Abstract
Modern medicine is given overarching importance to tackle disease in the human body than environmental determinants. Although, most of the literature confirms that the determinants of disease are there in the environment. Yet in the modern times what is being emphasized is highly limited and reductionist approach of curing ailments in the human body only, which is one of the desired interventions but is full of other side effects and risks leading to iatrogenic reactions. Most of the literature establishes that modern medicine is one of the major threats to the world health. Besides treating disease at the clinical level, rational, and well-thoughtout changes in the overall environment can positively impact the nature, extent, and distribution of disease.
Keywords: Adverse drug reaction, environment, iatrogenesis, India, over-medicalization, World Health Organization
What is Iatrogenesis?
The side effects and risks associated with the medical intervention are called iatrogenesis. These side effects are also called adverse drug reactions (ADRs). Iatrogenesis is composed of two Greek words, “iatros,” which means physicians and “genesis,” which means origin. Hence, iatrogenic ailments are those where doctors, drugs, diagnostics, hospitals, and other medical institutions act as “pathogens” or “sickening agents.”[1] According to the World Health Organization (WHO), “Iatrogenesis is any noxious, unintended, and undesired effect of a drug, which occurs at doses used in humans for prophylaxis, diagnosis, or therapy.”[2] The Joint Commission on the Accreditation of Healthcare Organizations defines an ADR as an undesired effect of a medication that either increases toxicity, decreases desired therapeutic effect, or both.[3] The WHO defined iatrogenesis does not give a clear picture of ADRs. This definition does not include in it the therapeutic failures, intentional and accidental poisoning, drug abuse, incorrect drug administration, and noncompliance.[4] This definition also tends to underestimate ADRs and incidences such as diagnostic procedures which include mechanical and radiological procedures, the therapeutic regimen which includes drugs, surgery, and invasive procedures, hospitalization and treating doctor himself/herself is also not accounted for. That too can cause iatrogenic effects.[3,5,6]
Periodicity of Iatrogenesis
The effect of iatrogenesis started in the 18th century. It showed an increase in the first half of the 19th century, which Nikola Schipkovenski calls clinical skepticism and therapeutic nihilism.[7] With the onset of bacteriology and serum related therapy, antibiotics, hormones, and sulfa drugs iatrogenic suspicion decreased. This can be categorized as the second wave of iatrogenesis. During the 1960s, iatrogenic suspicion again intensified due to thalidomides and psychopharmaceuticals. Erwin Ackeknecht (a historian of medicine) recognized this as the third wave of iatrogenesis. The American Medical Association also recognized this as the third wave of iatrogenesis.[8]
The groundbreaking work on iatrogenesis has been carried out by Ivan Illich. Illich, a leading critic of modern medicine has classified iatrogenesis into direct, caused by the medical care which can cause death, pain or sickness and indirect, wherein health policies themselves are responsible for illness, death, or disease. In his prestigious work named, “Medical Nemesis,” Illich opines that iatrogenesis is structural because it undermines people's agency and competence to deal with their own disease. He also classified iatrogenesis as social and cultural. According to him, social iatrogenesis results from the medicalization of life and cultural medicalization is the destruction of traditional ways of dealing with and making sense of death, pain, and sickness.[9] The intimidating nature of iatrogenesis has also been stated by Oliver Wendell Holmes, an American physician and a medical reformer of the 19th century when he stated:
“I firmly believe that if the whole materia medica, as used now could be sunk to the bottom of the sea, it would be all the better for mankind and all the worse for the fishes” (Holmes, 1891:19).[10]
These iatrogenic effects are viewed by Illich as overconsumption which is caused mainly by industrialization.[11] A. R. Smith assessing the intensity of the side effects of modern medicine states that the major threat to health in this world is modern medicine.[9] So says, Illich, that “the medical establishment has become a major threat to health.”[1]
Extent of Iatrogenesis
Iatrogenesis is the fifth leading cause of death in the world. There are about 5%–8% of deaths due to ADRs worldwide[12]. In many countries, ADRs are a leading cause of death.[13] About 1.4 million patients are affected by the infections at any given time due to the healthcare system. In the developed countries, the toll is 5%–10% of patients while in developing countries “as many as a quarter of all patients may be affected by a healthcare-associated infection.”[14] A study conducted in 2005 established communication problem as the major cause of 70% of sentinel events in a hospital-like setting.[14]
The unsafe injection practice (unsterilized syringes and needles) worldwide accounts for 40% of infections. In some of the countries, the unsafe injection practice is as high as 70%. “Unsafe injection practices cause an estimated 1.3 million deaths each year worldwide, a loss of 26 million years of life and an annual burden of US$ 535 million in direct medical costs.”[15] Unsafe blood transfusions contribute about 5%–15% of HIV infections. A study indicates that the donated blood was not at all screened for the infections such as HIV and Hepatitis in almost 60 countries worldwide.[15]
A study conducted by the WHO concluded that per capita medication usage was highest in the USA which exceeded Latin America and even Europe[16]. The report, compiled by (Life Extension Magazine) LEF estimates that every year in the USA, 2.2 million people experience ADRs and the death due to ADRs is 783,936. Although the USA spends 14% of its gross national product on healthcare yet, it is ironical that the American Medical System contributes to most of the deaths. The government-sanctioned medicine in the USA alone is responsible for 700,000 deaths every year.[17]
Leape in 1994[18] published his study called “Error in Medicine” in Journal of American Medical Association, in which he reported a study of Schimmel[19] in which he had estimated iatrogenic injury of 20% with 20% of fatalities. Leape also focused on the Harvard Medical Practice Study which was published in 1991 which suggested that 4% of iatrogenic illnesses occurred in New York City with 14% of fatalities. Hence, this way he estimated that people who get killed due to iatrogenic illness are about 180,000/year. However, he admitted that this number is a tip of iceberg due to the scarcity of actual data and underreporting of iatrogenic illnesses.[17] A meta-analysis of prospective studies was also done by Lazarou et al. to estimate the incidence of serious and fatal ADRs in hospital patients from 1966 to 1996. The overall incidence of serious ADRs was 6.7% and of fatal ADR was 0.32% of hospitalized patients, making them fourth and sixth leading causes of death.[3]
Among the European Union Member states, WHO concluded that the healthcare-related errors occur in 8% to 12% of hospitalizations. A report named “organisation of memory” estimated 850,000 ADRs occur each year. The statistics are more or less similar in Spain, France, and Denmark. If all these medical errors in the European Union would be prevented it will reduce the number of deaths by 95000/year.[20] A systematic review of the literature with regard to medical errors in the Middle-Eastern countries was found to be 7.1%–90.5% for prescription drugs and 9.4% to 80% for the drug administration.[21] In sub-Saharan Africa and Asian region at least 50% of injections given are unsafe due to which highest infections occur in these regions.[22]
Nature of Iatrogenesis
Due to thousands of drugs currently in usage and subsequently, their side effects it is a challenge to categorize and classify the nature of all the ADRs. The lack of common terminology has led to various categorizations and classifications of ADRs. They are, errors of omission, errors of execution, and errors of planning (names are suggestive). Healthcare settings such as hospitals and nursing homes and severity of the resultant injury such as “near miss,” “no harm events,” “sentinel events,” and “legal implications negligence” also form a basis of classification.[23]
On the basis of predictability, ADRs can be classified as predictable such as toxicity, side effects, superinfection, and drug interaction and unpredictable such as allergy, intolerance, pseudoallergy, and idiosyncrasy.[24] Some ADRs can occur right after the therapy or even during the course of therapy. In some cases, there are outright allergic reactions, hypersensitivity, and physiological idiosyncrasies.[23]
ADRs can also be classified on the basis of reactions which can take place during the administration of a particular drug. For example, Type A (Augmented): this ADR is dependent on the dose administered. The severity increases with the dose; Type B (Bizzare): Its mechanism is unknown. It can be fatal or serious. It cannot be predicted for example, hepatitis caused by halothane; Type C (Continuous use of drugs): this type of ADR occurs as a result of continuous drug use for example, dementia by anticholinergic medications; Type D (Delayed): this type of ADR occurs after the treatment is being stopped for example, corneal opacities after thioridazine; Type E (End of dose): this type of ADR occurs with the depressant drugs, after withdrawal; Type F (Failure of a therapy): this type of ADR mainly happens due to the failure of treatment or treatment being ineffective.[25]
Indian Experience
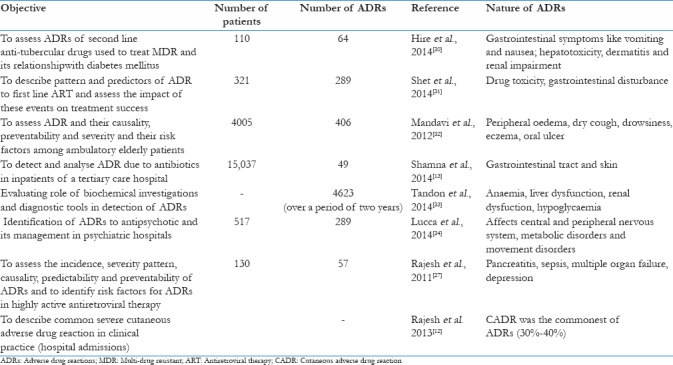
Although Indian studies in this regard are very few, the pattern of reactions seems to be similar to that of western experience.[26] A study of first of its kind was conducted in India in 2010 to assess the incidence, severity, pattern, causality, and predictability of ADRs and to identify risk factors for ADRs in highly active antiretroviral therapy. Monitoring of 130 retro-positive patients by active pharmacovigilance identified 74 ADRs from 57 patients. Anaemia and hepatotoxicity were the most commonly observed ADRs.[27] Another study done by Yadav et al., showed that the ADR of anti-tuberculosis drugs in the Medicine Department of Majeedia Hospital, Jamia Hamdard, over a period of 6 months. A total of 139 patients were studied. Nearly 46.7% of the patients faced the ADR to anti-tuberculosis drugs. It was concluded that ADR is the main factor of noncompliance during treatment and a reason for multi-drug resistance tuberculosis.[28] Another study was done across South Indian hospitals. A total of 270 suspected ADRs were reported and evaluated from 164 patients. A total of 3.7% of the hospitalized patients experienced an ADR, 0.7% of the admissions were due to ADRs and 1.8% had a fatal ADR. The gastrointestinal system (36.3%) was found to be most affected due to ADR. The drug class most commonly implicated with ADRs were the drugs used to treat cardiovascular ailments (18.3%).[29] Some additional studies from India on ADRs are listed in Table 1. And some of these studies reveal that ADRs have an effect on the skin of patients[12,13,30,32] Some studies show that drugs can cause ADRs in the form of psychological reactions as well like depression. Some of the studies indicated that ADRs can have severe effect on elderly people.[32] There are many reasons for the ADRs in India like a large number of patients, self-medication, presence of counterfiet drugs and the large amount of drug combinational products in the world.[26] These studies bring forth many factors which are confronted by pharmacovigilance presently in India.
Table 1.
Indian studies on adverse drug reactions
Drug Monitoring
The drug monitoring system started internationally in 1967 in the Twentieth World Health Assembly. The WHO started a pilot study in countries such as the Netherlands, Germany, Czechkoslavakia, Canada, Ireland, Denmark, the UK, the USA, Newzealand, and Australia which had established drug monitoring centers in their respective countries. A total of 3,00,000 cases of ADRs were found and fed to computers to be analyzed. Now the drug monitoring system across the world has improved with the main center in Uppsala, Sweden.[2] The Uppsala Monitoring Centre (UMC), Sweden is maintaining the international database of ADR reports. Currently, there are >4.7 million cases being reported by 96 member countries However, it is estimated that only 6%–10% of all ADRs are reported.[35]
Although, India is participating in the drug monitoring program, its contribution to UMC database is very little.[36] Pharmacovigilance is in its nascent stage in India. It lacks continuity. Reporting of ADRs due to medicines and other medical procedures is very less.[26] It is weak in terms of reporting by doctors, nurses, and pharmacists to the hospital ADR monitoring system. Since ADR is a professional obligation, but doctors and other staff do not carry out this responsibility seriously.[36] There is a lack of awareness and inadequate training about drug safety monitoring among healthcare professionals in India. Early detection of ADRs may help in preventing them.[25] Prevention and the detection of ADRs at the early stage are important because they have high healthcare costs in addition to morbidity and the mortality.[28] Ten percent of patients in acute care settings experience an adverse drug event which can be considerably prevented.[15] Despite five pharmacovigilance centers across India, the reporting to such centers annually is very low.[25] Reporting to the UMC by India is dismal.[36] There are also not efficient and well-developed mechanisms of reporting the side effects of medicines and other procedures related to the curative services. In India, often ADRs go unnoticed or are not reported.[26]
Causes of Iatrogenesis
Medical error/negligence
According to the WHO, “one of the major structural challenges for health systems is inadequate numbers and skills distribution of qualified health providers and the incomplete knowledge about safe practices.”[14] During the process of curing an ailment, the errors can happen at any stage be it diagnosis, treatment and at the level of preventive care as well. Silverman and Lee in their book Pills, Profits and Politics, remark that 2% to 8% of all drug doses given in a hospital setting is in error in terms of–“wrong drug,” “wrong dose,” “wrong route of administration,” “wrong patient,” or “failure to give the prescribed drug.”[37] The WHO assesses that almost 50% of the medicine prescribed and sold is inappropriate and 50% of patients take these drugs incorrectly.[38] There are issues of prescribing and delivering correct therapy to the patients. Another challenge faced by healthcare system is accurate and timely diagnosis of the ailment and management of preoperative care and minimizing medication errors.[14] There is dearth of manpower in healthcare system also which directly and indirectly also contributes to the error. “Developing and transitional countries have estimated the deficit of doctors, nurses, and midwives to ensure the safety of their healthcare systems to be in millions.”[14]
Malpractices
Sometimes health policies are a major factor contributing toward iatrogenic illness. For example, in the USA, there is a practice of defensive medicine which “occurs when doctors order tests, procedures, or visits, or avoid certain high-risk patients or procedures, primarily (but not necessarily solely) due to concern about malpractice liability.”[39] Hence, doctors recommend medical examinations and medications to their patients recklessly. The US office of technology assessment has concluded that fewer than 8% of all diagnostic tests are performed mainly due to the fear of malpractice.[40] The malpractice can occur at the level of diagnosis, prescribing drugs, ordering tests, recommending surgical procedures etc.
No prior warnings about possible Adverse Drug Reactions
There are substantial unmet needs concerning information about adverse drug effects and that is prominent among patients who have had prior experiences of adverse drug effects. A study concluded that 90% of the patients want to get information about the side effects of drugs.[22] Apart from the formal medical setting, not only in the hospitals alone, medical settings anywhere can cause problem of ADRs such as nursing homes, private doctors, and clinics.[41] The amount of risk which is associated with all the settings is not communicated to the patient in question. It has been observed that during clinical trials or animal studies ADRs show their effect afterwards. Furthermore, it happens that at the time of experimentation disease-drug interaction and drug interaction do not come to the fore.[42] The nuances of such experimentation and the amount of risk associated with such products is not disclosed.
Over medicalization of Ill health
The autonomy of patients dealing with their own illness with regard to modern medicine is compromised. Natural healing of a disease is questioned by the medical sciences. The cultural way of managing an illness is no longer considered relevant. The religious healing is rationalized. All the ways of traditional healing have been replaced by the over-medicalization of ill health.[1] “The power of modern drugs in treating specific symptoms absolves the individual from any responsibility in overcoming his illness.”[43]
Commercialization of medicine
Commercialization of medicine is one of the primary reasons for increased ADRs. There is a strong lobby between pharma industry and the medical institutions. Even studies are been funded by the pharma companies and it is most likely that they declare their drugs as effective. Furthermore, pharmaceutical companies have a transnational character so they transcend all the boundaries and show their presence everywhere.[44] Main destinations of these countries are developing nations due to cheap labor, cheap resources, and tax evasions. There are many companies which have tie-ups with petrochemical industry and obviously with financial companies as well. These companies are ready to provide huge loans for their unfettered profit motives. Ironically, the companies spend less on research and more on advertising.[45] The drugs supplied by the multinational corporations are huge, but they do not make explicit the amount of danger associated with drugs. “Since the founding of the WHO, the World Health Assembly has adopted many resolutions requesting the Organization to develop international standards, recommendations, and instruments to assure the quality of medicines, whether produced and traded nationally or internationally.”[46] In this regard, the multinational promotional schemes are denounced due to their failure to disclose ADRs.[47]
How to reduce iatrogenesis?
At clinical level
At the clinical level, iatrogenesis can be tackled by enhancing research base of drugs. The protocols have to be devised to enhance the knowledge regarding medical errors, malpractices, negligence. There is a need to establish a national focus and learning from errors. The medical care should raise the standard and expectations for improvement. There should be adequate safety systems being built inside the healthcare system to address the problem of ADRs.[22]
The WHO 2015 (18th edition) list of essential drugs should be implemented in all nations. Education about the right use of medicine should be advocated by the reputed institutions who do not take monetary favors from the companies and not buy the representatives of the drug companies. Research on human experimentation should be performed for the benefit of the public at large and not for the commercial purpose. The RCTs conducted should be safe and conducted openly. The WHO guidelines for the said purpose should be followed.[45] There should be a proper reporting mechanism where even a suspicion could be reported and causality of which could be established later on. The system of reporting should be easy to operate. There should be a mechanism to acknowledge the reporting of ADR whatever their nature be.[29] The doctors have a moral responsibility to stop taking favors from the pharmaceutical companies and give all the necessary information about drugs and therapies they give to their patients. Intensive and irrational use of technology has also to be minimized. Unnecessary surgical procedures, invasive diagnostics, and ruthless use of drugs have to be checked. The ever increasing new drugs in the market and the lack of formal system of drug monitoring add to the ADRs.[26] That has to be dealt with strongly by placing proper mechanisms at place.
At environmental level
The determinants of a disease are in the socio-cultural environment. Even the subtle imbalance in environment shows its effects in the human organism. There are various aspects to a healthy human organism. Paradoxically, the paradigm of bio-medicine defines treatment completely in bio-physical terms, disregarding cultural, and social factors.[47] The well-planned changes in the environment in terms of enhancing water quality, controlling vector borne diseases, reducing air pollution, checking toxic chemical exposure, tending to degrading urban environment, improving nutrition can have a long-lasting impact on the health of populations.[48] Rather focusing on environmental intervention, modern medicine instead focuses on medical technology and diagnostics, overuse surgical procedures, and unflinching dependence on pharmaceuticals.[17] The state has a responsibility to intervene at the environmental level to lessen the nature, extent, and distribution of diseases. Such measures should be incorporated in the plans and policies, so that they have an effective influence on the public health and this can save the humankind from the ruthless use of medicine and medical technologies and subsequently iatrogenic reactions.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Illich I. Medical Nemesis: Expropriation of Health. United States: Random House; 1975. [Google Scholar]
- 2.WHO. Technical Report No. 498: International Drug Monitoring: The Role of National Centres. Geneva, Switzerland: WHO; 1972. [PubMed] [Google Scholar]
- 3.Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: A meta-analysis of prospective studies. JAMA. 1998;279:1200–5. doi: 10.1001/jama.279.15.1200. [DOI] [PubMed] [Google Scholar]
- 4.WHO. International Drug, Monitoring: The Role of the Hospital. Technical Report Series No. 425. Switzerland, Geneva: WHO; 1996. [PubMed] [Google Scholar]
- 5.Krishnan NR, Kasthuri AS. Iatrogenic disorders. MJAFI. 2005;61:2–6. doi: 10.1016/S0377-1237(05)80107-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh S, Shakti GK, Arya S, Sharma DK, Aggarwal V. Adverse drug reaction policy in a tertiary care hospital. Int J Res Found Hosp Healthc Adm. 2015;3:41–7. [Google Scholar]
- 7.Schipnowensky, Nikola. Psychotherapy versus Iatrogeny: A Confrontation for Physicians. Detroit: Wayne State University Press; 1977. [Google Scholar]
- 8.Brown TM. A new wave of iatrogenic suspicion. Hastings Cent Rep. 1978;8:45–6. [Google Scholar]
- 9.Smith AR. Limits to medicine. Medical nemesis: The expropriation of health. J Epidemiol Community Health. 1979;57:12–928. doi: 10.1136/jech.57.12.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holmes OW. Medical Essays. Princeton: Princeton University Press; 1891. pp. 1842–82. [Google Scholar]
- 11.Siegmann AE, Elinson J. Newer sociomedical health indicators: Implications for evaluation of health services. Med Care. 1977;15:84–92. doi: 10.1097/00005650-197705001-00009. [DOI] [PubMed] [Google Scholar]
- 12.Rajesh V, Biju V, Vijendran P. Severe cutaneous adverse drug reactions. Med J Armed Forces India. 2013;69:375–83. doi: 10.1016/j.mjafi.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shamna M, Dilip C, Ajmal M, Linu Mohan P, Shinu C, Jafer CP, et al. A prospective study on adverse drug reactions of antibiotics in a tertiary care hospital. Saudi Pharm J. 2014;22:303–8. doi: 10.1016/j.jsps.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organisation. WHO Patient Safety Research: Topic, Better Knowledge for Safer Care. 2009. [Last accessed on 2016 Aug 18]. Available from: http://www.apps.who.int/iris/bitstream/10665/70145/1/WHO_IER_PSP_2009.10_eng.pdf .
- 15.World Health Organisation. Research for Patient Safety: Better Knowledge for Safer Care. 2008. [Last accessed on 2016 Aug 18]. Available from: http://www.who.int/patientsafety/information_centre/documents/ps_research_brochure_en.pdf .
- 16.Robert S, Fachinetti Neil J. Drug iatrogenesis and clinical pharmacy: The mutual fate of a social problem and a professional movement. Soc Probl. 1985;32:425–43. [Google Scholar]
- 17.Gary N, Carolyn D, Feldman M, Rosio D. Death by medicine. J Orthomol Med. 2005;20:21–34. [Google Scholar]
- 18.Leape LL. A new wave of iatrogenic suspicion, error in medicine. JAMA. 1994;272:1851–7. [PubMed] [Google Scholar]
- 19.Schimmel EM. The hazards of hospitalization. Ann Intern Med. 1964;60:100–10. doi: 10.7326/0003-4819-60-1-100. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organisation. A Brief Synopsis of Patient Safety: Regional Office for Europe: Denmark. 2010. [Last accessed on 2016 Aug 06]. Available from: http://www.euro.who.int/__data/assets/pdf_file/0015/111507/E93833.pdf .
- 21.Alsulami Z, Conroy S, Choonara I. Medication errors in the Middle East countries: A systematic review of the literature. Eur J Clin Pharmacol. 2013;69:995–1008. doi: 10.1007/s00228-012-1435-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kane A, Lloyd J, Zaffran M, Simonsen L, Kane M. Transmission of hepatitis B, hepatitis C and human immunodeficiency viruses through unsafe injections in the developing world: Model-based regional estimates. Bull World Health Organ. 1999;77:801–7. [PMC free article] [PubMed] [Google Scholar]
- 23.Enlund H, Vainio K, Wallenius S, Poston JW. Adverse drug effects and the need for drug information. Med Care. 1991;29:558–64. doi: 10.1097/00005650-199106000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Kishore K, Nagarkar KM. Adverse drug reaction. Hosp Today. 1996;61:35–41. [Google Scholar]
- 25.Bhatt AD. Drug-related problems and adverse drug events: Negligence, litigation and prevention. J Assoc Physicians India. 1999;47:715–20. [PubMed] [Google Scholar]
- 26.Dhikav V, Sindhu S, Anand KS. Adverse drug reaction monitoring in India. J Indian Acad Clin Med. 2004;5:27–33. [Google Scholar]
- 27.Rajesh R, Vidyasagar S, Nandakumar K. RETRACTED by plagiarism: Highly active antiretroviral therapy induced adverse drug reactions in Indian human immunodeficiency virus positive patients. Pharm Pract (Granada) 2011;9:48–55. doi: 10.4321/s1886-36552011000100008. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Yadav S, Pillai KK, Causality KP. Assessment, of suspected adverse drug reaction with anti-tubercular therapy by WHO probability scale. J Appl Pharm Sci. 2011;1:26–9. [Google Scholar]
- 29.Ramesh M, Pandit J, Parthasarathi G. Adverse drug reactions in a South Indian hospital – Their severity and cost involved. Pharmacoepidemiol Drug Saf. 2003;12:687–92. doi: 10.1002/pds.871. [DOI] [PubMed] [Google Scholar]
- 30.Hire R, Kale AS, Dakhale GN, Gaikwad N. A prospective, observational study of adverse reactions to drug regimen for multi-drug resistant pulmonary tuberculosis in central India. Mediterr J Hematol Infect Dis. 2014;6:e2014061. doi: 10.4084/MJHID.2014.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shet A, Antony J, Arumugam K, Kumar Dodderi S, Rodrigues R, DeCosta A, et al. Influence of adverse drug reactions on treatment success: Prospective cohort analysis of HIV-infected individuals initiating first-line antiretroviral therapy in India. PLoS One. 2014;9:e91028. doi: 10.1371/journal.pone.0091028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mandavi, D’Cruz S, Sachdev A, Tiwari P. Adverse drug reactions & their risk factors among Indian ambulatory elderly patients. Indian J Med Res. 2012;136:404–10. [PMC free article] [PubMed] [Google Scholar]
- 33.Tandon VR, Khajuria V, Raina K, Mahajan V, Sharma A, Gillani Z, et al. First Indian study evaluating role of biochemical investigations and diagnostic tools in detection of adverse drug reactions. J Clin Diagn Res. 2014;8:HC23–6. doi: 10.7860/JCDR/2014/8487.4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lucca JM, Madhan R, Parthasarathi G, Ram D. Identification and management of adverse effects of antipsychotics in a tertiary care teaching hospital. J Res Pharm Pract. 2014;3:46–50. doi: 10.4103/2279-042X.137063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feely J, Moriarty S, O’Connor P. Stimulating reporting of adverse drug reactions by using a fee. BMJ. 1990;300:22–3. doi: 10.1136/bmj.300.6716.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pankaj G, Aditya U. Adverse drug reaction reporting and pharmacovigilance: Knowledge, attitudes and perceptions amongst resident doctors. J Pharm Sci Res. 2011;3:1064–9. [Google Scholar]
- 37.Milton S, Lee Philip R. Pills, Profits, and Politics. California: University of California Press; 1974. [Google Scholar]
- 38.WHO. The Safety of Medicines in Public Health Programmes: Pharmacovigilance an Essential Tool. Geneva: Uppsala Monitoring Centre; 2006. [Google Scholar]
- 39.Office of Technology Assessment US Congress. Defensive Medicine and Medical Malpractices. Washington, D.C: US Government; 1994. [Google Scholar]
- 40.Dale TA, Wojtowycz M. Malpractice, defensive medicine and obstetric behaviour. Med Care. 1997;35:172–91. doi: 10.1097/00005650-199702000-00007. [DOI] [PubMed] [Google Scholar]
- 41.Kohn LT, Corrigan JM, Donaldson MS. Washington, D.C: Institute of Medicine, National Academy Press; 1996. To Err is Human: Building a Safer Health System Committee on Quality of Health Care in America. [PubMed] [Google Scholar]
- 42.Gardner P, Cluff LE. The epidemiology of ADRs: A review and perspective. Johns Hopkins Med J. 1970;126:77–87. [PubMed] [Google Scholar]
- 43.Paton A. Medical nemesis: Three views: Medicalization of health. Br Med J. 1974;5944:573–4. doi: 10.1136/bmj.4.5944.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dixon M, Bodenheimer T. Health Service in Crisis: Essays on Health Service under capitalism. San Francisco: Synthesis Publication; 1980. [Google Scholar]
- 45.Thomas BS. The transnational pharmaceutical industry and the health of the world's people. In: McKinlay JB, editor. Issues in the Political Economy of Health-Care. New York: Tavistock; 1985. [Google Scholar]
- 46.WHO. Quality Assurance of Pharmaceuticals: A Compendium of Guidelines and Related Materials: Good Manufacturing Practices and Inspection. 2nd ed. India: WHO; 2007. [Google Scholar]
- 47.Etkin LN. Side effects: Cultural constructions and reinterpretation of western pharmaceuticals. Med Anthropol Q. 1992;6:99–113. [Google Scholar]
- 48.WHO. Health and Environment: Tools for Effective Decision Making: Review of Initial Findings. WHO. UNEP Health and Environment Linkages Initiative (HELI) Geneva: WHO; 2004. [Google Scholar]


