Abstract
Background
Leptospirosis is postulated as a possible cause of Mesoamerican Nephropathy (MeN) in Central American workers.
Objectives
Investigate job-specific Leptospira seroprevalence and its association with kidney disease biomarkers.
Methods
In 282 sugarcane workers, 47 sugarcane applicants and 160 workers in other industries, we measured anti-leptospiral antibodies, serum creatinine, and urinary injury biomarkers, including neutrophil gelatinase-associated lipocalin (NGAL), interleukin-18 (IL-18), and N-acetyl-D-glucosaminidase (NAG).
Results
Leptospira seroprevalence differed among job categories and was highest among sugarcane cutters (59%). Seropositive sugarcane workers had higher NGAL concentrations (relative mean: 1.28; 95% CI: 0.94–1.75) compared to those who were seronegative, with similar findings among field and non-field workers.
Conclusions
Leptospira seroprevalence varied by job category. There was some indication that seropositivity was associated with elevated biomarker levels, but results were inconsistent. Additional studies may help establish whether Leptospira infection plays any role in MeN among Central American workers.
Keywords: Chronic kidney disease (CKD), Mesoamerican Nephropathy (MeN), Chronic kidney disease of nontraditional etiology (CKDnt), Leptospira, Ieptospirosis, Neutrophil gelatinase-associated lipocalin (NGAL), Interleukin-18 (IL-18), N-acetyl-D- glucosaminidase (NAG)
Introduction
In several regions of Central America, particularly the Pacific coast lowlands, there is an epidemic of chronic kidney disease (CKD) termed Mesoamerican Nephropathy (MeN) that mainly affects young male agricultural workers [1–7]. The etiology of the disease is unknown and it is not associated with common causes of CKD in the developed world such as diabetes, hypertension and obesity [3,5,7–15]. Although most attention has been focused on the potential relationship to heat stress and recurrent dehydration because of its occurrence in workers performing strenuous manual labor under hot environmental conditions, infectious diseases, particularly leptospirosis, were identified as one possible etiology requiring further study at the First International Workshop on MeN in 2012 [1,5,6,9,16,17].
Leptospirosis is a zoonotic disease caused by pathogenic spirochetes of the Leptospira spp. genus that is known to cause acute kidney injury (AKI); notably, both the geography and demographic characteristics of the population at risk of leptospirosis are similar to those of MeN [16,18–26]. Human infection usually occurs after contact with water or soil contaminated by the urine of animal reservoirs [20,21]. Leptospirosis has a wide spectrum of clinical manifestations [20,21,27–38] and, although known to cause AKI, there are only a few studies of its association with CKD [29,31,39–48]. The studies that have evaluated kidney recovery after leptospirosis-induced AKI show that normalization of serum creatinine occurs in the vast majority of patients, albeit with tubular dysfunction that can persist for several months [31,40]. Given the growing body of evidence showing an increased risk of developing CKD after an episode of AKI despite early normalization of serum creatinine [49,50] and a recent study showing an association between chronic human exposure to leptospires and CKD [51], investigations are warranted to assess whether clinically recognized leptospirosis is associated with CKD, as well as whether asymptomatic or mild leptospirosis, which is more likely to go unrecognized and is more frequent than the severe cases associated with overt AKI, can cause subclinical kidney injury that subsequently predisposes to CKD.
To explore these questions, we evaluated the prevalence of Leptospira seropositivity among workers employed in a region where MeN is common; estimated incident cases of leptospirosis among sugarcane workers within one harvest season; and determined whether Leptospira seropositivity was associated with biomarkers of kidney function and injury.
Methods
Study population
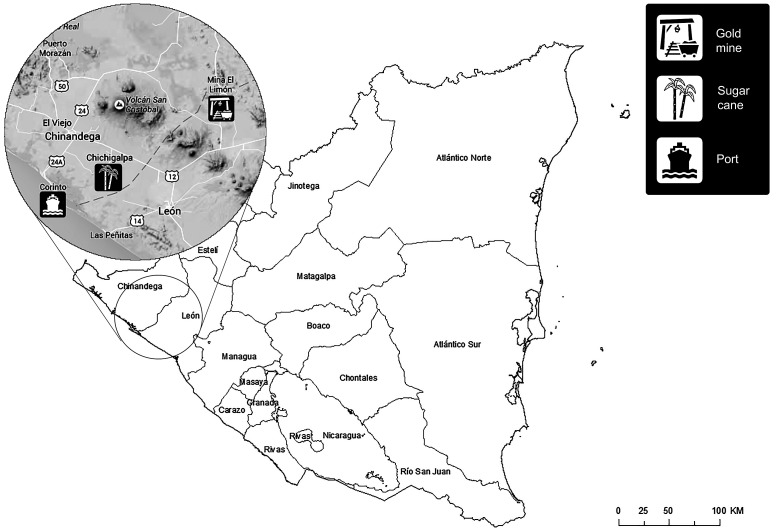
Data for this study was collected as part of a prospective study that evaluated biomarkers of kidney function and injury among a population of sugarcane workers in Nicaragua [14,52], including applicants for jobs as field workers who were found to have an elevated serum creatinine during employment screening, as well as a population of miners, construction workers, and port workers who had never worked in the sugarcane industry (Figure 1). All individuals were required to be at least 18 years of age to be eligible for participation. Study protocols were approved by the Institutional Review Boards at the Boston University Medical Center and the Nicaraguan Ministry of Health. All study participants provided written informed consent prior to enrollment in research activities.
Figure 1.
Location of industries in Nicaragua from which study population was recruited.
Notes: Area enlarged shows locations of sugarcane company, gold mine, and port from which study population of workers was recruited. Workers in the construction sector include workers from the entire enlarged area, involved in building of traditional houses and commercial buildings.
Sugarcane workers (n = 282) worked during the harvest at one company in northwestern Nicaragua in one of the following job categories: seed cutter, seeder, agrichemical applicator, irrigator, cane cutter, driver, or factory worker. Participants were enrolled at the pre-harvest screening during a mandatory medical exam of all potential employees (October to December 2010); during this time, questionnaires were administered and biospecimens collected. Workers were re-sampled approximately 4 to 6 months later, near the end of the harvest season (March to May 2011). The final study population was selected from the workers who had questionnaires and biospecimens collected at both time points (refer to Laws et al. 2015 for a detailed description of each job category and sampling methodology) [14].
Sugarcane applicants (n = 47) included individuals who had an elevated serum creatinine (≥1.4 mg/dl) at the pre-harvest mandatory testing conducted by the company. This creatinine threshold was used by the company to exclude individuals from being hired. At the time of enrollment, 59 individuals were identified as having an elevated serum creatinine, of these, we included all individuals with a creatinine of 1.6 mg/dl or above, and a random sample of those with creatinine levels of 1.4–1.5 mg/dl. Questionnaire and biospecimen collection for these individuals occurred at the time of the pre-harvest screening (October to December 2010).
Workers in other industries (n = 160) included miners, construction workers and port workers from northwestern Nicaragua who had never worked in the sugarcane industry. These workers were recruited because they were thought to have an increased prevalence of CKD based on a prior study [13] or anecdotal reports. Miners were recruited from those who worked in the largest gold mine in the region. Construction workers were recruited from laborers actively involved in various aspects of building traditional houses or commercial buildings. Port workers were recruited from those who were affiliated with unions and performed heavy manual labor in the port of Corinto, Nicaragua. Questionnaire and biospecimen collection was completed at their respective work locations at approximately the same time as the late-harvest sampling in sugarcane workers (March to May 2011).
For all workers, biospecimens consisted of blood and urine samples. Additionally, questionnaires were administered that asked about demographics, occupational history, and work practices. Sugarcane workers were sampled twice, before and near the end of the harvest, while the other two categories were sampled once.
Laboratory testing
All blood and urine samples were collected by trained personnel. On the day of collection, samples were processed at the local health center and stored at –20 °C. Within one week, they were transported to the ISO-certified Centro Nacional de Diagnóstico y Referencia (CNDR) in Managua, a division within the Ministry of Health, and stored at –80 °C.
Leptospira serology
Serum and urine samples were shipped to the Bacterial Special Pathogens Branch at the Centers for Disease Control and Prevention (CDC) (Atlanta, Georgia, USA). Serum samples were tested for the presence of anti-leptospiral antibodies using a microscopic agglutination test (MAT) following standard procedures. The CDC’s MAT panel was used, which includes 20 reference strains belonging to 17 serogroups [53]. The antigens were 5–7 day-old cultures grown in liquid Elinghausen Mc-Cullough Johnson Harris (EMJH) medium adjusted to the concentration of a 0.5 McFarland standard. MAT was done at doubling dilutions starting from 1:100. Positive samples were titrated up to end titers. The endpoint titer was reported as the highest dilution that agglutinated at least 50% of the cells for each strain tested.
Sugarcane workers were considered seronegative if specimens from both the pre- and late-harvest had titers of <100. Seroconversion or a fourfold rise in titers was considered evidence of recent or current leptospirosis. All titers ≥100 that did not represent either seroconversion or a fourfold rise in titers between paired samples, were considered evidence of past Leptospira infection. Some individuals had a negative titer at pre-harvest and a late-harvest titer of 100 or 200. It was unclear whether these represented true seroconversions given the low titers. Since there was a period of several months between samples collected at the pre- and late-harvest, peak titers could in theory be missed. To avoid misclassifying individuals with recent or current infection as past infection with Leptospira, anti-leptospiral IgM antibodies in serum were determined on all individuals that had low titer seroconversion or less than a fourfold rise in titers on the MAT using a dipstick ELISA kit (ImmunoDOT, GenBio, San Diego, CA). An ELISA IgM result of 2.0–2.5 dots was considered borderline positive; while 3.0–4.0 dots was considered positive. Since IgM antibodies can remain detectable for prolonged periods of time, only those individuals that had a negative IgM ELISA on the first sample (pre-harvest) and a positive IgM ELISA on the second sample (late-harvest) were considered to have recent or current infection; all others were categorized as past Leptospira infection.
Only a single serum sample was collected from sugarcane applicants and workers in other industries. These individuals were considered seronegative if the MAT titer was <100; a titer of ≥800 was considered evidence of recent or current leptospirosis [54]; all other titers were considered evidence of past Leptospira infection.
Leptospira urine PCR
Urine was also tested by polymerase chain reaction (PCR) to detect leptospiral DNA. For sugarcane workers, urine from the late-harvest sample was used. Frozen urine was thawed and centrifuged at 3000 ×g for 15 min at room temperature. Supernatants were decanted and 10 mL of PBS was added to wash the pelleted urine and centrifuged again. Washing was performed twice; upon decanting the final wash supernatant, pellets were resuspended in 500 μL PBS. DNA from the pellets was extracted with the QIAamp DNA Mini Kit using an automated QIAcube extraction instrument (Qiagen, Valencia, CA) following manufacturer’s directions. All urine samples were screened for the presence of Leptospira using a published TaqMan PCR assay targeting the gene for the leptospiral outer membrane protein LipL32 [55]; this was performed using an ABI 7500 Real Time System instrument (Applied Biosystems, Inc., San Francisco, CA).
Kidney injury biomarkers
Serum samples were analyzed for creatinine using the kinetic-rate Jaffe method at the Centro Nacional de Diagnóstico y Referencia (CNDR) (Managua, Nicaragua). Based on manufacturer specifications, 0.2 mg/dL was subtracted from serum creatinine results to calibrate to an isotope mass dilution spectroscopy standard prior to use of the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (with race considered “non-black”) to estimate glomerular filtration rate (eGFR) [56].
Urine samples were shipped to the Division of Nephrology and Hypertension at the Cincinnati Children’s Hospital Medical Center (Cincinnati, Ohio, USA) for analysis of creatinine, albumin, interleukin-18 (IL-18), neutrophil gelatinase-associated lipocalin (NGAL) and N-acetyl-D-glucosaminidase (NAG). IL-18, NGAL, and NAG are considered biomarkers of kidney tubular injury, and may provide earlier detection of kidney injury and better prognostication in CKD than can be achieved by measuring serum creatinine alone [57–69]. Urine creatinine and albumin were measured by immunoturbidimetry and a colorimetric modification of the Jaffe reaction, respectively. All reported values of urine creatinine (g/L) were above the limit of detection (LOD). The LOD for urine albumin was 1.3 mg/L. NGAL (Bioporto, Gentofte, Denmark) and IL-18 (MBL, Intl., Woburn, MA) were measured by ELISA. All reported values of NGAL (ng/ml) were above the LOD. The LOD for IL-18 was 4 pg/ml. NAG activity was measured using a colorimetric assay (Roche Diagnostics, USA) with a LOD of 0.003 U/L.
Data analysis
The distribution of each biomarker of kidney injury was examined using histograms, graphical displays, and summary statistics. For values below the LOD, the LOD/√2 was substituted. To account for urine dilution, urine biomarkers were normalized to urine creatinine (g/L) and expressed as follows: albumin to creatinine ratio (ACR) (mg/g creatinine), NGAL (μg/g creatinine), IL-18 (ng/g creatinine), and NAG (U/g creatinine).
The first set of analyses used logistic regression to assess job category as a predictor of Leptospira seropositivity. The second set of analyses used linear regression to assess Leptospira seropositivity as a predictor of eGFR, NGAL, IL-18, and NAG. In sugarcane workers, this association was examined at both pre-harvest and late-harvest. There were too few cases classified as recent or current infection to conduct separate analyses restricted to this category. Age, sex, and years employed at the company were included as covariates in all models to control for confounding; job category was included in models that were not already stratified by type of job.
Urine IL-18, NGAL, and NAG normalized to urine creatinine were natural log-transformed to satisfy normality assumptions, and the β estimates for IL-18, NGAL, and NAG were exponentiated (eβ) to show differences in means on the multiplicative scale or “relative means.”
Results
Study population
Among 489 individuals included in the study (93% men, mean age 35 years (SD = 10.6)), 282 were sugarcane workers (19 seed cutters, 35 seeders, 29 agrichemical applicators, 49 irrigators, 51 cane cutters, 41 drivers, and 58 factory workers), 47 were sugarcane applicants with elevated serum creatinine at pre-harvest, and 160 were workers in other industries (51 miners, 56 construction, and 53 port workers) (Table 1). The average number of years worked among sugarcane worker’s categories ranged from 3.7 to 14.4 years, seed cutters and cane cutters had worked for the fewest number of years, while drivers and factory workers had worked the longest.
Table 1.
Characteristics of study population, by job category.
| Job Category | n | Age Mean (SD) | Male n (%) | Years worked Mean (SD) |
|---|---|---|---|---|
| All workers | 489 | 35 (10.6) | 454 (93%) | _ |
| Sugarcane workers | 282 | 34 (10.4) | 249 (88%) | 9.6 (9.1) |
| Field Workers | 183 | 31 (9.4) | 152 (83%) | 7.0 (7.5) |
| Seed cutters | 19 | 30 (9.2) | 9 (47%) | 3.7 (4.4) |
| Seeders | 35 | 30 (8.8) | 14 (40%) | 5.0 (4.9) |
| Agrichemical applicators | 29 | 35 (8.2) | 29 (100%) | 12.0 (8.0) |
| Irrigators | 49 | 30 (8.8) | 49 (100%) | 9.7 (8.0) |
| Cane cutters | 51 | 31 (10.6) | 51 (100%) | 4.1 (6.8) |
| Non-Field Workers | 99 | 38 (10.6) | 97 (98%) | 14.3 (9.9) |
| Drivers | 41 | 41 (11.2) | 41 (100%) | 14.4 (8.9) |
| Factory workers | 58 | 37 (9.8) | 56 (97%) | 14.3 (10.6) |
| Sugarcane applicants with elevated serum creatinine | 47 | 33 (8.6) | 47 (100%) | 4.9 (3.5) |
| Miners | 51 | 38 (7.9) | 48 (94%) | * |
| Construction workers | 56 | 35 (12.3) | 56 (100%) | * |
| Port workers | 53 | 40 (11.6) | 53 (100%) | * |
No data available.
Leptospira serology and urine PCR
The proportion of seropositive individuals (MAT titer ≥ 100 at any time point), reflecting any prior infection with Leptospira, was 29% (n = 142) (Table 2). The highest seroprevalence was observed among cane cutters (59%), and the lowest among port workers (8%) and sugarcane factory workers (9%). Among sugarcane workers, field workers (seed cutters, seeders, agrichemical applicators, irrigators, and cane cutters) had a higher seroprevalence (40%) than non-field workers (drivers and factory workers) (21%), with an adjusted odds ratio of 3.2 (95% confidence interval (CI): 1.7–6.1). Among specific job categories, the odds of being seropositive for Leptospira was highest among cane cutters, seeders, irrigators, drivers, agrichemical applicators, sugarcane applicants, and miners, compared to port workers. Individuals in three job categories had evidence of recent or current leptospirosis: cane cutters (n = 2), sugarcane applicants (n = 3), and miners (n = 3).
Table 2.
Leptospira seropositivity by job category.
| Job category | Seropositive n (%)a | Unadjusted OR (95% CI) | Adjusted ORb (95% CI) |
|---|---|---|---|
| All workers | 142 (29%) | – | – |
| Sugarcane workers | 95 (34%) | – | – |
| Seed cutters | 3 (16%) | 2.3 (0.5–11.4) | 3.2 (0.6–17.6) |
| Seeders | 13 (37%) | 7.2 (2.1–24.7) | 10.6 (2.6–43.2) |
| Agrichemical applicators | 10 (35%) | 6.5 (1.8–23.1) | 6.9 (1.9–24.8) |
| Irrigators | 18 (37%) | 7.1 (2.2–23.0) | 8.0 (2.4–26.3) |
| Cane cutters | 30 (59%) | 17.5 (5.5–55.9) | 19.6 (6.0–64.2) |
| Drivers | 16 (39%) | 7.8 (2.4–26.0) | 7.8 (2.4–25.8) |
| Factory workers | 5 (9%) | 1.2 (0.3–4.6) | 1.2 (0.3–4.8) |
| Sugarcane applicants with elevated serum creatinine | 20 (43%) | 9.1 (2.8–29.3) | 9.8 (3.0–32.2) |
| Miners | 16 (31%) | 5.6 (1.7–18.2) | 5.9 (1.8–19.3) |
| Construction workers | 7 (13%) | 1.75 (0.5–6.4) | 1.8 (0.5–6.7) |
| Port workers | 4 (8%) | Ref | Ref |
“n” represents number of persons in category who are seropositive; “(%)” represents percentage of total number of people in category who are seropositive.
Adjusted for sex, age, and years worked.
The most frequently observed serological reactivity (highest titer by MAT) was to serovar Bratislava (n = 58, 40.8%), Canicola (n = 20, 14.1%), and Icterohaemorrhagiae (n = 11, 7.7%). More than one serovar with equally elevated titers was also frequently found (n = 14, 9.9%) (Table 3). All subjects tested had negative urine PCR results, suggesting the absence of chronic colonization of the kidney by leptospires.
Table 3.
Leptospira serovar distribution among seropositive individuals.a
| Serovar | n (%) |
|---|---|
| Alexi | 4 (2.8%) |
| Australis | 1 (0.7%) |
| Bataviae | 5 (3.5%) |
| Borincana | 1 (0.7%) |
| Bratislava | 58 (40.8%) |
| Canicola | 20 (14.1%) |
| Cynopteri | 2 (1.4%) |
| Djasiman | 8 (5.6%) |
| Georgia | 3 (2.1%) |
| Grippotyphosa | 2 (1.4%) |
| Icterohaemorrhagiae | 11 (7.7%) |
| Pomona | 5 (3.5%) |
| Pyrogenes | 3 (2.1%) |
| Tarassovi | 2 (1.4%) |
| Wolffi | 3 (2.1%) |
| More than one serovar with equally elevated titers | 14 (9.9%) |
Serovar with the highest titer by MAT is presented.
Leptospira seropositivity and kidney injury biomarkers
Leptospira seropositivity was not associated with eGFR except among sugarcane applicants with elevated creatinine. Those that were seropositive had lower mean eGFR (mean difference: –10.08; 95% CI: –24.12–3.96) compared to those who were seronegative.
At pre-harvest, there was no association between Leptospira seropositivity and concentrations of biomarkers of kidney injury among sugarcane workers (Table 4); however, IL-18 was somewhat elevated among seropositive field workers (relative mean (RM): 1.30; 95% CI: 0.89–1.88) compared to those that were seronegative. When stratified by job category, seropositive cane cutters had higher IL-18 (RM: 1.95; 95% CI: 0.98–3.87) and NGAL concentrations (RM: 1.30; 95% CI: 0.79–2.16) compared to those who were seronegative. Seropositive sugarcane applicants had higher NGAL (RM: 1.59; 95% CI: 0.81–3.11) and NAG (RM: 1.44; 95% CI: 0.85–2.43).
Table 4.
Leptospira seropositivitya as a predictor of kidney injury biomarkers, by job category in the sugarcane industry.
| eGFRb | IL-18c | NGALc | NAGc | |||||
|---|---|---|---|---|---|---|---|---|
| Job Category | Mean difference | Mean difference | Relative mean | Relative mean | Relative mean | Relative mean | Relative mean | Relative mean |
| Pre-harvest (95% CI) | Late-harvest (95% CI) | Pre-harvest (95% CI) | Late-harvest (95% CI) | Pre-harvest (95% CI) | Late-harvest (95% CI) | Pre-harvest (95% CI) | Late-harvest (95% CI) | |
| Sugarcane workers | 1.75 | 1.89 | 1.21 | 1.19 | 1.01 | 1.28 | 1.04 | 1.00 |
| n = 282 | (–1.95–5.44) | (–2.52–6.30) | (0.88–1.66) | (0.86–1.65) | (0.76–1.33) | (0.94–1.75) | (0.81–1.35) | (0.79–1.27) |
| Field workersa | 0.83 | 0.34 | 1.30 | 1.31 | 1.05 | 1.31 | 1.01 | 1.13 |
| n = 183 | (–3.60–5.26) | (-5.46–6.14) | (0.89–1.88) | (0.90–1.92) | (0.76–1.45) | (0.91–1.89) | (0.77–1.32) | (0.86–1.48) |
| Seed cutter | 4.33 | 11.73 | 0.65 | 1.75 | 1.60 | 2.09 | 0.76 | 1.40 |
| n = 19 | (–13.74–22.40) | (-13.07–36.53) | (0.10–4.23) | (0.29–10.48) | (0.51–5.00) | (0.73–5.97) | (0.34–1.69) | (0.29–6.75) |
| Seeder | 2.97 | 6.27 | 1.54 | 2.48 | 0.97 | 1.99 | 1.16 | 0.98 |
| n = 35 | (–9.73–15.65) | (–7.05–19.59) | (0.51–4.68) | (1.14–5.38) | (0.43–2.21) | (0.74–5.32) | (0.59–2.27) | (0.50–1.90) |
| Agrichemical applicator | 1.96 | 2.09 | 0.79 | 1.82 | 1.12 | 0.63 | 0.72 | 0.74 |
| n = 29 | (–5.96–9.89) | (–6.27–10.44) | (0.25–2.47) | (0.66–5.01) | (0.58–2.15) | (0.24–1.66) | (0.24–2.12) | (0.37–1.45) |
| Irrigator | 1.34 | 0.54 | 0.93 | 0.87 | 0.67 | 1.26 | 1.13 | 1.39 |
| n = 49 | (–6.71–9.40) | (–8.47–9.55) | (0.49–1.76) | (0.42–1.81) | (0.28–1.59) | (0.59–2.67) | (0.60–2.10) | (0.78–2.49) |
| Cane cutter | 1.91 | −0.87 | 1.95 | 0.69 | 1.30 | 0.84 | 1.03 | 0.80 |
| n = 51 | (–7.25–11.07) | (–15.93–14.19) | (0.98–3.87) | (0.32–1.50) | (0.79–2.16) | (0.45–1.58) | (0.67–1.58) | (0.52–1.22) |
| Non-field workersb | −2.00 | 1.61 | 1.28 | 1.56 | 0.83 | 1.66 | 1.08 | 1.08 |
| n = 99 | (–8.75–4.76) | (–3.87–7.09) | (0.72–2.28) | (0.84–2.88) | (0.50–1.40) | (0.92–2.97) | (0.63–1.86) | (0.68–1.72) |
| Driver | 2.21 | 1.80 | 0.94 | 1.20 | 0.84 | 1.52 | 0.96 | 0.84 |
| n = 41 | (–6.27–10.69) | (–5.10–8.70) | (0.42–2.06) | (0.56–2.60) | (0.48–1.46) | (0.90–2.57) | (0.44–2.12) | (0.49–1.44) |
| Factory | −6.90 | 2.30 | 1.12 | 1.53 | 0.79 | 2.84 | 1.37 | 1.60 |
| n = 58 | (–20.10–6.30) | (–8.65–13.25) | (0.42–2.95) | (0.46–5.10) | (0.27–2.37) | (0.81–10.03) | (0.55–3.45) | (0.64–4.03) |
| Sugarcane applicant with elevated serum creatinine | −10.08 | – | 0.84 | – | 1.59 | – | 1.44 | – |
| n = 47 | (–24.12–3.96) | (0.42–1.70) | (0.81–3.11) | (0.85–2.43) | ||||
eGFR = estimated glomerular filtration rate (ml/min/1.73 m2), IL-18 = Interleukin 18 (pg/ml), NGAL = Neutrophil gelatinase-associated lipocalin (ng/ml), NAG = N-acetyl-D-glucosaminidase (U/L).
Seronegative individuals in each job category are the reference group.
Results represent linear regression arithmetic differences in mean eGFR between individuals with exposure to Leptospira and seronegative individuals.
Results represent linear regression multiplicative differences (relative means) in mean IL-18, NGAL, and NAG between individuals with exposure to Leptospira and seronegative individuals.
Notes: Model adjusted for age, years worked (except for sugarcane applicants), and also adjusted for sex in categories with females (seed cutters, seeders, and factory); Sugarcane Workers also adjusted for job category.
At late harvest, NGAL, IL-18, and NAG were significantly higher among seropositive sugarcane workers as compared to seronegative workers when adjusting only for sex, age, and years worked (results not shown). However, additional adjustment for job category resulted in a decline of 15–22% in the relative means (Table 4). Including job category in the model, seropositive sugarcane workers had higher NGAL concentrations (RM: 1.28; 95% CI: 0.94–1.75) compared to those who were seronegative. When stratifying by fieldworker status, seropositive field workers had higher IL-18 (RM: 1.31 95% CI: 0.90–1.92) and NGAL (RM: 1.31; 95% CI: 0.91–1.89) concentrations compared to those who were seronegative. A similar result was evident among non-field workers (IL-18 RM: 1.56; 95% CI: 0.84–2.88; NGAL RM: 1.66; 95% CI: 0.92–2.97). When stratifying by individual job categories, higher NGAL concentrations were seen among seropositive seed cutters (RM: 2.09; 95% CI: 0.73–5.97), seeders (RM: 1.99; 95% CI: 0.74–5.32), drivers (RM: 1.52; 95% CI: 0.90–2.57), factory workers (RM: 2.84; 95% CI: 0.81–10.03), and higher IL-18 concentrations were observed among seropositive seeders (RM: 2.48; 95% CI: 1.14–5.38), as compared to seronegative workers in these jobs. Among workers in other industries, seropositive miners had higher IL-18 (RM: 1.48; 95% CI 0.74–2.95) and NAG (RM: 1.36; 95% CI 0.91–2.03) compared to those who were seronegative (Table 5).
Table 5.
Leptospira seropositivity as a predictor of kidney injury biomarkers, by job category among workers in other industries.a
| eGFRb | IL-18c | NGALc | NAGc | |
|---|---|---|---|---|
| Job category | Mean difference (95% CI) | Relative mean (95% CI) | Relative mean (95% CI) | Relative mean (95% CI) |
| Miner | 1.91 | 1.48 | 1.05 | 1.36 |
| n = 51 | (–9.11–12.93) | (0.74–2.95) | (0.65–1.69) | (0.91–2.03) |
| Construction | 10.70 | 1.50 | 0.87 | 0.53 |
| n = 56 | (–7.01–28.40) | (0.49–4.59) | (0.30–2.51) | (0.26–1.04) |
| Port worker | 13.97 | 0.87 | 0.50 | 0.63 |
| n = 53 | (–12.97–40.91) | (0.28–2.66) | (0.14–1.76) | (0.22–1.82) |
Notes: Model adjusted for age, and for sex in categories with females (miners). eGFR = estimated glomerular filtration rate (ml/min/1.73 m2), IL-18 = Interleukin 18 (pg/ml), NGAL = Neutrophil gelatinase-associated lipocalin (ng/ml), NAG = N-acetyl-D-glucosaminidase (U/L).
Seronegative individuals in each job category are the reference group.
Results represent linear regression arithmetic differences in mean eGFR between individuals with exposure to Leptospira and seronegative individuals.
Results represent linear regression multiplicative differences (relative means) in mean IL-18, NGAL, and NAG between individuals with exposure to Leptospira and seronegative individuals.
The median urine ACR among sugarcane workers was 2.4 mg/g at pre-harvest and 2.1 mg/g at late harvest (results not shown). At both time points fewer than 5% of workers had an ACR ≥ 30 mg/g (the clinical threshold for albuminuria). Sugarcane applicants had a median ACR of 3.2 mg/g, with 14.9% of them having an ACR ≥ 30 mg/g. Miners, construction workers, and port workers had a median ACR of 3.9, 3.3, and 1.3 mg/g, respectively. The percentage of workers with ACR ≥ 30 mg/g was 21.6% for miners, 10.9% for construction workers, and 7.5% for port workers. There was no association between urine ACR and Leptospira exposure (results not shown).
Discussion
Seropositivity to Leptospira was high among our study population, with significant differences by job category. We found some evidence that Leptospira seropositivity may be associated with elevated levels of biomarkers of kidney injury.
Despite infectious diseases being one of the hypothesized causes of the unexplained epidemic of MeN, there have been no prior studies evaluating them as a possible factor. Leptospirosis is a plausible candidate due to its widespread prevalence in Central America, high infection rates among the type of workers who have been identified as having elevated rates of MeN, and its established association with AKI.
Among sugarcane workers, field workers had higher seroprevalence than non-field workers, which is expected given the higher likelihood of occupational exposure to soil contaminated with Leptospira. Cane cutters had the highest seroprevalence at 59%. Among workers in other industries, miners were disproportionately affected. Drivers also had high seroprevalence, which was unexpected given that they have less occupational exposure to soil compared to field workers. However, workers were classified by their present occupation, and many drivers may have been field workers in the past or could be exposed non-occupationally.
There was evidence of recent or current leptospirosis in cane cutters, sugarcane applicants, and miners. Since cane cutters had samples from both pre- and late-harvest, recent or current leptospirosis represent incident cases (n = 2, 4%) during the harvest season. Given that Leptospira is endemic in regions of Central America, recent or current leptospirosis cases likely approximate the baseline incidence rate in this population, since samples were taken during a period when there was no outbreak. Sugarcane applicants and miners were sampled at only one time point, limiting the options for comparison with sugarcane cutters.
Some leptospiral serovars are commonly associated with particular animal reservoirs [20,21]. In this population, the serovars with the greatest number of seropositive individuals included Bratislava, typically associated with horses; Canicola, typically associated with dogs; and Icterohaemorrhagiae, typically associated with rats. All three animals are present in large numbers in the main geographic areas in which study participants live, but only rats are common in the specific occupational setting in which this study was conducted. However, highest MAT titer to a particular serovar does not necessarily represent the infecting serovar given the occurrence of paradoxical reactions and cross-reactions [70], so these data give only a broad idea of serovars present in this population of workers. Sources of exposure to Leptospira likely include contact with soil contaminated by rodent urine in the sugarcane fields and mines, in addition to non-occupational exposures, which are common in rural tropical environments [20,21].
Chronic colonization of the kidney by leptospires has been previously documented and seems to occur in a small percentage of individuals infected by Leptospira [71]. One recent study suggests that chronic colonization of kidney tubules by leptospires has a deleterious effect on kidney function [51]. Research on chronic colonization of renal tubules by leptospires and its role in the development of CKD is in its early stages, and the role of this pathophysiological mechanism in the development of MeN is still unknown. The negative urine PCR results in this study suggest the absence of chronic colonization of renal tubules by leptospires in our population, although low levels of leptospires in urine could have gone undetected. Given the apparent infrequent occurrence of chronic colonization among individuals infected by Leptospira, and the large scale of the CKD epidemic in Central America, we believe that it is important to consider alternative mechanisms of renal injury by which infection with Leptospira could lead to the development of CKD.
In acute leptospirosis urine PCR is expected to be positive since leptospires are actively shed in the urine. The eight individuals with evidence of recent or current leptospirosis had negative urine PCR results. Since individuals were tested at their respective work locations and did not show evidence of an acute illness, the precise timing of infection is uncertain. Two of the eight individuals with evidence of recent or current leptospirosis were sugarcane workers that had paired samples taken. Even though acute infection occurred within the six-month harvest season, since urine PCR is only positive for a few weeks after acute infection, it is possible that enough time had elapsed for leptospires to be undetectable in urine by the time of the late-harvest sample, explaining the negative urine PCR results.
Leptospira seropositivity was not associated with eGFR except among sugarcane applicants with elevated creatinine, who represent a group of individuals with some degree of kidney dysfunction at the time of testing. It is possible that no association between Leptospira seropositivity and eGFR was found among other job categories because the yearly pre-employment screening conducted by the company has created a selection process skewed toward healthier workers, excluding those individuals with elevated serum creatinine.
Biomarkers of kidney injury (IL-18, NGAL, and NAG) were significantly elevated among seropositive sugarcane workers at late-harvest when adjusting only for sex, age, and years worked. This association was attenuated by 15–22% when the model additionally included job category as a covariate, suggesting that job category is acting as a proxy for an unmeasured exposure that is causing the elevation in biomarkers of kidney injury. However, Leptospira infection may be acting as a kidney disease susceptibility factor, given the higher concentrations of biomarkers among seropositive workers. If leptospirosis, including the vast majority of cases that do not develop overt AKI, can cause some degree of underlying subclinical kidney injury, individuals that are seropositive to Leptospira could be more susceptible to additional kidney insults.
There are some limitations to this study. First, job category was determined based on present occupation, which could have been different at the time of infection with Leptospira if the worker held a different job category in the past. This is not a concern for recent or current leptospirosis among sugarcane cutters since infection occurred during the present harvest season. Second, a portion of sugarcane workers tested at pre-harvest were not tested at late-harvest (refer to Laws et al. 2015 for a detailed description of loss to follow-up (LTF) among sugarcane workers) [14]. If sugarcane workers with recent or current leptospirosis left their job due to symptoms and were LTF, the incidence of infection during the harvest season may have been greater than the one observed in this study. Finally, while the overall size of the study was not small, the need to conduct analyses among participants stratified into several job categories limited the precision of the results.
Future areas of research include conducting studies to confirm or disprove the association between Leptospira seropositivity and biomarkers of kidney injury seen in this study. In particular, it is important to obtain a better understanding of whether asymptomatic or mild leptospirosis can cause subclinical kidney injury that increases the risk of developing CKD if exposed to other kidney insults. Whether the timing of infection with Leptospira, particular serovar, or the occurrence of repeated infections with different serovars plays a role, is currently unknown. The possibility of an interaction between Leptospira infection and other hypothesized causes of MeN, such as heat exposure and strenuous work, requires further study.
Funding
Funding for the research conducted was the result of a mediation process convened by the Compliance Advisor/Ombudsman (CAO), the independent accountability mechanism for social and environmental issues of IFC/MIGA of the World Bank Group, between Nicaragua Sugar Estates Limited (NSEL) and Asociación de Chichigalpa por la Vida (ASOCHIVIDA). The funds were provided by the CAO and the Comité Nacional de Productores de Azúcar (CNPA), of which NSEL is a member. The CAO managed all funds and maintained the contract to conduct the research, with the agreement of both parties involved in the mediation. Additional funding was also provided through internal funds from the Boston University School of Public Health. ORR was also funded by the Enrique Najera predoctoral grant awarded by the Spanish Society of Epidemiology and the Instituto de Salud Carlos III. RLL was also funded by a National Institutes of Health training grant [grant number T32 ES014562]; a STAR Fellowship Assistance agreement No. FP-91764901-0 awarded by the U.S. Environmental Protection Agency (EPA). The EPA does not endorse any products or commercial services mentioned in this publication. The views expressed in this publication are solely those of the authors and do not necessarily represent the official position of the affiliated institutions. Donors have not reviewed or influenced the content of this paper.
Disclosure statement
Funding for additional studies has been provided by the CNPA and Los Azucareros Del Istmo Centroamericano (AICA) and was managed by the CDC Foundation. Dr. Parikh is listed as a co-inventor on a IL-18 patent granted to University of Colorado (no monetary value).
Acknowledgments
We thank the workers for their participation in this study. We also thank the Nicaraguan Ministry of Health for contributions to the field investigation and laboratory support.
References
- [1].Wesseling C, Crowe J, Hogstedt C, et al. Resolving the enigma of the Mesoamerican Nephropathy: a research workshop summary. Am J Kidney Dis. 2014;63(3):396–404. 10.1053/j.ajkd.2013.08.014 [DOI] [PubMed] [Google Scholar]
- [2].Ordunez P, Saenz C, Martinez R, et al. The epidemic of chronic kidney disease in Central America. Lancet Global Health. 2014;2(8):e440–e441. 10.1016/S2214-109X(14)70217-7 [DOI] [PubMed] [Google Scholar]
- [3].Orantes CM, Herrera R, Almaguer M, et al. Chronic kidney disease and associated risk factors in the Bajo Lempa region of El Salvador: Nefrolempa Study, 2009. MEDICC Rev. 2011;13(4):14–22. [DOI] [PubMed] [Google Scholar]
- [4].Orantes CM, Herrera R, Almaguer M, et al. 2014. Epidemiology of chronic kidney disease in adults of salvadoran agricultural communities. MEDICC Rev. 2014;16(2): 23–30. [DOI] [PubMed] [Google Scholar]
- [5].Weiner DE, McClean MD, Kaufman JS, et al. The Central American epidemic of CKD. Clin J Am Soc Nephrol. 2013;8(3):504–511. 10.2215/CJN.05050512 [DOI] [PubMed] [Google Scholar]
- [6].Correa-Rotter R, Wesseling C, Johnson RJ. CKD of unknown origin in Central America: the case for a Mesoamerican Nephropathy. Am J Kidney Dis. 2014;63(3):506–520. 10.1053/j.ajkd.2013.10.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Raines N, González M, Wyatt C, et al. Risk factors for reduced glomerular filtration rate in a Nicaraguan community affected by Mesoamerican Nephropathy. MEDICC Rev. 2014;16(2):16–22. [DOI] [PubMed] [Google Scholar]
- [8].Ramirez-Rubio O, Brooks DR, Amador JJ, et al. Chronic kidney disease in Nicaragua: a qualitative analysis of semi-structured interviews with physicians and pharmacists. BMC Public Health. 2013;13:247. 10.1186/1471-2458-13-350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ramirez-Rubio O, McClean MD, Amador JJ, et al. An epidemic of chronic kidney disease in Central America: an overview. J Epidemiology Community Health. 2013;67(1):1–3. 10.1136/jech-2012-201141 [DOI] [PubMed] [Google Scholar]
- [10].Wesseling C, Crowe J, Hogstedt C, et al. The epidemic of chronic kidney disease of unknown etiology in mesoamerica: a call for interdisciplinary research and action. Am J Public Health. 2013;103(11):1927–1930. 10.2105/AJPH.2013.301594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].O’Donnell JK, Tobey M, Weiner DE, et al. Prevalence of and risk factors for chronic kidney disease in rural Nicaragua. Nephrol Dial Transplant. 2011;26(9):2798–2805. 10.1093/ndt/gfq385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Peraza S, Wesseling C, Aragon A, et al. Decreased kidney function among agricultural workers in El Salvador. Am J Kidney Dis. 2012;59(4):531–540. 10.1053/j.ajkd.2011.11.039 [DOI] [PubMed] [Google Scholar]
- [13].Torres C, Aragón A, González M, et al. Decreased kidney function of unknown cause in Nicaragua: a community-based survey. Am J Kidney Dis. 2010;55:485–496. 10.1053/j.ajkd.2009.12.012 [DOI] [PubMed] [Google Scholar]
- [14].Laws RL, Brooks DR, Amador JJ, et al. Changes in kidney function among Nicaraguan sugarcane workers. Int J Occup Environ Health. 2015;21(3):241–250. 10.1179/2049396714Y.0000000102 Epub 2015 Jan 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wijkström J, Leiva R, Elinder CG, et al. Clinical and pathological characterization of Mesoamerican Nephropathy: a new kidney disease in Central America. Am J Kidney Dis. 2013;62(5):908–918. 10.1053/j.ajkd.2013.05.019 [DOI] [PubMed] [Google Scholar]
- [16].Wesseling C, Crowe J, Hogstedt C, et al., editors. Mesoamerican Nephropathy: report from the first international research workshop on MeN. Heredia: SALTRA/IRET-UNA; 2013:81–85. [cited 2015 Nov] Available from: http://www.saltra.una.ac.cr/index.php/sst-vol-10. [Google Scholar]
- [17].Cuadra SN, Jakobsson K, Hogstedt C, et al. Chronic kidney disease: assessment of current knowledge and feasibility for regional research collaboration in Central America. Work and Health Series, No. 2. Heredia: SALTRA, 20 IRET-UNA; 2006. [cited 2015 Nov]. Available from: http://www.saltra.una.ac.cr/index.php/sst-vol-2. [Google Scholar]
- [18].Pappas G, Papadimitriou P, Siozopoulou V, et al. The globalization of leptospirosis: worldwide incidence trends. Int J Infec Dis. 2008;12(4):351–357. 10.1016/j.ijid.2007.09.011 [DOI] [PubMed] [Google Scholar]
- [19].World Health Organization Leptospirosis Worldwide 1999. Weekly Epidemiological Record. 1999;74(29):237–244. [cited 2015 Nov] Available from: http://www.who.int/wer [Google Scholar]
- [20].Levett PN. Leptospirosis. Clin Microbiol Rev. 2001;14(2):296–326. 10.1128/CMR.14.2.296-326.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bharti AR, Nally JE, Ricaldi JN, et al. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis. 2003;3(12):757–771. 10.1016/S1473-3099(03)00830-2 [DOI] [PubMed] [Google Scholar]
- [22].Haake DA, Dundoo M, Cader R, et al. Leptospirosis, water sports, and chemoprophylaxis. Clin Infect Dis. 2002;34(9):e40–e43. 10.1086/cid.2002.34.issue-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Morgan J, Bornstein SL, Karpati AM, et al. Outbreak of leptospirosis among triathlon participants and community residents in Springfield, Illinois, 1998. Clin Infect Dis. 2002;34(12):1593–1599. 10.1086/cid.2002.34.issue-12 [DOI] [PubMed] [Google Scholar]
- [24].Sejvar J, Bancroft E, Winthrop K, et al. Leptospirosis in “Eco-Challenge” Athletes, Malaysian Borneo, 2000. Emerg Infect Dis. 2003;9(6):702–707. 10.3201/eid0906.020751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Waitkins SA. Leptospirosis as an occupational disease. Br J Ind Med. 1986;43(11):721–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Smythe L, Symonds M, Dohnt L, et al. Review of leptospirosis notifications in Queensland and Australia: January 1998–June 1999. Commun Dis Intell. 2000;24(6):153–157. [PubMed] [Google Scholar]
- [27].Bovet P, Yersin C, Merien F, et al. Factors associated with clinical leptospirosis: a population-based case-control study in the Seychelles (Indian Ocean). Int J Epidemiol. 1999;28(3):583–590. 10.1093/ije/28.3.583 [DOI] [PubMed] [Google Scholar]
- [28].Ashford DA, Kaiser RM, Spiegel RA, et al. Asymptomatic infection and risk factors for leptospirosis in Nicaragua. Am J Trop Med Hyg. 2000;63(5–6):249–254. [PubMed] [Google Scholar]
- [29].Abdulkader RC, Silva MV. The kidney in leptospirosis. Pediatr Nephrol. 2008;23(12):2111–2120. 10.1007/s00467-008-0811-4 Epub 2008 Apr 30. [DOI] [PubMed] [Google Scholar]
- [30].Katz AR, Ansdell VE, Effler PV, et al. Assessment of the clinical presentation and treatment of 353 cases of laboratory‐confirmed leptospirosis in Hawaii, 1974–1998. Clin Infect Dis. 2001;33(11):1834–1841. 10.1086/cid.2001.33.issue-11 [DOI] [PubMed] [Google Scholar]
- [31].De Francesco Daher EF, Zanetta DMT, Abdulkader RC. Pattern of renal function recovery after leptospirosis acute renal failure. Nephron Clin Pract. 2004;98(1):c8–c12. 10.1159/000079922 [DOI] [PubMed] [Google Scholar]
- [32].Coursin DB, Updike SJ, Maki DG. Massive rhabdomyolysis and multiple organ dysfunction syndrome caused by leptospirosis. Intensive Care Med. 2000;26(6):808–812. 10.1007/s001340051252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Vinetz JM, Glass GE, Flexner CE, et al. Sporadic urban leptospirosis. Ann Intern Med. 1996;125(10):794–798. 10.7326/0003-4819-125-10-199611150-00002 [DOI] [PubMed] [Google Scholar]
- [34].Heath CW Jr, Alexander AD, Galton MM. Leptospirosis in the United States. Analysis of 483 cases in man, 1949–1961. N Engl J Med. 1965;273(17):857–864, 915–922. 10.1056/NEJM196510142731606 [DOI] [PubMed] [Google Scholar]
- [35].O’Neil KM, Rickman LS, Lazarus AA. Pulmonary manifestations of leptospirosis. Rev Infect Dis. 1991;13(4):705–709. 10.1093/clinids/13.4.705 [DOI] [PubMed] [Google Scholar]
- [36].Dupont H, Dupont-Perdrizet D, Perie JL, et al. Leptospirosis: prognostic factors associated with mortality. Clin Infect Dis. 1997;25(3):720–724. 10.1086/cid.1997.25.issue-3 [DOI] [PubMed] [Google Scholar]
- [37].Marotto PC, Nascimento CM, Eluf-Neto J, et al. Acute lung injury in leptospirosis: clinical and laboratory features, outcome, and factors associated with mortality. Clin Infect Dis. 1999;29(6):1561–1563. 10.1086/313501 [DOI] [PubMed] [Google Scholar]
- [38].Takafuji ET, Kirkpatrick JW, Miller RN, et al. An efficacy trial of doxycycline chemoprophylaxis against leptospirosis. N Engl J Med. 1984;310(8):497–500. 10.1056/NEJM198402233100805 [DOI] [PubMed] [Google Scholar]
- [39].Yang CW, Wu MS, Pan MJ. Leptospirosis renal disease. Neprhol Dial Transplant. 2001;16(Suppl 5):73–77. 10.1093/ndt/16.suppl_5.73 [DOI] [PubMed] [Google Scholar]
- [40].Covic A, Goldsmith DJA, Gusbeth-Tatomir P, et al. A retrospective 5-year study in Moldova of acute renal failure due to leptospirosis: 58 cases and a review of the literature. Nephrol Dial Transplant. 2003;18(6):1128–1134. 10.1093/ndt/gfg095 [DOI] [PubMed] [Google Scholar]
- [41].Yang CW, Pan MJ, Wu MS, et al. Leptospirosis: an ignored cause of acute renal failure in Taiwan. Am J Kidney Dis. 1997;30(6):840–845. 10.1016/S0272-6386(97)90091-3 [DOI] [PubMed] [Google Scholar]
- [42].Jayakumar M, Ram Prabahar MR, Fernando EM, et al. Epidemiologic trend changes in acute renal failure – a tertiary center experience from South India. Ren Fail. 2006;28(5):405–410. 10.1080/08860220600689034 [DOI] [PubMed] [Google Scholar]
- [43].Visith S, Kearkiat P. Nephropathy in leptospirosis. J Postgrad Med. 2005;51(3):184–188. [PubMed] [Google Scholar]
- [44].Niwattayakul K, Homvijitkul J, Niwattayakul S, et al. Hypotension, renal failure, and pulmonary complications in leptospirosis. Ren Fail. 2002;24(3):297–305. 10.1081/JDI-120005363 [DOI] [PubMed] [Google Scholar]
- [45].Daher EF, Zanetta DMT, Cavalcante MB, et al. Risk factors for death and changing patterns in leptospirosis acute renal failure. Am J Trop Med Hyg. 1999;61(4):630–634. [DOI] [PubMed] [Google Scholar]
- [46].Cengiz K, Şahan C, Sünbül M, et al. Acute renal failure in leptospirosis in the black-sea reagion in Turkey. Int Urol Nephrol. 2002;33(1):133–136. 10.1023/A:1014494012062 [DOI] [PubMed] [Google Scholar]
- [47].Winearls CG, Chan L, Coghlan JD, et al. Acute renal failure due to leptospirosis: clinical features and outcome in six cases. Q J Med. 1984;53(212):487–495. [PubMed] [Google Scholar]
- [48].Herath NJ, Kularatne SA, Weerakoon KG, et al. Long term outcome of acute kidney injury due to leptospirosis? a longitudinal study in Sri Lanka. BMC Res Notes. 2014;7(1):398. 10.1186/1756-0500-7-398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Coca SG, Yusuf B, Shlipak MG, et al. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;53(6):961–973. 10.1053/j.ajkd.2008.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Newsome BB, Warnock DG, McClellan WM, et al. Long-term risk of mortality and end-stage renal disease among the elderly after small increases in serum creatinine level during hospitalization for acute myocardial infarction. Arch Int Med. 2008;168(6):609–616. 10.1001/archinte.168.6.609 [DOI] [PubMed] [Google Scholar]
- [51].Yang HY, Hung CC, Liu SH, et al. Overlooked risk for chronic kidney disease after leptospiral infection: a population based survey and epidemiological cohort evidence. PloS Negl Trop Dis. 2015;9(10):e0004105 10.1371/journal.pntd.0004105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Laws RL, Brooks DR, Amador JJ, et al. Biomarkers of kidney injury among Nicaraguan sugarcane workers. Am J Kidney Dis. 2015;pii: S0272-6386(15)01157-9. 10.1053/j.ajkd.2015.08.022 [Epub ahead of print]. [DOI] [Google Scholar]
- [53].Lelu M, Muñoz-Zanzi C, Higgins B, et al. Seroepidemiology of leptospirosis in dogs from rural and slum communities of Los Rios Region, Chile. BMC Vet Res. 2015;11:31 10.1186/s12917-015-0341-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Centers for Disease Control and Prevention (CDC) [Internet] Leptospirosis (Leptospira interrogans) 2013 Case Definition. Page last updated: May 06, 2015. [cited 2015 Nov] Available from: http://wwwn.cdc.gov/nndss/conditions/leptospirosis/case-definition/2013/
- [55].Stoddard RA. Detection of pathogenic Leptospira spp. through real-time PCR (qPCR) targeting the LipL32 gene. Methods Mol Biol. 2013;943:257–266. 10.1007/978-1-60327-353-4_17. [DOI] [PubMed] [Google Scholar]
- [56].Levey AS, Stevens LA, Schmid CH, et al. A New Equation to Estimate Glomerular Filtration Rate. Ann Intern Med. 2009;150(9):604–612. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Bolignano D, Lacquaniti A, Coppolino G, et al. Neutrophil gelatinase-associated lipocalin (NGAL) and progression of chronic kidney disease. Clin J Am Soc Nephrol. 2009;4(2):337–344. 10.2215/CJN.03530708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Bonventre JV, Vaidya VS, Schmouder R, et al. Next-generation biomarkers for detecting kidney toxicity. Nat Biotechnol. 2010;28(5):436–440. 10.1038/nbt0510-436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Charlton JR, Portilla D, Okusa MD. A basic science view of acute kidney injury biomarkers. Nephrol Dial Transplant. 2014;29(7):1301–1311. 10.1093/ndt/gft510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Devarajan P. Neutrophil gelatinase‐associated lipocalin (NGAL): a new marker of kidney disease. Scand J Clin Lab Invest Suppl. 2008;68:89–94. 10.1080/00365510802150158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Devarajan P. Biomarkers for the early detection of acute kidney injury. Curr Opin Pediatr. 2011;23(2):194–200. 10.1097/MOP.0b013e328343f4dd [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Liu Y, Guo W, Zhang J, et al. Urinary interleukin 18 for detection of acute kidney injury: a meta-analysis. Am J Kidney Dis. 2013;62(6):1058–1067. 10.1053/j.ajkd.2013.05.014 [DOI] [PubMed] [Google Scholar]
- [63].Parikh CR, Abraham E, Ancukiewicz M, et al. Urine IL-18 Is an early diagnostic marker for acute kidney injury and predicts mortality in the intensive care unit. J Am Soc Nephrol. 2005;16(10):3046–3052. 10.1681/ASN.2005030236 [DOI] [PubMed] [Google Scholar]
- [64].Smith ER, Lee D, Cai MM, et al. Urinary neutrophil gelatinase-associated lipocalin may aid prediction of renal decline in patients with non-proteinuric Stages 3 and 4 chronic kidney disease (CKD). Nephrol Dial Transplant. 2013;28(6):1569–1579. 10.1093/ndt/gfs586 [DOI] [PubMed] [Google Scholar]
- [65].Vaidya VS, Ferguson MA, Bonventre JV. Biomarkers of acute kidney injury. Annu Rev Pharmacol Toxicol. 2008;48:463–493. 10.1146/annurev.pharmtox.48.113006.094615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Obermüller N, Geiger H, Weipert C, et al. Current developments in early diagnosis of acute kidney injury. Int Urol and Nephrol. 2014;46(1):1–7. 10.1007/s11255-013-0448-5 [DOI] [PubMed] [Google Scholar]
- [67].Bedford M, Farmer C, Levin A, et al. Acute kidney injury and CKD: chicken or egg? Am J Kidney Dis. 2012;59(4):485–491. 10.1053/j.ajkd.2011.09.010 [DOI] [PubMed] [Google Scholar]
- [68].Venkatachalam MA, Griffin KA, Lan R, et al. Acute kidney injury: a springboard for progression in chronic kidney disease. Am J Physiol Renal Physiol. 2010;298(5):F1078–F1094. 10.1152/ajprenal.00017.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Yang L, Humphreys BD, Bonventre JV. Pathophysiology of acute kidney injury to chronic kidney disease: maladaptive repair. Contrib Nephrol. 2011;174:149–155. 10.1159/000329385 [DOI] [PubMed] [Google Scholar]
- [70].Levett PN. Usefulness of serologic analysis as a predictor of the infecting serovar in patients with severe leptospirosis. Clin Infect Dis. 2003;36(4):447–452. 10.1086/cid.2003.36.issue-4 [DOI] [PubMed] [Google Scholar]
- [71].Ganoza CA, Matthias MA, Saito M, et al. Asymptomatic renal colonization of humans in the peruvian Amazon by leptospira. PLoS Negl Trop Dis. 2010;4(2):e612. 10.1371/journal.pntd.0000612 [DOI] [PMC free article] [PubMed] [Google Scholar]