Abstract
Fungal contamination of indoor air is an issue of increasing public health concern. Essential oils have been demonstrated to have antifungal capabilities, but there are limited studies investigating the efficacy of essential oils against fungi relevant to air quality. This study provides a preliminary screening of the antifungal properties of clove, lavender and eucalyptus essential oils against a range of fungal species isolated from environmental air samples. The ability of the essential oils to inhibit fungal growth was examined using the disk diffusion assay on malt extract agar and was compared with vinegar, bleach and limonene, with phenol as a positive control. Results identified essential oils which demonstrated antifungal potential against species of environmental origin. Clove oil was found to be most efficacious, with eucalyptus and lavender oils showing some antifungal potential albeit less broad spectrum and with less persistence over time in this assay. All essentials oils performed better than traditional cleaning compounds such as vinegar. Clove oil would be a suitable candidate for further research to validate its use in improving indoor air quality. Further research should next take into consideration the practical application method, concentration and long-term persistence of antifungal properties.
Keywords: Plant extract, fungicide, environmental fungi, indoor air, air quality
Introduction
Indoor air quality is an issue of increasing public health significance [1]. Specifically, fungal contamination of indoor environments is responsible for a wide range of adverse health effects [2]. These include allergic responses (most common), toxic effects and infectious disease (less common) [3,4]. In addition, fungal contamination in indoor environments has also been linked to sick building syndrome [5]. Sick building syndrome is a term used to group a variety of non-specific adverse health issues associated with spending time in a particular building [6]. Symptoms can include various effects on the respiratory system (e.g. wheezing, coughing), dermal responses (e.g. dry skin, irritations), nervous system (e.g. headaches) and immunology (e.g. allergies) [5–7]. For these symptoms to be recognised as being associated with sick building syndrome, symptoms should improve when leaving the building under question [5]. There are many factors that can cause sick building syndrome including volatile organic compounds and toxins (e.g. mycotoxins), biological contaminants and general chemicals (e.g. formaldehyde) [4,8–11].
At present, there is inadequate quantitative knowledge correlating microbial concentrations in air, moisture levels within indoor environments and health effects [4,7]. Thus, a quantitative guideline for air quality focussing on fungi and micro-organisms in indoor environments is still being researched by WHO [7]. In the absence of appropriate guidelines, those assessing indoor air quality for bio-aerosols typically adopt a risk assessment process based on typical background concentrations and diversity for the particular geographic location and season [12]. In terms of remediating suspected contamination of indoor air, industry-adopted guidelines [13,14] recommend the use of two cleaning methods to remove mould from indoor contaminated surfaces; air-based methods (e.g. dislodging contaminants by vacuuming off with a HEPA filter), and liquid-based antifungal agents (e.g. mechanical cleaning action with a solution of vinegar, detergent or alcohol and water) [13].
Remediation of indoor environments with fungal contamination is essential for protecting human health [14]. This process should involve removal of material with visible mould contamination and treating surfaces which an antifungal treatment which should kill or inhibit the growth of fungi and/or fungal spores [13,15]. However, there is increasing concern regarding the use of synthetic chemicals in the home and as such there is increasing interest from consumers for perceived “natural” alternatives [4,14,16,17].
Essential oils are complex aromatic chemical products extracted from a diverse range of plants [18–20]. They are perceived by the general population to be “natural” and therefore more accepted for routine use in the home. Originally, essential oils or the source plants were used in traditional medicine for their antimicrobial properties [18–20]. However, essential oils are not harmless, with reports of toxicity by ingestion [21,22] and topical application [23,24].
Due to the complexity and variability of the compounds found in essential oils and between brands, being able to classify essential oils by their antimicrobial properties has been found to be difficult [25]. Currently, the knowledge surrounding many essential oils is limited or inconsistent, with little understanding about the mechanism of action or range of antifungal activity the oils have, particularly for non-clinical species [18,26,27].
Essential oils have been identified as potential antifungal treatments, although there are limited studies investigating the use of essential oils against fungi relevant to indoor air quality [27]. The aim of this preliminary study was to identify essential oils that demonstrate broad spectrum antifungal potential against species of environmental origin, and as such would be suitable candidates for further research investigating their potential to control indoor air quality.
Experimental procedures
Antifungal agent selection
Clove (Gold Cross, Victoria, Australia), lavender (Bosisto’s, Victoria, Australia), and eucalyptus (Perfect Potion, Queensland, Australia) oils were selected as test agents for potential antifungal properties against common fungal species isolated from indoor and outdoor air.
Environmental fungi sampling and identification
Environmental air samples were collected and isolated on malt extract agar (MEA) plates (Oxoid, SA, Australia), as described previously in Rogawansamy et al. [14]. Samples were collected from inside an office building with low humidity at Bedford Park, South Australia and from inside a single story office building with low humidity at Thebarton, South Australia, with outdoor samples collected 20 m adjacent to the building in a mixed residential and light commercial zone. Briefly, active air sampling was performed using a BioStage® single-stage viable cascade impactor, attached to a SKC QuickTakeTM 30 Air sampler [14], with a flowrate of 28.3 L/min. Settle plates (passive samples) were allowed to contaminate the plates over a 60 min period. The plates were wrapped in parafilm® and incubated for 5–7 days at 25 °C. A total of five individual isolates were yielded from the sampling of indoor and outdoor air environments. To isolate pure fungal cultures, a plug of a single colony was cut from the MEA plate and transferred onto the centre of a fresh MEA plate. Each single colony was subcultured in triplicate before being wrapped in parafilm® and incubated at 25 °C for 48 h.
For sequencing of fungal isolates, DNA was extracted using the PowerSoil® DNA extraction kit as per the manufacturer’s protocol (QIAGEN, Hilden, Germany). qPCR was performed using the Rotor-Gene® SYBR® Green PCR Kit (Qiagen, Victoria, Australia) and previously described primers [28] targeting the internal transcribed spacer regions which has been shown to be the best region for fungal barcoding [29]. The 25 μL reactions contained 12.5 μL of Rotor-Gene SYBR Green PCR Master Mix, 5 μL RNase-Free Water, 1 μM ITS5 (5′ -TCCTCCGCTTATTGATATGC-3′) and ITS4 (5′-GGAAGTAAAAGTCGTAACAAGG-3′ ) and 2.5 μL of template DNA. The cycling conditions included an initial hold at 95 °C for 5 min, followed by 40 cycles consisting of 95 °C for 5 s and 60 °C for 10 s. All PCR reactions were carried in a RotorGene 3000 (Corbett Research, Sydney, Australia) with data acquisition at 60 °C on the Green channel (excitation at 470 nm, detection at 510 nm) at a gain of 9.67. PCR products were purified and sequenced by the Australian Genome Research Facility Ltd (Adelaide, Australia). Sequences were analysed using the BLAST tool (NCBI) for identification to genus level.
The five fungal species identified were; Aspergillus sp., Ulocladium sp., Coprinellus sp., and two isolates of Penicillium sp. These represent the most common environmental saprophytes encountered in indoor and outdoor air [12,14].
Antifungal efficacy using disc diffusion assay
A disc diffusion assay was used to identify antifungal properties of the essential oils [30]. A spore suspension was prepared by flooding the isolated fungal culture MEA plates with 4 mL sterilised distilled water and using a sterilised spreader to agitate the colonies into releasing spores. One hundred microlitres of spore suspension was transferred and spread onto a fresh MEA plate before being left to dry. The inoculated plates were divided into quadrants. 20 μL of the test agent was pipetted onto a sterile filter paper disc approximately 11 mm in diameter and placed at the centre of the MEA plate where the quadrants met.
Phenol (88% solution) was used as a positive control for fungal growth with sterile distilled water being used as a negative control. Three additional test agents were used for comparison against the essential oils; vinegar (4% acetic acid) (Cornwell’s, NSW, Australia), bleach (3.3% (w/v) chlorine as sodium hypochlorite) (Foodland, SA, Australia), and limonene (97% purity) (Sigma-Aldrich, NSW, Australia). Six replicate plates were used for each test agent, against each isolated species. Each plate was sealed with parafilm® and incubated for 7 days at 25 °C.
To measure the zone of inhibition (defined as the concentric region devoid of growth around the paper disc saturated with the antifungal agent), the zone was measured along each quadrant from the edge of the filter paper with the mean of the quadrants being calculated. Measurements and observations were made at day 2–3, 4–5 and day 7. Time course observations were conducted to observe differences in growth rate and effectivity of each treatment over time. This method was repeated using each of the five species.
Results are expressed as mean zone of inhibition diameter in millimetres, with the maximum achievable being 35.5 mm as a function of the disc diameter.
One-way ANOVA was used to determine significant difference between zones of inhibition over time of each fungal species and treatment. Significance for all tests was set at a p value of ≤ 0.05 with statistical analyses being performed using Microsoft Excel software.
Results
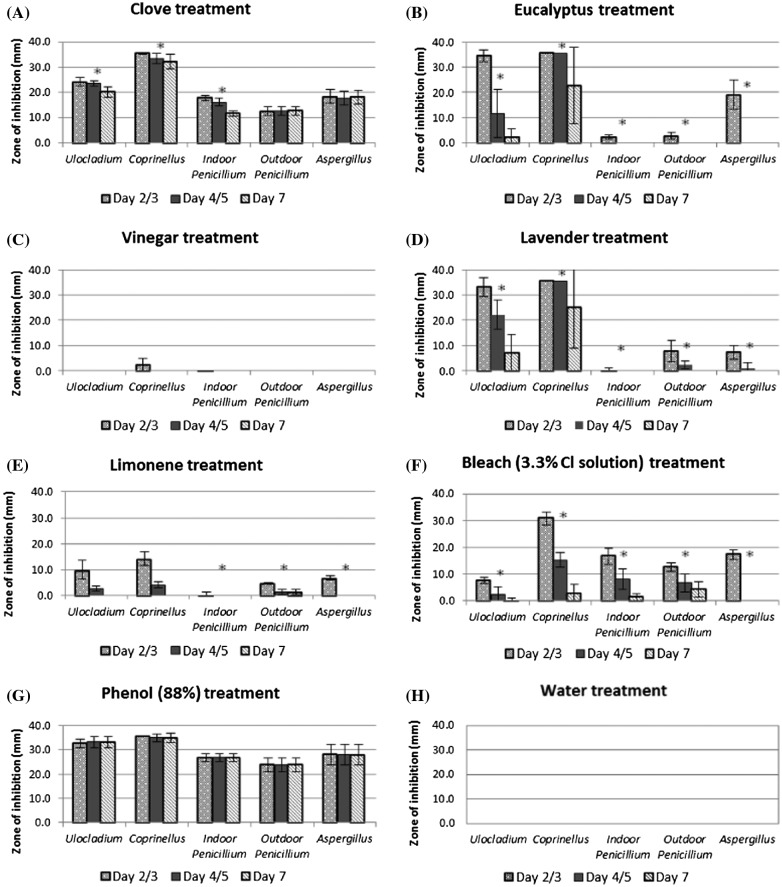
The ability of each essential oil to inhibit the growth of each fungal species is presented in Figure 1 (A–H). Clove oil was observed to be the essential oil with the greatest broad-spectrum inhibitory effect for growth across all fungal species (Figure 1(A)). After seven days of contact, Coprinellus sp. (mean inhibition diameter 32.2 ± 2.8 mm) and Ulocladium sp. (mean inhibition diameter 20.2 ± 2.0 mm) were found to have the highest sensitivity to the clove treatment. Aspergillus sp. growth was also inhibited by clove oil (mean inhibition diameter 18.2 ± 2.6 mm). The outdoor Penicillium sp. (mean inhibition diameter 12.8 ± 1.7 mm) and indoor Penicillium sp. (mean inhibition diameter 11.7 ± 1.1 mm) showed the lowest growth inhibition zones from clove treatment.
Figure 1.
Average growth inhibition zones (mm) for each fungal species by treatment agent. Measurements were taken at day 2/3, 4/5 and day 7 with standard deviation of measurements shown. *Variation of time significantly different p < 0.05.
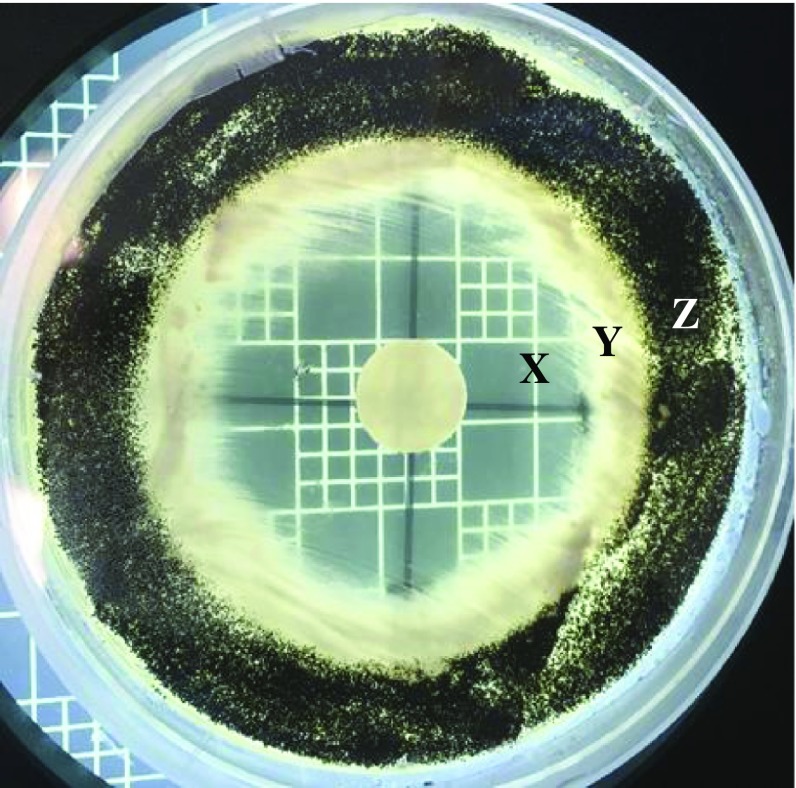
Eucalyptus oil was observed to have some antifungal efficacy against two species (Figure 1(B)), demonstrating growth inhibition. Ulocladium sp. was found to have growth inhibited by eucalyptus oil until day four (mean inhibition diameter 11.7 ± 9.6 mm) with lower inhibition of growth at day seven (mean inhibition diameter 2.2 ± 3.4 mm). Results for Coprinellus sp. also showed no growth on eucalyptus oil treated plates until day seven (mean inhibition diameter 22.8 ± 15.1 mm). While both Penicillium sp. were resistant to eucalyptus oil (mean inhibition diameter 2.3 ± 0.9 mm and 2.8 ± 1.2 mm at day two), Aspergillus sp. showed initial growth inhibition from eucalyptus oil treatment but only up to day two (mean inhibition diameter 19.0 ± 5.8 mm). An interesting observation was noted on eucalyptus oil treated groups relating to sporulation, with sporulation appearing partially inhibited in all species up to day four or five (see Figure 2). Sporulation inhibition is described as the absence or reduction of spores found on fungal growth. Sporulation inhibition differs from growth inhibition as only the formation of spores are inhibited rather than the overall growth of the fungi.
Figure 2.
The disk diffusion plate for Aspergillus treated with clove oil, showing the zone of clearing (X), inhibition of sporulation (Y) and normal growth (Z).
Results for lavender oil (Figure 1(D)) were comparable to eucalyptus oil. Lavender oil was found to inhibit the growth of two species, Coprinellus and Ulocladium. Coprinellus sp. growth inhibition was noted until day seven (mean inhibition diameter 25.1 ± 16.3 mm), whereas Ulocladium sp. growth was inhibited on day two but time reduced the effectivity of the treatment by day four and seven. The effect of time on growth inhibition of Ulocladium sp. between day two and day seven was significantly different (p = 6 × 10−6) with nearly no fungal growth at day two (mean inhibition diameter 33.1 ± 3.7 mm) and by day seven showing little growth inhibition from the treatment (mean diameter 7.0 ± 7.6 mm). Lavender oil treated fungal species were also found to have similar properties for inhibiting sporulation as eucalyptus, however with reduced effectivity.
Common bleach (Figure 1(F)) was found to have potent antifungal abilities for the species Coprinellus, Aspergillus and both indoor and outdoor Penicillium strains on day two to three post-treatment before the contact time effect significantly (p < 0.05) reduced its effectivity. Ulocladium sp. growth was reduced by bleach on day two (mean inhibition diameter 7.7 ± 1.0 mm) before contact time reduced the effectivity of the treatment (p = 1 × 10−5).
In contrast with the essential oils and bleach, both vinegar (Figure 1(C)) and limonene (Figure 1(E)) were found to have limited or no antifungal activity against the growth of fungal species tested.
Eucalyptus, lavender, bleach and limonene all showed significant differences (p < 0.05) in growth inhibition over time for the five fungal species, with eucalyptus oil (p = 0.034 to 4 × 10−8), limonene (p = 0.048 to 2 × 10−13) and bleach (p = 3 × 10−4 to 6 × 10−16) having greater variation over time compared with lavender oil (p = 0.122 to 6 × 10−6).
Discussion
This exploratory study of the antifungal activity of three essential oils against five common environmental air fungi identified three promising candidates worthy of further investigation. These were clove, eucalyptus and lavender oils. Each of the three essential oils exhibited antifungal potential with clove oil exhibiting broad spectrum antifungal properties over all five environmental species.
The antifungal efficacy of clove oil observed in this study is supported by previous studies (see also the review by Whiley et al. [27]). Levinskaitė and Paškevičius [31] used the disk diffusion assay method to demonstrate that clove oil had antifungal efficacy greater than other essential oils tested and comparable efficacy to a commercially available disinfectant (Biosheen). However, this study did not compare the efficacy to other commonly used commercial treatments such as vinegar or bleach. Another study, which was more translatable to a practical application, demonstrated that particle board dipped in 0.63% clove oil was able to prevent Aspergillus spp. and Trichothecium spp. growth for up to five weeks [32]. This supports the need for future work focusing on the practical application potential of clove oil as an antifungal.
An interesting finding of the study relates to contact time, and therefore when studies should measure the zone of inhibition. Notably, if measurements had been taken only at one single time point the overall conclusions regarding efficacy would be different. This highlights the need for a standardisation of methods, including contact time, when evaluating antifungal efficiency [27]. Depending on when inhibition is measured, there could be differences in the size of the zone of growth inhibition for fungal species, making comparing results from different studies difficult. This is particularly important when considering other treatment effects such as the ability of an essential oil to inhibit sporulation.
There are several reasons why some of the essential oils and other treatments demonstrated significant time effects on growth inhibition of fungal species. One possibility may be volatilisation over time of the more volatile essential oils, affecting the growth inhibition of the fungi. It is also unclear how the agar itself may affect the effectivity of the essential oils in their ability to inhibit growth. Similarly, the quenching of effectivity of the treatments may be from the fungi becoming less sensitive to the active compounds or due to the nutrient rich agar diluting the treatments, or quenching its concentration. This could mean that results showing significant time differences in growth inhibition (time effect) may not present the actual effectivity of the treatment but how the treatment was influenced when used on agar. Of all the treatments tested in the experiment, bleach was found to be particularly prone to decomposition over time. The differences observed over time have significant implications with regards to interpretation of results from previous studies, given the lack of a standardised methodology. This is further complicated given the differences in fungal species growth rates and suggests that future studies need to consider time as a significant variable when assessing potential antifungal activity [27].
Overall, clove oil was identified as having a general stable inhibitory effect against all fungal species with a significant time effect being found in three of the five species over the seven-day period. Ulocladium, Coprinellus, and Penicillium species were all found to have significant reduction in the zone of inhibition between the three measurements (time effect) whereas both outdoor isolates (Aspergillus and Penicillium) were found to have no significant difference recorded between measurements. Clove oil was found to still inhibit the growth of all five species after seven days of contact time.
Eucalyptus and lavender oils were found to have potential inhibitory qualities against certain species with an added effect of prevention of sporulation. While inhibition of sporulation does not affect the growth of the spores already present into fungi, it inhibits the production of new spores to create the next generation.
Limonene and vinegar were found to have little antifungal properties against the five species isolated. The results for these treatments were surprising as vinegar is a recommended treatment for mould remediation and limonene is used in a variety of domestic cleaning products [13]. Future studies are needed to investigate the translation of this laboratory-based study for practical application. These should focus on examining the efficacy of clove oil taking into consideration application methods and concentrations, as well as the long term persistence of its antifungal properties.
Conclusion
Clove oil was found to be the most effective and broad spectrum antifungal agent against fungal species of environmental origin. These results are preliminary and warrant further validation, particularly in terms of its practical application. An important outcome from a methodological perspective, was the significant variation of a number of the products over time, when studied using the disc diffusion assay. This has implications for the comparisons able to be made across different studies, or how standardised and optimised methods might be developed. This work demonstrates the need for an agreed method of testing the inhibition of fungal growth to allow cross-study comparisons. Essential oils have the potential for wide acceptance for indoor use as alternatives to traditional cleaning agents in improving indoor air.
Originality-significance statement
This is one of the first studies to investigate the antifungal efficacy of essential oils compared with other recommended chemical treatments against a broad spectrum of environmental fungi. Of particular note is that the fungal species chosen for this study were isolated from air rather than clinical isolates, which is important when considering air quality.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Mentese S, Mirici NA, Otkun MT, et al. Association between respiratory health and indoor air pollution exposure in Canakkale, Turkey. Build Environ. 2015. [cited [cited 2015 Nov 01]];93:72–83. DOI:10.1016/j.buildenv.2015.01.023. [DOI] [PubMed] [Google Scholar]
- [2].Méheust D, Le Cann P, Reboux G, et al. Indoor fungal contamination: health risks and measurement methods in hospitals, homes and workplaces. Crit Rev Microbiol. 2014. [cited [cited 2014 Aug 01]];40(3):248–260. DOI: 10.3109/1040841X.2013.777687. [DOI] [PubMed] [Google Scholar]
- [3].Nevalainen A, Täubel M, Hyvärinen A. Indoor fungi: companions and contaminants. Indoor Air. 2015;25(2):125–156. 10.1111/ina.2015.25.issue-2 [DOI] [PubMed] [Google Scholar]
- [4].Robbins CA, Swenson LJ, Nealley ML, et al. Health effects of mycotoxins in indoor air: a critical review. Appl Occup Environ Hyg. 2000;15(10):773–784. 10.1080/10473220050129419 [DOI] [PubMed] [Google Scholar]
- [5].Crook B, Burton NC. Indoor moulds, sick building syndrome and building related illness. Fungal Biol Rev. 2010;24(3–4):106–113. DOI: 10.1016/j.fbr.2010.05.001. [DOI] [Google Scholar]
- [6].Shoemaker RC, House DE. A time-series study of sick building syndrome: chronic, biotoxin-associated illness from exposure to water-damaged buildings. Neurotoxicol Teratol. 2005;27(1):29–46. 10.1016/j.ntt.2004.07.005 [DOI] [PubMed] [Google Scholar]
- [7].Hänninen OO. WHO guidelines for indoor air quality: dampness and mold. Fundamentals of mold growth in indoor environments and strategies for healthy living. Utrecht, The Netherlands: Springer; 2011. p. 277–302. [Google Scholar]
- [8].Portnoy JM, Kwak K, Dowling P, et al. Health effects of indoor fungi. Ann Allergy Asthma Immunol. 2005;94(3):313–320. 10.1016/S1081-1206(10)60982-9 [DOI] [PubMed] [Google Scholar]
- [9].Daisey JM, Angell WJ, Apte MG. Indoor air quality, ventilation and health symptoms in schools: an analysis of existing information. Indoor Air. 2003;13(1):53–64. 10.1034/j.1600-0668.2003.00153.x [DOI] [PubMed] [Google Scholar]
- [10].Singh J. Toxic moulds and indoor air quality. Indoor Built Environ. 2005;14(3–4):229–234. 10.1177/1420326X05054015 [DOI] [Google Scholar]
- [11].Hudson J, Kuo M, Vimalanathan S. The antimicrobial properties of cedar leaf (thuja plicata) oil; a safe and efficient decontamination agent for buildings. Int J Environ Res Publ Health. 2011;8(12):4477–4487. 10.3390/ijerph8124477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Taylor M, Gaskin S, Bentham R, et al. Airborne fungal profiles in office buildings in metropolitan Adelaide, South Australia: background levels, diversity and seasonal variation. Indoor Built Environ. 2014;23(7):1002–1011. 10.1177/1420326X13499172 [DOI] [Google Scholar]
- [13].Kemp P, Neumeister-Kemp H, Cheong C. Australian mould guideline. (AMG-2005-1). Osborne Park: Mycologia Australia Pty Ltd; 2005. [Google Scholar]
- [14].Rogawansamy S, Gaskin S, Taylor M, et al. An evaluation of antifungal agents for the treatment of fungal contamination in indoor air environments. Int J Environ Res Publ Health. 2015;12(6):6319–6332. 10.3390/ijerph120606319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chakravarty P, Kovar B. Engineering case report: evaluation of five antifungal agents used in remediation practices against six common indoor fungal species. J Occup Environ Hyg. 2013;10(1):D11–D16. 10.1080/15459624.2012.740987 [DOI] [PubMed] [Google Scholar]
- [16].Mehra T, Köberle M, Braunsdorf C, et al. Alternative approaches to antifungal therapies. Exp Dermatol. 2012;21(10):778–782. PMID: PMC3481189 DOI: 10.1111/exd.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Glegg GA, Richards JP. Chemicals in household products: problems with solutions [journal article]. Environ Manage. 2007;40(6):889–901. DOI: 10.1007/s00267-007-9022-1. [DOI] [PubMed] [Google Scholar]
- [18].Pinto E, Vale-Silva L, Cavaleiro C, et al. Antifungal activity of the clove essential oil from Syzygium aromaticum on candida, Aspergillus and dermatophyte species. J Med Microbiol. 2009;58(11):1454–1462. 10.1099/jmm.0.010538-0 [DOI] [PubMed] [Google Scholar]
- [19].Singh T, Chittenden C. Efficacy of essential oil extracts in inhibiting mould growth on panel products. Build Environ. 2010;45(10):2336–2342. 10.1016/j.buildenv.2010.03.010 [DOI] [Google Scholar]
- [20].Zabka M, Pavela R, Prokinova E. Antifungal activity and chemical composition of twenty essential oils against significant indoor and outdoor toxigenic and aeroallergenic fungi. Chemosphere. 2014;112:443–448. 10.1016/j.chemosphere.2014.05.014 [DOI] [PubMed] [Google Scholar]
- [21].Hartnoll G, Moore D, Douek D. Near fatal ingestion of oil of cloves. Arch Dis Child. 1993;69(3):392–393. 10.1136/adc.69.3.392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Patel S, Wiggins J. Eucalyptus oil poisoning. Arch Dis Child. 1980;55(5):405–406. 10.1136/adc.55.5.405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Darben T, Cominos B, Lee C. Topical eucalyptus oil poisoning. Australas J Dermatol. 1998;39(4):265–267. 10.1111/ajd.1998.39.issue-4 [DOI] [PubMed] [Google Scholar]
- [24].Prashar A, Locke IC, Evans CS. Cytotoxicity of clove (Syzygium aromaticum) oil and its major components to human skin cells. Cell Proliferat. 2006;39(4):241–248. 10.1111/cpr.2006.39.issue-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Delespaul Q, de Billerbeck VG, Roques CG, et al. The antifungal activity of essential oils as determined by different screening methods. J Essent Oil Res. 2000;12(2):256–266. [Google Scholar]
- [26].Su H-J, Chao C-J, Chang H-Y, et al. The effects of evaporating essential oils on indoor air quality. Atmos Environ. 2007;41(6):1230–1236. 10.1016/j.atmosenv.2006.09.044 [DOI] [Google Scholar]
- [27].Whiley H, Gaskin S, Ross K, et al. Antifungal properties of essential oils for improvement of indoor air quality: a review. Rev Environ Health. 2018;33(1):63–76 DOI:10.1515/reveh-2017-0023. [DOI] [PubMed] [Google Scholar]
- [28].White TJ, Bruns T, Lee S, et al. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics In: Innis MA, Gelfand DH, Sninsky JJ, et al., editors. PCR protocols: a guide to methods and applications. New York (NY): Academic Press; 1990. p. 315–322. [Google Scholar]
- [29].Schoch CL, Seifert KA, Huhndorf S, et al. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for fungi. Proc Natl Acad Sci. 2012;109(16):6241–6246. 10.1073/pnas.1117018109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ficker CE, Arnason J, Vindas P, et al. Inhibition of human pathogenic fungi by ethnobotanically selected plant extracts. Mycoses. 2003;46(1–2):29–37. 10.1046/j.1439-0507.2003.00838.x [DOI] [PubMed] [Google Scholar]
- [31].Levinskaitė L, Paškevičius A. Fungi in water-damaged buildings of vilnius old city and their susceptibility towards disinfectants and essential oils. Indoor Built Environ. 2013;22(5):766–775. 10.1177/1420326X12458514 [DOI] [Google Scholar]
- [32].Yingprasert W, Matan N, Matan N. Effects of surface treatment with cinnamon oil and clove oil on mold resistance and physical properties of rubberwood particleboards. Eur J Wood Wood Prod. 2015;73(1):103–109. 10.1007/s00107-014-0857-x [DOI] [Google Scholar]