Abstract
Introduction
The aim of the international CORE project was to explore the databases of the existing hernia registries and compare them in content and outcome variables.
Methods
The CORE project was initiated with representatives from all established hernia registries (Danish Hernia Database, Swedish Hernia Registry, Herniamed, EuraHS, Club Hernie, EVEREG, AHSQC) in March 2015 in Berlin. The following categories were used to compare the registries: initiation and funding, data collection and use for certification of hernia centers, patient data and data protection, operative data, registration of complications and follow-up data.
Results
The Danish Hernia Database is the only one to qualify as a genuine national registry where participation is compulsory for entry of all procedures by all surgeons performing a hernia operation. All other registries have to be considered as voluntary and completeness of data depends upon the participating hospitals and surgeons. Only the Danish Hernia Database and the Swedish Hernia Registry are publicly funded. All other registries are reliant on financial support from the medical technology industry. As an incentive for voluntary participation in a hernia registry, hospitals or surgeons are issued a certificate confirming that they are taking part in a quality assurance study for hernia surgery. Due to data protection and privacy regulations, most registries are obliged or have chosen to enter their patient data anonymously or coded. The Danish Hernia Database and Swedish Hernia Registry utilize a national personal patient code. In the Herniamed Registry, patient data are saved in a coded and anonymous format after obtaining the patient’s informed consent.
Conclusion
Despite the differences in the way data are collected for each of the listed hernia registries, the data are indispensable in clinical research.
keywords: Hernia registry, Hernia database, Clinical trial platform
Introduction
Randomized clinical trials (RCTs) and meta-analyses are considered the gold standard of evidence-based medicine nowadays [1]. The strength of RCTs rests on their excellent internal validity, which is based largely on the power of randomization to ensure that the only difference between two treatment arms is their exposure to the treatment of interest [2]. But the applicability of RCTs to the care of patients in routine practice is limited. In particular, patients, providers, and concurrent care in the general population are different from those in RCTs, and the generalizability or external validity of RCTs may be limited. Although observational research does not reach the same level of internal validity as RCTs, well-designed observational studies can offer high external validity and provide a unique opportunity to evaluate treatments and their outcomes in routine practice [2]. Many important clinical questions have not, cannot, and will not be addressed in the context of an RCT. In these situations, clinicians rely on information provided by observational research [2]. In a comparison of observational studies and RCTs, the estimates of the treatment effects from observational studies and RCTs were similar in most cases [3]. Registries are ongoing prospective observational data-collection repositories [4]. A registry is defined as a systematic collection of a clearly defined set of health and demographic data for patients with specific health characteristics, held in a central database for predefined purposes [5]. Medical registries can serve different purposes, for instance as a tool to monitor and improve the quality of care or as a resource for research [5]. To be useful, data in a medical registry must be of good quality [5]. To optimize the quality of medical registry data, the participating centers should follow certain procedures designed to minimize inaccurate and incomplete data [5]. The intended use of registry data determines the necessary properties of the data [5].
In 1992, surgeons from eight Swedish hospitals initiated a registry for inguinal and femoral hernia repair [6]. The aim of the registry was to report on the operative techniques used and to analyze outcome measures in order to stimulate quality improvement [6]. A number of national and international registries have since been added [6–12].
The aim of this manuscript is to explore the databases of these hernia registries and compare them in content and outcome variables.
Materials and methods
The CORE (Comparison of Hernia Registries in Europe) project was initiated with representatives from all established European hernia registries in March 2015 in Berlin. Initially perceived as a European project, the scope was broadened to also include the Americas Hernia Society Collaboration (AHSQC) Registry. Each registry representative was contacted to present and verify information regarding the registry (Table 1).
Table 1.
Representatives of the participating registries
| Representatives | Registries | Countries | Abbreviasions |
|---|---|---|---|
| William Hope | Americas Hernia Society Quality Collaboration Registry | United States | AHSQC |
| Jean Francois Gillion | Club Hernie | France | CH |
| Lars Nannestad Jørgensen | Danish Hernia Database | Denmark | DHDB |
| Iris Kyle-Leinhase Filip Muysoms |
EuraHS | Belgium | EuraHS |
| José Antonio Pereira Rodriguez | Registro Espaniol de Eventraciones | Spain | EVEREG |
| Ferdinand Köckerling | Herniamed | Germany, Austria, Switzerland | Herniamed |
| Agneta Montgomery | Swedish Hernia Registry | Sweden | SHR |
The following information was obtained: Country(ies) of use, start date of registry, procedures included, compulsory or voluntary data entry, overseeing body, funding, user cost, access route, language, number of active users, whether data are validated and by what method, data analysis provided, and how the data are published. The following categories were used to compare the registries: initiation and funding, data collection and use for certification of hernia centers, patient data and data protection, operative data, registration of complications and follow-up data.
Results
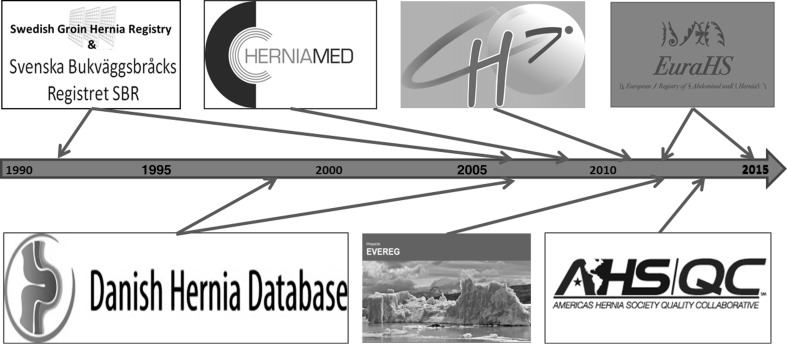
The timeline for launch of registries included in the CORE project is shown in Fig. 1. Prospective hernia surgery registration was pioneered by Erik Nilsson in 1992 with the Swedish Groin Hernia Registry (SGHR) [6]. In 1998 the Danish Groin Hernia Database (DGHD) was established and was subsequently extended to ventral hernias (Danish Hernia Database) in 2007 [7]. The German Herniamed Registry included both inguinal and ventral hernias and was launched in 2009 [9]. In France the Club Hernie (CH) started their ventral hernia registry in 2011 across 30 specialized hernia surgeons [10]. Two registries were launched in 2012: EuraHS [8], and the Spanish Registro Español de Eventraciones (EVEREG) [11]. The Americas Hernia Society Collaboration (AHSQC) Registry followed in 2013 [12].
Fig. 1.
Timeline of hernia registries
Compulsory or voluntary participation
The Danish Hernia Database is the only one to qualify as a genuine national registry where participation is compulsory for entry of all procedures by all surgeons performing a hernia operation. All other registries have to be considered as voluntary and completeness of data depends upon the participating hospitals and surgeons (Table 2).
Table 2.
Initiation and funding of registries
| Country | Routes | Release | Initiation | Compulsory or voluntary | Funding | |
|---|---|---|---|---|---|---|
| Swedish Hernia Registry | Sweden | Inguinal | 1992 | Non-profit team of surgeons | Voluntary | National Board of Health and Welfare |
| Ventral | 2007 | |||||
| Danish Hernia Database | Denmark | Inguinal | 1998 | Danish surgeons, non-profit | Compulsory | Public funding |
| Ventral | 2007 | |||||
| Herniamed | Germany, Austria and Switzerland | Inguinal, primary ventral, incisional, parastomal, hiatal | 2009 | Non-profit organization, German Hernia Society (DHG) | Voluntary | PFM medical, Storz, FEG, BARD, Ethicon, Braun, MenkeMed, Dahlhausen, Medtronic |
| Club Hernie | France | Inguinal, primary ventral, incisional, parastomal | 2011 | Non-profit surgeon incentive | Voluntary | Bard, Cousin, Medtronic, Peters |
| EuraHS | Europe | Primary ventral incisional, parastomal, | 2012 | Non-profit organization, European Hernia Society (EHS) | Voluntary | Medtronic, FEG, BARD, Ethicon |
| Hiatal, inguinal, open abdomen, abdominal wall closure, prophyl. meshes | 2015 | |||||
| Evereg | Spain | Incisional | 2012 | Surgeons’ incentive/B Braun | Voluntary | B. Braun |
| AHSQC | United States of America | Inguinal, primary ventral, parastomal | 2013 | Non-profit organization, Americas Hernia Society (AHS) | Voluntary | Bard, Allergan, Intuitive, Medtronic, W. L. Gore |
National vs international registries
Most hernia registries only record data on hernia operations conducted in their own country. The Herniamed Registry is used in the German-speaking countries Switzerland, Austria and Germany. EuraHS with a multilingual interface is intended for use at international level (Table 2).
Funding
Only the Danish Hernia Database and the Swedish Hernia Registry are publicly funded. All other registries are reliant on financial support from the medical technology industry (Table 2).
Case numbers
The case numbers in the various registries will of course greatly differ in accordance with how long a hernia registry has been in existence, the number of participating hospitals and surgeons as well as with the size of the respective country (Table 3).
Table 3.
Data collection and certification
| Routes | Language | Data entry | Active users | Registered cases | Percentage of all hernias in the country | Complete data necessary for inclusion in analyses | Certification for the surgeon/institution | |
|---|---|---|---|---|---|---|---|---|
| Swedish Hernia Registry | Inguinal | Swedish | Surgeon and follow-up by educated register secretary/nurse | 90 centers | Inguinal: > 240, 000 | > 95% | Yes | No certification is provided |
| Primary ventral, incisional, parastomal | > 10 centers | Primary ventral: > 2800 Incisional and parastomal: > 1800 |
15% | Yes | ||||
| Danish Hernia Database | Inguinal (including femoral) | Danish | Surgeon | > 300 | Inguinal: > 200,000 | 90% | Yes | No certification is provided |
| Ventral: incisional, umbilical, epigastric, port-site, parastomal, other (Spigeli, lumbar, etc.) | Ventral: > 45,000 (umbilical: > 22,500 Incisional: > 11,500 Epigastric: > 6500 Port: > 1500 Parastomal: > 1100) |
80% | Yes | |||||
| Herniamed | Incisional, parastomal, hiatal, inguinal, umbilical, epigastric | English, German | Surgeon | > 500 in Germany, Austria, Switzerland | Inguinal: > 290,000 Umbilical: > 70,000 Incisional: > 50,000 Epigastric: > 16,000 Hiatal: > 9000 Parastomal: > 2000 |
15–20% | Yes | User certificates defined by certain outcome criteria |
| Club Hernie | Primary ventral, incisional, inguinal, parastomal, giant incisional |
French | Surgeon and independent clinical research assistants | 50 | Inguinal: > 17,700 Ventral: > 7000 |
2–3% | Yes | Continuing medical education credits |
| EuraHS | Primary ventral, incisional, parastomal | English, German, French, Italian, Spanish, Polish | Surgeon | > 100 all over Europe | Incisional > 4175 Inguinal > 800 Hiatal: > 300 Open abdomen > 400 |
No data available for Europe | No | Certificate for registration from EuraHS |
| Hiatal, inguinal, open abdomen, abdominal wall closure, prophyl. meshes | English, German, Dutch | No data available for Europe | No | |||||
| Evereg | Only incisional hernias, no primary hernias | Spanish | Surgeon | 113 hospitals in Spain only | > 7300 | No data available for Spain | Yes | No certification is provided |
| AHSQC | Primary, incisional, parastomal, inguinal | English | Surgeon, clinical teams, patient | > 200 | > 20,000 | No data available for USA | Yes | American Board of Surgery Maintenance of Certification Part IV; Centers for Medicare and Medicaid Services Qualified Clinical Data Registry |
Certification of participation
As an incentive for voluntary participation in a hernia registry, hospitals or surgeons are issued a certificate (EuraHS, AHSQC, Herniamed) confirming that they are taking part in a quality assurance study for hernia surgery. Since participation in the Herniamed Registry constitutes a basic prerequisite for obtaining certification as a hernia center from the German Hernia Society (DHG), the DHG has defined certain outcome criteria (Table 3).
Data protection
Due to data protection and privacy regulations, most registries are obliged or have chosen to enter their patient data anonymously or coded. Registries often use only the patient’s age or year of birth and mostly only a unique case identification number. The DHDB and SHR use a national personal patient code. In the Herniamed Registry, patient data are saved in a coded and anonymous format after obtaining the patient’s informed consent. The latter can be deleted at any time upon the patient’s request. All data classified as sensitive may be read and edited only by the treating institution for follow-up of the patients (Table 4).
Table 4.
Patient data
| Routes | Indentification | Contact details | Date of birth | BMI | Occupation | Smoker | Sport/exercise | Risk factors | Comorbidities | |
|---|---|---|---|---|---|---|---|---|---|---|
| Swedish Hernia Registry | Inguinal | Anonymous, gender | No | Yes | Yes | No | Yes | No | Immunosuppression, collagen-related disease, increase risk for bleeding | Diabetes, pulmonary disease |
| Primary ventral, incisional, parastomal | Immunosuppression, collagen-related disease, bleeding disease, steroids | |||||||||
| Danish Hernia Database | Inguinal | National identity code (CPR) | Yes | Yes | No | No | No | No | No | No |
| Port-site, primary ventral, incisional, parastomal | Yes | Yes | Yes for incisional and parastomal hernia | |||||||
| Herniamed | Incisional, parastomal, hiatal, inguinal, umbilical, epigastric | No, only treating institution | No | No | Yes | No | Yes | No | Aneurysm, immunosuppression, thrombocyte aggregation inhibitors, coumarin derivate, coagulopathy, smoking | COPD, asthma, diabetes |
| Club Hernie | Primary ventral, incisional, inguinal, parastomal, giant incisional | Anonymous, gender | No | Age only | Yes | Yes | Yes | Yes | Aneurysm, immunosuppression, thrombocyte aggregation inhibitors, anticoagulant, personal history of hernia surgery, radiotherapy, chronic medical disease | ASA grading, diabetes, Hepatic disease, COPD, dysuria, constipation |
| EuraHS | Primary ventral, incisional, parastomal, hiatal, inguinal, open abdomen, abd. wall closure, prophyl. meshes | Anonymous, gender | No | Year only | Yes | Yes | Yes | Yes | Aneurysm, collagen-related disease, immunosuppression, thrombocyte aggregation inhibitors, personal history of hernia surgery | cardiac disease, COPD, diabetes, arterial hypertension, pulmonary disease, hepatic disease, renal disease, malignant disease |
| Evereg | Incisional | Anonymous, gender | No | Yes | Yes | No | Yes | No | Anticoag, antiplatelet, immunosupressants, smoking, personal history of hernia surgery | COPD, diabetes, cardiac disease, arterial hypertension, hepatic disease, renal disease, malignant disease |
| AHSQC | Primary ventral, incisional, parastomal, inguinal | Yes | Yes | Yes | Yes | No | Yes | Yes | Anticoagulant use, antiplatelet use, immunosuppressant use, nicotine use and route, history of hernia operation/open abdomen/myofascial release/surgical site infection, MRSA, currently active infection | Liver failure, ascites, HTN, diabetes, dialysis, COPD, dyspnea, inflammatory bowel disease, aneurysm |
COPD Chronic obstructive pulmonary disease, MRSA Multiresistant Staphylococcus aureus
Patient variables
In addition to the patient’s age and gender, most registries also record details of previous operations, risk factors and comorbidities (Tables 4, 5). Only a few registries record the patient’s occupation or information on sporting or exercise activities.
Table 5.
Operative data
| Routes | Pre-op data collection | Use of classifications (EHS) | Anatomical considerations | Operating time | Antibiotic use | Reducibility of the hernia | Defect closure | Registration of concomitant abdominal surgery | |
|---|---|---|---|---|---|---|---|---|---|
| Swedish Hernia Registry | Inguinal | Yes | Size and localization | Yes | Yes | Yes | Yes | No | Yes, but no report of type |
| Primary ventral, incisional, parastomal | Yes | Yes | |||||||
| Danish Hernia Database | Inguinal | No | No | Yes | No | No | No | Yes | Yes, but no report of type |
| Port-site, primary ventral, incisional, parastomal | Yes, only for incisional and parastomal | ||||||||
| Herniamed | Incisional, parastomal, hiatal, inguinal, umbilical, epigastric | Yes | Yes | Yes | Yes | Yes | No | Yes | No |
| Club Hernie | Primary ventral, incisional, inguinal, parastomal, giant incisional | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| EuraHS | Primary ventral, incisional, parastomal, hiatal, inguinal, open abdomen, abd. wall closure, prophyl. meshes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Evereg | Incisional | Yes | No | Yes | Yes | No | No | Yes | Yes |
| AHSQC | Primary ventral, incisional, parastomal, inguinal | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
Operative data
Most registries record details of the operation such as urgency of the operation, hernia classification, hernia localization, operating time, operative technique, anesthesia type, mesh type, fixation technique, defect closure, drain utilization and antibiotic prophylaxis (Table 5).
Intra- and postoperative complications
Intra- and postoperative surgical and general complications are recorded and vary among registries (Table 6).
Table 6.
Registration of complications
| Routes | Intraoperative wound contamination | Intraoperative complications | Postoperative complications | Mesh infection | Mesh removal | Post-surgical death | Intra-hospital pain | |
|---|---|---|---|---|---|---|---|---|
| Swedish Hernia Registry | Inguinal | No | Bleeding and injuries to other organs, cardiac and pulmonary, technical problems | Hematoma, urinary retention, infection, severe pain, reoperation (bleeding, infection, severe pain, ileus, other). Complication is graded: Mild, severe, life-threatening |
Superficial, deep and reoperation. | Yes | 30-day mortality | No |
| Primary ventral, incisional, parastomal | Yes | Bleeding and injuries to other organs, cardiac and pulmonary, technical problems, bladder injury, intestinal damage, severity of the injury and equipment failure | Bleeding, seroma/hematoma, SSI, mesh infection, intestinal injury, ileus, non-surgical complications, others | Superficial, deep and reoperation | No | 30-day mortality | No | |
| Danish Hernia Database | Inguinal | No | No | No (data obtained from the National Patient Registry) | No | No | 30-day mortality | No |
| Port-site, primary ventral, incisional, parastomal | Yes, only for incisional and parastomal | |||||||
| Herniamed | Incisional, parastomal, hiatal, inguinal, umbilical, epigastric | Yes | Bleeding and injuries to other organs | Complications within 30 days, non-surgical and surgical complications (bleeding, wound healing disorder, deep infection, seroma, hematoma), complication-related reoperations | Yes (deep infection) | No | Yes | Yes |
| Club Hernie | Primary ventral, incisional, inguinal, parastomal, giant incisional | Yes | Bleeding, adhesions, technical problems and injuries to other organs | Complications within 30 days, Clavien-Dindo grading, non-surgical complications, SSO, Surgical others, length of stay, ICU requirement, unplanned return to OR, Re-admissions within 30 days |
Yes | Yes | Yes | Yes |
| EuraHS | Primary ventral, incisional, parastomal, hiatal, inguinal, open abdomen, abd. wall closure, prophyl. meshes | Yes | Bleeding, adhesions, technical problems and injuries to other organs | Bleeding, intestinal injury, impaired wound healing, ileus, SSI, seroma, non-surgical complications; Clavien-Dindo grading | Superficial, deep and reoperation | Yes | Yes | Yes, but not for all routes |
| Evereg | Incisional | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| AHSQC | Primary, incisional, parastomal, inguinal |
Yes | Bleeding, adhesions, technical problems and injuries to other organs | Yes | Yes | Yes | Yes | No |
Follow-up data
Further variations are observed in the follow-up parameters and protocols as well as the follow-up achievements of the registries (Tables 7, 8). This can be explained by a huge variation in the structure of healthcare systems in different European countries. The quality and frequency of routine clinical follow-up varies due to clinical and financial limitations. Patients who experience postsurgical complications often do not present to the initial operating surgeons or institution.
Table 7.
Follow-up data part 1
| Routes | Time scale post-op follow-up | FU achievements | |
|---|---|---|---|
| Swedish Hernia Registry | Inguinal | 1 month, re-entry for a recurrence | > 90% |
| Primary ventral, incisional, parastomal | 1, 6 months | > 90%, respective 50% | |
| Danish Hernia Database | Inguinal, port-site, primary ventral, incisional, parastomal | Until patient death or emigration from data linking with the Danish Patient Registry | 100% for all included patients |
| HerniaMed | Incisional, parastomal, hiatal, inguinal, umbilical, epigastric | 1, 5, 10 years | Per contract with surgeon > 85% |
| Club Hernie | Primary ventral, incisional, inguinal, parastomal, giant incisional | 1 month by the surgeon clinically, 2 years and 5 years systematic control done by phone questionnaires by independent clinical research assistant blinded to the technique used. Additional if needed | > 85% at 2y FU for all correctly registered patients |
| EuraHS | Primary ventral, incisional, parastomal, hiatal, inguinal, open abdomen, abd. wall closure, prophyl. meshes | 1 month, 1 year, 2 years; additional time points between and after the fixed follow-up moments are possible | > 50% for 1 year; big differences in users |
| Evereg | Incisional | 1 month, 6 months, 1 year, 2 years. Additional if it’s needed | > 35% |
| AHSQC | Primary ventral, incisional, parastomal, inguinal | 1 month, 6 months, 1 year, 2 year, each year after operation | 90% 30 day; targeted long-term follow-up (based on individual populations of interest) |
Table 8.
Follow-up data part 2
| Routes | Post-operative complications | Post-operative pain | Seroma | Infection | Recurrence | Reoperation | Mortality | QoL measurements | |
|---|---|---|---|---|---|---|---|---|---|
| Swedish Hernia Registry | Inguinal | Registered by the coordinator | Yes | Yes | Yes | At reoperation | Yes | Yes | IPQ 2 |
| Primary ventral, incisional, parastomal | Yes | Yes and at reoperation | No | ||||||
| Danish Hernia Database | Inguinal, port-site, primary ventral, incisional, parastomal | Only if requiring reoperation or re-admission | No | Only if requiring reoper. or re-adm. | Only if requiring reoper. or re-adm. | Only if requiring reoper. or re-adm. | Yes | Yes, derived from national identity code | No |
| HerniaMed | Incisional, parastomal, hiatal inguinal, umbilical, epigastric | Secondary bleeding, intestinal lesion, wound healing disorder, ileus, deep infection | Pain (VAS scale) | Yes | Yes | Yes | Yes | Yes | No |
| Club Hernie | primary ventral, incisional, inguinal, parastomal, giant incisional | SSI, post-op bulging, mesh infection | Yes | Yes | SSI, post-op bulging, mesh infection | Yes | Yes | Yes | Club Hernie QoL Score |
| EuraHS | Primary ventral, incisional, parastomal, hiatal, inguinal, open abdomen, abd. wall closure, prophyl. meshes | SSI, post-op bulging, mesh infection | VAS, chronic pain: Cunningham classification | Yes | Yes | Yes | Yes | Yes | EuraHS QoL score, Giqli score |
| Evereg | Incisional | Yes | Chronic pain, VAS | Yes | Yes | Yes | Yes | Yes | No |
| AHSQC | Primary ventral, incisional, parastomal, inguinal | SSI, SSO, NSQIP complications | Yes | Yes | Yes | Yes | Yes | Yes | HerQLes, NIH PROMIS |
VAS visual analog scale, SSI surgical site infection, SSO surgical site occurance, NSQIP National Surgical Quality Improvement Program, QoL quality of life, HerQLes hernia-related quality-of-life survey, Gigli score gastrointestinal quality of life index, NIH PROMIS National Institute of Health patient-reported outcome measurement information system
Outcome measurement tools
All registries deliver feedback to their participating hospitals, surgeons and research groups via annual reports and Excel exported files (Table 9). Since registries have no proven system for checking the validy of entered data, they can suffer from selection and input bias. This is always a limitation of all data analyses from registries.
Table 9.
Provision of data and validation
| Routes | Data analysis provided | Validation | |
|---|---|---|---|
| Swedish Hernia Registry | Inguinal | Annual report on website and report to each center; individual surgeons get their results via the center; publication of data on the website, reports on national and international congresses | Random external validation; selected units are monitored each year by a specially educated team |
| Primary ventral, incisional, parastomal | Individual surgeons get their results via the center; publication of data on national and international congresses | Not at the moment, planned | |
| Danish Hernia Registry | Inguinal, port-site, primary ventral, incisional, parastomal | National education programs; feedback to surgeon; reports for research projects; publications in international papers; publication of data on international congresses | High validity has been demonstrated between patients´ files and entered data in the registry. Moreover, data are validated on an annual basis against certain quality standards, defined for groin and ventral hernia repair |
| HerniaMed | Incisional, parastomal, hiatal, inguinal, umbilical, epigastric | Study reporting per route and per section (demographic, status, surgery, mesh, complications, pain) possible. Excel export in real time for surgeons and groups; publication of data | Validation of the data via the German Hernia Society; 1st year: participant has to sign that he/she entered 90% of all hernia operations; after 3 years random audit |
| Club Hernie | Primary ventral, incisional, inguinal parastomal, giant incisional | Excel export in real time for surgeons and groups; real time comparisons with the group; publication of data on national and international congresses | Asking the patient, the clinical research assistant makes a retro-control of the surgeon’s input. In case of any difference, a control of the medical chart is done |
| EuraHS | Primary ventral, incisional, parastomal, hiatal, inguinal, open abdomen, abd. wall closure, prophyl. meshes | Excel export in real time per route or per case, case summary function, publication of data on international congresses. Annual report on website | Data validation is done by the constributing surgeons, as they are the owner of their data. |
| Evereg | Incisional | Excel export in real time for surgeons and groups; data report only for members of board; comparison with the group only available for the Executive Committee; publication of data on international congresses | Annual monitoring by an Executive Committee |
| AHSQC | Primary ventral, incisional, parastomal, inguinal | Real-time risk adjusted reports provided, comparing individual surgeon or hospital performance compared to collaborative; yearly individual surgeon reports; collaborative-wide analyses | Systematic data assurance including completion and accuracy |
Discussion
Within the scope of the CORE project, representatives from seven hernia registers gathered to compare different aspects of their hernia registers. The CORE project examined aspects such as financing, data collection, certification, patient data, operative data, complications and follow-up of the patients. As registries were developed during various time periods where hernia surgery techniques and focus on outcomes have differed over time, differences between registries can be found. Financial resources have also had an impact on the quality of registries as have the ideas of individual surgeons.
It would be desirable to directly compare and combine data from the various hernia registries; therefore, the present analysis suggests potential adjustments to the way data are collected to improve data comparability in the future. The recommendations for reporting outcomes should be given particular attention [13].
Despite the differences in the way data are collected for each of the listed hernia registries, the data are indispensable in clinical research. As a consequence of the numerous innovations in hernia surgery (surgical procedures, meshes, fixation devices), hardly any other area of surgical study has such a high need for clinical trials and data collection, comparison and analysis. Registries play a vital role in this innovation process [14]. In addition, there is insufficient public funding available to perform RCTs [15, 16]. Furthermore, the costs for conducting RCTs have increased dramatically over the last decades [17]. Therefore, RCTs should be more feasible embedded within registries [18].
It has been shown that the introduction of the Danish Hernia Database improved the quality of inguinal hernia surgery from a national perspective [19]. A review based on three European hernia registries demonstrated the range of insightful findings that can be gleaned from hernia registries [20]. Registries can also play an important role in monitoring new devices by the industry (post marketing surveillance) [21]. This is of paramount importance as registries are called upon to provide more data for this specific purpose, because in the context of the current regulation environment at least in the European Union countries, the need of post marketing surveillance of medical devices has increased. As the main aim of the new European Union Medical Device Regulation is better patient safety industry, insurance companies and governments should ultimately contribute to fund hernia registries.
Currently, over 170 analyses from various hernia registries (Danish Hernia Database—http://www.herniedatabasen.dk 84; Swedish Hernia Registry—http://www.svensktbrackregister.se 55; Herniamed—http://www.herniamed.de 22; EuraHS—http://www.eurahs.eu 5; AHSQC—http://www.ahsqc.org 5; Club Hernie—http://www.club-hernie.com 1; EVEREG—http://www.evereg.es 1) have been published. The number of published articles clearly indicates that RCTs and registry-based observational studies have become partners in the evolution of medical evidence in hernia surgery [20]. As there is a discrepancy between the actually published data from hernia registries and the number listed in PubMed the use of the registry name as key word for the publication should be obligatory.
Many important questions in the field of hernia surgery have only been studied in registry studies [20]. Thus, the registers in hernia surgery are of great importance for clinical research. One clear advantage of the registry concept is having the ability to detect and analyze low rate potentially clinically relevant or even catastrophic events. Due to the increasing complexity in hernia surgery, hernia centers are increasingly being established worldwide [22].
Public media are increasingly aware of the fact that surgery can only be improved if its results are known [23]; the registry data are increasingly used for quality control [24], for example, in the certification of hernia centers [25]. A hernia center should be required to participate in a registry and submit as complete as possible data on all hernia patients [25].
Limitation of all data analysis from registries is always selection and input bias. The American College of Surgeons National Surgical Quality Improvement Program (NSQIP) mandates that participating hospitals assigns a NSQIP trained clinical reviewer to collect data on a stratified sampling of patients. Ongoing education for the reviewers as well as auditing is designed to ensure data reliability. This can be a model for the future, but calls for adequate financial support. This model can also prevent misuse of a registry by participating hospitals for marketing purposes.
In summary, while the seven existing hernia registries worldwide may differ in structure, together they contribute to raising the quality of hernia surgery. Assurance of data quality is critical to registries. This aspect should be taken into account in the evaluation of registry data. It would be desirable to harmonize outcome variables. The registries are of great importance for clinical research and are complimentary to RCTs for quality assurance, monitoring innovations, and potential certification of hernia expert centers. Combining all registry data in a common database would be desirable to allow additional knowledge to be gained.
Authors’ contribution
All authors were responsible for their registry and provided all relevant information’s about their registry in the manuscript. All authors carefully checked the manuscript and gave advices for corrections. All authors gave their final approval for the current version of the manuscript.
Compliance with ethical standards
Conflict of interest
LNJ, AM and JAPR declare no conflict of interest. IKL declares conflict of interest directly related to the submitted work. JFG, WH and FM declare conflict of interest not directly related to the submitted work. FK declares conflict of interest directly and not directly related to the submitted work.
Ethical approval
This study did not need approval from an ethic committee.
Human and animal rights
This study does not contain any studies with participants or animals performed by any of the authors.
Informed consent
Informed consent was not required for this study.
Footnotes
I. Kyle-Leinhase and F. Köckerling contributed equally to this publication.
References
- 1.Demange MK, Fregni F. Limits to clinical trials in surgical areas. Clinics. 2011;66(1):159–161. doi: 10.1590/S1807-59322011000100027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Booth CM, Tannock IF. Randomised controlled trials and population-based observational research: partners in the evolution of medical evidence. BJC. 2014;110:551–555. doi: 10.1038/bjc.2013.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benson K, Hartz AJ. A comparison of observational studies and randomized, controlled trials. N Engl J Med. 2000;342(25):1878–1886. doi: 10.1056/NEJM200006223422506. [DOI] [PubMed] [Google Scholar]
- 4.McNeil JJ, Evans SM, Johnson NP, Cameron PA. Clinical-quality registries: their role in quality improvement. MJA. 2010;192(5):244–245. doi: 10.5694/j.1326-5377.2010.tb03499.x. [DOI] [PubMed] [Google Scholar]
- 5.Arts DGT, de Keizer NF, Scheffer GJ. Defining and improving data quality in medical registries: a literature review, case study, and generic framework. J Am Med Inform Assoc. 2002;9:600–611. doi: 10.1197/jamia.M1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nilsson E, Haapaniemi S. The Swedish hernia register: an 8 years experience. Hernia. 2000;4:286–289. doi: 10.1007/BF01201085. [DOI] [Google Scholar]
- 7.Helgstrand F, Rosenberg J, Bay-Nielsen M, Friis-Andersen H, Wara P, Jorgensen LN, Kehlet H, Bisgaard T. Establishment and initial experinces from the Danish Ventral Hernia Database. Hernia. 2010;14:131–135. doi: 10.1007/s10029-009-0592-0. [DOI] [PubMed] [Google Scholar]
- 8.Muysoms F, Campanelli G, Champault GG, DeBeaux AC, Dietz UA, Jeekel J, Klinge U, Köckerling F, Mandala V, Montgomery A, Morales Conde S, Puppe F, Simmermacher RKJ, Smietanski M, Miserez M. EuraHS: the development of an international online platform for registration and outcome measurement of ventral abdominal wall hernia repair. Hernia. 2012;16:239–250. doi: 10.1007/s10029-012-0912-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stechemesser B, Jacob DA, Schug-Pass C, Köckerling F. Herniamed: an internet-based registry for outcome research in hernia surgery. Hernia. 2012;16:269–276. doi: 10.1007/s10029-012-0908-3. [DOI] [PubMed] [Google Scholar]
- 10.Gillion JF, Fromount G, Lepère M, Letoux N, Dabrowski A, Zaranis C, Barrat C, The Hernia-Club Members Laparoscopic ventral hernia repair using a novel intraperitoneal lightweight mesh coated with hyaluronic acid: 1-year follow-up from a case–control study using the Hernia-Club registry. Hernia. 2016;20:711–722. doi: 10.1007/s10029-016-1501-y. [DOI] [PubMed] [Google Scholar]
- 11.Pereira JA, López-Cano M, Hernández-Granados P, Feliu X, on behalf of the EVEREG group Initial results of the National Registry of incisional hernia. CIR ESP. 2016;94(10):595–602. doi: 10.1016/j.ciresp.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Poulose BK, Roll S, Murphy JW, Matthews BD, Todd Heniford B, Voeller G, Hope WW, Goldblatt ML, Adrales GL, Rosen MJ. Design and implementation of the Americas Hernia Society Quality Collaborative (AHSQC): improving value in hernia care. Hernia. 2016;20:177–189. doi: 10.1007/s10029-016-1477-7. [DOI] [PubMed] [Google Scholar]
- 13.Muysoms FE, Deerenberg EB, Peeters E, Agresta F, Berrevoet F, Campanelli G, Ceelen W, Champault GG, Corcione F, Cuccurullo D, DeBeaux AC, Dietz UA, Fitzgibbons RJ, Jr, Gillion JF, Hilgers RD, Jeekel J, Kyle-Leinhase I, Köckerling F, Mandala V, Montgomery A, Morales Conde S, Simmermacher RKJ, Schumpelick V, Smietanski M, Walgenbach M, Miserez M. Recommendations for reporting outcome results in abdominal wall hernia. Hernia. 2013;17:423–433. doi: 10.1007/s10029-013-1108-5. [DOI] [PubMed] [Google Scholar]
- 14.Köckerling F. The need for registries in the early scientific evaluation of surgical innovations. Front Surg. 2014 doi: 10.3389/fsurg.2014.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rangel SJ, Efron B, Moss RL. Recent trends in national institutes of health funding of surgical research. Ann Surg. 2002;236(3):277–287. doi: 10.1097/00000658-200209000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Royal College of Surgeons of England (2013) Brifing of house of lords short debate on the impact of NHS innovation and research strategies. RCS Publications, London
- 17.Shore BJ, Nasreddine AY, Kocher MS. Overcoming the funding challenge: the cost of randomized controlled trials in the next decade. J Bone Jt Surg Am. 2012;94(Supplement 1):101–106. doi: 10.2106/JBJS.L.00193. [DOI] [PubMed] [Google Scholar]
- 18.James S, Rao SV, Granger CB. Registry-based randomized clinical trials—a new clinical trial paradigm. Nat Rev Cardiol. 2015;12(5):312–316. doi: 10.1038/nrcardio.2015.33. [DOI] [PubMed] [Google Scholar]
- 19.Kehlet H, Bay-Nielsen M, For the Danish Hernia Database Collaboration Nationwide quality improvement of groin hernia repair from the Danish Hernia Database of 87,840 patients from 1998 to 2005. Hernia. 2008;2008(12):1–7. doi: 10.1007/s10029-007-0285-5. [DOI] [PubMed] [Google Scholar]
- 20.Köckerling F. Data and outcome of inguinal hernia repair in hernia registers—a review of the literature. Innov Surg Sci. 2017 doi: 10.1515/iss-2016-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Köckerling F, Simon T, Hukauf M, et al. The importance of registries in the postmarketing surveillance of surgical meshes. Ann Surg. 2017 doi: 10.1097/SLA.0000000000002326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kulacoglu H, Oztuna D. Current status of hernia centres around the globe. Indian J Surg. 2015;77(Suppl 3):1023–1026. doi: 10.1007/s12262-014-1115-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landro L (2015) How to make surgery safer. Wall Street Journal (Newspaper/Magazine Article)
- 24.Rosser D, Lilford R (2013) Using surgeons’ outcome data for quality control. Health Service Journal, HSJ is part of Wilmington Healthcare Limited, Southfields, Essex
- 25.Köckerling F, Berger D, Jost JO. What is a certified hernia center? The example of the German Hernia Society and German Society of general and visceral surgery. Front Surg. 2014;1:26. doi: 10.3389/fsurg.2014.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]