Abstract
It is widely known that the incidence of pulmonary arterial hypertension (PAH) is higher in female, whereas prognosis is poorer in male patients. However, sex differences in hemodynamic response to and long-term prognosis with PAH-targeted treatment in the modern era remain to be fully elucidated. We examined the long-term prognosis of 129 consecutive PAH patients (34 males and 95 females) diagnosed in our hospital from April 1999 to October 2014, and assessed hemodynamic changes in response to PAH-targeted therapy. Female patients had better 5-year survival compared with male patients (74.0 vs. 53.4%, P = 0.003); however, higher age quartiles in females were associated with poor outcome. Follow-up examination after medical treatment showed significant decreases in mean pulmonary arterial pressure (mPAP), pulmonary vascular resistance (PVR), and pulmonary arterial capacitance (PAC) in both sexes (both P < 0.05), whereas only females had a significant improvement in right ventricular end-diastolic pressure (RVEDP), right atrial pressure (RAP), cardiac index, and mixed venous oxygen saturation (SvO2) (all P < 0.05). Baseline age significantly correlated with the hemodynamic changes only in female patients; particularly, there were significant sex interactions in RVEDP and RAP (both P < 0.10). The multivariable analysis showed that SvO2 at baseline and mPAP and SvO2 at follow-up were significant prognostic factors in males, whereas the changes in mPAP, PVR, and PAC and use of endothelin-receptor antagonist in females. These results indicate that female PAH patients have better long-term prognosis than males, for which better improvements of right ventricular functions and hemodynamics may be involved.
Keywords: Pulmonary arterial hypertension, Sex difference, Right ventricular function, Pulmonary hemodynamics, Prognosis
Introduction
Pulmonary arterial hypertension (PAH) is a disease characterized by progressive pulmonary vascular remodeling that increases pulmonary arterial pressure and finally leads to right heart failure and premature death [1]. In the past 2 decades, PAH-targeted medical therapy for major 3 pathways have been developed, including prostacyclin, endothelin-1 (ET-1), and nitric oxide (NO), with resultant marked improvement of survival of PAH patients [2–5]. Responses to the PAH-targeted treatment are heterogeneous, and epoprostenol, an intravenous prostacyclin analog, and macitentan, a dual endothelin-receptor antagonist, have only been shown to improve survival of PAH patients in randomized controlled trials [6–8]. Furthermore, it is widely known that the incidence of PAH is higher in females than in males, whereas long-term prognosis of PAH patients is poorer in males than in females [3, 9, 10]. This paradoxical phenomenon could be partially explained by the detrimental vs. protective effects of sex hormones in PAH patients; however, it remains to be examined what mechanism(s) is involved in such sex differences.
Responses of the right ventricle (RV) in response to increased hemodynamic load, such as right atrial pressure (RAP) and cardiac index (CI), are known to be significant prognostic factors of PAH [7, 11–13]. These clinical parameters of RV functions are also be poorer in male PAH patients at diagnosis [10, 14, 15]. However, sex differences in the relationship between hemodynamic parameters and long-term prognosis after PAH-specific therapy also remain to be elucidated. This point is important when developing personalized medicine by sex for each PAH patient. In the present study, we thus examined sex differences in the hemodynamic responses to and long-term prognosis with PAH-specific medical therapy in PAH patients.
Methods
Study population and treatment
The present study was approved by the Ethics Committee of Tohoku University Graduate School of Medicine (2012-1-301, 2016-1-582) and all patients provided written informed consent. From April 1999 to October 2014, we enrolled 129 consecutive patients with PAH who were diagnosed in our hospital. The diagnosis of PAH was made based on the established approaches, including physical examination, blood tests, echocardiography, pulmonary function test, chest X-ray, computed tomography (CT), ventilation-perfusion scanning, and right heart catheterization [16]. PAH subtype was classified based on the Nice classification [17]. PAH-specific drug therapy during the study period in Japan included oral and intravenous prostacyclin analogues since 1999, endothelin-receptor antagonists (ERAs) since 2005, and phosphodiesterase type-5 (PDE-5) inhibitors since 2008. These drugs were chosen by a physician in charge as a monotherapy or combination therapy. PAH-specific drugs were listed for the present analysis when the largest number of drugs were used during the follow-up. Baseline demographic data were collected from the medical records of each patient. Follow-up hemodynamic data were obtained at least 12 weeks after right heart catheterization at baseline in 97 out of 129 patients. Follow-up was completed in November 2016. The primary outcome was the composite end-point of all-cause death and lung transplantation [8, 18, 19].
Statistical analysis
Continuous variables are expressed as the mean ± SD and categorical variables as the number (%). Means and percentages were compared using paired t test, Wilcoxon signed-rank test, χ2 test, or Fisher exact test, as appropriate. Event-free survival time was calculated from the date of diagnostic catheterization to the date of any cause of death, lung transplantation, or last follow-up. A Kaplan–Meier curve was used to estimate the overall event-free survival, and differences between survival curves were assessed using the log-rank test. Univariable and multivariable Cox proportional hazard models were used to estimate the hazard ratios and 95% confidence intervals. P values of < 0.05 were regarded to be statistically significant. All analyses were performed with JMP Pro 12.2.0 (Japanese version, SAS Institute Inc., Tokyo, Japan).
Results
Clinical characteristics of PAH patients
The number of patients with clinical subtypes of PAH was as follows; idiopathic/heritable PAH (IPAH/HPAH) in 45, connective tissue diseases (CTD) in 41, congenital heart disease (CHD) in 31, portal hypertension in 11, and drug- and toxin-induced in one. Clinical characteristics of the 129 patients are shown in Table 1. Mean age was 45 ± 18 years and 34 (26%) were male. Among them, 30 (23%) were treated with monotherapy, 84 (65%) with combination therapy with 2–3 PAH-specific drugs, and 40 (31%) with intravenous prostacyclin. During the mean observation period of 5.9 years, 43 (33%) patients died and 11 (9%) underwent lung transplantation.
Table 1.
Sex differences in clinical characteristics, hemodynamics, and medical therapy in PAH patients
| Overall | Male | Female | P value | |
|---|---|---|---|---|
| N | 129 | 34 | 95 | |
| Age (years) | 45 ± 18 | 43 ± 20 | 45 ± 17 | 0.65 |
| Time between baseline and follow-up (years) | 1.2 ± 1.5 | 1.4 ± 2.0 | 1.1 ± 1.4 | 0.60 |
| Mean follow-up duration (years) | 5.9 ± 4.3 | 4.8 ± 3.6 | 6.3 ± 4.4 | 0.09 |
| Subtype of PAH | ||||
| IPAH, n (%) | 45 (35) | 13 (38) | 32 (34) | |
| Drug and toxin, n (%) | 1 (1) | 0 (0) | 1 (1) | |
| CTD, n (%) | 41 (32) | 5 (15) | 36 (38) | |
| Portal HT, n (%) | 11 (9) | 4 (12) | 7 (7) | |
| CHD, n (%) | 31 (24) | 12 (35) | 19 (20) | |
| WHO-FC III or IV, n (%) | 52 (40) | 13 (38) | 39 (41) | 0.84 |
| BNP (pg/mL) | 273 ± 389 | 210 ± 170 | 295 ± 440 | 0.96 |
| Hemodynamics | ||||
| mPAP (mmHg) | 50.6 ± 20.0 | 52.4 ± 20.0 | 50.0 ± 20.1 | 0.45 |
| PAWP (mmHg) | 8.5 ± 3.8 | 9.5 ± 3.8 | 8.2 ± 3.7 | 0.09 |
| RVEDP (mmHg) | 9.8 ± 4.6 | 10.1 ± 4.8 | 9.6 ± 4.5 | 0.60 |
| RAP (mmHg) | 6.8 ± 4.2 | 7.5 ± 4.1 | 6.5 ± 4.2 | 0.21 |
| CI (L/min/m2) | 2.79 ± 0.88 | 2.85 ± 0.96 | 2.76 ± 0.86 | 0.65 |
| PVR (dyn/s/cm5) | 933 ± 731 | 892 ± 727 | 948 ± 736 | 0.53 |
| Heart rate (bpm) | 79.8 ± 14.7 | 80.4 ± 14.1 | 79.6 ± 14.9 | 0.78 |
| Pulmonary pulse pressure (mmHg) | 44.2 ± 17.6 | 43.1 ± 17.9 | 44.7 ± 17.6 | 0.69 |
| PAC (mL/mmHg) | 1.52 ± 0.94 | 1.67 ± 0.96 | 1.46 ± 0.93 | 0.20 |
| SvO2 (%) | 67.7 ± 10.2 | 68.3 ± 11.8 | 67.4 ± 9.6 | 0.69 |
| Medical therapy | ||||
| Epoprostenol, n (%) | 40 (31) | 8 (24) | 32 (34) | 0.39 |
| Beraprost, n (%) | 53 (41) | 14 (41) | 39 (41) | 1.00 |
| ERA, n (%) | 83 (64) | 22 (65) | 61 (64) | 1.00 |
| PDE-5 inhibitor, n (%) | 77 (60) | 22 (65) | 55 (58) | 0.54 |
| No PAH-targeted drug, n (%) | 15 (12) | 5 (15) | 10 (11) | 0.54 |
| Monotherapy, n (%) | 30 (23) | 7 (21) | 23 (24) | 0.81 |
| Double combination therapy, n (%) | 29 (22) | 7 (21) | 22 (23) | 0.82 |
| Triple combination therapy, n (%) | 55 (43) | 15 (44) | 40 (42) | 0.84 |
Continuous variables are expressed as mean ± SD, n (%)
BNP brain natriuretic peptide, CHD congenital heart disease, CI cardiac index, CTD connective tissue diseases, ERA endothelin-receptor antagonist, IPAH idiopathic pulmonary arterial hypertension, mPAP mean pulmonary arterial pressure, PAC pulmonary arterial capacitance, PAWP pulmonary artery wedge pressure, PDE-5 phosphodiesterase type-5, Portal HT portal hypertension, PVR pulmonary vascular resistance, RAP right atrial pressure, RVEDP right ventricular end-diastolic pressure, SvO2 mixed venous oxygen saturation, WHO-FC World Health Organization-functional class
Long-term prognosis of PAH patients
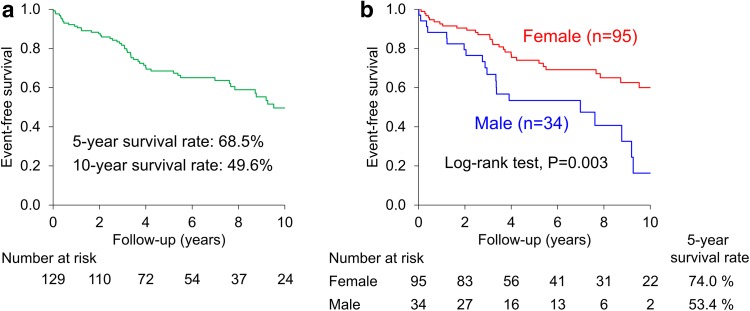
Event-free survival in all PAH patients was 68.5% at 5 years and 49.6% at 10 years (Fig. 1a). Multivariable analysis at baseline showed that male sex, elderly age older than 60 years, World Health Organization-functional class (WHO-FC) III or IV, and higher mixed venous oxygen saturation (SvO2) at baseline were significant predictors for mortality (Table 2).
Fig. 1.
Long-term prognosis of PAH patients. a Event-free survival was 68.5% at 5 years and 49.6% at 10 years in all PAH patients. b Female patients had a better survival compared with male patients (P = 0.003)
Table 2.
Univariable and multivariable Cox proportional hazards model of PAH patients
| Candidate variables | Univariable | Multivariable | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Male | 2.29 (1.28–4.00) | 0.006 | 2.63 (1.41–4.80) | 0.003 |
| Age < 32 years | Reference | Reference | ||
| 32 ≤ age < 43 years | 0.70 (0.31–1.57) | 0.38 | 0.86 (0.36–2.01) | 0.72 |
| 43 ≤ age < 60 years | 1.13 (0.51–2.51) | 0.76 | 2.04 (0.87–4.75) | 0.10 |
| Age ≥ 60 years | 2.03 (0.97–4.35) | 0.06 | 3.16 (1.45–7.07) | 0.004 |
| WHO-FC | ||||
| I or II | Reference | Reference | ||
| III or IV | 2.44 (1.42–4.24) | 0.001 | 3.03 (1.71–5.47) | 0.0001 |
| BNP (per 100 pg/mL) | 1.00 (0.91–1.07) | 0.91 | ||
| mPAP (mmHg) | 1.00 (0.99–1.02) | 0.40 | ||
| PAWP (mmHg) | 1.02 (0.94–1.09) | 0.64 | ||
| RVEDP (mmHg) | 1.06 (0.998–1.12) | 0.06 | ||
| RAP (mmHg) | 1.03 (0.97–1.08) | 0.38 | ||
| CI (L/min/m2) | 0.92 (0.66–1.27) | 0.62 | ||
| PVR (per 100 dyn/s/cm5) | 1.02 (0.98–1.05) | 0.42 | ||
| PAC (mL/mmHg) | 0.79 (0.54–1.09) | 0.16 | ||
| SvO2 (%) | 0.97 (0.94–0.998) | 0.04 | 0.96 (0.93–0.99) | 0.01 |
See Table 1 for abbreviations
Sex differences in clinical characteristics and long-term prognosis of PAH patients
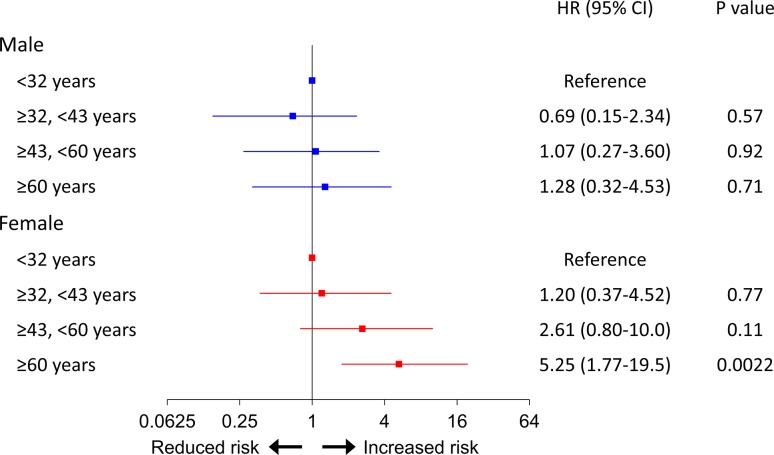
There were no significant sex differences in age, hemodynamic parameters, or severity of PH at baseline (Table 1). No sex difference was also noted for the use of PAH-specific drugs or the prevalence of combination therapy. However, female patients had better survival compared with male patients (5-year event-free survival rate, 74.0 vs. 53.4%, P = 0.003) (Fig. 1b). At baseline, when the patients were divided into 4 quartiles by age, elderly age (≥ 60 years) was significantly associated with poor outcome in the multivariable analysis adjusted for WHO-FC in females but not in males (Fig. 2).
Fig. 2.
Multivariable Cox proportional hazard model of baseline age divided into 4 quartiles adjusted for WHO-FC. Elderly group was significantly associated with poor outcome in females but not in males
Hemodynamic changes in response to optimal medical therapy
Follow-up examination after medical therapy showed a significant decrease in mean pulmonary arterial pressure (mPAP) and pulmonary vascular resistance (PVR) and a significant increase in pulmonary arterial capacitance (PAC) in both sexes (both P < 0.05), whereas only female patients had significant decreases in right ventricular end-diastolic pressure (RVEDP) and right atrial pressure (RAP) and significant increases in cardiac index (CI) and SvO2 (Table 3). Importantly, a significant sex difference was noted in terms of change in RVEDP and tendency in that of RAP (Table 3).
Table 3.
Sex differences in the hemodynamic changes after medical therapy in PAH patients
| Male | Female | P value for sex | |||
|---|---|---|---|---|---|
| Change | P value between baseline and follow-up | Change | P value between baseline and follow-up | ||
| mPAP (mmHg) | − 6.1 ± 9.0 | 0.004 | − 7.1 ± 10.7 | < 0.0001 | 0.70 |
| RVEDP (mmHg) | 0.4 ± 3.6 | 0.40 | − 1.2 ± 4.4 | 0.008 | 0.06 |
| RAP (mmHg) | − 0.1 ± 2.5 | 0.93 | − 1.3 ± 3.9 | 0.007 | 0.11 |
| CI (L/min/m2) | 0.14 ± 0.99 | 0.11 | 0.29 ± 0.91 | 0.023 | 0.90 |
| PVR (dyn/s/cm5) | − 320 ± 569 | 0.01 | − 257 ± 454 | < 0.0001 | 0.78 |
| PAC (mL/mmHg) | 0.43 ± 0.76 | 0.02 | 0.54 ± 0.72 | < 0.0001 | 0.55 |
| SvO2 (%) | 1.1 ± 8.1 | 0.56 | 2.5 ± 8.8 | 0.027 | 0.52 |
See Table 1 for abbreviations
Baseline age significantly correlated with mPAP in male patients, and mPAP, RVEDP, and PVR in female patients; however, there were no significant sex interactions for the correlations (Table 4). In contrast, baseline age significantly correlated with the hemodynamic changes in mPAP, RAP, CI, PVR, and SvO2 only in female patients; particularly, there were significant sex interactions for the correlations between age and hemodynamic changes in RVEDP and RAP (Table 4).
Table 4.
Sex differences in the correlation between baseline age and hemodynamics
| Male | Female | P value for interaction | |||
|---|---|---|---|---|---|
| R | P value | R | P value | ||
| Baseline | |||||
| mPAP (mmHg) | − 0.49 | 0.003 | − 0.50 | < 0.0001 | 0.63 |
| RVEDP (mmHg) | − 0.19 | 0.28 | − 0.32 | 0.002 | 0.39 |
| RAP (mmHg) | − 0.13 | 0.46 | − 0.18 | 0.08 | 0.69 |
| CI (L/min/m2) | 0.08 | 0.66 | 0.17 | 0.11 | 0.62 |
| PVR (dyn/s/cm5) | − 0.30 | 0.10 | − 0.36 | 0.001 | 0.54 |
| PAC (mL/mmHg) | 0.19 | 0.28 | 0.19 | 0.07 | 0.89 |
| SvO2 (%) | − 0.03 | 0.86 | 0.19 | 0.07 | 0.26 |
| Changes | |||||
| ΔmPAP (mmHg) | − 0.014 | 0.95 | 0.27 | 0.02 | 0.23 |
| ΔRVEDP (mmHg) | − 0.26 | 0.28 | 0.22 | 0.06 | 0.08 |
| ΔRAP (mmHg) | − 0.31 | 0.18 | 0.25 | 0.03 | 0.05 |
| ΔCI (L/min/m2) | − 0.26 | 0.25 | − 0.27 | 0.02 | 0.95 |
| ΔPVR (dyn/s/cm5) | 0.22 | 0.35 | 0.30 | 0.01 | 0.95 |
| ΔPAC (mL/mmHg) | − 0.12 | 0.61 | − 0.13 | 0.26 | 0.97 |
| ΔSvO2 (%) | 0.043 | 0.86 | − 0.32 | 0.01 | 0.14 |
Δ indicates change in each hemodynamics. See Table 1 for abbreviations
Prognostic effects of hemodynamics and PAH-specific medical therapy
The multivariable analysis adjusted for WHO-FC showed that SvO2 at baseline and mPAP and SvO2 at follow-up were significant prognostic factors in male patients, while the decrease in mPAP and PVR and the increase in PAC in female patients (Table 5). In addition, the use of ERA or PDE-5 inhibitors significantly correlated with a better prognosis in females but not in males (Table 5). Interestingly, a significant sex interaction was noted only for the use of ERA (Table 5). No significant correlation was noted between survival and the use of prostacyclin analogues, such as beraprost and epoprostenol, in both sexes (Table 5).
Table 5.
Multivariable Cox proportional hazard models of the sex differences in hemodynamics and their changes in response to medical therapy, and PAH-targeted medical therapy adjusted for WHO-FC
| Male | Female | P value for interaction | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Baseline | |||||
| mPAP per 10 mmHg | 1.30 (0.995–1.726) | 0.054 | 0.91 (0.74–1.10) | 0.35 | 0.08 |
| RAP per mmHg | 1.01 (0.89–1.14) | 0.82 | 0.98 (0.90–1.05) | 0.58 | 0.64 |
| CI per L/min/m2 | 0.87 (0.53–1.35) | 0.54 | 1.19 (0.71–1.95) | 0.51 | 0.47 |
| PVR per 100 dyn/s/cm5 | 1.05 (0.98–1.12) | 0.12 | 0.99 (0.92–1.04) | 0.70 | 0.24 |
| PAC per mL/mmHg | 0.74 (0.42–1.18) | 0.22 | 0.88 (0.51–1.40) | 0.63 | 0.93 |
| SvO2 per 10% | 0.53 (0.30–0.90) | 0.02 | 0.92 (0.63–1.39) | 0.69 | 0.10 |
| Follow-up | |||||
| mPAP per 10 mmHg | 1.60 (1.04–2.48) | 0.04 | 1.13 (0.85–1.47) | 0.38 | 0.14 |
| RAP per mmHg | 1.14 (0.94–1.39) | 0.18 | 1.08 (0.92–1.25) | 0.33 | 0.60 |
| CI per L/min/m2 | 1.20 (0.47–2.79) | 0.69 | 0.65 (0.31–1.27) | 0.21 | 0.16 |
| PVR per 100 dyn/s/cm5 | 1.28 (0.97–1.70) | 0.08 | 1.05 (0.96–1.15) | 0.27 | 0.20 |
| PAC per mL/mmHg | 0.49 (0.13–1.29) | 0.17 | 0.61 (0.29–1.11) | 0.11 | 0.87 |
| SvO2 per 10% | 0.34 (0.12–0.86) | 0.02 | 0.99 (0.59–1.76) | 0.96 | 0.05 |
| Changes | |||||
| Decrease in mPAP per 10 mmHg | 0.61 (0.26–1.35) | 0.24 | 0.55 (0.33–0.88) | 0.013 | 0.89 |
| Decrease in RAP per mmHg | 0.97 (0.72–1.20) | 0.80 | 0.98 (0.88–1.08) | 0.66 | 0.64 |
| Increase in CI per L/min/m2 | 1.07 (0.59–2.37) | 0.83 | 0.68 (0.35–1.27) | 0.24 | 0.22 |
| Decrease in PVR per 100 dyn/s/cm5 | 1.10 (0.95–1.26) | 0.19 | 0.88 (0.77–0.99) | 0.034 | 0.02 |
| Increase in PAC per mL/mmHg | 0.67 (0.22–1.83) | 0.44 | 0.29 (0.09–0.78) | 0.013 | 0.20 |
| Increase in SvO2 per 10% | 0.62 (0.21–1.58) | 0.32 | 1.04 (0.66–1.62) | 0.85 | 0.33 |
| Beraprost | 2.03 (0.72–5.84) | 0.18 | 1.09 (0.53–2.17) | 0.82 | 0.30 |
| Epoprostenol | 0.78 (0.26–2.03) | 0.62 | 0.72 (0.33–1.48) | 0.37 | 0.94 |
| ERA | 2.02 (0.75–6.37) | 0.17 | 0.42 (0.21–0.87) | 0.02 | 0.02 |
| PDE-5 inhibitor | 0.73 (0.28–1.97) | 0.52 | 0.45 (0.22–0.89) | 0.02 | 0.65 |
See Table 1 for abbreviations
Discussion
The novel findings of the present study are as follows: (1) event-free survival at 5 years in Japanese PAH patients was 68.5%, where female patients had superior survival compared with male patients, (2) aging was significantly associated with poor outcome in females but not in males, (3) in response to optimal medical therapy, several parameters, particularly RVEDP and RAP, were ameliorated in females but not in males, where significant sex interactions were noted in terms of the correlation between age and the changes in RVEDP and RAP, (4) significant prognostic factors were hemodynamics at baseline and follow-up in males but were hemodynamic changes in females, and (5) the uses of ERA and PDE-5 inhibitor were related to better prognosis in females but not in males. To the best of our knowledge, this is the first study demonstrating the sex differences in hemodynamic responses and long-term survival in response to optimal medical therapy in PAH patients.
Sex differences in clinical characteristics in PAH
The prevalence of PAH is higher in females than in males in the general population [3, 9, 10], which was also the case in the present study. A number of experimental and clinical studies implicated the aggravating roles of estrogen in the pathogenesis of PAH, relating to tryptophan hydroxylase-1, 5-hydroxytryptamine, serotonin transporter, cytochrome P450 1B1, and mutations in bone morphogenetic protein receptor type 2 [20–23]. Through these pathways, estrogen accelerates cell proliferation and forming pulmonary artery lesions, leading to the development of PAH.
Although recent registry studies of IPAH patients showed that males had higher mPAP, PVR and RAP, and lower CI at diagnosis [9, 10, 15], no significant sex differences were noted in the present study. Since we enrolled patients of group 1 PAH with various etiologies, this may have resulted in the heterogeneity in hemodynamics as in the previous studies [24, 25].
Sex differences in hemodynamic responses to optimal medical therapy in PAH
Increased RVEDP and RAP reflect RV overload or ischemia [14, 26, 27]. Hemodynamic and morphological parameters of RV functions are also important predictors of long-term survival of PAH patients [9, 18, 28]. Although it is generally known that female PAH patients tend to have more favorable RV function than males at diagnosis [3, 9, 10, 14], sex differences in RV functions in response to optimal medical therapy remain to be fully elucidated. The present study indicates that sex differences in hemodynamic responses to optimal medical therapy largely result from adaptation of RV function to pressure overload.
A number of studies showed that estrogens exert protective cardiovascular effects, which are dramatically reduced after menopause [29, 30]. Recent epidemiological studies also showed that higher estrogen levels were associated with better RV systolic function [31, 32]. In addition, experimental studies showed that estrogen exerted beneficial effects against PAH by preventing RV dysfunction and hypertrophy through inhibition of inflammation, fibrosis, and apoptotic signaling and improving mitochondrial function and RV contractility [33–37].
While estrogen has attracted much attention, androgens may also have essential parts in PAH. In a rodent model, androgens affect mal-adaptiveness for RV hypertrophy and fibrosis [38]. In addition, epidemiological studies showed that males had accelerated cardiomyocyte apoptosis, lower RV ejection fraction, and larger RV mass [39, 40], and that higher androgen levels were associated with poor RV functions [31].
In addition to the direct effects of sex hormones, PAH-targeted drugs may also have different effects for RV functions by sex. It was reported that PDE-5 inhibitor efficacy was estrogen-dependent in female mouse heart, which was mediated by enhanced cardiac synthesis of cyclic guanosine monophosphate (cGMP) through endothelial NO synthase and soluble guanylyl cyclase pathway [41]. This result supports our present finding of better RV responses after optimal medical therapy in female PAH patients compared with male patients.
Sex differences in long-term prognosis with optical medical therapy in PAH
In the present study, although female PAH patients had worse hemodynamic conditions compared with male patients, they paradoxically had a better survival as in the previous studies [3, 9, 10]. However, sex differences in the prognostic factors in PAH patients remain to be examined. In the present study, aging was associated with worse prognosis only in female patients, suggesting negative effects of menopause. Moreover, a recent study demonstrated that there were sex differences in RV responses to medical therapy in PAH patients, which could explain a significant portion of the sex difference in survival in those patients [19, 42]. Taken together, these findings suggest that RV functions in response to optimal medical therapy are a key determinant of long-term prognosis of PAH patients. Indeed, in the present study, greater improvement in RV function-related parameters after optimal medication was associated with a better prognosis in females compared with males.
We also found that there are sex differences in the prognostic effects of PAH-targeted drugs, especially ERAs. It was previously reported that ERAs ameliorated 6-min walk distance (6MWD) and outcomes, especially in females [43]. This sex difference in clinical responses to ERAs may be related, at least in part, to higher levels of circulating ET-1 and greater vasoconstriction mediated by ET receptor type A in males [44, 45]. Sex differences in the effects of PDE-5 inhibitors have also been reported; male and premenopausal female patients were able to achieve clinically relevant responses in 6MWD and health-related quality of life [46, 47]. These findings may be explained by sexual dimorphism in NO metabolism, as urinary 15N nitrate excretion, which reflects total NO biosynthesis, was significantly higher in females compared with males, suggesting that males may benefit more from the NO pathway and PDE-5 inhibitors than females [48]. In the present study, no significant sex interaction was noted for PDE-5 inhibitors use as in the previous study [47]. This is probably because medication is effective for both sexes in different ways. Further studies are needed to explore the mechanisms of the sex differences in clinical responses to PAH-targeted drugs.
Limitations
Several limitations should be mentioned for the present study. First, the present study is a single center one with possible selection bias. Event-free survival of PAH patients in the present study was poor compared with other 2 recent studies with Japanese IPAH/HPAH patients [42, 49]. This difference in mortality rate may be due to that in intravenous prostacyclin use (present study, 64% vs. Ogawa et al. 79%) [42] and that in enrollment period (present study, 1999–2014 vs. Tamura et al., 2008–2013) [49]. Second, in the present study, we enrolled all patients with Group 1 PAH; in particular, there was a relatively large number of CTD-PAH in female patients. However, event-free survival in systemic sclerosis-associated PAH (4 males, 7 females) showed no sex difference in the present study (log-rank test, P = 0.38) as in the previous study [50]. Even if we excluded patients with CTD-PAH, female patients showed a better prognosis (data not shown). Thus, we consider that the present findings could be generalizable to all forms of PAH. Third, there were 32 (25%) cases without follow-up right heart catheterization in the present study. However, among the patients without follow-up catheterization (13 males and 19 females), there was no sex difference in the long-term prognosis (log-rank test, P = 0.63). Finally, it is possible that long-term survivors had more chance to be treated with newly approved drugs during the follow-up period. Thus, further prospective studies are needed to examine the efficacy of the combination therapy of PAH-targeted drugs.
Conclusions
In the present study, we were able to demonstrate that female PAH patients, particularly in younger patients, have better prognosis, for which a better improvement of RV functions in response to PAH-specific medical therapy may be involved. Further development of individual treatment may ameliorate long-term prognosis of PAH patients.
Acknowledgements
The authors thank the clinical research coordinators for enrolling patients, collecting data, and supporting the project and all the patients for their participation in the present study.
Funding
This work was supported in part by a Grant-in-Aid (15K09116, 24591032) from the Japanese Ministry of Education, Culture, Sports, Science and Technology, Tokyo, Japan.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
References
- 1.Voelkel NF, Gomez-Arroyo J, Abbate A, Bogaard HJ, Nicolls MR. Pathobiology of pulmonary arterial hypertension and right ventricular failure. Eur Respir J. 2012;40:1555–1565. doi: 10.1183/09031936.00046612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D’Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Kernis JT, Levy PS, Pietra GG, Reid LM, Reeves JT, Rich S, Vreim CE, Williams GW, Wu M. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115:343–349. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 3.Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier JF, Chabot F, Dromer C, Pison C, Reynaud-Gaubert M, Haloun A, Laurent M, Hachulla E, Cottin V, Degano B, Jais X, Montani D, Souza R, Simonneau G. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation. 2010;122:156–163. doi: 10.1161/CIRCULATIONAHA.109.911818. [DOI] [PubMed] [Google Scholar]
- 4.Lee WT, Ling Y, Sheares KK, Pepke-Zaba J, Peacock AJ, Johnson MK. Predicting survival in pulmonary arterial hypertension in the UK. Eur Respir J. 2012;40:604–611. doi: 10.1183/09031936.00196611. [DOI] [PubMed] [Google Scholar]
- 5.Benza RL, Miller DP, Barst RJ, Badesch DB, Frost AE, McGoon MD. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest. 2012;142:448–456. doi: 10.1378/chest.11-1460. [DOI] [PubMed] [Google Scholar]
- 6.Barst RJ, Rubin LJ, Long WA, McGoon MD, Rich S, Badesch DB, Groves BM, Tapson VF, Bourge RC, Brundage BH, Koerner SK, Langleben D, Keller CA, Murali S, Uretsky BF, Clayton LM, Jobsis MM, Blackburn SD, Shortino D, Crow JW, Primary Pulmonary Hypertension Study Group A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med. 1996;334:296–301. doi: 10.1056/NEJM199602013340504. [DOI] [PubMed] [Google Scholar]
- 7.Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, Aboyans V, Vaz Carneiro A, Achenbach S, Agewall S, Allanore Y, Asteggiano R, Paolo Badano L, Albert Barbera J, Bouvaist H, Bueno H, Byrne RA, Carerj S, Castro G, Erol C, Falk V, Funck-Brentano C, Gorenflo M, Granton J, Iung B, Kiely DG, Kirchhof P, Kjellstrom B, Landmesser U, Lekakis J, Lionis C, Lip GY, Orfanos SE, Park MH, Piepoli MF, Ponikowski P, Revel MP, Rigau D, Rosenkranz S, Voller H, Luis Zamorano J. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Heart J. 2016;37:67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 8.Pulido T, Adzerikho I, Channick RN, Delcroix M, Galie N, Ghofrani HA, Jansa P, Jing ZC, Le Brun FO, Mehta S, Mittelholzer CM, Perchenet L, Sastry BK, Sitbon O, Souza R, Torbicki A, Zeng X, Rubin LJ, Simonneau G, SERAPHIN Investigators Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med. 2013;369:809–818. doi: 10.1056/NEJMoa1213917. [DOI] [PubMed] [Google Scholar]
- 9.Benza RL, Miller DP, Gomberg-Maitland M, Frantz RP, Foreman AJ, Coffey CS, Frost A, Barst RJ, Badesch DB, Elliott CG, Liou TG, McGoon MD. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL) Circulation. 2010;122:164–172. doi: 10.1161/CIRCULATIONAHA.109.898122. [DOI] [PubMed] [Google Scholar]
- 10.Shapiro S, Traiger GL, Turner M, McGoon MD, Wason P, Barst RJ. Sex differences in the diagnosis, treatment, and outcome of patients with pulmonary arterial hypertension enrolled in the registry to evaluate early and long-term pulmonary arterial hypertension disease management. Chest. 2012;141:363–373. doi: 10.1378/chest.10-3114. [DOI] [PubMed] [Google Scholar]
- 11.van Wolferen SA, Marcus JT, Boonstra A, Marques KM, Bronzwaer JG, Spreeuwenberg MD, Postmus PE, Vonk-Noordegraaf A. Prognostic value of right ventricular mass, volume, and function in idiopathic pulmonary arterial hypertension. Eur Heart J. 2007;28:1250–1257. doi: 10.1093/eurheartj/ehl477. [DOI] [PubMed] [Google Scholar]
- 12.Yamada Y, Okuda S, Kataoka M, Tanimoto A, Tamura Y, Abe T, Okamura T, Fukuda K, Satoh T, Kuribayashi S. Prognostic value of cardiac magnetic resonance imaging for idiopathic pulmonary arterial hypertension before initiating intravenous prostacyclin therapy. Circ J. 2012;76:1737–1743. doi: 10.1253/circj.CJ-11-1237. [DOI] [PubMed] [Google Scholar]
- 13.Baggen VJ, Leiner T, Post MC, van Dijk AP, Roos-Hesselink JW, Boersma E, Habets J, Sieswerda GT. Cardiac magnetic resonance findings predicting mortality in patients with pulmonary arterial hypertension: a systematic review and meta-analysis. Eur Radiol. 2016;26:3771–3780. doi: 10.1007/s00330-016-4217-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawut SM, Al-Naamani N, Agerstrand C, Berman Rosenzweig E, Rowan C, Barst RJ, Bergmann S, Horn EM. Determinants of right ventricular ejection fraction in pulmonary arterial hypertension. Chest. 2009;135:752–759. doi: 10.1378/chest.08-1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ventetuolo CE, Praestgaard A, Palevsky HI, Klinger JR, Halpern SD, Kawut SM. Sex and haemodynamics in pulmonary arterial hypertension. Eur Respir J. 2014;43:523–530. doi: 10.1183/09031936.00027613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoeper MM, Bogaard HJ, Condliffe R, Frantz R, Khanna D, Kurzyna M, Langleben D, Manes A, Satoh T, Torres F, Wilkins MR, Badesch DB. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D42–D50. doi: 10.1016/j.jacc.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 17.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, Krishna Kumar R, Landzberg M, Machado RF, Olschewski H, Robbins IM, Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D34–D41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 18.Nickel N, Golpon H, Greer M, Knudsen L, Olsson K, Westerkamp V, Welte T, Hoeper MM. The prognostic impact of follow-up assessments in patients with idiopathic pulmonary arterial hypertension. Eur Respir J. 2012;39:589–596. doi: 10.1183/09031936.00092311. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs W, van de Veerdonk MC, Trip P, de Man F, Heymans MW, Marcus JT, Kawut SM, Bogaard HJ, Boonstra A, Vonk Noordegraaf A. The right ventricle explains sex differences in survival in idiopathic pulmonary arterial hypertension. Chest. 2014;145:1230–1236. doi: 10.1378/chest.13-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wallace E, Morrell NW, Yang XD, Long L, Stevens H, Nilsen M, Loughlin L, Mair KM, Baker AH, MacLean MR. A sex-specific microRNA-96/5-hydroxytryptamine 1B axis influences development of pulmonary hypertension. Am J Respir Crit Care Med. 2015;191:1432–1442. doi: 10.1164/rccm.201412-2148OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White K, Dempsie Y, Nilsen M, Wright AF, Loughlin L, MacLean MR. The serotonin transporter, gender, and 17β oestradiol in the development of pulmonary arterial hypertension. Cardiovasc Res. 2011;90:373–382. doi: 10.1093/cvr/cvq408. [DOI] [PubMed] [Google Scholar]
- 22.White K, Johansen AK, Nilsen M, Ciuclan L, Wallace E, Paton L, Campbell A, Morecroft I, Loughlin L, McClure JD, Thomas M, Mair KM, MacLean MR. Activity of the estrogen-metabolizing enzyme cytochrome P450 1B1 influences the development of pulmonary arterial hypertension. Circulation. 2012;126:1087–1098. doi: 10.1161/CIRCULATIONAHA.111.062927. [DOI] [PubMed] [Google Scholar]
- 23.Austin ED, Cogan JD, West JD, Hedges LK, Hamid R, Dawson EP, Wheeler LA, Parl FF, Loyd JE, Phillips JA., 3rd Alterations in oestrogen metabolism: implications for higher penetrance of familial pulmonary arterial hypertension in females. Eur Respir J. 2009;34:1093–1099. doi: 10.1183/09031936.00010409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hurdman J, Condliffe R, Elliot CA, Davies C, Hill C, Wild JM, Capener D, Sephton P, Hamilton N, Armstrong IJ, Billings C, Lawrie A, Sabroe I, Akil M, O’Toole L, Kiely DG. ASPIRE registry: assessing the Spectrum of Pulmonary hypertension Identified at a REferral centre. Eur Respir J. 2012;39:945–955. doi: 10.1183/09031936.00078411. [DOI] [PubMed] [Google Scholar]
- 25.McGoon MD, Miller DP. REVEAL: a contemporary US pulmonary arterial hypertension registry. Eur Respir Rev. 2012;21:8–18. doi: 10.1183/09059180.00008211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gomez A, Bialostozky D, Zajarias A, Santos E, Palomar A, Martinez ML, Sandoval J. Right ventricular ischemia in patients with primary pulmonary hypertension. J Am Coll Cardiol. 2001;38:1137–1142. doi: 10.1016/S0735-1097(01)01496-6. [DOI] [PubMed] [Google Scholar]
- 27.Ghio S, Pazzano AS, Klersy C, Scelsi L, Raineri C, Camporotondo R, D’Armini A, Visconti LO. Clinical and prognostic relevance of echocardiographic evaluation of right ventricular geometry in patients with idiopathic pulmonary arterial hypertension. Am J Cardiol. 2011;107:628–632. doi: 10.1016/j.amjcard.2010.10.027. [DOI] [PubMed] [Google Scholar]
- 28.Querejeta Roca G, Campbell P, Claggett B, Solomon SD, Shah AM. Right atrial function in pulmonary arterial hypertension. Circ Cardiovasc Imaging. 2015;8:e003521. doi: 10.1161/CIRCIMAGING.115.003521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayward CS, Kelly RP, Collins P. The roles of gender, the menopause and hormone replacement on cardiovascular function. Cardiovasc Res. 2000;46:28–49. doi: 10.1016/S0008-6363(00)00005-5. [DOI] [PubMed] [Google Scholar]
- 30.Murphy E. Estrogen signaling and cardiovascular disease. Circ Res. 2011;109:687–696. doi: 10.1161/CIRCRESAHA.110.236687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ventetuolo CE, Ouyang P, Bluemke DA, Tandri H, Barr RG, Bagiella E, Cappola AR, Bristow MR, Johnson C, Kronmal RA, Kizer JR, Lima JA, Kawut SM. Sex hormones are associated with right ventricular structure and function: the MESA-right ventricle study. Am J Respir Crit Care Med. 2011;183:659–667. doi: 10.1164/rccm.201007-1027OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ventetuolo CE, Mitra N, Wan F, Manichaikul A, Barr RG, Johnson C, Bluemke DA, Lima JA, Tandri H, Ouyang P, Kawut SM. Oestradiol metabolism and androgen receptor genotypes are associated with right ventricular function. Eur Respir J. 2016;47:553–563. doi: 10.1183/13993003.01083-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tofovic SP, Zhang X, Jackson EK, Dacic S, Petrusevska G. 2-Methoxyestradiol mediates the protective effects of estradiol in monocrotaline-induced pulmonary hypertension. Vasc Pharmacol. 2006;45:358–367. doi: 10.1016/j.vph.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 34.Umar S, Iorga A, Matori H, Nadadur RD, Li J, Maltese F, van der Laarse A, Eghbali M. Estrogen rescues preexisting severe pulmonary hypertension in rats. Am J Respir Crit Care Med. 2011;184:715–723. doi: 10.1164/rccm.201101-0078OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lahm T, Albrecht M, Fisher AJ, Selej M, Patel NG, Brown JA, Justice MJ, Brown MB, Van Demark M, Trulock KM, Dieudonne D, Reddy JG, Presson RG, Petrache I. 17β-Estradiol attenuates hypoxic pulmonary hypertension via estrogen receptor-mediated effects. Am J Respir Crit Care Med. 2012;185:965–980. doi: 10.1164/rccm.201107-1293OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frump AL, Goss KN, Vayl A, Albrecht M, Fisher A, Tursunova R, Fierst J, Whitson J, Cucci AR, Brown MB, Lahm T. Estradiol improves right ventricular function in rats with severe angioproliferative pulmonary hypertension: effects of endogenous and exogenous sex hormones. Am J Physiol Lung Cell Mol Physiol. 2015;308:L873–L890. doi: 10.1152/ajplung.00006.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu A, Schreier D, Tian L, Eickhoff JC, Wang Z, Hacker TA, Chesler NC. Direct and indirect protection of right ventricular function by estrogen in an experimental model of pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol. 2014;307:H273–H283. doi: 10.1152/ajpheart.00758.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hemnes AR, Maynard KB, Champion HC, Gleaves L, Penner N, West J, Newman JH. Testosterone negatively regulates right ventricular load stress responses in mice. Pulm Circ. 2012;2:352–358. doi: 10.4103/2045-8932.101647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mallat Z, Fornes P, Costagliola R, Esposito B, Belmin J, Lecomte D, Tedgui A. Age and gender effects on cardiomyocyte apoptosis in the normal human heart. J Gerontol A Biol Sci Med Sci. 2001;56:M719–M723. doi: 10.1093/gerona/56.11.M719. [DOI] [PubMed] [Google Scholar]
- 40.Kawut SM, Lima JA, Barr RG, Chahal H, Jain A, Tandri H, Praestgaard A, Bagiella E, Kizer JR, Johnson WC, Kronmal RA, Bluemke DA. Sex and race differences in right ventricular structure and function: the multi-ethnic study of atherosclerosis-right ventricle study. Circulation. 2011;123:2542–2551. doi: 10.1161/CIRCULATIONAHA.110.985515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sasaki H, Nagayama T, Blanton RM, Seo K, Zhang M, Zhu G, Lee DI, Bedja D, Hsu S, Tsukamoto O, Takashima S, Kitakaze M, Mendelsohn ME, Karas RH, Kass DA, Takimoto E. PDE5 inhibitor efficacy is estrogen dependent in female heart disease. J Clin Investig. 2014;124:2464–2471. doi: 10.1172/JCI70731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ogawa A, Satoh T, Tamura Y, Fukuda K, Matsubara H. Survival of Japanese patients with idiopathic/heritable pulmonary arterial hypertension. Am J Cardiol. 2017;119:1479–1484. doi: 10.1016/j.amjcard.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 43.Gabler NB, French B, Strom BL, Liu Z, Palevsky HI, Taichman DB, Kawut SM, Halpern SD. Race and sex differences in response to endothelin receptor antagonists for pulmonary arterial hypertension. Chest. 2012;141:20–26. doi: 10.1378/chest.11-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Polderman KH, Stehouwer CD, van Kamp GJ, Dekker GA, Verheugt FW, Gooren LJ. Influence of sex hormones on plasma endothelin levels. Ann Intern Med. 1993;118:429–432. doi: 10.7326/0003-4819-118-6-199303150-00006. [DOI] [PubMed] [Google Scholar]
- 45.Stauffer BL, Westby CM, Greiner JJ, Van Guilder GP, Desouza CA. Sex differences in endothelin-1-mediated vasoconstrictor tone in middle-aged and older adults. Am J Physiol Regul Integr Comp Physiol. 2010;298:R261–R265. doi: 10.1152/ajpregu.00626.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mathai SC, Hassoun PM, Puhan MA, Zhou Y, Wise RA. Sex differences in response to tadalafil in pulmonary arterial hypertension. Chest. 2015;147:188–197. doi: 10.1378/chest.14-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rusiecki J, Rao Y, Cleveland J, Rhinehart Z, Champion HC, Mathier MA. Sex and menopause differences in response to tadalafil: 6-minute walk distance and time to clinical worsening. Pulm Circ. 2015;5:701–706. doi: 10.1086/683829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Forte P, Kneale BJ, Milne E, Chowienczyk PJ, Johnston A, Benjamin N, Ritter JM. Evidence for a difference in nitric oxide biosynthesis between healthy women and men. Hypertension. 1998;32:730–734. doi: 10.1161/01.HYP.32.4.730. [DOI] [PubMed] [Google Scholar]
- 49.Tamura Y, Kumamaru H, Satoh T, Miyata H, Ogawa A, Tanabe N, Hatano M, Yao A, Abe K, Tsujino I, Fukuda K, Kimura H, Kuwana M, Matsubara H, Tatsumi K, Japan PHRN. Effectiveness and outcome of pulmonary arterial hypertension-specific therapy in Japanese patients with pulmonary arterial hypertension. Circ J. 2017 doi: 10.1253/circj.CJ-17-0139. [DOI] [PubMed] [Google Scholar]
- 50.Pasarikovski CR, Granton JT, Roos AM, Sadeghi S, Kron AT, Thenganatt J, Moric J, Chau C, Johnson SR. Sex disparities in systemic sclerosis-associated pulmonary arterial hypertension: a cohort study. Arthritis Res Ther. 2016;18:30. doi: 10.1186/s13075-016-0933-1. [DOI] [PMC free article] [PubMed] [Google Scholar]