The problem of new psychoactive substance (NPS) abuse, which includes synthetic cannabinoids, is emerging globally, and the cardiotoxicity of these synthetic cannabinoids has not yet been evaluated extensively.
The problem of new psychoactive substance (NPS) abuse, which includes synthetic cannabinoids, is emerging globally, and the cardiotoxicity of these synthetic cannabinoids has not yet been evaluated extensively.
Abstract
The problem of new psychoactive substance (NPS) abuse, which includes synthetic cannabinoids, is emerging globally, and the cardiotoxicity of these synthetic cannabinoids has not yet been evaluated extensively. In the present study, we investigated the effects of synthetic cannabinoids on the cytotoxicity, human Ether-à-go-go-related gene (hERG) channel, action potential duration (APD), and QT interval. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay showed that JWH-030 was more cytotoxic than JWH-210, JWH-250, and RCS4 in H9c2 cells at 0.1 μM. In addition, the cytotoxicity was associated with its pro-apoptotic effects as evidenced by the increase in caspase-3 levels. We demonstrated that a cannabinoid receptor type 2 (CB2) antagonist, AM630, inhibited JWH-030-induced cytotoxicity, whereas a CB1 antagonist, rimonabant, did not. Furthermore, fluorescence polarization assay showed JWH-030 to block the hERG channel (half-maximal inhibitory concentration, IC50 was 88.36 μM). JWH-030 significantly reduced the APD at 90% repolarization (APD90) in rabbit Purkinje fibers and decreased the left ventricular end diastolic pressure (LVEDP) in Langendorff-perfused Sprague-Dawley (SD) rat hearts at 30 μM. In addition, the electrocardiogram (ECG) measurement revealed that the intravenous injection of JWH-030 (0.5 mg kg–1) prolonged the QT interval in SD rats. These results suggest that JWH-030 is cytotoxic and its cytotoxicity is mediated by its action on the CB2 receptor; it prolongs the QT interval by regulating ion current channels and APD.
Introduction
New psychoactive substances (NPS) have diverse adverse effects including cardiovascular, neurological, gastrointestinal, and pulmonary effects. Currently, there is a dearth of knowledge on many types of NPS. Most of the available data on NPS-induced toxicity are derived from retro- or prospectively analyzed cases of intoxication as well as from interviews with drug users, and therefore, are of limited scientific value.1 Therefore, to evaluate the life-threatening adverse effect of NPS, preclinical studies are required to study their toxicity. However, most preclinical research studies have investigated the dependence potential and neuropsychiatric effects of NPS. Synthetic cannabinoids are one of the most abused NPS and have dependence liability potential that is similar to that of natural and botanical components. A few studies reported that synthetic cannabinoids induced cardiovascular symptoms including tachycardia and myocardial infarction.2 However, the cardiotoxicity of synthetic cannabinoids has not yet been evaluated extensively. Arrhythmia is one of the most life-threatening adverse cardiovascular effects and monitoring telemetry for arrhythmia is recommended for patients who use synthetic cannabinoids. Certain drugs may cause undesirable effects by delaying cardiac repolarization, which can be defined as a prolongation of the QT interval on a surface electrocardiogram (ECG).3 The QT interval, which represents the duration of ventricular depolarization and subsequent repolarization, is measured from the beginning of the QRS complex to the end of the T wave. The QT interval has been used as a surrogate biomarker to assess drug-induced ventricular repolarization effects, although it inaccurately predicts proarrhythmia. The molecular mechanism underlying the adverse QT-prolonging effects of these drugs is thought to be mediated via the blockade of the delayed rectifier potassium channel (IKr), encoded by the human Ether-à-go-go-related gene (hERG).4 In the present study, we investigated the cardiotoxicity and proarrhythmic effects of the synthetic cannabinoids, JWH-210, JWH-030, JWH-250, and RCS4 in vitro, ex vivo, and in vivo. The cytotoxicity and pro-apoptotic effects of the synthetic cannabinoids were evaluated in H9c2 cells. We also performed hERG channel binding assays, APD measurements in rabbit Purkinje fibers, evaluated cardiac function in isolated hearts, and analyzed the QT interval obtained from the ECG of Sprague-Dawley (SD) rats. Furthermore, a pharmacological rescue study of the cytotoxicity of the agents was performed using CB1 and CB2 antagonists.
Experimental
Animals
All experimental procedures were approved by the Animal Ethics Committee, National Institute of Food and Drug Safety Evaluation, and complied with the Guide for the Care and Use of Laboratory Animals (National Research Council, NRC, 1996). All efforts were made to minimize animal distress and prevent suffering. Male SD rats (7-week-old) were obtained from the Ministry of Food and Drug Safety (Association for Assessment and Accreditation of Laboratory Animal Care, AAALAC member, Osong, Republic of Korea) and male New Zealand white rabbits (2890–3380 g) were obtained from Samtako Inc., (Osan, Republic of Korea). The rats and rabbits were maintained in an animal facility where they were transferred to cages on arrival and were housed in adequate group sizes. They were allowed to acclimatize for 1 week before being used in the experiments. The animal holding rooms were maintained at a temperature of 21–24 °C and 40–60% relative humidity with a 12 h light/dark cycle (light on 08:00 to 20:00). The animals received a solid diet and tap water ad libitum.
Materials
All the synthetic cannabinoids tested (JWH-210, JWH-030, JWH-250, and RCS4, illustrated in Fig. 1) were purchased from Cayman Chemical (Ann Arbor, MI, USA). The rimonabant hydrochloride and AM630 were purchased from Sigma-Aldrich (St Louis, MO, USA) while the H9c2 (CRL-1446) cell line was obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). The other routine chemicals were purchased from Sigma-Aldrich (St Louis, MO, USA) unless otherwise specified. The normal Tyrode solution used contained sodium chloride (NaCl, 143 mM), magnesium chloride (MgCl2, 0.5 mM), HEPES (5.0 mM), sodium dihydrogen phosphate (NaH2PO4, 0.33 mM), glucose (10 mM), and calcium chloride (CaCl2 1.8 mM). The Krebs–Henseleit buffer contained NaCl (118 mM), potassium chloride (KCl, 4.7 mM), CaCl2 (1.5 mM), magnesium sulfate (MgSO4, 1.66 mM), sodium bicarbonate (NaHCO3, 24.88 mM), potassium dihydrogen phosphate (KH2PO4, 1.18 mM), glucose (5.55 mM), Na-pyruvate (2 mM), and bovine albumin (0.1% w/v).
Fig. 1. Chemical structures of synthetic cannabinoids.
Effects of synthetic cannabinoids on cell viability
The H9c2 cells were maintained in Dulbecco's modified Eagle's medium (DMEM, ATCC) supplemented with 10% fetal bovine serum (FBS, Invitrogen, Carlsbad, CA, USA) and 1% antibiotics/antimycotics (Invitrogen, Carlsbad, CA, USA) under an atmosphere of 95% air and 5% CO2 using standard culture methods. The cells were plated in a 96-well plate (2 × 104 cells per well) for the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. After overnight incubation, the medium was removed and replaced with fresh medium containing varying concentrations (0.1–100 μM) of JWH-210, JWH-250, JWH-030, and RCS4. The cells were also co-treated with rimonabant or AM630 (CB1 or CB2 receptor antagonist, respectively). The final concentration of dimethyl sulfoxide (DMSO) in all cases did not exceed 0.5% (v/v), which was found to be nontoxic to all the cell lines tested. After incubation, the cells were treated with MTT (0.5 mg mL–1) for 4 hours under an atmosphere of 95% air and 5% CO2 at 37 °C, and then the dark-purple crystals that had formed were dissolved by adding 100 μL of solubilization buffer to each well. Subsequently, the plates were incubated overnight under an atmosphere of 95% air and 5% CO2 at 37 °C, and the optical density (OD) of the reaction solution was measured at 570 nm using a micro-plate reader (SpectraMAX M5, Molecular Device, Sunnyvale, CA, USA). The cell viability was calculated as a percentage of the value of the control cells incubated with fresh medium only.
Effects of synthetic cannabinoids on caspase-3 levels
The H9c2 cells were plated in a 96-well plate (2 × 104 cells per well) with 100 μL fresh medium containing 100 μM of JWH-030. The caspase assay was performed using the ApoTox-Glo™ Triplex assay (Promega, Madison, WI, USA) according to the manufacturer's protocol. In brief, after incubation, the cells were treated with 20 μL of the viability/cytotoxicity reagent for 1 hour at 37 °C, and the fluorescence was measured at 485 and 520 nm. Then, 100 μL of the caspase-Glo 3/7 reagent was added to each well, followed by 30 minutes of incubation at 27 °C and then the luminescence was measured.
hERG channel binding assay
The propensity of the test substances to block the hERG potassium channel was measured by using a Predictor™ hERG Fluorescence Polarization assay (PV5365, Invitrogen, Carlsbad, CA, USA). The test substances were prepared at 100× in DMSO and then diluted to 4 × (4% DMSO) in assay buffer. The wells contained 100 μL assay buffer while 100 μL of the hERG membrane samples was used for the blank wells. Then, 50 μL of the test substances (JWH-210 and JWH-030) were dispensed into a 96-well plate at a final concentration of 0.01–2500 μM into each well. Then, 100 μL of 2× Predictor hERG membranes were dispensed into each well, followed by 50 μL of 4× Predictor hERG Tracer Red. Each substance was tested in the absence and presence of 30 μM E-4031 to correct for possible test compound interference. The plates were incubated for 4 hours prior to measuring the fluorescence polarization using a SpectraMAX iX3 (Molecular Device, Sunnyvale, CA, USA) at excitation and emission wavelengths of 530 and 585 nm, respectively. The half-maximal inhibitory concentration (IC50) of each substance was calculated using SoftPro 6.2.2 (Molecular Devices, Sunnyvale, CA, USA).
hERG channel patch clamp assay
A patch clamp assay was used to measure hERG current as described in our previous study by Korea Research Institute of Chemical Technology (KRICT, Daejeon, Korea).3 Briefly, human embryonic kidney (HEK293) cells stably expressing hERG (HEK293-hERG cells; Genionics, Switzerland)5 were used to assess cardiac toxicity. The cells were grown at 37 ± 2 °C in the presence of 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (Invitrogen, Carlsbad, CA, USA) and 1 mM glutamine. When the cells had reached 80% confluency, the medium was removed with an aspirator and the cells were washed twice with phosphate-buffered saline (PBS). Next, 3 mL of 0.05% trypsin–EDTA : Vergene (1 : 1, v/v, Invitrogen, Carlsbad, CA, USA) solution was added to the flask, and the cells were incubated for 1–2 min. The cell suspension was transferred to a sterile tube, and the cells were spun down in a centrifuge for 1 min (1100 rpm, 25 °C). After fresh medium was added to the tube, the cells were counted using a hemocytometer, and the density was adjusted to 2–3 × 105 cells per mL with medium. For the patch clamp experiment, the cells were centrifuged and resuspended in 150 μL of extracellular solution. The cells were then applied to the AutoPatch system (PatchXpress 7000A, Molecular Devices, CA, USA). hERG currents were elicited by 1 s voltage pulses to +20 mV, from a holding potential of –80 mV followed by repolarization back to –50 mV for 1 s and returned to a holding potential of –80 mV. Data were collected at a frequency of 0.1 Hz. All experiments included a buffer control without the test compound to enable accurate IC50 determination. JWH-030 was applied from a low to a high concentration, and the tail current was monitored continuously. The data were saved automatically into the PatchXpress database, and the IC50 values were derived automatically from curve-fitting plots with data from six concentrations.
Effects of synthetic cannabinoids on APD
The APD assay was performed by Korea Institute of Toxicology (KIT, Daejeon, Korea). The hearts were isolated from the ventricles of the New Zealand white rabbits (Yac:NZW) as described in a previous study.6 The rabbits were anesthetized using sodium pentobarbital (100 mg kg–1, intravenous, i.v., Hanlim Pharmaceuticals, Seoul, Republic of Korea), then the Purkinje fibers were removed from the hearts, and this procedure was performed in normal Tyrode solution. Then, the tissues were fixed to the bottom of a chamber using thin pins and continuously superfused with oxygenated normal Tyrode solution at a flow rate of 5 mL min–1. The temperature in the chamber was maintained at 37.0 ± 1.0 °C. The Purkinje fibers were stimulated at 1 Hz for 2 ms, 13–30 V for 1 hour. The intracellular potential was recorded using glass microelectrodes filled with 3 M potassium chloride (KCl) with tip resistances of 15.2–41.1 MΩ. Then, the tissues were stabilized with normal Tyrode at a rate of 5 mL min–1 for 40 minutes. After the baseline values had been stabilized, JWH-210 or JWH-030 (0.3, 3, or 30 μM) was superfused with the normal Tyrode solution in the chamber, and the data were recorded at 20 and 40 minutes from the stabilization period. The APD at 50% repolarization (APD50) and APD90 (in ms), resting membrane potential, total amplitude (TA), and the maximum upstroke velocity (Vmax) of the phase 0max depolarization values were determined from the recordings.
Effects of synthetic cannabinoids on isolated rat hearts
The effects of the synthetic cannabinoids on the isolated rat heart were measured using a Langendorff apparatus. The SD rats were anesthetized with sodium pentobarbital (50 mg kg–1, i.v.), the abdominal cavities were opened by making a transverse incision using scissors, and then the isolated hearts were placed into ice-cold modified Krebs–Henseleit buffer. Then, the isolated hearts were rapidly set up in the Langendorff perfusion apparatus through the aortas. The modified Krebs–Henseleit buffer saturated with carbogen (95% O2 and 5% CO2) was previously set at a constant flow rate (9.7 ± 0.5 mL min–1). To measure the pharmacodynamic response, a latex balloon was tied to the end of a polyethylene tube, which was connected to a pressure transducer, and was carefully inserted into the left ventricle of the isolated heart. JWH-210 and JWH-030 were added to the K–H buffer at final concentrations of 3 or 30 μM. The data were analyzed at 1, 3, and 5 minutes. The three pharmacodynamic endpoints, which were the left ventricular systolic pressure (LVSP), left ventricular end-diastolic pressure (LVEDP) and left ventricular developed pressure (LVDP) were measured and monitored continuously using a physiological recording system (AD Instrument, Spain). Additionally, the LVDP was defined as LVDP = LVSP – LVEDP.
Effects of synthetic cannabinoids on surface ECG
The standard lead III ECG was recorded before and after administration of the drugs. The SD rats were anesthetized with sodium pentobarbital (50 mg kg–1, intraperitoneal, i.p.) followed by inhalation of 1% isoflurane (100% O2). Then, the unconscious rats were restrained in the supine position and their body temperature was maintained by placing a rubber bag filled with warm water under each animal. Before the ECGs were recorded, the ventral thoracic region of the anesthetized rats was carefully shaved using an electric clipper. The three lead surface ECG was recorded with the needle electrodes placed under the chest skin at optimized positions to obtain maximal amplitude recording to ensure the accurate measurement of the QT interval. The ECG signals were acquired and analyzed by using an Animal Bio Amp and PowerLab 8/35 and the LabChart software (AD Instrument, Colorado Springs, CO, USA). LabChart software calculates the QRS complex, isoelectric level, P wave, and T wave as follows. The R wave is identified as the most positive value in the neighborhood of the beat marker. The start and end of the QRS complex are determined on each side of the R wave for regions where the slope (dV/dt) falls to sufficiently low values. The isoelectric level is the median of all data values preceding QRSstart. The P peak is the point of greatest absolute deviation from the isoelectric level in an interval from pre-P to just before QRSstart. The T wave is the first significant peak of either sign, starting from a point after QRSend. If a suitable peak is found, a straight line is fitted by least squares to the tail of the wave, over a data range 70–30% of the T peak. The intersection of this line with the isoelectric level is Tend.
After an acclimatization of at least 15 minutes, the vehicle (DMSO : saline, 1 : 9) or JWH-030 (0.1 or 0.5 mg kg–1) was administered i.v. at an injection rate of 1 mL kg–1 min–1. We chose the i.v. administration route according to a previous report7 and assumed that the dose of JWH-030 (0.5 mg kg–1) may have induced a blood concentration of approximately 30 μM, which is in the dose range of APD assay and isolated heart experiments, according to the equation, total blood volume (mL) = 0.06 × body weight + 0.77, of Lee and Blaufox.8 The QT intervals represented the mean value of the 1 minute measurements. The QT intervals were corrected by using Bazett's equation: QTc = QT/(RR)1/2. The difference between the basal QTc interval (average QTc interval of ten consecutive recordings before the drug injection, QTcbasal) and each QTc interval (QTceach) was designated as delta QTc (ΔQTc = QTceach – QTcbasal).
Quantification of JWH-030 in rat serum
We measured the serum concentration of JWH-030 according to previous reports with modification.9 Rats (JWH-030, 0.5 mg kg–1 group) were decapitated and 1.2 mL of blood was collected with 30 μL of 100 mM EDTA immediately after the final measurement of ECG. Samples were centrifuged (10 min, 14 000 rpm, 4 °C) and supernatants were stored at –80 °C until analysis. To the 0.5 mL serum, 0.25 mL trizma buffer (242 g trizma base in 1 L deionized water, pH adjusted to 9.2 with hydrochloric acid) and 1.25 mL of 1 chlorobutane containing 10% of isopropanol were added and mixed thoroughly. The sample was extracted for 6 min on a shaker at 1400 rpm. After a brief centrifugation to separate layers, the solvent layer was transferred to an Eppendorf tube and evaporated to dryness using a centrifugal vaporizer (CUE-200D, EYELA, Tokyo, Japan) operated at 27 °C. The residue was reconstituted in 250 μL of methanol and 5 μL of the final extract was injected into the LC-MS/MS system: Agilent 6460 triple quadrupole mass spectrometer operated in the electrospray ionization (ESI) and Agilent 1200 series HPLC system (Agilent, Palo Alto, CA, USA). Gradient elution was performed on a Luna C18 (2.0 × 150 mm, 3 μm, 100 Å, Phenomenex, Torrance, CA, USA) column. The mobile phase consisted of 0.1% formic acid in deionized water (eluent A) and 0.1% formic acid in acetonitrile (eluent B). The flow rate was set at 0.3 mL min–1, the gradient was programmed as follows: 0.00–1.00 min, 5% eluent B, 1.01–4.0 min, eluent B increasing to 95%, 4.01–6.00 min, 95% eluent B, 6.01–6.10 min, eluent B decreasing to 5%, 6.11–10.00 min, starting conditions (5% eluent B) to re-equilibrate the column. The mass spectral data were acquired with the following ESI inlet conditions: nebulizing gas was at a flow rate of 5 L min–1, and the capillary voltage was set to 3.5 kV. The mass spectrometer was operated in the positive mode of multiple reaction monitoring (MRM). The JWH-030 transitions monitored were m/z 292.2 > 127 and the collision energy was 40 V.
Data analysis
The data were presented as the means ± standard error (SE) and the statistically significant differences between the drug-treated and vehicle-treated groups were analyzed using the Student's t-test. In addition, a one-way, two-way, or two-way repeated measures analysis of variance (ANOVA) and the Bonferroni's test for equal variance data were performed, followed by Dunnett's rank test for non-equal variance data using the SigmaPlot 13 software (Sigmaplot, Chicago, IL, USA). Furthermore, a one-way ANOVA followed by Dunnett's test was used for the APD analysis (GraphPad Instat, version 3.10, La Jolla, CA, USA) and p-values <0.05 were considered statistically significant.
Results
Synthetic cannabinoids reduced cell viability
To investigate the effects of the synthetic cannabinoids on cell viability, H9c2 cells were treated with 0 (vehicle control), 0.1, 1, 10, or 100 μM of the drugs for 16 hours and an MTT assay was performed. We observed that all the synthetic cannabinoids reduced the cell viability. Especially, JWH-030 reduced the H9c2 cell viability at 0.1 μM while JWH-210, JWH-250, and RCS4 reduced it at 1, 1, and 100 μM, respectively (Fig. 2).
Fig. 2. Effects of synthetic cannabinoids on H9c2 cell viability. Cell viability was measured using MTT assay after the treatment of H9c2 cells with substances for 16 hours at the indicated concentration. Data are means ± standard error (SE, n = 6). Cell viability was expressed as a percentage of that of the control; *p < 0.05 and **p < 0.01 vs. each vehicle-treated control group using one-way analysis of variance (ANOVA), Bonferroni's test. MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide. Naïve, no treatment.
JWH-030 decreased the viability and increased the caspase-3 levels of H9c2 cells
We aimed to determine if JWH-030, which was the most cytotoxic substance tested, induced cell apoptosis. The results revealed that treatment with JWH-030 (100 μM, 4 hours) increased the caspase-3 levels, showed cytotoxicity, and reduced the viability of H9c2 cells (Fig. 3); however, the caspase-3 level was not upregulated after a longer incubation duration (16 hours, data not shown). These results suggest that JWH-030 induced cytotoxicity through apoptosis, at least in part.
Fig. 3. Effects of JWH-030 on H9c2 cytotoxicity and caspase-3 levels. (A) Cell viability, (B) cytotoxicity, and (C) caspase-3 levels were measured using ApoTox-Glo™ Triplex assay. Cells were treated with JWH-030 (100 μM) for 4 hours. Data are means ± standard error (SE, n = 8); *p < 0.05 and **p < 0.01 vs. each vehicle-treated control group, one-way analysis of variance (ANOVA) and Bonferroni's test.
AM630 rescued the JWH-030-induced decrease in H9c2 cell viability
To examine the role of CB1 and CB2 in the decreased cell viability, the H9c2 cells were treated with the specific antagonist for these receptors and JWH-030 (10 and 100 μM for 4 and 16 hours, respectively), and then the MTT assay was performed. As shown in Fig. 4, the JWH-030 (10 μM for 4 hours)-induced decrease in cell viability was rescued by co-treatment with AM630 but not rimonabant (20 μM). Furthermore, AM630 (20 μM) inhibited the decrease in cell viability of the JWH-030 treated cells (16 hours at 100 μM). These results suggest that CB2 may play a role in the decrement of cell viability induced by JWH-030.
Fig. 4. Effects of cannabinoid receptor antagonists on JWH-030-induced decrease in cell viability. Cell viability was measured using MTT assay after the treatment of H9c2 cells with substances for 4 or 16 hours at the indicated concentration (JWH-030, 10 or 100 μM; rimonabant and AM630, 20 μM). Data are means ± standard error (SE, n = 12–24); *p < 0.05 and ##p < 0.01 vs. vehicle-treated control and JWH-030-treated groups, respectively; one-way analysis of variance (ANOVA) and Bonferroni's test; **p < 0.01 and ##p < 0.01 vs. vehicle-treated control and JWH-030 treated groups, respectively; two-way ANOVA and Bonferroni's test.
JWH-030 significantly bound to and blocked the hERG channel
To investigate the binding properties of JWH-210 and JWH-030 to the hERG K+ channel, the hERG fluorescence polarization assay was performed. The cells were incubated with the drugs at concentrations of 0.001–2500 μM and the IC50 values of JWH-030 and JWH-210 were calculated to be 91.72 μM and 3670 μM, respectively (Fig. 5). Based on the binding property of JWH-030 to the hERG channel, we used the patch clamp assay to measure the inhibition property of JWH-030 to the hERG current. The IC50 value of JWH-030 was calculated to be 88.36 μM (Fig. 5).
Fig. 5. Human Ether-à-go-go-related gene (hERG) channel binding and inhibition properties of JWH-210 and JWH-030. (A) Representative graph shows the concentration–response curve of JWH-210 and JWH-030 in fluorescence polarization assay. The half-maximal inhibitory concentration (IC50) values of JWH-030 and JWH-210 were 91.72 μM and 3670 μM, respectively. (B) Representative graph shows the concentration–response curve of JWH-030 in patch clamp assay. The half-maximal inhibitory concentration (IC50) value of JWH-030 was 88.36 μM.
JWH-030 reduced APD90
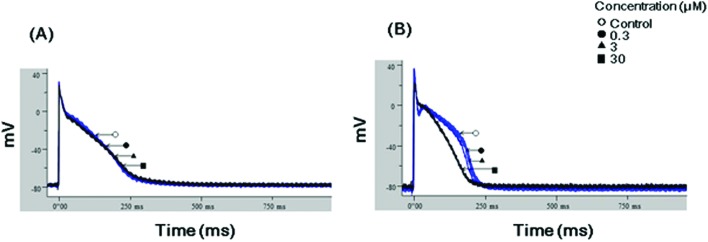
To clarify the effects of JWH-210 and JWH-030 on repolarization, the APD assay was performed in rabbit Purkinje fibers. As Fig. 6 shows, a continuous flow of JWH-030 (30 μM) significantly reduced the APD90 but had no effects on the APD50, RMP, Vmax, and TA of the isolated rabbit Purkinje fibers (Tables 1 and 2); however, JWH-210 did not alter the APD90.
Fig. 6. Effects of JWH-030 and JWH-210 on the action potential duration at 90% repolarization (APD90). Representative graphs show APD90 for (A) JWH-210 and (B) JWH-030 in isolated rabbit Purkinje fibers. JWH-030 significantly reduced APD at 30 μM (refer to Table 1).
Table 1. Concentration-dependent effects of JWH-210 and JWH-030 on the action potential duration (APD) of rabbit Purkinje fibers.
| JWH-210 |
JWH-030 |
|||
| Concentrations | APD50 (ms) | APD90 (ms) | APD50 (ms) | APD90 (ms) |
| Vehicle control | 158.4 ± 25.2 | 244.4 ± 14.5 | 170.1 ± 15.1 | 229.2 ± 6.2 |
| 0.3 μM | 158.7 ± 26.2 | 247.1 ± 14.2 | 168.0 ± 15.9 | 226.7 ± 6.6 |
| 3 μM | 156.1 ± 25.7 | 245.4 ± 13.2 | 151.9 ± 15.0 | 215.4 ± 5.9 |
| 30 μM | 152.9 ± 26.6 | 245.2 ± 13.5 | 117.5 ± 6.0 | 188.6 ± 4.8** |
Table 2. Concentration-dependent effects of JWH-210 and JWH-030 on the resting membrane potential (RMP), maximum upstroke velocity (Vmax), and total amplitude (TA) in rabbit Purkinje fibers.
| JWH-210 | |||
| Concentrations | RMP (mV) | TA (mV) | V max (V s–1) |
| Vehicle control | –81.9 ± 2.1 | 116.2 ± 4.3 | 328.5 ± 54.5 |
| 0.3 μM | –81.4 ± 1.7 | 116.5 ± 4.1 | 326.1 ± 56.3 |
| 3 μM | –81.4 ± 1.8 | 115.8 ± 4.8 | 319.6 ± 57.7 |
| 30 μM | –80.0 ± 1.7 | 115.0 ± 5.4 | 307.3 ± 56.9 |
| JWH-030 | |||
| Concentrations | RMP (mV) | TA (mV) | V max (V s–1) |
| Vehicle control | –83.6 ± 0.5 | 122.0 ± 1.3 | 362.8 ± 16.7 |
| 0.3 μM | –84.2 ± 2.4 | 119.9 ± 0.7 | 347.0 ± 6.2 |
| 3 μM | –82.1 ± 1.9 | 114.2 ± 3.4 | 288.8 ± 15.3 |
| 30 μM | –84.5 ± 2.5 | 114.9 ± 5.3 | 325.5 ± 36.0 |
JWH-030 reduced the LVEDP of isolated rat hearts
We further examined the effect of JWH-210 and JWH-030 on the isolated rat hearts by using a Langendorff apparatus. We measured the LVSP, LVDP, heart rate, and LVEDP and discovered that JWH-030 reduced the LVEDP at 30 μM but showed no effects on the LVSP, LVDP, and heart rate (Fig. 7 and ESI 1†). In contrast, JWH-210 had no effect on LVEDP.
Fig. 7. Effects of JWH-030 and JWH-210 on isolated heart function. The left ventricular end diastolic pressure (LVEDP) was measured after treatment with substances at the indicated concentration in Langendorff perfused Sprague-Dawley (SD) rat hearts. Data are means ± standard error (SE, n = 4); *p < 0.05 vs. the vehicle-treated control group; one-way analysis of variance (ANOVA) and Bonferroni's test.
JWH-030 induced QT interval prolongation in anesthetized rats
To clarify the effects of JWH-030 on the heart function in vivo, we examine whether JWH-030 changed the QT interval in rats. We demonstrated that the i.v. administration of 0.5 mg kg–1 JWH-030 but not 0.1 mg kg–1 JWH-030 and vehicle prolonged the QTcB and ΔQTcB of SD rats compared to values at 1 minute before the injection (Fig. 8, and ESI 2 and 3†). Furthermore, JWH-030 prolonged the ΔQTcB of SD rats compared to values of the vehicle group at 64, 65, and 66 minutes after the injection. However, JWH-030 had no effect on the RR interval (ESI 2†). In addition, we detected and quantified JWH-030 in the serum of rats (0.5 mg kg–1 group) immediately after the final measurement of ECG. The mean serum concentration of JWH-030 was 10.2 pg mL–1 (ESI 7†).
Fig. 8. Effect of JWH-030 on the QT interval of rats. Surface electrocardiograms (ECGs) were recorded before and after treatment with JWH-030 or vehicle. (A) Representative ECG tracings show electrocardiographic patterns and changes in basal (upper left) and JWH-030 (0.5 mg kg–1) treatment (lower left). The averaged ECG signal and QT interval is illustrated (upper right and lower right). (B) JWH-030 (0.5 mg kg–1) but not 0.1 mg kg–1 of JWH-030 and vehicle treatment prolonged the QTcB interval compared to that of the basal interval. JWH-030 treatment prolonged the QTcB interval compared to that of the vehicle at 64, 65, and 66 minutes after treatment. Open arrow indicates the drug injection time. Data are means ± standard error (SE, n = 4–5); *p < 0.05 vs. –1 (1 minute before treatment), #p < 0.05 vs. vehicle; two-way repeated measures analysis of variance (ANOVA) and Bonferroni's test.
Discussion
Synthetic cannabinoids are one of the most abused novel psychoactive substances. Currently, there is very little information available on the toxicity and pharmacological activity of synthetic cannabinoids, which may mislead people to abuse these substances without considering the potential health risks. We previously reported that the JWH series of compounds induced conditioned place preference in parallel with their CB1R binding affinity.10 However, the cardiotoxicity of synthetic cannabinoids has not yet been fully elucidated. In the present study, we demonstrated the severe harmful effects of JWH-030 on the cardiovascular system. The MTT assay revealed that 0.1 μM JWH-030 reduced the viability of H9c2 cells while JWH-210, JWH-250, and RCS4 also reduced cell viability at 1, 1, and 100 μM respectively. JWH-210 has been detected in the serum/plasma sample of individuals that abuse this drug at concentrations of up to 0.5 μM.11 The 0.1 μM drug concentration used in the MTT assay may be within the range that has been detected in abusers and therefore, we opined that the toxicity of JWH-030, in particular, needed to be further evaluated.
Consequently, we demonstrated that apoptosis might mediate the early events involved in cellular cytotoxicity induced by JWH-030, at least in part. The JWH-030-induced increase in the caspase-3 levels of the H9c2 cells indicates that the caspase cascades may play an important role in the apoptosis induced by synthetic cannabinoids as previously reported.12 Therefore, next, we sought to verify the role of the cannabinoid receptors in JWH-030-induced cytotoxicity. AM630, a CB2 receptor antagonist, rescued the JWH-030-induced decrease in the viability of H9c2 cell but rimonabant, a CB1 antagonist, did not. The synthetic cannabinoid ligands may modulate the myocardial or cerebral ischemia-reperfusion-induced tissue damage via CB2 receptors.13 These results suggest that the CB2 receptor may play a role in the synthetic cannabinoid-induced harmful effects on the cardiovascular system.
According to Hermanns-Clausen et al.14 cardiovascular effects are reported in up to 80% of the patients who used synthetic cannabinoids and, therefore, they might alter blood pressure and heart rate, as well as the ECG. We demonstrated that JWH-030 had a hERG channel binding and inhibiting properties at similar IC50 values. Furthermore, JWH-030 reduced the APD90 in rabbit Purkinje fibers and induced deficits in cardiac functions of rat isolated hearts. However, JWH-210 had no effects on the hERG channel, APD90, and cardiac functions. From the safety pharmacology viewpoint, a significant hERG inhibiting property and APD effects are putative biomarkers for pro-arrhythmia. Furthermore, a hERG channel blockade may induce QT prolongation and consequently evoke torsade de pointes in the ECG.15 It has been suggested that hERG channel inhibition is associated with prolonged APD; however, our APD assay revealed that JWH-030 shortened, rather than prolonged the APD. Other ion channels such as the Na+ and Ca2+ current channels are also related to the induction of APD. Although we did not elucidate the exact mechanisms underlying the effects of JWH-030 on other ion channels, it may induce myocardial action potential shortening. Furthermore, JWH-030 decreased the LVEDP of the isolated rat hearts while JWH-210 had no effects, which indicates that JWH-030 induced the deficit in the ventricular performance. We also observed similar effects of JWH-030 on the beating rates of mice primary cardiomyocytes (ESI 4 and 5†).
It is also important to monitor drug-induced QT prolongation because this effect is associated with a potential risk for ventricular tachycardia and fibrillation.16 Therefore, we next evaluated the in vivo effects of JWH-030 by measuring the ECG of rats. At first, we demonstrated the superiority of Bazett's (QTcB) correction method over that of Fridericia's (QTcF, ESI 6†). The RR–QTcB results showed that Bazett's correction method allowed the detection of relevant effects on the QT/QTc interval in the present study. The QTcB interval was prolonged by i.v. JWH-030 while the vehicle did not change the QT interval. The serum concentration of JWH-030 treated rats was 10.02 pg mL–1 (0.04 μM), which is lower than would be expected from the in vitro and ex vivo studies. Cannabinoids, such as JWH-030 are highly lipid-soluble agents and may be accumulated in the adipose tissue.17,18 Therefore, detection of cannabinoids from serum is usually difficult.18 However, JWH-030 treatment-induced QT prolongation is in accordance with the QT patterns of E-4031 (IKr blocker, 0.2 mg kg–1, i.v.) in SD rats (ESI 3†). The in vitro study also revealed that JWH-030 has the hERG channel binding and blocking properties. Therefore, JWH-030 may prolong the QT interval by blocking the hERG channel (IKr).
Conclusions
Our present study demonstrates that synthetic cannabinoids have harmful effects on the cardiovascular system. All four of the synthetic cannabinoids (JWH-210, JWH-250, JWH-030, and RCS4) investigated in this study, showed varying levels of cytotoxicity against the H9c2 cell. Particularly, JWH-030 induced significant changes in the hERG currents, APD, and heart function. Furthermore, JWH-030 prolonged the QT interval, which may be associated with its adverse cardiovascular effects in synthetic cannabinoid abusers.
Conflict of interest
There are no conflicts of interest to declare.
Supplementary Material
Acknowledgments
We sincerely thank The Animal Facility of NIFDS for generously providing us with laboratory space and animal supply assistance throughout the entire timeline of this study. This study was funded by the Ministry of Food and Drug Safety (grant number 16181MFDS415 and 1518MFDS482).
Footnotes
†Electronic supplementary information (ESI) available. See DOI: 10.1039/c6tx00259e
References
- Hohmann N., Mikus G., Czock D. Dtsch. Arztebl. Int. 2014;111:139–147. doi: 10.3238/arztebl.2014.0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath T. S., Burroughs Z., Thompson A. J., Tecklenburg F. W. J. Pediatr. Pharmacol. Ther. 2012;17:177–181. doi: 10.5863/1551-6776-17.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun J., Hwangbo E., Lee J., Chon C. R., Kim P. A., Jeong I. H., Park M., Park R., Kang S. J., Choi D. Cardiovasc. Toxicol. 2015;15:197–202. doi: 10.1007/s12012-014-9285-8. [DOI] [PubMed] [Google Scholar]
- Giorgi M. A., Bolanos R., Gonzalez C. D., Di Girolamo G. Curr. Drug Saf. 2010;5:54–57. doi: 10.2174/157488610789869148. [DOI] [PubMed] [Google Scholar]
- Steiner F., Ghose S., Thomet U. Methods Mol. Biol. 2010;617:209–221. doi: 10.1007/978-1-60327-323-7_17. [DOI] [PubMed] [Google Scholar]
- Lu H. R., Vlaminckx E., Van Ammel K., De Clerck F. Eur. J. Pharmacol. 2002;452:183–192. doi: 10.1016/s0014-2999(02)02246-x. [DOI] [PubMed] [Google Scholar]
- Tampus R., Yoon S. S., De la Pena J. B., Botanas C. J., Kim H. J., Seo J. W., Jeong E. J., Jang C. G., Cheong J. H. Biomol. Ther. 2015;23:590–596. doi: 10.4062/biomolther.2015.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. B., Blaufox M. D. J. Nucl. Med. 1985;26:72–76. [PubMed] [Google Scholar]
- Ammann J., McLaren J. M., Gerostamoulos D., Beyer J. J. Anal. Toxicol. 2012;36:372–380. doi: 10.1093/jat/bks048. [DOI] [PubMed] [Google Scholar]
- Cha H. J., Lee K. W., Song M. J., Hyeon Y. J., Hwang J. Y., Jang C. G., Ahn J. I., Jeon S. H., Kim H. U., Kim Y. H., Seong W. K., Kang H., Yoo H. S., Jeong H. S. Biomol. Ther. 2014;22:363–369. doi: 10.4062/biomolther.2014.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karinen R., Tuv S. S., Oiestad E. L., Vindenes V. Forensic Sci. Int. 2015;246:98–103. doi: 10.1016/j.forsciint.2014.11.012. [DOI] [PubMed] [Google Scholar]
- Tomiyama K., Funada M. Toxicol. Appl. Pharmacol. 2014;274:17–23. doi: 10.1016/j.taap.2013.10.028. [DOI] [PubMed] [Google Scholar]
- Steffens S., Pacher P. Br. J. Pharmacol. 2012;167:313–323. doi: 10.1111/j.1476-5381.2012.02042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermanns-Clausen M., Kithinji J., Spehl M., Angerer V., Franz F., Eyer F., Auwarter V. Drug Test. Anal. 2016 doi: 10.1002/dta.1936. [DOI] [PubMed] [Google Scholar]
- Vargas H. M., Bass A. S., Koerner J., Matis-Mitchell S., Pugsley M. K., Skinner M., Burnham M., Bridgland-Taylor M., Pettit S., Valentin J. P. Br. J. Pharmacol. 2015;172:4002–4011. doi: 10.1111/bph.13207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah R. R. Br. J. Pharmacol. 2010;159:58–69. doi: 10.1111/j.1476-5381.2009.00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rom S., Persidsky Y. J. Neuroimmune Pharmacol. 2013;8:608–620. doi: 10.1007/s11481-013-9445-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namera A., Kawamura M., Nakamoto A., Saito T., Nagao M. Forensic Toxicol. 2015;33:175–194. doi: 10.1007/s11419-015-0270-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.