 This review discusses mechanisms of toxicity, particularly those observed in liver tissue, mediated by microcystins (MCs) produced by cyanobacteria.
This review discusses mechanisms of toxicity, particularly those observed in liver tissue, mediated by microcystins (MCs) produced by cyanobacteria.
Abstract
Microcystins, such as microcystin-leucine arginine (MC-LR), are some of the most toxic and prevalent cyanotoxins produced by cyanobacteria in freshwater and saltwater algal blooms worldwide. Acute and chronic exposures to microcystins are primarily known to cause hepatotoxicity; cellular damage and genotoxicity within mammalian livers. However, in vivo studies indicate that similar damage may occur in other mammalian organs and tissues, such as the kidney, heart, reproductive systems, and lungs – particularly following chronic low-dose exposures. Mechanisms of toxicity of mycrocystins are reviewed herein; including cellular uptake, interaction with protein phosphatases PP1 and PP2A, cytoskeletal effects, formation of oxidative stress and induction of apoptosis. In general, the mode of action of toxicity by MCs in mammalian organs are similar to those that have been observed in liver tissues. A comprehensive understanding of the toxic mechanisms of microcystins in mammalian tissues and organs will assist in the development of risk assessment approaches to public health protection strategies and the development of robust drinking water policies.
Introduction
Algal blooms occur in fresh and saltwater bodies around the world and pose a threat to human and ecological health. Many water bodies, including Lake Erie, suffer from persistent noxious algal blooms.1,2 These blooms can produce cyanotoxins and negatively impact the drinking water supplies to surrounding communities, as well as tourism and fishery economies.
Algal blooms are caused by an overgrowth of photosynthetic microorganisms known as cyanobacteria. Cyanobacteria include numerous genera capable of producing a variety of intracellular cyanotoxins that can harm aquatic, livestock, and human health (Table 1).3–5 Cell-bound (i.e. intracellular) toxins may be released into the water (i.e. extracellular) column if the cyanobacterial cell wall is disrupted during cell death or certain drinking water treatment processes.6
Table 1. Cyanotoxins, cyanobacteria genera and primary organ targets.
| Type | Toxin compounds | Toxin-producing genera | Primary organ target(s) |
| Hepatotoxins | Microcystins (e.g. MC-LR, -LA, -YR, -RR) | • Microcystis aeruginosa | • Liver damage |
| • Anabaena sp. | • Indications of tumour promotion in rodent studies | ||
| • Oscillatoria sp. | |||
| • Nostoc sp. | |||
| Nodularin | • Nodularia spumigena | ||
| Cylindrospermopsin | • Cylindrospermopsis raciborskii | • Cytotoxic | |
| • Aphanizomenon ovalisporum | • Liver, kidney, spleen, intestine, heart, thymus | ||
| • Anabaena bergii | • Genotoxic | ||
| Neurotoxins | Anatoxin-a | • Anabaena sp. | • Nervous system; paralysis and respiratory failure |
| • Aphanizomenon sp. | |||
| • Oscillatoria sp. | |||
| • Cylindrospermum sp. | |||
| Saxitoxins | • Anabaena circinalis | • Nerve axons | |
| • Aphanizomenon sp. | |||
| • Cylindrospermopsis raciborskii | |||
| • Lyngbya wollei | |||
| Cytotoxins | Cylindrospermopsin | • Cylindrospermopsis sp. | • Liver, kidney, spleen, intestine, heart |
| • Umezakia sp. | |||
| Dermatotoxins | Lungbyatoxins-a | • Lyngbya | • Skin |
| Irritants | Lipopolysaccharides | • Most cyanobacteria (cellular wall component) | • Exposed tissue, gastro-intestinal disorders, respiratory symptoms |
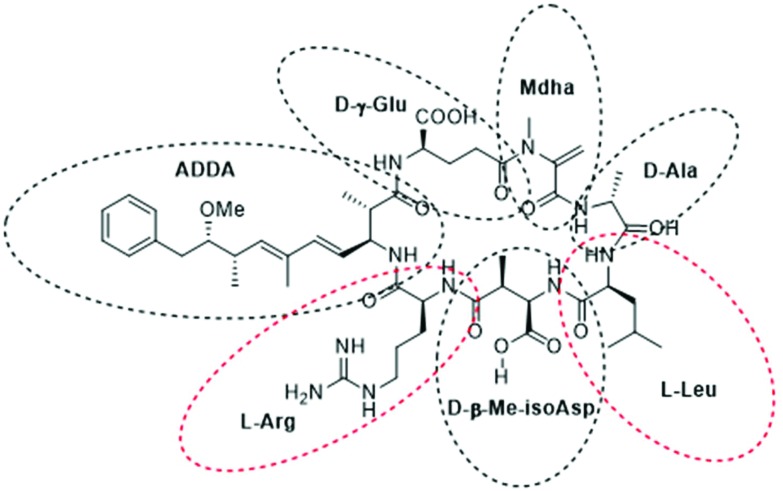
Microcystins (MCs), a class of known hepatotoxins, are the most common cyanotoxins detected in Lake Erie and in freshwaters worldwide.1,7 MCs are monocyclic peptide toxins of which there are >100 known variants.8–11 The ∼1000 Da structure of MCs include seven amino acids in a ring formation with a unique β-amino acid side chain (ADDA group). The nomenclature for each variant emanates from two variable positions that are always occupied by l-amino acids (e.g.l-leucine and l-arginine in the case of MC-LR). The variants can differ in their polarity; some contain hydrophobic amino acid residues such as MC-LF (with phenylalanine) and MC-LW (with tryptophan); while MC-LR is more hydrophilic.11 The consistent amino acids include d-alanine (d-ala), d-β-methyl-isoaspartate (d-β-Me-isoAsp) and d-γ-glutamate (d-glu) and are shown in Fig. 1 of MC-LR. The d-glu group and ADDA side chain have been implicated to play critical roles in the mode of action (MOA) of microcystins binding and inhibiting protein phosphatases (PP1 and PP2A) in mammalian cells.9,11
Fig. 1. Chemical structure of MC-LR highlighting the seven amino acid residues.
A recent review by Health Canada found that further research is required to determine the carcinogenic and tumour promoting potential of microcystins in mammals.12 A tolerable daily intake (TDI) of 0.056 μg per kg body weight per day was derived from a lowest-observed-adverse-effect level (LOAEL) of 50 μg per kg body weight per day based on increased liver weight and slight to moderate liver lesions with hemorrhages in rats.13 The TDI was established using an uncertainty factor of 900 (10× for intraspecies variation, 10× for interspecies variation, 3× for database deficiencies, and 3× for the use of a LOAEL in place of a NOAEL). Additional uncertainty factor(s) were not considered necessary for the limited evidence of carcinogenicity in animals. Subsequently, a maximum acceptable concentration (MAC) for MC-LR in drinking water was derived at 1.5 μg L–1.12 The World Health Organization recommends a lower provisional guideline value of 1.0 μg L–1 for MC-LR.14 This is based on a 13-week MC-LR study in mice where a NOAEL of 40 μg per kg of body weight per day was determined for pathology of the liver.15 An uncertainty factor of 1000 (100 for intraspecies and interspecies variation and 10 for database limitations on chronic toxicity and carcinogenic endpoints) was used to calculate the TDI of 0.04 μg per kg of body weight per day.14,15
Mammalian plasma and albumin has been found to have greater binding affinities for microcystins (MC-LR and MC-RR) than that of other species (p < 0.01), such as fish.16 Furthermore, human serum albumin has been found to have the highest binding rates to microcystins of six species of mammals and fish (bovine, porcine, bighead carp, silver carp, and crucian carp); which is likely a result of differences in the composition of plasma and albumin binding characteristics between species. These findings emphasize the importance of uncertainty factors for intra-species variation and the need for experimentation in appropriate animal models.
Human exposures to microcystins
Nation-wide studies have found that the concentrations of MCs in treated drinking water in Australia and the United States do not exceed guideline values.17,18 However, there have been several instances in Ohio, USA, and parts of China, where MC-LR has been detected in the drinking water supply and alternative drinking water sources have been required, such as bottled water.19–21 Isolated cases of hepatotoxicity as a result of MC toxicity have been documented in exposed renal dialysis patients in Brazil22 and in Chinese fisherman as a result of their diet.23
Cyanotoxins may pose a threat to recreational bathers. Although MC-LR has been found to degrade in aquatic environments, the rate is non-linear and studies suggest MC-LR has a half-life up to 7 d in natural waters.24 The acute symptoms of ingestion of cyanobacteria and cyanotoxins were observed following an incident in Argentina in 2007. A male surfer became immersed in an algal bloom and ingested several mouthfuls of the contaminated water (48.6 μL L–1 MC-LR).25 The symptoms were similar to that of other poisonings: nausea, abdominal pain, and fever. Three days later, the patient developed indigestion and pneumonia. A week later, he developed hepatotoxicosis. Complete recovery was observed within three weeks.
Acute exposures to cyanotoxins are rare and do not typically result in death; though the effects of chronic exposure to cyanotoxins on mammalian organ function should not be overlooked. Chronic long-term exposure to relatively low levels of MCs in tap water has been linked to elevated liver cancer and high mortality rates in China.26 A relationship between cancer rates and chronic exposure to cyanotoxins in drinking water sources has also been suspected in Slovenia.27
Understanding the mechanisms of toxicity of the most common and toxic cyanotoxins will help to build robust risk assessment models for human exposure.28 MC-LR is primarily known as a hepatotoxin (i.e. causing cellular damage and carcinogenicity to the liver) that also has genotoxic attributes.29 Recent studies suggest that MCs may cause similar cellular damage to other mammalian organs, such as the kidney, heart, and reproductive systems.30–32
General toxic mechanisms
Toxicokinetics have an important role in the complex cellular uptake of MCs. The absorption of MC-LR occurs in the gastrointestinal tract (7 to 10%) or in the blood stream following ingestion or intravenous exposure, respectively.33 Following intravenous injection, approximately 50 to 70%, 1 to 5%, and <1% of MC-LR has been found to distribute in the liver, kidneys and other organs (brain, heart, lungs, and reproductive organs), respectively. Therefore, while MC primarily accumulates in the liver and kidneys, toxic responses may be seen in other organs.
MCs cause toxic responses via multiple cellular pathways. They are relatively large hydrophilic molecules that are believed to enter cells via transmembrane organic anion transporter peptides (OATP) that are responsible for sodium-independent solute passage.34 Research suggests that OATPs translocate the MC substrate through a central positively charged pore.33,35 Specifically, OATP1B1, OATP1B2, OATP1B3 and OATP1A2 have been shown to be involved in MC-LR uptake and the variability in expression of these peptides on organ tissues drives the unbalanced uptake throughout the body.36 OATP1B1 and OAT1B3 are abundant in liver cells, as they are responsible for the uptake of endogenous and exogenous chemicals into the liver; while OATP2B1 is expressed in several tissues such as the kidney.10,34 OATP1A2 is thought to mediate the transport of MC-LR across the blood–brain barrier and the kidneys.34,37
As a result of the abundance of OATPs expressed in the liver, the majority of ingested MC accumulates in hepatocyte cells. This is mostly excreted by the biliary route, though about 9% is filtered by the kidneys and is passed in the urine.33 Approximately 15% of ingested MC-LR is eliminated in the faeces.
Fischer et al. (2005)34 studied the uptake of radiolabelled MC-LR by human and rodent OATPs expressed by the Xenopus laevis oocyte expression system in vivo. The transport of MC-LR was between 2 to 5 fold higher than the control (water) for oocytes expressing rat OATP1b2, human OATP1B1, human OATP1B3, and human OATP1A1; while no uptake was observed for oocytes expressing OATP1a1, OATP1a4, and OATP2B1. Therefore, the expression of various OATPs by mammalian organs can impact the uptake of MC-LR. MCs may enter mammalian cells of other organisms via alternative pathways. RAW 264.7 macrophages lacking transmembrane transporters for MCs (OATPs 1A1, 1A, and 1B2) were observed in vitro to present stress responses such as stimulated tumour necrosis factor alpha (TNFα) and interleukin 6 (IL-6) following exposure of MC-LR; likely via the Toll-like receptors (TLRs).38
To discern the cellular uptake of MC-LR, Komatsu et al. (2007) studied the response of HEK293 (human embryonic kidney) cells that were transfected to over-express OATP surface proteins. Using OATP inhibitors and a low dose of MC-LR (1 μM MC-LR), they found that OATP1B1 and OATP1B3 were essential for the uptake of intact MC-LR.39 Mice lacking expression of OATP have been observed to resist MC-LR hepatotoxicity; providing in vivo evidence for the kinetic uptake of MC-LR by OATP on cellular membranes.40 Furthermore, MC-LR can be taken up in a dose-dependent manner and the route of exposure (e.g. oral or injection) can affect the toxicokinetic results.41 For example, when MC-LR is injected at a range of doses, it has not been observed to inhibit PP1 and PP2A.42 Therefore, oral administration is an important experimental control to determine the potential toxic response in mammals to MCs in drinking water and food. Cellular detoxification of MCs is understood to occur via glutathione conjucation, which would decrease cellular uptake of the parent MC.43–45
Two conjugates of MC-LR can form upon cellular uptake: an MC-cysteine conjugate and an MC-LR glutathione conjugate.46 These two metabolites have been found to be approximately 1/12 as toxic as MC-LR alone on PP2A activity in vitro in mice. Ito et al. (2002)46 found that the affinity of MC-LR to the PP2A subunit was approximately 20 times greater than that of PP-1C or PP-1γ. The authors suggested that these observations may be the result of incomplete cellular uptake of the conjugates relative to free MC-LR.
Select in vitro studies
In vitro studies of the toxic mechanisms of MC-LR on mammalian cells are often conducted on rat hepatocytes or murine cell lines, while others have been performed using human cells to highlight the various MOA.47 The sensitivity of various mammalian cell lines to MCs may differ.33 For example, established cell lines can lose their expression of OATPs; though HepG2 cells have been demonstrated to maintain the OATP transport system. Therefore, cellular toxicological responses should be evaluated in a variety of mammalian cell lines and in vivo species to determine their broad effects and a comprehensive understanding of MOAs. Meier-Abt et al. (2005) showed that Vero-E6 (African green monkey kidney epithelial) cell lines presented similar dose response decreases in cell viability to liver-derived cell lines of HepG2 and AML12, and could be a promising in vitro model for the study of MC-LR cellular toxicity mechanisms.33 Later, Alverca et al. (2009) also found that Vero-E6 cellular response to MC-LR exposure was a function of the dose and exposure time. After 24 h of exposure to concentrations greater than 30 μM, cell viability substantially decayed and lysosome destabilization was followed by mitochondrial disruption. Further, ultrastructural analysis showed early autophagy in response to MC-LR uptake proceeded by the induction of endoplasmic reticulum vacuolization. Doses of more than 40 and 75 μM increased apoptosis and necrotic cells. These results suggest that the endoplasmic reticulum is the primary target for MC-LR in Vero cells.48
Ikehara et al. (2015) found that the response to MC-LR exposure in two human cell lines, normal human hepatocytes (h-Nheps) and human hepatoma cells (HepG2), were different. The results suggested that the HepG2 cell line was not an appropriate assay for evaluating the toxicity of MC-LR because it did not reveal morphological or cytotoxic effects.49 Furthermore, Teneva et al. (2016) detected differences in the response to MC-LR by three non-hepatic adhesive cell lines; human lung carcinoma (A549), human liver adenocarcinoma (SK-Hep-1), and human amniotic normal cells (FL). Overall, the effects of MC-LR on these mammalian cell lines was found to be dose-dependent; though the cellular toxicological effects were most notable in the human lung carcinoma cell line.50 Further, all three cell lines presented reduced mitochondrial membrane potential following MC-LR treatment though lysosomes appeared to be unaffected.50 While in vitro studies such as these can provide an indication of the cellular toxicity mechanisms of MC-LR, mammalian in vivo studies can also elucidate the comprehensive effects of MC-LR exposure.
The uptake kinetics and immune response of human and chicken peripheral blood lymphocytes to MC-LR exposure from 12 to 72 h at environmentally representative doses (from 1 to 25 μg mL–1) were evaluated in vitro by Lankoff et al. (2004). They found that MC-LR exposures led to modification of immune system production of cytokine and diminished lymphocyte functions as a result of induction of apoptosis and necrosis. Specifically, all doses of MC-LR inhibited B-cell proliferation and the highest dose resulted in decreased T-cell proliferation. Furthermore, MC-LR stimulated the production of IL-6 and decreased production of IL-2.51
The toxic effects of MC-LR exposure on protein phosphatase subunits in human normal liver cells (HL7702) were first analysed by Sun et al. (2013) during a 24 h incubation. MC-LR was taken up by the cells, PP2A activity decreased relative to the MC-LR dose, and PP2A subunits were assessed. The highest dose of MC-LR slightly increased the detection of the B55α regulatory subunit of PP2A.47 Additionally, exposure to MC-LR did not initiate apoptosis of the HL7702 cells, though slight alterations of cellular shape were observed suggesting cytoskeletal modifications.
The uptake and molecular toxicity of MC-LR into primary murine whole brain cells (mWBC) was studied by Feurstein et al. (2009) over 48 h using MC-western blot analysis, immunocytochemistry, cell viability and protein phosphatase (PP) inhibition assays. MC-LR uptake was mOATP-dependent, concentration-dependent, and MC-LR covalently bound and inhibited the PP of mWBC cells.37 It was found that at least five mOATPs were expressed in the mWBC, including mOATP1b2. Immunoblotting using antibodies against PP1α and PP2A/C suggested that MC-LR covalently bound to mWBC PP units, and subsequent MC-incubation studies confirmed the association between MC-LR and PP1 and PP2A subunits.52 Similarly, Ufelmann et al. (2012) observed the inhibition of PP1 and PP2A subunits in human and rat hepatocyte toxicity resulting from exposure to naturally derived MC. It was found that MC-LR was cytotoxic in the 20 to 600 nM range.53
Cytoskeletal disruption has also been reported in a human liver cell line (HL7702) in response to MC-LR exposure. Sun et al. (2011) observed rearrangement of filamentous actins and microtubules as well as decreased activity of PP2A. The mechanism of cytoskeletal disruption was suggested to follow a cascade of events from inhibition of PP2A, phospho-activation of the MAPK superfamily (p38 MAPK, JNK and ERK1/2), and leads to phosphorylation of HSP27. HSP27 is an ATP-independent molecular chaperone and heat shock protein involved in cellular stress protection, anti-oxidative stress, anti-apoptosis, and actin stabilization.54
The use of murine brain cell lines was employed to observe the effects of low doses of MC-LR from 0.001 to 10 μM by Takser et al. (2016). An ELISA assay determined that TNF-α was increased and the activity of caspases 3/7 (CASP3 and CASP7) were elevated after 24 h; which are involved in the induction of apoptosis.55 Liu et al. (2016) found that apoptosis was the primary (or only) toxic response from short-term low doses of MC to HL7702 cells. Later work showed that prolonged exposure to MC-LR activated the Akt/S6K1 cascade and led to cell proliferation while hyperphosphorylation of transcription factors c-MYC and c-Jun and apoptosis proteins Bcl-2 and Bad were also observed.56
Inhibition of protein phosphatases
The main MOA of MC toxicity involves inhibition of serine/threonine protein phosphatases (PP) via interaction with PP subunits leading to unregulated phosphorylation of PP substrates.57 PPs regulate a suite of cellular processes in mammalian cells such as cell proliferation, division, signal transduction and gene expression.58 It has been suggested that MC-LR has fairly equal inhibition potency against protein phosphatase 1 (PP1) and protein phosphatase 2A (PP2A), while an in vivo mouse study of 28 d using intraperitoneal pumps into male rats suggests that the effects on PP2A were greater than on PP1.58,59 PP2A is an important cellular enzyme responsible for the phosphorylation of numerous proteins for cellular function; including tumour suppression. PP2A occurs in mammalian cells as either PP2A/C which is a heterodimeric core enzyme with a scaffold subunit (A or PR65 subunit) and catalytic subunit (C), or a heterotrimeric haloenzyme.57 There are two isoforms of subunits A and C, namely α and β, and the α form is more abundant within mammalian cells. The heterotrimeric haloenzyme forms when the PP2A core enzyme interacts with the regulatory subunit (B). MC-LR has been reported to primarily bind to the PP2A/C subunit pocket.47 The affinity of MC-LR to PP2B subunit has been shown to be far less substantial.57
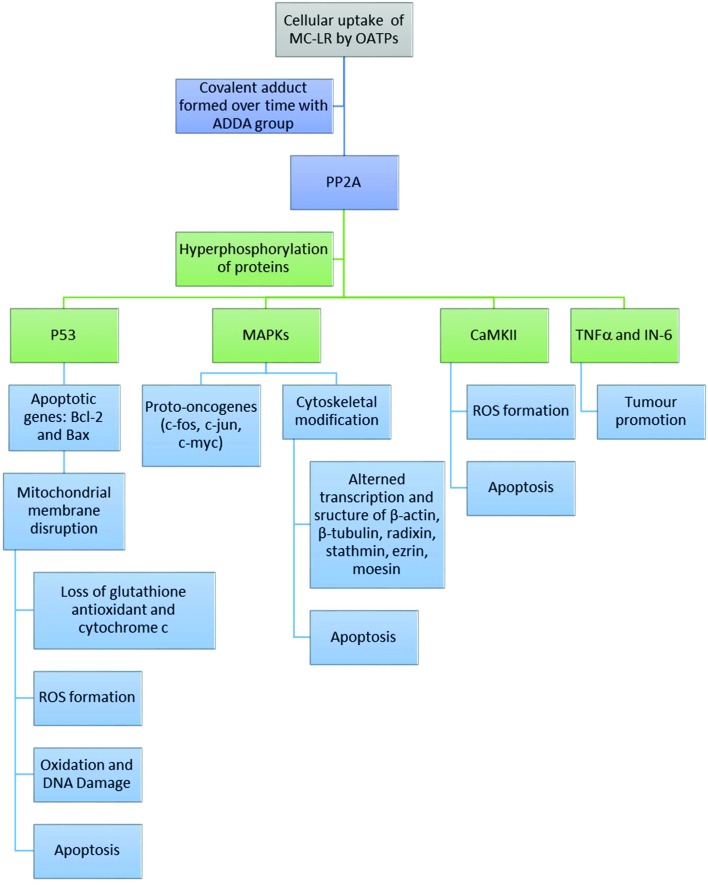
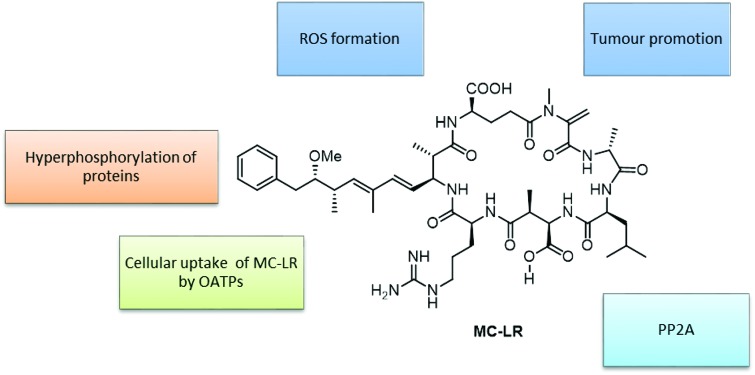
Following cell entry, MCs inhibits the serine/threonine protein phosphatases PP1 and PP2A through covalent binding between a terminal carbon of the Mdha side chain and a protein cysteine (Cys269 of the PP2A/C subunit).60,61 Serine phosphorylation is involved in the regulation of functional proteins for cell proliferation, metabolism and cell death. Hyperphosphorylation of PP2A by MCs induces a cascade of negative effects on cellular functions. These include: (1) regulation of phosphoproteins (e.g. P53 and MAPKs) resulting in tumour promotion and apoptosis, and (2) creation of reactive oxygen species (ROS) resulting in oxidative stress.62 Therefore, cellular toxicity in response to acute and chronic MC exposure can be assessed by monitoring the activity of PP2A.29
Adduct formation and phosphoprotein regulation
MC-LR interacts with PP1 and PP2A in a two-step process; first, the toxin binds to the enzyme followed by formation of a covalent adduct.29 The structural interactions between MC-LR and PP2A have been observed using crystallization.63 The active site of PP2A contains two manganese atoms within the binding pocket that facilitate covalent linkages with MC-LR. The long hydrophobic ADDA side chain of MC-LR interacts with four amino acids (Cln122, Ile123, His191, and Trp200) on one side of the catalytic subunit of PP2A that form a hydrophobic cage. The other end of the PP2A binding pocket effectively forms multiple van der Waals interactions with the hydrophobic portion of MC-LR (Fig. 2). This binding pocket of PP2A was found to bind to another tumour promoting toxin, okadaic acid, and effectively inhibited MC-LR binding.63 These results further indicate that PP2A is the active site for MC-LR adduct formation.
Fig. 2. Series of mechanisms of toxicity of MC-LR in mammalian tissues.
In general, lethal doses of MC-LR can result in measurable inhibition of cellular enzymatic activity. P53 is a substrate of PP2A, and therefore MC-LR/PP2A adducts interfere with P53 activities (Fig. 2). P53 is an important nuclear phosphoprotein involved in the regulation of apoptosis, tumour suppression pathways, and transcriptional transactivation of DNA repair. P53 activity, along with expression of apoptosis proteins Bcl-2 and Bax, are induced in response to cellular stress. MC-LR inhibition of protein phosphatases (e.g. PP2A) can lead to hyperphosphorylation of P53 and induce P53-independent apoptosis within minutes of lethal dosing; as has been shown in mouse hepatocytes.64
It is critical to validate in vitro cell models in lieu of in vivo studies (that may be more costly or cumbersome in many cases). Fu et al. (2005) found that rat liver tissues exposed to MC-LR both in vivo and in vitro significantly increased the expression of P53 and Bax apoptosis proteins; while Bcl-2 expression was decreased in vitro and was not significantly changed in vivo. Bcl-2 and Bax proteins are involved in mitochondrial membrane permeability and homeostasis and these results imply that their ratio may be important for apoptosis regulation. The observed increase in apoptosis proteins P53 and Bax in response to the presence of MC-LR may have been a secondary result from increased phosphorylation of proteins other than p53 (e.g. those that require functioning p53).65 Investigation of the multiple effects of MC-LR on phosphorylation of proteins related to apoptosis requires further study.
Exposure to MC-LR has also been found to affect the regulation of mutagen-activated protein kinases (MAPK) phosphoproteins, the expression of proto-oncogenes and disrupt DNA repair (e.g. as a result of oxidative stress).39,66 MC-LR inhibition of PP2A can result in the activation of MAPKs; thereby activating proto-oncogenes, such as c-Jun, c-Fos, and c-Myc, that are responsible for initiating transcription of differentiation and growth genes (Fig. 2). These observations were made by Komatsu et al. (2007)39in vitro (in the HEK293 cell line) and verified by Li et al. (2009)66 in various tissues of male Winstar rats. Li et al. (2009) studied the expression profiles and mRNA levels of proto-oncogenes following MC-LR intravenous injection in vivo. The authors stressed the lack of correlation between mRNA quantification and protein expression in cells under toxicological stress; which should be taken into consideration for studies solely quantifying mRNA to provide an indication of protein expression.66 The results showed significantly elevated mRNA synthesis profiles for each proto-oncogene in rat liver, kidney and testis tissues. Furthermore, the protein levels for c-Jun and c-Fos were moderately elevated compared to the control. The expression of c-Jun indicated positive regulation of cellular proliferation and c-Fos is known to have oncogenic activity with overexpression in tumour cells. These results suggest that MC-LR may induce enhanced expression of proto-oncogenes in mammalian organ tissues and contribute to tumour formation. Activated MAPK may be transferred to the nucleus and activates c-fos and c-jun genes. However, it is possible that these genes could be induced by oxidative stress that is also caused by MCs.67
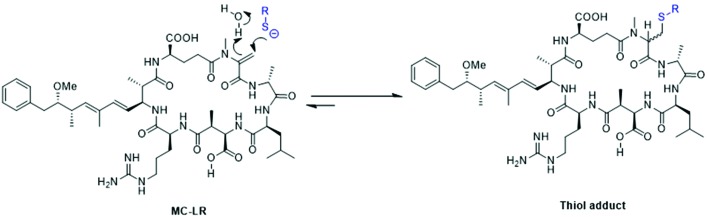
MCs have the ability to react with thiols (Fig. 3).68 Thiol modification of proteins by MCs is well-known,69,70 and may provide a mechanism for hepatotoxicity. For example, Tylenol (acetaminophen) can be hepatotoxic by undergoing metabolism to a quinoneimine (NAPQI) which targets proteins and reacts with cysteine residues. The chemistry of the interaction between thiols and MCs is complex.71 Some MCs such as MC-LR contain an α,β-unsaturated amide (i.e. carbonyl) group within the N-methyldehydroalanine (Mdha, Fig. 3) residue that has been shown to react with amino acids, peptides and proteins of thiols in vivo and in vitro in mammalian tissues.68 Eight covalent bonds are altered during the interaction between phosphoprotein phosphatases and MCs. In general, cysteine residues of phosphoprotein phosphatases can covalently bind with the methylene group (Mdha) of MCs in a similar chemical reaction to that of non-enzymatic conjugations between glutathione and MC. During thiol modification, the sulphur atom of the cysteine residue side-chain of phosphoprotein phosphatase may form a covalent bond with the β-carbon of the α,β-unsaturated carbonyl group of the Mdha residue of MCs; possibly via a Michael-type addition.71 During the Michael-type reaction, the thiol hydrogen is simultaneously abstracted by a water molecule, which transfers a hydrogen molecule to the Mdha carbonyl oxygen during the addition of sulphur to MC. This may occur in a two-step process, following an initial non-covalent interaction that inactivates the phosphatase enzymes. Phosphoprotein phosphatases vary in their available cysteine residues in the β12–β13 loop necessary to form a covalent bond.
Fig. 3. Depiction of the conjugation of the Mdha group of LC-MR with a thiolate.
Miles et al. (2016) recently found this reaction to be reversible by thiol-derivatization-based LC-MS methods. MC variants were shown to conjugate with mercaptoethanol, methanethiol, cysteine, and glutathione; where deconjugation was also observed. These observations further complicate the understanding of interactions between MCs and thiols. The authors note that conjugated MCs in structural proteins could lend to extended tissue exposure to free MCs over time depending on the local physical–chemical conditions. Additionally, it is hypothesized that MCs could conjugate with thiols in the diet prior-to or during ingestion; which may also lead to elongated MC exposures in mammalian tissues.68
The covalent interaction between MCs and glutathione was previously found to occur as a result of enzyme action using LC-MS at representative pH conditions, and not due to a Michael reaction.72 Previous observations that indicated a Michael reaction were conducted at elevated (i.e. basic) pH conditions.
Cytoskeletal modification
A primary toxic response of mammalian cells to MC exposure is cytoskeletal disruption.57 MC-LR has been found to affect the homeostasis of several cytoskeletal genes in the liver, kidney and spleen of male Winstar rats treated with 80 μg kg–1.73 β-Actin and β-tubulin are involved in cell structure integrity; radixin is thought to be involved in actin binding to the cell membrane; stathmin is an important regulatory protein of eukaryotic microtubule filaments; and ezrin and moesin are intermediates between the plasma membrane and the actin cytoskeleton that have key roles in cell surface structure and organization. In rats treated with MC-LR, β-actin genes were substantially altered in the liver, while stathmin and radixin genes were affected to a lesser extent in the kidney and spleen, respectively.73 Observed changes in the transcription of ezrin, moesin and stathmin (tumour-associated skeletal genes) suggest that MC-LR was involved in tumour-promoting activities in these organs as well. Cytoskeletal modifications through the mitochondrial and endoplasmic reticulum pathways, coupled with oxidative stress, has been shown to lead to cellular apoptosis in response to MC exposure.57,74
Oxidative stress
Oxidative stress has been commonly observed during MC-LR exposure studies. Oxidative stress results from two pathways: the development of ROS species and a disruption in glutathione (GSH) homeostasis as a result of interaction with MC.57 The toxic responses as a result of increased ROS include cytoskeletal modifications, general oxidative damage as well as DNA damage (possibly leading to tumour formation) and apoptosis. The mechanisms of ROS formation are poorly characterized relative to the other known mechanisms of cyanotoxin toxicity.29 One approach to assessing the formation of ROS species as a result of cyanotoxin exposure is to monitor malondialdehyde (MDA) and superoxide dismutase (via the SOD assay).75 MDA can be used as a marker for oxidative damage and SOD is an antioxidant enzyme that recognized as the primary response to ROS damage. Li et al. (2011) observed the effects of acute and sub-chronic intraperitoneal injections of MC-LR on liver cells of Kumming mice. When compared to the controls, significant increases in MDA (p < 0.01), and no significant differences with respect to SOD activity, were observed.19 However, decreased CAT activity (p < 0.05) was observed in the highest MC dosed group (12 lyophilized algae cells per kg BW); which may have caused the observation of elevated MDA concentrations.
Mitochondria are primarily responsible for energy production (i.e. ATP) through oxidative phosphorylation (i.e. respiration). The maintenance of the mitochondrial membrane potential (MMP) is also important to give rise to the chemical electrical gradient that permits ATP formation. This is generated as a result of an asymmetric distribution of protons and other ions (e.g. Ca2+) on either side of the inner membrane.76 Chen et al. (2005) challenged the conventional theory of mitochondrial-induced ROS formation as a result of disrupted membrane potential from low-dose MC-LR damage (Fig. 2). The authors hypothesized that mitochondrial membrane disruption only occurs in acute cases, and that ROS formation from chronic exposure to MC-LR is related to iron pathways. They suggest that MC-LR induces cellular generation of iron and prolongs the activation of iron regulatory protein (IRP) (likely through MC-LR-PP2A adducts).77 The extended activity of IRP suppresses ferratin, an iron sequestering protein, and allows free iron to persist and participate in oxygen radical formation. These steps likely precede those described in Fig. 2; as the formation of intracellular ROS has been linked to increased cytosolic calcium.78 Zhang et al. (2008) studied the effects of MC (MC-RR) on fish lymphocytes (in vitro) to discern the mechanisms to mitochondrial damage resulting in apoptosis. ROS generation was significantly elevated as early as 1 h following incubation with 10 nmol L–1 MC-RR (p < 0.005; approximately two fold higher during the entire 4–6 h incubation), and significantly elevated intracellular Ca2+ concentrations were observed after 0.5 h incubation (p < 0.001) when compared to un-treated controls.79 Additionally, a time-dependent reduction in mitochondrial membrane potential (i.e. permeability) was observed (p < 0.001; decreased by 20% compared to the controls). Finally, cellular ATP content also decreased with MC-RR contact time beginning at 0.25 h. Overall, this study contributed to elucidating the MC-LR-induced apoptosis-related events in fish lymphocytes that may explain the toxicity pathways in other cells and tissues (Fig. 2).
Apoptosis
Cellular apoptosis is the outcome of several events resulting from the initial inhibition of PP2A by MC exposure and hyperphosphorylation of proteins such as P53 (Fig. 2). Increased phosphorylation of P53 can induce apoptosis via mitochondrial disruption, phosphorylation of MAPKs leading to cytoskeletal modifications, and production of ROS species as a result of CaMKII phosphorylation.
Chen et al. (2005) used transcriptomic and proteomic data from chronic and acutely dosed mice (50 μg per kg bw and 70 μg per kg bw, respectively) in combination with computer simulations. The results showed that apoptosis was induced primarily by proto-oncogene activity (Bax and Bcl-2) in the low-dose group; while apoptosis in the high-dose group was caused by ROS accumulation.77 Therefore, it is suggested that the pathway for induction of apoptosis is dose-dependent. Additionally, apoptosis resulting from MC-LR exposure is assisted by the serine/threonine protein kinase CaMKII (Ca2+/calmodulin-dependent protein kinase II) leading to increase ROS formation.29
Hence, there are multiple pathways by which MC-LR induces cellular toxicity. Additionally, cellular response pathways may differ as a result of chronic or acute exposure to MCs. Analysing the response of other organ tissues to MC-LR exposure in vivo may aid in addressing these gaps in understanding the mechanisms of toxicity of MCs, and to form more comprehensive analyses of risks to human health from algal blooms.49
Effects in other organs and tissues
Organ uptake
Although MCs are known to primarily affect the liver, there is evidence to suggest that they accumulate in other mammalian tissues and organs and cause cellular damage via similar mechanisms. Wang et al. (2008) examined the distribution of MCs taken up in various tissues of Winstar rats during intravenous injection with 80 μg per kg bw MC-LR (∼1 LD50) for a 24 h period. During this acute exposure simulation, each tissue was analysed at hourly intervals (1, 2, 4, 6, 12, and 24 h). Peaks of MC were detected in the kidney at 2 and 24 h, and the maximum content of MC in the entire rat body was 2.9% at 2 h.31 These results suggest that microcystins were excreted from the body via the rat kidney. Overall, the highest concentrations were found in the kidney, followed by the lung, stomach, and liver; while less than 0.2% of the injected microcystins was detected in the heart, intestine, spleen, brain, and gonads.31 These relatively low accumulations in various tissues do not imply that cellular damage is unlikely since it is possible that MCs could accumulate in organ tissues over the course of a lifetime of chronic, low-dose, exposures.
Researchers from the University of Ljubljana in Slovenia have conducted several well-controlled in vivo studies on the effects of chronic exposure to MCs on rat organs.30,80,81 Recent Chinese studies from Nanjing University and environmental groups have also explored the effects of exposure to MCs on mammalian cardiac and reproductive organs. Collectively, these studies lend to elucidating the mechanisms of toxicity by MCs on various mammalian organs and tissues.
Renal toxicity
Chronic exposures to low doses of MCs may pose a considerable risk to renal toxicity in mammals. MC-LR is thought to impair renal function, cause vascular and glomerular lesions, and promote alterations to kidney tissues.82 The key toxic MOA of MC-LR in kidneys involves disruption of mitochondria and enhanced ROS attack.
Milutinović et al. (2003) used a variety of technical assays to demonstrate that cellular changes induced by acute exposure of liver tissues to MC-LR are similar to those observed following chronic exposure to MC-LR in kidney tissues at the cellular level. Rats received low-dose injections of MC-LR (10 μg per kg bw) every-other day for 8 months (n = 5), while control rats received a “vehicle” (0.8% ethanol, 0.2% methanol, and 0.9% saline). In general, rats in the treatment group presented hunched postures, reduced motor activity, and lacked resistance to handling.80 They also had significantly reduced body weight when compared to the controls at the end of the study (–13% ± 3; p < 0.05).
Histopathological evaluations of the kidney renal corpuscles were performed using staining with haematoxylin and eosin (HE). In the kidney, the renal corpuscle is the initial blood-filtering component of the nephron and is composed of two structures: the glomerulus and a Bowman's capsule. This assay revealed collapsed glomeruli with thickened basement membranes and dialated tubuli filled with proteinaceous material.80 The Bowman's space also appeared widened. The percentage of injured renal corpuscles was significantly higher in the treatment group than in the control group (13% ± 10 p < 0.05). A TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling) assay was used to detect apoptotic cells that have undergone extensive DNA degradation during the final stages of programmed cell death. Following MC-LR treatment, the kidney cortex and medulla showed an increase in number of TUNEL positive cells when compared to the control (p < 0.05). Finally, fluorescent rhodamine-phalloidin labeling was used to visualize cytoskeletal damage to actin filaments. This assay revealed that the MC-LR treated rats showed renal tissues with increased granular appearance of the cytoplasm of the epithelial cells in the proximal and distal tubes (p < 0.05). These observations may have been caused by condensation of actin filaments into intracytoplasmic aggregates.80 While this study reveals substantial changes at the cellular level of kidney tissues in response to MC-LR exposure, it does not reveal key pathways in the mode of action for nephrotoxicity.
A Portugal-based research group further elucidated the key role of mitochondria in MC-LR renal toxicity by studying isolated mitochondrial bodies from rat kidneys.76 A sensitive TPP+ (tetraphenylphosphonium) electrode was used to estimate changes to the mitochondrial membrane potential (MMP). MC-LR exposure was found to decrease the MMP (in a dose-dependent manner), and at higher doses (90 nmol mg–1 protein) it was found to increase the inner mitochondrial permeability to protons. The authors suggest that this was caused by the translocation of ROS from within the mitochondria. Lower doses of MC-LR (30 nmol mg–1 protein) caused a reduction in the amount of cytochrome c contained within the mitochondria and may have further enhanced oxidative stress and ATP synthesis (which were reduced by 40–80% depending on the MC-LR dose). Finally, they found that MC-LR induced MPT in extracted rat kidney mitochondria; which led to an influx of Ca2+ ions and disrupted homeostasis of this organelle.76 Although these results were not validated in vivo, they suggest that the MOA of MC-LR on nephrotoxicity involves inhibition of the mitochondrial respiratory chain as described in Fig. 2.
Pulmonary toxicity
MCs have been found to accumulate in mammalian lung tissues via the circulation of blood.83 Consequently, damage to lung tissues may result in mammals exposed to long-term low-doses of MCs. Wang et al. (2008) found that the second highest concentration of MCs in treated rats, following the kidney, was found in the lungs at 0.007 to 0.067 μg per g dry weight; with maximum concentrations after 2 h of exposure to an intravenous dose of 80 μg per kg BW. Therefore, approximately 0.04% of the MC dose accumulated in the lung tissue of rats within just a few hours.31 Chronic low-dose oral administration of MC-LR to mice (0, 1, 10 and 40 μg L–1 over the course of 6 months) lead to alveolar collapse, apoptosis of lung cells, and damage to cell junction integrity upon analysis of MC-LR uptake into ATII cells.83
Histopathological studies suggest that exposures to MCs indirectly caused lung damage in adult male Swiss mice in vivo.84 Following a 60 m exposure to 160 μg L–1 MC-LR in rats, large quantities of hepatocellular debris were observed in pulmonary tissues using transmission electron microscopy.85 It was hypothesized that, following hepatocyte damage, microemboli debris were released into circulation and larger particles accumulated in the pulmonary vasculature while others were transported to the renal capillaries. Substantial damage to pulmonary tissues was not observed in rats following acute exposures to MC-LR.
Pulmonary cells of mice that received chronic persistent exposure to MC-LR (1, 5, 10, 20, and 40 μg L–1) over a one year period presented distressed redox systems, reduced mitrochondrial DNA stability, and modified expression of mitochondrial genes.75 Exposure to MC-LR affected the level of inflammatory cytokines and thickening of the alveolar septa when compared to the controls. The SOD assay showed increased activity in the 1 μg L–1 exposure group, which provides evidence that antioxidant enzymes were activated after exposure to even low doses of MC-LR in pulmonary tissues. In the higher-dosed groups, SOD activity was diminished; possibly because considerable ROS had formed as antioxidant defense mechanisms were impaired. Collectively, these results suggest that mammalian lungs are susceptible, perhaps indirectly, to chronic exposures to MC-LR.
Inhalation of cyanotoxins from prolific algal blooms is a potential alternative exposure route to oral ingestion that may pose a risk to respiratory health in mammals. In two Brazilian studies, adult mice were injected intraperitoneally (to simulate inhalation) with a sub-lethal dose of 40 μg kg–1 MC-LR, and compared to a control subjects that received saline via the same method.84,86 After 2 and 8 h of exposure, collapse of the alveolar was observed, though no inhibition of PP1 or PP2A or accumulation of micricystins was detected (by ELISA) in mouse lung tissues. Similar results were observed by Picanço et al. (2004)84 in young and adult mice lungs (4 weeks and 12 weeks old, respectively). Therefore, inhalation of microcystins from algal blooms may cause irritation to lung cells presented as inflammation and possible accumulation of ROS species, thought the potential for more severe responses needs to be further explored.
Cardiac toxicity
Chronic exposure to relatively low doses of MC-LR may pose a risk to cardiac injury. Milutinović et al. (2006) examined the effects of chronic exposure to MC-LR on rat cardiac tissues in vivo for the first time. Again, substantial cytoskeletal alterations were observed with histopathological assays (e.g. fibrosis, loss of cell cross-striations, enlargement of cardiomyocytes, enlarged and unusual nuclei).30 However, TUNEL staining of heart sections showed no signs of apoptosis. Qiu et al. (2009) further studied rat cardiac response to both high (1 LD50; 87 μg per kg bw MC) and low (0.16 LD50; 14 μg per kg bw) doses of MC-LR. Dead mice from the high dose group showed signs of myocardial infraction and cardiac cell injury; as elevated levels of troponin I were observed (p < 0.01).87 However, increases in troponin I can also result from renal failure so these results are inconclusive. However, both dose groups showed increased creatine kinase (140% in the low-dose group) and lipid peroxide levels (p < 0.01); which indicate cardiac cell injury and high levels of oxidative stress, respectively.
Reproductive toxicity
The cellular uptake of MCs into male and female reproductive organs remains unclear. Steroli cells and whole testes do not express OATP1 mRNA;88 though they have been shown to express the mRNA of numerous other OATPs.89 The expression of OATP3A1 in rat spermatogonia has been shown to be affected by MC-LR exposure and a specific western blot assay has indicated that MC-LR was taken up by rat spermatogonia. Further evidence is required to indicate the cellular uptake of MCs in both male and female reproductive organs.
Observations at the cellular level have highlighted the possible mechanisms of toxicity in mammalian reproductive organs. Chen et al. (2013) injected male rats with low doses of MC-LR (1 and 10 μg per kg bw) for 50 days. Histopathology revealed a dose-dependent effect on morphological changes to seminiferous tubules; including reduced cytoplasm, cell membrane blebbing, swollen mitochondria and deformed nuclei (p < 0.01).90 Additionally, cellular ROS levels increased by approximately 15% in the higher dose group (p < 0.05). Exposure to MC-LR has also been found to disrupt the transcription of cytoskeletal genes in rat gonads.90 The transcription of several cytoskeletal genes (β-actin and β-tubulin, stathmin, ezrin, and moesin) was significantly increased following MC-LR dosing (p < 0.01). Transcription of mitochondrial genes was also increased. The authors suggest that this response in elevated cytoskeletal and mitochondrial gene transcription was the result of cellular compensatory mechanism to the stress induced by MC-LR entry and activity.
Wang et al. (2013) assessed the up-regulation of apoptosis-related genes in testicular cells in mice exposed to MC-LR (1/16 LD50 and ½ LD50) for 1 to 4 d. TUNEL assays revealed dose-dependent and duration-dependent apoptosis responses in treated mice testicular cells.91 Reverse-transcription PCR (RT-PCR) showed that transcription of proto-oncogenes (c-myc, c-ju, c-fos) and apoptosis genes (e.g. Bax) were up-regulated following MC-LR exposure. Lastly, phosphorylation of apoptosis-regulating genes, P53 and Bcl-2, were increased in MC-LR treated mice. These results remarkably mimic those that have been found in historical investigations into hepatotoxicity mechanisms (Fig. 2) and demonstrate that the MOAs of MCs in mammalian tissues and organs may be similar to those that occur within the liver. However, Chen et al. (2016) recently reviewed the reproductive toxicity of microcystins in mammals and other organisms and found that MCs also indirectly affected endocrine regulators and sex hormones by causing damage to the hypothalamic-pituitary-gonad (HPG) axis.32 Further, the potential for trans-generational toxicity has been reported,32 and hepatotoxicity and neurotoxicity of offspring has been observed following prenatal exposures to MCs in mice studies.92
Studies exploring the reproductive toxicity of MC-LR on female mammals are limited. Acute toxicity exposures to MC-LR in mice (of 20 μg kg–1 injections over four weeks) demonstrated that MC-LR could accumulate in ovarian tissues.93 Wu et al. (2015) reported that chronic oral exposure to MC-LR in drinking water at representative environmental concentration (0, 1, 10 and 40 μg L–1 for three and six months) to female mice (n = 10 for each assay) caused a reduction in the number of litters and pups (i.e. subfertility).14,94 Specifically, the in vitro observations included decreased follicle development, and a reduction of the gonadosomatic index (GSI) that were significant after three months of exposure at the 40 μg L–1 dose (p < 0.05), and after six months of exposure at the 10 μg L–1 dose (p < 0.05) and 40 μg L–1 dose (p < 0.01). The GSI is a calculation that determines the relative weight of ovaries to body weight. Additionally, the uptake of MC-LR into mouse granulosa (i.e. ovarian) cells was observed in vivo. Preliminary studies suggest that a possible MOA for the toxicity of MC-LR in reproductive tissues involves disruption to microRNAs (miRNA).95,96 These results suggest that following acute and chronic MC-LR exposures, the toxin could target ovarian granulosa cells and possibly lead to female subfertility in mammals.
Conclusions
Although the primary effects of MC-LR are observed as hepatotoxicity, the toxic response in mammalian organs and tissues extends beyond that of the liver. Recent studies suggest that MC-LR damage extend to the kidneys, brain, heart, reproductive organs and possibly elsewhere (e.g. intestine and spleen). The key pathways of MC-LR toxicity are highly complex and therefore remain poorly described, despite preliminary investigations into the effects of exposure to MCs on other mammalian organs. It is evident that MCs cause cellular damage primarily through interaction with the subunits of PP2A by disrupting mitochondrial respiratory chain homeostasis and the oxidative phosphorylation system.29,61 MC-LR has also repeatedly been found to increase ROS formation within mammalian cells; however the precise mechanism (either through MMP disruption or increased iron concentrations) remains unclear. Eventually, MC-LR disrupts the mitochondrial and endoplasmic reticulum pathways, upregulates apoptosis genes, and induces apoptosis. Additionally, several mammalian cytoskeletal and cytoskeleton-associated proteins can be affected by MC-LR.57 MCs also present carcinogenic potential in several organs; demonstrated by the observed up-regulation of proto-oncogenes. While MC-LR has been shown to present tumour-promoting toxic mechanisms, possible mutagenic or genotoxic effects remain unclear.62,97,98
The mechanisms of acute and chronic toxicity appear to be similar at the cellular level in the various tissues studied; although new research suggests that the mechanisms creating ROS and inducing apoptosis may differ between the two exposure types. A standardized animal model approach to assessing both chronic and acute exposure to cyanotoxins would allow for direct comparisons between studies36 and build the weight of evidence to support risk assessments.
While MCs are considered the most common and toxic cyanotoxins in Canada, the presence of other cyanotoxins such as anatoxin-a and cynlindrospermopsin should not be ignored4 (Table 1). The current Canadian Guidelines for Drinking Water Quality only provide information on the control of total microcystins, since health and treatment information on health impacts of other cyanotoxins is relatively lacking. As cyanobacteria are capable of producing multiple cyanotoxins and typical algal blooms contain more than one cyanotoxin, the effect of cyanotoxin mixtures on cell function and toxicity should be evaluated. Further, new cyanotoxins are continuously being discovered and this work should continue to remain at the forefront of protecting human and ecological health.99,100 For example, Mankiewics-Boczek et al. (2015) recently found that cellular biosensors detected toxic bioactivity in cyanobacterial extracts that exceeded toxicity levels for MCs; including effects on lipopolysaccharides and cellular wall components.100 Finally, the toxicity of degradation products of MCs should be explored.101,102
Conflict of interest
There are no conflicts of interest to declare.
Biographies

Nicole L. McLellan
Nicole L. McLellan is a Ph.D. Candidate at the University of Guelph in the program of Environmental Science + Toxicology. She received a B.Sc. in Honours Biology (2007) and M.A.Sc. in Civil Engineering (2013) from the University of Waterloo (Waterloo, Canada). Ms. McLellan has worked for over 5 years at Stantec Consulting Ltd where her responsibilities include optimizing municipal drinking water treatment for cyanotoxin removal. Her research is sponsored by an NSERC Industrial Post-Graduate Scholarship, and she was selected by Health Canada for the FSWEP (2016) to gain experience in the development of the guidelines for Canadian drinking water quality.

Richard A. Manderville
Dr Richard A. Manderville is Professor of Chemistry and Director of the Toxicology Program at the University of Guelph. He received a B.Sc. (1986) and Ph.D. (1992) from Queen's University (Kingston, Canada) in physical-organic chemistry under the supervision of Prof. Erwin Buncel. After postdoctoral studies with Prof. Sidney Hecht at the University of Virginia (Charlottesville, VA), he launched his independent career at Wake Forest University (Winston-Salem, NC) in 1995. In 2004 he returned to Canada and continued his research program at Guelph on the structural and biochemical implications of DNA base modification and their potential applications.
References
- Steffen M. M., Belisle B. S., Watson S. B., Boyer G. L., Wilhelm S. W. J. Great Lakes Res. 2014;40:215–225. [Google Scholar]
- Zhou Y., Michalak A. M., Beletsky D., Rao Y. R., Richards R. P. Environ. Sci. Technol. 2015;49:800–807. doi: 10.1021/es503981n. [DOI] [PubMed] [Google Scholar]
- Mankiewicz J., Tarczynska M., Walter Z., Zalewski M. Acta Biol. Cracov., Ser. Bot. 2003;45:9–20. [Google Scholar]
- Aranda-Rodriguez R., Benoit F. M. and Giddings M., Current approaches to cyanotoxin risk assessment, risk management and regulations in different countries, Fed. Environmental Agency Berlin, 2005. [Google Scholar]
- Jia J., Luo W., Lu Y., Giesy J. P. Sci. Total Environ. 2014;487:224–232. doi: 10.1016/j.scitotenv.2014.04.037. [DOI] [PubMed] [Google Scholar]
- Hoeger S. J., Dietrich D. R., Bettina C., Hitzfeld C. Environ. Health Perspect. 2002;110:1127–1132. doi: 10.1289/ehp.021101127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer I. R., Bartram J., Chorus I., Kuiper-Goodman T., Utkilen H. and Codd G., Safe levels and practices, WHO, London, 1999. [Google Scholar]
- Meriluoto J. A., Spoof L. E. Adv. Exp. Med. Biol. 2008;619:483–499. doi: 10.1007/978-0-387-75865-7_21. [DOI] [PubMed] [Google Scholar]
- Gupta N., Pant S. C., Vijayaraghavan R., Rao P. V. L. Toxicology. 2003;188:285–296. doi: 10.1016/s0300-483x(03)00112-4. [DOI] [PubMed] [Google Scholar]
- Feurstein D., Stemmer K., Kleinteich J., Speicher T., Dietrich D. R. Toxicol. Sci. 2011;124:424–431. doi: 10.1093/toxsci/kfr243. [DOI] [PubMed] [Google Scholar]
- Vesterkvist P. S., Misiorek J. O., Spoof L. E., Toivola D. M., Meriluoto J. A. Toxins. 2012;4:1008–1023. doi: 10.3390/toxins4111008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canada Health, Guidelines for Canadian drinking water quality: Cyanobacterial toxins in drinking water, Document for public consultation, Water, Air and Climate Change Bureau, Health Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario, Feb. 2016. Available at: https://www.canada.ca/en/health-canada/programs/cyanobacterial-toxins-drinking-water/cyanobacterial-toxins-drinking-water.html#p2_12.
- Heinz R. Environ. Toxicol. 1999;14:57–60. [Google Scholar]
- WHO, Guidelines for drinking-water quality, WHO Press, Geneva, Switzerland, 4th edn, 2011, pp. 344–346. Available at: http://www.who.int/water_sanitation_health/publications/2011/dwq_guidelines/en/.
- Fawell J. K., James C. P. and James H. A., Toxins from blue-green algae: toxicological assessment of microcystin-LR and a method for its determination in water, Water Research Centre, Medmenham, UK, 1994. [Google Scholar]
- Zhang W., Liang G., Wu L., Tuo X., Wang W., Chen J., Xie P. Ecotoxicology. 2013;22:1012–1019. doi: 10.1007/s10646-013-1086-5. [DOI] [PubMed] [Google Scholar]
- Hoeger S. J., Shaw G., Hitzfeld B. C., Dietrich D. R. Toxicon. 2004;43:639–649. doi: 10.1016/j.toxicon.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Haddix P. L., Hughley C. J., Lechevallier M. W. J. - Am. Water Works Assoc. 2007;99:118–152. [Google Scholar]
- Liu Y., Chen W., Li D., Huang Z., Shen Y., Liu Y. J. Environ. Sci. 2011;23:575–581. doi: 10.1016/s1001-0742(10)60450-0. [DOI] [PubMed] [Google Scholar]
- Tian D., Zheng W., Wei X., Sun X., Liu L., Chen X., Zhang H., Zhou Y., Chen H., Zhang H., Wang X., Zhang R., Jiang S., Zheng Y., Yang G., Qu W. Chemosphere. 2013;91:1064–1071. doi: 10.1016/j.chemosphere.2013.01.051. [DOI] [PubMed] [Google Scholar]
- Ho J. C., Michalak A. M. J. Great Lakes Res. 2015:317–325. [Google Scholar]
- Azevedo S. M., Carmichael W. W., Jochimsen E. M., Rinehart K. L., Lau S., Shaw G. R., Eaglesham G. K. Toxicology. 2002;181–182:441–446. doi: 10.1016/s0300-483x(02)00491-2. [DOI] [PubMed] [Google Scholar]
- Chen J., Xie P., Li L., Xu J. Toxicol. Sci. 2009;108:81–89. doi: 10.1093/toxsci/kfp009. [DOI] [PubMed] [Google Scholar]
- Bourne D. G., Blakeley R. L., Riddles P., Jones G. J. Water Res. 2006;40:1294–1302. doi: 10.1016/j.watres.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Giannuzzi L., Sedan D., Echenique R., Andrinolo D. Mar. Drugs. 2011;9:2164–2175. doi: 10.3390/md9112164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno Y., Nagata S., Tsutsumi T., Hasegawa A., Watanabe M. F., Park H. D., Chen G. C., Chen G., Yu S. Z. Carcinogenesis. 1996;17:1317–1321. doi: 10.1093/carcin/17.6.1317. [DOI] [PubMed] [Google Scholar]
- Svircev Z., Drobac D., Tokodi N., Luzanin Z., Munjas A. M., Nikolin B., Vuleta D., Meriluoto J. J. Environ. Sci. Health, Part C: Environ. Carcinog. Ecotoxicol. Rev. 2014;32:319–337. doi: 10.1080/10590501.2014.967053. [DOI] [PubMed] [Google Scholar]
- Burch M. and Humpage A., Regulation and Management of Cyanobacteria, Fed. Environmental Agency Berlin, 2005. [Google Scholar]
- Campos A., Vasconcelos V. Int. J. Mol. Sci. 2010;11:268–287. doi: 10.3390/ijms11010268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milutinović A., Zorc-Pleskovic R., Petrovic D., Zorc M., Suput D. Folia Biol. 2006;52:116–118. doi: 10.14712/fb2006052040116. [DOI] [PubMed] [Google Scholar]
- Wang Q., Xie P., Chen J., Liang G. Toxicon. 2008;52:721–727. doi: 10.1016/j.toxicon.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Chen L., Chen J., Zhang X., Xie P. J. Hazard. Mater. 2016;301:381–399. doi: 10.1016/j.jhazmat.2015.08.041. [DOI] [PubMed] [Google Scholar]
- Meier-Abt F., Mokrab Y., Mizuguchi K. J. Membr. Biol. 2005;208:213–227. doi: 10.1007/s00232-005-7004-x. [DOI] [PubMed] [Google Scholar]
- Fischer W. J., Altheimer S., Cattori V., Meier P. J., Dietrich D. R., Hagenbuch B. Toxicol. Appl. Pharmacol. 2005;203:257–263. doi: 10.1016/j.taap.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Leuthold S., Hagenbuch B., Mohebbi N., Wagner C. A., Meier P. J., Stieger B. Am. J. Physiol.: Cell Physiol. 2009;296:C570–C582. doi: 10.1152/ajpcell.00436.2008. [DOI] [PubMed] [Google Scholar]
- Menezes C., Alverca E., Dias E., Sam-Bento F., Pereira P. Toxicol. in Vitro. 2013;27:138–148. doi: 10.1016/j.tiv.2012.09.009. [DOI] [PubMed] [Google Scholar]
- Feurstein D., Holst K., Fischer A., Dietrich D. R. Toxicol. Appl. Pharmacol. 2009;234:247–255. doi: 10.1016/j.taap.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Adamovsky O., Moosova Z., Pekarova M., Basu A., Babica P., Svihalkova Sindlerova L., Kubala L., Blaha L. Environ. Sci. Technol. 2015;49:12457–12464. doi: 10.1021/acs.est.5b02049. [DOI] [PubMed] [Google Scholar]
- Komatsu M., Furukawa T., Ikeda R., Takumi S., Nong Q., Aoyama K., Akiyama S., Keppler D., Takeuchi T. Toxicol. Sci. 2007;97:407–416. doi: 10.1093/toxsci/kfm054. [DOI] [PubMed] [Google Scholar]
- Lu H., Choudhuri S., Ogura K., Csanaky I. L., Lei X., Cheng X., Song P. Z., Klaassen C. D. Toxicol. Sci. 2008;103:35–45. doi: 10.1093/toxsci/kfn038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiwaki R., Ohta T., Sueoka E., Suganuma M., Harada K., Watanabe M. F., Fujiki H. Cancer Lett. 1994;83:283–289. doi: 10.1016/0304-3835(94)90331-x. [DOI] [PubMed] [Google Scholar]
- Harada K., Imanishi S., Kato H., Mizuno M., Ito E., Tsuji K. Toxicon. 2004;44:107–109. doi: 10.1016/j.toxicon.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Dittmann E., Wiegand C. Mol. Nutr. Food Res. 2006;50:7–17. doi: 10.1002/mnfr.200500162. [DOI] [PubMed] [Google Scholar]
- Buratti F. M., Testai E. Toxicol. Lett. 2015;232:133–140. doi: 10.1016/j.toxlet.2014.10.020. [DOI] [PubMed] [Google Scholar]
- Gehringer M. M., Shephard E. G., Downing T. G., Wiegand C., Neilan B. A. Int. J. Biochem. Cell Biol. 2004;36:931–941. doi: 10.1016/j.biocel.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Ito E., Takai A., Kondo F., Masui H., Imanishi S., Harada K. Toxicon. 2002;40:1017–1025. doi: 10.1016/s0041-0101(02)00099-5. [DOI] [PubMed] [Google Scholar]
- Sun Y., Zheng Q., Sun Y. T., Huang P., Guo Z. L., Xu L. H. Environ. Toxicol. 2014;29:1236–1244. doi: 10.1002/tox.21854. [DOI] [PubMed] [Google Scholar]
- Alverca E., Andrade M., Dias E., Sam Bento F., Batoreu M. C., Jordan P., Silva M. J., Pereira P. Toxicon. 2009;54:283–294. doi: 10.1016/j.toxicon.2009.04.014. [DOI] [PubMed] [Google Scholar]
- Ikehara T., Nakashima J., Nakashima S., Yasumoto T. Toxicon. 2015;105:4–9. doi: 10.1016/j.toxicon.2015.08.025. [DOI] [PubMed] [Google Scholar]
- Teneva I., Klaczkowska D., Batsalova T., Kostova Z., Dzhambazov B. Toxicon. 2016;111:50–57. doi: 10.1016/j.toxicon.2015.12.006. [DOI] [PubMed] [Google Scholar]
- Lankoff A., Carmichael W. W., Grasman K. A., Yuan M. Toxicology. 2004;204:23–40. doi: 10.1016/j.tox.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Monks N. R., Liu S., Xu Y., Yu H., Bendelow A. S., Moscow J. A. Mol. Cancer Ther. 2007;6:587–598. doi: 10.1158/1535-7163.MCT-06-0500. [DOI] [PubMed] [Google Scholar]
- Ufelmann H., Krüger T., Luckas B., Schrenk D. Toxicology. 2012;293:59–67. doi: 10.1016/j.tox.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Sun Y., Meng G.-M., Guo Z.-L., Xu L.-H. Toxicol. Lett. 2011;207:270–277. doi: 10.1016/j.toxlet.2011.09.025. [DOI] [PubMed] [Google Scholar]
- Takser L., Benachour N., Husk B., Cabana H., Gris D. Toxicol. Rep. 2016;3:180–189. doi: 10.1016/j.toxrep.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Wang H., Wang B., Chen T., Wang X., Huang P., Xu L., Guo Z. Toxicol. Lett. 2016;240:214–225. doi: 10.1016/j.toxlet.2015.10.015. [DOI] [PubMed] [Google Scholar]
- Zhou M., Tu W. W., Xu J. Toxicon. 2015;101:92–100. doi: 10.1016/j.toxicon.2015.05.005. [DOI] [PubMed] [Google Scholar]
- Rossini G. P., Toxins and Biologically Active Compounds from Microalgae, CRC Press, 2014, vol. 1. [Google Scholar]
- Solter P. F., Wollenberg G. K., Huang X., Chu F. S., Runnegar M. T. Toxicol. Sci. 1998;44:87–96. doi: 10.1006/toxs.1998.2478. [DOI] [PubMed] [Google Scholar]
- Svircev Z., Baltic V., Gantar M., Jukovic M., Stojanovic D., Baltic M. J. Environ. Sci. Health, Part C: Environ. Carcinog. Ecotoxicol. Rev. 2010;28:39–59. doi: 10.1080/10590500903585382. [DOI] [PubMed] [Google Scholar]
- Liu J., Sun Y. Toxicol. Lett. 2015;236:1–7. doi: 10.1016/j.toxlet.2015.04.010. [DOI] [PubMed] [Google Scholar]
- Vichi S., Buratti F. M. and Testai E., Microcystins: Toxicological Profile, Marine and Freshwater Toxins, pp. 2016, 219–238. [Google Scholar]
- Xing Y., Xu Y., Chen Y., Jeffrey P. D., Chao Y., Lin Z., Li Z., Strack S., Stock J. B., Shi Y. Cell. 2006;127:341–353. doi: 10.1016/j.cell.2006.09.025. [DOI] [PubMed] [Google Scholar]
- Guzman R. E., Solter P. F., Runnegar M. T. Toxicon. 2003;41:773–781. doi: 10.1016/s0041-0101(03)00030-8. [DOI] [PubMed] [Google Scholar]
- Fu W. Y., Chen J. P., Wang X. M., Xu L. H. Toxicon. 2005;46:171–177. doi: 10.1016/j.toxicon.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Li H., Xie P., Li G., Hao L., Xiong Q. Toxicon. 2009;53:169–175. doi: 10.1016/j.toxicon.2008.10.027. [DOI] [PubMed] [Google Scholar]
- Bouaicha N., Maatouk I. Toxicol. Lett. 2004;148:53–63. doi: 10.1016/j.toxlet.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Miles C. O., Sandvik M., Nonga H. E., Ballot A., Wilkins A. L., Rise F., Jaabaek J. A., Loader J. I. Chem. Res. Toxicol. 2016;29:860–870. doi: 10.1021/acs.chemrestox.6b00028. [DOI] [PubMed] [Google Scholar]
- Jaeschke H., Gores G. J., Cederbaum A. I., Hinson J. A., Pessayre D., Lemasters J. J. Toxicol. Sci. 2002;65:166–176. doi: 10.1093/toxsci/65.2.166. [DOI] [PubMed] [Google Scholar]
- Bessems J. G., Vermeulen N. P. Crit. Rev. Toxicol. 2001;31:55–138. doi: 10.1080/20014091111677. [DOI] [PubMed] [Google Scholar]
- Pereira S. R., Vasconcelos V. M., Antunes A. FEBS J. 2013;280:674–680. doi: 10.1111/j.1742-4658.2011.08454.x. [DOI] [PubMed] [Google Scholar]
- Takenaka S. Environ. Toxicol. Pharmacol. 2001;9:135–139. doi: 10.1016/s1382-6689(00)00049-1. [DOI] [PubMed] [Google Scholar]
- Hao L., Xie P., Li H., Li G., Xiong Q., Wang Q., Qiu T., Liu Y. Toxicon. 2010;55:1378–1386. doi: 10.1016/j.toxicon.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Huang X., Chen L., Liu W., Qiao Q., Wu K., Wen J., Huang C., Tang R., Zhang X. Aquat. Toxicol. 2015;165:41–50. doi: 10.1016/j.aquatox.2015.05.009. [DOI] [PubMed] [Google Scholar]
- Li X., Xu L., Zhou W., Zhao Q., Wang Y. Environ. Pollut. 2016;210:48–56. doi: 10.1016/j.envpol.2015.12.001. [DOI] [PubMed] [Google Scholar]
- La-Salete R., Oliveira M. M., Palmeira C. A., Almeida J., Peixoto F. P. J. Appl. Toxicol. 2008;28:55–62. doi: 10.1002/jat.1251. [DOI] [PubMed] [Google Scholar]
- Chen T., Wang Q., Cui J., Yang W., Shi Q., Hua Z., Ji J., Shen P. Mol. Cell. Proteomics. 2005;4:958–974. doi: 10.1074/mcp.M400185-MCP200. [DOI] [PubMed] [Google Scholar]
- Meneghini R. Free Radicals Biol. Med. 1997;23:783–792. doi: 10.1016/s0891-5849(97)00016-6. [DOI] [PubMed] [Google Scholar]
- Zhang H., Zhang J., Chen Y., Zhu Y. Fish Physiol. Biochem. 2008;34:307–312. doi: 10.1007/s10695-007-9189-7. [DOI] [PubMed] [Google Scholar]
- Milutinović A., Zivin M., Zorc-Pleskovic R., Sedmak B., Suput D. Toxicon. 2003;42:281–288. doi: 10.1016/s0041-0101(03)00143-0. [DOI] [PubMed] [Google Scholar]
- Milutinović A., Zorc-Pleskovic R., Zivin M., Vovk A., Sersa I., Suput D. Mar. Drugs. 2013;11:2785–2798. doi: 10.3390/md11082785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobre A. C., Coelho G. R., Coutinho M. C., Silva M. M., Angelim E. V., Menezes D. B., Fonteles M. C., Monteiro H. S. Toxicon. 2001;39:721–724. doi: 10.1016/s0041-0101(00)00193-8. [DOI] [PubMed] [Google Scholar]
- Wang C., Gu S., Yin X., Yuan M., Xiang Z., Li Z., Cao H., Meng X., Hu K., Han X. Toxicon. 2016;115:81–88. doi: 10.1016/j.toxicon.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picanco M. R., Soares R. M., Cagido V. R., Azevedo S. M. D. O., Rocco P. R. N., Zin W. A. Braz. J. Med. Biol. Res. 2004;37:1225–1229. doi: 10.1590/s0100-879x2004000800013. [DOI] [PubMed] [Google Scholar]
- Hooser S. B., Beasley V. R., Basgall E. J., Carmichael W. W., Haschek W. M. Vet. Pathol. 1990;27:9–15. doi: 10.1177/030098589002700102. [DOI] [PubMed] [Google Scholar]
- Soares R. M., Cagido V. R., Ferraro R. B., Meyer-Fernandes J. R., Rocco P. R., Zin W. A., Azevedo S. M. Toxicon. 2007;50:330–338. doi: 10.1016/j.toxicon.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Qiu T., Xie P., Liu Y., Li G., Xiong Q., Hao L., Li H. Toxicology. 2009;257:86–94. doi: 10.1016/j.tox.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Augustine L. M., Markelewicz Jr. R. J., Boekelheide K., Cherrington N. J. Drug Metab. Dispos. 2005;33:182–189. doi: 10.1124/dmd.104.001024. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Yuan J., Wu J., Han X. Toxicol. Lett. 2012;212:48–56. doi: 10.1016/j.toxlet.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Chen L., Zhang X., Zhou W., Qiao Q., Liang H., Li G., Wang J., Cai F. PLoS One. 2013;8:e53949. doi: 10.1371/journal.pone.0053949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Chen Y., Zuo X., Ding N., Zeng H., Zou X., Han X. Food Chem. Toxicol. 2013;60:309–317. doi: 10.1016/j.fct.2013.07.039. [DOI] [PubMed] [Google Scholar]
- Li D., Liu Z., Cui Y., Li W., Fang H., Li M., Kong Z. Ecotoxicology. 2011;20:1018–1025. doi: 10.1007/s10646-011-0693-2. [DOI] [PubMed] [Google Scholar]
- Wu J., Shao S., Zhou F., Wen S., Chen F., Han X. Environ. Toxicol. Pharmacol. 2014;37:1–6. doi: 10.1016/j.etap.2013.10.012. [DOI] [PubMed] [Google Scholar]
- Wu J., Yuan M., Song Y., Sun F., Han X. Toxins. 2015;7:5212–5223. doi: 10.3390/toxins7124872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Zhuang X., Xu T., Mao M., Wang C., Chen Y., Han X., Wu J. Toxicon. 2017;129:11–19. doi: 10.1016/j.toxicon.2017.01.022. [DOI] [PubMed] [Google Scholar]
- Meng X., Zhang L., Chen X., Xiang Z., Li D., Han X. Toxins. 2016;8:260. doi: 10.3390/toxins8090260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramsson-Zetterberg L., Sundh U. B., Mattsson R. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2010;699:5–10. doi: 10.1016/j.mrgentox.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Dias E., Louro H., Pinto M., Santos T., Antunes S., Pereira P., Silva M. J. BioMed Res. Int. 2014;2014:949521. doi: 10.1155/2014/949521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paerl H. W. Life. 2014;4:988–1012. doi: 10.3390/life4040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankiewicz-Boczek J., Karwaciak I., Ratajewski M., Gagala I., Jurczak T., Zalewski M., Pulaski L. Aquat. Toxicol. 2015;168:1–10. doi: 10.1016/j.aquatox.2015.09.004. [DOI] [PubMed] [Google Scholar]
- Rodriguez E. M., Acero J. L., Spoof L., Meriluoto J. Water Res. 2008;42:1744–1752. doi: 10.1016/j.watres.2007.10.039. [DOI] [PubMed] [Google Scholar]
- Svrcek C., Smith D. W. J. Environ. Eng. Sci. 2004;3:155–185. [Google Scholar]