Abstract
Multiple risk factors for rheumatoid arthritis (RA) have been studied, but there is a dearth of research on occupational noise, which is highly prevalent in the United States (U.S.). This study aimed to determine whether occupational noise exposure was associated with an elevated risk of prevalent RA in the U.S. general population. Data from the 2011 to 2012 cross-sectional, population-based National Health and Nutrition Examination Survey were used for secondary analysis. Self-reported lifetime exposure to very loud noise was linked to self-reported doctor-diagnosed RA in a sample of 4192 participants. Weighted logistic regression was used to obtain nationally representative prevalence odds ratios (OR). The main and fully adjusted models yielded OR = 3.98 (95% CI: 1.74, 9.11) and OR = 2.84 (95% CI: 1.23, 6.57) for participants exposed for ≥ 15 years compared to never exposed participants. Excluding those diagnosed with RA more than five years before the interview, the effect dropped to OR = 3.67 (95% CI: 1.06, 12.75) in the main model, and was no longer significant in the fully adjusted model (OR = 2.68, 95% CI: 0.80, 8.96). The only significant effect modifier was race/ethnicity, with higher risk in Non-Hispanic whites. To conclude, long-term occupational noise exposure might be a modifiable risk factor for RA, but currently, the evidence base is very thin and tenuous.
Keywords: Autoimmune disease, rheumatic disease, work environment, stress, dust, fumes, air pollution
Introduction
During 2013–2015, 54.4 million (22.7%) adults in the United States (U.S.) reported doctor-diagnosed arthritis [1], and this proportion is expected to increase to 78 million (26%) by 2040 [2]. Rheumatoid arthritis (RA) is the most common type of adult autoimmune arthritis in the U.S. [3] and its incidence might actually be rising among women [4]. RA is associated with limited physical functioning [5], higher co-morbidity [6], impaired quality of life [7], and some $39.2 billion total annual societal costs [8]. In the past decades, its global burden increased from 3.3 million to 4.8 million disability-adjusted life years [9].
Multiple genetic, environmental, and lifestyle factors are involved in the pathogenesis of RA [10,11], with genetics having relatively low contribution compared with other autoimmune diseases [12]. Research has shown that smoking is the strongest and most consistent environmental/lifestyle factor [13]. There is also higher risk among people exposed to silica [13], vibration [14], and ambient air pollution [15].
Little evidence exists of the risk associated with noise exposure, though there is an underlying mechanistic hypothesis. Noise engenders a stress response in the body, increasing glucocorticoids and catecholamines, thereby modifying the immune system [16,17]. Sleep disturbance caused by noise [17] might also increase the risk of RA [18]. Furthermore, noise might promote smoking behavior [19,20] and indirectly lead to RA [13]. Given that 22 million Americans are exposed to hazardous noise above 85 dB(A) [21], its potential effect on RA should be of interest to both rheumatologists and occupational therapists. Only one study, however, has looked at this relationship and it found no significant risk (OR = 1.07; 95% CI: 0.57, 2.01) for occupational exposure > 95 dB(A)-year versus < 85 dB(A)-year [16]. A community study that focused on environmental noise exposure also failed to support the above-described hypothesis [22]. Overall, research in this field is scarce and previous studies were not carried out in the U.S., therefore their results may not be generalizable to the U.S. population. In the present study, we aimed to determine whether occupational noise exposure was associated with an elevated risk of prevalent RA in the U.S. general population.
Methods
Study population
The study population came from the 2011 to 2012 wave of the National Health and Nutrition Examination Survey (NHANES). NHANES is an ongoing series of cross-sectional surveys repeated every two years in the U.S. by the National Center for Health Statistics (NCHS). In order to ensure a nationally representative sample, NHANES employs multistage random sampling of the non-institutionalized civilian population resident in the U.S. at the time of enrollment [23,24]. In 2011–2012, 13,431 persons were selected from 30 different study locations. Of those selected, 9756 completed the interview (72.6% response rate). After weighting adjustments, the non-response bias analyses performed by the NCHS demonstrated little bias; there was similarity of the 2011–2012 NAHNES estimates with comparable estimates from previous waves of NHANES and with estimates from another population-based U.S. survey, the National Health Interview Survey (NHIS) [25]. All variables used in the present study were self-reported, except for body mass index (BMI). These data were collected during a household interview.
Ethics
NHANES was approved by the NCHS Research Ethics Review Board (Protocol #2011–17). All information collected in the survey was kept strictly confidential and privacy was protected by public laws. It followed the Helsinki Declaration and participants provided informed consent. Access to the data were granted for scientific research. No additional ethics approval was necessary for our secondary analysis.
Exposure assessment
We used the data-set of the 2011–2012 wave of NHANES because it contains information on lifetime noise exposure elicited form two questions. The first one identified individuals ever exposed to “very loud noise” (“In your work were you exposed to very loud noise? Very loud noise is noise that is so loud you have to shout in order to be understood by someone standing 3 feet away from you.”). The second question determined overall lifetime exposure (This next question is about your work in jobs where there was very loud noise for 4 or more hours a day, several days a week. Please give me the total number of months or years for all jobs where this has happened.)
From these questions, we constructed a variable with five levels: Never exposed (reference group), <5 years, 5–9 years, 10–14 years, and ≥15 years. The question on very loud noise exposure is a valid proxy for exposure to 80–85 dB(A) or more [26–30].
Outcome assessment
The outcome variable was self-reported doctor diagnosis with RA. Participants reporting other type of arthritis (e.g. osteoarthritis or degenerative arthritis, psoriatic arthritis) or who were unsure about their status (i.e. responded “other” or “don’t know”) were excluded. Song [16] used a similar definition of RA.
Other covariates
We gathered data on confounders such as age, sex, race/ethnicity, educational attainment, annual family income, smoking, alcohol drinking, BMI (derived from objectively measured weight and height), lifetime occupational exposures to mineral dust, organic dust, exhaust fumes, other fumes. The wording of the questions asking about occupational air pollutants was similar to that of the noise questions. Questions on hearing status and hearing protection use during the past year were also used.
Statistical analysis
Participants with missing data on continuous variables were dropped, whereas missing values on categorical predictor variables were coded in a separate category (“Missing data”), and included in the main analysis to make better use of the small sample. Such category was not created for the variable education level, because it had missing data in minors, who were a priori excluded from the study.
For the univariate tests, we used Pearson’s chi-square test, ANOVA, or t-test. Bonferroni correction for multiple comparisons was not applied [31,32]. The multivariate associations between lifetime noise exposure and RA were examined using logistic regression with increasing adjustments; all covariates were included in the models a priori based on previous knowledge. The crude model (Model 1) was adjusted for sex and age; the main model (Model 2) was additionally adjusted for race/ethnicity, education, annual family income, smoking, alcohol drinking, and BMI; and the fully adjusted model (Model 3) was further adjusted for lifetime exposures to mineral dust, organic dust, exhaust fumes, and other fumes. No multicollinearity was detected in the main model (tolerance > 0.2 and Variance Inflation Factor < 5).
Several sensitivity analyses were performed to check the robustness of the results. First, Models 2 and 3 were repeated after participants diagnosed with RA more than 5 years before the interview were excluded, so there was greater possibility that noise exposure preceded their diagnosis.
Next, the main analysis (Model 2) was stratified by sex, age (< 50 vs. ≥ 50 years), race/ethnicity (Non-Hispanic white vs. Other), annual family income (< $ 35,000 vs. ≥ $ 35,000), smoking status (Ever vs. Never smoker), hearing status (Excellent/good vs. Impaired), and hearing protection use (Always/Usually vs. Half of the time or less often), in order to identify potential effect modifiers. Interactions were formally tested at the p < 0.05 level. For the interaction tests, we included in the model the product of the dichotomized noise variable (i.e. > 15 years vs. Never exposed) and the dichotomized moderator (e.g. income < $ 35,000 vs. ≥ $ 35,000) and report the p-value for the Wald’s test. The main effects of the exposure and moderator variables were also kept in the model when testing the respective product term. When performing stratified analysis, the respective moderator by which the sample was stratified was removed from the model. Also, since the category that was created for some categorical variables with missing data (i.e. the “Missing data” category) had few cases and we could not use it as a separate subgroup, it was removed from the stratified analysis (i.e. those cases were dropped).
To account for the complex sampling of NHANES (i.e. multilevel nature of the data, people sampled from different geographic clusters) and to generate estimates representative of the general population, we accounted for the survey design in all analyses using strata, primary sampling units, and weigh variables (“2-year Interview weight” in all analyses not involving BMI, and “2-year Interview and MEC weight” for analyses involving BMI) [33]. Data were processed with the SPSS Complex Samples module. Results were considered statistically significant at the p < 0.05 level.
Results
Of all participants in the study, 3892 were minors and were excluded. Of the remaining 5864 participants, 1116 had been diagnosed with another type of arthritis and were also excluded. After dropping another 314 participants with missing data on RA and 242 with missing data on BMI, we were left with a sample size of 4 192 for further analysis. Participants included in the analyses were somewhat older (44.00 years vs. 37.48 years) and more likely to have never been exposed to occupational noise (73.2% vs. 33.4%). There was no significant sex- or racial/ethnic difference though.
Participants’ mean age was 44.00 years (SE = 0.84, range 20–80 years) and 50.3% were male; 4.6% reported RA. From Table 1, the prevalence of RA was significantly associated with participants’ age, sex, race/ethnicity, education, income, BMI, and occupational exposures to noise and air pollutants.
Table 1.
Sample characteristics and stratification by participants’ rheumatoid arthritis status.
| Rheumatoid arthritis | |||
|---|---|---|---|
| No (N = 3 956, 95.4%) | Yes (N = 236, 4.6%) | p-value | |
| Age (mean years, 95% CI) | 43.40 (41.57, 45.23) | 56.53 (54.42, 58.64) | < 0.001 |
| Men (n, %) | 2080 (50.8) | 93 (39.2) | 0.015 |
| Race/ethnicity (n, %) | 0.008 | ||
| Non-Hispanic White | 1329 (63.7) | 78 (66.7) | |
| Mexican American | 423 (8.7) | 22 (6.7) | |
| Other Hispanic | 402 (7.0) | 23 (5.5) | |
| Non-Hispanic Black | 1012 (11.8) | 91 (16.4) | |
| Other race | 790 (8.8) | 22 (4.8) | |
| Missing data | 0 (0.0) | 0 (0.0) | |
| Education (n, %) | 0.001 | ||
| <9th grade | 309 (4.8) | 36 (11.3) | |
| 9–11th grade | 529 (10.2) | 39 (14.1) | |
| High school graduate/GED | 820 (19.6) | 53 (25.1) | |
| Some college or AA degree | 1189 (32.0) | 80 (35.5) | |
| College graduate or above | 1108 (33.4) | 26 (14.1) | |
| Missing data | 0 (0.0) | 0 (0.0) | |
| Annual family income (n, %) | 0.043 | ||
| < $35 000 | 1715 (34.4) | 139 (49.2) | |
| $35 000–$75 000 | 1011 (28.8) | 45 (25.7) | |
| > $75 000 | 940 (31.7) | 32 (19.9) | |
| Missing data | 290 (5.1) | 20 (5.2) | |
| Smoking (n, %) | 0.099 | ||
| Never smoker | 2407 (59.6) | 117 (45.5) | |
| Former smoker | 755 (20.9) | 66 (26.7) | |
| Current smoker | 788 (19.4) | 53 (27.9) | |
| Missing data | 6 (0.1) | 0 (0.0) | |
| Alcohol drinking (n, %) | 0.111 | ||
| Lifetime abstainer | 493 (9.0) | 39 (10.4) | |
| Former drinker | 384 (7.8) | 35 (11.5) | |
| Current drinker | 2571 (72.9) | 145 (72.8) | |
| Missing data | 508 (10.4) | 17 (5.3) | |
| BMI (mean kg/m2, 95% CI) | 28.23 (27.82, 28.65) | 29.97 (28.69, 31.24) | 0.005 |
| Very loud noise exposure (n, %) | < 0.001 | ||
| Never | 2903 (74.1) | 119 (52.7) | |
| < 5 years | 381 (11.1) | 19 (7.9) | |
| 5–9 years | 104 (2.7) | 9 (2.6) | |
| 10–14 years | 76 (1.8) | 5 (2.2) | |
| ≥ 15 years | 140 (3.5) | 18 (14.4) | |
| Missing data | 352 (6.7) | 66 (20.2) | |
| Mineral dust exposure (n, %) | 0.116 | ||
| Never | 2683 (69.0) | 145 (64.0) | |
| ≤ 5 years | 453 (12.31) | 24 (9.7) | |
| 6–10 years | 185 (1.2) | 11 (3.3) | |
| 11–15 years | 116 (3.0) | 5 (2.8) | |
| > 15 years | 203 (6.0) | 17 (10.5) | |
| Missing data | 316 (5.4) | 34 (9.7) | |
| Organic dust exposure (n, %) | 0.002 | ||
| Never | 2987 (76.4) | 145 (60.7) | |
| ≤ 5 years | 338 (9.3) | 22 (12.3) | |
| 6–10 years | 113 (3.0) | 14 (6.7) | |
| 11–15 years | 65 (1.9) | 5 (1.8) | |
| > 15 years | 142 (4.1) | 17 (9.1) | |
| Missing data | 311 (5.3) | 33 (9.5) | |
| Exhaust fumes exposure (n, %) | < 0.001 | ||
| Never | 2892 (74.9) | 145 (58.8) | |
| ≤ 5 years | 346 (9.5) | 18 (9.8) | |
| 6–10 years | 157 (3.9) | 14 (4.2) | |
| 11–15 years | 84 (2.21) | 5 (3.3) | |
| > 15 years | 166 (4.2) | 21 (14.4) | |
| Missing data | 311 (5.2) | 33 (9.5) | |
| Other fumes exposure (n, %) | 0.013 | ||
| Never | 2683 (69.4) | 130 (59.5) | |
| ≤ 5 years | 481 (12.9) | 23 (10.4) | |
| 6–10 years | 195 (4.7) | 9 (3.6) | |
| 11–15 years | 91 (2.4) | 10 (3.6) | |
| > 15 years | 196 (5.3) | 31 (13.3) | |
| Missing data | 310 (5.2) | 33 (9.5) | |
Data source: CDC/NCHS – NHANES, 2011 – 2012.
Reported coefficients (except raw number of cases) are weighted to obtain nationally representative estimates. The “Interview weight” is applied to all univariate tests except for that involving body mass index (BMI), for which the “Interview and MEC Exam weight” is used.
Percentages are reported within columns.
p-values are associated with Pearson chi-square test, ANOVA, or t-test.
Table 2 reports multivariate models for RA. In the crude model, the OR was 5.22 (95% CI: 2.26, 12.06). It was strongly attenuated when adjusted for sociodemographic and lifestyle factors (OR = 3.98; 95% CI: 1.74, 9.11), and further, for occupational air pollutants (OR = 2.84; 95% CI: 1.23, 6.57).
Table 2.
Multivariate association between occupational lifetime exposure to very loud noise and prevalent rheumatoid arthritis (odds ratio with 95% confidence interval).
| Model 1a,d | Model 2b,e | Model 3c,e | ||||
|---|---|---|---|---|---|---|
| Exposure duration | OR | 95% CI | OR | 95% CI | OR | 95% CI |
| Never | 1.00 | 1.00 | 1.00 | |||
| < 5 years | 1.45 | 0.62, 3.37 | 1.13 | 0.46, 2.76 | 0.97 | 0.38, 2.49 |
| 5–9 years | 2.07 | 0.77, 5.55 | 1.46 | 0.55, 3.87 | 1.21 | 0.51, 2.88 |
| 10–14 years | 1.88 | 0.71, 4.97 | 1.30 | 0.46, 3.63 | 1.06 | 0.36, 3.11 |
| ≥ 15 years | 5.22 | 2.26, 12.07 | 3.98 | 1.74, 9.11 | 2.84 | 1.23, 6.57 |
| Missing data | 0.82 | 0.38, 1.75 | 0.70 | 0.37, 1.33 | 0.73 | 0.40, 1.36 |
Data source: CDC/NCHS – NHANES, 2011 – 2012.
Models are based on weighted logistic regression to obtain nationally representative estimates. The unweighted sample size for these models is N = 4 192. Bold coefficients are significant at p < 0.05.
Model 1: adjusted for age and sex.
Model 2: Model 1 + additional adjustment for race/ethnicity, education, annual family income, smoking, alcohol drinking, and body mass index.
Model 3: Model 2 + additional adjustment for mineral dust, organic dust, exhaust fumes, and other fumes at the workplace.
Model 1: The “Interview weight” is applied.
Model 2 and Model 3: The “Interview and MEC Exam weight” is applied.
Excluding participants diagnosed with RA more than five years before the interview, the OR in the highest exposure category (i.e. ≥ 15 years) dropped to 3.67 (95% CI: 1.06, 12.75) in the main model (n = 4 036), and was no longer significant in the fully adjusted model (OR = 2.68, 95% CI: 0.80, 8.96).
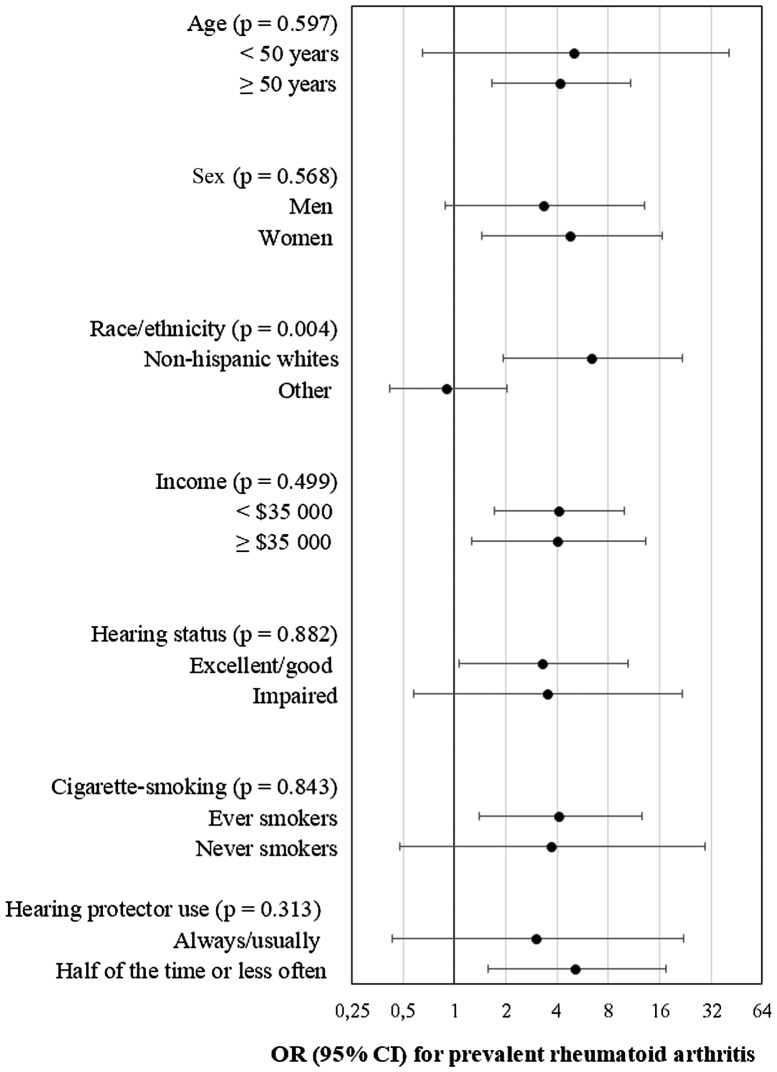
Figure 1 shows results of stratified analysis exploring potential effect modifiers. The only significant interaction was for race/ethnicity. That is, noise exposure for 15 years or more was associated with stronger effect in Non-Hispanic whites (OR = 6.43, 95% CI: 1.92, 21.54) compared to other races/ethnicities (OR = 0.92, 95% CI: 0.42, 2.02). Also of note, the effect was statistically significant in participants over 50 years of age, in women, in those with excellent/good hearing, in ever smokers, and in those who did not regularly use hearing protection while exposed to very loud noise at work.
Figure 1.
Multivariate association between occupational lifetime exposure to very loud noise (≥ 15 years vs Never) and prevalent rheumatoid arthritis (odds ratio with 95% confidence interval) – Stratified analysis. Data source: CDC/NCHS – NHANES, 2011–2012. Models are based on weighted logistic regression to obtain nationally representative estimates. Models are adjusted for age, sex, race/ethnicity, education, annual family income, smoking, alcohol drinking, and body mass index. The “Interview and MEC Exam weight” is applied.
Discussion
Key findings
This study examined the association between lifetime exposure to very loud occupational noise and prevalent RA in the U.S. general population. The overall findings indicated elevated risk in workers exposed for more than 15 years. However, this evidence is tentative because the effect was appreciably attenuated when we excluded people diagnosed with RA more than five years before the interview to increase the chance for noise exposure preceding the diagnosis. On the one hand, that finding could be due to biased recall of noise exposure by RA patients (see the Strengths and limitations section); on the other hand, it may be attributed to the lower number of RA cases included in that sensitivity analysis compared to the main analysis (< 2 vs. 4.6%).
The only significant effect modifier in the main model was race/ethnicity, with higher risk in Non-Hispanic whites. It should be noted that, although in some of the other subgroups the effect estimates were statistically significant, their confidence intervals were wide, therefore, we consider them unstable.
Our findings contrast with the only other study on occupational noise and RA, which found no effect (OR = 1.07, 95% CI: 0.57–2.01) for exposure to over 95 dB(A)-year [16]. Several methodological differences may contribute to the contrasting findings. First, our sample was larger and we had more cases with RA than Song, who only had 91 cases and 455 controls [16]. Second, Song used a job-exposure matrix to derive average noise levels based on occupational taxonomy, white our measure was self-reported at the individual level. Song acknowledged that the potential for exposure misclassification could have biased the results towards the null [16]. Finally, Song’s noise variable represented cumulative noise exposure [16], whereas ours represented duration of noise exposure. A previous case-control study on traffic noise and RA also found no effect [22].
Our results lend tentative support to the hypothesis that occupational noise exposure to over 85 dB(A) might be a risk factor for RA. One experimental study in rodents indicated a significant association between noise exposure (90 dB(A)) and prevalence and severity of collagen arthritis [34]. However, the mechanistic hypothesis in humans is still a conjecture. Noise could lead to RA if it suppresses the hypothalamic–pituitary–adrenal axis but that “association could be quite complex” [16]. In addition to the neuro-immunological effect of noise, we suggest that indirect mechanisms could also be important. For instance, noise exposure might promote smoking [19,20] and inhibit physical activity [35]; simultaneously, both smoking [13] and physical inactivity [36] might increase the risk of RA. Going further, while residential noise has been linked to sleep disturbance, occupational noise might also impinge on sleep architecture. Short- and long-term exposure to over 75 dB(A) significantly decrease rapid eye movement (REM) sleep, slow wave sleep, REM onset latency, and total sleep duration [37,38], thereby possibly leading to autoimmune diseases such as RA [18]. Alternatively, noise stress might not cause RA, but rather provoke its manifestation, thus leading to its diagnosis [39]. To support this explanation, it should be noted that elevated anti-cyclic citrullinated peptide antibodies (found in seropositive RA patients) can go unnoticed for 5–10 years prior to clinical diagnosis of RA [40–42].
A spurious relationship cannot be ruled-out either. That is, occupational noise could simply be highly correlated with some unmodelled occupational factors, aside from dust and fumes.
Strengths and limitations
This was the second study on occupational noise and RA. Its population-based sample of around 4000 participants and the comprehensive adjustment are clear advantages over the study of Song [16]. However, several limitations should be acknowledged.
This was not a case-control (i.e. analytic) study like Song’s [16], rather a cross-sectional one, making it difficult to claim causal relationships between the variables. To address the issue of what comes first (the exposure or the outcome), we used information on lifetime noise exposure and also repeated the analysis in a subgroup of people diagnosed within the preceding five years, which appreciably attenuated the effect.
Both the exposure and the outcome variables were self-reported. There is a possibility that any association observed could be due to biased recall, with current cases being more likely to report past exposure than non-cases. Furthermore, noise perception may be affected by psychological disorders such as depression and anxiety [43], and persons with autoimmune diseases may have higher rates of mental ill-health [44]. Reassuringly, the validity of self-reported very loud noise exposure has been demonstrated repeatedly [26–30]. Conversely, the validity of self-reported RA is uncertain and could have biased the results, despite the fact that other types of arthritis were excluded [45–47].
Although the sample itself was not that small, the low number of RA cases across exposure categories could explain the wide confidence intervals of the risk estimates. Also, non-response bias diagnostics suggested few reasons to suspect selection bias affecting the results. That could have inflated the association between noise and RA because participants included in the analysis were somewhat older and more likely to never have been exposed to occupational noise, which could have made the contrast between exposed and unexposed cases sharper. Going further, instead of imputing missing values, which was hampered by analytic limitations and the need to incorporate the complex survey design into the imputation algorithm, we created a separate category for missing data on categorical predictor variables and included those cases in the main analysis. In spite of that, there is still a possibility of selection bias due to missing data that was not eliminated by the addition of categories for missing responses.
We did not have data on potential effect modifiers such as noise sensitivity, heredity, psychological stress, (night) shift-work, and other co-exposures at the workplace. We also lacked data on participants’ residential addresses (because all geographic identifiers had been removed before making the NHANES data publically available), therefore, we could not account for the fact that persons living in suburban areas might have been more likely to work jobs with increased exposure to occupational noise and be exposed to residential factors that increased their risk of RA. Owing to the crude variables available and the possibility for recall bias when participants were asked about smoking and sleep quality, we could not shed light on whether those factors acted as mediators.
Future studies on the subject should ensure sufficient number of cases and controls, objectively measured noise and RA, and long-term follow-up of participants.
Conclusion
Long-term occupational noise exposure might be a modifiable risk factor for RA, as suggested in our cross-sectional study of NHANES data, but currently, the evidence base is very thin and tenuous. Future research in this field is warranted.
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgments
Authors are grateful to the National Center for Health Statistics of the United States for making the National Health and Nutrition Examination Survey data available. However, they retain sole responsibility for the analyses, interpretations and conclusions based on these data, which do not necessarily represent the views of the Centers for Disease Control and Prevention and the National Center for Health Statistics.
References
- [1].Barbour KE, Helmick CG, Boring M, et al. Vital signs: prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation – United States, 2013–2015. MMWR Morb Mortal Wkly Rep. 2017;66(9):246–253. 10.15585/mmwr.mm6609e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hootman JM, Helmick CG, Barbour KE, et al. Updated projected prevalence of self-reported doctor-diagnosed arthritis and arthritis-attributable activity limitation among US adults, 2015–2040. Arthritis Rheumatol. 2016;68(7):1582–1587. 10.1002/art.v68.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Helmick CG, Felson DT, Lawrence RC. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Arthritis Rheum. 2008;58(1):15–25. 10.1002/(ISSN)1529-0131 [DOI] [PubMed] [Google Scholar]
- [4].Myasoedova E, Crowson CS, Kremers HM, et al. Is the incidence of rheumatoid arthritis rising?: Results from Olmsted County, Minnesota, 1955–2007. Arthritis Rheum. 2010;62(6):1576–1582. 10.1002/art.27425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pincus T, Callahan LF, Sale WG, et al. Severe functional declines, work disability, and increased mortality in seventy-five rheumatoid arthritis patients studied over nine years. Arthritis Rheum. 1984;27:864–872. 10.1002/(ISSN)1529-0131 [DOI] [PubMed] [Google Scholar]
- [6].Symmons DP, Gabriel SE. Epidemiology of CVD in rheumatic disease, with a focus on RA and SLE. Nat Rev Rheumatol. 2011;7(7):399–408. 10.1038/nrrheum.2011.75 [DOI] [PubMed] [Google Scholar]
- [7].Chorus AM, Miedema HS, Boonen A, et al. Quality of life and work in patients with rheumatoid arthritis and ankylosing spondylitis of working age. Ann Rheum Dis. 2003;62(12):1178–1184. 10.1136/ard.2002.004861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Birnbaum H, Pike C, Kaufman R, et al. Societal cost of rheumatoid arthritis patients in the US. Curr Med Res Opin. 2010;26(1):77–90. 10.1185/03007990903422307 [DOI] [PubMed] [Google Scholar]
- [9].Cross M, Smith E, Hoy D, et al. The global burden of rheumatoid arthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(7):1316–1322. 10.1136/annrheumdis-2013-204627 [DOI] [PubMed] [Google Scholar]
- [10].Tobón GJ, Youinou P, Saraux A. The environment, geo-epidemiology, and autoimmune disease: rheumatoid arthritis. J Autoimmun. 2010;35(1):10–14. 10.1016/j.jaut.2009.12.009 [DOI] [PubMed] [Google Scholar]
- [11].Hoovestol RA, Mikuls TR. Environmental exposures and rheumatoid arthritis risk. Curr Rheumatol Rep. 2011;13(5):431–439. 10.1007/s11926-011-0203-9 [DOI] [PubMed] [Google Scholar]
- [12].Cooper GS, Miller FW, Pandey JP. The role of genetic factors in autoimmune disease: implications for environmental research. Environ Health Perspect. 1999;107(5):693–700. 10.1289/ehp.99107s5693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Farhat SC, Silva CA, Orione MA, et al. Air pollution in autoimmune rheumatic diseases: a review. Autoimmun Rev. 2011;11(1):14–21. 10.1016/j.autrev.2011.06.008 [DOI] [PubMed] [Google Scholar]
- [14].Olsson AR, Skogh T, Axelson O, et al. Occupations and exposures in the work environment as determinants for rheumatoid arthritis. Occup Environ Med. 2004;61(3):233–238. 10.1136/oem.2003.007971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Dzhambov AM, Dimitrova DD, Turnovska TH. Long-term residential ambient air pollution and rheumatoid arthritis: a systematic review. Health Scope. Forthcoming. DOI: 10.17795/jhealthscope-33053. [DOI] [Google Scholar]
- [16].Song C. Occupational noise exposure and the risk of diabetes, rheumatoid arthritis, and cardiovascular disease [master’s thesis]. Vancouver: The University of British Columbia; 2013. Available from: http://www.rdc-cdr.ca/occupational-noise-exposure-and-risk-diabetes-rheumatoid-arthritis-and-cardiovascular-disease. [Google Scholar]
- [17].Recio A, Linares C, Banegas JR, et al. Road traffic noise effects on cardiovascular, respiratory, and metabolic health: an integrative model of biological mechanisms. Environ Res. 2016;146:359–370. 10.1016/j.envres.2015.12.036 [DOI] [PubMed] [Google Scholar]
- [18].Hsiao YH, Chen YT, Tseng CM, et al. Sleep disorders and increased risk of autoimmune diseases in individuals without sleep apnea. Sleep. 2015;38(4):581–586. 10.5665/sleep.4574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cherek DR. Effects of acute exposure to increased levels of background industrial noise on cigarette smoking behavior. Int Arch Occup Environ Health. 1985;56:23–30. 10.1007/BF00380697 [DOI] [PubMed] [Google Scholar]
- [20].Kim YJ. Impact of work environments and occupational hazards on smoking intensity in Korean workers. Workplace Health Saf. 2016;64:103–113. 10.1177/2165079915616397 [DOI] [PubMed] [Google Scholar]
- [21].Tak S, Davis RR, Calvert GM. Exposure to hazardous workplace noise and use of hearing protection devices among US workers – NHANES, 1999–2004. Am J Ind Med. 2009;52:358–371. 10.1002/ajim.v52:5 [DOI] [PubMed] [Google Scholar]
- [22].De Roos AJ, Koehoorn M, Tamburic L, et al. Proximity to traffic, ambient air pollution, and community noise in relation to incident rheumatoid arthritis. Environ Health Perspect. 2014;122(10):1075–1080. 10.1289/ehp.1307413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Centers for Disease Control and Prevention (CDC) , National Center for Health Statistics (NCHS). National health and nutrition examination survey data. Hyattsville (MD): Department of Health and Human Services, Centers for Disease Control and Prevention. 2011–2012. [[cited cited 2016 July 9]]. Available from: http://www.cdc.gov/nchs/nhanes.htm [Google Scholar]
- [24].Centers for Disease Control and Prevention (CDC) , National center for health statistics (NCHS). About the national health and nutrition examination survey. 2006. [[cited cited 2016 July 9]]. Available from: http://www.cdc.gov/nchs/nhanes/about_nhanes.htm
- [25].National center for health statistics, centers for disease control and prevention National health and nutrition examination survey: analytic guidelines. 2011–2012. [[cited cited 2017 Dec 23]]. Available from: https://www.cdc.gov/nchs/data/nhanes/analytic_guidelines_11_12.pdf
- [26].Ahmed HO, Dennis JH, Ballal SG. The accuracy of self-reported high noise exposure level and hearing loss in a working population in Eastern Saudi Arabia. Int J Hyg Environ Health. 2004;207:227–234. 10.1078/1438-4639-00291 [DOI] [PubMed] [Google Scholar]
- [27].Neitzel RL, Daniell WE, Sheppard L, et al. Evaluation and comparison of three exposure assessment techniques. J Occup Environ Hyg. 2011;8:310–323. 10.1080/15459624.2011.568832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Neitzel RL, Svensson EB, Sayler SK, et al. A comparison of occupational and nonoccupational noise exposures in Sweden. Noise Health. 2014;16:270–278. 10.4103/1463-1741.140503 [DOI] [PubMed] [Google Scholar]
- [29].Neitzel RL, Andersson M, Andersson E. Comparison of multiple measures of noise exposure in paper mills. Ann Occup Hyg. 2016;60:581–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Schlaefer K, Schlehofer B, Schuz J. Validity of self-reported occupational noise exposure. Eur J Epidemiol. 2009;24:469e75. [DOI] [PubMed] [Google Scholar]
- [31].Perneger TV. What’s wrong with bonferroni adjustments. BMJ. 1998;316(7139):1236–1238. 10.1136/bmj.316.7139.1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. 10.1097/00001648-199001000-00010 [DOI] [PubMed] [Google Scholar]
- [33].National Center for Health Statistics, Centers for Disease Control and Prevention The National Health and Nutrition Examination Survey (NHANES) Analytic and Reporting Guidelines; [[cited cited 2016 July 9]]. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/nhanes_analytic_guidelines_dec_2005.pdf
- [34].Rogers MP, Trentham DE, Dynesius-Trentham R, et al. Exacerbation of collagen arthritis by noise stress. J Rheumatol. 1983;10:651–654. [PubMed] [Google Scholar]
- [35].Roswall N, Ammitzbøll G, Christensen JS, et al. Residential exposure to traffic noise and leisure-time sports – a population-based study. Int J Hyg Environ Health. 2017;220(6):1006–1013. 10.1016/j.ijheh.2017.05.010 [DOI] [PubMed] [Google Scholar]
- [36].Di Giuseppe D, Bottai M, Askling J, et al. Physical activity and risk of rheumatoid arthritis in women: a population-based prospective study. Arthritis Res Ther. 2015;17:40. 10.1186/s13075-015-0560-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Gitanjali B, Ananth R. Effect of acute exposure to loud occupational noise during daytime on the nocturnal sleep architecture, heart rate, and cortisol secretion in healthy volunteers. J Occup Health. 2003;45(3):146–152. 10.1539/joh.45.146 [DOI] [PubMed] [Google Scholar]
- [38].Gitanjali B, Dhamodharan R. Effect of occupational noise on the nocturnal sleep architecture of healthy subjects. Indian J Physiol Pharmacol. 2004;48(1):65–72. [PubMed] [Google Scholar]
- [39].Herrmann M, Schölmerich J, Straub RH. Stress and rheumatic diseases. Rheum Dis Clin North Am. 2000;26(4):737–763, viii 10.1016/S0889-857X(05)70167-8 [DOI] [PubMed] [Google Scholar]
- [40].Rantapää-Dahlqvist S, de Jong BA, Berglin E, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003;48(10):2741–2749. 10.1002/art.11223 [DOI] [PubMed] [Google Scholar]
- [41].Nielen MM, van Schaardenburg D, Reesink HW, et al. Simultaneous development of acute phase response and autoantibodies in preclinical rheumatoid arthritis. Ann Rheum Dis. 2006;65(4):535–537. 10.1136/ard.2005.040659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Chibnik LB, Mandl LA, Costenbader KH, et al. Comparison of threshold cutpoints and continuous measures of anti-cyclic citrullinated peptide antibodies in predicting future rheumatoid arthritis. J Rheumatol. 2009;36(4):706–711. 10.3899/jrheum.080895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].van Kamp I, van Kempen E, Baliatsas C, et al. , editors. Mental health as context rather than health outcome of noise: competing hyptheses regarding the role of sensitivity, perceived soundscapes and restoration. INTER-NOISE Conference Proceedings; Innsbruck; Institute of Noise Control Engineering; 2013. [Google Scholar]
- [44].Dickens C, McGowan L, Clark-Carter D, et al. Depression in rheumatoid arthritis: a systematic review of the literature with meta-analysis. Psychosom Med. 2002;64(1):52–60. 10.1097/00006842-200201000-00008 [DOI] [PubMed] [Google Scholar]
- [45].Star VL, Scott JC, Sherwin R, et al. Validity of self-reported rheumatoid arthritis in elderly women. J Rheumatol. 1996;23(11):1862–1865. [PubMed] [Google Scholar]
- [46].Kvien TK, Glennås A, Knudsrød OG, et al. The validity of self-reported diagnosis of rheumatoid arthritis: results from a population survey followed by clinical examinations. J Rheumatol. 1996;23(11):1866–1871. [PubMed] [Google Scholar]
- [47].Formica MK, McAlindon TE, Lash TL, et al. Validity of self-reported rheumatoid arthritis in a large cohort: results from the black women’s health study. Arthritis Care Res (Hoboken). 2010;62(2):235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]