Abstract
Aims/hypothesis
After coronary artery bypass graft (CABG) surgery in individuals with type 2 diabetes, there remains a considerable residual cardiovascular risk. In the EMPA-REG OUTCOME® trial in participants with type 2 diabetes and established cardiovascular disease, empagliflozin reduced the risk of cardiovascular death by 38%, all-cause mortality by 32%, hospitalisation for heart failure by 35% and incident or worsening nephropathy by 39% vs placebo when given in addition to standard of care. The aim of this post hoc analysis of the EMPA-REG OUTCOME® trial was to determine the effects of the sodium glucose cotransporter 2 inhibitor empagliflozin on cardiovascular events and mortality in participants with type 2 diabetes and a self-reported history of CABG surgery.
Methods
The EMPA-REG OUTCOME® trial was a randomised, double-blind, placebo-controlled trial. Participants with type 2 diabetes and established cardiovascular disease were randomised 1:1:1 to receive placebo, empagliflozin 10 mg or empagliflozin 25 mg, once daily, in addition to standard of care. In subgroups by self-reported history of CABG (yes/no) at baseline, we assessed: cardiovascular death; all-cause mortality; hospitalisation for heart failure; and incident or worsening nephropathy (progression to macroalbuminuria, doubling of serum creatinine, initiation of renal replacement therapy or death due to renal disease). Differences in risk between empagliflozin and placebo were assessed using a Cox proportional hazards model.
Results
At baseline, 25% (1175/4687) of participants who received empagliflozin and 24% (563/2333) of participants who received placebo had a history of CABG surgery. In participants with a history of CABG surgery, HRs (95% CI) with empagliflozin vs placebo were 0.52 (0.32, 0.84) for cardiovascular mortality, 0.57 (0.39, 0.83) for all-cause mortality, 0.50 (0.32, 0.77) for hospitalisation for heart failure and 0.65 (0.50, 0.84) for incident or worsening nephropathy. Results were consistent between participants with and without a history of CABG surgery (p > 0.05 for treatment by subgroup interactions).
Conclusions/interpretation
In participants with type 2 diabetes and a self-reported history of CABG surgery, treatment with empagliflozin was associated with profound reductions in cardiovascular and all-cause mortality, hospitalisation for heart failure, and incident or worsening nephropathy. These data have important implications for the secondary prevention of cardiovascular events after CABG in individuals with type 2 diabetes.
Trial registration:
Electronic supplementary material
The online version of this article (10.1007/s00125-018-4644-9) contains peer-reviewed but unedited supplementary material, which is available to authorised users.
Keywords: Cardiovascular disease, Coronary artery bypass graft, Coronary revascularisation, Diabetes mellitus, Empagliflozin, Sodium glucose cotransporter 2 inhibition, Type 2 diabetes

Introduction
Compared with individuals without diabetes, individuals with diabetes have a higher rate, extent and severity of obstructive coronary artery disease [1]. Individuals with diabetes and multi-vessel coronary artery disease derive greater benefit from coronary artery bypass graft (CABG) surgery than percutaneous coronary intervention (PCI) [2], and CABG is regarded as a preferred strategy in these individuals [3]. However, despite advances in surgical techniques, perioperative management and pharmacotherapy, there remains considerable residual cardiovascular risk in individuals with diabetes after CABG. The 5 year event rate of major adverse cardiovascular events (MACE) after CABG in participants with diabetes was 19% in the FREEDOM trial and 29% in the SYNTAX trial [4, 5]. In addition to residual ischaemic cardiovascular risk, there remains a substantial risk of heart failure after CABG, with 2 year rates of heart failure hospitalisation ranging from 12.9% (in participants with ejection fraction ≥50%) to 36.9% (in those with ejection fraction <25%) [6].
Following Food and Drug Administration (FDA) guidance [7], results are available from large trials evaluating the cardiovascular effects of glucose-lowering agents in participants with type 2 diabetes. One such trial was the EMPA-REG OUTCOME® trial (ClinicalTrials.gov NCT01131676), in which participants with type 2 diabetes and established cardiovascular disease were randomised to receive the sodium glucose cotransporter 2 (SGLT2) inhibitor empagliflozin or placebo in addition to standard of care [8]. The EMPA-REG OUTCOME® trial was the first placebo-controlled trial to report a benefit of a glucose-lowering agent on major cardiovascular outcomes, with risk reductions in cardiovascular and all-cause mortality of 38% and 32%, respectively [8]. Data from this trial led to the FDA approval of an indication for empagliflozin to include reducing the risk of cardiovascular death in participants with type 2 diabetes and established cardiovascular disease—the first such approval for a glucose-lowering agent [9]. These data have also led to recommendations in clinical practice guidelines that empagliflozin should be considered in the treatment of individuals with type 2 diabetes and cardiovascular disease [10, 11].
There remains controversy regarding pharmacological cardiovascular risk reduction approaches in individuals following surgical revascularisation, with very limited data on this group available from large randomised clinical trials [12, 13]. Approximately one quarter of the 7020 participants enrolled in the EMPA-REG OUTCOME® trial had a self-reported history of CABG at baseline. We investigated the effect of empagliflozin on cardiovascular outcomes and mortality in this subgroup.
Methods
Trial design and population
The design of the EMPA-REG OUTCOME® trial has been described and the study protocol has been published [8]. Briefly, eligible participants had type 2 diabetes (with HbA1c 53–75 mmol/mol [7.0–9.0%] for drug-naive participants and 53–86 mmol/mol [7.0–10.0%] for those on stable glucose-lowering therapy), established cardiovascular disease and estimated (e)GFR, according to modification of diet in renal disease (MDRD) ≥30 ml min−1 1.73 m−2. Participants were identified as having a history of CABG at baseline based on case report forms in which the investigator noted a history of CABG (based on the participant’s self-report) using a yes/no checkbox. Participants were randomised to receive empagliflozin 10 mg, empagliflozin 25 mg or placebo, once daily. Background glucose-lowering therapy was to remain unchanged for 12 weeks. After week 12, investigators were encouraged to adjust glucose-lowering therapy to achieve glycaemic control according to local guidelines. Investigators were encouraged to treat cardiovascular risk factors to achieve the best standard of care according to local guidelines throughout the trial. Serum creatinine and urinary albumin were measured from spot urine samples obtained during study visits at screening (creatinine only), during the placebo run-in period, at baseline, at weeks 12, 28 and 52 and then every 14 weeks until the final visit. Events that were consistent with changes in the albuminuria category were captured if any laboratory assessment during the trial fulfilled the given criteria on at least one occasion. The trial was to continue until ≥691 participants experienced an adjudicated event included in the primary outcome (three-point MACE: composite of cardiovascular death, non-fatal myocardial infarction and non-fatal stroke). Participants who prematurely discontinued study medication continued to be followed for ascertainment of cardiovascular outcomes, adverse events and vital status.
The EMPA-REG OUTCOME® trial was registered with ClinicalTrials.gov (NCT01131676) and carried out in compliance with the protocol and the principles of the Declaration of Helsinki, and in accordance with the International Conference on Harmonization Harmonized Tripartite Guideline for Good Clinical Practice. All participants provided signed and dated informed consent. The trial was conducted at 590 sites in 42 countries.
Outcomes
All cardiovascular outcome events and deaths were prospectively adjudicated by Clinical Events Committees who were blinded to treatment allocation. The following outcomes were analysed in subgroups by history of CABG (yes/no) at baseline: three-point MACE (composite of cardiovascular death, non-fatal myocardial infarction or non-fatal stroke); fatal or non-fatal myocardial infarction; fatal or non-fatal stroke; cardiovascular death; four-point MACE (composite of cardiovascular death, non-fatal myocardial infarction, non-fatal stroke or hospitalisation for unstable angina); hospitalisation for heart failure; composite of heart failure hospitalisation or cardiovascular death; all-cause mortality; incident or worsening nephropathy (defined as progression to macroalbuminuria [urine albumin-to-creatinine ratio >300 mg/g], doubling of serum creatinine accompanied by eGFR [MDRD] ≤45 ml min−1 1.73 m−2, initiation of renal replacement therapy or death due to renal disease). Safety was assessed based on adverse events that occurred during treatment or within 7 days after the last dose of study drug.
Analyses
Analyses in subgroups by CABG at baseline were post hoc and based on participants who received ≥1 dose of study drug, except for incident or worsening nephropathy. This outcome was analysed in participants who received ≥1 dose of study drug who did not have macroalbuminuria at baseline, who had serum creatinine measurements at baseline and after baseline, and who had post-baseline urine albumin-to-creatinine ratio measurements (unless participants who did not fulfil these criteria had at least one of the other components of the composite renal outcome).
Differences between empagliflozin (pooled) and placebo in the risk of an outcome were assessed using a Cox proportional hazards model, with factors for age, sex, baseline BMI, baseline HbA1c, baseline eGFR, region, treatment, CABG and treatment by CABG interaction, using events observed from randomisation to the end of the trial. Adjustment for these factors is consistent with the pre-specified analyses in subgroups by other baseline characteristics [8].
The p values for treatment by subgroup interaction were obtained from tests of homogeneity of treatment group differences among subgroups, with no adjustment for multiple testing. Data from participants who did not have an event were censored on the last day they were known to be free of the outcome. Kaplan–Meier estimates are presented for three-point MACE, cardiovascular death, hospitalisation for heart failure, all-cause mortality and incident or worsening nephropathy.
In participants with a history of CABG, we assessed cardiovascular death, hospitalisation for heart failure and all-cause mortality in subgroups by time from CABG to randomisation (≤5, >5 to ≤10, >10 years) and the proportion of participants with different numbers of coronary revascularisation (redo CABG; redo and/or new PCI) procedures during the trial. Outcomes in subgroups by time from CABG to randomisation and the proportion of participants with different numbers of coronary revascularisation procedures during the trial were assessed descriptively. Adverse events in subgroups by history of CABG were assessed descriptively, consistent with how adverse events in other subgroups were to be analysed according to the statistical analysis plan [8].
Results
Baseline characteristics
Between 1 September 2010 and 22 April 2013, 7028 participants were randomised and 7020 participants received the study drug (electronic supplementary material [ESM] Fig. 1). At baseline, a history of CABG was present in 25% (1175/4687) of participants in the empagliflozin (pooled) group and 24% (563/2333) of participants in the placebo group. Baseline characteristics were balanced between the empagliflozin and placebo groups in participants with and without CABG (Table 1). At baseline, mean (SD) age of participants with a history of CABG was 65 (8) years, HbA1c was 64 (9.2) mmol/mol (8.05 [0.84]%), 81% were male, 51% had a history of myocardial infarction, 11% had a history of heart failure, 15%, 52% and 33% had eGFR ≥90, 60 to <90 and <60 ml min−1 [1.73 m]−2, respectively, mean (SD) systolic blood pressure was 135.6 (17.1) mmHg and diastolic blood pressure was 75.3 (10.0) mmHg. The median observation time was 3.1 years.
Table 1.
Baseline characteristics by history of CABG at baseline
| Characteristic | Participants with a history of CABG | Participants without a history of CABG | ||
|---|---|---|---|---|
| Empagliflozin (n = 1175) | Placebo (n = 563) | Empagliflozin (n = 3512) | Placebo (n = 1770) | |
| Age, years | 64.5 ± 8.2 | 65.5 ± 8.0 | 62.6 ± 8.6 | 62.5 ± 8.9 |
| Male | 945 (80.4) | 459 (81.5) | 2391 (68.1) | 1221 (69.0) |
| Race | ||||
| White | 922 (78.5) | 441 (78.3) | 2481 (70.6) | 1237 (69.9) |
| Asian | 191 (16.3) | 78 (13.9) | 815 (23.2) | 433 (24.5) |
| Black/African-American | 51 (4.3) | 35 (6.2) | 186 (5.3) | 85 (4.8) |
| Other/Missing | 11 (0.9) | 9 (1.6) | 30 (0.9) | 15 (0.9) |
| Region | ||||
| Europe | 399 (34.0) | 189 (33.6) | 1527 (43.5) | 770 (43.5) |
| North America (plus Australia and New Zealand) | 389 (33.1) | 201 (35.7) | 543 (15.5) | 261 (14.7) |
| Asia | 138 (11.7) | 58 (10.3) | 759 (21.6) | 392 (22.1) |
| Latin America | 154 (13.1) | 72 (12.8) | 567 (16.1) | 288 (16.3) |
| Africa | 95 (8.1) | 43 (7.6) | 116 (3.3) | 59 (3.3) |
| Weight, kg | 88.8 ± 18.6 | 90.5 ± 19.7 | 85.3 ± 18.9 | 85.4 ± 18.7 |
| BMI, kg/m2 | 30.8 ± 5.1 | 31.2 ± 5.2 | 30.5 ± 5.3 | 30.5 ± 5.2 |
| Time since CABG | ||||
| ≤1 year | 86 (7.3) | 33 (5.9) | n/a | n/a |
| >1 to 5 years | 386 (32.9) | 174 (30.9) | n/a | n/a |
| >5 to 10 years | 373 (31.7) | 170 (30.2) | n/a | n/a |
| >10 years | 322 (27.4) | 181 (32.1) | n/a | n/a |
| CV disease | ||||
| Coronary artery disease | 1175 (100.0) | 563 (100.0) | 2370 (67.5) | 1200 (67.8) |
| Multi-vessel coronary artery disease | 869 (74.0) | 420 (74.6) | 1310 (37.3) | 680 (38.4) |
| History of myocardial infarction | 598 (50.9) | 286 (50.8) | 1592 (45.3) | 797 (45.0) |
| History of strokea | 140 (11.9) | 59 (10.5) | 944 (26.9) | 494 (27.9) |
| Peripheral artery diseaseb | 216 (18.4) | 107 (19.0) | 766 (21.8) | 372 (21.0) |
| Single vessel coronary artery diseasea | 60 (5.1) | 25 (4.4) | 438 (12.5) | 213 (12.0) |
| Cardiac failurec | 119 (10.1) | 70 (12.4) | 343 (9.8) | 174 (9.8) |
| HbA1c, mmol/mold | 64 ± 9.1 | 64 ± 9.3 | 65 ± 9.3 | 65 ± 9.2 |
| HbA1c, %d | 8.05 ± 0.83 | 8.04 ± 0.85 | 8.08 ± 0.85 | 8.09 ± 0.84 |
| Time since diagnosis of type 2 diabetes | ||||
| ≤1 year | 18 (1.5) | 7 (1.2) | 110 (3.1) | 45 (2.5) |
| >1 to 5 years | 125 (10.6) | 63 (11.2) | 587 (16.7) | 308 (17.4) |
| >5 to 10 years | 277 (23.6) | 112 (19.9) | 898 (25.6) | 459 (25.9) |
| >10 years | 755 (64.3) | 381 (67.7) | 1917 (54.6) | 958 (54.1) |
| Glucose-lowering therapy | ||||
| Medication taken alone or in combination | ||||
| Metformin | 840 (71.5) | 411 (73.0) | 2619 (74.6) | 1323 (74.7) |
| Sulfonylurea | 440 (37.4) | 220 (39.1) | 1574 (44.8) | 772 (43.6) |
| Insulin | 675 (57.4) | 318 (56.5) | 1577 (44.9) | 817 (46.2) |
| Monotherapy | 340 (28.9) | 171 (30.4) | 1040 (29.6) | 520 (29.4) |
| Dual therapy | 570 (48.5) | 252 (44.8) | 1689 (48.1) | 896 (50.6) |
| Anti-hypertensive therapy | 1127 (95.9) | 550 (97.7) | 3319 (94.5) | 1671 (94.4) |
| Angiotensin-converting enzyme inhibitor/angiotensin receptor blocker | 961 (81.8) | 452 (80.3) | 2837 (80.8) | 1416 (80.0) |
| Beta-blocker | 902 (76.8) | 434 (77.1) | 2154 (61.3) | 1064 (60.1) |
| Diuretic | 572 (48.7) | 281 (49.9) | 1475 (42.0) | 707 (39.9) |
| Calcium channel blocker | 359 (30.6) | 187 (33.2) | 1170 (33.3) | 601 (34.0) |
| Mineralocorticoid receptor antagonist | 96 (8.2) | 36 (6.4) | 209 (6.0) | 100 (5.6) |
| Renin inhibitor | 10 (0.9) | 5 (0.9) | 17 (0.5) | 14 (0.8) |
| Other | 88 (7.5) | 50 (8.9) | 295 (8.4) | 141 (8.0) |
| Lipid-lowering therapy | 1025 (87.2) | 501 (89.0) | 2795 (79.6) | 1363 (77.0) |
| Statin | 974 (82.9) | 481 (85.4) | 2656 (75.6) | 1292 (73.0) |
| Fibrate | 116 (9.9) | 50 (8.9) | 315 (9.0) | 149 (8.4) |
| Ezetimibe | 65 (5.5) | 26 (4.6) | 124 (3.5) | 55 (3.1) |
| Niacin | 40 (3.4) | 13 (2.3) | 51 (1.5) | 22 (1.2) |
| Other | 137 (11.7) | 69 (12.3) | 228 (6.5) | 106 (6.0) |
| Anticoagulant | 1098 (93.4) | 536 (95.2) | 3064 (87.2) | 1554 (87.8) |
| Acetylsalicylic acid | 1035 (88.1) | 496 (88.1) | 2841 (80.9) | 1431 (80.8) |
| Clopidogrel | 98 (8.3) | 48 (8.5) | 396 (11.3) | 201 (11.4) |
| Vitamin K antagonist | 79 (6.7) | 59 (10.5) | 187 (5.3) | 97 (5.5) |
| Systolic blood pressure, mmHg | 135.5 ± 17.0 | 136.1 ± 17.3 | 135.2 ± 16.9 | 135.7 ± 17.2 |
| Diastolic blood pressure, mmHg | 75.3 ± 9.8 | 75.2 ± 10.6 | 77.1 ± 9.7 | 77.3 ± 10.0 |
| Total cholesterol, mmol/le | 4.1 ± 1.0 | 4.0 ± 1.1 | 4.3 ± 1.2 | 4.2 ± 1.1 |
| LDL-cholesterol, mmol/lf | 2.1 ± 0.9 | 2.1 ± 0.9 | 2.3 ± 1.0 | 2.2 ± 0.9 |
| HDL-cholesterol, mmol/le | 1.1 ± 0.3 | 1.1 ± 0.3 | 1.2 ± 0.3 | 1.2 ± 0.3 |
| Triacylglycerol, mmol/le | 1.8 ± 1.1 | 1.9 ± 1.4 | 2.0 ± 1.6 | 1.9 ± 1.4 |
| eGFR (MDRD), ml min−1 1.73 m−2 | 69.8 ± 20.2 | 68.9 ± 20.1 | 75.6 ± 21.8 | 75.4 ± 21.1 |
| ≥90 | 183 (15.6) | 77 (13.7) | 867 (24.7) | 411 (23.2) |
| 60 to <90 | 612 (52.1) | 290 (51.5) | 1811 (51.6) | 948 (53.6) |
| <60 | 380 (32.3) | 196 (34.8) | 832 (23.7) | 411 (23.2) |
Data are n (%) or mean ± SD in participants treated with ≥1 dose of study drug
aInformation was not available for one participant in the placebo group
bInformation was not available for one participant in the placebo group and one participant in the empagliflozin group
cBased on the narrow standardised Medical Dictionary for Regulatory Activities (MedDRA) query ‘cardiac failure’
dInformation was not available for one participant in the empagliflozin group
eEmpagliflozin n = 1162 and placebo n = 555 for participants with a history of CABG; empagliflozin n = 3464 and placebo n = 1754 for participants without a history of CABG
fEmpagliflozin n = 1161 and placebo n = 555 for participants with a history of CABG; empagliflozin n = 3462 and placebo n = 1754 for participants without a history of CABG
CV, cardiovascular; n/a, not applicable
Background medication use was balanced between the empagliflozin and placebo groups in participants with and without CABG (Table 1). In individuals with a history of CABG, 96% were using any anti-hypertensive therapy (96% in the empagliflozin group and 98% in the placebo group), 81% were using angiotensin-converting enzyme inhibitor/angiotensin receptor blocker therapy (82% in the empagliflozin group and 80% in the placebo group) and 8% were using mineralocorticoid receptor antagonists (8% in the empagliflozin group and 6% in the placebo group). In addition, 84% of individuals were using statins (83% in the empagliflozin group and 85% in the placebo group), 94% were using antiplatelet/anticoagulant therapy (93% in the empagliflozin group and 95% in the placebo group) and 57% were taking insulin (57% in the empagliflozin group and 56% in the placebo group) (Table 1).
Cardiovascular outcomes, mortality and renal outcomes
In participants with a history of CABG at baseline, empagliflozin was associated with a 48% reduction in the risk of cardiovascular death (HR 0.52 [95% CI 0.32, 0.84]) (Figs 1 and 2a). This was consistent with the overall trial population (HR 0.62 [95% CI 0.49, 0.77]), and there was no significant difference between participants with and without a history of CABG at baseline (p = 0.3976 for treatment by subgroup interaction) (Fig. 1). Results appeared to be consistent across subgroups by time from CABG to randomisation (ESM Table 1). In participants with a history of CABG at baseline, empagliflozin reduced the risk of all-cause mortality by 43% (HR 0.57 [95% CI 0.39, 0.83]) (p = 0.2695 for interaction between subgroups of participants with and without CABG at baseline) (Figs 1 and 2b).
Fig. 1.
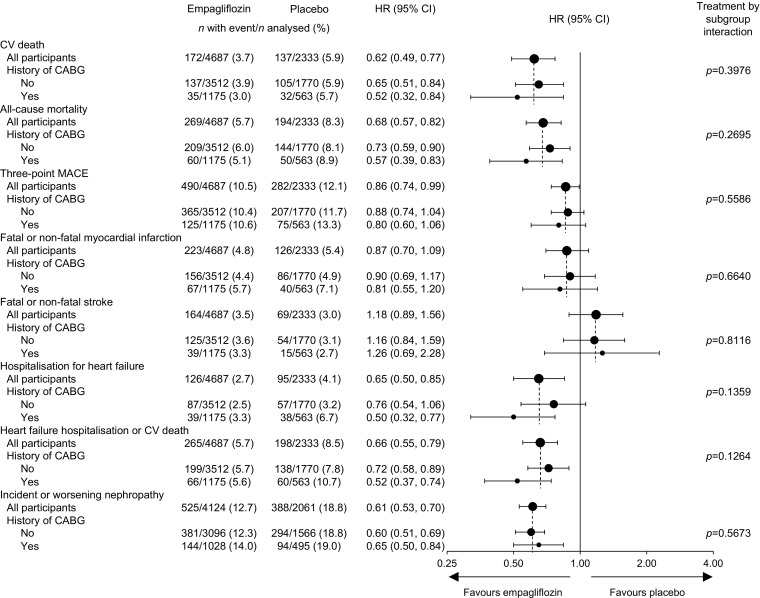
Cardiovascular outcomes, all-cause mortality and incident or worsening nephropathy by history of CABG surgery. Cox regression analysis in participants treated with ≥1 dose of study drug, except for incident or worsening nephropathy, which was analysed in participants who received ≥1 dose of study drug who did not have macroalbuminuria at baseline, had serum creatinine measurements at baseline and after baseline, and had post-baseline urine albumin-to-creatinine ratio measurements. Interaction p value is for test of homogeneity of treatment group difference among subgroups (test for treatment by subgroup interaction) with no adjustment for multiple tests. CV; cardiovascular
Fig. 2.
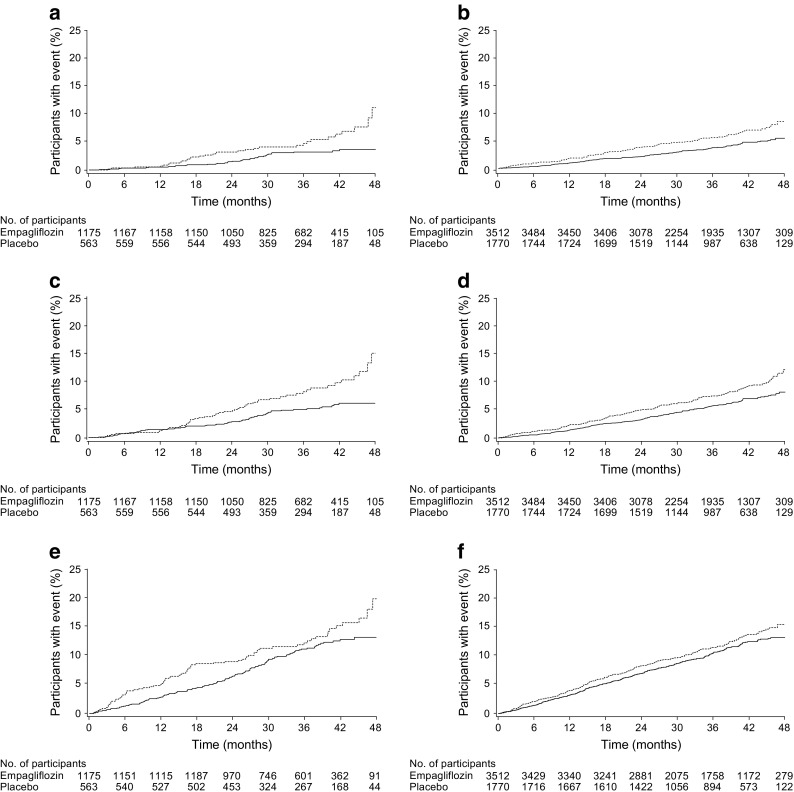
Time to cardiovascular death, all-cause mortality and three-point MACE by history of CABG. (a) Cardiovascular death in participants with a history of CABG (HR 0.52 [95% CI 0.32, 0.84]). (b) Cardiovascular death in participants without a history of CABG (HR 0.65 [95% CI 0.51, 0.84]). (c) All-cause mortality in participants with a history of CABG (HR 0.57 [95% CI 0.39, 0.83]). (d) All-cause mortality in participants without a history of CABG (HR 0.73 [95% CI 0.59, 0.90]). (e) Three-point MACE in participants with a history of CABG (HR 0.80 [95% CI 0.60, 1.06]). (f) Three-point MACE in participants without a history of CABG (HR 0.88 [95% CI 0.74, 1.04]). Kaplan–Meier estimates in participants treated with ≥1 dose of study drug. HR and 95% CI are based on Cox regression analyses. Solid line, empagliflozin; dashed line, placebo. No., number
Consistent with the overall trial population, the HR for three-point MACE with empagliflozin vs placebo was 0.80 (95% CI 0.60, 1.06) in participants with a history of CABG at baseline (Figs 1 and 2c). This was consistent with participants without a history of CABG (HR 0.88 [95% CI 0.74, 1.04]) (p = 0.5586 for treatment by subgroup interaction). There was no difference between empagliflozin and placebo in the risk of myocardial infarction or stroke in participants with or without a history of CABG (Fig. 1). The HR for four-point MACE with empagliflozin vs placebo was 0.89 (95% CI 0.68, 1.15) in participants with a history of CABG at baseline, and this was consistent with participants without a history of CABG (HR 0.89 [95% CI 0.76, 1.04]) (p = 0.9933 for treatment by subgroup interaction). Of participants with a history of CABG at baseline, a smaller proportion treated with empagliflozin (7.7%) than placebo (9.8%) underwent ≥1 coronary revascularisation procedure during the trial. Of participants who underwent coronary revascularisation, most had only one such procedure (ESM Table 2).
Consistent with the overall trial population, empagliflozin reduced the risk of hospitalisation for heart failure by 50% in participants with a history of CABG at baseline (HR 0.50 [95% CI 0.32, 0.77]). Risk reductions were consistent between participants with and without a history of CABG (p = 0.1359 for treatment by subgroup interaction) (Figs 1 and 3a). Consistent with the overall trial population, the HR for the composite of heart failure hospitalisation or cardiovascular death in participants with a history of CABG was 0.52 (95% CI 0.37, 0.74). Risk reductions were consistent between participants with and without a history of CABG (p = 0.1264 for treatment by subgroup interaction) (Fig. 1). Also consistent with the overall trial population, empagliflozin reduced the risk of incident or worsening nephropathy by 35% in participants with a history of CABG at baseline (HR 0.65 [95% CI 0.50, 0.84]). This was consistent between participants with and without a history of CABG (p = 0.5673 for interaction) (Figs 1 and 3b).
Fig. 3.
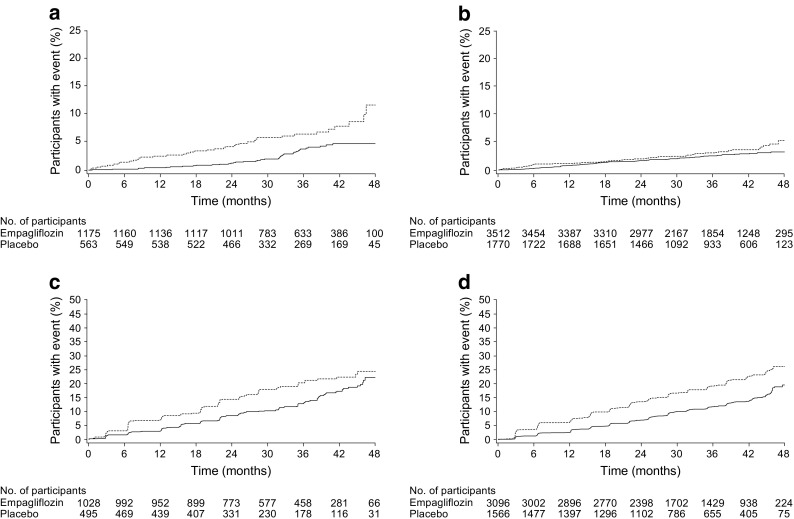
Time to hospitalisation for heart failure and incident or worsening nephropathy by history of CABG. (a) Time to hospitalisation for heart failure in participants with a history of CABG (HR 0.50 [95% CI 0.32, 0.77]). (b) Time to hospitalisation for heart failure in participants without a history of CABG (HR 0.76 [95% CI 0.54, 1.06]). (c) Incident or worsening nephropathy in participants with a history of CABG (HR 0.65 [95% CI 0.50, 0.84]). (d) Incident or worsening nephropathy in participants without a history of CABG (HR 0.60 [95% CI 0.51 0.69]). Kaplan–Meier estimates in participants treated with ≥1 dose of study drug, except for incident or worsening nephropathy, which was analysed in participants who received ≥1 dose of study drug who did not have macroalbuminuria at baseline, had serum creatinine measurements at baseline and after baseline and had post-baseline urine albumin-to-creatinine ratio measurements. HR and 95% CI are based on Cox regression analyses. Solid line, empagliflozin; dashed line, placebo. No., number
Adverse events
Adverse events are summarised in Table 2. Compared with placebo, the proportions of participants with serious adverse events or adverse events leading to discontinuation were lower or similar with empagliflozin in participants with and without a history of CABG. The proportions of participants with confirmed hypoglycaemic adverse events and hypoglycaemic adverse events requiring assistance were greater in participants with a history of CABG, but were similar between empagliflozin and placebo.
Table 2.
Adverse events
| Event | Participants with a history of CABG | Participants without a history of CABG | ||
|---|---|---|---|---|
| Empagliflozin (n = 1175) | Placebo (n = 563) | Empagliflozin (n = 3512) | Placebo (n = 1770) | |
| Adverse event | 1092 (92.9) | 532 (94.5) | 3138 (89.4) | 1607 (90.8) |
| Serious adverse eventa | 490 (41.7) | 286 (50.8) | 1299 (37.0) | 702 (39.7) |
| Adverse event leading to discontinuation of study drug | 215 (18.3) | 122 (21.7) | 598 (17.0) | 331 (18.7) |
| Confirmed hypoglycaemic adverse eventb | 409 (34.8) | 208 (36.9) | 894 (25.5) | 442 (25.0) |
| Requiring assistance | 23 (2.0) | 11 (2.0) | 40 (1.1) | 25 (1.4) |
| Adverse event consistent with urinary tract infectionc | 187 (15.9) | 78 (13.9) | 655 (18.7) | 345 (19.5) |
| Adverse event consistent with genital infectiond | 91 (7.7) | 12 (2.1) | 210 (6.0) | 30 (1.7) |
| Adverse event consistent with volume depletione | 80 (6.8) | 36 (6.4) | 159 (4.5) | 79 (4.5) |
| Acute renal failuref | 68 (5.8) | 62 (11.0) | 178 (5.1) | 93 (5.3) |
| Acute kidney injury | 14 (1.2) | 21 (3.7) | 31 (0.9) | 16 (0.9) |
| Thromboembolic eventf | 9 (0.8) | 6 (1.1) | 21 (0.6) | 14 (0.8) |
| Bone fractureg | 49 (4.2) | 24 (4.3) | 130 (3.7) | 67 (3.8) |
| Lower limb amputationh | 27 (2.3) | 14 (2.5) | 61 (1.7) | 29 (1.6) |
Data are n (%) of participants treated with ≥1 dose of study drug in whom ≥1 such adverse event was reported. Participants can be included in ≥1 category. Events that occurred during treatment or ≤7 days after the last dose of study drug are presented
aDefined as an adverse event that resulted in death, was immediately life-threatening, resulted in persistent or marked disability/incapacity, required or prolonged participant hospitalisation, was a congenital anomaly/birth defect or was deemed serious for any other reason
bPlasma glucose ≤3.39 mmol/l and/or requiring assistance
cBased on 79 Medical Dictionary for Regulatory Activities (MedDRA) preferred terms
dBased on 88 MedDRA preferred terms
eBased on eight MedDRA preferred terms
fBased on one standardised MedDRA query
gBased on 62 MedDRA preferred terms
hIdentified from events reported as adverse events, those reported as a ‘medical procedure’ in electronic case report forms or in investigator comments describing adverse events, and via a systematic search of serious adverse event narratives
A greater proportion of participants treated with empagliflozin than placebo had adverse events consistent with genital infections. The proportion of participants with adverse events consistent with volume depletion was greater in participants with a history of CABG, but was similar between empagliflozin and placebo. In participants with a history of CABG, the proportion of participants with acute renal failure (including acute kidney injury) was lower in the empagliflozin group (5.8%) than the placebo group (11.0%) (although statistical tests were not performed). The proportion of participants with lower limb amputations was balanced between the empagliflozin and placebo groups in participants with and without CABG.
Discussion
In this post hoc subanalysis of data from the EMPA-REG OUTCOME® trial, we report findings in participants with type 2 diabetes and a history of CABG that are consistent with those in the overall trial population [8, 14]. In participants with a history of CABG, cardiovascular death was reduced by 48%, all-cause mortality by 43%, hospitalisation for heart failure by 50% and incident or worsening nephropathy by 35% with empagliflozin vs placebo.
In addition to a greater burden of atherosclerosis, diabetes predisposes individuals to diffuse coronary lesions that are more vulnerable and often not amenable to complete revascularisation [15]. People with diabetes also have specific myocardial defects and are known to exhibit high rates of diastolic dysfunction, placing them at high risk of heart failure [6, 16, 17]. Although much attention has focused on reducing macrovascular events through antiplatelet, antithrombotic and cholesterol-lowering approaches, limited success has been achieved in the prevention of heart failure and mortality following CABG in individuals with type 2 diabetes. Our analyses illuminate the benefits of empagliflozin in individuals with type 2 diabetes and a history of CABG, demonstrating clinically important reductions in important adverse cardiovascular and renal outcomes in this high-risk population when empagliflozin was given in addition to standard of care.
Several mechanisms have been suggested to underlie the beneficial effects of empagliflozin on heart failure and cardiovascular death [18, 19]. As an SGLT2 inhibitor, empagliflozin promotes renal glycosuria and natriuresis [20, 21]. Whereas glycosuria leads to an improvement in glycaemic control, it has been proposed that the ensuing natriuresis and osmotic diuresis reduces preload and ventricular stress [18, 22]. Individuals with type 2 diabetes and cardiovascular disease have a higher body sodium content and, in many instances, are in pre-clinical heart failure or volume overload; thus, recalibration of sodium/volume balance may improve outcomes. In a cohort of ten post-CABG participants with type 2 diabetes, empagliflozin reduced indices of diastolic dysfunction within a 3 month period [23]. In non-clinical models, empagliflozin has been shown to prevent worsening of cardiac failure [24, 25]. Empagliflozin may also improve myocardial energetics through increasing ketone production, yielding ATP at a more efficient rate than other substrates, although this concept remains to be proven [26]. Further, empagliflozin may have SGLT2-independent effects that attenuate myocardial sodium-hydrogen exchange, promoting calcium efflux through cardiomyocytes [27]. The renal benefits of empagliflozin appear to stem from natriuresis-induced tubuloglomerular feedback, which is believed to cause afferent arteriolar vasoconstriction, reducing intraglomerular hypertension [28–30]. Large clinical studies will examine the role of empagliflozin in the treatment of cardiac failure both in participants with reduced and preserved ejection fraction (NCT03057977, NCT03057951) and in the treatment of participants with chronic kidney disease [31].
Guidelines published by the American Heart Association highlight that the majority of evidence for secondary prevention in individuals who have undergone CABG has been derived and/or extrapolated from studies of broader populations of participants with coronary disease [32]. Very few subanalyses of large trials examining subgroups of participants after CABG are available to help guide practice. The only Class IIa recommendation (level of evidence B) that exists for people who have had CABG with respect to diabetes is to strive to achieve an HbA1c of 53 mmol/mol (7%) [32]. There are no specific recommendations on the type of glucose-lowering agents that should be used. Our data provide additional information to help guide clinicians about the choice of glucose-lowering agent for secondary prevention in individuals with type 2 diabetes after CABG. From a practical point of view, surgeons are to be reminded that SGLT2 inhibitors are not indicated in individuals with type 1 diabetes, although trials are ongoing to establish the benefit:risk in this population. Moreover, although hypoglycaemia does not occur with these agents per se, they may increase hypoglycaemia when combined with a sulfonylurea or insulin. Thus, in certain clinical scenarios, a collaborative approach between surgeons and endocrinologists or primary care clinicians will be required. Empagliflozin was not associated with an increased risk of lower limb amputation in participants with or without a history of CABG, similar to observations in the overall trial population [33] and in subgroups by peripheral artery disease at baseline [34].
The EMPA-REG OUTCOME® trial had several strengths. It was a large international trial in which participants received the study drug in addition to standard of care. Cardiovascular events and deaths were adjudicated by blinded committees. Vital status was available for 99% of participants [8]. Participants were identified as having CABG at baseline based directly on case report forms. However, a few limitations to our findings in CABG subgroups must be acknowledged. These analyses were post hoc. Data specific to surgical revascularisation (e.g. number of grafts, use of arterial or venous grafts, on- or off-pump) are not available, nor are biomarker data (e.g. B-type natriuretic peptide). No echocardiograms were performed and baseline indices of left ventricular systolic or diastolic function are unknown. Our analyses were not adjusted for changes in background glucose-lowering therapy or cardiovascular medications.
Conclusions
In participants with type 2 diabetes and a history of CABG, empagliflozin given in addition to standard of care was associated with significant reductions in cardiovascular and all-cause mortality, hospitalisation for heart failure and incident or worsening nephropathy compared with placebo. The relative risk reductions with empagliflozin were consistent with those observed in the overall EMPA-REG OUTCOME® trial population. Absolute risk reductions, particularly for hospitalisation for heart failure, were numerically greater in participants with a history of CABG, reflecting the higher event rates in these participants (although no statistical tests were performed to compare absolute risk reductions). These data have important implications for secondary prevention pharmacotherapy after CABG in individuals with type 2 diabetes.
Electronic supplementary material
(PDF 153 kb)
Acknowledgements
Medical writing assistance, supported financially by Boehringer Ingelheim, was provided by E. Ng and W. Morris (FleishmanHillard Fishburn, London, UK) during the preparation of this article. The authors were fully responsible for all content and editorial decisions and were involved at all stages of manuscript development and have approved the final version.
Abbreviations
- CABG
Coronary artery bypass graft
- eGFR
estimated GFR
- FDA
Food and Drug Administration
- MACE
Major adverse cardiovascular events
- MDRD
Modification of diet in renal disease
- PCI
Percutaneous coronary intervention
- SGLT2
Sodium–glucose cotransporter 2
Contribution statement
SV contributed to the analysis planning and interpretation of data, drafted the article and approved the final version. EP contributed to the analysis planning and interpretation of data, revised the article critically for important intellectual content and approved the final version. CDM, DF, SEI, JTG and BZ contributed to the interpretation of data, revised the article critically for important intellectual content and approved the final version. The authors were fully responsible for all content and editorial decisions and were involved at all stages of manuscript development and have approved the final version. EP is the guarantor of this work.
Funding
The EMPA-REG OUTCOME® trial was funded by the Boehringer Ingelheim and Eli Lilly and Company Diabetes Alliance. Boehringer Ingelheim had a role in study design, data collection, data analysis, data interpretation, and writing of the report. Eli Lilly and Company had no role in study design, data collection, data analysis, data interpretation or writing of the report.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Duality of interest
SV has received research grants and/or speaking honoraria from Boehringer Ingelheim, Eli Lilly, AstraZeneca, Janssen, Merck, Amgen, Sanofi, Valeant, Bayer, Pfizer and Valeant, holds a tier 1 Canada Research Chair, is national lead investigator for the DAPA-HF trial (Study to Evaluate the Effect of Dapagliflozin on the Incidence of Worsening Heart Failure or Cardiovascular Death in Patients with Chronic Heart Failure), the EMPagliflozin outcomE tRial in Patients With chrOnic heaRt failure with Reduced ejection fraction (EMPEROR-Reduced) and the EMPagliflozin outcomE tRial in Patients With chrOnic heaRt failure with Preserved ejection fraction (EMPEROR-Preserved), and is a member of the steering committees of the Cardiovascular Inflammation Reduction Trial (CIRT) and the Behavior of Valve Leaflets (BELIEVE) trial. CDM has received consulting fees from Amgen, Boehringer Ingelheim and OctaPharma. DF reports honoraria from Sanofi, Merck & Co., Amgen, AstraZeneca, Eli Lilly and Company and Boehringer Ingelheim. SEI has consulted for Janssen, vTv Therapeutics and Alere, served on Clinical Trial Steering/Executive Committees for Boehringer Ingelheim, AstraZeneca, Novo Nordisk, Sanofi/Lexicon Pharmaceuticals, Daiichi-Sankyo and Eisai (Thrombolysis in Myocardial Infarction [TIMI]) and served on Data Monitoring Committees for Intarcia Therapeutics, Inc. BZ has received research grants awarded to his institution from Boehringer Ingelheim, AstraZeneca and Novo Nordisk, and honoraria from Janssen, Sanofi, Eli Lilly and Company, Boehringer Ingelheim, Novo Nordisk and Merck. EP and JTG are employees of Boehringer Ingelheim.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s00125-018-4644-9) contains peer-reviewed but unedited supplementary material, which is available to authorised users.
References
- 1.Rana JS, Dunning A, Achenbach S, et al. Differences in prevalence, extent, severity, and prognosis of coronary artery disease among patients with and without diabetes undergoing coronary computed tomography angiography: results from 10,110 individuals from the CONFIRM (COronary CT Angiography EvaluatioN For Clinical Outcomes): an InteRnational Multicenter Registry. Diabetes Care. 2012;35:1787–1794. doi: 10.2337/dc11-2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verma S, Farkouh ME, Yanagawa B, et al. Comparison of coronary artery bypass surgery and percutaneous coronary intervention in patients with diabetes: a meta-analysis of randomised controlled trials. Lancet Diabetes Endocrinol. 2013;1:317–328. doi: 10.1016/S2213-8587(13)70089-5. [DOI] [PubMed] [Google Scholar]
- 3.Ryden L, Grant PJ, Anker SD, et al. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD) Eur Heart J. 2013;34:3035–3087. doi: 10.1093/eurheartj/eht108. [DOI] [PubMed] [Google Scholar]
- 4.Farkouh ME, Domanski M, Sleeper LA, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367:2375–2384. doi: 10.1056/NEJMoa1211585. [DOI] [PubMed] [Google Scholar]
- 5.Kappetein AP, Head SJ, Morice MC, et al. Treatment of complex coronary artery disease in patients with diabetes: 5-year results comparing outcomes of bypass surgery and percutaneous coronary intervention in the SYNTAX trial. Eur J Cardiothorac Surg. 2013;43:1006–1013. doi: 10.1093/ejcts/ezt017. [DOI] [PubMed] [Google Scholar]
- 6.Moreyra AE, Deng Y, Wilson AC, Cosgrove NM, Kostis WJ, Kostis JB. Incidence and trends of heart failure admissions after coronary artery bypass grafting surgery. Eur J Heart Fail. 2013;15:46–53. doi: 10.1093/eurjhf/hfs154. [DOI] [PubMed] [Google Scholar]
- 7.U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research. Guidance for Industry. Diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. Available from www.fda.gov/downloads/Drugs/Guidances/ucm071627.pdf. Accessed 9 Jan 2018
- 8.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 9.Boehringer Ingelheim. Jardiance® (empagliflozin) tablets prescribing information. Available from http://bidocs.boehringer-ingelheim.com/BIWebAccess/ViewServlet.ser?docBase=renetnt&folderPath=/Prescribing+Information/PIs/Jardiance/jardiance.pdf. Accessed 9 Jan 2018
- 10.American Diabetes Association (2017) Pharmacologic approaches to glycemic treatment. Sec. 8. In Standards of Medical Care in Diabetes—2017. Diabetes Care 40(Suppl 1):S64–S74 [DOI] [PubMed]
- 11.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee Pharmacologic management of type 2 diabetes: 2016 interim update. Can J Diabetes. 2016;40:484–486. doi: 10.1016/j.jcjd.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Okrainec K, Platt R, Pilote L, Eisenberg MJ. Cardiac medical therapy in patients after undergoing coronary artery bypass graft surgery: a review of randomized controlled trials. J Am Coll Cardiol. 2005;45:177–184. doi: 10.1016/j.jacc.2004.09.065. [DOI] [PubMed] [Google Scholar]
- 13.Rouleau JL, Warnica WJ, Baillot R, et al. Effects of angiotensin-converting enzyme inhibition in low-risk patients early after coronary artery bypass surgery. Circulation. 2008;117:24–31. doi: 10.1161/CIRCULATIONAHA.106.685073. [DOI] [PubMed] [Google Scholar]
- 14.Fitchett D, Zinman B, Wanner C, et al. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME® trial. Eur Heart J. 2016;37:1526–1534. doi: 10.1093/eurheartj/ehv728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mancini GBJ, Farkouh M, Verma S. Revascularization for patients with diabetes mellitus and stable ischemic heart disease: an update. Curr Opin Cardiol. 2017;32:608–616. doi: 10.1097/HCO.0000000000000421. [DOI] [PubMed] [Google Scholar]
- 16.Seferovic PM, Paulus WJ. Clinical diabetic cardiomyopathy: a two-faced disease with restrictive and dilated phenotypes. Eur Heart J. 2015;36:1718–1727. doi: 10.1093/eurheartj/ehv134. [DOI] [PubMed] [Google Scholar]
- 17.Ledru F, Ducimetiere P, Battaglia S, et al. New diagnostic criteria for diabetes and coronary artery disease: insights from an angiographic study. J Am Coll Cardiol. 2001;37:1543–1550. doi: 10.1016/S0735-1097(01)01183-4. [DOI] [PubMed] [Google Scholar]
- 18.Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016;134:752–772. doi: 10.1161/CIRCULATIONAHA.116.021887. [DOI] [PubMed] [Google Scholar]
- 19.Verma S, McMurray JJV, Cherney DZI. The metabolodiuretic promise of sodium-dependent glucose cotransporter 2 inhibition: the search for the sweet spot in heart failure. JAMA Cardiol. 2017;2:939–940. doi: 10.1001/jamacardio.2017.1891. [DOI] [PubMed] [Google Scholar]
- 20.Heise T, Jordan J, Wanner C, et al. Acute pharmacodynamic effects of empagliflozin with and without diuretics in patients with type 2 diabetes. Clin Ther. 2016;38:2248–2264. doi: 10.1016/j.clinthera.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 21.Heise T, Jordan J, Wanner C, et al. Pharmacodynamic effects of single and multiple doses of empagliflozin in patients with type 2 diabetes. Clin Ther. 2016;38:2265–2276. doi: 10.1016/j.clinthera.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Sattar N, McLaren J, Kristensen SL, Preiss D, McMurray JJ. SGLT2 inhibition and cardiovascular events: why did EMPA-REG outcomes surprise and what were the likely mechanisms? Diabetologia. 2016;59:1333–1339. doi: 10.1007/s00125-016-3956-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verma S, Garg A, Yan AT, et al. Effect of empagliflozin on left ventricular mass and diastolic function in individuals with diabetes: an important clue to the EMPA-REG OUTCOME trial? Diabetes Care. 2016;39:e212–e213. doi: 10.2337/dc16-1312. [DOI] [PubMed] [Google Scholar]
- 24.Byrne NJ, Parajuli N, Levasseur JL, et al. Empagliflozin prevents worsening of cardiac function in an experimental model of pressure overload-induced heart failure. JACC Basic Transl Sci. 2017;2:347–354. doi: 10.1016/j.jacbts.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi X, Verma S, Yun J, et al. Effect of empagliflozin on cardiac biomarkers in a zebrafish model of heart failure: clues to the EMPA-REG OUTCOME trial? Mol Cell Biochem. 2017;433:97–102. doi: 10.1007/s11010-017-3018-9. [DOI] [PubMed] [Google Scholar]
- 26.Lopaschuk GD, Verma S. Empagliflozin’s fuel hypothesis: not so soon. Cell Metab. 2016;24:200–202. doi: 10.1016/j.cmet.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 27.Baartscheer A, Schumacher CA, Wust RC, et al. Empagliflozin decreases myocardial cytoplasmic Na+ through inhibition of the cardiac Na+/H+ exchanger in rats and rabbits. Diabetologia. 2017;60:568–573. doi: 10.1007/s00125-016-4134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–334. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 29.Cherney DZI, Zinman B, Inzucchi SE, et al. Effects of empagliflozin on the urinary albumin-to-creatinine ratio in patients with type 2 diabetes and established cardiovascular disease: an exploratory analysis from the EMPA-REG OUTCOME randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017;5:610–621. doi: 10.1016/S2213-8587(17)30182-1. [DOI] [PubMed] [Google Scholar]
- 30.Cherney DZ, Perkins BA, Soleymanlou N, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014;129:587–597. doi: 10.1161/CIRCULATIONAHA.113.005081. [DOI] [PubMed] [Google Scholar]
- 31.Boehringer Ingelheim. Empagliflozin (Jardiance®) to be studied in chronic kidney disease. Available from www.boehringer-ingelheim.com/press-release/empagliflozin-be-studied-chronic-kidney-disease. Accessed 9 Jan 2018
- 32.Kulik A, Ruel M, Jneid H, et al. Secondary prevention after coronary artery bypass graft surgery: a scientific statement from the American Heart Association. Circulation. 2015;131:927–964. doi: 10.1161/CIR.0000000000000182. [DOI] [PubMed] [Google Scholar]
- 33.Inzucchi SE, Iliev H, Pfarr E, Zinman B. Empagliflozin and assessment of lower-limb amputations in the EMPA-REG OUTCOME trial. Diabetes Care. 2018;41:e4–e5. doi: 10.2337/dc17-1551. [DOI] [PubMed] [Google Scholar]
- 34.Verma S, Mazer CD, Al-Omran M, et al. Cardiovascular outcomes and safety of empagliflozin in patients with type 2 diabetes mellitus and peripheral artery disease: a subanalysis of EMPA-REG OUTCOME. Circulation. 2018;137:405–407. doi: 10.1161/CIRCULATIONAHA.117.032031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 153 kb)
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.


