. Furthermore, mice lacking MCU show normal basal cardiac function in terms of ejection fraction, fractional shortening, stroke volume, and chamber size, both in adulthood (12-month-old mice) and in aging (20-month-old mice). In addition, no differences between WT and MCU−/− mice were observed in the left ventricular cardiac output at baseline and after isoproterenol stimulus, which mimics the “fight or flight” response, i.e., an episode of high-energy demand triggered by catecholamine-induced heart acceleration. Also, when mice were subjected to surgical transverse aortic constriction (TAC), as a model of chronic stress, MCU−/− hearts showed the same cardiac parameters measured in the WT [41]. Taken together, these data suggest that mitochondrial Ca2+ accumulation is dispensable both for the basal cardiac function and during acute and chronic increased workload. Further experiments were carried out to assess the role of MCU during ischemia-reperfusion (I-R) injury. MCU−/− hearts show no sign of I-R injury protection [75]. In detail, measurements of the rate pressure product and direct assessment of the infarct area in post-ischemic recovery period indicate no difference between MCU−/− animals and the controls. Interestingly, when treated with cyclosporine A (CsA), that inhibits Ca2+-dependent cell death mediated by the opening of mPTP, WT hearts were protected from I-R injury, while MCU−/− hearts were not. This result suggests that an mPTP-independent death pathway occurs in the absence of MCU [75]. It is noteworthy that the birth ratio of the MCU−/− mice, which are in an outbred strain composed by a mix of CD1 and C57/BL6 backgrounds, is lower than expected [75] and that, in an inbred strain, MCU deletion is embryonically lethal [68]. These data demonstrate a crucial role of mitochondrial Ca2+ uptake during embryonic development, which is hidden in the mixed strain.
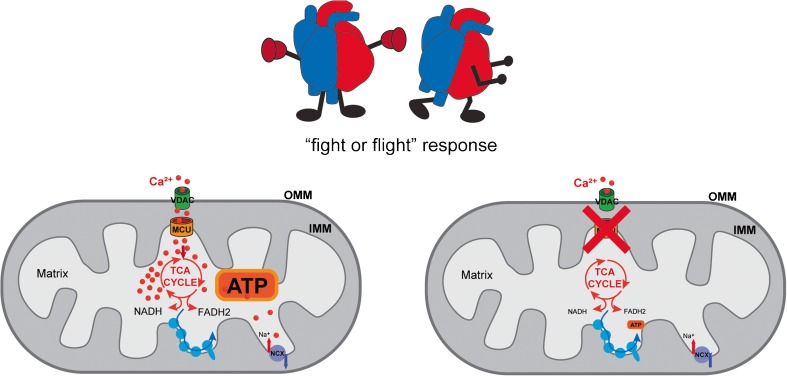
The first heart-specific model generated was a transgenic mouse expressing a dominant-negative MCU isoform, MCUD260Q,E263Q (DN-MCU), in the same mixed background of the constitutive MCU−/− model [120]. When expressed in cultured cells, DN-MCU does not completely abolish organelle Ca2+ accumulation [21], although the mitochondria from DN-MCU-expressing hearts have no measurable mitochondrial Ca2+ uptake. DN-MCU mice have normal heart rate; however, they display an impairment in the “fight or flight” response (Fig. 3). In detail, when sinus atrial node (SAN) cells undergo an extreme physiological stress, ATP generation is required to fuel SERCA activity to maintain the proper Ca2+ load of the SR. DN-MCU mice are not able to increase the heart rate under physiological stress indicating that the ATP production is MCU dependent. ATP dialysis in cardiac pacemaker cells is sufficient to recover the phenotype. Additionally, oxygen consumption rate (OCR) is increased in DN-MCU-isolated perfused heart, but not in permeabilized fibers or isolated mitochondria [89]. Moreover, DN-MCU heart has a higher diastolic cytosolic [Ca2+], consistent with the loss of mitochondrial buffering. This cytosolic Ca2+ increase is partially rescued by the addition of ATP, suggesting that these cardiomyocytes display an extramitochondrial adaptation that depends on the reduced ATP availability. Importantly, similar to the MCU−/− model, DN-MCU hearts are not protected against I-R injury.
Fig. 3.
Dominant-negative MCU (DN-MCU) transgenic mice and inducible heart-specific MCU−/− mice are characterized by impaired “fight or flight” response, due to lack of ATP production required for heart rate increase
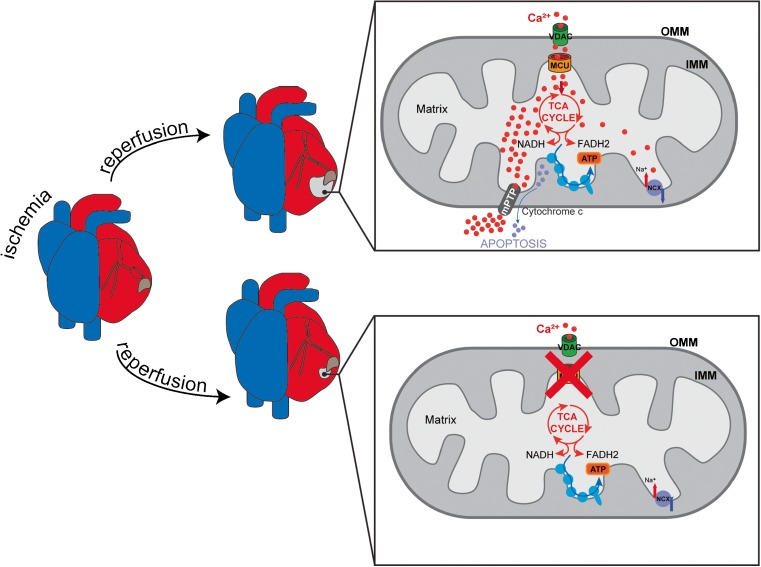
Next, a mouse model with two LoxP sites flanking exons 5 and 6 of the MCU gene was mated with animals expressing a tamoxifen-inducible Cre recombinase driven by a cardiomyocyte-specific promoter. MCU gene deletion was induced in adult mice, and the cardiac function was evaluated [50, 54]. Firstly, MCU ablation in adult heart led to a great reduction in mitochondrial Ca2+ accumulation, although the basal mitochondrial [Ca2+] was not affected. Similar to DN-MCU mice and total MCU−/− mice, cardiac-specific inducible MCU−/− mice are indistinguishable from WT in normal conditions and after cardiac pressure overload. Cardiomyocytes derived from these mice present normal respiration rate in basal conditions, although a decrease in oxygen consumption rate was detected after isoproterenol treatment [50, 54]. However, as opposed to previously reported total MCU knockout and DN-MCU mouse models [75, 120], in the inducible cardiac-specific model, MCU ablation strongly protects hearts from I-R injury [50, 54] (Fig. 4). Finally, studies on the cardiac-specific adult MCU−/− mouse confirmed the impairment in the “fight or flight” response triggered by β-adrenergic stimulation [50, 54] (Fig. 3), as observed in the DN-MCU transgenic mouse model [120].
Fig. 4.
Inducible cardiac-specific MCU deletion confers protection from ischemia-reperfusion (I-R)-induced damage associated to mitochondrial Ca2+ overload and mPTP opening
Mitochondrial [Ca2+] is regulated by the coordinated activity of influx and efflux pathways [101]. To assess the contribution of mitochondrial Ca2+ efflux to heart pathophysiology, Elrod’s group recently developed an inducible mouse model with a cardiomyocyte-specific deletion of the Slc8b1 gene [55], which was previously demonstrated to encode the mitochondrial Na+/Ca2+ exchanger (NCLX) [74]. These mice present a severe phenotype. Indeed, the heart-specific deletion of the exchanger causes acute myocardial dysfunction and fulminant heart failure with a survival rate of only 13%. The hearts of the knockout mice display increased mass and cardiac fibrosis. In addition, echocardiographic analyses show ventricular dilatation and decreased left ventricular function. Finally, the NCLX−/− hearts present a great sarcomere disorganization. Regarding the molecular mechanism, knockout adult cardiomyocytes show a faster rate of mitochondrial swelling compared to control, increased superoxide generation, and compromised sarcolemmal integrity. Heart-specific NCLX−/− mice crossed with cyclophilin D (CypD)-null mice showed an almost complete rescue of the phenotype, demonstrating that the sudden death induced by Slc8b1 deletion was due to an mPTP-dependent mechanism. The mRNA expression of Slc8b1 and of MICU1 was increased in left ventricular biopsies of explanted failing hearts of transplant recipients [55]. To determine the biological relevance of these findings, a cardiac-restricted doxycycline-controlled NCLX overexpressing mouse model was generated (NCLX-Tg). NCLX-Tg adult cardiomyocytes present increased mitochondrial Ca2+ efflux compared to controls which is sufficient to reduce mPTP activity. In addition, NCLX-Tg hearts show a reduction in infarct size and enhanced contractile function upon I-R injury, suggesting a cardioprotective role of increased mitochondrial Ca2+ efflux [55]. These data demonstrate that mitochondrial Ca2+ efflux capacity is necessary for the maintenance of mitochondrial homeostasis and heart cell survival.
Skeletal muscle
Ca2+ represents a powerful intracellular messenger in skeletal muscle fibers, being able not only to trigger contractions by binding to troponin C but also to activate protein phosphorylation or dephosphorylation by binding to calmodulin and stimulating substrate oxidation by the mitochondria [100].
The rise of Ca2+ in the sarcoplasm is a key requirement for skeletal muscle contraction. This event is initiated by the excitation-contraction coupling mechanism that couples muscular action potentials to myofibril contraction [99]. This process relies on a direct coupling between two proteins, the SR Ca2+-releasing channel ryanodine receptor (RyR1) and the voltage-gated L-type Ca2+ channels (dihydropyridine receptors, DHPRs), located on the sarcolemma of the transverse tubule. As the RyR1s open, Ca2+ is released from the SR and diffuses into the bulk cytoplasm generating a Ca2+ spark. The reversible binding of Ca2+ ions to TnC triggers the cross-bridge cycling, thus producing force. TnC, together with troponin T (TnT), troponin I (TnI), and tropomyosin (TM), forms a regulatory unit that controls the dependency from Ca2+ to muscle contraction [100].
Not only Ca2+ links excitation to contraction but also conjugates excitation to transcription, thus accounting for the huge molecular heterogeneity of muscle cells [100]. For example, binding of Ca2+ to calmodulin is known to activate signaling pathways typical of the slow-oxidative phenotype [100]. Indeed, Ca2+- and thus activity-dependent transcriptional regulation via calcineurin (calmodulin-dependent phosphatase 2A) and NFAT is associated with the translation of fast and slow motor neuron activity into muscle fiber type-specific transcriptional programs [16].
The cross-bridge cycle between myosin and actin is not only dependent on Ca2+ but also on ATP hydrolysis that liberates energy for the mechanical work. ATP consumption increases by approximately 100-fold during contraction, and thus, high demand cannot be fulfilled by the finite amount of ATP normally stored inside the muscle [35].
It has been extensively shown that the Ca2+ waves during contraction are transmitted to the mitochondria both in vitro [14] and in vivo [95], which respond by activating Ca2+-sensitive dehydrogenases that are key rate-controlling enzymes in the TCA cycle [23, 35]. This tight coupling is achieved by the mitochondria being located adjacent to Ca2+ stores (SR) and in proximity of release sites (Ca2+ release units [CRUs]) [11].
The role of mitochondrial Ca2+ uptake in skeletal muscle physiology is being vigorously investigated, as detailed hereafter. The MCU−/− mice which, as explained above, are characterized by a mild phenotype, the most affected tissue is the skeletal muscle [75]. Specifically, resting matrix Ca2+ levels of skeletal muscle mitochondria of MCU−/− mice are diminished by approximately 75% compared to controls. In addition, the phosphorylation levels of PDH are increased, and accordingly, PDH activity is decreased, in line with the Ca2+-dependent regulation of PDP1. In workload tests, MCU−/− mice have significant impairment in exercise capacity, in line with the role of mitochondrial Ca2+ accumulation to regulate ATP production necessary to maintain a normal muscle functionality.
To avoid the compensatory effects acting during embryonic development, the role of mitochondrial Ca2+ homeostasis in skeletal muscle has been further investigated by local and postnatal administration of AAV (adeno-associated viral) vectors overexpressing or silencing MCU [60]. MCU overexpression and downregulation triggered muscle hypertrophy and atrophy, respectively (Fig. 5). Most importantly, MCU overexpression is protected from denervation-induced muscle atrophy caused by sciatic nerve excision. These effects are independent from the control of aerobic metabolism, as demonstrated by various lines of evidence. Firstly, PDH activity, although defective in MCU-silenced muscles, was unaffected in MCU overexpressing muscles. Second, hypertrophy was comparable in both oxidative and glycolytic muscles, and finally, semiquantitative analyses of aerobic metabolism revealed no major alterations. The control of skeletal muscle mass by mitochondrial Ca2+ modulation is due to the activity of two major hypertrophic pathways of skeletal muscle, PGC-1α4 and IGF1-AKT/PKB. Taken together, these results demonstrate that the modulation of mitochondrial Ca2+ accumulation after birth contributes to skeletal muscle trophism and that a Ca2+-dependent mitochondria-to-nucleus signaling route links organelle physiology to the control of muscle mass [60].
Fig. 5.
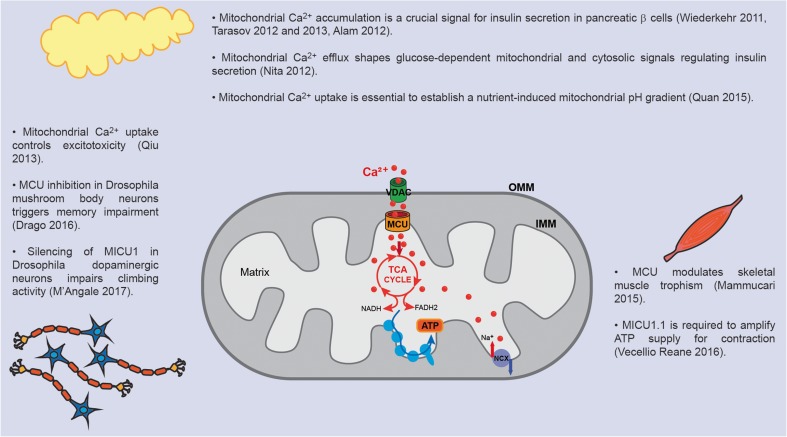
The importance of proper mitochondrial Ca2+ homeostasis in different organs, like the skeletal muscle, endocrine pancreas, and brain, is highlighted by the dysfunctional phenotype of specific animal models, as detailed in the figure
The control of oxidative phosphorylation by Ca2+ is particularly crucial in skeletal muscle, one of the most ATP-consuming organs of the body. It is thus not surprising that, compared to other tissues, skeletal muscle mitochondria display high Ca2+ conductance [29] and that skeletal muscle expresses a unique MCU Ca2+ uptake machinery [111]. Indeed, recently, an alternative splice variant of MICU1, that was named MICU1.1, was discovered [111]. This isoform, characterized by the addition of a micro-exon coding for four amino acids, greatly modifies the properties of the MCU. In detail, MICU1.1 binds Ca2+ one order of magnitude more efficiently than MICU1 and, when heterodimerized with MICU2, activates MCU current at lower Ca2+ concentrations than MICU1-MICU2 heterodimers. In vivo injection of antisense oligonucleotides mediating exon skipping of the MICU1.1 extra exon, and thus forced expression of MICU1, demonstrated that MICU1.1 is required for maintaining sufficient levels of mitochondrial Ca2+ uptake to provide the ATP needed for contraction [111] (Fig. 5). These results demonstrate a novel mechanism of the molecular plasticity of the MCU Ca2+ uptake machinery. Future studies will likely unravel other tissue-specific regulatory mechanisms of mitochondrial Ca2+ uptake.
Pancreatic β cells
The initial concept that Ca2+ controls the release of insulin by β cells goes back to the seminal observation that the release of this hormone is blocked in the absence of Ca2+ [119]. Notably, β cells, the sole source of circulating insulin, convert small fluctuations in blood glucose concentration into large changes in insulin secretion within minutes. These cells are electrically excitable cells that respond to increases in glucose concentration with enhanced metabolism, closure of ATP-sensitive K+ channels, and electrical spiking [97]. In detail, glucose induces the secretion of insulin through the stimulation of oxidative metabolism, an elevation in cytosolic ATP/ADP ratio, and the closure of ATP-sensitive K+ channels (KATP). The subsequent depolarization of the plasma membrane results in oscillatory Ca2+ influx through voltage-gated Ca2+ channels, which is the main and necessary signal for insulin release through secretory granule exocytosis [31, 118]. Importantly, defects in the generation of Ca2+ oscillations, and thus in pulsatile insulin secretion, are associated with the loss of normal glucose homeostasis in type 2 diabetes [103]. Ca2+ influx through voltage-dependent Ca2+ channels has been shown to create Ca2+ microdomains beneath the β-cell plasma membrane with high [Ca2+] that might be crucial for insulin exocytosis and to open Ca2+-activated K+ channels (see below and [96]). Therefore, in pancreatic β cells, ATP acts as a signaling molecule initiating plasma membrane electrical activity linked to Ca2+ influx. Of note, the mitochondria play a central role in this process by connecting glucose metabolism to insulin release [57]. Specifically, in single primary β cells, cytosolic Ca2+ oscillations triggered by electrical stimulation cause stable increases in both [Ca2+]m and cytosolic ATP/ADP ratio which depend on MCU activity [105]. Respiratory chain inhibitors and uncouplers strongly inhibit insulin release [44], and chelation of mitochondrial matrix Ca2+ [117] or silencing of either MICU1 or MCU [1, 104] causes defective insulin secretion in β-cell lines (Fig. 5). Finally, it has been proposed that mitochondrial Ca2+ accumulation is essential to establish a nutrient-induced mitochondrial pH gradient which is crucial to sustain ATP synthesis and metabolism secretion coupling in insulin-releasing cells [86]. In summary, a two-phase model has been proposed, according to which soon after glucose stimulation, cytosolic [ATP] increases independently of any increase in cytosolic or mitochondrial [Ca2+]. Subsequently, an increase in [Ca2+]cyt occurs, which is followed by a rise in mitochondrial Ca2+ signal. This, in turn, stimulates mitochondrial metabolism and therefore ATP production. Importantly, mitochondrial Ca2+ efflux mediated by the Na+/Ca2+ exchanger NCLX contributes to the regulation of insulin secretion in β cells by shaping glucose-dependent mitochondrial and cytosolic Ca2+ signals [70] (Fig. 5).
Neurons
Neurons require extremely precise spatial-temporal control of Ca2+-dependent processes since they regulate vital functions such as transmission of depolarizing signals, synaptic plasticity, and metabolism [15]. Neurons have thus developed complex pathways to couple Ca2+ signals to their physiological response. Therefore, neurons are extremely sensitive to [Ca2+] levels and even small defects in Ca2+ homeostasis can lead to destructive consequences and alter normal neuronal activity, such as in aging [49] and neurodegeneration [15]. Ca2+ influx into neurons occurs through plasma membrane receptors and voltage-dependent ion channels. Furthermore, the release of Ca2+ from the ER also contributes to the elevation of [Ca2+]cyt. Overall, a complex and highly compartmentalized Ca2+ signaling system in neuronal cells allows the activation of different spatially separated Ca2+-dependent processes at the same time [79]. The wide range of neuronal functions regulated by intracellular Ca2+ signals raises the question of how selectivity is encoded by such a universal messenger. One answer to this question is the presence of local Ca2+ signals, or Ca2+ microdomains, developed rapidly near open Ca2+ channels, creating spatial Ca2+ gradients of high [Ca2+] near the open pores [69]. The major sources of intracellular Ca2+ include Ca2+ influx through ligand-gated glutamate receptors, such as N-methyl-d-aspartate (NMDA) receptor (NMDAR) or various voltage-dependent Ca2+ channels (VDCCs), as well as the release of Ca2+ from intracellular stores [6]. The relative contribution of these sources will depend on neuron size, transmitter system, and location in neural circuits (i.e., excitatory or inhibitory) [6].
As for the Ca2+ entry through neuron plasma membrane channels, the activation of presynaptic neurons leads to the release of neurotransmitters into the synaptic cleft via the entry of Ca2+ through the voltage-operated Ca2+ channels (VOCCs). The released neurotransmitters in the synaptic cleft, in turn, activate receptors in the postsynaptic PM, thus initiating signal transmission. In postsynaptic neurons, the activation of neurotransmitter receptors results in the generation of Ca2+ signals that trigger responses that are specific to the type of receptor (reviewed in [15]).
Importantly, in addition to regulating the physiological functions of mature neurons, Ca2+ signaling also plays essential roles in the neurogenesis from neural stem/progenitor cells which proliferate, migrate, and ultimately differentiate into billions of neurons and glia that populate the brain. There is increasing evidence that Ca2+ signaling controls specific genetic programs that establish the structures of the nervous system through Ca2+-dependent signaling pathways such as the calcineurin-NFAT signaling axis that has been shown to be critical for axonal growth as well as presynaptic development, dendritogenesis, and neuronal survival (reviewed in [107]).
Neurons are responsible for a huge oxygen consumption in resting conditions in humans. Indeed, the brain uses about 20% of the total oxygen consumed at rest but represents only 2% of the body mass [64]. Importantly, neurons are almost exclusively dependent on mitochondrial oxidative phosphorylation (OXPHOS) as a main source of ATP, and Ca2+ entry into the mitochondria guarantees activity-dependent regulation of cellular energy metabolism [52]. As for skeletal muscle contraction [35], also neuronal activity not only contributes significantly to ATP consumption but also rapidly adapts to increased activity stimulating ATP synthesis through a Ca2+-dependent increase in OXPHOS [88].
The mitochondria exert other neuron-specific functions. Indeed, their cellular distribution contributes to the accumulation of a large amount of Ca2+ in a defined subcellular domain, promoting large local cytoplasmic Ca2+ rises. Importantly, Ca2+ sequestration by the mitochondria profoundly affects neurotransmitter release, being strategically located in the proximity of Ca2+ channels such as NMDAR at the synaptic terminal [9, 62]. In general, mitochondria recruitment to neuronal soma, synapses, and dendritic spines is crucial for the regulation of nerve activity, and any change in the positioning of the mitochondria to subcellular domains affects neuron physiology and might contribute to the pathogenesis of neurodegeneration [102].
For a long time, one of the outstanding questions among neurologists has been whether modulation of mitochondrial Ca2+ uptake impinges on the neurotoxic effects of the excitatory neurotransmitter glutamate. In this respect, it was clear that the cause of neuronal dysfunction and death was the excessive Ca2+ influx in neuron through the NMDA subtype of glutamate receptor. Experiments performed by modulating MCU allowed to directly determine the role of mitochondrial Ca2+ uptake in response to excitotoxic stimuli [85]. In detail, MCU overexpression exacerbated NMDA-induced loss of mitochondrial membrane potential and cell death, while MCU knockdown protected the mitochondria from NMDA-induced depolarization and increased resistance to excitotoxicity. Endogenous MCU expression is controlled by neuroprotective synaptic activity, which negatively regulates MCU transcription, with a mechanism that implies nuclear Ca2+ and CaM kinase-dependent induction of the transcription factor Npas4 [85] (Fig. 5).
Following a report of a siRNA library screening that identified MCU and MICU1 as important factors for proper memory formation [114], a study performed in Drosophila melanogaster established that MCU-mediated mitochondrial Ca2+ uptake during development is of fundamental importance for olfactory memory formation but not for learning. Decreased mitochondrial Ca2+ accumulation, triggered by overexpression of a dominant-negative isoform of MCU in mushroom body neurons, causes axon lengthening accompanied by decreased synaptic vesicle content [25]. A recent report focused on the role of mitochondrial Ca2+ uptake in dopaminergic neurons. Silencing of D. melanogaster CG4495 gene, identified as MICU1 homolog, in dopaminergic neurons impaired climbing activity, which was worsened with aging, and shortened life span [56] (Fig. 5).
Concluding remarks
The physiological role of mitochondrial Ca2+ uptake has been extensively studied in the last few years thanks to the availability of transgenic animal models. Either constitutive or conditional deletion of MCU, as well as overexpression of MCUb, has been achieved in vivo. In addition, tissue-specific transgenic animals have been produced. The effects of MCU activity modulation on organs particularly relying on mitochondrial metabolism for their energy demand, including the heart, skeletal muscle, neurons, and pancreas, have been dissected. These studies have highlighted the importance of MCU in physiologic organ functions and in the protection from damaging insults, but also the existence of compensatory mechanisms. Intriguingly, cardiac-specific deletion or overexpression of the NCLX demonstrates the essential role of proper mitochondrial Ca2+ efflux for heart function and, in general, the requirement of fine-tuned mitochondrial Ca2+ dynamics for proper organ physiology. In the future, comprehensive studies will unravel still obscure aspects of mitochondrial Ca2+ homeostasis on cell and tissue functions.
Funding information
Research in Rizzuto Laboratory is supported by funding from the Italian Telethon Foundation (GGP16029 to RR and GGP16026 to AR); the Italian Association for Cancer Research (IG 18633 to RR); the Italian Ministry of Education, University, and Research (to CM); the French Muscular Dystrophy Association (AFM-Téléthon) (18857 to CM and 19471 to AR); and the National Institutes of Health (NIH) (to RR).
Footnotes
This article is part of the special issue on Mitochondrial Signalling in Pflügers Archiv – European Journal of Physiology
Contributor Information
Cristina Mammucari, Phone: +390498276481, Email: cristina.mammucari@unipd.it.
Rosario Rizzuto, Phone: +390498273001, Email: rosario.rizzuto@unipd.it.
References
- 1.Alam MR, Groschner LN, Parichatikanond W, Kuo L, Bondarenko AI, Rost R, Waldeck-Weiermair M, Malli R, Graier WF. Mitochondrial Ca2+ uptake 1 (MICU1) and mitochondrial Ca2+ uniporter (MCU) contribute to metabolism-secretion coupling in clonal pancreatic β-cells. J Biol Chem. 2012;287:34445–34454. doi: 10.1074/jbc.M112.392084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bassi MT, Manzoni M, Bresciani R, Pizzo MT, Della Monica A, Barlati S, Monti E, Borsani G. Cellular expression and alternative splicing of SLC25A23, a member of the mitochondrial Ca2+-dependent solute carrier gene family. Gene. 2005;345:173–182. doi: 10.1016/j.gene.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 3.Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL, Koteliansky V, Mootha VK. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernardi P, Vassanelli S, Veronese P, Colonna R, Szabó I, Zoratti M. Modulation of the mitochondrial permeability transition pore. Effect of protons and divalent cations. J Biol Chem. 1992;267:2934–2939. [PubMed] [Google Scholar]
- 5.Bernardi P, Rasola A, Forte M, Lippe G. The mitochondrial permeability transition pore: channel formation by F-ATP synthase, integration in signal transduction, and role in pathophysiology. Physiol Rev. 2015;95:1111–1155. doi: 10.1152/physrev.00001.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- 7.Bers DM. Cardiac excitation–contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 8.Bick AG, Calvo SE, Mootha VK. Evolutionary diversity of the mitochondrial calcium uniporter. Science. 2012;336:886. doi: 10.1126/science.1214977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Billups B, Forsythe ID. Presynaptic mitochondrial calcium sequestration influences transmission at mammalian central synapses. J Neurosci. 2002;22:5840–5847. doi: 10.1523/JNEUROSCI.22-14-05840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boehning D, Patterson RL, Sedaghat L, Glebova NO, Kurosaki T, Snyder SH. Cytochrome c binds to inositol (1,4,5) trisphosphate receptors, amplifying calcium-dependent apoptosis. Nat Cell Biol. 2003;5:1051–1061. doi: 10.1038/ncb1063. [DOI] [PubMed] [Google Scholar]
- 11.Boncompagni S, Rossi AE, Micaroni M, Beznoussenko GV, Polishchuk RS, Dirksen RT, Protasi F. Mitochondria are linked to calcium stores in striated muscle by developmentally regulated tethering structures. Mol Biol Cell. 2009;20:1058–1067. doi: 10.1091/mbc.E08-07-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonora M, Morganti C, Morciano G, Pedriali G, Lebiedzinska-Arciszewska M, Aquila G, Giorgi C, Rizzo P, Campo G, Ferrari R, Kroemer G, Wieckowski MR, Galluzzi L, Pinton P. Mitochondrial permeability transition involves dissociation of F 1 F O ATP synthase dimers and C-ring conformation. EMBO Rep. 2017;18:1077–1089. doi: 10.15252/embr.201643602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyman L, Chikando AC, Williams GSB, Khairallah RJ, Kettlewell S, Ward CW, Smith GL, Kao JPY, Lederer WJ. Calcium movement in cardiac mitochondria. Biophys J. 2014;107:1289–1301. doi: 10.1016/j.bpj.2014.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brini M, De Giorgi F, Murgia M, Marsault R, Massimino ML, Cantini M, Rizzuto R, Pozzan T. Subcellular analysis of Ca2+ homeostasis in primary cultures of skeletal muscle myotubes. Mol Biol Cell. 1997;8:129–143. doi: 10.1091/mbc.8.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brini M, Calì T, Ottolini D, Carafoli E. Neuronal calcium signaling: function and dysfunction. Cell Mol Life Sci. 2014;71:2787–2814. doi: 10.1007/s00018-013-1550-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calabria E, Ciciliot S, Moretti I, Garcia M, Picard A, Dyar KA, Pallafacchina G, Tothova J, Schiaffino S, Murgia M. NFAT isoforms control activity-dependent muscle fiber type specification. Proc Natl Acad Sci U S A. 2009;106:13335–13340. doi: 10.1073/pnas.0812911106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung J-H, Biesiadecki BJ, Ziolo MT, Davis JP, Janssen PML. Myofilament calcium sensitivity: role in regulation of in vivo cardiac contraction and relaxation. Front Physiol. 2016;7:562. doi: 10.3389/fphys.2016.00562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colombini M. The VDAC channel: molecular basis for selectivity. Biochim Biophys Acta - Mol Cell Res. 2016;1863:2498–2502. doi: 10.1016/j.bbamcr.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 19.Csordás G, Golenár T, Seifert EL, Kamer KJ, Sancak Y, Perocchi F, Moffat C, Weaver D, de la Fuente Perez S, Bogorad R, Koteliansky V, Adijanto J, Mootha VK, Hajnóczky G. MICU1 controls both the threshold and cooperative activation of the mitochondrial Ca2+ uniporter. Cell Metab. 2013;17:976–987. doi: 10.1016/j.cmet.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Das AM, Harris DA. Control of mitochondrial ATP synthase in heart cells: inactive to active transitions caused by beating or positive inotropic agents. Cardiovasc Res. 1990;24:411–417. doi: 10.1093/cvr/24.5.411. [DOI] [PubMed] [Google Scholar]
- 21.De Stefani D, Raffaello A, Teardo E, Szabò I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deluca HF, Engstrom GW. Calcium uptake by rat kidney mitochondria. Proc Natl Acad Sci U S A. 1961;47:1744–1750. doi: 10.1073/pnas.47.11.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Denton RM. Regulation of mitochondrial dehydrogenases by calcium ions. Biochim Biophys Acta. 2009;1787:1309–1316. doi: 10.1016/j.bbabio.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Di Lisa F, Bernardi P. A CaPful of mechanisms regulating the mitochondrial permeability transition. J Mol Cell Cardiol. 2009;46:775–780. doi: 10.1016/j.yjmcc.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Drago I, Davis RL. Inhibiting the mitochondrial calcium uniporter during development impairs memory in adult Drosophila. Cell Rep. 2016;16:2763–2776. doi: 10.1016/j.celrep.2016.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drago I, De Stefani D, Rizzuto R, Pozzan T. Mitochondrial Ca2+ uptake contributes to buffering cytoplasmic Ca2+ peaks in cardiomyocytes. Proc Natl Acad Sci. 2012;109:12986–12991. doi: 10.1073/pnas.1210718109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eisner V, Csordas G, Hajnoczky G. Interactions between sarco-endoplasmic reticulum and mitochondria in cardiac and skeletal muscle—pivotal roles in Ca2+ and reactive oxygen species signaling. J Cell Sci. 2013;126:2965–2978. doi: 10.1242/jcs.093609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eisner DA, Caldwell JL, Kistamás K, Trafford AW. Calcium and excitation-contraction coupling in the heart. Circ Res. 2017;121:181–195. doi: 10.1161/CIRCRESAHA.117.310230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fieni F, Lee SB, Jan YN, Kirichok Y. Activity of the mitochondrial calcium uniporter varies greatly between tissues. Nat Commun. 2012;3:1317. doi: 10.1038/ncomms2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghafourifar P, Schenk U, Klein SD, Richter C. Mitochondrial nitric-oxide synthase stimulation causes cytochrome c release from isolated mitochondria. Evidence for intramitochondrial peroxynitrite formation. J Biol Chem. 1999;274:31185–31188. doi: 10.1074/jbc.274.44.31185. [DOI] [PubMed] [Google Scholar]
- 31.Gilon P, Chae H-Y, Rutter GA, Ravier MA. Calcium signaling in pancreatic β-cells in health and in type 2 diabetes. Cell Calcium. 2014;56:340–361. doi: 10.1016/j.ceca.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 32.Giorgio V, von Stockum S, Antoniel M, Fabbro A, Fogolari F, Forte M, Glick GD, Petronilli V, Zoratti M, Szabo I, Lippe G, Bernardi P. Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc Natl Acad Sci. 2013;110:5887–5892. doi: 10.1073/pnas.1217823110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giorgio V, Burchell V, Schiavone M, Bassot C, Minervini G, Petronilli V, Argenton F, Forte M, Tosatto S, Lippe G, Bernardi P. Ca 2+ binding to F-ATP synthase β subunit triggers the mitochondrial permeability transition. EMBO Rep. 2017;18:1065–1076. doi: 10.15252/embr.201643354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giorgio V, Guo L, Bassot C, Petronilli V, Bernardi P (2017) Calcium and regulation of the mitochondrial permeability transition. Cell Calcium. 10.1016/j.ceca.2017.05.004 [DOI] [PubMed]
- 35.Glancy B, Willis WT, Chess DJ, Balaban RS. Effect of calcium on the oxidative phosphorylation cascade in skeletal muscle mitochondria. Biochemistry. 2013;52:2793–2809. doi: 10.1021/bi3015983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He J, Carroll J, Ding S, Fearnley IM, Walker JE. Permeability transition in human mitochondria persists in the absence of peripheral stalk subunits of ATP synthase. Proc Natl Acad Sci. 2017;114:9086–9091. doi: 10.1073/pnas.1711201114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He J, Ford HC, Carroll J, Ding S, Fearnley IM, Walker JE. Persistence of the mitochondrial permeability transition in the absence of subunit c of human ATP synthase. Proc Natl Acad Sci. 2017;114:3409–3414. doi: 10.1073/pnas.1702357114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hempel N, Trebak M. Crosstalk between calcium and reactive oxygen species signaling in cancer. Cell Calcium. 2017;63:70–96. doi: 10.1016/j.ceca.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoffman NE, Chandramoorthy HC, Shanmughapriya S, Zhang XQ, Vallem S, Doonan PJ, Malliankaraman K, Guo S, Rajan S, Elrod JW, Koch WJ, Cheung JY, Madesh M. SLC25A23 augments mitochondrial Ca2+ uptake, interacts with MCU, and induces oxidative stress-mediated cell death. Mol Biol Cell. 2014;25:936–947. doi: 10.1091/mbc.E13-08-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holmström KM, Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat Rev Mol Cell Biol. 2014;15:411–421. doi: 10.1038/nrm3801. [DOI] [PubMed] [Google Scholar]
- 41.Holmström KM, Pan X, Liu JC, Menazza S, Liu J, Nguyen TT, Pan H, Parks RJ, Anderson S, Noguchi A, Springer D, Murphy E, Finkel T. Assessment of cardiac function in mice lacking the mitochondrial calcium uniporter. J Mol Cell Cardiol. 2015;85:178–182. doi: 10.1016/j.yjmcc.2015.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hung V, Zou P, Rhee H-W, Udeshi ND, Cracan V, Svinkina T, Carr SA, Mootha VK, Ting AY. Proteomic mapping of the human mitochondrial intermembrane space in live cells via ratiometric APEX tagging. Mol Cell. 2014;55:332–341. doi: 10.1016/j.molcel.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hüser J, Blatter LA, Sheu S-S. Mitochondrial calcium in heart cells: beat-to-beat oscillations or slow integration of cytosolic transients? J Bioenerg Biomembr. 2000;32:27–33. doi: 10.1023/a:1005556227425. [DOI] [PubMed] [Google Scholar]
- 44.Hutton JC, Sener A, Herchuelz A, Atwater I, Kawazu S, Boschero AC, Somers G, Devis G, Malaisse WJ. Similarities in the stimulus-secretion coupling mechanisms of glucose- and 2-keto acid-induced insulin release*. Endocrinology. 1980;106:203–219. doi: 10.1210/endo-106-1-203. [DOI] [PubMed] [Google Scholar]
- 45.Joiner MA, Koval OM, Li J, He BJ, Allamargot C, Gao Z, Luczak ED, Hall DD, Fink BD, Chen B, Yang J, Moore SA, Scholz TD, Strack S, Mohler PJ, Sivitz WI, Song L-S, Anderson ME. CaMKII determines mitochondrial stress responses in heart. Nature. 2012;491:269–273. doi: 10.1038/nature11444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kamer KJ, Mootha VK. MICU1 and MICU2 play nonredundant roles in the regulation of the mitochondrial calcium uniporter. EMBO Rep. 2014;15:299–307. doi: 10.1002/embr.201337946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kamer KJ, Grabarek Z, Mootha VK. High-affinity cooperative Ca2+ binding by MICU1–MICU2 serves as an on–off switch for the uniporter. EMBO Rep. 2017;18:1397–1411. doi: 10.15252/embr.201643748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kovács-Bogdán E, Sancak Y, Kamer KJ, Plovanich M, Jambhekar A, Huber RJ, Myre MA, Blower MD, Mootha VK. Reconstitution of the mitochondrial calcium uniporter in yeast. Proc Natl Acad Sci U S A. 2014;111:8985–8990. doi: 10.1073/pnas.1400514111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kumar A, Bodhinathan K, Foster TC. Susceptibility to calcium dysregulation during brain aging. Front Aging Neurosci. 2009;1:2. doi: 10.3389/neuro.24.002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kwong JQ, Lu X, Correll RN, Schwanekamp JA, Vagnozzi RJ, Sargent MA, York AJ, Zhang J, Bers DM, Molkentin JD. The mitochondrial calcium uniporter selectively matches metabolic output to acute contractile stress in the heart. Cell Rep. 2015;12:15–22. doi: 10.1016/j.celrep.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee Y, Min CK, Kim TG, Song HK, Lim Y, Kim D, Shin K, Kang M, Kang JY, Youn H-S, Lee J-G, An JY, Park KR, Lim JJ, Kim JH, Kim JH, Park ZY, Kim Y-S, Wang J, Kim DH, Eom SH. Structure and function of the N-terminal domain of the human mitochondrial calcium uniporter. EMBO Rep. 2015;16:1318–1333. doi: 10.15252/embr.201540436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Llorente-Folch I, Rueda CB, Pardo B, Szabadkai G, Duchen MR, Satrustegui J. The regulation of neuronal mitochondrial metabolism by calcium. J Physiol. 2015;593:3447–3462. doi: 10.1113/JP270254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lu X, Ginsburg KS, Kettlewell S, Bossuyt J, Smith GL, Bers DM. Measuring local gradients of intramitochondrial [Ca(2+)] in cardiac myocytes during sarcoplasmic reticulum Ca(2+) release. Circ Res. 2013;112:424–431. doi: 10.1161/CIRCRESAHA.111.300501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Luongo TS, Lambert JP, Yuan A, Zhang X, Gross P, Song J, Shanmughapriya S, Gao E, Jain M, Houser SR, Koch WJ, Cheung JY, Madesh M, Elrod JW. The mitochondrial calcium uniporter matches energetic supply with cardiac workload during stress and modulates permeability transition. Cell Rep. 2015;12:23–34. doi: 10.1016/j.celrep.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luongo TS, Lambert JP, Gross P, Nwokedi M, Lombardi AA, Shanmughapriya S, Carpenter AC, Kolmetzky D, Gao E, van Berlo JH, Tsai EJ, Molkentin JD, Chen X, Madesh M, Houser SR, Elrod JW. The mitochondrial Na(+)/Ca(2+) exchanger is essential for Ca(2+) homeostasis and viability. Nature. 2017;545:93–97. doi: 10.1038/nature22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.M’Angale PG, Staveley BE (2017) Inhibition of mitochondrial calcium uptake 1 in Drosophila neurons. Genet Mol Res 16. 10.4238/gmr16019436 [DOI] [PubMed]
- 57.Maechler P, Wollheim CB. Mitochondrial function in normal and diabetic β-cells. Nature. 2001;414:807–812. doi: 10.1038/414807a. [DOI] [PubMed] [Google Scholar]
- 58.Mallilankaraman K, Cárdenas C, Doonan PJ, Chandramoorthy HC, Irrinki KM, Golenár T, Csordás G, Madireddi P, Yang J, Müller M, Miller R, Kolesar JE, Molgó J, Kaufman B, Hajnóczky G, Foskett JK, Madesh M. MCUR1 is an essential component of mitochondrial Ca2+ uptake that regulates cellular metabolism. Nat Cell Biol. 2012;14:1336–1343. doi: 10.1038/ncb2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mallilankaraman K, Doonan P, Cárdenas C, Chandramoorthy HC, Müller M, Miller R, Hoffman NE, Gandhirajan RK, Molgó J, Birnbaum MJ, Rothberg BS, Mak D-OD, Foskett JK, Madesh M. MICU1 is an essential gatekeeper for MCU-mediated mitochondrial Ca2+ uptake that regulates cell survival. Cell. 2012;151:630–644. doi: 10.1016/j.cell.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mammucari C, Gherardi G, Zamparo I, Raffaello A, Boncompagni S, Chemello F, Cagnin S, Braga A, Zanin S, Pallafacchina G, Zentilin L, Sandri M, De Stefani D, Protasi F, Lanfranchi G, Rizzuto R. The mitochondrial calcium uniporter controls skeletal muscle trophism in vivo. Cell Rep. 2015;10:1269–1279. doi: 10.1016/j.celrep.2015.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martell JD, Deerinck TJ, Sancak Y, Poulos TL, Mootha VK, Sosinsky GE, Ellisman MH, Ting AY. Engineered ascorbate peroxidase as a genetically encoded reporter for electron microscopy. Nat Biotechnol. 2012;30:1143–1148. doi: 10.1038/nbt.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Medler K, Gleason EL. Mitochondrial Ca(2+) buffering regulates synaptic transmission between retinal amacrine cells. J Neurophysiol. 2002;87:1426–1439. doi: 10.1152/jn.00627.2001. [DOI] [PubMed] [Google Scholar]
- 63.Mildaziene V, Baniene R, Nauciene Z, Bakker BM, Brown GC, Westerhoff HV, Kholodenko BN. Calcium indirectly increases the control exerted by the adenine nucleotide translocator over 2-oxoglutarate oxidation in rat heart mitochondria. Arch Biochem Biophys. 1995;324:130–134. doi: 10.1006/abbi.1995.9918. [DOI] [PubMed] [Google Scholar]
- 64.Mink JW, Blumenschine RJ, Adams DB. Ratio of central nervous system to body metabolism in vertebrates: its constancy and functional basis. Am J Phys. 1981;241:R203–R212. doi: 10.1152/ajpregu.1981.241.3.R203. [DOI] [PubMed] [Google Scholar]
- 65.Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- 66.Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966;41:445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- 67.Morciano G, Giorgi C, Bonora M, Punzetti S, Pavasini R, Wieckowski MR, Campo G, Pinton P. Molecular identity of the mitochondrial permeability transition pore and its role in ischemia-reperfusion injury. J Mol Cell Cardiol. 2015;78:142–153. doi: 10.1016/j.yjmcc.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 68.Murphy E, Pan X, Nguyen T, Liu J, Holmström KM, Finkel T. Unresolved questions from the analysis of mice lacking MCU expression. Biochem Biophys Res Commun. 2014;449:384–385. doi: 10.1016/j.bbrc.2014.04.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Neher E. Vesicle pools and Ca2+ microdomains: new tools for understanding their roles in neurotransmitter release. Neuron. 1998;20:389–399. doi: 10.1016/s0896-6273(00)80983-6. [DOI] [PubMed] [Google Scholar]
- 70.Nita II, Hershfinkel M, Fishman D, Ozeri E, Rutter GA, Sensi SL, Khananshvili D, Lewis EC, Sekler I. The mitochondrial Na+/Ca2+ exchanger upregulates glucose dependent Ca2+ Signalling linked to insulin secretion. PLoS One. 2012;7:e46649. doi: 10.1371/journal.pone.0046649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Oxenoid K, Dong Y, Cao C, Cui T, Sancak Y, Markhard AL, Grabarek Z, Kong L, Liu Z, Ouyang B, Cong Y, Mootha VK, Chou JJ. Architecture of the mitochondrial calcium uniporter. Nature. 2016;533:269–273. doi: 10.1038/nature17656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pagliarini DJ, Calvo SE, Chang B, Sheth SA, Vafai SB, Ong S-E, Walford GA, Sugiana C, Boneh A, Chen WK, Hill DE, Vidal M, Evans JG, Thorburn DR, Carr SA, Mootha VK. A mitochondrial protein compendium elucidates complex I disease biology. Cell. 2008;134:112–123. doi: 10.1016/j.cell.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Paillard M, Csordás G, Szanda G, Golenár T, Debattisti V, Bartok A, Wang N, Moffat C, Seifert EL, Spät A, Hajnóczky G. Tissue-specific mitochondrial decoding of cytoplasmic Ca2+ signals is controlled by the stoichiometry of MICU1/2 and MCU. Cell Rep. 2017;18:2291–2300. doi: 10.1016/j.celrep.2017.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Palty R, Silverman WF, Hershfinkel M, Caporale T, Sensi SL, Parnis J, Nolte C, Fishman D, Shoshan-Barmatz V, Herrmann S, Khananshvili D, Sekler I. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc Natl Acad Sci. 2010;107:436–441. doi: 10.1073/pnas.0908099107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pan X, Liu J, Nguyen T, Liu C, Sun J, Teng Y, Fergusson MM, Rovira II, Allen M, Springer DA, Aponte AM, Gucek M, Balaban RS, Murphy E, Finkel T. The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter. Nat Cell Biol. 2013;15:1464–1472. doi: 10.1038/ncb2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Park MK, Ashby MC, Erdemli G, Petersen OH, Tepikin AV. Perinuclear, perigranular and sub-plasmalemmal mitochondria have distinct functions in the regulation of cellular calcium transport. EMBO J. 2001;20:1863–1874. doi: 10.1093/emboj/20.8.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Patron M, Checchetto V, Raffaello A, Teardo E, Vecellio Reane D, Mantoan M, Granatiero V, Szabò I, De Stefani D, Rizzuto R. MICU1 and MICU2 finely tune the mitochondrial Ca2+ uniporter by exerting opposite effects on MCU activity. Mol Cell. 2014;53:726–737. doi: 10.1016/j.molcel.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Paupe V, Prudent J, Dassa EP, Rendon OZ, Shoubridge EA. CCDC90A (MCUR1) is a cytochrome c oxidase assembly factor and not a regulator of the mitochondrial calcium uniporter. Cell Metab. 2015;21:109–116. doi: 10.1016/j.cmet.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 79.Pchitskaya E, Popugaeva E, Bezprozvanny I (2017) Calcium signaling and molecular mechanisms underlying neurodegenerative diseases. Cell Calcium. 10.1016/j.ceca.2017.06.008 [DOI] [PMC free article] [PubMed]
- 80.Perocchi F, Gohil VM, Girgis HS, Bao XR, McCombs JE, Palmer AE, Mootha VK. MICU1 encodes a mitochondrial EF hand protein required for Ca(2+) uptake. Nature. 2010;467:291–296. doi: 10.1038/nature09358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Petronilli V, Cola C, Bernardi P. Modulation of the mitochondrial cyclosporin A-sensitive permeability transition pore. II. The minimal requirements for pore induction underscore a key role for transmembrane electrical potential, matrix pH, and matrix Ca2+ J Biol Chem. 1993;268:1011–1016. [PubMed] [Google Scholar]
- 82.Petrungaro C, Zimmermann KM, Küttner V, Fischer M, Dengjel J, Bogeski I, Riemer J. The Ca(2+)-dependent release of the Mia40-induced MICU1-MICU2 dimer from MCU regulates mitochondrial Ca(2+) uptake. Cell Metab. 2015;22:721–733. doi: 10.1016/j.cmet.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 83.Plovanich M, Bogorad RL, Sancak Y, Kamer KJ, Strittmatter L, Li AA, Girgis HS, Kuchimanchi S, De Groot J, Speciner L, Taneja N, Oshea J, Koteliansky V, Mootha VK. MICU2, a paralog of MICU1, resides within the mitochondrial uniporter complex to regulate calcium handling. PLoS One. 2013;8:e55785. doi: 10.1371/journal.pone.0055785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Porporato PE, Payen VL, Pérez-Escuredo J, De Saedeleer CJ, Danhier P, Copetti T, Dhup S, Tardy M, Vazeille T, Bouzin C, Feron O, Michiels C, Gallez B, Sonveaux P. A mitochondrial switch promotes tumor metastasis. Cell Rep. 2014;8:754–766. doi: 10.1016/j.celrep.2014.06.043. [DOI] [PubMed] [Google Scholar]
- 85.Qiu J, Tan Y-W, Hagenston AM, Martel M-A, Kneisel N, Skehel PA, Wyllie DJA, Bading H, Hardingham GE. Mitochondrial calcium uniporter Mcu controls excitotoxicity and is transcriptionally repressed by neuroprotective nuclear calcium signals. Nat Commun. 2013;4:2034. doi: 10.1038/ncomms3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Quan X, Nguyen TT, Choi S-K, Xu S, Das R, Cha S-K, Kim N, Han J, Wiederkehr A, Wollheim CB, Park K-S. Essential role of mitochondrial Ca2+ uniporter in the generation of mitochondrial pH gradient and metabolism-secretion coupling in insulin-releasing cells. J Biol Chem. 2015;290:4086–4096. doi: 10.1074/jbc.M114.632547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Raffaello A, De Stefani D, Sabbadin D, Teardo E, Merli G, Picard A, Checchetto V, Moro S, Szabò I, Rizzuto R. The mitochondrial calcium uniporter is a multimer that can include a dominant-negative pore-forming subunit. EMBO J. 2013;32:2362–2376. doi: 10.1038/emboj.2013.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rangaraju V, Calloway N, Ryan TA. Activity-driven local ATP synthesis is required for synaptic function. Cell. 2014;156:825–835. doi: 10.1016/j.cell.2013.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rasmussen TP, Wu Y, Joiner MA, Koval OM, Wilson NR, Luczak ED, Wang Q, Chen B, Gao Z, Zhu Z, Wagner BA, Soto J, McCormick ML, Kutschke W, Weiss RM, Yu L, Boudreau RL, Abel ED, Zhan F, Spitz DR, Buettner GR, Song L-S, Zingman LV, Anderson ME. Inhibition of MCU forces extramitochondrial adaptations governing physiological and pathological stress responses in heart. Proc Natl Acad Sci U S A. 2015;112:9129–9134. doi: 10.1073/pnas.1504705112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ringer S. A third contribution regarding the influence of the inorganic constituents of the blood on the ventricular contraction. J Physiol. 1883;4:222–225. doi: 10.1113/jphysiol.1883.sp000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rizzuto R, Simpson AW, Brini M, Pozzan T. Rapid changes of mitochondrial Ca2+ revealed by specifically targeted recombinant aequorin. Nature. 1992;358:325–327. doi: 10.1038/358325a0. [DOI] [PubMed] [Google Scholar]
- 92.Rizzuto R, Brini M, Murgia M, Pozzan T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science. 1993;262:744–747. doi: 10.1126/science.8235595. [DOI] [PubMed] [Google Scholar]
- 93.Rizzuto R, De Stefani D, Raffaello A, Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol. 2012;13:566–578. doi: 10.1038/nrm3412. [DOI] [PubMed] [Google Scholar]
- 94.Rottenberg H, Scarpa A. Calcium uptake and membrane potential in mitochondria. Biochemistry. 1974;13:4811–4817. doi: 10.1021/bi00720a020. [DOI] [PubMed] [Google Scholar]
- 95.Rudolf R. In vivo monitoring of Ca2+ uptake into mitochondria of mouse skeletal muscle during contraction. J Cell Biol. 2004;166:527–536. doi: 10.1083/jcb.200403102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rutter GA, Tsuboi T, Ravier MA. Ca2+ microdomains and the control of insulin secretion. Cell Calcium. 2006;40:539–551. doi: 10.1016/j.ceca.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 97.Rutter GA, Hodson DJ, Chabosseau P, Haythorne E, Pullen TJ, Leclerc I. Local and regional control of calcium dynamics in the pancreatic islet. Diabetes Obes Metab. 2017;19:30–41. doi: 10.1111/dom.12990. [DOI] [PubMed] [Google Scholar]
- 98.Sancak Y, Markhard AL, Kitami T, Kovács-Bogdán E, Kamer KJ, Udeshi ND, Carr SA, Chaudhuri D, Clapham DE, Li AA, Calvo SE, Goldberger O, Mootha VK. EMRE is an essential component of the mitochondrial calcium uniporter complex. Science. 2013;342:1379–1382. doi: 10.1126/science.1242993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sandow A. Excitation-contraction coupling in muscular response. Yale J Biol Med. 1952;25:176–201. [PMC free article] [PubMed] [Google Scholar]
- 100.Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev. 2011;91:1447–1531. doi: 10.1152/physrev.00031.2010. [DOI] [PubMed] [Google Scholar]
- 101.Sekler I. Standing of giants shoulders the story of the mitochondrial Na+Ca2+ exchanger. Biochem Biophys Res Commun. 2015;460:50–52. doi: 10.1016/j.bbrc.2015.02.170. [DOI] [PubMed] [Google Scholar]
- 102.Sheng Z-H, Cai Q. Mitochondrial transport in neurons: impact on synaptic homeostasis and neurodegeneration. Nat Rev Neurosci. 2012;13:77–93. doi: 10.1038/nrn3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet. 2005;365:1333–1346. doi: 10.1016/S0140-6736(05)61032-X. [DOI] [PubMed] [Google Scholar]
- 104.Tarasov AI, Semplici F, Ravier MA, Bellomo EA, Pullen TJ, Gilon P, Sekler I, Rizzuto R, Rutter GA. The mitochondrial Ca2+ uniporter MCU is essential for glucose-induced ATP increases in pancreatic β-cells. PLoS One. 2012;7:e39722. doi: 10.1371/journal.pone.0039722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tarasov AI, Semplici F, Li D, Rizzuto R, Ravier MA, Gilon P, Rutter GA. Frequency-dependent mitochondrial Ca(2+) accumulation regulates ATP synthesis in pancreatic β cells. Pflugers Arch. 2013;465:543–554. doi: 10.1007/s00424-012-1177-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tosatto A, Sommaggio R, Kummerow C, Bentham RB, Blacker TS, Berecz T, Duchen MR, Rosato A, Bogeski I, Szabadkai G, Rizzuto R, Mammucari C. The mitochondrial calcium uniporter regulates breast cancer progression via HIF-1α. EMBO Mol Med. 2016;8:569–585. doi: 10.15252/emmm.201606255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Toth AB, Shum AK, Prakriya M. Regulation of neurogenesis by calcium signaling. Cell Calcium. 2016;59:124–134. doi: 10.1016/j.ceca.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tretter L, Takacs K, Kövér K, Adam-Vizi V. Stimulation of H(2)O(2) generation by calcium in brain mitochondria respiring on alpha-glycerophosphate. J Neurosci Res. 2007;85:3471–3479. doi: 10.1002/jnr.21405. [DOI] [PubMed] [Google Scholar]
- 109.Vais H, Mallilankaraman K, Mak D-OD, Hoff H, Payne R, Tanis JE, Foskett JK. EMRE is a matrix Ca2+ sensor that governs gatekeeping of the mitochondrial Ca2+ uniporter. Cell Rep. 2016;14:403–410. doi: 10.1016/j.celrep.2015.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vasington FD, Murphy JV. Ca ion uptake by rat kidney mitochondria and its dependence on respiration and phosphorylation. J Biol Chem. 1962;237:2670–2677. [PubMed] [Google Scholar]
- 111.Vecellio Reane D, Vallese F, Checchetto V, Acquasaliente L, Butera G, De Filippis V, Szabò I, Zanotti G, Rizzuto R, Raffaello A. A MICU1 splice variant confers high sensitivity to the mitochondrial Ca2+ uptake machinery of skeletal muscle. Mol Cell. 2016;64:760–773. doi: 10.1016/j.molcel.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 112.Viola HM, Hool LC. How does calcium regulate mitochondrial energetics in the heart?—new insights. Heart Lung Circ. 2014;23:602–609. doi: 10.1016/j.hlc.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 113.Waldeck-Weiermair M, Malli R, Parichatikanond W, Gottschalk B, Madreiter-Sokolowski CT, Klec C, Rost R, Graier WF. Rearrangement of MICU1 multimers for activation of MCU is solely controlled by cytosolic Ca(2) Sci Rep. 2015;5:15602. doi: 10.1038/srep15602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Walkinshaw E, Gai Y, Farkas C, Richter D, Nicholas E, Keleman K, Davis RL. Identification of genes that promote or inhibit olfactory memory formation in Drosophila. Genetics. 2015;199:1173–1182. doi: 10.1534/genetics.114.173575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang L, Yang X, Li S, Wang Z, Liu Y, Feng J, Zhu Y, Shen Y. Structural and mechanistic insights into MICU1 regulation of mitochondrial calcium uptake. Embo. 2014;33:2014–2604. doi: 10.1002/embj.201386523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wernette ME, Ochs RS, Lardy HA. Ca2+ stimulation of rat liver mitochondrial glycerophosphate dehydrogenase. J Biol Chem. 1981;256:12767–12771. [PubMed] [Google Scholar]
- 117.Wiederkehr A, Szanda G, Akhmedov D, Mataki C, Heizmann CW, Schoonjans K, Pozzan T, Spät A, Wollheim CB. Mitochondrial matrix calcium is an activating signal for hormone secretion. Cell Metab. 2011;13:601–611. doi: 10.1016/j.cmet.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 118.Wiser O, Trus M, Hernández A, Renström E, Barg S, Rorsman P, Atlas D. The voltage sensitive Lc-type Ca2+ channel is functionally coupled to the exocytotic machinery. Proc Natl Acad Sci U S A. 1999;96:248–253. doi: 10.1073/pnas.96.1.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wollheim CB, Sharp GW. Regulation of insulin release by calcium. Physiol Rev. 1981;61:914–973. doi: 10.1152/physrev.1981.61.4.914. [DOI] [PubMed] [Google Scholar]
- 120.Wu Y, Rasmussen TP, Koval OM, Joiner M-L, Hall DD, Chen B, Luczak ED, Wang Q, Rokita AG, Wehrens XHT, Song L-S, Anderson ME. The mitochondrial uniporter controls fight or flight heart rate increases. Nat Commun. 2015;6:6081. doi: 10.1038/ncomms7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yamamoto T, Yamagoshi R, Harada K, Kawano M, Minami N, Ido Y, Kuwahara K, Fujita A, Ozono M, Watanabe A, Yamada A, Terada H, Shinohara Y. Analysis of the structure and function of EMRE in a yeast expression system. Biochim Biophys Acta. 2016;1857:831–839. doi: 10.1016/j.bbabio.2016.03.019. [DOI] [PubMed] [Google Scholar]