Abstract
The purpose of this study was to identify the clinical features and laboratory factors that are predictive of intravenous immunoglobulin (IVIG)-resistant Kawasaki disease. Multiple databases were searched for relevant studies on IVIG-resistant Kawasaki disease published from January 2002 to April 2017. Eligible studies were retrieved by manual review of the references. Stata 12 was used for the meta-analysis. Weighted mean differences and odds ratios with 95% confidence intervals were calculated for several indices. Twenty-eight studies involving 26,260 patients comprising 4442 IVIG-resistant Kawasaki disease patients and 21,818 IVIG-sensitive Kawasaki disease patients were included. The meta-analysis showed that the erythrocyte sedimentation rate (ESR) in the IVIG-resistant group was significantly higher than that in the IVIG-sensitive group, and that platelet count and hemoglobin levels were significantly lower in the IVIG-resistant group. The patients with oral mucosa alterations, cervical lymphadenopathy, swelling of the extremities, polymorphous rash, and initial administration of IVIG ≤ 4.0 days after the onset of symptoms were more likely to be IVIG resistant.
Conclusion: The initial administration of IVIG ≤ 4.0 days after the onset of symptoms increased ESR and decreased hemoglobin and platelet counts, oral mucosa alterations, cervical lymphadenopathy, swelling of the extremities, and polymorphous rash and are the risk factors for IVIG-resistant Kawasaki disease.
|
What is Known:
• Recent reports on this topic are about aspartate aminotransferase (AST), alanine aminotransferase (ALT), gammaglutamyl transferase, total bilirubin, white blood cells, platelets, erythrocyte sedimentation rate (ESR), polymorphonuclear leukocytes (PMN), C-reactive protein (CRP), pro-brain natriuretic peptide (BNP), albumin, and sodium as the risk factors in the IVIG-resistant Kawasaki disease; however, no studies have been published on clinical features as predictors of IVIG resistance. | |
|
What is New:
• This meta-analysis identified the clinical features, the initial administration of IVIG ≤ 4.0 days after the onset of symptoms, and much more comprehensive laboratory indicators, such as hemoglobin, as predictors of IVIG-resistant Kawasaki disease. |
Electronic supplementary material
The online version of this article (10.1007/s00431-018-3182-2) contains supplementary material, which is available to authorized users.
Keywords: Kawasaki disease, Intravenous immunoglobulin, Meta-analysis, Risk
Introduction
Kawasaki disease is an acute, self-limiting, systemic vascular inflammation that mainly affects the small arteries, especially the coronary arteries [39]. It is believed that during the acute period, administering large doses of immunoglobulin can reduce the risk of damage to the coronary arteries; however, 15–20% [30] of patients have intravenous immunoglobulin (IVIG)-resistant Kawasaki disease, and research [3] has shown that the probability of IVIG-resistant Kawasaki disease patients also having coronary artery lesions is nine times greater than that for IVIG-sensitive patients. Because the probability of coronary artery damage associated with IVIG-resistant Kawasaki disease is higher than that with IVIG-sensitive Kawasaki disease, if patients with IVIG-resistant Kawasaki disease can be detected and appropriately treated before additional IVIG treatments, the probability of damage to the coronary arteries would decrease, as well as the cost and hospitalization time.
There are many studies about the risk of IVIG-resistant Kawasaki disease. Japanese scholars Kobayashi et al. [20], Sano et al. [36], and Egami et al. [11] summarized the standards for the prediction of IVIG-resistant Kawasaki disease; American scholars Tremoulet et al. [42], Loomba et al. [27], and Davies et al. [7] also proposed a prediction system for the disease. At Beijing Children’s Hospital, Fu et al. [13], Yan et al. [44], and Choi et al. [6] created a scoring system based on single-center research results. Although insightful, these prediction systems lacked unity.
The aim of this study was to perform a systematic review and meta-analysis of pediatric patients reported over the past 15 years in studies published in several databases to investigate the risk factors associated with IVIG-resistant Kawasaki disease. Our results are expected to be helpful for identifying high-risk factors, providing early treatment, and reducing the occurrence of coronary artery injury in patients affected by this disease.
Materials and methods
Database search
Relevant multicenter or single-center studies conducted from January 2002 to April 2017 on patients with IVIG-resistant Kawasaki disease were searched. The study group was identified as those patients with IVIG-resistant Kawasaki disease, and the control group was patients with IVIG-sensitive Kawasaki disease.
Electronic databases were searched (foreign language databases, PubMed, Medline, OvidMedline, SpringerLink, China Academic Journals Full-text Database, Wanfang Data, VIP Data, and dissertation databases). The search strategy involved studies conducted from January 2002 to April 2017. Keywords used were namely “Kawasaki disease” and “IVIG resistance” or “IVIG unresponsiveness.” A manual search was conducted using reference lists of original articles. Each publication was independently reviewed and relevant information was extracted by two authors (LX and CY).
Study selection and data extraction
The inclusion criteria were as follows: (1) diagnosed with Kawasaki disease according to Japanese diagnostic criteria and the 2017 American Heart Association common standards [14, 31] (i.e., IVIG resistance was defined as persistent or recrudescent fever (T ≥ 38.0 °C) at least 36 h after completion of the first IVIG infusion), (2) odds ratios (ORs) and 95% confidence intervals (CIs) provided for categorical variables in the original data and number and standard deviation provided for continuous variables, and (3) clear description of statistical methods and correct statistical analyses.
The exclusion criteria were as follows: (1) animal studies; (2) defective or poor-quality study design; (3) ORs and 95% CIs not provided for categorical variables and mean and standard deviation not provided directly or indirectly for continuous variables; and (4) review, duplicate, or unpublished literature.
The observation indices were the number of cases and control groups, days of initial administration of IVIG, hemoglobin, platelet count, erythrocyte sedimentation rate (ESR), oral mucosa, conjunctival congestion, cervical lymphadenopathy, swelling of extremities, and polymorphous rash.
A meta-analysis conducted in 2016 by Baek et al. [2] revealed that higher total bilirubin, PMN, BNP, AST, alanine transaminase (ALT), and CRP levels, and lower sodium and albumin levels are predictive of IVIG-resistant Kawasaki disease, but white blood cell count, platelet count, and ESR had no effect as predictors of IVIG-resistant Kawasaki disease. This meta-analysis showed the same results for higher total bilirubin, PMN, pro-BNP, AST, ALT, and CRP levels, and lower sodium, albumin level, and white blood cells has no effect (Supplementary 1). Considering the length of this paper, the data for these measures are not exhibited here. This paper presents the results for the clinical features and laboratory predictive factors as follows: hemoglobin, which Baek et al. [2] did not study, and platelet count and ESR, for which the results differed from those of Baek et al. [2].
Statistical analyses
Statistical analyses were performed using Stata v. 12.0 (STATA Corp, College Station, TX, USA). Both categorical and continuous variable meta-analyses were performed. The continuous variables included platelet count, hemoglobin, and ESR. Analyses determined the relative risk of the disease for specific groups of patients (OR and 95% CI). The categorical variables included the days of initial administration of IVIG, oral mucosa alteration, conjunctival congestion, cervical lymphadenopathy, swelling of extremities, and polymorphous rash. The mean and standard deviation for each group of continuous variables were used to calculate the weighted mean difference (WMD) and 95% CI. Heterogeneity tests were performed with the use of Q and I2 statistics [16]. Values of p ≤ .10 and I2 > 50% suggested there was high statistical heterogeneity among the studies. The random-effects model was used for analysis. When p > .10 and I2 ≤ 50%, there was little or no statistical heterogeneity among the studies; therefore, we chose the fixed-effects model for analysis. A sensitivity analysis was conducted by omitting one study at a time to examine the influence of a single study on the overall effect sizes. Egger’s test was used to investigate publication bias [12]. If the Egger’s test revealed the P value of bias ≥ .1, there was no publication bias. To explore the influence of different regions on IVIG-resistant Kawasaki disease, a series of subgroup analyses were performed with meta-regression. Subgroups were selected based on different regions, such as Asian and non-Asian populations.
Results
Characteristics of included studies
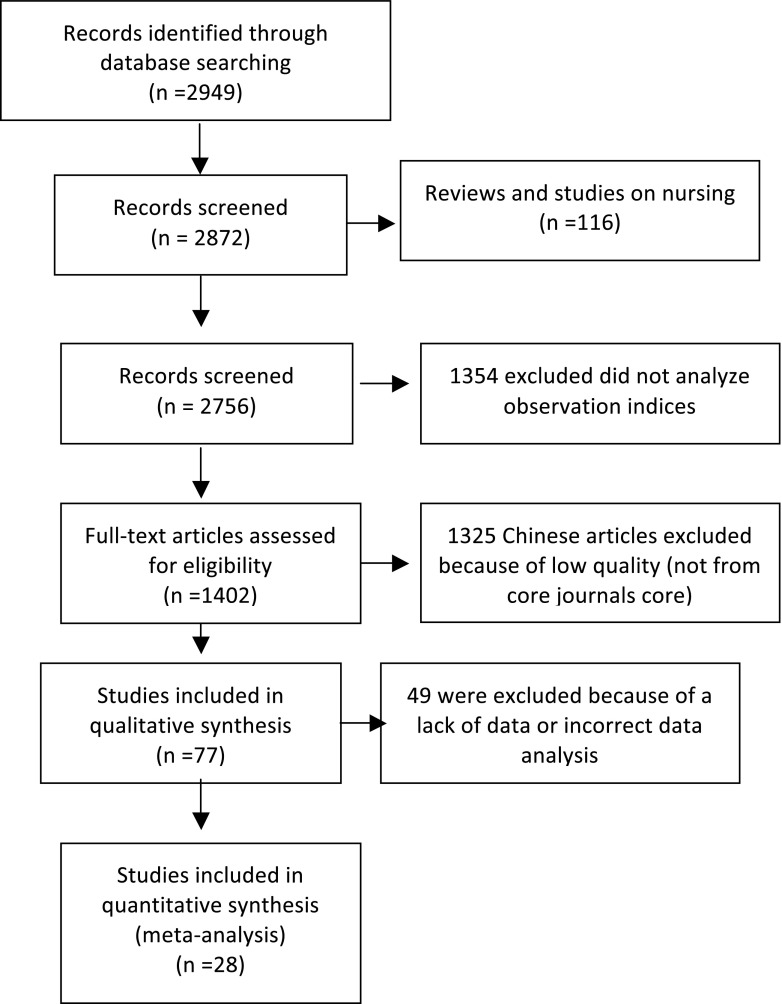
Our database search retrieved 2949 papers comprising 1108 in Chinese journals and 1841 in other journals. We excluded 2872 papers that were reviews, studies on nursing, or duplicate studies, as well as those that did not analyze observation indices, provide detailed data, or conform to our inclusion criteria. Among the 77 papers remaining, 49 were excluded because of a lack of data or incorrect data analysis. Finally, 28 papers with 26,260 cases were selected, with 4442 cases in the IVIG-resistant group and 21,818 in the IVIG-sensitive group (Fig. 1). The studies were conducted in Japan (n = 7), China (n = 10, including four articles from Chinese Taipei, North America (n = 5)), and Korea (n = 6). The general characteristics of the groups from the selected literature are shown in Table 1.
Fig. 1.
Literature selection for the meta-analysis
Table 1.
Characteristics of patients included in the study
| Author | Year | Mean age (months) | Sex (female/male) | Number of patients of IVIG-resistant, n | Number of patients of IVIG-sensitive, n | The rate of IVIG-resistant(%) | Location |
|---|---|---|---|---|---|---|---|
| Durongpisitkul et al. [10] | 2003 | Average 26.35 | 52/68 | 14 | 106 | 11.67% | China |
| Kobayashi et al. [20] | 2006 | 29.1 ± 22.1 | 231/315 | 112 | 434 | 20.51% | Japan |
| Sano et al. [36] | 2007 | Average 27.24 | 59/53 | 22 | 90 | 19.64% | Japan |
| Egami et al. [11] | 2006 | Average 27.6 | 136/184 | 41 | 279 | 12.81% | Japan |
| Muta et al. [28] | 2006 | Average 30.61 | 4822/6544 | 1855 | 9511 | 16.32% | Japan |
| Du et al. [9] | 2006 | 31.2 ± 26.4 | 370/682 | 135 | 917 | 12.83% | China |
| Cha et al. [4] | 2008 | Average 35.37 | 17/34 | 18 | 33 | 35.29% | Korea |
| Uehara et al. [43] | 2008 | – | 2643/3687 | 1286 | 5044 | 20.32 | Japan |
| Ashouri et al. [1] | 2008 | 35.13 | 83/113 | 40 | 156 | 20.41 | North America |
| Tremoulet et al. [42] | 2008 | – | – | 60 | 302 | 16.57 | North America |
| Piao et al. [33] | 2009 | 25.48 | 75/147 | 37 | 185 | 16.67 | China |
| Rigante et al. [35] | 2010 | 23.8 | 12/20 | 5 | 27 | 15.63 | North America |
| Kuo et al. [22] | 2010 | 19.25 | 39/92 | 20 | 111 | 15.27 | China |
| Hwang et al. [17] | 2011 | 28.77 | 103/126 | 23 | 206 | 10.04 | Korea |
| Sleeper et al. [38] | 2011 | 39.22 | 74/124 | 27 | 171 | 13.64 | North America |
| Liu et al. [26] | 2012 | – | 110/268 | 24 | 354 | 6.35 | China |
| Yan et al. [44] | 2012 | 29.92 | 77/142 | 21 | 198 | 9.59 | China |
| Sato et al. [37] | 2013 | 26.7 | 43/62 | 21 | 84 | 20.00 | Japan |
| Fu et al. [13] | 2013 | – | 431/746 | 211 | 966 | 17.93 | China |
| Kim et al. [18] | 2013 | 29.24 | 51/84 | 22 | 113 | 16.30 | North America |
| Ou-Yang et al. [32] | 2013 | 19.86 | 23/40 | 5 | 58 | 7.94 | China |
| Choi et al. [6] | 2014 | 33.47 | 231/342 | 158 | 415 | 27.57 | Korea |
| Lee et al. [24] | 2014 | 38.22 | 44/47 | 11 | 80 | 12.09 | Korea |
| Lin et al. [25] | 2016 | 22.8 | 74/107 | 22 | 159 | 12.15 | China |
| Nakagama et al. [29] | 2016 | 33.75 | 62/109 | 54 | 117 | 31.58 | Japan |
| Kim et al. [19] | 2016 | 31.9 | 302/401 | 118 | 585 | 16.79 | Korea |
| Lee et al. [23] | 2016 | – | 128/159 | 34 | 253 | 11.85 | Korea |
| Tang et al. [41] | 2016 | – | 584/326 | 46 | 864 | 5.05 | China |
Indices of high-risk factors
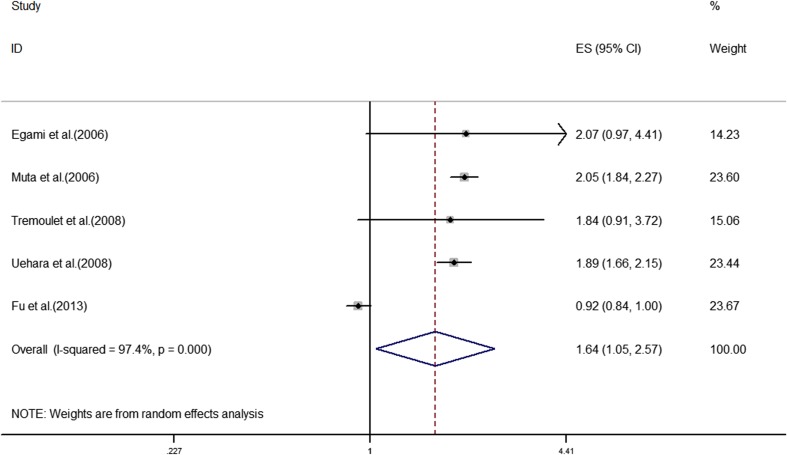
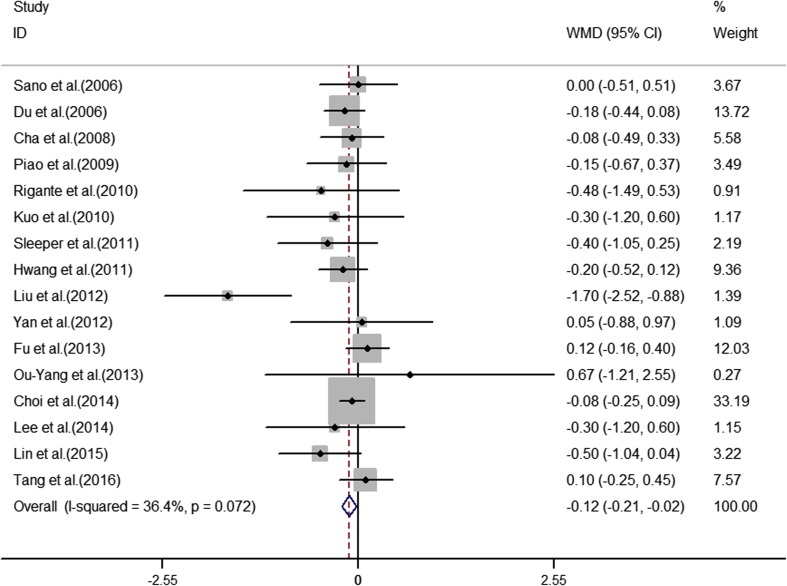
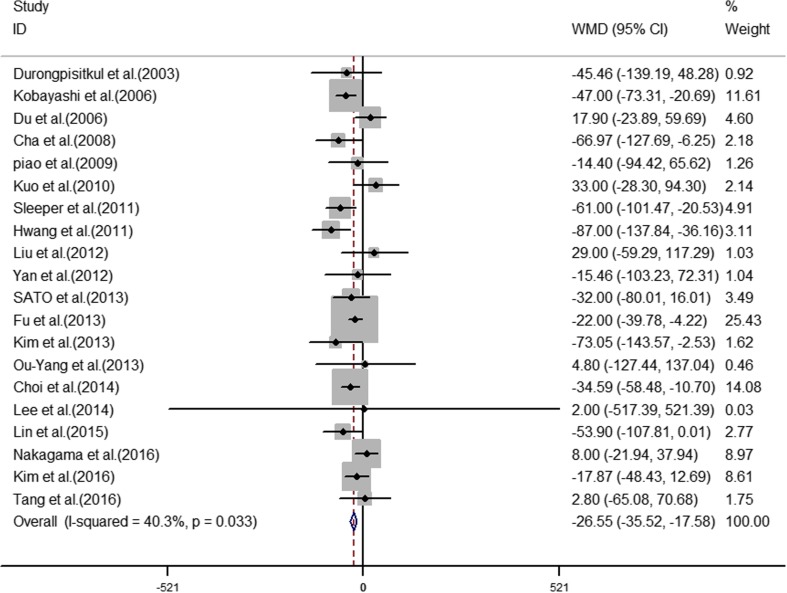
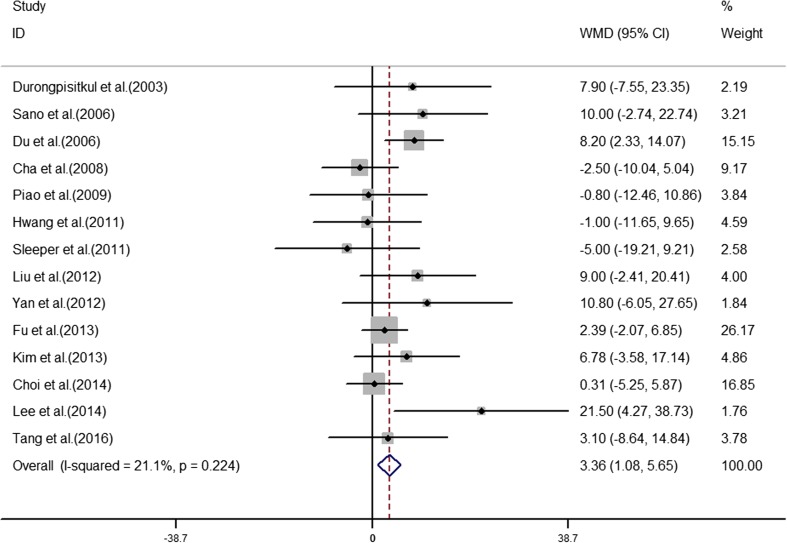
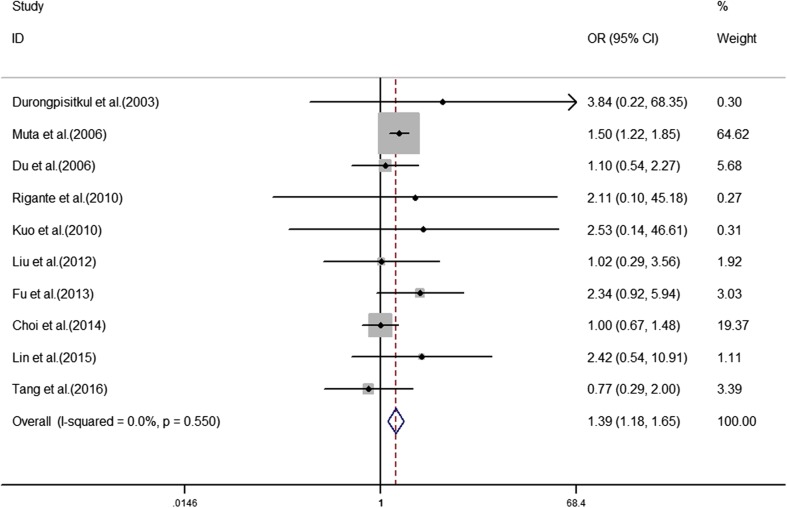
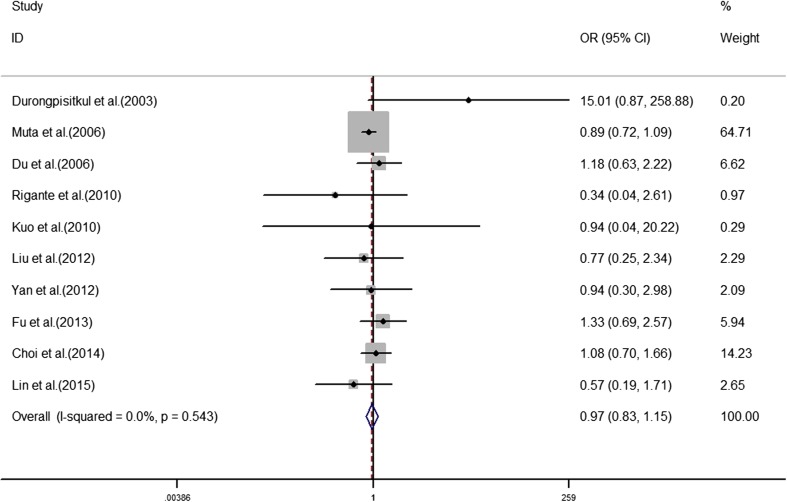
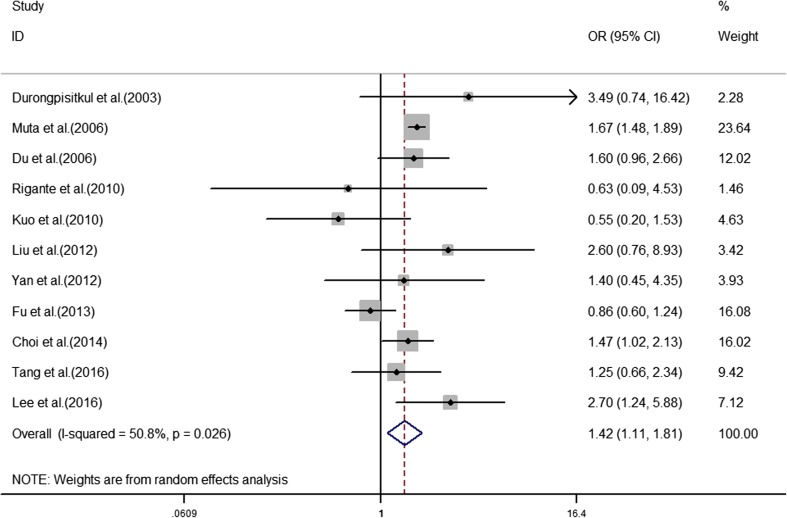
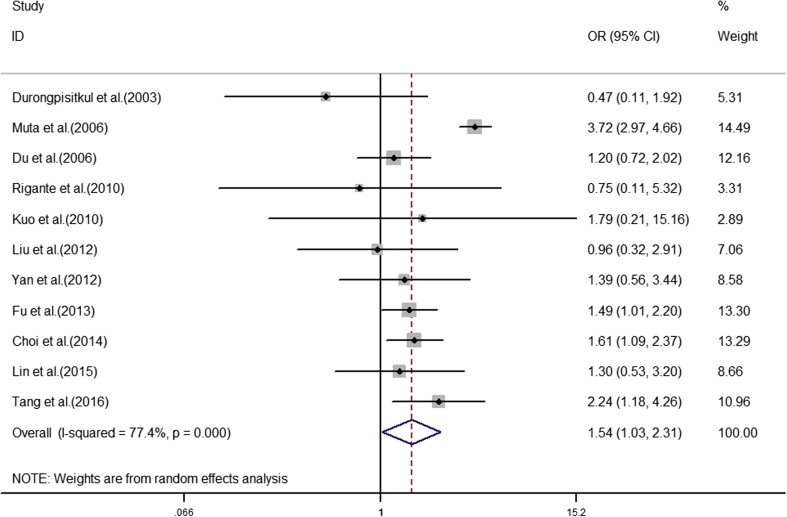
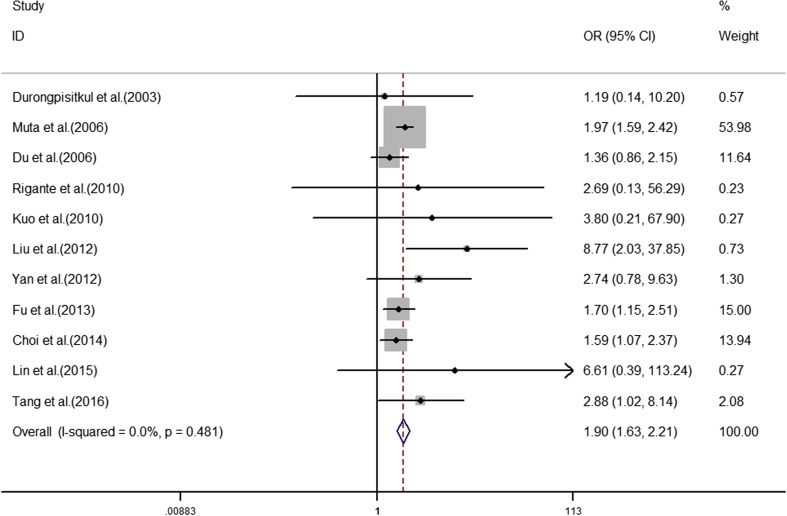
The analyses of the high-risk factors are shown in Table 2. Treatment time ≤ 4.0 days was more likely to not result in IVIG-sensitive Kawasaki disease, with an OR value of 1.64 and 95% CI of 1.05–2.57 (Fig. 2). IVIG-resistant patients had a significantly lower hemoglobin value than IVIG-sensitive patients (Fig. 3), with WMD = − 26.55 and 95% CI = − 35.52, − 17.58. IVIG-resistant patients had significantly lower platelet counts than IVIG-sensitive patients (Fig. 4), with WMD = − 26.55 and 95% CI = − 35.52, − 17.58. IVIG-resistant patients had significantly higher ESR values than IVIG-sensitive patients (Fig. 5), with WMD = 3.36 and 95% CI = 1.08–5.65. IVIG-resistant patients were more likely to have changes in oral mucosa than IVIG-sensitive patients (Fig. 6), with OR = 1.39 and 95% CI = 1.18–1.65. The difference in conjunctival congestion in each group was not statistically significant (Fig. 7), with OR = 0.97 and 95% CI = 0.83–1.15. IVIG-resistant patients were more likely to have cervical lymphadenopathy than IVIG-sensitive patients (Fig. 8), with OR = 1.42 and 95% CI = 1.11–1.81. IVIG-resistant patients were more likely to have swelling of the extremities than IVIG-sensitive patients (Fig. 9), with OR = 1.54 and 95% CI = 1.03–2.31. Patients with polymorphous rash were significantly more likely not to be sensitive to IVIG (Fig. 10), with OR = 1.90, and 95% CI = 1.63–2.21.
Table 2.
Pooled estimates of indices of high-risk factors on intravenous immunoglobulin (IVIG) resistance
| Variables | Number of trials | I2 (%) | Net change (95%CI) | p |
|---|---|---|---|---|
| Initiation of IVIG treatment | 5 | 97.4 | 1.64 (1.05, 2.57) | 0.03 |
| Hemoglobin | 16 | 36.4 | − 0.12 (− 0.21, − 0.02) | 0.02 |
| Platelet count | 20 | 40.3 | − 26.55 (− 35.52, − 17.58) | < 0.001 |
| Erythrocyte sedimentation rate | 14 | 21.1 | 3.36 (1.08, 5.65) | 0.004 |
| Changes in oral mucosa | 10 | 0 | 1.39 (1.18, 1.65) | < 0.001 |
| Conjunctival congestion | 10 | 0 | 0.97 (0.83, 1.15) | 0.755 |
| Cervical lymphadenopathy | 11 | 50.8 | 1.42 (1.11, 1.81) | 0.06 |
| Swelling of extremities | 11 | 77.4 | 1.54 (1.03, 2.31) | < 0.001 |
| Polymorphous rash | 11 | 0 | 1.90 (1.63, 2.21) | < 0.001 |
Fig. 2.
Prevalence of intravenous immunoglobulin (IVIG)-resistant Kawasaki disease among patients who received IVIG treatment ≤ 4.0 days after the onset of symptoms
Fig. 3.
Hemoglobin as a predictive index for resistance to intravenous immunoglobulin therapy in Kawasaki disease
Fig. 4.
Platelet count as a predictive index for intravenous immunoglobulin resistance in Kawasaki disease
Fig. 5.
Erythrocyte sedimentation rate (ESR) as a predictive index for intravenous immunoglobulin resistance in Kawasaki disease
Fig. 6.
Changes in oral mucosa as a predictive index for intravenous immunoglobulin resistance in Kawasaki disease
Fig. 7.
Conjunctival congestion as a predictive index for intravenous immunoglobulin resistance in Kawasaki disease
Fig. 8.
Cervical lymphadenopathy as a predictive index for intravenous immunoglobulin resistance in Kawasaki disease
Fig. 9.
Swelling of the extremities as a predictive index for intravenous immunoglobulin resistance in Kawasaki disease
Fig. 10.
Polymorphous rash as a predictive index for intravenous immunoglobulin resistance in Kawasaki disease
Sensitivity analyses and publication bias
Omitting one study at a time for the platelet count data produced WMD values from − 29.956 (95% CI − 39.354, −20.557) to − 23.864 (95% CI − 33.402, − 14.327). Omitting one study at a time for the erythrocyte sedimentation rate data gave WHD values from 2.498 (95% CI 0.019–4.977) to 3.981 (95% CI 1.477–6.485). Omitting one study at a time for cervical lymphadenopathy data showed OR values from 1.309 (95% CI 1.078–1.591) to 1.640 (95% CI 1.473–1.825). Omitting one study at a time for swelling of the extremities data gave OR values from 1.461 (95% CI 1.189–1.795) to 1.657 (95% CI 1.111–2.471). Omitting one study at a time for polymorphous rash data showed OR values from 1.814 (95% CI 1.455–2.261) to 1.967 (95% CI 1.674–2.310). Egger’s tests for hemoglobin and platelet count showed no publication bias (p = .18 and p = .97, respectively).
Subgroup analyses
The results of our subgroup analyses are shown in Table 3. Subgroups were selected based on different regions, such as Asian and non-Asian. Hemoglobin, platelet count, ESR, oral mucosa, conjunctival congestion, cervical lymphadenopathy, swelling of extremities, and polymorphous rash were analyzed. For the initiation of IVIG treatment, we had only five studies; therefore, we did not conduct a subgroup analysis for this variable. The summary WMDs for hemoglobin were 0.14 (95% CI − 0.29, 0.01) and − 0.42(95% CI − 0.97, 0.13) for the studies in Asian and non-Asian populations, respectively, without any significant region-specific differences (Pdifference = 0.07). The summary WMDs for platelet count were − 23.22 (95% CI − 36.88, − 9.55) and − 63.99 (95% CI − 40.57, − 13.58) for the studies in Asian and non-Asian populations, respectively, indicating there was a significant region-specific difference (Pdifference = 0.03). Asian patients with high ESR are more likely to be IVIG resistant, but there was no significant difference between IVIG-sensitive and IVIG-resistant patients in non-Asian patients. Asian patients with changes in oral mucosa, cervical lymphadenopathy, swelling of the extremities, and polymorphous rash were more likely to be IVIG resistant, but there was no significant difference between IVIG-sensitive and IVIG-resistant patients in non-Asian patients. For conjunctival congestion, there was no difference in Asian and non-Asian patients between those who were IVIG-sensitive and those who were IVIG resistant.
Table 3.
Subgroup analyses for meta-analysis of the risk of intravenous immunoglobulin (IVIG)-resistant Kawasaki disease
| Factor | Geographic area | Number of studies | WMD (95% CI) | Pheterogeneity | I2(%) | Pdifference |
|---|---|---|---|---|---|---|
| Hemoglobin | Asian [4, 6, 9, 13, 17, 22, 24–26, 32, 33, 36, 41, 44] | 14 | − 0.14 (− 0.29, 0.01) | 0.05 | 41.8 | 0.07 |
| Non-Asian [35, 38] | 2 | − 0.42 (− 0.97, 0.13) | 0.90 | 0 | ||
| Platelet count | Asian [4, 6, 9, 10, 13, 17, 19, 20, 22, 24–26, 29, 32, 33, 37, 41, 44] | 18 | − 23.22 (− 36.88, − 9.55) | 0.06 | 37.1 | 0.03 |
| Non-Asian [18, 38] | 2 | − 63.99 (−40.57, − 13.58) | 0.77 | 0 | ||
| Erythrocyte sedimentation rate | Asian [4, 6, 9, 10, 13, 17, 18, 24, 26, 33, 36, 41, 44] | 12 | 3.76 (0.76, 6.77) | 0.20 | 25.3 | 0.22 |
| Non-Asian [18, 38] | 2 | 1.94 (− 9.43, 13.30) | 0.19 | 42.0 | ||
| Changes in oral mucosa | Asian [6, 9, 10, 13, 22, 25, 26, 28, 41] | 9 | 1.37 (1.16, 1.62) | 0.46 | 0 | 0.55 |
| Non-Asian [35] | 1 | 2.11 (0.10, 45.18) | NA | NA | ||
| Conjunctival congestion | Asian [6, 9, 10, 13, 22, 25, 26, 28, 41, 44] | 9 | 0.98 (0.83, 1.16) | 0.54 | 0 | 0.54 |
| Non-Asian [35] | 1 | 0.34 (0.83, 1.15) | NA | NA | ||
| Cervical lymphadenopathy | Asian [6, 9, 10, 13, 22, 23, 26, 28, 41, 44] | 10 | 1.43 (1.12,1.84) | 0.02 | 53.9 | 0.03 |
| Non-Asian [35] | 1 | .63 (0.09, 4.53) | NA | NA | ||
| Swelling of extremities | Asian [6, 9, 10, 13, 22, 23, 25, 26, 28, 41, 44] | 10 | 1.58 (1.05, 2.38) | 0.00 | 79.1 | 0.00 |
| Non-Asian [35] | 1 | 0.75 (0.11, 5.32) | NA | NA | ||
| Polymorphous rash | Asian [6, 9, 10, 13, 22, 23, 25, 26, 28, 41, 44] | 10 | 1.84 (1.55, 2.18) | 0.39 | 5.2 | 0.48 |
| Non-Asian [35] | 1 | 2.69 (0.13, 56.30) | NA | NA |
WMD weighted mean difference, 95%CI 95% confidence intervals
Our results on platelet count and ESR differed from those of Baek et al. [2]. We included more studies, for a longer study period, with subgroup analyses (Table 4). The results revealed that the difference in platelet count among Chinese, Korean, Japanese, and non-Asian patients was significant, and the difference in erythrocyte sedimentation rate was statistically significant in Chinese patients, but not in Korean, Japanese, and non-Asian patients.
Table 4.
Subgroup analyses for meta-analysis of platelet count and erythrocyte sedimentation rate
| Factor | Geographic area | Number of studies | WMD (95% CI) | Pheterogeneity | I2(%) | Pdifference |
|---|---|---|---|---|---|---|
| Platelet count | Japan | 2 | − 43.535 (−66.61, − 20.46) | 0.59 | 0 | .03 |
| China | 10 | − 14.221 (−28.32, − 0.13) | 0.43 | 0.4 | ||
| Korea | 6 | − 32.851 (−60.42, − 5.29) | 0.02 | 61.6 | ||
| Non-Asian | 2 | − 63.985 (95% CI − 99.09, − 28.88) | 0.77 | 0 | ||
| Erythrocyte sedimentation rate | Japan | 1 | 10.000 (− 2.74, 22.74) | – | – | .22 |
| China | 6 | 4.588 (1.50, 7.67) | 0.50 | 42.0 | ||
| Korea | 5 | 2.162 (− 3.75, 8.08) | 0.12 | 44.7 | ||
| Non-Asian | 2 | 1.936 (− 9.43, 13.30) | 0.190 | 21.1 |
Discussion
This meta-analysis showed that differences in the timing of initiation of IVIG treatment (≤ 4.0 days), hemoglobin level, platelet count, ESR, oral mucosa features, cervical lymphadenopathy, swelling of the extremities, and polymorphous rash between IVIG-resistant and IVIG-sensitive patients were statistically significant.
Initial administration of IVIG
Our study found that initial administration of IVIG ≤ 4.0 days rather than > 4.0 days after the onset of symptoms resulted in Kawasaki disease that was more likely to be IVIG resistant (p = .03). Among the included studies, Fu et al. [13], Egami et al. [11], and Tremoulet et al. [42] concluded that initial administration of IVIG ≤ 4.0 days after the onset of symptoms might not correlate with IVIG resistance; whereas, two other studies showed a relationship, and the overall combined effect revealed the relationship between the initial administration of IVIG and IVIG resistance. The symptoms of Kawasaki disease always appear after fever; therefore, if the patient has confirmed Kawasaki disease for ≤ 4.0 days, it suggests the severity of the disease, which is perhaps why the patients treated with IVIG ≤ 4.0 days were more susceptible to IVIG resistance. In the acute phase of Kawasaki disease, the inflammatory reaction continues, and the early use of IVIG cannot block the inflammatory mediators that continue to be released. If IVIG is used within 4 days, the inflammatory reaction will continue; therefore, the possibility of continuous fever might be likely.
Hemoglobin and ESR
Straface et al. [40] found that the inflammatory reaction in patients with Kawasaki disease changes in the serum redox state, with increased expression of inducible nitric oxide synthase in monocytes and neutrophils. It has been suggested that this pro-oxidant status of the blood could also alter the homeostasis of red blood cells (RBCs), resulting in a type of premature aging in these circulating cells that could lead to anemia and the formation of blood clots. Decreased glycophorin A and CD47 expression, as well as the externalization of phosphatidylserine, were measured in RBCs from patients with Kawasaki disease during the early phase of the disease. The number of RBCs, hemoglobin values, mean corpuscular volume, and hematocrit were significantly decreased in these patients. Alterations in RBC structure and function might independently and synergistically impair blood flow and induce vascular occlusion, whereas premature aging of RBCs and their consequent removal from circulation might be a risk factor for anemia. RBC aging, inflammation, and thrombosis result in increased ESR. In this study, the IVIG-resistant patients had a significantly lower hemoglobin level and significantly higher ESR than IVIG-sensitive patients; however, the differences were not significant among each subgroup. The results for hemoglobin in each subgroup showed no relationship to IVIG resistance; however, a relationship was observed in the combined results. One Korean study [24] and one Chinese study [9] showed a strong relationship between ESR and Kawasaki disease; the other 12 studies showed a weak relationship.
Platelet count
Del et al. [8] suggested that the formation of heterotypic platelet–leucocyte aggregates, which is dependent on platelet activation, and leucocyte–RBC–platelet aggregates could at least partially be associated with the release of pro-aggregating factors (e.g., arachidonate) and/or with changes in the expression of molecules on the cell surface, including P-selectin. This crosstalk between activated platelets and leucocytes operates through several systems, including the interaction of P-selectin with P-selectin glycoprotein ligand-1 (PSGL-1). P-selectin and PSGL-1 are vascular adhesion molecules that play an important role in the inflammatory response by mediating the interaction of leucocytes, which stimulates endothelium and platelets bound within the vicinity of vascular injury. P-selectin captures leucocytes from the blood to bring them into contact with the endothelial cell surface on the blood vessel wall where P-selectin–PSGL-1 interaction supports leucocyte rolling, platelet activation, and aggregation, which leads to a cascade of reactions that promote inflammation and thrombosis; therefore, we can conclude that the platelet count is associated with the inflammatory reaction of Kawasaki disease and can speculate that the number of platelets is positively correlated with the severity of inflammation.
This study also confirmed that platelet count can predict IVIG-resistant Kawasaki disease. The results of 20 studies were combined in this study comprising 18 Asian studies [4, 6, 9, 10, 13, 17, 19, 20, 22, 24–26, 29, 32, 33, 37, 41, 44] and 2 North American studies [18, 38]. Each subgroup analysis showed the results had statistical significance; however, Baek et al. [2] reported that ESR and platelet count could not predict IVIG-resistant Kawasaki disease from their statistical meta-analysis, which is contrary to the results of the present study. Baek et al. [2] included 7 papers reporting ESR and 10 papers reporting platelet count, where we included 14 papers reporting ESR and 20 papers reporting platelets, giving the present study a larger sample size with higher reliability. In addition, the subgroup analyses of platelet count showed that the differences in platelet count in Chinese, Korean, Japanese, and non-Asian patients were significant. For erythrocyte sedimentation rate, the difference was significant in Chinese, but not in Korean, Japanese, and non-Asian patients. The results differed by ethnicity.
Clinical features
IVIG-resistant Kawasaki disease with fever over a long period can have different clinical features. Ram et al. [34] found that prolonged fever, wider dispersion of symptoms, and pyuria were significantly associated with the development of coronary lesions, all of which the Kawasaki disease patients had. Choi et al. [6] found that cervical lymphadenopathy is a risk factor for IVIG resistance, and Fu et al. [13] believed that polymorphous rash and perianal change are risk factors for IVIG resistance. Yan et al. [44] suggested that clinical features cannot predict IVIG-resistant Kawasaki disease; however, this study found that changes in oral mucosa, cervical lymphadenopathy, swelling of extremities, and polymorphous rash can predict IVIG-resistant Kawasaki disease, whereas conjunctival congestion cannot. Hartas et al. [15] showed that patients with Kawasaki disease who also have acute arthritis are at high risk for being IVIG-resistant, but because of the lack of relevant studies, we did not include this factor in our meta-analysis.
In subgroup analyses, Asian patients with changes in oral mucosa, cervical lymphadenopathy, swelling of the extremities, and polymorphous rash were more likely to be IVIG resistant, but in non-Asian patients, there was no significant difference among these symptoms and IVIG resistance. For conjunctival congestion, neither Asian nor non-Asian patients exhibited any difference between IVIG sensitivity and IVIG resistance.
This study was aimed to explore the risk factors associated with IVIG-resistant Kawasaki disease through studying clinical features and laboratory index, which would provide evidences for treatment regimens in these patients. Chen et al. [5] and Yang et al. [45] conducted the meta-analysis, which pointed out that the early application of intravenous immunoglobulin plus corticosteroid can reduce the incidence of coronary artery abnormalities. A prospective study was conducted by Kobayashi et al. [21] and after putting forward the Kobayashi score, they found that among IVIG-resistant high-risk patients (Kobayashi score, 5 or higher), the incidences of treatment failure and coronary artery abnormalities were more frequent in the IVIG group than in the IVIG + PSL group. The clinical and coronary outcomes were similar among low-risk patients (Kobayashi score 0–4). A prediction model to select the appropriate treatment and alleviate complications in IVIG-resistant Kawasaki disease was warranted in the future.
Study limitations
There were several limitations to our study. First, most studies used were retrospective and few multicenter studies were included. Second, because of language constraints, few Japanese articles were included. Third, because the original articles did not provide the data on age-adjusted z-scores of hemoglobin, we had no statistic on zHgb.
Conclusion
The risk factors for IVIG-resistant Kawasaki disease are the initial administration of IVIG ≤ 4.0 days after the onset of symptoms, increased ESR, decreased hemoglobin and platelet count, changes in oral mucosa, cervical lymphadenopathy, swelling of extremities, and polymorphous rash.
Electronic supplementary material
(DOCX 4290 kb)
(DOC 67 kb)
Abbreviations
- BNP
Brain natriuretic peptide
- ALT
Alanine transaminase
- AST
Aspartate aminotransferase
- CI
Confidence interval
- CRP
C-reactive protein
- IVIG
Intravenous immunoglobulin
- ESR
Erythrocyte sedimentation rate
- OR
Odds ratio
- PMN
Polymorphonuclear leukocytes
- WMD
Weighted mean difference
Authors’ contributions
Xuan Li and Ye Chen contributed to the study design, data collection, interpretation of data, and drafting the report; Yunjia Tang, Yueyue Ding, Lin Sun, and Weiguo Qian contributed to the data collection and statistical analysis and reviewed the report; Guanghui Qian, Liqiang Qin, and Haitao Lv contributed to the study design, interpretation of data, and drafting the report; and Xuan Li is the guarantor.
Funding
This work was financially supported by the Chinese Natural Science Foundation (No. 81370217 and No. 8157021282), the National Natural Science Foundation for Youth (Nos. 31600695, 81600391), the Universities Natural Science Foundation of Jingasu Province (No. 16KJB310014), the Applied Foundational Research of Medical and Health Care of Suzhou City (No. SYS201642), Jiangsu Provincial Science Foundation of Young (No. BK20150291), Jiangsu Provincial Medical Young Talents (QNRC 2016756), and the Suzhou Science and Technology Bureau (Nos. SYS201557, SYS201633, KJXW2014015 and SYS201443).
Compliance with ethical statements
Conflict of interest
The authors declare that they have no conflicts of interest.
Informed consent
None.
Footnotes
Xuan Li and Ye Chen contributed equally to this work and should be considered co-first authors.
Electronic supplementary material
The online version of this article (10.1007/s00431-018-3182-2) contains supplementary material, which is available to authorized users.
Contributor Information
Xuan Li, Email: 879991662@qq.com.
Ye Chen, Email: chenye20080921@sina.com.
Yunjia Tang, Email: 824432264@qq.com.
Yueyue Ding, Email: dyyqd79@hotmail.com.
Qiuqin Xu, Email: xuqiuqin922@163.com.
Lin Sun, Email: Sunny70mail@163.com.
Weiguo Qian, Email: qianweiguo1974@sina.com.
Guanghui Qian, Email: guanghui_qian@163.com.
Liqiang Qin, Email: qinliqiang@suda.edu.cn.
Haitao Lv, Phone: +8618913597948, Email: haitaosz@163.com.
References
- 1.Ashouri N, Takahashi M, Dorey F, Mason W. Risk factors for nonresponse to therapy in Kawasaki disease. J Pediatr. 2008;153(3):365–368. doi: 10.1016/j.jpeds.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 2.Baek JY, Song MS. Meta-analysis of factors predicting resistance to intravenous immunoglobulin treatment in patients with Kawasaki disease. Korean J Pediatr. 2016;59(2):80–90. doi: 10.3345/kjp.2016.59.2.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell AJ, Burns JC. Adjunctive therapies for Kawasaki disease. J Inf Secur. 2016;72(Suppl):S1–S5. doi: 10.1016/j.jinf.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 4.Cha S, Yoon M, Ahn Y, Han M, Yoon KL. Risk factors for failure of initial intravenous immunoglobulin treatment in Kawasaki disease. J Korean Med Sci. 2008;23(4):718–722. doi: 10.3346/jkms.2008.23.4.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen S, Dong Y, Yin Y, Krucoff MW. Intravenous immunoglobulin plus corticosteroid to prevent coronary artery abnormalities in Kawasaki disease: a meta-analysis. Heart. 2013;99(2):76–82. doi: 10.1136/heartjnl-2012-302126. [DOI] [PubMed] [Google Scholar]
- 6.Choi MH, Park CS, Kim DS. Prediction of intravenous immunoglobulin nonresponse Kawasaki disease in Korea. Korean Journal of Pediatric Infectious Diseases. 2014;21:29–36. doi: 10.14776/kjpid.2014.21.1.29. [DOI] [Google Scholar]
- 7.Davies S, Sutton N, Blackstock S, Gormley S, Hoggart CJ, Levin M, Herberg JA. Predicting IVIG resistance in UK Kawasaki disease. Arch Dis Child. 2015;100(4):366–368. doi: 10.1136/archdischild-2014-307397. [DOI] [PubMed] [Google Scholar]
- 8.Del Principe D, Pietraforte D, Gambardella L, Marchesi A, Tarissi de Jacobis I, Villani A, Malorni W, Straface E. Pathogenetic determinants in Kawasaki disease: the haematological point of view. J Cell Mol Med. 2017;21(4):632–639. doi: 10.1111/jcmm.12992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du ZD, Zhang YL, Zhao D, Du JB, Lu S, Yi JM, et al. Retreatment and risk factors of IVIG nonresponsiveness. Chinese Journal of Practical Pediatrics. 2006;21(10):738–741. [Google Scholar]
- 10.Durongpisitkul K, Soongswang J, Laohaprasitiporn D, Nana A, Prachuabmoh C, Kangkagate C. Immunoglobulin failure and retreatment in Kawasaki disease. Pediatr Cardiol. 2003;24(2):145–148. doi: 10.1007/s00246-002-0216-2. [DOI] [PubMed] [Google Scholar]
- 11.Egami K, Muta H, Ishii M, Suda K, Sugahara Y, Iemura M, Matsuishi T. Prediction of resistance to intravenous immunoglobulin treatment in patients with Kawasaki disease. J Pediatr. 2006;149(2):237–240. doi: 10.1016/j.jpeds.2006.03.050. [DOI] [PubMed] [Google Scholar]
- 12.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu PP, Du ZD, Pan YS. Novel predictors of intravenous immunoglobulin resistance in Chinese children with Kawasaki disease. Pediatr Infect Dis J. 2013;32(8):e319–e323. doi: 10.1097/INF.0b013e31828e887f. [DOI] [PubMed] [Google Scholar]
- 14.Group JCSJW Guidelines for diagnosis and management of cardiovascular sequelae in Kawasaki disease (JCS 2013). Digest version. Circ J. 2014;78(10):2521–2562. doi: 10.1253/circj.CJ-66-0096. [DOI] [PubMed] [Google Scholar]
- 15.Hartas GA, Hashmi SS, Pham-Peyton C, Tsounias E, Bricker JT, Gupta-Malhotra M. Immunoglobulin resistance in Kawasaki disease. Pediatr Allergy Immunol Pulmonol. 2015;28(1):13–19. doi: 10.1089/ped.2014.0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 17.Hwang JY, Lee KY, Rhim JW, Youn YS, Oh JH, Han JW, Lee JS, Burgner D. Assessment of intravenous immunoglobulin non-responders in Kawasaki disease. Arch Dis Child. 2011;96(11):1088–1090. doi: 10.1136/adc.2010.184101. [DOI] [PubMed] [Google Scholar]
- 18.Kim SY, Han MY, Cha SH, Jeon YB. N-terminal pro-brain natriuretic peptide (NT proBNP) as a predictive indicator of initial intravenous immunoglobulin treatment failure in children with Kawasaki disease: a retrospective study. Pediatr Cardiol. 2013;34(8):1837–1843. doi: 10.1007/s00246-013-0724-2. [DOI] [PubMed] [Google Scholar]
- 19.Kim BY, Kim D, Kim YH, Ryoo E, Sun YH, Jeon IS, Jung MJ, Cho HK, Tchah H, Choi DY, Kim NY. Non-responders to intravenous immunoglobulin and coronary artery dilatation in Kawasaki disease: predictive parameters in Korean children. Korean Circ J. 2016;46(4):542–549. doi: 10.4070/kcj.2016.46.4.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobayashi T, Inoue Y, Takeuchi K, Okada Y, Tamura K, Tomomasa T, Kobayashi T, Morikawa A. Prediction of intravenous immunoglobulin unresponsiveness in patients with Kawasaki disease. Circulation. 2006;113(22):2606–2612. doi: 10.1161/CIRCULATIONAHA.105.592865. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi T, Inoue Y, Otani T, Morikawa A, Kobayashi T, Takeuchi K, Saji T, Sonobe T, Ogawa S, Miura M, Arakawa H. Risk stratification in the decision to include prednisolone with intravenous immunoglobulin in primary therapy of Kawasaki disease. Pediatr Infect Dis J. 2009;28(6):498–502. doi: 10.1097/INF.0b013e3181950b64. [DOI] [PubMed] [Google Scholar]
- 22.Kuo HC, Liang CD, Wang CL, Yu HR, Hwang KP, Yang KD. Serum albumin level predicts initial intravenous immunoglobulin treatment failure in Kawasaki disease. Acta Paediatr. 2010;99(10):1578–1583. doi: 10.1111/j.1651-2227.2010.01875.x. [DOI] [PubMed] [Google Scholar]
- 23.Lee HY, Song MS. Predictive factors of resistance to intravenous immunoglobulin and coronary artery lesions in Kawasaki disease. Korean J Pediatr. 2016;59(12):477–482. doi: 10.3345/kjp.2016.59.12.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee SM, Lee JB, Go YB, Song HY, Lee BJ, Kwak JH. Prediction of resistance to standard intravenous immunoglobulin therapy in Kawasaki disease. Korean Circ J. 2014;44(6):415–422. doi: 10.4070/kcj.2014.44.6.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin MT, Chang CH, Sun LC, Liu HM, Chang HW, Chen CA, Chiu SN, Lu CW, Chang LY, Wang JK, Wu MH. Risk factors and derived Formosa score for intravenous immunoglobulin unresponsiveness in Taiwanese children with Kawasaki disease. J Formos Med Assoc. 2016;115(5):350–355. doi: 10.1016/j.jfma.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 26.Liu F, Ding Y, Yin W. Clinical analysis of Kawasaki disease with non-responsiveness immunoglobulin therapy. J Appl Clin Pediatr. 2012;27(21):1670–1671. [Google Scholar]
- 27.Loomba RS, Raskin A, Gudausky TM, Kirkpatrick E. Role of the Egami score in predicting intravenous immunoglobulin resistance in Kawasaki disease among different ethnicities. Am J Ther. 2016;23(6):e1293–e1299. doi: 10.1097/MJT.0000000000000045. [DOI] [PubMed] [Google Scholar]
- 28.Muta H, Ishii M, Furui J, Nakamura Y, Matsuishi T. Risk factors associated with the need for additional intravenous gamma-globulin therapy for Kawasaki disease. Acta Paediatr. 2006;95(2):189–193. doi: 10.1080/08035250500327328. [DOI] [PubMed] [Google Scholar]
- 29.Nakagama Y, Inuzuka R, Hayashi T, Shindo T, Hirata Y, Shimizu N, Inatomi J, Yokoyama Y, Namai Y, Oda Y, Takamizawa M, Harita Y, Oka A. Fever pattern and C-reactive protein predict response to rescue therapy in Kawasaki disease. Pediatr Int. 2016;58(3):180–184. doi: 10.1111/ped.12762. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura Y, Yashiro M, Uehara R, Sadakane A, Tsuboi S, Aoyama Y, Kotani K, Tsogzolbaatar EO, Yanagawa H. Epidemiologic features of Kawasaki disease in Japan: results of the 2009-2010 nationwide survey. J Epidemiol. 2012;22(3):216–221. doi: 10.2188/jea.JE20110126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, Shulman ST, Bolger AF, Ferrieri P, Baltimore RS, Wilson WR, Baddour LM, Levison ME, Pallasch TJ, Falace DA, Taubert KA, Committee on Rheumatic Fever E, Kawasaki Disease CoCDitYAHA Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the committee on rheumatic fever, endocarditis, and Kawasaki disease, council on cardiovascular disease in the young, American Heart Association. Pediatrics. 2004;114(6):1708–1733. doi: 10.1542/peds.2004-2182. [DOI] [PubMed] [Google Scholar]
- 32.Ou-Yang MC, Kuo HC, Lin IC, Sheen JM, Huang FC, Chen CC, Huang YH, Lin YJ, Yu HR. Plasma clusterin concentrations may predict resistance to intravenous immunoglobulin in patients with Kawasaki disease. ScientificWorldJournal. 2013;2013:382523. doi: 10.1155/2013/382523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piao JH, Jin LH, Sun JH, Jin CJ, Zhai SB, Lv J. Clinical analysis of Kawasaki disease patients with non-responsiveness to high-dose gammaglobulin therapy. Journal of Clinical Pediatrics. 2009;27(5):480–483. [Google Scholar]
- 34.Ram Krishna M, Sundaram B, Dhanalakshmi K. Predictors of coronary artery aneurysms in Kawasaki disease. Clin Pediatr (Phila) 2014;53(6):561–565. doi: 10.1177/0009922814530802. [DOI] [PubMed] [Google Scholar]
- 35.Rigante D, Valentini P, Rizzo D, Leo A, De Rosa G, Onesimo R, De Nisco A, Angelone DF, Compagnone A, Delogu AB. Responsiveness to intravenous immunoglobulins and occurrence of coronary artery abnormalities in a single-center cohort of Italian patients with Kawasaki syndrome. Rheumatol Int. 2010;30(6):841–846. doi: 10.1007/s00296-009-1337-1. [DOI] [PubMed] [Google Scholar]
- 36.Sano T, Kurotobi S, Matsuzaki K, Yamamoto T, Maki I, Miki K, Kogaki S, Hara J. Prediction of non-responsiveness to standard high-dose gamma-globulin therapy in patients with acute Kawasaki disease before starting initial treatment. Eur J Pediatr. 2007;166(2):131–137. doi: 10.1007/s00431-006-0223-z. [DOI] [PubMed] [Google Scholar]
- 37.Sato S, Kawashima H, Kashiwagi Y, Hoshika A. Inflammatory cytokines as predictors of resistance to intravenous immunoglobulin therapy in Kawasaki disease patients. Int J Rheum Dis. 2013;16(2):168–172. doi: 10.1111/1756-185X.12082. [DOI] [PubMed] [Google Scholar]
- 38.Sleeper LA, Minich LL, McCrindle BM, Li JS, Mason W, Colan SD, Atz AM, Printz BF, Baker A, Vetter VL, Newburger JW, Pediatric Heart Network I. Evaluation of Kawasaki disease risk-scoring systems for intravenous immunoglobulin resistance. J Pediatr. 2011;158(5):831–835 e833. doi: 10.1016/j.jpeds.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Son MB, Newburger JW. Kawasaki disease. Pediatr Rev. 2013;34(4):151–162. doi: 10.1542/pir.34-4-151. [DOI] [PubMed] [Google Scholar]
- 40.Straface E, Marchesi A, Gambardella L, Metere A, Tarissi de Jacobis I, Viora M, Giordani L, Villani A, Del Principe D, Malorni W, Pietraforte D. Does oxidative stress play a critical role in cardiovascular complications of Kawasaki disease? Antioxid Redox Signal. 2012;17(10):1441–1446. doi: 10.1089/ars.2012.4660. [DOI] [PubMed] [Google Scholar]
- 41.Tang Y, Yan W, Sun L, Huang J, Qian W, Ding Y, Lv H. Prediction of intravenous immunoglobulin resistance in Kawasaki disease in an East China population. Clin Rheumatol. 2016;35(11):2771–2776. doi: 10.1007/s10067-016-3370-2. [DOI] [PubMed] [Google Scholar]
- 42.Tremoulet AH, Best BM, Song S, Wang S, Corinaldesi E, Eichenfield JR, Martin DD, Newburger JW, Burns JC. Resistance to intravenous immunoglobulin in children with Kawasaki disease. J Pediatr. 2008;153(1):117–121. doi: 10.1016/j.jpeds.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uehara R, Belay ED, Maddox RA, Holman RC, Nakamura Y, Yashiro M, Oki I, Ogino H, Schonberger LB, Yanagawa H. Analysis of potential risk factors associated with nonresponse to initial intravenous immunoglobulin treatment among Kawasaki disease patients in Japan. Pediatr Infect Dis J. 2008;27(2):155–160. doi: 10.1097/INF.0b013e31815922b5. [DOI] [PubMed] [Google Scholar]
- 44.Yan H, Wan H, Du JB, Chen YH, Li WZ, Liu XQ, Jin HF. Risk factors and prediction analysis of intravenous immunoglobulin resistant Kawasaki disease. J Appl Clin Pediatr. 2012;27(21):1637–1640. [Google Scholar]
- 45.Yang TJ, Lin MT, Lu CY, Chen JM, Lee PI, Huang LM, Wu MH, Chang LY (2017) The prevention of coronary arterial abnormalities in Kawasaki disease: a meta-analysis of the corticosteroid effectiveness. J Microbiol Immunol Infect. 10.1016/j.jmii.2017.08.012 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 4290 kb)
(DOC 67 kb)