Abstract
Nipah virus (NiV) encephalitis first reported in “Sungai Nipah” in Malaysia in 1999 has emerged as a global public health threat in the Southeast Asia region. From 1998 to 2018, more than 630 cases of NiV human infections were reported. NiV is transmitted by zoonotic (from bats to humans, or from bats to pigs, and then to humans) as well as human-to-human routes. Deforestation and urbanization of some areas have contributed to greater overlap between human and bat habitats resulting in NiV outbreaks. Common symptoms of NiV infection in humans are similar to that of influenza such as fever and muscle pain and in some cases, the inflammation of the brain occurs leading to encephalitis. The recent epidemic in May 2018 in Kerala for the first time has killed over 17 people in 7 days with high case fatality and highlighted the importance of One Health approach. The diagnosis is often not suspected at the time of presentation and creates challenges in outbreak detection, timely control measures, and outbreak response activities. Currently, there are no drugs or vaccines specific for NiV infection although this is a priority disease on the World Health Organization's agenda. Antivirals (Ribavirin, HR2-based fusion inhibitor), biologicals (convalescent plasma, monoclonal antibodies), immunomodulators, and intensive supportive care are the mainstay to treat severe respiratory and neurologic complications. There is a great need for strengthening animal health surveillance system, using a One Health approach, to detect new cases and provide early warning for veterinary and human public health authorities.
Keywords: Global health security, Kerala, Nipah virus, One Health, paramyxovirus, Pteropus bat species
Introduction
Nipah virus (NiV) encephalitis is an emerging infectious disease of public health importance in the World Health Organization (WHO) Southeast Asia region. NiV is an enveloped, negative-sense, single-stranded RNA virus in the family Paramyxoviridae, genus henipavirus. The name of the virus and disease is from the village of “Sungai Nipah” in Malaysia where the virus was recognized in 1999 during an outbreak among pig farmers.[1] Both animal-to-human and human-to-human transmission have been documented. From 1998 to 2015, more than 600 cases of NiV human infections were reported. Subsequent outbreaks in India and Bangladesh have occurred with high case fatality. A total of 276 cases were reported with 106 fatalities (38%) in Malaysia, but case fatalities in later outbreaks in India and Bangladesh were associated with significantly higher case fatality rates of 43–100%.[2] NiV infection in humans has a range of clinical presentations, from asymptomatic infection to acute respiratory syndrome and fatal encephalitis. The natural reservoir of the virus consists of the widely distributed fruit bats from the Pteropodidae family.[3] Virus transmission from bats to humans occurs through inhalation, contact, or consumption of NiV contaminated foods.[4] NiV is transmitted by zoonotic (from bats to humans, or from bats to pigs, and then to humans) as well as human-to-human routes. Human-to-human transmission is particularly notable in the outbreaks in India and Bangladesh, where it has been reported to account for 75 and 51% of cases, respectively. At present no vaccines or antiviral drugs are available for NiV disease and the treatment is just supportive.[5] Current prevention strategies focus on raising disease awareness in affected areas.
Historical Perspective: From 1998 to 2018
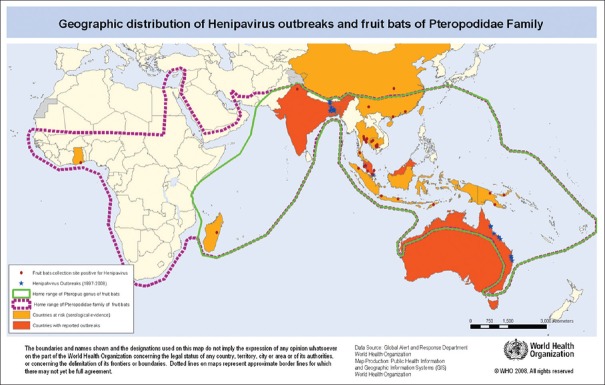
NiV was first recognized in 1999 during an outbreak among pig farmers in Kampung Sungai Nipah, Malaysia. Human NiV infection was first recognized in a large outbreak of 276 reported cases in Peninsular Malaysia and Singapore from September 1998 through May 1999.[6,7,8] But there are no new outbreaks that have been reported in Malaysia and Singapore since 1999. Three years later, a genetically distinct NiV independently emerged in India as well as in Bangladesh, where human NiV outbreak events have been reported nearly every year since. A putative NiV also caused an outbreak of the disease in horses and people in the Philippines in 2014. NiV was first recognized in Bangladesh in 2001 and nearly annual outbreaks have occurred in that country with periodic disease events in eastern India bordering to Bangladesh. Other regions may be at risk for NiV infection, as serologic evidence for NiV has been found in the known natural reservoir namely Pteropus bats. There are over 50 species of Pteropus bats in South and Southeast Asian region,[9] including Cambodia, Thailand, Indonesia, Madagascar, Ghana, and the Philippines, as shown in Figure 1.
Figure 1.
A schematic representation of the distribution of Pteropus genus (green line) and Pteropodidae family of fruit bats. Source: WHO
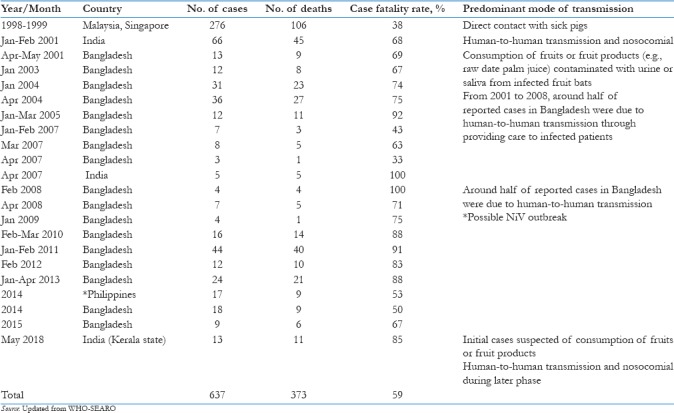
Cases of NiV are reported in Bangladesh almost every year, with high mortality and constituting a public health threat. Up to March 31, 2012, a total of 209 human cases of NiV infection in Bangladesh were reported; 161 (77%) of them died. India reported two outbreaks of NiV encephalitis in the eastern state of West Bengal, bordering Bangladesh, in 2001 and 2007. Seventy-one cases with 50 deaths (70% of the cases) were reported in two outbreaks. During January and February 2001, an outbreak of febrile illness with neurological symptoms was observed in Siliguri, West Bengal.[10] The clinical material obtained during the Siliguri outbreak was retrospectively analyzed for evidence of NiV infection. NiV-specific immunoglobulin M (IgM) and IgG antibodies were detected in 9 out of 18 patients. Reverse transcription-polymerase chain reaction (RT-PCR) assays detected RNA from NiV in urine samples from five patients. A second outbreak was reported in 2007 in Nadia district of West Bengal. Thirty cases of fever with acute respiratory distress and/or neurological symptoms were reported and five cases were fatal. All five fatal cases were found to be positive for NiV by RT-PCR. The morbidity and mortality data of human NiV infection in Malaysia, India, and Bangladesh from 1999 to May 23, 2018 are presented in Table 1.
Table 1.
Morbidity and mortality and modes of transmission of Nipah virus encephalitis, Southeast Asia region, 1999 to May 23, 2018
Outbreaks of Nipah in Southeast Asia have a strong seasonal pattern and a limited geographical range. All the outbreaks occurred during winter and spring (December–May). This could be associated with several factors such as the breeding season of the bats, increased shedding of virus by the bats, and the date palm sap harvesting season.[10]
Virology
NiV was first isolated by Chua et al. in 1999 after a severe outbreak of viral encephalitis among pig farmers in Malaysia.[11] The virus, cultured from the cerebrospinal fluid of two patients, was causing the syncytial formation of Vero cells after 5 days, and it was found to be a previously undescribed paramyxovirus related to the Hendra virus (HeV). The henipavirus genus in the subfamily Paramyxovirinae (family Paramyxoviridae) was then created for these two pathogenic viruses, HeV and NiV.[12] Subsequently, other viruses were added to this genus. NiV is an enveloped, negative-sense, single-stranded RNA virus. The genome is unusually large, comprising more than 18,000 nucleotides. Its six genes code are the nucleocapsid (N), phosphoprotein (P), matrix protein (M), fusion glycoprotein (F), attachment glycoprotein (G), and the large polymerase. The viral G protein attaches to the host cell ephrin B2 and/or B3 receptor, and activates the F protein to initiate viral envelope and host membrane fusion and viral entry.[13] A relative heterogeneity has been observed among nucleotide sequences obtained from Bangladesh/India as compared with sequences from samples obtained during the initial Malaysian outbreak. This observation led to the classification of two distinct lineages of NiV. Currently, the available sequences obtained from Malaysia and Cambodia were designated genotype M, while sequences obtained from Bangladesh and India were designated genotype B.[14,15] A 729 nucleotide region of the N protein gene of NiV has been proposed that can be used for such genotyping. Predicted amino acid identities between NiV and the NiV diversity observed among isolates from bats in Malaysia (NiV-M) and Thailand (NiV-T) were associated with co-circulation of multiple strains within populations rather than co-evolutionary patterns.[15] NiV-M and NiV-B range from 92 to 100%.[16] In Bangladesh, genetic heterogeneity in human isolates suggests multiple introductions of NiV in the human population from different colonies of fruit bats.
Reservoir of virus
Fruit bats of the genus Pteropus have been identified as natural reservoirs of NiV. A seroepidemiologic study in Malaysia implicated four fruit bat species, Pteropus hypomelanus, Pteropus vampyrus, Cynopterus brachyotis, Eonycteris spelaea, and an insectivorous bat, Scotophilus kuhlii.[17] NiV has been isolated from the brain and spinal fluid of victims in Malaysia.[18] Infective virus has also been isolated from environmental samples of bat urine and partially eaten fruit in Malaysia.[19] Given the distribution of the locally abundant fruit bats in South Asia, NiV outbreaks are likely to continue to occur in affected countries. The bats are migratory.[20] This has generated intensive surveillance for evidence of NiV infection in bats in these countries. Evidence of NiV could be demonstrated in Pisaster giganteus in Bangladesh.[21] NiV has been isolated from Lyle's flying fox (Pteropus lylei) in Cambodia[22] and viral RNA found in urine and saliva from Pteropus lylei and Horsfield's roundleaf bat (Hipposideros larvatus) in Thailand.[23] Antibodies to a Nipah-like virus have been found in sera from fruit bats collected in India, Indonesia, and Timor-Leste.[24] The status of NiV infection in other countries of the Southeast Asia region is not known.
Mode of transmission
Infected bats shed virus in their excretion and secretion such as saliva, urine, semen, and excreta, but they are symptomless carriers. The NiV is highly contagious among pigs, spread by coughing. Direct contact with infected pigs was identified as the predominant mode of transmission in humans when it was first recognized in a large outbreak in Malaysia in 1999.[25] Ninety percent of the infected people in the 1998–1999 outbreaks were pig farmers or had contact with pigs.
There is strong evidence that emergence of bat-related viral infection communicable to humans and animals has been attributed to the loss of natural habitats of bats. As the flying fox habitat is destroyed by human activity, the bats get stressed and hungry, their immune system gets weaker, their virus load goes up, and a lot of virus spills out in their urine and saliva.[26] Similar fluctuation of virus shedding may be associated with the stressful physiological conditions or seasons. Evidence of seasonal preference of transmission in P. lylei was recently demonstrated in a study in Thailand. The period April–June was the time (highest in May) when viral RNA could be mainly detected in urine, which was associated with a fluctuation of population numbers that was observed only in May and correlated with young bats leaving to fly.
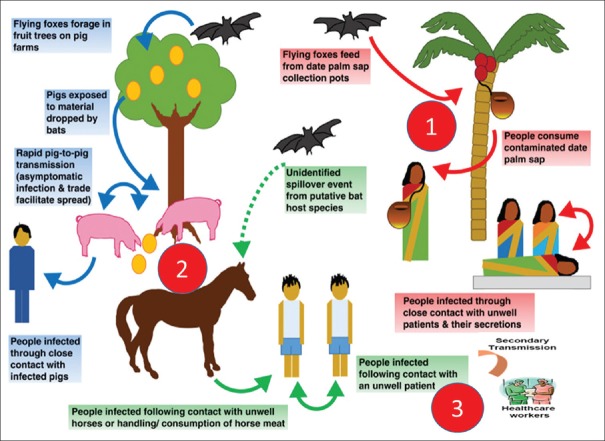
There were focal outbreaks of NiV in Bangladesh and India in 2001 during winter. Drinking of fresh date palm sap, possibly contaminated by fruit bats (P. giganteus) during the winter season, may have been responsible for indirect transmission of NiV to humans.[27] The consumption of date palm sap (which is also known as toddy, kallu, tuak, and tuba in other countries) is popular in a number of Southeast Asian countries, including Bangladesh, India, Indonesia, and Thailand as well as countries such as Malaysia and the Philippines. Fruit bats also consume date palm sap and can contaminate it with saliva, urine, and faeces. This is the means by which NiV is thought to be transmitted from infected fruit bats to humans.[28] There is circumstantial evidence of human-to-human transmission in India in 2001. During the outbreak in Siliguri, 33 health workers and hospital visitors became ill after exposure to patients hospitalized with NiV illness, suggesting nosocomial infection.[29] During the Bangladesh outbreak, the virus is suggested to have been transmitted either directly or indirectly from infected bats to humans. Strong evidence indicative of human-to-human transmission of NiV was found in Bangladesh in 2004. The various modes of transmission are described below in Figure 2.[30]
Figure 2.
Schematic representation of three modes of Nipah virus spread: (1) bat-to-human, (2) animal-to-human, and (3)human-to- human (including nosocomial)
Clinical Presentation and Diagnosis
Symptoms of NiV infection in humans are similar to that of influenza such as fever and muscle pain. In some cases, inflammation of the brain occurs leading to disorientation or coma. Encephalitis may present as acute or late onset. The latter may be difficult to diagnose because exposure may have taken place several months earlier. Further, those who may have recovered from an acute episode may also have a relapse. Nevertheless, magnetic resonance of the brain is helpful in differentiating Nipah encephalitis from other encephalitis as well as in defining between acute and late onset or a relapsed form of the disease. The brain magnetic resonance imaging in relapsing encephalitis shows more extensive and confluent hyperintense cortical lesions.
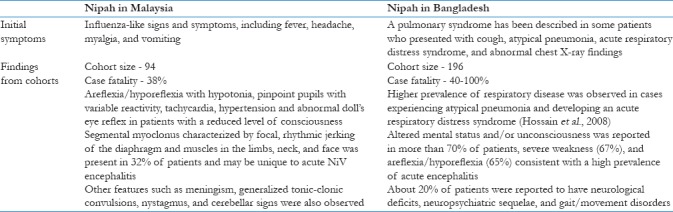
In the majority of cases, the incubation period of Nipah has been reported to be 5 days to 2 weeks; however, a maximum delay of 2 months between exposure and the onset of illness has also been observed during the outbreak in Malaysia.[25] There was a high case fatality in the recurrent epidemics in Bangladesh.[31] The characteristics of these epidemics are shown in Table 2.
Table 2.
Characteristics of Nipah virus disease in Malaysia and Bangladesh
In a large cohort of patients who survived, the majority had no or few sequelae. However, approximately 20% of patients were reported to have neurological deficits, neuropsychiatric sequelae, and gait/movement disorders. The most intriguing complication of Nipah is probably relapsing encephalitis, which may occur weeks to years after symptomatic infection and even after asymptomatic NiV infection. So far, more than 20 cases of relapsing NiV encephalitis have been reported, one of which occurred 11 years after an asymptomatic infection. Clinical and radiological findings suggest that relapsing NiV encephalitis is distinct from acute NiV encephalitis. Pathological features include disseminated, multiorgan vasculopathy comprising endothelial infection/ulceration, vasculitis, vasculitis-induced thrombosis/occlusion, parenchymal ischemia/microinfarction, and parenchymal cell infection in the central nervous system (CNS), lung, kidney, and other major organs. This unique dual pathogenic mechanism of vasculitis-induced microinfarction and neuronal infection causes severe tissue damage in the CNS.
Diagnosis
Initial signs and symptoms of NiV infection are nonspecific, and the diagnosis is often not suspected at the time of presentation. This can hinder accurate diagnosis and creates challenges in outbreak detection and institution of effective and timely infection control measures and outbreak response activities. In addition, clinical sample quality, quantity, type, the timing of collection, and the time necessary to transfer samples from patients to the laboratory can affect the accuracy of laboratory results.[32]
NiV infection can be diagnosed together with clinical history during the acute and convalescent phase of the disease. Main tests including RT-PCR from bodily fluids as well as antibody detection via enzyme-linked immunosorbent assay (ELISA). Different tests include:
ELISA
PCR assay
Virus isolation by cell culture.
NiV is classified internationally as a biosecurity level 4 agent,[5] and the laboratory at National Institute of Virology (ICMR), Pune, India is prepared to diagnose NiV in the country.
Clinical Management
There are currently no drugs or vaccines specific for NiV infection, although this is a priority disease on the WHO R&D Blueprint. Intensive supportive care is recommended to treat severe respiratory and neurologic complications.[32]
Antiviral drugs
Ribavirin
Ribavirin is a guanosine analog and broad-spectrum nucleoside antimetabolite antiviral drug that features on the WHO Essential Medicines List. An inhalation solution of ribavirin is also indicated for the treatment, in young children, of severe lower respiratory tract infections due to the respiratory syncytial virus, another paramyxovirus.
Other antiviral drugs
In view of the questionable efficacy of ribavirin and/or chloroquine and the severity of NiV infections in people, a 36 amino acid HR2-based fusion inhibitor (NiV-Fc2), analogous to the approved HIV-specific therapeutic peptide enfuvirtide, has been proposed as a specific therapy against henipaviruses.[33]
Biologicals
Convalescent plasma
The recent pathogen outbreaks, such as Ebola viral disease or Middle East respiratory syndrome coronavirus, have renewed attention to convalescent plasma and immunoglobulins. In case of severe disease, when no treatment with a proven record of safety and efficacy is available, they may appear as the only available therapeutic option.
Monoclonal antibodies
Monoclonal antibodies targeting the surface glycoproteins of HeV have shown efficacy against both HeV and NiV as pre- and postexposure prophylaxis in animal models, but as these antibodies must be administered before the onset of clinical signs, they are unlikely to be useful for treating symptomatic patients, while probably beneficial for postexposure prophylaxis in potentially exposed individuals.[34]
Host-directed interventions
Immunomodulators
The innate immune response to NiV infection is thought to alter the pathogenic process that is induced, offering the option of a therapeutic approach based on immunomodulation. A derivative of synthetic polyinosinic: polycytidylic acid (poly-IC12U or Rintatolimod), an analogue of double-stranded RNA which strongly activates IFN production, has been shown effective in limiting disease and increasing survival of NiV-infected hamsters. When administered at 3 mg/kg of body weight daily from the day of infection to 10 days postinfection, prevented the mortality in 5 of 6 infected animals.[35]
Adjunctive therapies
As for other severe diseases of viral origin, aggressive supportive care may help improve patient survival. NiV infections, especially as seen in Bangladesh, are associated to respiratory disease and respiratory failure. Oxygen supplementation and eventually transfer to ICU are part of the management guidelines of this infection. Ensuring patient access to the best medical practices in this area should remain a priority.
NIV Surveillance and Prevention Strategies
Controlling NiV in domestic animals
Currently, there are no vaccines available against NiV. Routine and thorough cleaning and disinfection of pig farms (with appropriate detergents) may be effective in preventing infection. If an outbreak is suspected, the animal premises should be quarantined immediately. Culling of infected animals – with close supervision of burial or incineration of carcasses – may be necessary to reduce the risk of transmission to people. Restricting or banning the movement of animals from infected farms to other areas can reduce the spread of the disease. As NiV outbreaks in domestic animals have preceded human cases, establishing an animal health surveillance system, using a One Health approach, to detect new cases is essential in providing early warning for veterinary and human public health authorities.[32]
Reducing the risk of infection in people
In the absence of a licensed vaccine, the only way to reduce infection in people is by raising awareness of the risk factors and educating people about the measures they can take to reduce exposure to and decrease infection from NiV. Public health education should focus on the strategies listed in Table 3.
Table 3.
Strategies for reducing the risk of Nipah virus infection

Controlling infection in healthcare settings
Healthcare workers caring for patients with suspected or confirmed NiV infection, or handling specimens from them, should implement standard infection control precautions for all patients at all times. As human-to-human transmission in particular nosocomial transmission has been reported, contact and droplet precautions should be used in addition to standard precautions. Samples taken from people and animals with suspected NiV infection should be handled by trained staff working in suitably equipped laboratories.
“One Health” Approach for NiV Epidemic
The One Health initiative is a growing movement to promote collaboration between the fields of medicine, veterinary medicine, and environmental sciences to improve the interconnected health of people, animals, and ecosystems.[36] The importance of such an approach is particularly obvious in the field of infectious disease, as 75% of all emerging infectious diseases are zoonotic.[37] NiV exemplifies the need for a One Health approach. The first known outbreak in Malaysia in 1998, killed 105 people and required the culling of over one million pigs.[38] The virus initially causes fever, headaches, and vomiting in infected people, which can progress to severe encephalitis (inflammation of the brain), respiratory disease, and often death.[39] Outbreaks of NiV also began to be recorded approximately annually in Bangladesh starting in 2001, with an average fatality rate of 74.5%.[40] The natural reservoir hosts of NiV are large fruit bats known as flying foxes, which are not known to suffer clinical disease when infected.[41]
Interactions between humans, animals, and the environment are key factors behind NiV outbreaks. Deforestation and urbanization of some areas in Bangladesh have contributed to greater overlap between human and bat habitats. By promoting human–bat interactions, this overlap can increase the risk of “spillover” events, with NiV crossing the species barrier and infecting people. For example, NiV can be shed on fruit discarded by infected bats. Domesticated animals which consume this fruit can become a vector for NiV transmission to people. In isolated cases, cows, pigs, and goats have been implicated in NiV outbreaks in Bangladesh in this manner.[42] However, a more important route through which humans acquire NiV from bats is the consumption of raw date palm sap, a delicacy in Bangladesh. The sap is collected in jars underneath trees, where it can be contaminated by virus-containing secretions from bats. Once infected, people can transmit NiV to each other, which may cause local epidemics.
Vaccines are a potentially powerful tool to directly prevent and limit NiV outbreaks in Bangladesh and beyond, but their role should be evaluated in light of the ecological complexities of NiV transmission, including deforestation, human–bat interactions, and human-to-human transmission.
The current May 2018 outbreak in Kerala, southern India
The latest outbreak occurred in Kerala state, which has a population of 34 million. The first index case was detected in Perambra (Kozhikode district) where the three members of a family have died in the process of cleaning the old well infected by the bats. Along with infection from bats, there are some nosocomial infections and a total of 11 (8 from Kozhikode District and 3 from Malappuram District) people died out of 13 confirmed cases.[43] A multidisciplinary Central team from National Centre for Disease Control, including Epidemiologist, Pulmonologist, Emergency Medicine specialist, an expert in Zoonosis and Animal Husbandry, was set up for investigation. Some bats have been caught and have been sent for lab examination and a total of 60 different samples have been collected from the spot. The field team has advised hospitals to follow intracranial pressure guidelines; use personal protective equipment (PPE) for healthcare workers and sample collection; assist in enhancing active fever surveillance in the community; strengthen contact tracing in close contacts of cases, relatives, healthcare workers; ensure isolation facilities, ventilator support, and hospital infection control practices; and coordinate with animal sector and enhance surveillance for unusual illness and deaths in animals. Appropriate steps to contain this virus have been taken among domestic animals such as pigs.[44] All the contacts are under observation and steps to avoid exposure through animal vectors have been taken and efforts are in place to reduce the panic created by social media by educating people that NiV is not an airborne infection and there is no risk of contracting disease without coming in contact with an infected person.
Global Initiatives and Implications for Global Health Security
Deadly epidemics have been threatening humanity since our earliest days. The recent 2013–2016 outbreak of Ebola virus disease[45] in West Africa and the Zika virus in South America in 2016[46] taught us a lesson: Epidemics can only be prevented when health systems are prepared for them. In 2017, with US $540 million, the Coalition for Epidemic Preparedness Innovations (CEPI) was launched and aims to develop vaccines for diseases identified by the WHO that have high epidemic potential but no vaccine or curative options such as NiV.[47,48] The Center for Infectious Disease Research and Policy (CIDRAP) at the University of Minnesota, with support from the Wellcome Trust and in collaboration with the WHO, has been tasked with facilitating the collaborative development of a draft “Nipah R&D Roadmap” to prioritize the development of diagnostics, therapeutics, and vaccines that are most needed by Nipah-affected countries.[49]
The WHO's International Health Regulations (IHR) aim to provide a legal framework for the prevention, detection, and containment of public health risks at source, before they spread across borders, through the collaborative actions of States Parties and WHO. There is a close link between globalization, urbanization, and the behavior of emerging viruses in the modern era, which can be addressed well through “One Health.” Approaches to such a potential global health security threat should be consistent, proactive, and should involve coordinated, multipronged, multilateral collaborative efforts that actively engage local, regional, national, and global levels.[50,51]
Conclusions
Currently, NiV is an emerging infectious disease of public health significance for the countries in the Southeast Asia region, which is a natural habitat for the fruit bats. As NiV can be transmitted by various methods, there is a potential public health threat globally. Because NiV is an issue to be addressed by multiple stakeholders to promote health to all citizens, the concept of global health diplomacy holds a great promise to address the needs of global health security through its binding or nonbinding instruments enforced by the global governance institutions (e.g., the WHO's IHR).[52] The ministries of health and stakeholders (e.g. CEPI, CIDRAP) need to work together to develop a vaccine and ensure health security from this bat-borne disease. There is a great need to strengthen intersectoral coordination, review the treatment procedures, infection control practices, and ensure use of PPE and availability of drugs to handle the suspected cases in a better way.
Acknowledgement
The authors would like to acknowledge all the authors and researchers of the articles that were reviewed in preparing this manuscript.
References
- 1.Anno Outbreak of Hendra-like virus – Malaysia and Singapore, 1998–99. Morb Mortal Wkly Rep. 1999;48:265–9. [PubMed] [Google Scholar]
- 2.WHO. Nipah virus outbreaks in the WHO South. East Asia Region. Surveillance and outbreak Alert. [Last accessed on 2018 May 25]. Available from: http://www.searo.who.int/entity/emerging_diseases/links/nipah_virus_outbreaks_sear/en .
- 3.Yob JM, Field H, Rashdi AM, Morrissy C, van der Heide B, Rota P, et al. Nipah virus infection in bats (order Chiroptera) in peninsular Malaysia. Emerg Infect Dis. 2001;7:439–41. doi: 10.3201/eid0703.010312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chua KB, Koh CL, Hooi PS, Wee KF, Khong JH, Chua BH, et al. Isolation of Nipah virus from Malaysian Island flying-foxes. Microbes and Infection. 2002;4:145–51. doi: 10.1016/s1286-4579(01)01522-2. [DOI] [PubMed] [Google Scholar]
- 5.WHO SEARO. Nipah Virus Infection. [Last accessed on 2018 May 25]. Available from: http://www.searo.who.int/entity/emerging_diseases/links/CDS_Nipah_Virus.pdf?ua=1 .
- 6.Chua KB, Bellini WJ, Rota PA, Harcourt BH, Tamin A, Lam SK, et al. Nipah virus: A recently emergent deadly paramyxovirus. Science. 2000;288:1432–5. doi: 10.1126/science.288.5470.1432. [DOI] [PubMed] [Google Scholar]
- 7.Paton NI, Leo YS, Zaki SR, Auchus AP, Lee KE, Ling AE, et al. Outbreak of Nipah-virus infection among abattoir workers in Singapore. Lancet. 1999;354:1253–6. doi: 10.1016/S0140-6736(99)04379-2. [DOI] [PubMed] [Google Scholar]
- 8.Chua KB. Nipah virus outbreak in Malaysia. J Clin Virol. 2003;26:265–75. doi: 10.1016/s1386-6532(02)00268-8. [DOI] [PubMed] [Google Scholar]
- 9.Nowak RM, Walker EP, Kunz TH, Pierson ED. Walker's bats of the world. JHU Press; 1994. [Google Scholar]
- 10.Surveillance and outbreak. Nipah virus outbreaks in the WHO South-East Asia Region. [Last accessed on 2018 May 25]. Available from: http://www.searo.who.int/entity/emerging_diseases/links/nipah_virus_outbreaks_sear/en .
- 11.Chua KB, Goh KJ, Wong KT, Kamarulzaman A, Tan PS, Ksiazek TG, et al. Fatal encephalitis due to Nipah virus among pig-farmers in Malaysia. Lancet. 1999;354:1257–9. doi: 10.1016/S0140-6736(99)04299-3. [DOI] [PubMed] [Google Scholar]
- 12.Wang L, Harcourt BH, Yu M, Tamin A, Rota PA, Bellini WJ, et al. Molecular biology of Hendra and Nipah viruses. Microbes Infect. 2001;3:279–87. doi: 10.1016/s1286-4579(01)01381-8. [DOI] [PubMed] [Google Scholar]
- 13.Ong KC, Wong KT. Henipavirus encephalitis: Recent developments and advances. Brain Pathol. 2015;25:605–13. doi: 10.1111/bpa.12278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lo MK, Rota PA. Molecular virology of the henipaviruses. Curr Top Microbiol Immunol. 2012;359:41–58. doi: 10.1007/82_2012_211. [DOI] [PubMed] [Google Scholar]
- 15.Angeletti S, Lo Presti A, Cella E, Ciccozzi M. Molecular epidemiology and phylogeny of Nipah virus infection: A mini review. Asian Pac J Trop Med. 2016;9:630–4. doi: 10.1016/j.apjtm.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rockx B, Winegar R, Freiberg AN. Recent progress in henipavirus research: Molecular biology, genetic diversity, animal models. Antiviral Res. 2012;95:135–49. doi: 10.1016/j.antiviral.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Yob JM, Field H, Rashdi AM, Morrissy C, van der Heide B, Rota P, et al. Nipah virus infection in bats (order Chiroptera) in Peninsular Malaysia. Emerg Infect Dis. 2001;7:439–41. doi: 10.3201/eid0703.010312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chua KB, Bellini WJ, Rota PA, Harcourt BH, Tamin A, Lam SK, et al. Nipah virus: A recently emergent deadly paramyxovirus. Science. 2000;288:1432–5. doi: 10.1126/science.288.5470.1432. [DOI] [PubMed] [Google Scholar]
- 19.Chua KB, Koh CL, Hooi PS, Wee KF, Khong JH, Chua BH, et al. Isolation of Nipah virus from Malaysian Island flying-foxes. Microbes Infect. 2002;4:145–51. doi: 10.1016/s1286-4579(01)01522-2. [DOI] [PubMed] [Google Scholar]
- 20.Eaton BT, Broder CC, Middleton D, Wang LF. Hendra and Nipah viruses: Different and dangerous. Nat Rev Microbiol. 2006;4:23–35. doi: 10.1038/nrmicro1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsu, VP, Hossain, MJ, Parashar UD, Ali MM, Ksiazek TG, Kuzmin I, et al. Nipah virus encephalitis reemergence, Bangladesh. Emerg Infect Dis. 2004;10:2082–7. doi: 10.3201/eid1012.040701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reynes JM, Counor D, Ong S, Faure C, Seng V, Molia S, et al. Nipah virus in Lyle's flying foxes, Cambodia. Emerging Infectious Diseases. 2005;11:1042. doi: 10.3201/eid1107.041350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wacharapluesadee S, Lumlertdacha B, Boongird K, Wanghongsa S, Chanhome L, Rollin P, et al. Bat Nipah virus, Thailand. Emerg Infect Dis. 2005;11:1949–51. doi: 10.3201/eid1112.050613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heymann DL. 19th edition. American Public Health Association; 2008. Henipavirus: Hendra and Nipah viral diseases. Control of Communicable Diseases Manual; pp. 275–8. [Google Scholar]
- 25.Goh KJ, Tan CT, Chew NK, Tan PSK, Kamarulzaman A, Sarji SA, et al. Clinical features of Nipah virus encephalitis among pig farmers in Malaysia. N Engl J Med. 2000;342:1229–35. doi: 10.1056/NEJM200004273421701. [DOI] [PubMed] [Google Scholar]
- 26.Halpin K, Young PL, Field HE, Mackenzie JS. Isolation of Hendra virus from pteropid bats: A natural reservoir of Hendra virus. J General Virology. 2000;81:1927–32. doi: 10.1099/0022-1317-81-8-1927. [DOI] [PubMed] [Google Scholar]
- 27.Luby SP, Rahman M, Hossain MJ, Blum LS, Husain MM, Gurley E, et al. Foodborne transmission of Nipah virus, Bangladesh. Emerg Infect Dis. 2006;12:1888–94. doi: 10.3201/eid1212.060732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.ICDDR B. Nipah outbreak in Faridpur District, Bangladesh, 2010. HSB (Health Science Bulletin) 2010:8. [Google Scholar]
- 29.Chadha MS, Comer JA, Lowe L, Rota PA, Rollin PE, Bellini WJ, et al. Nipah virus-associated encephalitis outbreak, Siliguri, India. Emerg Infect Dis. 2006;12:235–40. doi: 10.3201/eid1202.051247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gurley E, Montgomery JM, Hossain MJ, Bell M, Azad AK, Islam MR, et al. Person-to-person transmission of Nipah virus in a Bangladeshi community. Emerg Infect. 2007;13:1031–7. doi: 10.3201/eid1307.061128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rahman M, Chakraborty A. Nipah virus outbreaks in Bangladesh: A deadly infectious disease. WHO South-East Asia J Public Health. 2012;1:208–12. doi: 10.4103/2224-3151.206933. [DOI] [PubMed] [Google Scholar]
- 32.WHO. Nipah Virus Fact Sheets. [Last accessed on 2018 May 24]. Available from: http://www.who.int/news-room/fact-sheets/detail/nipah-virus .
- 33.Steffen DL, Xu K, Nikolov DB, Broder CC. Henipavirus mediated membrane fusion, virus entry and targeted therapeutics. Viruses. 2012;4:280–308. doi: 10.3390/v4020280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Satterfield BA, Cross RW, Fenton KA, Agans KN, Basler CF, Geisbert TW, et al. The immunomodulating V and W proteins of Nipah virus determine disease course. Nat Commun. 2015;6:7483. doi: 10.1038/ncomms8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Georges-Courbot MC, Contamin H, Faure C, Loth P, Baize S, Leyssen P, et al. Poly(I)-poly(C12U) but not ribavirin prevents death in a hamster model of Nipah virus infection. Antimicrob Agents Chemother. 2006;50:1768–72. doi: 10.1128/AAC.50.5.1768-1772.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.One Health Commission. [Last accessed on 2018 May 22]. https://www.onehealthcommission.org/
- 37.Vorou RM, Papavassiliou VG, Tsiodras S. Emerging zoonoses and vector-borne infections affecting humans in Europe. Epidemiol Infect. 2007;135:1231–47. doi: 10.1017/S0950268807008527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chua KB, Bellini WJ, Rota PA, Harcourt BH, Tamin A, Lam SK, et al. Nipah virus: A recently emergent deadly paramyxovirus. Science. 2000:288. doi: 10.1126/science.288.5470.1432. [DOI] [PubMed] [Google Scholar]
- 39.Looi L-M, Chua K-B. Lessons from the Nipah virus outbreak in Malaysia. Malays J Pathol. 2007;29:63–7. [PubMed] [Google Scholar]
- 40.WHO, Nipah virus outbreaks in the WHO South-East Asia Region. [Last accessed on 2017 Apr 30]. http://www.searo.who.int/entity/emerging_diseases/links/nipah_virus_outbreaks_sear/en/
- 41.Mire CE, Satterfield BA, Geisbert JB, Agans KN, Borisevich V, Yan L, et al. Pathogenic differences between Nipah virus Bangladesh and Malaysia strains in primates: Implications for antibody therapy. Sci Rep. 2016;6:30916. doi: 10.1038/srep30916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luby SP, Gurley ES, Hossain MJ. Transmission of human infection with Nipah virus. Clin Infect Dis. 2009;49:1743–8. doi: 10.1086/647951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ministry of Health and Family Welfare. Press release 23 May. 2018. [Last accessed on 2018 May 25]. Available from: http://www.searo.who.int/india/topics/emergencies/mohfw-niv-press-release-23may2018.pdf .
- 44.Ministry of Health and Family Welfare. Press release 22 May. 2018. [Last accessed on 2018 May 25]. Available from: http://www.searo.who.int/india/topics/emergencies/mohfw-niv-press-release-22may2018.pdf .
- 45.Chattu VK. Politics of Ebola and the critical role of global health diplomacy for the CARICOM. J Family Med Primary Care. 2017;6:463–7. doi: 10.4103/jfmpc.jfmpc_75_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sikka V, Chattu VK, Popli RK, Galwankar SC, Kelkar D, Sawicki SG, et al. The emergence of zika virus as a global health security threat: A review and a consensus statement of the INDUSEM Joint Working Group (JWG) J Global Infect Dis. 2016;8:3–15. doi: 10.4103/0974-777X.176140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.CEPI. [Last accessed on 2018 May 22]. http://www.cepi.net/mission .
- 48.WHO, WHO publishes list of top emerging diseases likely to cause major epidemics. [Last accessed on 2018 May 22]. http://www.who.int/medicines/ebola-treatment/WHO-list-of-top-emerging-diseases/en/
- 49.WHO. Nipah R&D. Available from: http://www.who.int/blueprint/priority-diseases/key-action/nipah/en .
- 50.Kalra S, Kelkar D, Galwankar SC, Papadimos TJ, Stawicki SP, Arquilla B, et al. The emergence of Ebola as a global health security threat: From “lessons learned” to coordinated multilateral containment efforts. J Glob Infect Dis. 2014;6:164–77. doi: 10.4103/0974-777X.145247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wojda TR, Valenza PL, Cornejo K, McGinley T, Galwankar SC, Kelkar D, et al. The Ebola outbreak of 2014-2015: From coordinated multilateral action to effective disease containment, vaccine development, and beyond. J Glob Infect Dis. 2015;7:127–38. doi: 10.4103/0974-777X.170495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chattu VK. The rise of global health diplomacy: An interdisciplinary concept linking health and international relations. Indian J Public Health. 2017;61:134–6. doi: 10.4103/ijph.IJPH_67_16. [DOI] [PubMed] [Google Scholar]