Abstract
Fluconazole is a triazole antifungal medication used in the treatment of various fungal infections. It is available in both oral and parenteral formulations. Liver damage has been reported with fluconazole use, but most commonly it is benign elevated liver transaminases. Acute liver failure (ALF) in fluconazole use is rare, with cases being reported sporadically in literature and large cohorts describing incidence rates of acute liver injury ranging from 0.0 to 31.6/10,000 patients. We present a case of a 45-year-old African-American male with no history of liver disease who presented with superficial candidiasis and superimposed bacterial cellulitis. He was subsequently started on intravenous fluconazole and clindamycin. Shortly after he developed ALF and a drug-induced liver injury (DILI) was suspected. Fluconazole was stopped, and the clinical picture improved shortly afterward, leading to a diagnosis of fluconazole-induced ALF. Patient underwent laboratory and clinical evaluation to exclude competing etiologies of liver injury as well as a standardized assessment for causality and disease severity such as Roussel Uclaf Causality Assessment Method/Council for International Organizations of Medical Sciences score, which concluded a “Highly Probable” DILI, and a Naranjo score identifying adverse drug reaction (ADR) which concluded a “Definite ADR.” Due to the severity of ALF and the routine use of fluconazole in clinical practice, clinicians should be aware that fluconazole can be a causative agent of ALF, even in low-risk populations.
Keywords: Failure, fluconazole, liver
Introduction
Fluconazole is a commonly used triazole drug in the treatment of various fungal infections. It is available in both oral and parental formulations and is unique from other azoles as it is metabolized primarily by the kidneys and not by the liver like the other azoles.[1] Despite this, transient elevations of transaminases may be seen in fluconazole usage.[1,2] There have also been rare isolated reports in literature of fluconazole-induced acute liver failure (ALF). Below we present a patient with no history of chronic liver disease (CLD) or use of hepatotoxic drugs who developed ALF shortly after beginning intravenous (IV) fluconazole therapy.
Case Report
A 45-year-old African-American male presented to emergency department with complaints of fever and a pruritic inguinal rash. He had medical history of hypertension, diabetes mellitus, dyslipidemia, bilateral Stage 3 gluteal decubitus ulcer, and paraplegia after gunshot wound to the spine 26 years ago. Patient noticed reddish-brown itchy rash with odor in the groin for the past 4 days. He denied any cough, chest pain, dyspnea, palpitations, dysuria, diarrhea, nausea, or vomiting. Patient had a history of tobacco and alcohol abuse but no illicit drug abuse. Patient reported asymptomatic elevations in liver function tests (LFTs) with prior 7-day course of oral fluconazole for candiduria approximately a year ago, after which the drug was discontinued.
On admission, his vitals were remarkable for temperature of 102.4°F, pulse of 117 beats/min, and blood pressure of 170/106 mmHg. Physical examination revealed an elevated, erythematous, and tender rash in inguinal area with marks of skin excoriations consistent with intense pruritus. There was a bilateral Stage 3 sacral decubitus ulcer visible with no signs of infection. There were no signs of asterixis, spider angioma, or organomegaly. A sepsis workup including blood cultures, urine cultures, sputum cultures, lactic acid, and chest X-ray was ordered. His initial serum electrolytes, LFTs, comprehensive metabolic panel, and coagulation panel were all within reference range. Hematological investigations showed a leukocytosis of 21,000 white blood cell/uL (reference 4500–11,000) with normochromic and normocytic anemia. On the day of admission, his aspartate aminotransferase (AST) was 12 IU/L (reference 8–46 IU/L), alanine aminotransferase (ALT) 18 IU/L (reference 7–55 IU/L), alkaline phosphatase 100 IU/L (reference 45–115 IU/L), total bilirubin 0.9 (reference 0.1–1.2 mg/dL), and direct bilirubin 0.2 IU/L (reference <0.3 mg/dL).
A diagnosis of sepsis was made, likely due to inguinal candidiasis with superimposed bacterial cellulitis. Patient was started on IV normal saline, IV fluconazole 200 mg daily, and IV clindamycin. Cultures were reported negative, and the patient had no further febrile episodes. On day 3 of hospitalization, the patient developed ALF with an AST of 25000 IU/L (reference 8–46 IU/L), an ALT of 6500 IU/L (reference 7–55 IU/L), a GGT of 210 IU/L (reference 0–65 IU/L), an alkaline phosphatase of 130 IU/L (reference 45–115 IU/L), a total bilirubin 2.3 mg/dL (reference 0.1–1.2 mg/dL), and a direct bilirubin 0.4 mg/dL (reference <0.3 mg/dL). With an ALT/alkaline phosphatase ratio >5, this drug-induced liver injury (DILI) was classified as “hepatocellular.” Coagulation studies noted an international normalized ratio of 3.2 (0.8–1.1), a prothrombin time of 25 s (reference 11–14), and a partial thromboplastin time 28 s (reference 25–35). Hepatitis panel, Epstein–Barr virus, cytomegalovirus, and HIV tests resulted negative. He had no history of chronic liver disease (CLD). An abdominal ultrasound showed mild hepatomegaly. Considering the temporal association combined with no other probable etiologies for the patient's worsening ALF, DILI secondary to fluconazole was suspected, and fluconazole was stopped. Three days after stopping the drug, the LFTs [Figure 1] and coagulation studies [Figure 2] improved. The patient's prompt clinical recovery after withholding the drug corroborated our diagnosis of fluconazole-mediated ALF.
Figure 1.
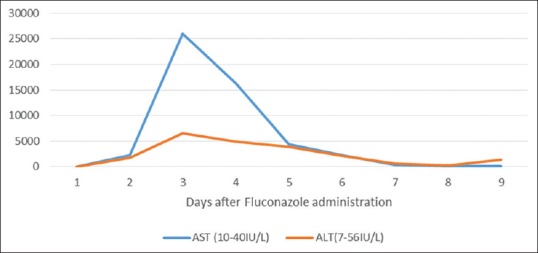
Liver enzymes after fluconazole administration.
Figure 2.
Prothrombin and internationalized normal ratio after fluconazole administration.
Discussion
Fluconazole is a commonly used antifungal drug that can be administered both orally and parenterally. Elevations in LFTs are a known side effect of taking fluconazole.[2,3] One publication summarized that 0.7% of patients had to be discontinued from fluconazole as a result of hepatotoxicity.[1] In vivo rat studies show that fluconazole is associated with hepatocyte degeneration at high doses of 100 mg/kg/day over 14 days, but inflammation and necrosis were still absent at that dosage group. Conversely, the same model showed that itraconazole had hepatocyte damage along with inflammation and necrosis at the 100 mg/kg/day group.[4] The discrepancy might be ascribable to the unique metabolism of fluconazole in the azole class, as it is primarily metabolized through the kidneys, compared to the other azoles which are usually metabolized hepatically.[1] While the exact mechanism of liver damage in fluconazole use is unknown, the damage is thought to be dose dependent.[4]
Although antimicrobials are the most common class of drug to cause liver injury,[5] ALF is a rare outcome with fluconazole usage, with varying incidences reported in literature. We examined three studies describing acute liver injury rates in oral antifungal use and describe the findings pertinent to our case. The first cohort had 171,806 patients, without any history of CLD, taking oral fluconazole and concluded a cumulative incidence for severe liver disease at a rate of 0.3/1000 patients.[3] The second cohort reported 35,833 patients receiving oral fluconazole and reported zero cases of ALF, providing an incidence rate of 0/10,000 patients.[6] The smallest of the three studies was a Taiwanese cohort with 3793 patients taking oral fluconazole and concluded an acute liver injury rate of 31.6 per 10,000 patients with six fatalities. Of note, all patients who expired in the Taiwanese cohort were older than 60 years, possible implicating age as a factor for poor prognosis.
Fluconazole is rarely a causative agent of ALF, but there may be associations correlated with fluconazole-induced ALF. CLD may also be a contributing comorbidity, as the rates for severe liver injury increase remarkably in patients with preexisting CLD in one of the cohorts.[3] Another possible factor is renal impairment, as fluconazole is renally excreted[1] and can, therefore, increasing the risk of toxicity in renal impairment. One case in literature described a similar event, a patient who was recovering oral fluconazole concurrently with amphotericin B, and subsequently developed ALF.[7] Although this patient did not have any intrinsic renal pathology, amphotericin B can impair renal function, therefore increasing the risk of fluconazole toxicity. A possible infectious factor, HIV, may be seen as increasing the risk of liver injury in patients taking fluconazole as shown in one review.[1] Jacobson et al. described a patient with AIDS who developed ALF after initiating oral fluconazole and eventually expired, with biopsy revealing hepatic necrosis.[8]
There are considerations to be made when comparing our patient with other cases in our review of literature. Foremost, the majority of literature involving antifungals involve oral administration; a contrast from our patient who received IV fluconazole. In addition, the patient also had a history of asymptomatic elevated LFTs related to oral fluconazole, leading to a complex decision as clinicians must be prudent with a rechallenge involving potential hepatotoxicity. The decision to initiate fluconazole was decided, and the dose was adjusted appropriately for weight. Another key difference is the sudden onset of hepatotoxicity within 72 h of initiating fluconazole, providing support for the hypothesis that our patient was unique from the dose-dependent hepatotoxicity described in literature. Finally, comorbidities characterized in the literature linked to fluconazole-induced ALF including HIV, CLD, and renal impairment were all notably absent in our patient. As such, this case is paramount as fluconazole-induced ALF occurred in a patient without any evident risk factors. Although the patient does have a history of alcohol abuse, at the time of presentation, there were no symptomatic or clinical findings of any hepatic dysfunction ruling out CLD. There was confirmation that an ADR occurred and a casualty established between the initiation of fluconazole and the subsequent hepatotoxicity.
The ADR was confirmed by Naranjo Algorithm as follows: previous reports positive (+1), adverse events appeared after the suspected drug was given (+2), with the transaminases improving after the discontinuation of the drug (+1), and the adverse reaction appearing after the re-administration of the drug (+2), with no alternative causes to explain this adverse reaction (+2), placebo not been given (0), drug levels not done (0), without changing the administered dose of the drug (0), similar reaction in the past with the same drug (+1), and the adverse event confirmed by objective evidence (+1) with a total score of 10 which is >9, thus “Definite ADR.”[9]
The causality of fluconazole inducing hepatotoxicity was established by Roussel Uclaf Causality Assessment Method/Council for International Organizations of Medical Sciences score as below: hepatocellular, second exposure, onset of <5 days (+1), time from withdrawal of drug until reaction onset <15 days (+1), risk factors being alcohol (+1), age <55 (0), >50% improvement in 8 days (+3), no concomitant therapy (0), excluded nondrug related causes: rule out (+2), response to re-administration positive (+3) with total score of 11 indicating “Highly Probable” (>8).[10]
Conclusion
Fluconazole is a known cause of elevated LFTs but has rarely been reported in literature as an agent associated with ALF. While acute liver injury has been linked with fluconazole usage, it can be associated with concurrent risks such as CLD, renal impairment, and HIV and tends to cause hepatotoxicity in a dose-dependent manner. Our case illustrates that a patient on fluconazole can develop ALF without any of these apparent risk factors. Further investigations are warranted to establish etiologies, specifically with IV administration. Meanwhile, clinicians should be aware of fluconazole as a cause of ALF, even in low-risk patients.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understand that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Tverdek FP, Kofteridis D, Kontoyiannis DP. Antifungal agents and liver toxicity: A complex interaction. Expert Rev Anti Infect Ther. 2016;14:765–76. doi: 10.1080/14787210.2016.1199272. [DOI] [PubMed] [Google Scholar]
- 2.Wang JL, Chang CH, Young-Xu Y, Chan KA. Systematic review and meta-analysis of the tolerability and hepatotoxicity of antifungals in empirical and definitive therapy for invasive fungal infection. Antimicrob Agents Chemother. 2010;54:2409–19. doi: 10.1128/AAC.01657-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lo Re V 3rd, Carbonari DM, Lewis JD, Forde KA, Goldberg DS, Reddy KR, et al. Oral azole antifungal medications and risk of acute liver injury, overall and by chronic liver disease status. Am J Med. 2016;129:283–91.e5. doi: 10.1016/j.amjmed.2015.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Somchit N, Norshahida AR, Hasiah AH, Zuraini A, Sulaiman MR, Noordin MM, et al. Hepatotoxicity induced by antifungal drugs itraconazole and fluconazole in rats: A comparative in vivo study. Hum Exp Toxicol. 2004;23:519–25. doi: 10.1191/0960327104ht479oa. [DOI] [PubMed] [Google Scholar]
- 5.Reuben A, Koch DG, Lee WM, Acute Liver Failure Study Group Drug-induced acute liver failure: Results of a U.S. Multicenter, prospective study. Hepatology. 2010;52:2065–76. doi: 10.1002/hep.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.García Rodríguez LA, Duque A, Castellsague J, Pérez-Gutthann S, Stricker BH. A cohort study on the risk of acute liver injury among users of ketoconazole and other antifungal drugs. Br J Clin Pharmacol. 1999;48:847–52. doi: 10.1046/j.1365-2125.1999.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crerar-Gilbert A, Boots R, Fraenkel D, MacDonald GA. Survival following fulminant hepatic failure from fluconazole induced hepatitis. Anaesth Intensive Care. 1999;27:650–2. doi: 10.1177/0310057X9902700616. [DOI] [PubMed] [Google Scholar]
- 8.Jacobson MA, Hanks DK, Ferrell LD. Fatal acute hepatic necrosis due to fluconazole. Am J Med. 1994;96:188–90. doi: 10.1016/0002-9343(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 9.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–45. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 10.Andrade RJ, Robles M, Fernández-Castañer A, López-Ortega S, López-Vega MC, Lucena MI, et al. Assessment of drug-induced hepatotoxicity in clinical practice: A challenge for gastroenterologists. World J Gastroenterol. 2007;13:329–40. doi: 10.3748/wjg.v13.i3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]


