Abstract
The International Society for Clinical Electrophysiology of Vision (ISCEV) standard for full-field electroretinography (ERG) describes a minimum procedure, but encourages more extensive testing. This ISCEV extended protocol describes an extension to the ERG standard, namely the dark-adapted (DA) red flash ERG. The DA red flash ERG can be incorporated conveniently within the ISCEV standard ERG protocol after a minimum of 20-min DA and recorded after the DA 0.01 ERG to a flash strength of 0.3 phot cd s m−2, eliciting a waveform with two positive peaks in healthy individuals. The first positive component is the cone-mediated x-wave with a peak at 30–50 ms; the second is a rod-mediated b-wave with a peak time of approximately 100 ms. Shorter DA times may be desirable to shorten the recording time or to alter the prominence of the early cone-mediated x-wave relative to the rod-mediated b-wave. The DA red flash ERG is used to aid the diagnosis of achromatopsia (rod monochromacy), cone dystrophy and other forms of cone system dysfunction, including “Bradyopsia” (RGS9/R9AP-retinopathy), when the DA red flash ERG x-wave is preserved in the absence of ISCEV standard LA ERGs. The DA red flash ERG can also help determine the origin of residual DA ERGs in cases of severe rod dysfunction, for example in disorders such as vitamin A deficiency, fundus albipunctatus (RDH5-retinopathy), Oguchi disease (SAG- or GRK1-retinopathy) and some rod-cone dystrophies. To shorter DA periods, the x-wave may be elicited without the following rod b-wave, shown to be helpful in abbreviated protocols for children.
Keywords: Clinical standards, Electroretinogram (ERG), Full-field ERG, International Society of Clinical Electrophysiology of Vision (ISCEV), Dark-adapted (DA), Red flash ERG, Retinal dystrophy
Introduction
The International Society for Clinical Electrophysiology of Vision (ISCEV) standard for full-field electroretinography (ERG) describes a minimum set of tests, but encourages the use of additional ERG protocols for clinical ERG testing [1]. This extended protocol describes the dark-adapted (DA) red ERG, as a specialized procedure which is well established and broadly accepted by experts in the field. The protocol was prepared by the authors in accordance with ISCEV procedures (http://www.iscev.org/standards/index.html) and was approved by the ISCEV Board of Directors on March 25, 2018.
Scope and applications
The ISCEV ERG standard [1] describes a minimum protocol to test generalized rod and cone system function in the outer and inner retina. The DA red flash ERG can be used to distinguish the function of DA rod and cone systems and can help determine the origins of abnormal standard flash ERGs, which may be important for accurate characterization of retinal function and to establish some diagnoses. This extended protocol describes parameters for the dark-adapted (DA) red flash ERG that may be added to the ISCEV standard ERG protocol.
The normal cone system contributes to the full-field ERG under DA as well as light-adapted (LA) conditions. This occurs in DA ERGs evoked by flash strengths greater than 0.1 cd s m−2 [2], including the ISCEV standard DA 3 (“combined rod cone”) and DA 10 (“strong flash”) ERGs. Early investigations revealed the contribution of DA cones in the ERG waveform by using colored flashes that exploited differences in the spectral sensitivities of rods and cones [3–5]. These studies showed that the DA ERG waveform to a red flash has two distinct positive peaks. The first, named the x-wave, occurred within 30–50 ms and was attributed to DA cone activity. The x-wave was followed by a rod-mediated b-wave [3]. The x-wave is larger than the b-wave during the early stages of dark adaptation when the rod system threshold is high. As dark adaptation proceeds, the x- and b-wave amplitudes become similar and finally the b-wave exceeds the x-wave [6].
The DA red flash ERG has several clinical applications, and the circumstances and diagnoses that may benefit from testing are outlined below:
The DA red flashes are usually well tolerated by patients of all ages, and the test is therefore useful if photophobia or photo-aversion confounds the recording of standard LA ERGs. This can occur in the presence of cone dysfunction, but also, for example, in the presence of media opacity or strong Bell’s phenomenon.
In cases of generalized cone system dysfunction such as rod- and S-cone monochromacy and cone dystrophy, the DA red flash ERG x-wave may be undetectable, markedly attenuated and/or delayed [7–10].
In cases of generalized retinal dysfunction, the relative involvement of the DA red flash ERG x-wave and b-wave may suggest predominant dysfunction of cone or rod systems, not always obvious by comparing standard DA and LA ERGs.
In cases of severe or selective rod dysfunction, the DA red flash ERG can help determine the causes and origins of abnormal or residual DA bright flash ERGs. This occurs, for example, in vitamin A deficiency [11], fundus albipunctatus (RDH5-retinopathy) [12] and Oguchi disease (SAG- or GRK1-retinopathy) [13] and in some cases of rod-cone dystrophy including early stages of Bothnia dystrophy (RLBP1-retinopathy). In these disorders, the DA 3 and DA 10 ERGs have reduced a-waves indicating rod photoreceptor dysfunction, but there may also be reduction in the b:a ratio and shortening of b-wave peak time in the absence of a rod system contribution. The reduced b:a ratio may arise from strong stimulation of the relatively preserved DA cone system, analogous to the photopic hill phenomenon, and produces a b-wave which resembles the DA red flash ERG x-wave.
“Bradyopsia” (RGS9- and R9AP-retinopathy). The DA red flash ERG is normal, but LA cone-mediated ERGs are extinguished by repetitive flashes [14, 15]. The combination of a preserved DA red flash ERG x-wave and undetectable or severely abnormal standard LA ERGs is pathognomonic for the disorder.
The red flash ERG has been used to detect color vision deficiencies and has been reported to be absent [9, 16] or subnormal [10] in protanopia. The implication is that around 1/100 males would have an absent red flash ERG although this has not been established for an ISCEV DA red flash ERG extended protocol.
Patient population
Patients of all ages, referred for investigation of possible retinal dysfunction, retinal dystrophy, generalized cone or rod system dysfunction or patients with photophobia may benefit from the DA red flash ERG.
Technical issues
The DA red flash ERG will follow the specifications of the current ISCEV standard full-field ERG and for most applications may be embedded within the standard protocol [1].
Additional considerations include the following:
The spectral characteristics of the red flash. Both peak wavelength and bandwidth may affect the DA red flash ERG. Physical filters, e.g., Kodak Wratten filters 26 (dominant wavelength 619 nm) or 29 (dominant wavelength 630 nm), were used in many older studies, but have been largely superseded by LEDs, e.g. peak wavelengths 635 or 655 nm, and choice may be equipment dependent. It is noted that peak wavelengths shorter than 620 nm may be perceived as orange, and wavelengths longer than 630 nm provide slightly better separation of x-wave and b-wave, and that for wavelengths longer than 650 nm waveforms have been reported with a third positive wave, later than the rod b-wave [6].
The units of flash strength. The relative (effective) strength of a colored flash depends upon the adaptation and hence spectral sensitivity of the eye. Absolute measures are radiant energy, but for uniformity of clinical use and consistency with other flash stimuli, photometric units defined in phot cd s m−2 are recommended.
- Duration of dark adaptation. The choice of dark adaptation duration and flash strength depends upon one of three aims (Fig. 1):
- To separate the x- and b-wave peak times: if an ISCEV standard period of at least 20-min dark adaptation is used, weaker red flash strengths of around 0.03–0.3 cd s m−2 allow maximum separation in time of the cone- and rod-mediated components.
- To match the amplitudes of the DA red flash ERG b-wave with the ISCEV standard DA 0.01 ERG (rod ERG) b-wave, different red flash strengths may be needed depending upon the patient’s age, as the DA red flash rod b-wave diminishes with age, relative to the DA red flash x-wave [18].
Frequency of red flash presentation. The inter-stimulus interval will influence the light adaptation of the retina and shape of the DA red flash ERG waveform [19]. The ISCEV standard for the DA 0.01 ERG is less than or equal to 1 flash every 2 s, and a similar frequency may be appropriate for flash strengths that elicit responses of similar amplitude to the DA 0.01 ERG.
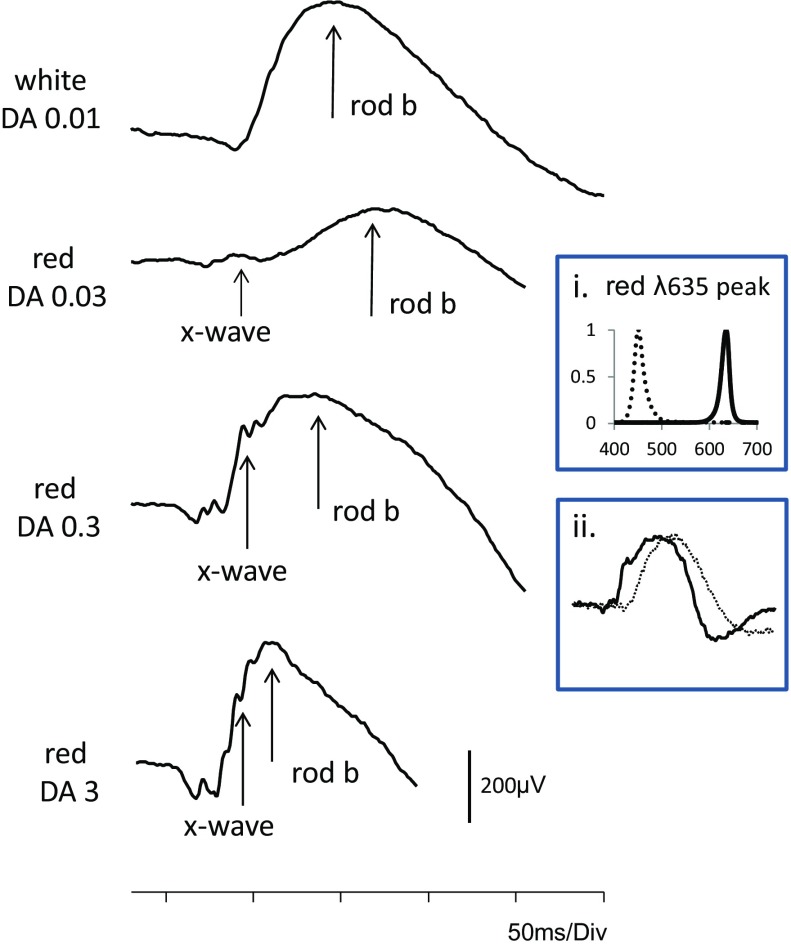
Fig. 1.
An ISCEV standard DA 0.01 ERG is shown at the top of the figure to compare the waveform of the rod driven b-wave with that of DA red flash ERGs produced by three different flash strengths of wavelength 635 nm after 20-min dark adaptation. Note the separation in peak time of the x-wave and b-wave to DA 0.03 cd s m−2 (dim) flashes, the enlargement of the x-wave to DA 0.3 cd s m−2 and the merging of x- and b-waves at DA 3 in a control subject. Insert i shows the spectral characteristics of the red (solid line) and blue (dotted line) LEDs in the Ganzfeld. Insert ii shows the DA red flash ERG to 0.3 cd s m−2 in a second subject compared with a DA blue flash ERG of “scotopically matched” b-wave amplitude, in this case DA blue 0.0003 cd s m−2 (the red flash response may also be “matched” to a DA dim white flash ERG). DA red ERG is shown as a solid black lines and DA blue flash ERG as a dotted line
Calibration
Calibration is in accordance with the ISCEV ERG standard [1]. A spectral photometer is required to determine the spectral characteristics of the red flash. Stimulators may use different combinations of LEDs for different flash strengths, so equal spectral characteristics should not be assumed.
Protocol specification
Patient preparation follows that of the current ISCEV ERG standard [1] and the DA red flash ERG may be embedded within the standard ERG protocol. Additional specifications are listed below:
Stimulus wavelength For routine diagnostic applications, LEDs with a peak wavelength of between 635 nm (Fig. 1) and 650 nm (Fig. 2) are suggested to allow separation of x- and b-waves. If Xenon flashes and filters are used, a dominant wavelength of 619 nm (e.g., Wratten 26) or 630 nm (e.g., Wratten 29) may be used. The peak wavelength and bandwidth at half-height of the stimulus and method of generation (optical filter or LED) should be stated.
Flash strength The minimum dark-adapted red flash protocol includes a red flash strength of 0.3 cd s m−2. This does not preclude the recording of additional red flash ERGs (ranging around 0.3 cd s m−2; see Fig. 2), but care should be taken to avoid light-adapting the retina with higher flash strengths, and it may be necessary to increase the inter-stimulus interval. If an additional red flash is defined as that required to elicit a DA red flash ERG rod system b-wave of equal or similar amplitude to the DA 0.01 ERG or to a DA blue flash ERG, this should be acknowledged and the corresponding flash stimuli stated in phot cd s m−2.
Duration of dark adaptation The duration of dark adaptation required to record a dark-adapted red flash depends on the aims. The DA red flash ERG can be incorporated conveniently within the ISCEV standard ERG protocol after a minimum of 20-min DA, after the standard DA 0.01 ERG. Shorter DA times may be desirable to shorten the recording time or specifically to reduce the rod system b-wave and increase the prominence of the cone system x-wave (see above). Mesopic cone–rod interactions associated with shorter DA may increase the variability of the DA red ERG b-wave amplitude.
Frequency of red flash presentation A flash rate of ≤ 0.5/s is recommended (inter-stimulus interval ≥ 2 s). This conforms to the current ISCEV standard for the DA 0.01 ERG. Longer inter-stimulus intervals may be needed for stronger red flashes.
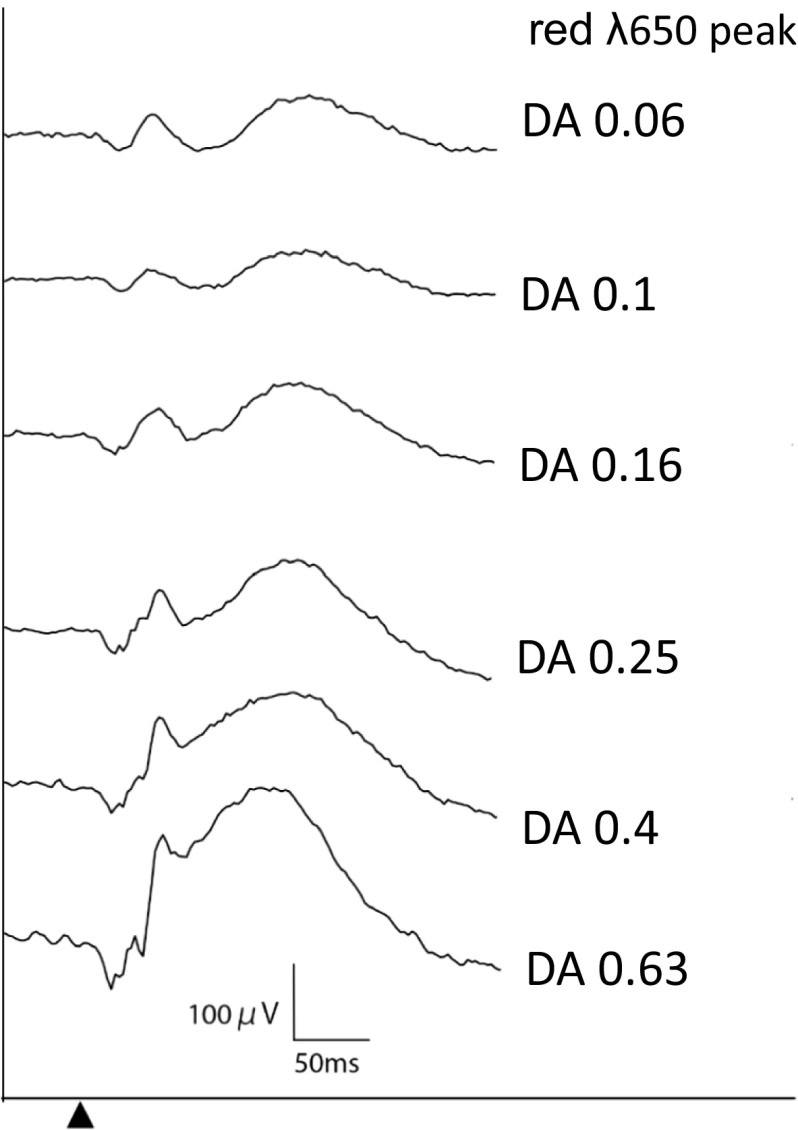
Fig. 2.
The gradual change in waveform of DA red flash ERG to a range of flash strengths weaker or stronger than 0.3 cd s m−2, recorded after 20 min DA with a 650 nm red flash
Response evaluation
Examples are shown in Figs. 1 and 2 of the DA red flash ERG waveforms produced by different flash strengths delivered using an LED (peak wavelength 635 or 650 nm). For routine testing, it is recommended that the x-wave and b-wave peak times and amplitudes are measured and reported. Peak times are measured from the flash (mid-point) and amplitudes from the baseline or a-wave (earliest negative trough) if present.
Reporting
Reporting the DA red flash should follow the recommendations of the ISCEV ERG protocol. The flash stimulus characteristics (LED or filter), peak wavelength or filter specification (e.g., Wratten 26 or 29) should be stated. The flash strength should be stated. Unless already embedded within the ISCEV standard ERG protocol, pupil size and duration of dark adaption should be stated. The amplitude of the a-wave, x-wave and b-wave and their respective time to peaks may be reported along with age-appropriate laboratory reference data. It is acknowledged that in studies involving ISCEV standard ERGs it may be sufficiently informative to describe the relative reduction or preservation of x-wave and/or b-waves relative to each other and reference ranges.
Acknowledgements
We would like to thank the members of ISCEV and BriSCEV and in particular Donnell Creel, Chris Hogg, Michael F. Marmor, Daphne McCulloch, Yves Sauvé and Mineo Kondo for their valuable discussion during the consultation period.
Appendix: Justification for the protocol details
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [20] when writing this report. The search strategy aimed to identify reports of scotopic red flash ERGs in order to extract stimulus parameters of wavelength, flash strength, stimulus duration, temporal frequency, dark adaptation period and amass evidence of its clinical application and range of response expected in normal and clinical cases.
A systematic literature review was performed to find publications that reported the scotopic red flash ERG initially from the period January 1942 to April 10, 2017 using MEDLINE, EMBASE and Cochrane reviews. Exclusion criteria were animal studies and absence of any stimulus specification. In summary, red flash strengths used, included 0.05, 0.1 [7], 0.17 [21], 0.25, 0.5, 0.75, 1 & 1.5 [22], 2.37 [23], 2.4 [2], 1.5 and 2.5 [24], and 8 cd s m−2 [25] (personal communication Sauve 2017) after 20 min DA. A range of Grass stroboscope flash settings have been stated as 1, 4, 8 and 16 e.g. gr4 white PS22 ~ 3.7 × 105 candles [26] or gr4 + Wratten 26 filter = 0.02 Log μJ/cm2-steradian [18]. Some studies report flash strengths “such that in a normal subject the amplitude of the rod component to the red flash ERG is equivalent to that of the rod-specific response to a dim white flash (dark-adapted 0.01 cd s m−2)” [11, 12, 15, 27] or report that the red flash luminance empirically be set to elicit a b-wave of ~ 200μV [28]. The DA red flash ERG x-wave is reported to be stable between 10–70 years of age whilst the rod-driven b-wave diminishes with increasing age, [18]. To match b-wave amplitudes in the DA 0.01 and DA red flash ERGs it may be necessary to increase the red flash strength in older individuals. The main published parameters are summarized in Table 1.
Table 1.
A summary of published DA red flash ERG parameters
| Data | Peak λ | Flash strength | DA duration | LED/Xenon |
|---|---|---|---|---|
| Auerbach and Burian [6] | Wratten 29 635 nm Wratten 70 650 nm |
6 and 12 cd s m−2 | 5 min | Xenon @30 cm |
| Francois et al. [9] | Neon 570 nm | 0.1 J | Neon 0.2 s (orange) | |
| x-wave 25–60 μV@40 ms | ||||
| Iiyami and Yamaguchi [29] | Wratten 29 > 600 nm |
86–112 cd s m−2 | 30 min | |
| Kellner and Foerster [10] | Wratten 29 623 nm |
Not stated | Xenon in Ganzfeld | |
| Lovasik et al. [23] | Wratten 26 > 600 nm |
2.37 cd s m−2 | Not stated | Xenon in Ganzfeld |
| From figure | 90 μV@50 ms | |||
| Mizunoya et al. [21] | LED 660 nm | 0.17 cd s m−2 | 20 min | Contact lens Ganzfeld |
| Verdon et al. [19] | Wratten 26 > 600 nm |
Xenon in Ganzfeld | ||
| From figure | @40 ms | |||
| Lim and Ohn [2] | Wratten 26 = 605 nm (Scot match—14Db blue) | 2.4 cd s m−2 | 45 min | Xenon in Ganzfeld |
| Control data | 172.4 µV@46 ms | N = 52 adult | ||
| Weleber [18] | Wratten 26 > 600 nm |
Gr1, 4 and 16 | 30 min | Xenon in Ganzfeld |
| Control data | Burian Allen | Gr1 = 50 µV (25–75) @40–50 ms Gr4 = 150 µV@50 ms Gr16 = 325 µV (200–400) @ 50 ms |
N = 24 adult | |
| Kriss et al. [8] | Grass red | Gr4 | 5 min | Grass @30 cm |
| Peak 670 nm | ||||
| Control data | Skin electrode | 14.3 (SD 4.9) µV@ 40.4 ms (SD 2.6) lower limit 4.5 µV @46.9 | N = 30 over 5 m and adult | |
| Chen et al. [22] abs | Espion | 0.25 cd s m−2 | 20 min | LED |
| color dome | ||||
| Peak 635 nm | ||||
| Control data | a-wave | 17.6 µV@19.8 ms | N = 37 adult | |
| x-wave | 64 µV@50.7 ms | |||
| b-wave | 68 µV@72.9 ms | |||
| Hamilton and Graham [17] | Skin electrode | 0.2–2.0 cd s m−2 | 20 min | N = 16 adults |
Compliance with ethical standards
Conflict of interest
The authors have no conflicts of interest.
Informed consent
As this article does not contain any studies with human participants performed directly by any of the authors, the concept of informed consent is not applicable.
Statement of human rights
The study involved no research on Human participants and consent is not applicable.
Statement on the welfare of animals
The study involved no research on animals.
References
- 1.McCulloch DL, Marmor MF, Brigell MG, Hamilton R, Holder GE, Tzekov R, et al. ISCEV Standard for full-field clinical electroretinography (2015 update) Doc Ophthalmol. 2015;130(1):1–12. doi: 10.1007/s10633-014-9473-7. [DOI] [PubMed] [Google Scholar]
- 2.Lim SH, Ohn YH. Study of blue and red flash in dark-adapted electroretinogram. Korean J Ophthalmol. 2005;19(2):106–111. doi: 10.3341/kjo.2005.19.2.106. [DOI] [PubMed] [Google Scholar]
- 3.Motokawa K, Mita T. Über eine einfachere Untersuchungsmethode und Eigenschaften der Aktionsströme der Netzhaut des Menschen. Tohoku J Exp Med. 1942;42:114–133. doi: 10.1620/tjem.42.114. [DOI] [Google Scholar]
- 4.Adrian ED. The electric response of the human eye. J Physiol. 1945;104(1):84–104. doi: 10.1113/jphysiol.1945.sp004109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adrian ED. The rod and cone components in the electrical response of the human eye. J Physiol. 1946;104:84–104. doi: 10.1113/jphysiol.1945.sp004109. [DOI] [Google Scholar]
- 6.Auerbach E, Burian HM. Studies on the photopic-scotopic relationships in the human electroretinogram. Am J Ophthalmol. 1955;40(5):42–60. doi: 10.1016/0002-9394(55)91836-5. [DOI] [PubMed] [Google Scholar]
- 7.Miyake Y. Electrodiagnosis of retinal disease. Japan: Springer; 2006. pp. 16–19. [Google Scholar]
- 8.Kriss A, Jeffrey B, Taylor D. The electroretinogram in infants and young children. J Clin Neurophysiol. 1992;9(3):373–393. doi: 10.1097/00004691-199207010-00005. [DOI] [PubMed] [Google Scholar]
- 9.Francois J, Verriest G, De Rouck A. Pathology of the x-wave of the human electroretinogram. I. Red-blindness and other congenital functional abnormalities. Br J Ophthalmol. 1956;40(7):439–443. doi: 10.1136/bjo.40.7.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kellner U, Foerster MH. Color electroretinography. A method for separation of dysfunctions of cones. Doc Ophthalmol Adv Ophthalmol. 1992;80(1):13–23. doi: 10.1007/BF00161227. [DOI] [PubMed] [Google Scholar]
- 11.McBain VA, Egan CA, Pieris SJ, Supramaniam G, Webster AR, Bird AC, Holder GE. Functional observations in vitamin A deficiency: diagnosis and time course of recovery. Eye. 2007;21:367–376. doi: 10.1038/sj.eye.6702212. [DOI] [PubMed] [Google Scholar]
- 12.Sergouniotis PI, Sohn EH, Li Z, McBain VA, Wright GA, Moore AT, et al. Phenotypic variability in RDH5 retinopathy (fundus albipunctatus) Ophthalmology. 2011;118(8):1661–1670. doi: 10.1016/j.ophtha.2010.12.031. [DOI] [PubMed] [Google Scholar]
- 13.Sergouniotis PI, Davidson AE, Sehmi K, Webster AR, Robson AG, Moore AT. Mizuo-Nakamura phenomenon in Oguchi disease due to a homozygous nonsense mutation in the SAG gene. Eye (Lond) 2011;25(8):1098–1101. doi: 10.1038/eye.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michaelides M, Li Z, Rana NA, Richardson EC, Hykin PG, Moore AT, Holder GE, Webster AR. Novel mutations and electrophysiologic findings in RGS9- and R9AP-associated retinal dysfunction (Bradyopsia) Ophthalmology. 2010;117(1):120–127. doi: 10.1016/j.ophtha.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 15.Cheng JYC, Luu CD, Yong VHK, Mathur R, Aung T, Vithana EN. Bradyopsia in an Asian man. Arch Ophthalmol. 2007;125:1138–1140. doi: 10.1001/archopht.125.8.1138. [DOI] [PubMed] [Google Scholar]
- 16.Von Schubert G, Bornschein H. Beitrag zur Analyse des menschlichen Elektroretinogramms. Ophthalmologica. 1952;123(6):396–412. doi: 10.1159/000301211. [DOI] [PubMed] [Google Scholar]
- 17.Hamilton R, Graham K. Dark-adapted red flash ERGs in healthy adults. Doc Ophthalmol. 2018 doi: 10.1007/s10633-018-9642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weleber RG. The effect of age on human cone and rod ganzfeld electroretinograms. Invest Ophthalmol Vis Sci. 1981;20(3):392–399. [PubMed] [Google Scholar]
- 19.Verdon WA, Schneck ME, Haegerstrom-Portnoy G. A comparison of three techniques to estimate the human dark-adapted cone electroretinogram. Vis Res. 2003;43(19):2089–2099. doi: 10.1016/S0042-6989(03)00330-4. [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mizunoya S, Kuniyoshi K, Arai M, Tahara K, Hirose T. Electroretinogram contact lens electrode with tri-color light-emitting diode. Acta Ophthalmol Scand. 2001;79(5):497–500. doi: 10.1034/j.1600-0420.2001.790514.x. [DOI] [PubMed] [Google Scholar]
- 22.Chen L, Png R, Mathur R, Chia A (2015) Scotopic red ERG findings. In: 53rd symposium of International Society for Clinical Electrophysiology of Vision (ISCEV). Springer, Heidelberg, p 31
- 23.Lovasik JV, Kothe AC, Kergoat H. Improving the diagnostic power of electroretinography by transient alteration of the ocular perfusion pressure. Optom Vis Sci. 1992;69(2):85–94. doi: 10.1097/00006324-199202000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Chia A, Png R, Mathur R (eds) (2014) Scotopic red response: rod and cone components. In: International Society for Clinical Electrophysiology of Vision Symposium. Doc Ophthalmol, Boston
- 25.Freund PR, Watson J, Gilmour GS, Gaillard F, Sauve Y. Differential changes in retina function with normal aging in humans. Doc Ophthalmol Adv Ophthalmol. 2011;122(3):177–190. doi: 10.1007/s10633-011-9273-2. [DOI] [PubMed] [Google Scholar]
- 26.Kriss A, Russell-Eggitt I. Electrophysiological assessment of visual pathway function in infants. Eye (Lond). 1992;6(Pt 2):145–153. 10.1038/eye.1992.30 [DOI] [PubMed]
- 27.Vincent A, Robson AG, Holder GE. Pathognomonic (diagnostic) ERGs. A review and update. Retina. 2013;33(1):5–12. doi: 10.1097/IAE.0b013e31827e2306. [DOI] [PubMed] [Google Scholar]
- 28.Creel DJ (ed) (2013) Scotopic dim blue and red ERG stimuli. Doc Ophthalmol
- 29.Iijima H, Yamaguchi S. Adaptational changes in cone electroretinograms in man. Nippon Ganka Gakkai Zasshi Acta Soc Ophthalmol Jpn. 1990;94(11):987–992. [PubMed] [Google Scholar]