Abstract
This review compiles the combined chemical and enzymatic synthesis of aromatic l-amino acids (l-phenylalanine, l-tyrosine, l-DOPA, l-tryptophan, and their derivatives and precursors) specifically labeled with carbon and hydrogen isotopes, which were elaborated in our research group by the past 20 years. These compounds could be then employed to characterize the mechanisms of enzymatic reactions via kinetic and solvent isotope effects methods.
Keywords: Amino acid, Bioamine, Deuterium, Enzyme, Labeling, Tritium
Introduction
This review deals with combined chemical and enzymatic synthesis of aromatic l-amino acids and bioamines labeled specifically with carbon and hydrogen isotopes. These compounds play an essential role in biochemical processes of life. Therefore, in the past the majority of very laborious syntheses have been carried out to provide these biologically active compounds, which were used as analytical, diagnostic, or therapeutic agents. However, the main impact on searches for new improved methods of synthesis comes from nuclear medicine, biochemistry, and pharmacy. Information on these methods are scattered, although a large knowledge may be taken starting from the large monograph published quite a long time ago [1], or from subsequently issued book [2–4]. In response to the growing demands for the labeled compounds, recently enzymatic methods were introduced, leading to the formation of needed biologically active products. However, there are no literature reviews devoted only to the synthesis of labeled compounds of particular relevance to the field of life science.
Our research group investigates the mechanisms of reactions catalyzed by enzymes. We employ isotopic techniques, particularly kinetic isotope effect (KIE) and solvent isotope effect (SIE) methods [5, 6], which require the use of selectively labeled compounds. For the abovementioned purposes the combined chemical and enzymatic synthesis of isotopomers of l-aromatic amino acids, its precursors, and derived bioamines, selectively labeled with isotopes of carbon and hydrogen were elaborated. In this paper we review previously published methods of synthesis of isotopomers of l-phenylalanine, l-tyrosine, l-DOPA, l-tryptophan, their derivatives, and precursors, all of which are specifically labeled with isotopes of hydrogen and carbon.
Synthesis
Synthesis of l-phenylalanine labeled with hydrogen and carbon isotopes
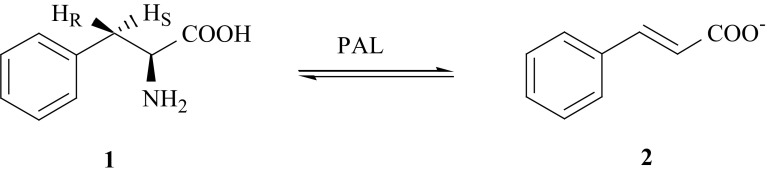
The synthesis of isotopomers of l-phenylalanine, l-Phe (1), specifically labeled with isotopes of carbon and hydrogen were elaborated by us to study the mechanism of elimination of ammonia from l-Phe catalyzed by enzyme phenylalanine ammonia lyase (PAL, EC 4.3.1.5), leading to formation of (E)-cinnamic acid (2) [7–12] according to Fig. 1.
Fig. 1.
Reaction catalyzed by ammonia lyase
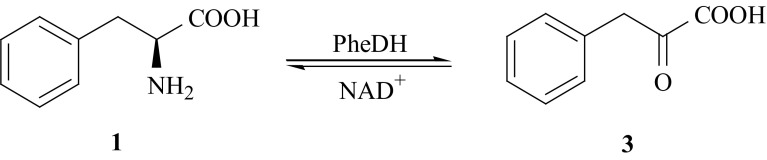
The metabolism of l-Phe is also connected with one of the human genetic disease—phenylketonuria (PKU), which is accompanied by elevated levels of l-Phe (1) metabolites such as phenylpyruvate and phenyllactate in body fluids. The knowledge about the mechanism of enzymatic conversion of l-Phe (1) into phenylpyruvic acid, PPA (3) is essential for proper therapy of PKU patients. One of the metabolic paths of conversion of (1) into (3) is reversible, oxidative deamination catalyzed by enzyme l-phenylalanine dehydrogenase (PheDH, EC 1.4.1.20) [13, 14] (Fig. 2).
Fig. 2.
Oxidative deamination catalyzed by enzyme l-phenylalanine dehydrogenase
The above two multistep reactions involve several intermediates, and therefore it is important to determine the structure of active complexes formed in the rate determining step. The number of arising questions can be minimized by determining kinetic isotope effects, KIE, of carbon 14, deuterium and tritium, as well as, the deuterium solvent isotope effects, SIE. Aforementioned studies require the use of the optically active forms of (1) specifically labeled with deuterium or tritium in desired (3R) and (3S) positions. The introduction of label in these specific positions only by chemical methods is a very tedious, time consuming, and sometimes even impossible, therefore, the combined chemical and enzymatic approaches were used.
For the preparation of labeled enantiomers of phenylalanine, the experimental procedures described in the literature resulted in multilabeled products or those labeled specifically with deuterium in irrelevant positions [15–19]. Also in the earlier reported studies on the synthesis of stereoisomers of [3-2H]- and [3-3H]-Phe the desired products were obtained as a result of tedious, multi step chemical synthesis [20–24]. Furthermore, often the enzymatic approach was applied to separate l- and d-isomers as the last step.
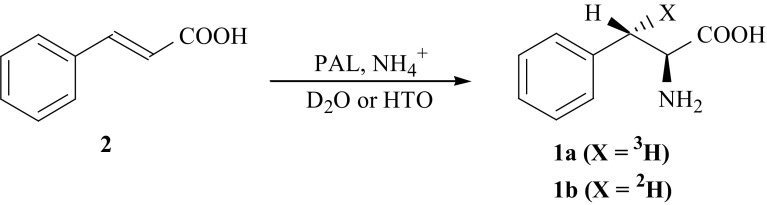
For synthesis of specifically labeled isotopomer, [(3S)-3H]-l-Phe (1a) properties of the enzyme PAL were used. This enzyme, under proper conditions, catalyzes addition of ammonia to (E)-cinnamic acid (2) resulting in formation of l-Phe (1) [7]. The synthesis of (1a) was performed according to Fig. 3. Addition of ammonia to cinnamic acid, catalyzed by PAL, was carried out in the buffer containing tritiated water, HTO, leading to formation of (1a) [25, 26].
Fig. 3.
Synthesis of l-Phe isotopomers labeled with hydrogen isotopes in the (3S) position
The same approach was taken to obtain deuterium labeled [(3S)-2H]-l-Phe (1b). In this case, addition of ammonia was carried out in fully deuterated phosphate buffer.
The synthesis of tritiated isotopomer [(3R)-3H]-l-Phe (1d), was carried out according to Fig. 4. The compound (1d) was obtained by combining chemical and enzymatic methods. In the first step, benzaldehyde (4) labeled with tritium in the formyl group {[7-3H]-benzaldehyde} (4a) was prepared. There are several synthetic protocols for obtaining the benzaldehyde, however, most of them require expensive reagents and long reaction time. The method chosen by us involved the step in which benzil, (PhCO)2 (5), was cleaved by cyanide ion in tritiated water, HTO. The tritiated benzaldehyde (4a) was condensed with malonic acid (6) in pyridine solvent using catalytic amounts of piperidine giving [3-3H]-cinnamic acid (2a). Compound 2a then was converted into (1d) by addition of ammonia catalyzed by PAL [25] (Fig. 4).
Fig. 4.
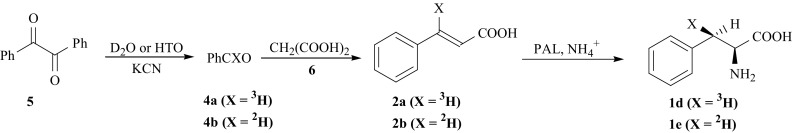
Synthesis of l-Phe isotopomers labeled with hydrogen isotopes in the (3R) position
Deuterium labeled [(3R)-2H]-l-Phe (1e) was synthesized the same way by cleaving benzil in heavy water (99.9% D2O) and adding ammonia to resulted [3-2H]-cinnamic acid (2b).
In the literature there are several procedures describing the synthesis of l-Phe labeled with isotopes of carbon. Isotopomers [3-11C]-dl-Phe [27] and [3-14C]-D-Phe [28] were obtained using multi step chemical path. Also, [3-11C]-l-Phe was synthesized via combined chemo- and enzymatic method [29]. 13C- or doubly labeled [2H, 13C]- phenylalanines were obtained using purified enzymes [24] or intact microorganisms [19, 30] in the key step of reaction. However, these methods yielded irreverently labeled products only, useful for spectroscopic studies.
Our studies have required different isotopomers of l-Phe specifically labeled with isotopes of carbon in desired positions. For KIE studies the novel enzymatic pathways to obtain the desired labeled compounds i.e. [1-14C]-l-Phe (1 h) and [1-13C]-l-Phe (1i) using sodium [1-14C]-acetate (7a) or sodium [1-13C]-acetate (7b) as a source of carbon label were elaborated [31]. The syntheses were carried out according to Fig. 5. Labeled sodium acetates (7a or 7b) were converted into labeled acetic anhydrides (8a, 8b) and then reacted with benzaldehyde (4) yielding labeled [1-14C]- (2c) or [1-13C]-cinnamic acid (2d). These compounds were converted into desired isotopomers (1h) and (1i) by enzymatic addition of ammonia catalyzed by the enzyme PAL.
Fig. 5.
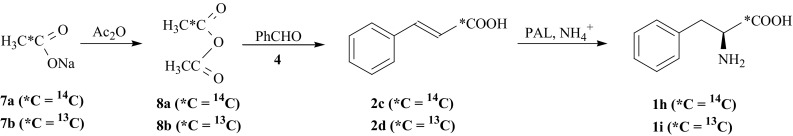
Synthesis of l-Phe isotopomers labeled with carbon isotopes
Another synthetic route to obtain the isotopomer of (1) specifically labeled with 14C, i.e., [2-14C]-l-Phe (1j), consists of combination of chemical and enzymatic methods [32]. As a source of 14C commercially available [2-14C]-malonic acid (6a) was used. Then the labeled [2-14C]-cinnamic acid (2e) was prepared by Knovenagel condensation. In the last step of the synthesis leading to the pure l-enantiomer of phenylalanine (1j), the activity of enzyme PAL was used (Fig. 6).
Fig. 6.
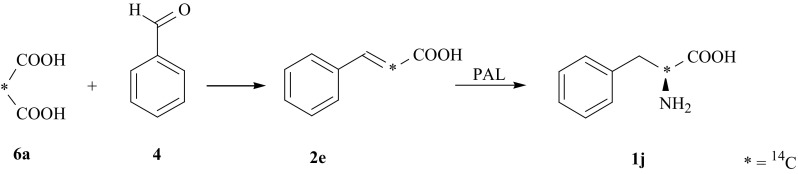
Chemo-enzymatic synthesis of [2-14C]-l-Phe
For synthesis of [3-14C]-l-Phe (1k), [1-14C]-benzaldehyde (4c) and unlabeled malonic acid (6) were applied. [1-14C]-Benzaldehyde (4c) was prepared using the following reaction sequence: carbonation of Grignard’s reagent PhMgBr (9) with [14C]carbon dioxide (10), hydrolysis resulted Ph14COOMgBr (11) to [7-14C]-benzoic acid (12), which was reduced with LiAlH4 yielded [7-14C]-benzyl alcohol (13). Next (13) was enzymatically converted into [1-14C]-benzaldehyde (4c) using yeast alcohol dehydrogenase (YADH, EC 1.1.1.1), which condensed with malonic acid (6) gave [3-14C]-cinnamic acid (2f). Finally, the addition of ammonia to (2f) catalyzed by PAL leads to (1k) (Fig. 7) [32, 33].
Fig. 7.
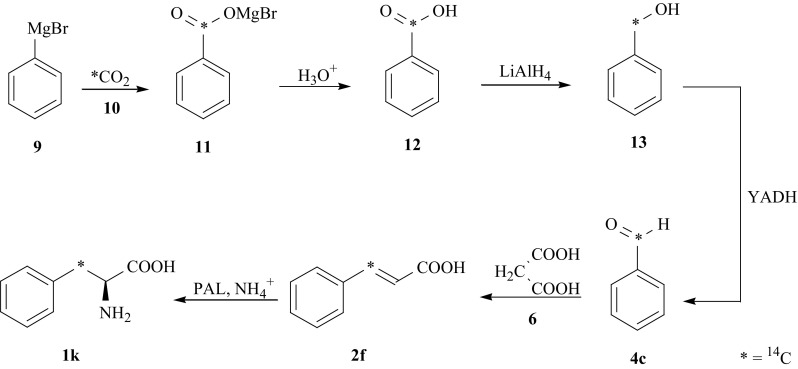
Chemo-enzymatic synthesis of [3-14C]-l-Phe
The characteristic of isotopomers of l-Phe (1) are collected in Table 1.
Table 1.
The characteristic of l-Phe isotopomers
Synthesis of l-tyrosine labeled with hydrogen and carbon isotopes
The metabolism of l-tyrosine, l-Tyr (14), is a key step in many biological processes of living organisms. A number of questions cannot be answered without understanding the mechanisms of the reversible conversion of l-Tyr (14) to phenol (15), pyruvate (16) and ammonia, a reaction that is catalyzed by the enzyme β-tyrosinase (tyrosine phenol lyase, EC 4.1.99.2) [34–38] and enzymatic conversion of l-Tyr (14) to l-DOPA (17), catalyzed by tyrosinase (EC 1.14.18.1) [39–41] (Fig. 8). Such studies using KIE and SIE methods [42–45] require different isotopomers of l-Tyr (14), specifically labeled with carbon and hydrogen isotopes in the positions of interest.
Fig. 8.
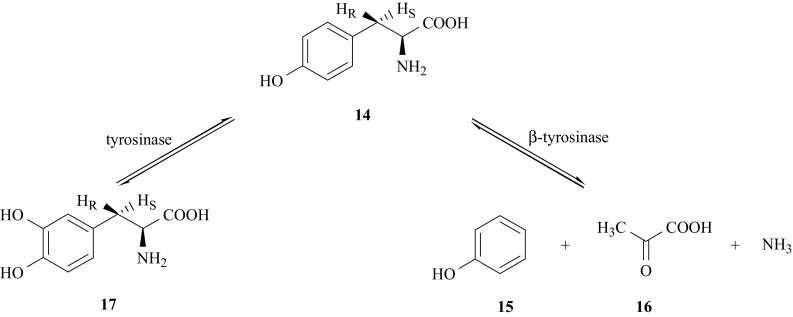
Reactions catalyzed by tyrosinase and β-tyrosinase
l-Tyrosine multilabeled with deuterium in several positions has been prepared by the different routes including chemical [46–48] and enzymatic [17, 49] mainly for spectroscopic studies. However, to study the mechanisms of enzymatic reactions, doubly labeled [3-2H/3H]-l-Tyr [50] and deuterated [(2S)-2H]-, [(2R)-2H]-l-Tyr [51] were obtained as a result of multistep chemical synthesis.
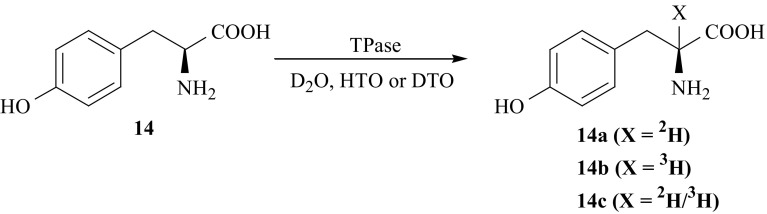
For our KIE studies the isotopomers of l-Tyr (14), labeled in the second position of the side chain, i.e., [2-2H]-l-Tyr (14a) and [2-3H]-l-Tyr (14b) were synthesized using the simpler way (Fig. 9). The label (deuterium or tritium) was introduced into 2-position of (14) by enzymatic isotopic exchange between incubation medium (containing D2O or HTO) and (14), catalyzed by enzyme tryptophanase (TPase, EC 4.1.99.1) from E. coli [52]. Under some conditions this enzyme causes labilization of hydrogen attached to α-carbon of many native l-amino acids and facilitates the H/D(T) exchange [53]. Doubly labeled isotopomer [2-2H/3H]-l-Tyr (14c) was obtained the same manner using fully deuterated buffer with DTO added.
Fig. 9.
Enzymatic synthesis of l-Tyr isotopomers labeled with hydrogen isotopes in the second position of the side chain
[2-3H]-l-Tyr (14b) was also synthesized from [2-3H]-l-Phe (1f), according to the reaction route shown in Fig. 10. The tritium label was introduced into the methylene group of malonic acid (6), as a result of isotopic exchange of (6) and tritiated water at elevated temperature [54]. Knovenagel condensation of [2-3H]-malonic acid (6b) with benzaldehyde (4) leads to [2-3H]-cinnamic acid (2g), which in turn was converted by enzyme PAL to [2-3H]-l-Phe (1f). Finally, tritiated l-Phe incubated in medium containing the enzyme l-phenylalanine 4′-monooxygenase (EC 1.14.16.1) from rat liver, produces [2-3H]-l-Tyr (14b). To stimulate the hydroxylation of l-Phe to l-Tyr, the reaction was carried out in the presence of d,l-6-methyl-5,6,7,8-tetrahydropterine (cofactor) and d,l-dithiothreitol. The medium also contained the enzyme catalase (EC 1.11.1.6) that protects lTyr from H2O2 formed during the course of incubation. The general protocol of this step was described earlier [55].
Fig. 10.
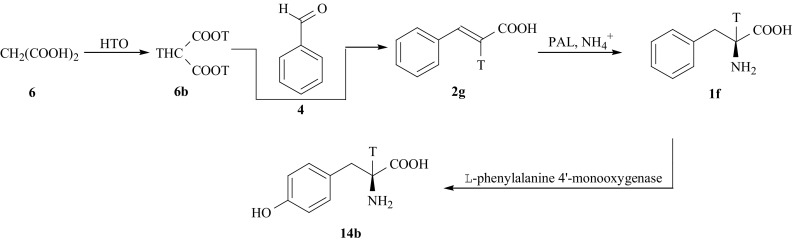
Chemo-enzymaic synthesis of [2-3H]-l-Tyr
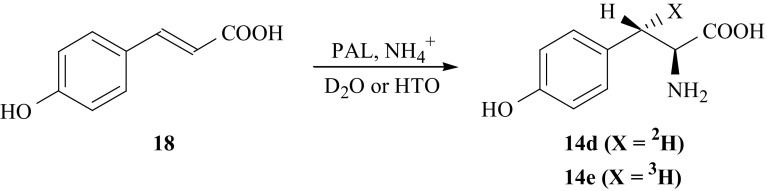
The enzyme PAL was also used for the synthesis of [(3S)-2H]- (14d) and [(3S)-3H]-lTyr (14e) [52, 55] (Fig. 11). Under proper conditions this enzyme catalyzes addition of ammonia and hydrogen isotope (deuterium or tritium, depending on incubation medium) into pro-S position of p-coumaric acid (18) yielding (14d) or (14e). The yield of this synthetic route is very small, however it is the simplest way to obtain [(3S)-2H]- (14d) and [(3S)-3H]-lTyr (14e).
Fig. 11.
Synthesis of l-Tyr isotopomers labeled with hydrogen isotopes in the (3S) position
[(3R)-3H]-lTyr (14f) was synthesized by enzymatic hydroxylation of [(3R)-3H]-lphenylalanine (1d) obtained earlier [25] catalyzed by l-phenylalanine 4′-monooxygenase (Fig. 12). The hydroxylation of l-Phe to l-Tyr was carried out in the presence of d,l-6-methyl-5,6,7,8-tetrahydropterine (cofactor) and the enzyme catalase (EC 1.11.1.6) [55].
Fig. 12.
Synthesis of l-Tyr isotopomers labeled with hydrogen isotopes in the (3R) position
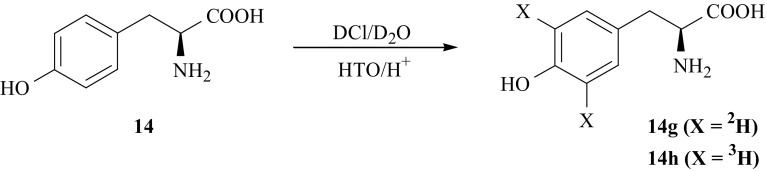
Isotopomers of l-tyrosine (14) labeled with deuterium in the 3′ and 5′ positions of the ring were obtained using isotopic exchange between heavy water and l-tyrosine. Under the acid catalyzed conditions, at elevated temperature, the exchange between D2O and l-Tyr (14) introduces deuterium exclusively into orto position [56, 57] (respectively to ring hydroxyl group) in (14), yielding [3′,5′-2H2]-l-Tyr (14g) [58, 59]. The same method was used to synthesize [3′,5′-3H2]-l-Tyr (14h). As a source of hydrogen isotope, tritiated water was used (Fig. 13).
Fig. 13.
Synthesis of l-Tyr isotopomers labeled with hydrogen isotope in the 3′ and 5′ positions of aromatic ring
Synthetic route of [2′,6′-3H2]-l-Tyr (14i) which consists of a combination of chemical and enzymatic methods [60] is shown in Fig. 14. First, the key intermediate i.e., [3,5-3H2]-phenol (15b) was obtained as a result of H/T exchange between phenol (15) and tritiated water. The literature data [56, 57, 61] show that phenol can be catalytically exchanged with deuterated or tritiated water selectively in the o- and p-positions or per labeled. By the reverse acid catalyzed exchange of uniformly tritiated phenol [U-3H]-PhOH (15a) with water it is possible to prepare [3,5-3H2]-phenol (15b), which in turn condensed with S-methyl-l-cysteine (19) using the enzyme β-tyrosinase (EC 4.1.99.2) from Citrobacter freundii yielded (14i).
Fig. 14.
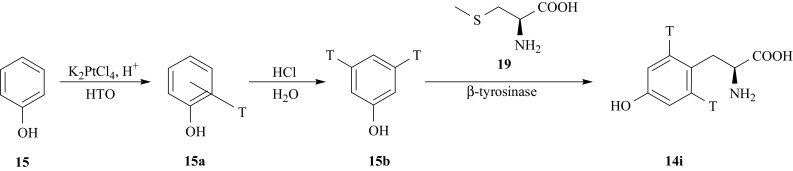
Synthesis of [2′,6′-3H2]-l-Tyr
In the literature there are reports of preparation of isotopomers of l-Tyr labeled with stable and radioactive carbon isotopes using classical chemical methods. Doubly labeled stereoisomers, i.e., threo- and erythro-[1-13C, 2,3-2H2]-l-Tyr, used for subsequent spectroscopic studies, were afforded in the multistep chemical synthesis [19]. Similarly, the pure chemical approach was applied for synthesis o [2-11C]-l-Tyr [62]. The demand for compounds labeled with short-lived 11C that are used as a diagnostic in nuclear medicine (i.e., positron emission tomography, PET) has prompted the efforts to synthesize amino acids labeled with this nuclide. Using 11CO2 as a source of label and applying the combined chemo- and multienzymatic methods the following isotopomers labeled in side chain were obtained: [1-11C]-l-Tyr [63], [2-11C]-l-Tyr [64] and [3-11C]-l-Tyr [43].
For our KIE studies three isotopomers of l-Tyr (14) specifically labeled with 14C in the 1-, 2- and 3-positions of the side chain {[1-14C]- (14j), [2-14C]- (14k) and [3-14C]-l-Tyr (14l)}, have been prepared using combined chemical and multienzymatic methods. For these syntheses, as intermediates, isotopomers of [1-14C]- (2c) [2-14C]- (2e), and [3-14C]-cinnamic acid (2f), have been converted into [1-14C]- (1h), [2-14C]- (1j), and [3-14C]-l-phenylalanine (1k), in the presence of the enzyme PAL (Fig. 15). In the next step, labeled l-Phe was oxidized to l-Tyr using an enzyme phenylalanine 4′-monooxygenase from rat liver [52, 65]. The hydroxylation of l-Phe to l-Tyr was carried out in the presence of a cofactor and the enzyme catalase (EC 1.11.1.6) that protects l-Tyr from hydrogen peroxide formed during incubation. The general route for the synthesis of labeled l-Tyr is shown in Fig. 15.
Fig. 15.
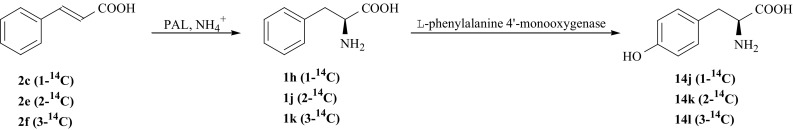
Synthesis of [1-14C]-, [2-14C]- and [3-14C]-l-Tyr
Another compound, [1′-14C]-l-Tyr (14m), specifically labeled with 14C in the 1′ position in the ring, has been prepared in 6 step reaction sequence [66]. For this synthesis, as a starting substrate and a source of 14C label, [2-14C]-malonic acid (6a), was used. It was converted via its silver salt (20), in diethyl [2-14C]-malonate (21) [67]. The ring closure reaction of (21) with 4H-pyran-4-on afforded ethyl [1′-14C]-p-hydroxybenzoate (22), which was hydrolyzed to [1′-14C]-p-hydroxybenzoic acid (23). Its thermal decomposition yielded [4-14C]-phenol (15c) [68], which in turn was coupled [42] with S-methyl-l-cysteine (19) catalyzed by the enzyme β-tyrosinase from Citrobacter freundii yielding desired [1′-14C]-l-Tyr (14m) (Fig. 16).
Fig. 16.
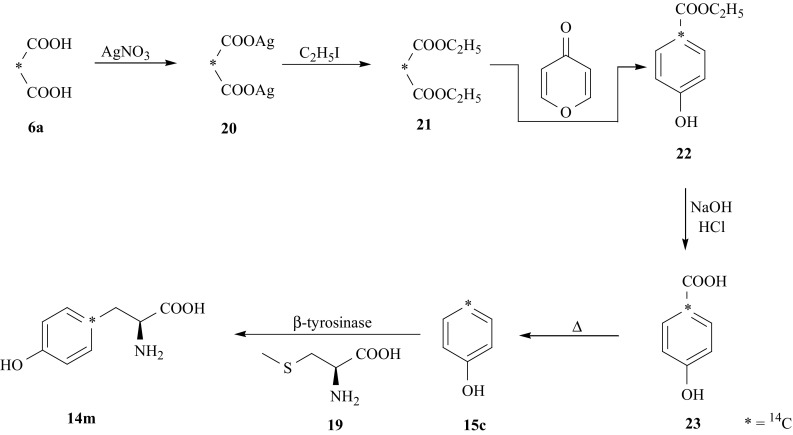
Synthesis of [1′-14C]-l-Tyr
The characteristic of l-Tyr (14) isotopomers are collected in Table 2.
Table 2.
The characteristic of l-Tyr isotopomers
| Compound | Specific activity (Bq/mmol) | Chemical yield (%) | References |
|---|---|---|---|
| [2-3H]-l-Tyr (14b) | 3.58 × 108 | – | [52] |
| [(3S)-2H]-l-Tyr (14d) | – | 1 | [52] |
| [(3S)-3H]-l-Tyr (14e) | 3.7 × 108 | – | [52] |
| [(3R)-3H]-l-Tyr (14f) | 4.64 × 106 | – | [55] |
| [3′,5′-3H2]-l-Tyr (14 h) | 5.5 × 106 | 75 | [58] |
| [2′,6′-3H2]-l-Tyr (14i) | 6.27 × 107 | 18 | [60] |
| [1-14C]-l-Tyr (14j) | 6.8 × 106 | – | [65] |
| [2-14C]-l-Tyr (14k) | 1.88 × 106 | – | [65] |
| [3-14C]-l-Tyr (14l) | 2.8 × 106 | – | [52] |
| [1′-14C]-l-Tyr (14m) | 1.83 × 106 | 15 | [66] |
Synthesis of l-DOPA labeled with hydrogen and carbon isotopes
l-DOPA (3′,4′-dihydroxy-l-phenylalanine) (17), plays a significant role in many metabolic processes [69]. It is a precursor of biogenic amine—dopamine, DA, (25)—an important neurotransmitter in the nervous system of mammals. DA is formed in the brain as a result of decarboxylation of l-DOPA catalyzed by enzyme aromatic l-amino acid decarboxylase (EC 4.1.1.28) [70, 71] (Fig. 17). The mechanism of decarboxylation is not clear up to now, so for KIE and SIE studies specifically labeled isotopomers of l-DOPA are needed.
Fig. 17.
Enzymatic decarboxylation of l-DOPA
The original literature data concerning the synthesis of dl-DOPA specifically labeled with deuterium and tritium in different positions of ring and side chain are dated [72, 73] and yielded products useless for biological studies.
Needed for our purpose isotopomer [(3S)-3H]-l-DOPA (17a), selectively labeled with tritium in the (3S) position of the side chain, was obtained from the appropriate isotopomer of l-Tyr (14e) via enzymatic pathway [58] (Fig. 18). Enzyme tyrosinase from mushrooms Neurospora Crassa (EC 1.14.18.1) selectively introduces a hydroxyl group into the 3′-ring position of l-Tyr (14) [74], and also immediately mediates oxidation of l-DOPA to dopaquinone (26) [75]. However, in the presence of ascorbic acid (27), the oxidation of l-DOPA is a reversible process [76]. Ascorbic acid reduces dopaquinone to l-DOPA, and itself undergoes oxidation to dehydroascrobic acid (28).
Fig. 18.
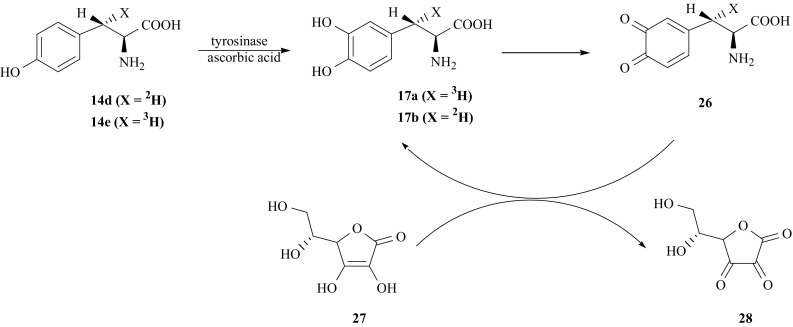
Synthesis of [(3S)-2H]- or [(3S)-3H]-l-DOPA
For the synthesis of isotopomer [(3S)-2H]-l-DOPA (17b) labeled with deuterium in (3S) position of the side chain instead of (14e) as starting substrate (14d) was used (Fig. 18).
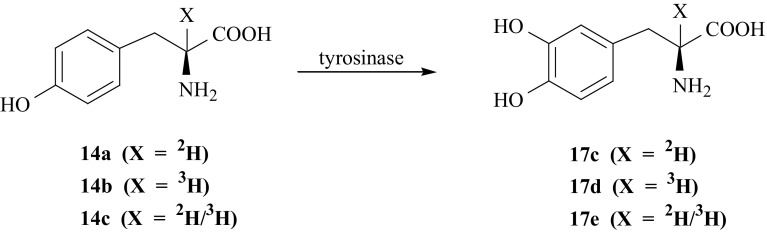
The same enzyme tyrosinase was used to obtain isotopomers of l-DOPA selectively labeled in the second position of the side chain, i.e., [2-2H]- (17c), [2-3H]- (17d) and [2-2H/3H]-l-DOPA (17e). Appropriate isotopomers of l-Tyr (14a, 14b, 14c) [52, 59, 77] were converted to l-DOPA by enzymatic hydroxylation catalyzed by enzyme tyrosinase in presence of ascorbic acid (Fig. 19).
Fig. 19.
Enzymatic synthesis of isotopomers of l-DOPA labeled with hydrogen isotopes in the second position of the side chain
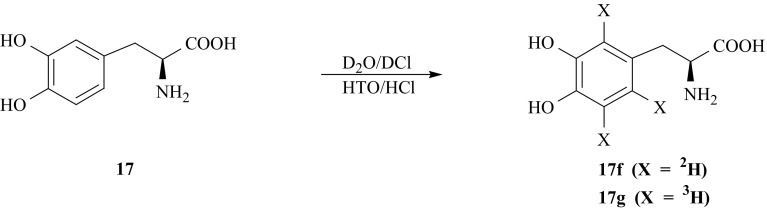
The ring deuteration of l-DOPA (17) was carried out using acid catalyzed isotope exchange method at elevated temperature [78] (Fig. 20). No significant change of proton NMR signal integrations, corresponding to methylene and methine groups of the side chain, have been noticed in the course of experiments. The incorporation of deuterium takes place only into the aromatic ring of l-DOPA (17) yielding [2′,5′,6′-2H3]-l-DOPA (17f). Also the rates of H/D exchange are practically the same for the protons in 2′, 5′, and 6′ ring positions. Tritiation of (17) carried out in the same conditions using HTO as a source of 3H-label leads to [2′,5′,6′-3H3]-l-DOPA (17 g).
Fig. 20.
Synthesis of l-DOPA isotopomers labeled with hydrogen isotopes in the aromatic ring
l-DOPA labeled with 14C in carboxyl group, needed as internal radiometric standard, was synthesized [79] from [1-14C]-l-Tyr (14j) according to Fig. 18. The literature data concerning the chemical and combined chemo- enzymatic synthesis of l-DOPA bearing 11 C-label are very tedious and were designed to obtain the products for PET diagnosis. Chemical [80, 81] and chemo-enzymatic [63] routes are applied for synthesis of [1-14C]-l-DOPA, as well as for obtaining of [2-11C]-l-DOPA [64, 82]. The old paper reports on the synthesis of uniformly ring labeled [U-14C]-l-DOPA using [U-14C]-phenol as a substrate [83].
The characteristic of l-DOPA (17) isotopomers are collected in Table 3.
Table 3.
The characteristic of l-DOPA isotopomers
Synthesis of l-tryptophan labeled with hydrogen and carbon isotopes
The important metabolic reaction of l-tryptophan, l-Trp (29) in living organisms is its decomposition to the corresponding indole (30), pyruvate (16), and ammonia. This reaction is catalyzed by the enzyme tryptophanase (l-tryptophan indole lyase, TPase, EC 4.1.99.1) [84–86] (Fig. 21).
Fig. 21.
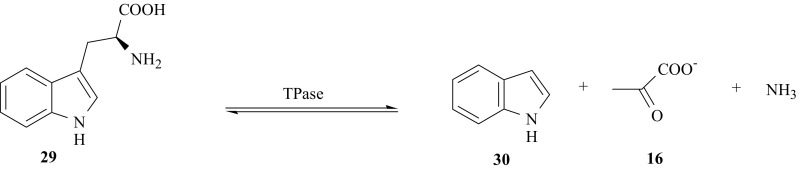
Enzymatic decomposition of l-Trp catalyzed by tryptophanase
Under some experimental conditions the enzyme TPase catalyses the condensation of the indonyl moiety [(30) or 5-hydroxyindole (31)] with S-methyl-l-cysteine (19), leading to the synthesis of (29) or 5′-hydroksy-l-tyrptophan, 5′-OH-l-Trp (32), respectively [87]. The enzymatic labilization of hydrogen attached to the α-carbon (occurred in this reaction) facilitates the H/D or H/T exchange with the solvent (deuterated or tritiated water in this case).
The suggested mechanism of decomposition of l-Trp postulates proton transfer from the side chain to the C-3 carbon atom of the indole ring. This hypothesis should be verified by measuring the KIE for deuterium, tritium and carbon-14, as well as, the deuterium solvent isotope effects, SIE. For such kind of studies there is a need for isotopomers of l-Trp and 5′-OH-l-Trp specifically labeled with deuterium and tritium at the α-carbon position. Unfortunately, while the literature provides several synthetic methods leading to preparation of different isotopomers of tryptophan and its hydroxyl derivative labeled with deuterium and tritium specifically or nonspecifically, these reports are of little value for this purpose. Perdeuterated in indonyl moiety [2H5]-l-Trp [88] and [4,5,6,7 -2H4]-l-Trp were obtained by H/D exchange in D2O/CF3COOD solvent [46]. The [(2RS)-2H]-Trp was afforded by exchange with D2O by racemization/acylation procedure. This intermediate was resolved with acylase yielding [(2S)-2H]-Trp [89]. Four isotopomers of l-Trp labeled with deuterium specifically in indole ring have been obtained by coupling labeled indoles with l-serine catalyzed by extracts of E. coli cells containing enzyme tryptophan synthetase [90]. Also, the various isotopomers of tryptophan labeled with deuterium and tritium at the 2- and 3-positions of side chain were synthesized by chemical methods [91–93]. [5′-2H]-dl-Trp and [5′-3H]-dl-Trp were synthesized by reduction of 5-bromo-dl-Trp with gaseous deuterium or tritium [94]. In turn, 5′-hydroxy-[4′-3H]-dl-Trp was obtained by H/D exchange between 5′-hydroxy-dl-Trp and HTO [94]. 5′-Hydroxy-[4′-3H]-Trp was prepared by enzymatic hydroxylation of [4′-3H]-Trp [95]. In addition, the isotopomers doubly labeled with deuterium and 13C were prepared [19, 96] for spectroscopic studies.
The coupling reaction (Fig. 22) was used by us to obtain l-Trp (29) and 5′-OH-l-Trp (32) labeled with the isotopes of hydrogen at the α-carbon position [97]. For the synthesis of [2-2H]-l-Trp (29a), and 5′-OH-[2-2H]-l-Trp (32a), all reagents were dissolved in fully deuterated phosphate buffer. For the synthesis of [2-3H]-l-Trp (29b), and 5′-OH-[2-3H]-l-Trp (32b) the reaction was carried out in phosphate buffer contained tritiated water (HTO). Doubly labeled [2-2H/3H]-l-Trp (29c), and 5′-OH-[2-2H/3H]-l-Trp (32c) have been obtained using a medium composed with fully deuterated phosphate buffer to which DTO was added. In all cases 2-mercaptoethanol was used to prevent the growth of bacteria and fungi during incubation.
Fig. 22.
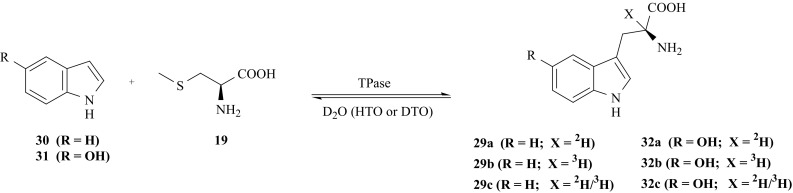
Synthesis of l-Trp and 5′-OH-l-Trp labeled with hydrogen isotopes at the α-carbon position
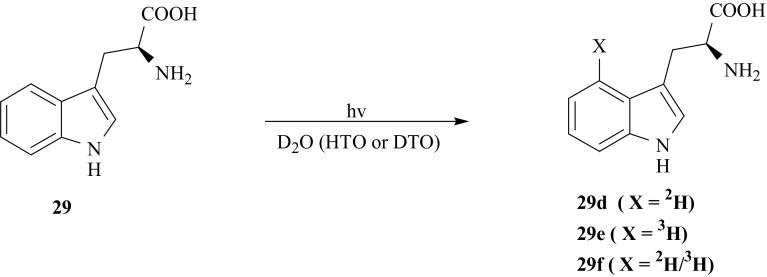
The isotopomer [4′-2H]-l-Trp (29d) was obtained by irradiation of the unbuffered solution of l-Trp (29) in heavy water with light from a 250 W mercury lamp filtered by Pyrex glass [98, 99]. The rate and degree of deuterium incorporation was monitored by 1H NMR spectroscopy. The results obtained in the course of deuteration allowed us to elaborate the exchange procedures for indole ring tritiation of (29). The tritiated [4′-3H]-l-Trp (29e) was obtained in one-step H/T exchange between (29) and tritiated water irradiated with UV light. The doubly labeled [4′-2H/3H-]-l-Trp (29f) was obtained in the same way by tritiation of deuterated (29d) dissolved in DTO (Fig. 23).
Fig. 23.
Synthesis of l-Trp labeled with hydrogen isotopes in the 4′ position of the aromatic ring
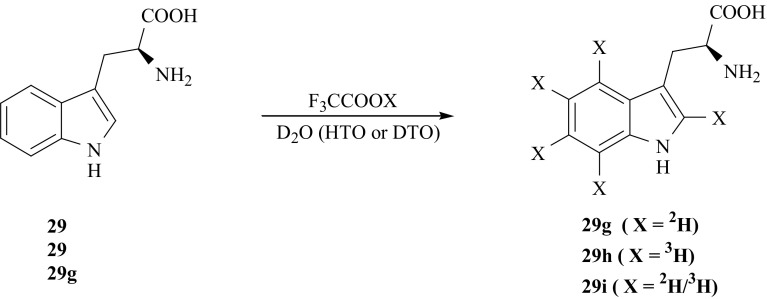
The deuterated in the whole indole ring isotopomer, i.e., [2′,4′,5′,6′,7′-2H5]-l-Trp (29g) was obtained as a result of H/D exchange between (29) dissolved in a mixture of D2O and CF3COOD (1: 2, v/v). The reaction was carried out in darkness for 3 days at room temperature [47, 100]. The obtained product was isolated and the exchange procedure was repeated twice. After each step the extent of deuterium enrichment of (29g) was checked by means of 1H NMR. The whole indole ring tritiated [2′,4′,5′,6′,7′-3H5]-l-Trp (29h) was obtained as a result of one-step isotope exchange between (29) and the CF3COOH/HTO mixture. Also, the doubly labeled [2′,4′,5′,6′,7′-(2H/3H)5]-l-Trp (29i) was synthesized by one-step D/T exchange between deuterated (29g) and DTO [99] (Fig. 24).
Fig. 24.
Synthesis of l-Trp uniformly labeled with hydrogen isotopes in the aromatic ring
For purposes of KIE studies, the specifically labeled with 14C isotopomers of l-Trp and 5′-OH-l-Trp were needed as an internal radiometric standard. In the literature there are descriptions of several methods of synthesis of the l-Trp and 5′-OH-l-Trp labeled with 11C or 13C. The 13C-indoles have been converted to the corresponding isotopomers of l-Trp using E. coli cells containing enzyme tryptophane synthetase [90, 101–103]. Also, the 11C-labeling of l-Trp [63, 104–106] have been reported for tumor diagnosis using PET. Additionally, several tedious chemical procedures of synthesis the different isotopomers of 14C-labeled dl-Trp have been described about 50 years ago [1].
[1-14C]-l-Trp (29j), and 5′-OH-[1-14C]-l-Trp (32d) specifically labeled with 14C in the carboxyl group, have been prepared using a combination of chemical and multienzymatic methods [107]. For this synthesis we applied, as an intermediate, a racemic mixture of [1-14C]-dl-alanine (33), which was obtained in a multistep synthesis that has been previously reported [108, 109]. 14CO2 (10) was used as a source of 14C-label. It has been converted in turn into (33) via [1-14C]-propionic acid (carbonation of the Grignard reagent C2H5MgI with 14CO2 and decomposition of the complex formed), 2-bromo[1-14C]propionic acid, followed by ammonolysis. In a one-pot multienzymatic synthesis (33) was converted into [1-14C]-pyruvic acid (16a) using the enzymes: D-amino acid oxidase (D-AAO, EC 1.4.3.3), catalase (EC 1.11.1.6) and glutamic-pyruvate transaminase (GPT, EC 2.6.1.2) [110]. In turn (16a) was coupled with indole (30) or 5-hydroxyindole (31) by the enzyme TPase (EC 4.1.99.1) giving (29j) or (32d), respectively (Fig. 25). Enzyme D-AAO rapidly transforms d-alanine to pyruvic acid only, therefore, to avoid the loss of half of radioactivity from the l-enantiomer we also used the second enzyme, GPT, converting l-alanine into pyruvic acid only. The medium also contained the enzyme catalase (EC 1.11.1.6) (removing the H2O2 formed) and coenzymes for D-AAO and GPT, i.e., flavin adenine dinucleotide, FAD, and pyridoxal 5′-phosphate, PLP, respectively [107].
Fig. 25.
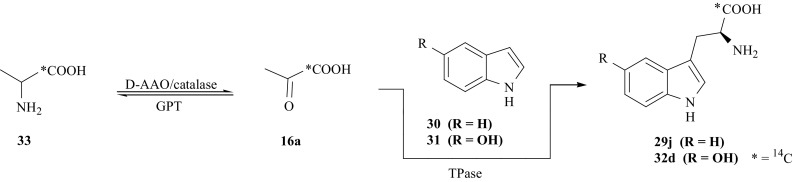
Synthesis of [1-14C]-l-Trp and 5′-OH-[1-14C]-l-Trp
In the same manner the isotopomers of [3-14C]-l-Trp (29k) and 5-OH-[3-14C]-l-Trp (32e) were obtained in one-pot multienzymatic synthesis in presence of the same four enzymes as above, and by using as substrates [3-14C]-dl-alanine (33a) and indole (30) or 5-hydroxyindole (31) respectively [111] (Fig. 26).
Fig. 26.
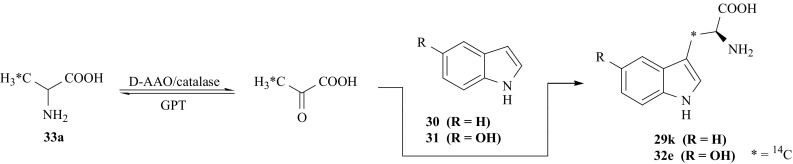
Synthesis of [3-14C]-l-Trp and 5′-OH-[3-14C]-l-Trp
The characteristic of l-Trp (29) and 5′-OH-l-Trp (32) isotopomers are collected in Table 4.
Table 4.
The characteristic of l-Trp and 5′-OH-l-Trp isotopomers
| Compound | Specific activity (Bq/mmol) | References |
|---|---|---|
| of [2-3H]-l-Trp (29b) | 4.5 × 106 | [97] |
| [2-2H/3H]-l-Trp (29c) | 4.4 × 106 | [97] |
| [4′-3H]-l-Trp (29e) | 8.9 × 107 | [99] |
| [4′-2H/3H-]-l-Trp (29f) | 4.25 × 107 | [99] |
| [2′,4′,5′,6′,7′-3H5]-l-Trp (29h) | 3 × 108 | [99] |
| [2′,4′,5′,6′,7′-(2H/3H)5]-l-Trp (29i) | 1.4 × 108 | [99] |
| [1-14C]-l-Trp (29j) | 1.03 × 106 | [107] |
| 5′-OH-[2-3H]-l-Trp (32b) | 4.38 × 106 | [97] |
| 5′-OH-[2-2H/3H]-l-Trp (32c) | 4.2 × 106 | [97] |
| 5′-OH-[1-14C]-l-Trp (32d) | 1.02 × 106 | [107] |
Synthesis of dopamine labeled with hydrogen isotopes
The biogenic amine, dopamine, DA, (25) plays an important role in many physiological functions as a neurotransmitter in the nervous system of mammals [112, 113]. DA (25) is also involved as a precursor in the synthetic enzymatic route of the other catecholamines as noradrenaline (34) and adrenaline (35) [114, 115].
The mechanism of β-hydroxylation of DA, leading to formation of noradrenaline, catalyzed by the enzyme dopamine β-hydroxylase (EC 1.14.17.1) (Fig. 27) are not completely clear up to now.
Fig. 27.
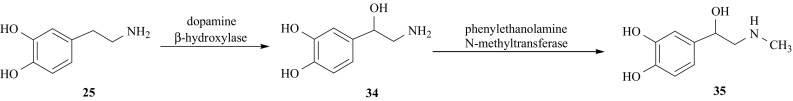
Enzymatic route of noradrenaline and adrenaline
The literature data on the synthesis of labeled DA is very old and scarce. Dideutero [2-2H2]-DA was obtained by reduction of 3,4-dimethoxyphenylacetonitrile with LiAlD4 as [1-2H2]-DA was prepared from homoveratric acid by incorporation of deuterium into the side chain with exchange procedure [116]. The different isotopomers of DA tritiated in the 2- and 3-positions were obtained from (dihydroksyphenyl)ethyl alcohols as the result of three step chemical procedures [117]. Also, the very old data reports on chemo-enzymatic preparation of DA labeled with deuterium and tritium in the side chain [118, 119]. Deuterated [(1S)-2H]-DA and [(1R)-2H]-DA were obtained by enzymatic decarboxylation of [2-2H]-l-DOPA and l-DOPA, respectively [120].
Therefore, to study processes in Fig. 27 using KIE and SIE methods, a new simpler synthesis of deuterium or tritium labeled isotopomers of DA was elaborated.
Isotopomers of (25) specifically labeled in the side chain, i.e., [(1R)-2H]- (25a) and [(1R)-3H]-DA (25b) were obtained by enzymatic decarboxylation of l-DOPA (17) catalyzed by the enzyme tyrosine decarboxylase (EC 4.1.1.25) from Steptococcus faecalis [121], and carried out in fully deuterated or tritiated medium respectively [122]. [(1R)-2H/3H]-DA (25c) was synthesized in fully deuterated incubation medium with DTO added [77] (Fig. 28).
Fig. 28.
Enzymatic synthesis of dopamine isotopomers labeled with hydrogen isotopes in (1R) position
Previous studies have shown that enzymatic decarboxylation of l-amino acids occurs with retention of configuration at the α-carbon [123, 124]. This fact has been used to obtain two (1S)-isotopomers of (25) labeled with deuterium or tritium by enzymatic decarboxylation of specifically labeled isotopomers of l-DOPA (17) i.e., [2-2H]- (17c) and [2-3H]- (17d) and [2-2H/3H]-l-DOPA (17e) obtained earlier. According to this rule, the deuterium atom at Cα retains (1S)-configuration in [(1S)-2H]-DA (25d) obtained by enzymatic decarboxylation of (17c). Consequently when (17d) and (17e) are the substrates—[(1S)-3H]- (25e) and [(1S)-2H/3H]-DA (25f) were obtained [77] (Fig. 29). For these reactions enzyme tyrosine decarboxylase (EC 4.1.1.25) was used.
Fig. 29.
Enzymatic synthesis of dopamine isotopomers labeled with hydrogen isotopes in (1S) position
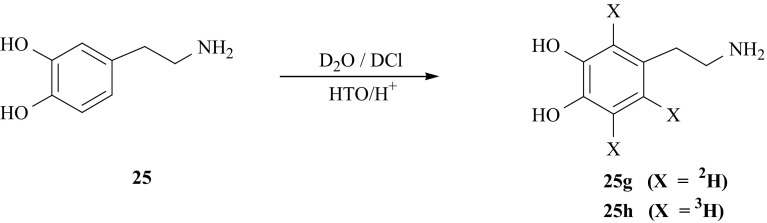
Isotopomers of DA (25) ring labeled with hydrogen isotopes, i.e., [2′,5′,6′-2H3]- (25g) and [2′,5′,6′-3H3]-DA (25 h) were obtained using isotopic exchange method between heavy water and (25). Under the acid catalyzed conditions, at elevated temperature, the exchange between D2O and (25) takes place only into the aromatic ring [122] (Fig. 30). Tritiation of (25) was carried out in the same conditions but instead of D2O, tritiated water was used.
Fig. 30.
Synthesis of dopamine isotopomers labeled with hydrogen isotopes in aromatic ring using isotopic exchange
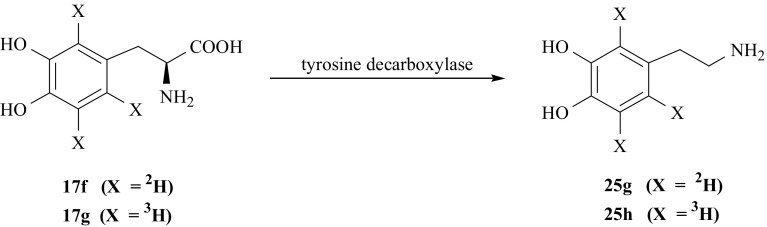
For the synthesis of DA ring labeled with hydrogen isotopes, we have also used previously synthesized isotopomers of l-DOPA (17) [78]. [2′,5′,6′-2H3]- (25g) and [2′,5′,6′-3H3]-DA (25h) were obtained by enzymatic decarboxylation of [2′,5′,6′-2H3]- (17f) and [2′,5′,6′-3H3]-l-DOPA (17g), respectively, in presence of enzyme tyrosine decarboxylase (Fig. 31).
Fig. 31.
Enzymatic synthesis of dopamine isotopomers labeled with hydrogen isotopes in aromatic ring
The characteristic of DA (25) isotopomers are collected in Table 5.
Table 5.
The characteristic of DA isotopomers
| Compound | Specific activity (Bq/mmol) | Chemical yield (%) | References |
|---|---|---|---|
| [(1R)-2H]-DA (25a) | – | 81 | [122] |
| [(1R)-3H]-DA (25b) | 3.05 × 107 | 82 | [122] |
| [(1R)-2H/3H]-DA (25c) | 2.33 × 107 | 63 | [77] |
| [(1S)-2H]-DA (25d) | – | 80 | [77] |
| [(1S)-3H]-DA (25e) | 7.75 × 106 | 78 | [77] |
| [(1S)-2H/3H]-DA (25f) | 3.5 × 106 | 84 | [77] |
| [2′,5′,6′-2H3]-DA (25g) | – | 83.5 | [78] |
| [2′,5′,6′-3H3]-DA (25h) | 1.56 × 108 | 63.5 | [78] |
Synthesis of tyramine labeled with hydrogen isotopes
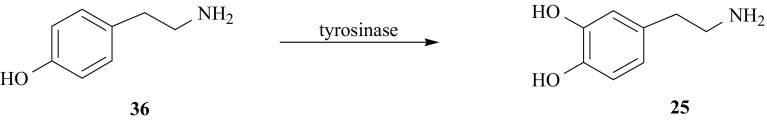
Tyramine, TA (36), a biogenic amine, plays an important role in many metabolic processes. It is one of the trace amines in the central nervous system in humans [125, 126]. TA may also be a substrate for enzymatic hydroxylation to another important neurotransmitter such as DA (25), catalyzed by enzyme tyrosinase (EC 1.14.18.1), Fig. 32.
Fig. 32.
Synthesis of dopamine catalyzed by tyrosinase
Some isotopomers of TA labeled with deuterium, tritium and 14C have been obtained during the study on the stereochemistry of enzymatic elimination of ammonia [127] and decarboxylation of l-Tyr [51]. Unfortunately, these chemical multistep syntheses are very labor intensive. For our purposes, to better understand the reaction of hydroxylation, specifically labeled isotopomers of (36), needed for KIE and SIE studies, were synthesized.
TA (36), specifically labeled with hydrogen isotopes in (1S) position, was obtained by enzymatic decarboxylation of labeled l-Tyr, catalyzed by tyrosine decarboxylase [59]. In the course of decarboxylation of l-Tyr (14) labeled in the 2-position of side chain, a solvent proton replaces the carboxyl group with retention of configuration [123, 124]. Therefore, the products obtained by enzymatic decarboxylation of isotopomers of l-Tyr {(14a), (14b), and (14c)} retain the label (deuterium or tritium) at configuration S, yielding [(1S)-2H]- (36a), [(1S)-3H]- (36b), and [(1S)-2H/3H]-TA (36c) (Fig. 33).
Fig. 33.
Enzymatic synthesis of tyramine isotopomers labeled with hydrogen isotopes in (1S) position
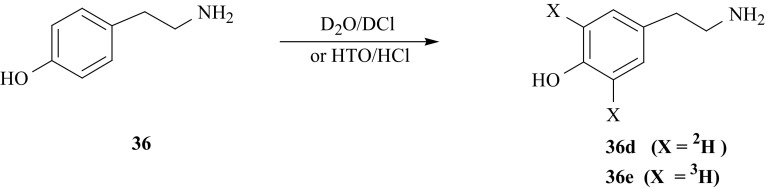
TA ring labeled with hydrogen isotopes, i.e., [3′,5′-2H2]-TA (36d) was synthesized via two different routes. In the first, enzymatic decarboxylation of labeled l-Tyr (14g), catalyzed by tyrosine decarboxylase (EC 4.1.1.25), gives desired product (36d) [59] (Fig. 34).
Fig. 34.
Enzymatic synthesis of tyramine isotopomer labeled with deuterium in 3′ and 5′ positions
In the second direct synthetic route, deuterated [3′,5′-2H2]-TA (36d), as well as, tritiated [3′,5′-3H2]-TA (36e), were obtained in the course of acid catalyzed isotopic exchange carried out between deuterated or tritiated water and unlabeled tyramine (Fig. 35). In this case, the deuterium or tritium is incorporated exclusively into 3′ and 5′ ring position of TA (36).
Fig. 35.
Synthesis of tyramine isotopomers labeled with hydrogen isotopes in aromatic ring using isotopic exchange
The characteristic of TA (36) isotopomers are collected in Table 6.
Table 6.
The characteristic of TA isotopomers
Synthesis of histamine labeled with hydrogen isotopes
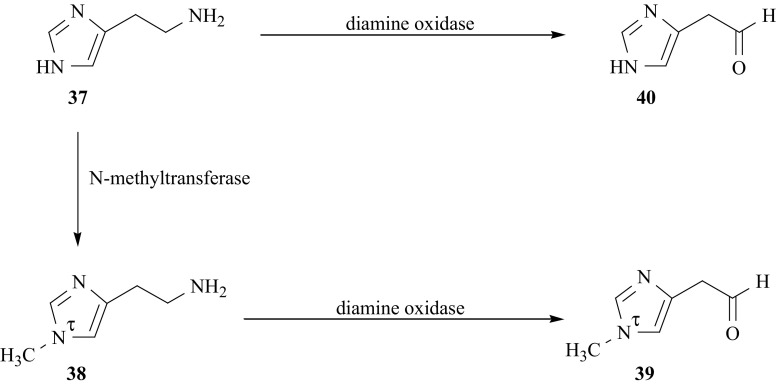
The biogenic amine histamine, HA (37) plays an important role in various physiological function as a key mediator of cell growth, gastric secretion, acute allergic inflammation, and neurotransmitter for blood pressure [128–130]. In humans and experimental animals HA is mainly metabolized on the two pathways, Fig. 36 [131–133]. In humans about three quarters of HA is methylated to Nτ-methylhistamine, τMeHA (38) by enzyme N-methyltransferase (EC 2.1.1.8), and subsequently this intermediate is oxidized to Nτ-methylimidasole acetalaldehyde (39) by enzyme diamine oxidase (DAO, EC 1.4.3.6). The remaining quarter of HA, however, is indirectly biotransferred into imidasole acetalaldehyde (40) by DAO. (According to the recommendation of IUPAC [134], the nitrogen atoms of the imidasole ring are denoted by π and τ, carbon atoms in the side chain as α, and β and ring carbon atoms as 2, 4, 5).
Fig. 36.
The fragment of metabolic pathway of histamine
Despite of many studies the mechanism of the removal of excess of HA (37) from human body is not completely understood. Therefore, we planned experiments to investigate some details of methylation and oxidation reactions presented in Fig. 36, by applying the KIE and SIE methods. For this kind of study the isotopomers of HA and N-methyl-HAs specifically labeled with deuterium and tritium are needed. In the literature there is description of the synthesis of τMeHA and πMeHA tritiated selectively in the methyl group [135]. The product obtained consists of two (τ and π) isomers, which separation was unsuccessful. Also, the preparation of tritiated (Nτ-C[3H3])-HA from [3H]CH3I by chemical method is described [136], as well as the synthesis of deuterated (Nτ-C[2H3])-HA [137].
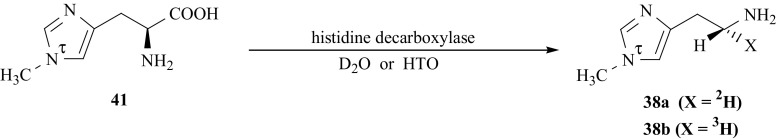
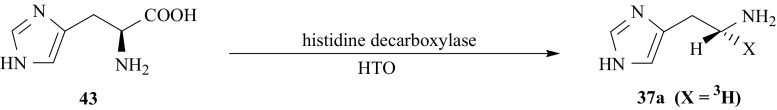
For KIE assays isotopomers [(αR)-2H]- (38a) and [(αR)-3H]-τMeHA (38b) specifically labeled with deuterium and tritium were obtained by enzymatic decarboxylation of Nτ-methyl-l-histidine (41), catalyzed by the enzyme histidine decarboxylase (HDC, EC 4.1.1.22) from Lactobacillus 30a, in the presence of cofactor PLP. This enzyme introduces deuterium or tritium from incubation medium (D2O or HTO) directly into the (αR) position of corresponding amine (Fig. 37).
Fig. 37.
Enzymatic synthesis of τMeHA labeled with hydrogen isotopes in (αR) position
Isotopomers of Nπ-methylhistamine, πMeHA (42) specifically labeled with hydrogen isotopes in the (αR) position i.e., [(αR)-2H]- (42a) and [(αR)-3H]-πMeHA (42b) were obtained in the same manner as in Fig. 37 by enzymatic decarboxylation of Nπ-methyl-l-histidine [138].
Enzymatic decarboxylation of native l-histidine (43) carried out in incubation medium containing HTO leads to formation of [(αR)-3H]-HA (37a) [139] (Fig. 38). In the literature, there are also reports on the synthesis of [2-2H]-, and [2-3H]-HA and doubly labeled with tritium and 14C [2-2H, 2-14C]-HA obtained by decarboxylation of labeled l-His catalyzed by the enzyme HDC [46, 140].
Fig. 38.
Synthesis of [(αR)-3H]-HA
The characteristic of HA (37) isotopomers are collected in Table 7.
Table 7.
The characteristic of HA isotopomers
Synthesis of phenylpyruvic acid labeled with hydrogen and carbon isotopes
Phenylpyruvic acid, PPA (3) is a product of oxidative deamination reaction of l-Phe (1) presented in Fig. 2. In the course of this reaction some tautomerization of PPA takes place, and in the process the stereospecific abstraction of proton from 3-position of PPA is involved [141]. The numerical values of isotope effects allowed us to elucidate the intrinsic details of this mechanism. This kind of studies require the use of isotopomers of PPA labeled with deuterium and tritium in 3 position, and also the 14C-labeled isotopomer of PPA used as internal radiometric standard in the course of KIE assays. In the literature there are a few papers that describe the synthesis of deuterium-, [141] tritium-, [142] and 14C-labeled [143] isotopomers of PPA. Most of them yielding isotopomers bearing the label in position not useful for study of mechanism of reaction presented in Fig. 2 using KIE and SIE methods.
Desired isotopomers of (3) labeled with isotopes of hydrogen were synthesized according to the reaction route shown in Fig. 39. Three isotopomers of l-Phe i.e., [(3S)-2H]- (1b), [(3S)-3H]-l-Phe (1a) [15] and [(3S)-2H/3H]-l-Phe (1c) [144] were converted into corresponding isotopomers of PPA, i.e., [(3S)-2H]- (3a), [(3S)-3H]- (3b), and [(3S)-2H/3H]-PPA (3c) by oxidative deamination, catalyzed by enzyme l-phenylalanine dehydrogenase (PheDH, EC 1.4.1.20) [144].
Fig. 39.
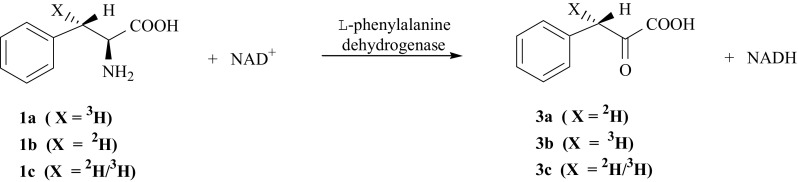
Synthesis of phenylpyruvic acid isotopomers labeled with hydrogen isotopes in (3S) position
Isotopomer [1-14C]-PPA (3d) was synthesized as above using [1-14C]-l-Phe (1 h) as a substrate [144].
The characteristic of PPA (3) isotopomers are collected in Table 8.
Table 8.
The characteristic of PPA isotopomers
Synthesis of halogen derivatives of l-Phe, l-Tyr and l-Trp labeled with hydrogen isotopes
Halogenated derivatives of l-Phe (1), l-Tyr (14) and l-Trp (29), labeled with short-lived radioisotopes i.e., 18F, 123I, 125I or 76Br are recently applied in nuclear medicine for diagnosis of many types of tumours and neurodegenerative diseases using positron emission tomography (PET) or single-photon emission computed tomography (SPECT). 2′-[18F]fluoro-l-Tyr is used for glioma imaging [145]. 2′-[76Br]bromo-α-methyl-l-Phe is a potential PET tumor tracer [146]. 3′-[125I]iodo-α-methyl-l-Tyr as well as 4′-[123I]iodo-l-Phe are validated for visualization by SPECT [147, 148] and 5′-[18F]fluoro-α-methyl-l-Trp holds great potential for cancer imaging using PET [149]. From a medical perspective, it is crucial to elucidate the influence of halogen substituents on kinetics of metabolic pathways of l-Phe (1), l-Tyr (14) and l-Trp (29) using KIE and SIE studies.
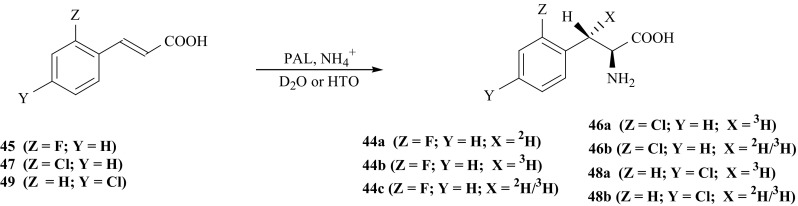
For KIE studies isotopomers of 2′-fluoro-l-Phe (44) i.e., 2′-fluoro-[(3S)-2H]- (44a), 2′-fluoro-[(3S)-3H]- (44b) and 2′-fluoro-[(3S)-2H/3H]-l-Phe (44c) were synthesized according to Fig. 40. Addition of ammonia to 2′-fluoro-(E)-cinnamic acid (45), catalyzed by PAL, was carried out in deuterated or tritiated buffer solutions [150, 151].
Fig. 40.
Enzymatic synthesis of halogen derivatives of l-Phe labeled with hydrogen izotopes
The same procedure was applied for synthesis of 2′-chloro-l-Phe (46) isotopomers, labeled with tritium and doubly labeled with deuterium and tritium in the (3S) position of the side chain i.e., 2′-chloro-[(3S)-3H]- (46a) and 2′-chloro-[(3S)-2H/3H]-l-Phe (46b). In case of this synthesis 2′-chloro-(E)-cinnamic acid (47) was used as a substrate. The isotopomers of 4′-chloro-l-Phe (48) were synthesized the same way using 4′-chloro-(E)-cinnamic acid (49) as substrate, yielding 4′-chloro-[(3S)-3H]- (48a) and 4′-chloro-[(3S)-2H/3H]-l-Phe (48b) [151] Fig. 40.
The characteristic of halogenated derivatives of l-Phe (44, 46, 48) are collected in Table 9.
Table 9.
The characteristic of halogenated l-Phe isotopomers
| Compound | Specific activity (Bq/mmol) | Chemical yield (%) | References |
|---|---|---|---|
| 2′-fluoro-[(3S)-2H]-l-Phe (44a) | – | 54 | [150] |
| 2′-fluoro-[(3S)-3H]-l-Phe (44b) | 5.7 × 107 | 40 | [151] |
| 2′-fluoro-[(3S)-2H/3H]-l-Phe (44c) | 4.5 × 106 | 39 | [151] |
| 2′-chloro-[(3S)-3H]-l-Phe (46a) | 5.5 × 107 | 45 | [151] |
| 2′-chloro-[(3S)-2H/3H]-l-Phe (46b) | 1.4 × 107 | 40 | [151] |
| 4′-chloro-[(3S)-3H]-l-Phe (48a) | 7.1 × 107 | 48 | [151] |
| 4′-chloro-[(3S)-2H/3H]-l-Phe (48b) | 1.86 × 107 | 38 | [151] |
Desired isotopomers of 3′-fluoro- (50), 3′-chloro- (51) and 3′-iodo-l-Tyr (52) i.e., 3′-fluoro-[2-2H]- (50a), 3′-fluoro-[2-3H]- (50b), 3′-fluoro-[2-2H/3H]- (50c), 3′-chloro-[2-2H]- (51a), 3′-chloro-[2-3H]- (51b), 3′-chloro-[2-2H/3H]- (51c), 3′-iodo-[2-2H]- (52a), 3′-iodo-[2-3H]- (52b), 3′-iodo-[2-2H/3H]-l-Tyr (52c),were synthesized by isotopic exchange between (50), (51) or (52) and deuterated or tritiated water [150–152], catalyzed by enzyme TPase according to Fig. 41. This enzyme causes labilization of hydrogen in the second position of the side chain of various aromatic amino acids [53] and facilitates isotopic exchange.
Fig. 41.

Enzymatic synthesis of halogen derivatives of l-Tyr labeled with hydrogen izotopes in the side chain
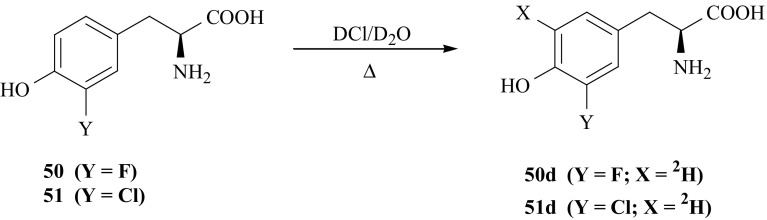
Ring labelled isotopomers of (50) and (51) i.e., 3′-fluoro-[5′-2H]- (50d) and 3′-chloro-[5′-2H]-l-Tyr (51d) were synthesized by acid catalyzed isotopic exchange between (50) and (51) and deuterium from incubation medium at high temperature [153], Fig. 42.
Fig. 42.
Synthesis of halogen derivatives of l-Tyr ring labeled with deuterium
The structure of the compounds was checked by 1H NMR spectroscopy. Obtained data revealed that deuterium incorporation takes place only at 5′ position of (50) and (51) aromatic ring and reached close to 100% deuterium enrichment.
The characteristic of halogenated derivatives of l-Tyr (50, 51, 52) are collected in Table 10.
Table 10.
The characteristic of halogenated l-Tyr isotopomers
| Compound | Specific activity (Bq/mmol) | Chemical yield (%) | References |
|---|---|---|---|
| 3′-fluoro-[2-2H]-l-Tyr (50a) | – | 98 | [150] |
| 3′-fluoro-[2-3H]-l-Tyr (50b) | 6.8 × 106 | 88 | [151] |
| 3′-fluoro-[2-2H/3H]-l-Tyr (50c) | 5.7 × 106 | 84 | [151] |
| 3′-fluoro-[5′-2H]-l-Tyr (50d) | – | 58 | [153] |
| 3′-chloro-[2-2H]-l-Tyr (51a) | – | 97 | [150] |
| 3′-chloro-[2-3H]-l-Tyr (51b) | 6.1 × 106 | 92 | [151] |
| 3′-chloro-[2-2H/3H]-l-Tyr (51c) | 5.2 × 106 | 86 | [151] |
| 3′-chloro-[5′-2H]-l-Tyr (51d) | – | 70 | [153] |
| 3′-iodo-[2-2H]-l-Tyr (52a) | – | 68 | [152] |
| 3′-iodo-[2-3H]-l-Tyr (52b) | 5.73 × 106 | 75 | [152] |
| 3′-iodo-[2-2H/3H]-l-Tyr (52c) | 1.77 × 106 | 97 | [152] |
We have also developed the method for synthesis of halogenated derivatives of l-Trp (29), selectively labeled with hydrogen isotopes at the α-position of the side chain i.e., 5′-bromo-[2-2H]- (53a), 5′-bromo-[2-3H]- (53b), 5′-bromo-[2-2H/3H]- (53c), 5′-fluoro-[2-2H]- (54a), 5′-fluoro-[2-3H]- (54b), 5′-fluoro-[2-2H/3H]- (54c), 6′-fluoro-[2-2H]- (55a), 6′-fluoro-[2-3H]- (55b) and 6′-fluoro-[2-2H/3H]-l-Trp (55c). Coupling of the S-methyl-l-cysteine (19) with 5-bromo- (56), 5-fluoro- (57) or 6-fluoroindole (58) was catalyzed by TPase [150, 151] and carried out in deuterated or tritiated incubation medium, according to Fig. 43. In all cases 2-mercaptoethanol was used to prevent the growth of bacteria and fungi during incubation.
Fig. 43.
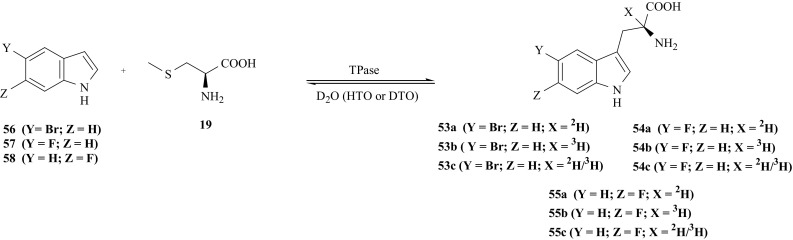
Enzymatic synthesis of halogen derivatives of l-Trp labeled with hydrogen izotopes
The characteristic of halogenated derivatives of l-Trp (53, 54, 55) are collected in Table 11.
Table 11.
The characteristic of halogenated l-Trp isotopomers
| Compound | Specific activity (Bq/mmol) | Chemical yield (%) | References |
|---|---|---|---|
| 5′-bromo-[2-2H]-l-Trp (53a) | – | 27 | [150] |
| 5′-bromo-[2-3H]-l-Trp (53b) | 3.5 × 108 | 35 | [151] |
| 5′-bromo-[2-2H/3H]-l-Trp (53c) | 6.1 × 107 | 22 | [151] |
| 5′-fluoro-[2-2H]-l-Trp (54a) | – | 23 | [150] |
| 5′-fluoro-[2-3H]-l-Trp (54b) | 3 × 108 | 38 | [151] |
| 5′-fluoro-[2-2H/3H]-l-Trp (54c) | 3 × 107 | 32 | [151] |
| 6′-fluoro-[2-2H]-l-Trp (55a) | – | 23 | [150] |
| 6′-fluoro-[2-3H]-l-Trp (55b) | 3.2 × 108 | 34 | [151] |
| 6′-fluoro-[2-2H/3H]-l-Trp (55c) | 2.1 × 107 | 29 | [151] |
Conclusions
Taking into account the advantages of enzymatic synthetic methods, it is foreseeable that this type of reactions will gain a stronger presence in preparation of biologically active labeled compounds. While introduction of isotopic carbon atom to the backbone of a molecule may create some synthesis challenges, in majority of cases however, enzymatic syntheses are still easier to carry out than classic multistep organic syntheses. Furthermore this issue is greatly minimized when dealing with the substitution of the stable atom for radioactive one bonded to backbone of molecule (either isotopes of hydrogen and halogens) or addition of functional group bearing isotopic (for instance 11C- 13C- or 14C-) label.
Abbreviations
- Ac
Acetyl group (CH3CO–)
- D
Deuterium, 2H
- DA
Dopamine
- d-AAO
d-amino acid oxidase
- DAO
Diamine oxidase
- EC
Enzyme Commission
- FAD
Flavine adenine dinucleotyde
- GPT
Glutamic-pyruvate transaminase
- HA
Histamine
- HDC
Histidine decarboxylase
- KIE
Kinetic isotope effect
- l-DOPA
3′,4′-Dihydroxy-l-phenylalanine
- l-His
l-Histidine
- l-Phe
l-Phenylalanine
- l-Trp
l-Tryptophan
- 5′-OH-l-Trp
5′-Hydroxy-l-tryptophan
- l-Tyr
l-Tyrosine
- NAD+, NADH
Nicotinamide adenine dinucleotide (oxidized or reduced form)
- PAL
Phenylalanine ammonia lyase
- PET
Positron emission tomography
- Ph
Phenyl
- PheDH
Phenylalanine dehydrogenase
- PKU
Phenylketonuria
- PLP
Pyridoxal 5′-phosphate
- PPA
Phenylpyruvic acid
- SIE
Solvent isotope effect
- SPECT
Single-photon emission computed tomography
- T
Tritium, 3H
- TA
Tyramine
- TPase
Tryptophanase
- YADH
Yeast alcohol dehydrogenase
- πMeHA
Nπ-Methylhistamine
- τMeHA
Nτ-Methylhistamine
References
- 1.Murray A, III, Williams DL. Organic synthesis with isotopes, part I and part II. New York: Interscience Publishers; 1958. [Google Scholar]
- 2.Evans EA. Tritium and its compounds. London: Butterworth; 1974. [Google Scholar]
- 3.Buncel E, Jones JR. Isotopes in the physical and biomedical sciences. Amsterdam: Elsevier; 1987. [Google Scholar]
- 4.Voges R, Heys JR, Moenius T. Preparation of compounds labeled with tritium and carbon-14. Chichester: Wiley; 2009. [Google Scholar]
- 5.Fry A. In: Isotope effects in reaction rates. Collins CJ, Bowman NS, editors. New York: Van Nostrand Reinhold Book Co.; 1970. [Google Scholar]
- 6.Cook PF. Enzyme mechanism from isotope effects. Boca Raton: CRS Press; 1991. [Google Scholar]
- 7.Havir EA, Hanson KR. l-Phenylalanine ammonia lyase. II. Mechanism and kinetic properties of the enzyme from potato tubers. Biochemistry. 1968;7:1904–1914. doi: 10.1021/bi00845a039. [DOI] [PubMed] [Google Scholar]
- 8.Havir EA, Hanson KR. l-Phenylalanine ammonia-lyase (maize and potato and Rhodotorula glutinis). Studies of the prosthetic group with nitromethane. Biochemistry. 1975;14:1620–1626. doi: 10.1021/bi00679a012. [DOI] [PubMed] [Google Scholar]
- 9.Hodgins DS. Yeast phenylalanine ammonia lyase. J Biol Chem. 1971;246:2977–2985. [PubMed] [Google Scholar]
- 10.Schuster B, Retey J. The mechanism of action of phenylalanine ammonia-lyase: the role of prosthetic dehydroalanine. Proc Natl Acad Sci USA. 1995;92:8433–8437. doi: 10.1073/pnas.92.18.8433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Globe A, Langer B, Poppe L, Retey J. The behavior of substrate analogues and secondary deuterium isotope effect in the phenylalanine ammonia-lyase reaction. Arch Biochem Biophys. 1998;359:1–7. doi: 10.1006/abbi.1998.0860. [DOI] [PubMed] [Google Scholar]
- 12.Lewandowicz A, Jemielity J, Kanska M, Zoń J, Paneth P. Tritium secondary kinetic isotope effect on phenylalanine ammonia-lyase catalyzed reaction. Arch Biochem Biophys. 1999;370:216–221. doi: 10.1006/abbi.1999.1402. [DOI] [PubMed] [Google Scholar]
- 13.Brunhuber NM, Banerjee A, Jacobs WR, Jr, Blanchard JS. Cloning, sequencing, and expression of Rhodococcusl-phenylalanine dehydrogenase. Sequence comparisons to amino-acid dehydrogenases. J Biol Chem. 1994;269:16203–16211. [PubMed] [Google Scholar]
- 14.Brunhuber NM, Thoden JB, Blanchard JS, Vanhooke JL. Rhodococcusl-phenylalanine dehydrogenase: kinetics, mechanism, and structural basis for catalytic specificity. Biochemistry. 2000;39:9174–9187. doi: 10.1021/bi000494c. [DOI] [PubMed] [Google Scholar]
- 15.Tachibana Y, Ando M, Kuzuhara H. Asymmetric synthesis of α-deuteriated α-amino acids through nonenzymatic transamination reaction and the determination of their enantiomeric excesses. Bull Chem Soc Jpn. 1983;56:3652–3656. [Google Scholar]
- 16.Tanimura K, Kato T, Waki M, Lee S, Kodera Y, Izumiya N. Facile synthesis of (2R,3R)-phenylalanine-2,3-d2 and NMR study on deuterated Gramicidin S. Bull Chem Soc Jpn. 1984;57:2193–2197. [Google Scholar]
- 17.Wishart DS, Sykes BD, Richards FM. Improved synthetic methods for the selective deuteration of aromatic amino acids: applications of selective protonation towards the identification of protein folding intermediates through nuclear magnetic resonance. Biochim Biophys Acta. 1993;1164:36–46. doi: 10.1016/0167-4838(93)90109-5. [DOI] [PubMed] [Google Scholar]
- 18.Easton CJ, Hutton CA. Synthesis of each stereoisomer of [3-2H1]phenylalanine and evaluation of the stereochemical course of the reaction of (R)-phenylalanine with (S)-phenylalanine ammonia-lyase. J Chem Soc Perkin Trans. 1994;1:3545–3547. [Google Scholar]
- 19.Oba M, Ueno R, Fukuoka M, Kainosho M, Nishiiyama K. Synthesis of l-threo- and l-erythro-[1-13C,2,3,-2H]amino acids: novel probes for conformational analysis of peptide side chains. J Chem Soc Perkin Trans. 1995;1:1603–1609. [Google Scholar]
- 20.Ife R, Haslam E. The shikimate pathway. Part III. The stereochemical course of the l-phenylalanine ammonia lyase reaction. J Chem Soc(C) 1971;1:2818–2821. doi: 10.1039/j39710002818. [DOI] [PubMed] [Google Scholar]
- 21.Kirby GW, Michael J. Labelling of aromatic amino-acids stereoselectively with tritium in the β-methylene group: the stereochemistry of hydroxylation in the biosynthesis of haemanthamine. Chem Commun. 1971;8:187–188. [Google Scholar]
- 22.Wightman RH, Staunton J, Battersby AR. Studies of enzyme-mediated reactions. Part I. Syntheses of deuterium- or tritium-labelled (3S)- and (3R)-phenylalanines: stereochemical course of the elimination catalysed by l-phenylalanine ammonia-lyase. J Chem Soc Perkin Trans. 1972;1:2355–2364. doi: 10.1039/p19720002355. [DOI] [PubMed] [Google Scholar]
- 23.Bartl K, Cavalar C, Krebs T, Ripp E, Retey J, Hull WE, Günther H, Simon H. Synthesis of stereospecifically deuterated phenylalanines and determination of their configuration. Eur J Biochem. 1977;72:247–250. doi: 10.1111/j.1432-1033.1977.tb11246.x. [DOI] [PubMed] [Google Scholar]
- 24.Hädener A, Tamm Ch. Synthesis of specifically labeled l-phenylalanines using phenylalanine ammonia lyase activity. J Label Compd Radiopharm. 1987;24:1291–1306. [Google Scholar]
- 25.Jemielity J, Kanski R, Kanska M. Synthesis of tritium labeled [3R-3H]-, and [3S-3H]-l-phenylalanine. J Label Compd Radiopharm. 2001;44:295–304. [Google Scholar]
- 26.Kańska M, Boroda E, Augustyniak W, Kański R. In: Synthesis and applications of isotopically labelled compounds. Dean DC, Filer CN, McCarthy KE, editors. Chichester: Wiley; 2004. [Google Scholar]
- 27.Fasth K-J, Antoni G, Långström B. Asymmetric synthesis of l-[3-11C]alanine and l-[3-11C]phenylalanine by a phase-transfer alkylation reaction. J Chem Soc Perkin Trans. 1988;1:3081–3084. [Google Scholar]
- 28.Koltai E, Alexin A, Gy R, Tóth-Sarudy É. Synthesis of optically pure (D)-phenyl[3-14C]alanine. J Label Compd Radiopharm. 1998;41:977–982. [Google Scholar]
- 29.Halldin C, Långström B. Synthesis of [3-11C]phenylpyruvic acid and its use in an enzymatic transamination to [3-11C]phenylalanine. J Label Compd Radiopharm. 1986;23:715–722. [Google Scholar]
- 30.LeMaster DM, Cronan E. Biosynthetic production of 13C-labeled amino acids with site-specific enrichment. J Biol Chem. 1982;257:1224–1230. [PubMed] [Google Scholar]
- 31.Jemielity J, Kańska M, Kański R. Enzymatic synthesis of [1-13C]- and [1-14C]-l-phenylalanine. Isotopes Environ Health Stud. 1998;34:335–339. [Google Scholar]
- 32.Augustyniak W, Bukowski J, Jemielity J, Kański R, Kańska M. Enzymatic synthesis of carbon-14 labeled isotopomers of l-phenylalanine. J Radioanal Nucl Chem. 2001;247:371–374. [Google Scholar]
- 33.Bukowski J, Szul J, Kański R, Kańska M. Enzymatic synthesis of [3-14C]-cinnamic acid. J Radioanal Nucl Chem. 2000;243:635–638. [Google Scholar]
- 34.Kumagai H, Yamada H, Matsui H, Ohkishi H, Ogata K. Tyrosine phenol lyase. I. Purification, crystallization, and properties. J Biol Chem. 1970;245:1767–1772. [PubMed] [Google Scholar]
- 35.Yamada H, Kumagai H. Synthesis of l-tyrosine-related amino acids by β-tyrosinase. Adv Appl Microbiol. 1975;19:249–288. doi: 10.1016/s0065-2164(08)70431-3. [DOI] [PubMed] [Google Scholar]
- 36.Kumagai H, Utagawa T, Yamada H. Studies on tyrosine phenol lyase. Modification of essential histidyl residues by diethylpyrocarbonate. J Biol Chem. 1975;250:1660–1667. [PubMed] [Google Scholar]
- 37.Nagasawa T, Utagawa T, Goto J, Kim CJ, Tani Y, Kumagai H, Yamada H. Syntheses of l-tyrosine-related amino acids by tyrosine phenol-lyase of Citrobacter intermedius. Eur J Biochem. 1981;117:33–40. doi: 10.1111/j.1432-1033.1981.tb06299.x. [DOI] [PubMed] [Google Scholar]
- 38.Palcic MM, Shen S-J, Schleicher E, Kumagai H, Sawada S, Yamada H, Floss HG. Stereochemistry and mechanism of reactions catalyzed by tyrosine phenol-lyase from Escherichia intermedia. Z Naturforsch C. 1987;42:307–318. doi: 10.1515/znc-1987-0401. [DOI] [PubMed] [Google Scholar]
- 39.Nagatsu T, Levitt M, Udenfriend S. Conversion of l-tyrosine to 3,4-dihydroxyphenylalanine by cell-free preparations of brain and sympathetically innervated tissues. Biochem Biophys Res Commun. 1964;14:543–549. doi: 10.1016/0006-291x(64)90266-9. [DOI] [PubMed] [Google Scholar]
- 40.Pomerantz SH. Tyrosine hydroxylation catalyzed by mammalian tyrosinase: an improved method of assay. Biochem Biophys Res Commun. 1964;16:188–194. doi: 10.1016/0006-291x(64)90359-6. [DOI] [PubMed] [Google Scholar]
- 41.Pomerantz SH. The tyrosine hydroxylase. Activity of mammalian tyrosinase. J Biol Chem. 1966;241:161–168. [PubMed] [Google Scholar]
- 42.Kiick DM, Phillips RS. Mechanistic deductions from kinetic isotope effects and pH studies of pyridoxal phosphate dependent carbon-carbon lyases: Erwinia herbicola and Citrobacter freundii tyrosine phenol-lyase. Biochemistry. 1988;27:7333–7338. doi: 10.1021/bi00419a023. [DOI] [PubMed] [Google Scholar]
- 43.Axelsson BS, Bjurling P, Matsson O, Langstrom B. 11C/14C kinetic isotope effects in enzyme mechanism studies. 11C/14C kinetic isotope effects of the tyrosine phenol-lyase catalyzed α,β-elimination of l-tyrosine. J Am Chem Soc. 1992;114:1502–1503. [Google Scholar]
- 44.Augustyniak W, Kański R, Kańska M. In: Synthesis and applications of isotopically labelled compounds. Dean DC, Filer CN, McCarthy KE, editors. Chichester: Wiley; 2004. [Google Scholar]
- 45.Augustyniak W, Kański R, Kańska M. Carbon isotope effects in the studies of the mechanism of action of tyrosine phenol-lyase. Nukleonika. 2006;51(Suppl. 2):S7–S11. [Google Scholar]
- 46.Griffiths DV, Feeney J, Roberts GCK, Burgen ASV. Preparation of selectively deuterated aromatic amino acids for use in 1H NMR studies of proteins. Biochim Biophys Acta. 1976;446:479–485. doi: 10.1016/0005-2795(76)90014-3. [DOI] [PubMed] [Google Scholar]
- 47.Matthews HR, Matthews KS, Opella SJ. Selectively deuterated amino acid and analogues synthesis, incorporation into proteins and NMR properties. Biochim Biophys Acta. 1977;497:1–13. doi: 10.1016/0304-4165(77)90134-9. [DOI] [PubMed] [Google Scholar]
- 48.Kendall JT. Synthesis of [2,3,3,2′3′,5′6′-2H7]-l-tyrosine from phenol-d6. J Label Compd Radiopharm. 2000;43:917–924. [Google Scholar]
- 49.Walker TE, Matheny C, Storm CB, Hayden H. An efficient chemomicrobiological synthesis of stable isotope-labeled l-tyrosine and l-phenylalanine. J Org Chem. 1986;51:1175–1179. [Google Scholar]
- 50.Asano Y, Lee JJ, Shieh TL, Spreafico F, Kowal C, Floss HG. Steric course of the reactions catalyzed by 5-enolpyruvylshikimate-3-phosphate synthase, chorismate mutase, and anthranilate synthase. J Am Chem Soc. 1985;107:4314–4320. [Google Scholar]
- 51.Battersby AR, Chrystal EJ, Staunton J. Studies of enzyme-mediated reactions. Part 12. Stereochemical course of the decarboxylation of (2S)-tyrosine to tyramine by microbial, mammalian, and plant systems. J Chem Soc Perkin Trans. 1980;1:31–42. doi: 10.1039/p19800000031. [DOI] [PubMed] [Google Scholar]
- 52.Augustyniak W, Suchecki P, Kański R, Kańska M. In: Synthesis and applications of isotopically labelled compounds. Dean DC, Filer CN, McCarthy KE, editors. Chichester: Wiley; 2004. [Google Scholar]
- 53.Kawata Y, Tani S, Sato M, Katsube Y, Tokushige M. Preliminary X-ray crystallographic analysis of tryptophanase from Escherichia coli. FEBS Lett. 1991;284:270–272. doi: 10.1016/0014-5793(91)80701-4. [DOI] [PubMed] [Google Scholar]
- 54.Murray A, III, Williams DL. Organic synthesis with isotopes, part II. New York: Interscience; 1958. [Google Scholar]
- 55.Augustyniak W, Suchecki P, Jemielity J, Kański R, Kańska M. Synthesis of tritium labeled isotopomers of l-tyrosine. J Label Compd Radiopharm. 2002;45:559–567. [Google Scholar]
- 56.Werstiuk NH, Kadai T. The high temperature and dilute acid (HTDA) procedure as a general method of replacing aromatic hydrogen by deuterium. II. Can J Chem. 1974;52:2169–2171. [Google Scholar]
- 57.Werstiuk NH. In: Isotopes in the Physical and Biomedical Sciences. Buncel E, Jones JR, editors. Amsterdam: Elsevier; 1987. [Google Scholar]
- 58.Kozłowska M, Kański R, Kańska M. Enzymatic synthesis of tritium-labelled isotopomers of l-DOPA. J Label Compd Radiopharm. 2005;48:235–240. [Google Scholar]
- 59.Panufnik E, Kańska M. Enzymatic synthesis of isotopomers of tyramine labeled with deuterium and tritium. J Label Compd Radiopharm. 2007;50:85–89. [Google Scholar]
- 60.Augustyniak W, Kański R, Kańska M. Synthesis of tritium labeled [2′,6′]-l-tyrosine. J Label Compd Radiopharm. 2004;47:977–981. [Google Scholar]
- 61.Garnett JL. π-Complex intermediates in homogeneous and heterogeneous catalytic exchange reactions of hydrocarbons and derivatives with metals. Catal Rev. 1971;5:229–268. [Google Scholar]
- 62.Fasth KJ, Långström B. Asymmetric synthesis of l-[β-11C]amino acids using a chiral nickel complex of the schiff base of (S)-o-[(N-benzylprolyl)-amino]benzophenone and glycine. Acta Chem Scand. 1990;44:720–725. [Google Scholar]
- 63.Bjurling P, Antoni G, Watanabe Y, Långström B. Enzymatic synthesis of carboxy-11C-labelled l-tyrosine, l-DOPA, l-tryptophan, and 5-hydroxy-l-tryptophan. Acta Chem Scand. 1990;44:178–182. [Google Scholar]
- 64.Bjurling P, Watanabe Y, Oka SW, Nagasawa T, Yamada H, Långström B. Multi-enzymatic synthesis of β-11C-labelled l-tyrosine and l-DOPA. Acta Chem Scand. 1990;44:183–188. [Google Scholar]
- 65.Augustyniak W, Kański R, Kańska M. Synthesis of carbon-14 labeled [1-14C]- and [2-14C]-l-tyrosine. J Label Compd Radiopharm. 2001;44:553–560. [Google Scholar]
- 66.Kański R, Augustyniak W, Kańska M. Synthesis of ring labeled [1′-14C]-l-tyrosine. Nukleonika. 2006;51(Suppl. 2):S13–S16. [Google Scholar]
- 67.Murray A, III, Williams DL. Organic synthesis with isotopes, part I. New York: Interscience; 1958. pp. 438–439. [Google Scholar]
- 68.Aronoff S. Techniques of radiochemistry. Ames: The Iowa State College Press; 1957. [Google Scholar]
- 69.Hornykiewicz O. l-DOPA: from a biologically inactive amino acid to a successful therapeutic agent. Amino Acids. 2002;23:65–70. doi: 10.1007/s00726-001-0111-9. [DOI] [PubMed] [Google Scholar]
- 70.Lovenberg W, Weissbach H, Udenfriend S. Aromatic l-amino acid decarboxylase. J Biol Chem. 1962;237:89–93. [PubMed] [Google Scholar]
- 71.Sourkes TL. Aromatic l-amino acid decarboxylase. Methods Enzymol. 1987;147:170–178. doi: 10.1016/s0076-6879(87)42026-0. [DOI] [PubMed] [Google Scholar]
- 72.Kirby GW, Ogunkoya L (1965) Deuterium and tritium exchange of phenols and the synthesis of labelled 3,4-dihydroxyphenylalanines. J Chem Soc 6914–6922
- 73.Binns F, King JAG, Percival A, Robson NC, Swan GA (1970) Studies related to the chemistry of melanins. Part IX. Synthesis of specifically deuteriated 3,4-dihydroxyphenylethylamines and (±)-3,4-dihydroxyphenylalanines. J Chem Soc (C) 1134–1138 [DOI] [PubMed]
- 74.Seo SY, Sharma VK, Sharma N. Mushroom tyrosinase: recent prospects. J Agric Food Chem. 2003;51:2837–2853. doi: 10.1021/jf020826f. [DOI] [PubMed] [Google Scholar]
- 75.Marumo K, Waite JH. Optymalization of hydroxylation of tyrosine and tyrosine-containing peptides by mushroom tyrosinase. Biochim Biophys Acta. 1986;872:98–103. doi: 10.1016/0167-4838(86)90152-4. [DOI] [PubMed] [Google Scholar]
- 76.Ros JR, Rodríguez-López JN, García-Cánovas F. Effect of l-ascorbic acid on the monophenolase activity of tyrosinase. Biochem J. 1993;295:309–312. doi: 10.1042/bj2950309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pająk M, Kańska M. Synthesis of isotopomers of l-DOPA and dopamine labeled with hydrogen isotopes in the side chain. J Radioanal Nucl Chem. 2009;281:365–370. [Google Scholar]
- 78.Pająk M, Kańska M. Enzymatic synthesis of dopamine ring labeled with hydrogen isotopes. J Radioanal Nucl Chem. 2009;279:455–458. doi: 10.1007/s10967-013-2816-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kozłowska M, Kańska M. Synthesis of deuterium, tritium and 14-carbon labelled isotopomers of l-DOPA. Polish J Chem. 2007;81:1763–1769. [Google Scholar]
- 80.Bolster JM, Vaalburg W, Van Veen W, Van Dijk T, Van der Molen HD, Wynberg H, Woldring MG. Synthesis of no-carrier-added l- and d-[1-11C]-DOPA. Int J Appl Radiat Isot. 1983;34:1650–1652. doi: 10.1016/0020-708x(83)90019-4. [DOI] [PubMed] [Google Scholar]
- 81.Adam MJ, Grierson JR, Ruth TJ, Pedersen K, Pate BD. Routineynthesis of carbon-11-carboxyl-labeled l-Dopa. J Nucl Med. 1987;28:1599–1603. [PubMed] [Google Scholar]
- 82.Kuznetsova OF, Mosevich IK, Korsakov MV, Fedorova OS, Krasikova RN. Asymmetric synthesis of l-[β-11C]-3,4-dihydroxyphenylalanine via the chiral Ni(II) complex of the Schiff base derived from (S)-o-N-(N′-benzylprolyl)aminobenzophenone and glycine (Ni-BPB-Gly) Radiochemistry. 2002;44:582–587. [Google Scholar]
- 83.Ellis BE, Major G, Zenk MH. Preparation of l-tyrosine-ring-14C, l-DOPA-ring-14C and related metabolites. Anal Biochem. 1973;53:470–477. doi: 10.1016/0003-2697(73)90096-1. [DOI] [PubMed] [Google Scholar]
- 84.Wood WA, Gunsalus JC, Umbreit WW. Function of pyridoxal phosphate: resolution and purification of the tryptophanase enzyme of Escherichia coli. J Biol Chem. 1947;170:313–321. [Google Scholar]
- 85.Watanabe T, Snell EE. Reversibility of the tryptophanase reaction: synthesis of tryptophan from indole, pyruvate, and ammonia. Proc Natl Acad Sci USA. 1972;69:1086–1090. doi: 10.1073/pnas.69.5.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Snell EE. Tryptophanase: structure, catalytic activities, and mechanism of action. Adv Enzymol Relat Areas Mol Biol. 1975;42:287–333. doi: 10.1002/9780470122877.ch6. [DOI] [PubMed] [Google Scholar]
- 87.Kiick DM, Phillips RS. Mechanistic deductions from multiple kinetic and solvent deuterium isotope effects and pH studies of pyridoxal phosphate dependent carbon-carbon lyases: Escherichia coli tryptophan indole-lyase. Biochemistry. 1988;27:7339–7344. doi: 10.1021/bi00419a024. [DOI] [PubMed] [Google Scholar]
- 88.Bak B, Led JJ, Pedersen EJ. Isotopic labelling of tryptophan and tryptophan residues in polypeptides. Acta Chem Scand. 1969;23:3051–3054. doi: 10.3891/acta.chem.scand.23-3051. [DOI] [PubMed] [Google Scholar]
- 89.Ross FC, Botting NP. Synthesis of 2S-[2-2H]-kynurenine and use in kinetic isotope effect studies with kynureninase. Tetrahedron. 1997;46:15761–15770. [Google Scholar]
- 90.Van den Berg EMM, van Liemt WBS, Heemskerk B, Lugtenburg J. Synthesis of indoles and l-tryptophans specifically 2H- or 13C-labelled in the six-membered ring. Recl Trav Chim Pays-Bas. 1989;108:304–313. [Google Scholar]
- 91.Jesus AE, Steyn PS. Biosynthesis of α-cyclopiazonic acid. Steric course of proton removal during the cyclisation of β-cyclopiazonic acid in Penicillium griseofulvum. J Chem Soc Perkin Trans. 1981;1:3292–3294. [Google Scholar]
- 92.Kirby GW, Varley MJ. Synthesis of tryptophan stereoselectively labeled with tritium and deuterium in the β-methylene group: the steric course of hydroxylation in sporidesmin biosynthesis. J Chem Soc Chem Commun. 1974;20:833–834. [Google Scholar]
- 93.Amir-Heidari B, Thirlway J, Micklefield J. Stereochemical course of tryptophan dehydrogenation during biosynthesis of the calcium-dependent lipopeptide antibiotics. Org Lett. 2007;9:1513–1516. doi: 10.1021/ol0701619. [DOI] [PubMed] [Google Scholar]
- 94.Renson J, Daly J, Weissbach H, Witkop B, Udenfriend S. Enzymatic conversion of 5-tritiotryptophan to 4-tritio-5-hydroxytryptophan. Biochem Biophys Res Commun. 1966;25:504–513. [Google Scholar]
- 95.Daly JW, Witkop B. Selective exchange of nuclear protons in hydroxyindoles. J Am Chem Soc. 1967;89:1032–1033. doi: 10.1021/ja00980a054. [DOI] [PubMed] [Google Scholar]
- 96.Amir-Heidari B, Micklefield J. NMR confirmation that tryptophan dehydrogenation occurs with syn stereochemistry during the biosynthesis of CDA in Streptomyces coelicolor. J Org Chem. 2007;72:8950–8953. doi: 10.1021/jo701660v. [DOI] [PubMed] [Google Scholar]
- 97.Boroda E, Rakowska S, Kański R, Kańska M. Enzymatic synthesis of l-tryptophan and 5′-hydroxy-l-tryptophan labeled with deuterium and tritium at the α-carbon position. J Label Compd Radiopharm. 2003;46:691–698. [Google Scholar]
- 98.Sato I, Sugiyama H, Yamamoto A, Maramatsu S, Matsura T. Photochemical hydrogen-deuterium exchange reaction of tryptophan. The role of nonradiative decay of singlet tryptophan. J Am Chem Soc. 1984;106:4286–4287. [Google Scholar]
- 99.Winnicka E, Kańska M. Synthesis of l-tryptophan labeled with hydrogen isotopes in the indole ring. J Radioanal Nucl Chem. 2009;279:675–678. [Google Scholar]
- 100.Roepe P, Gray D, Lugtenburg J, Van den Berg EMM, Herzfeld J, Rothschild KJ. FTIR evidence for tryptophan perturbations during the bacteriorhodopsin photocycle. J Am Chem Soc. 1988;110:7223–7224. [Google Scholar]
- 101.Van den Berg EMM, Baldew AU, de Goede ATJ, Raap J, Lugtenburg J. Synthesis of three isotopomers of l-tryptophan via combination of organic synthesis and biotechnology. Recl Trav Chim Pays-Bas. 1988;107:73–81. [Google Scholar]
- 102.Van den Berg EMM, Jansen FJHM, de Goede ATJ, Baldew AU, Lugtenburg J. Chemo-enzymatic synthesis and characterization of l-tryptophans selectively 13C-enriched or hydroxylated in the six-membered ring using transformed Escherichia coli cells. Recl Trav Chim Pays-Bas. 1990;109:287–297. [Google Scholar]
- 103.Malthouse JPG, Fitzpatrick TB, Milne JJ, Grehn L, Ragnarsson U. Enzymatic synthesis of isotopically labelled serine and tryptophan for application in peptide synthesis. J Pept Sci. 1997;3:361–366. doi: 10.1002/(sici)1099-1387(199709)3:5<361::aid-psc112>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 104.Bjurling P, Watanabe Y, Tokushige M, Oba T, Långström B. Synthesis of β-11C-labelled l-tryptophan and 5-hydroxy-l-tryptophan using a multi-enzymatic reaction route. J Chem Soc Perkin Trans. 1989;1:1331–1334. [Google Scholar]
- 105.Sasaki M, Ikemoto M, Mutoh M, Haradahira T, Tanaka A, Watanabe Y, Suzuki K. Automatic synthesis of l-[β-11C]amino acids using an immobilized enzyme column. Appl Radiat Isot. 2000;52:199–204. doi: 10.1016/s0969-8043(99)00118-9. [DOI] [PubMed] [Google Scholar]
- 106.Harada N, Nishiyama S, Sato K, Tsukada H. Developement of an automated synthesis apparatus for l-[3-11C] labeled aromatic amino acids. Appl Radiat Isot. 2000;52:845–850. doi: 10.1016/s0969-8043(99)00099-8. [DOI] [PubMed] [Google Scholar]
- 107.Boroda E, Kański R, Kańska M. Synthesis of [14C]-l-tryptophan and [14C]-5′-hydroxy-l-tryptophan labeled in the carboxyl group. J Label Compd Radiopharm. 2003;46:441–447. [Google Scholar]
- 108.Ostwald R, Adams PT, Tolbert BM. Synthesis of several carbon-14 labeled dl-alanines. J Am Chem Soc. 1952;74:2425–2427. [Google Scholar]
- 109.Murray III A, Williams DL (1958) Organic synthesis with isotopes, part I. Interscience, New York, pp 95–99, pp 164–167
- 110.Kloster G, Laufer P. Enzymatic synthesis and chromatographic purification of l-3-[11C]-lactic acid via d, l-3-[11C]-alanine. J Label Compd Radiopharm. 1980;17:889–894. [Google Scholar]
- 111.Winnicka E, Kańska M. Synthesis of [3-14C]-l-tryptophan and 5′-hydroxy-[3-14C]-l-tryptophan. J Radioanal Nucl Chem. 2009;280:79–84. [Google Scholar]
- 112.Premont RT, Gainetdinov RR, Caron MG. Following the trace of elusive amines. Proc Natl Acad Sci USA. 2001;98:9474–9475. doi: 10.1073/pnas.181356198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nieoullon A, Coquerel A. Dopamine: a key regulator to adapt action, emotion, motivation and cognition. Curr Opin Neurol Suppl. 2003;2:S3–S9. [PubMed] [Google Scholar]
- 114.Diliberto EJ, Jr, Allen PL. Mechanism of dopamine-beta-hydroxylation. Semidehydroascorbate as the enzyme oxidation product of ascorbate. J Biol Chem. 1981;256:3385–3393. [PubMed] [Google Scholar]
- 115.Friedman S, Kaufman S. 3,4-dihydroxyphenylethylamine beta-hydroxylase. Physical properties, copper content, and role of copper in the catalytic activity. J Biol Chem. 1965;240:4763–4773. [PubMed] [Google Scholar]
- 116.Perel JM, Dawson DK, Dayton PG, Goldberg LI. α, α’- and β,β′-Deuterium-labeled dopamine. Synthesis and pharmacologic actions. J Med Chem. 1972;15:714–716. doi: 10.1021/jm00277a005. [DOI] [PubMed] [Google Scholar]
- 117.Farnum MF, Klinman JP. Stereochemical probes of bovine plasma amine oxidase: evidence for mirror image processing and a syn abstraction of hydrogens from C-1 and C-2 of dopamine. Biochemistry. 1986;25:6028–6036. doi: 10.1021/bi00368a029. [DOI] [PubMed] [Google Scholar]
- 118.Battersby AR, Sheldrake PW, Staunton J, Williams DC. Studies of Enzyme-mediated reactions. Part VII. Stereospecific syntheses of tritium-labeled (2R)- and (2S)-dopamines: stereochemical course of hydroxylation of dopamine by dopamine β-hydroxylase (EC 1.14.17.1) J Chem Soc Perkin Trans. 1976;1:1056–1062. doi: 10.1039/p19760001056. [DOI] [PubMed] [Google Scholar]
- 119.Summers MC, Markovic R, Klinman JP. Stereochemistry and kinetic isotope effects in the bovine plasma amine oxidase catalyzed oxidation of dopamine. Biochemistry. 1979;18:1969–1979. doi: 10.1021/bi00577a019. [DOI] [PubMed] [Google Scholar]
- 120.Yu PH, Bailey BA, Durden DA, Boulton AA. Stereospecific of deuterium substitution at the α-carbon position of dopamine and its effect on oxidative deamination catalyzed by MAO-A and MAO-B from different tissues. Biochem Pharmacol. 1986;35:1027–1036. doi: 10.1016/0006-2952(86)90094-8. [DOI] [PubMed] [Google Scholar]
- 121.Vederas JC, Reingold ID, Sellers HW. Stereospecificity of sodium borohydride reduction of tyrosine decarboxylase from Streptococcus faecalis. J Biol Chem. 1979;254:5053–5057. [PubMed] [Google Scholar]
- 122.Pająk M, Kańska M. Synthesis of isotopomers of dopamine labeled with deuterium or tritium. J Label Compd Radiopharm. 2006;49:1061–1067. [Google Scholar]
- 123.Dunathan HC. Stereochemical aspects of pyridoxal phosphate catalysis. Adv Enzymol Relat Areas Mol Biol. 1971;35:79–134. doi: 10.1002/9780470122808.ch3. [DOI] [PubMed] [Google Scholar]
- 124.Belleau B, Burba J. The stereochemistry of the enzymic decarboxylation of amino acids. J Am Chem Soc. 1960;82:5751–5752. [Google Scholar]
- 125.Burchett SA, Hicks TP. The mysterious trace amines: protean neuromodulators of synaptic transmission in mammalian brain. Prog Neurobiol. 2006;79:223–246. doi: 10.1016/j.pneurobio.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 126.Berry MD. Mammalian central nervous system trace amines. Pharmacologic amphetamines, physiologic neuromodulators. J Neurochem. 2004;90:257–271. doi: 10.1111/j.1471-4159.2004.02501.x. [DOI] [PubMed] [Google Scholar]
- 127.Strange PG, Staunton J, Wiltshire HR, Battersby AR. Studies of enzyme-mediated reactions. Part II. Stereochemistry of the elimination of ammonia from l-tyrosine catalyzed by the enzyme from maize. J Chem Soc Perkin Trans. 1972;1:2364–2372. doi: 10.1039/p19720002364. [DOI] [PubMed] [Google Scholar]
- 128.Rocha e Silva M. Histamine and anti-histaminics, part I. Handbook of experimental pharmacology. Berlin: Springer; 1966. [Google Scholar]
- 129.Rocha e Silva M. Histamine and anti-histaminics, part I. Handbook of experimental pharmacology. Berlin: Springer; 1978. [Google Scholar]
- 130.Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77:1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- 131.Brown DD, Axelrod J, Tomchick R. Enzymatic N-methylation of histamine. Nature. 1959;183:680. doi: 10.1038/183680a0. [DOI] [PubMed] [Google Scholar]
- 132.Beall GN, Vanarsdel PP., Jr Histamine metabolism in human disease. J Clin Invest. 1960;39:676–683. doi: 10.1172/JCI104083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Waldmeier PC, Feldtrauer JJ, Maître L. Methylhistamine: evidence for selective deamination by MAO B in the rat brain. J Neurochem. 1977;29:785–790. doi: 10.1111/j.1471-4159.1977.tb10719.x. [DOI] [PubMed] [Google Scholar]
- 134.IUPAC Nomenclature and symbolism for amino acids and peptides, recommendation 1983. Pure Appl Chem. 1984;56:595–624. [Google Scholar]
- 135.Iwashina T, Scott PG, Tredget EE. Synthesis of tritium-labelled Nτ-methylhistamine for the improvement of extraction efficiency of Nτ-methylhistamine from biological fluids. Appl Radiat Isot. 1997;48:1187–1191. doi: 10.1016/s0969-8043(96)00310-7. [DOI] [PubMed] [Google Scholar]
- 136.Knight RI, Morecombe DJ, Saunders D, Smith IR. The preparation of Nτ-[3H]methylhistamine dihydrochloride and Nτ-methyl[α, β,2,4,5–14C5]histamine dihydrochloride. J Label Compd Radiopharm. 1979;16:761–769. [Google Scholar]
- 137.Tredget EE, Iwashina T, Scott PG, Ghahary A. Determination of plasma Nτ-methylhistamine in vivo by isotope dilution using benchtop gas chromatography-mass spectrometry. J Chromatogr B. 1997;694:1–9. doi: 10.1016/s0378-4347(97)00122-9. [DOI] [PubMed] [Google Scholar]
- 138.Šamonina J, Kańska M. Enzymatic synthesis of N-methylhistamine labeled with deuterium and tritium. J Label Compd Radiopharm. 2009;52:372–375. [Google Scholar]
- 139.Panufnik E, Kański R, Kanska M. Enzymatic synthesis of tritium labelled isotopomers of histamine. J Label Compd Radiopharm. 2005;48:45–50. [Google Scholar]
- 140.Battersby AR, Nicoletti M, Staunton J, Vleggaar R. Studies of Enzyme-mediated Reactions. Part 13. Stereochemical course of the formation of histamine by decarboxylation of (2S)-histidine with enzymes from Clostridium welchii and Lactobacillus 30a. J Chem Soc Perkin Trans. 1980;1:43–51. doi: 10.1039/p19800000043. [DOI] [PubMed] [Google Scholar]
- 141.Rétey J, Bartl K, Ripp E, Hull WE. Stereospecificity of phenylpyruvate tautomerase. A convenient method for the preparation of chirally labelled phenylpyruvates. Eur J Biochem. 1977;72:251–257. doi: 10.1111/j.1432-1033.1977.tb11247.x. [DOI] [PubMed] [Google Scholar]
- 142.Christopherson RI, Morrison JF. Synthesis and separation of tritium-labeled intermediates of shikimate pathway. Arch Biochem Biophys. 1983;220:444–450. doi: 10.1016/0003-9861(83)90434-4. [DOI] [PubMed] [Google Scholar]
- 143.Larsen PO, Wieczorkowska E. Syntheses of 14C-labeled cinnamic, mandelic, phenylacetic, phenylglyoxylic, and phenylpyruvic acids. J Label Compd Radiopharm. 1974;10:287–296. [Google Scholar]
- 144.Skowera K, Kańska M. Enzymatic synthesis of phenylpyruvic acid labeled with deuterium, tritium and carbon-14. J Label Compd Radiopharm. 2008;51:321–324. [Google Scholar]
- 145.Gulyas B, Halldin C. New PET radiopharmaceuticals beyond FDG for brain tumor imaging. Q J Nucl Med Imaging. 2012;56:173–190. [PubMed] [Google Scholar]
- 146.Hanaoka H, Ohshima Y, Suzuki Y, Yamaguchi A, Watanabe S, Uehara T, Nagamori S, Kanai Y, Ishioka NS, Tsushima Y, Endo K, Arano Y. Development of a widely usable amino acid tracer: 76Br-α-methyl-phenylalanine for tumor PET imaging. J Nucl Med. 2015;56:791–797. doi: 10.2967/jnumed.114.152215. [DOI] [PubMed] [Google Scholar]
- 147.Shikano N, Kanai Y, Kawai K, Ishikawa N, Endou H. Characterization of 3-[125I]iodo-α-methyl-l-tyrosine transport via human l-type amino acid transporter 1. Nucl Med Biol. 2003;30:31–37. doi: 10.1016/s0969-8051(02)00350-5. [DOI] [PubMed] [Google Scholar]
- 148.Hellwig D, Ketter R, Romeike BFM, Schaefer A, Farmakis G, Grgic A, Moringlane JR, Steudel W-I, Kirsch C-M, Samnic S. Prospective study of p-[123I]iodo-l-phenylalanine and SPECT for the evaluation of newly diagnosed cerebral lesions: specific confirmation of glioma. Eur J Nucl Med Mol Imaging. 2010;37:2344–2353. doi: 10.1007/s00259-010-1572-4. [DOI] [PubMed] [Google Scholar]
- 149.Giglio BC, Fei H, Wang M, Wang H, He L, Feng H, Wu Z, Lu H, Li Z. Synthesis of 5-[18F]Fluoro-α-methyl tryptophan: new Trp based PET agents. Theranostic. 2017;7:1524–1530. doi: 10.7150/thno.19371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Winnicka E, Pająk M, Pałka K, Czerwińska K, Kańska M. Enzymatic synthesis of halogen derivatives of aromatic amino acids labeled with hydrogen isotopes. J Chem Chem Eng. 2014;8:54–60. [Google Scholar]
- 151.Pająk M, Pałka K, Winnicka E, Kańska M. Syntheses of halogen derivatives of l-tryptophan, l-tyrosine and l-phenylalanine labeled with hydrogen isotopes. J Label Compd Radiopharm. 2016;59:4–8. doi: 10.1002/jlcr.3357. [DOI] [PubMed] [Google Scholar]
- 152.Pająk M, Kańska M. Enzymatic syntheses of 3′-halotyramines, selectively 2H- and 3H-labeled in the side chain. Appl Radiat Isot. 2018;131:8–12. doi: 10.1016/j.apradiso.2017.10.032. [DOI] [PubMed] [Google Scholar]
- 153.Pająk M, Kańska M. Isotope effects in mechanistic studies of l-tyrosine halogen derivatives hydroxylation catalyzed by tyrosinase. J Radioanal Nucl Chem. 2017;314:2123–2128. doi: 10.1007/s10967-017-5526-1. [DOI] [PMC free article] [PubMed] [Google Scholar]