Abstract
Objective
The aim of this study was to establish a reliable and routine method for the preparation of 4-[10B]borono-2-[18F]fluoro-l-phenylalanine (l-[18F]FBPA) for boron neutron capture therapy-oriented diagnosis using positron emission tomography.
Methods
To produce l-[18F]FBPA by electrophilic fluorination of 4-[10B]borono-l-phenylalanine (l-BPA) with [18F]acetylhypofluorite ([18F]AcOF) via [18F]F2 derived from the 20Ne(d,α)18F nuclear reaction, several preparation parameters and characteristics of l-[18F]FBPA were investigated, including: pre-irradiation for [18F]F2 production, the carrier F2 content in the Ne target, l-BPA-to-F2 ratios, separation with high-performance liquid chromatography (HPLC) using 10 different eluents, enantiomeric purity, and residual trifluoroacetic acid used as the reaction solvent by gas chromatography-mass spectrometry.
Results
The activity yields and molar activities of l-[18F]FBPA (n = 38) were 1200 ± 160 MBq and 46–113 GBq/mmol, respectively, after deuteron-irradiation for 2 h. Two 5 min pre-irradiations prior to [18F]F2 production for 18F-labeling were preferable. For l-[18F]FBPA synthesis, 0.15–0.2% of carrier F2 in Ne and l-BPA-to-F2 ratios > 2 were preferable. HPLC separations with five of the 10 eluents provided injectable l-[18F]FBPA without any further formulation processing, which resulted in a synthesis time of 32 min. Among the five eluents, 1 mM phosphate-buffered saline was the eluent of choice. The l-[18F]FBPA injection was sterile and pyrogen-free, and contained very small amounts of D-enantiomer (< 0.1% of l-[18F]FBPA), l-BPA (< 1% of l-FBPA), and trifluoroacetic acid (< 0.5 ppm).
Conclusions
l-[18F]FBPA injection was reliably prepared by the electrophilic fluorination of l-BPA with [18F]AcOF followed by HPLC separation with 1 mM phosphate-buffered saline.
Keywords: l-[18F]FBPA, [18F]F2 production, Quality control, PET, BNCT
Introduction
4-[10B]borono-2-[18F]fluoro-l-phenylalanine (l-[18F]FBPA) was developed in 1991 as a probe for positron emission tomography (PET) to evaluate in vivo 4-[10B]borono-l-phenylalanine (l-BPA) used in boron neutron capture therapy (BNCT) for patients with malignant tumors [1]. Based on several basic studies that verified the usefulness of l-[18F]FBPA [2–5], l-[18F]FBPA PET has been clinically applied for this purpose [6–9] and expanded in limited numbers of PET facilities over the past 20 years [10–13], mainly because BNCT is performed using a nuclear reactor for neutron irradiation. Recently, a cyclotron that acts as an epithermal-neutron source for BNCT and that can be installed in the hospitals has been developed [14, 15]. Phase I and II clinical trials of l-BPA BNCT using this cyclotron are in progress at two institutes in Japan, including the Southern Tohoku BNCT Research Center at the Southern TOHOKU Research Institute for Neuroscience. Therefore, the importance of l-[18F]FBPA PET is increasing, and further basic studies on the characterization of l-[18F]FBPA have been reported in recent years [15–23].
l-[18F]FBPA has been synthesized by the electrophilic fluorination of l-BPA with carrier-added [18F]F2 or [18F]acetylhypofluorite ([18F]AcOF) produced by three different routes. [18F]F2 was originally produced by deuteron irradiation of carrier F2-containing Ne, termed the 20Ne(d,α)18F nuclear reaction [1]. However, the activity yields of [18F]F2 and the resultant l-[18F]FBPA were low. For example, Wang et al. prepared 444–518 MBq of l-[18F]FBPA from 5.55 GBq of [18F]F2 after deuteron irradiation for 2 h [24]. The molar activity of l-[18F]FBPA was also low because of carrier F2: 30–60 MBq/µmol [1]. The second and third routes used the 18O(p,n)18F nuclear reaction for carrier-added [18F]F2 production. In the former, [18F]F2 was produced by proton irradiation of highly enriched [18O]O2 gas followed by a second proton irradiation step for the release of [18F]F2 [25, 26]. In the latter, [18F]fluoride produced by proton irradiation of [18O]H2O was converted to [18F]F2 via [18F]fluoromethane [27]. The 18O(p,n)18F reaction can produce potentially large amounts of 18F compared with the 20Ne(d,α)18F reaction; therefore, the activity yields of l-[18F]FBPA synthesized via the second route (2 GBq [25] to 5.3 GBq [26]), and the molar activity (257 MBq/µmol [26]) has been improved. The third route was especially devised to give less carrier-added [18F]F2. Consequently, the molar activity of l-[18F]FBPA was the highest (3700 MBq/µmol), but the activity yields have not been clearly described [28].
In the future, the synthesis of l-[18F]FBPA by nucleophilic fluorination using no-carrier-added [18F]fluoride will be developed to obtain higher activity yields and higher molar activities of l-[18F]FBPA, as the synthesis of 2-deoxy-2-[18F]fluoro-d-glucose has progressed from the method using [18F]F2 to that using no-carrier-added [18F]fluoride. However, at present such radiosynthesis is still under development, although a preliminary synthesis was reported recently [29].
At the present stage of l-[18F]FBPA PET for l-BPA BNCT-oriented diagnosis, l-[18F]FBPA PET is applied to only a few patients per l-[18F]FBPA preparation and not to mass screening; therefore, a steady and reliable synthesis of l-[18F]FBPA is required. The molar activity of l-[18F]FBPA is not a critical issue. Among the three methods of l-[18F]FBPA synthesis described previously, Ne-derived [18F]F2 is produced simply and cost-effectively compared with 18O-derived [18F]F2. Therefore, the original method of l-[18F]FBPA synthesis has been adapted clinically to date; however, detailed procedures, the optimization of each process, and technical knowhow have not been described sufficiently in previous reports on the three methods [1, 8, 24–26, 28]. In the present study, we aimed to elaborate on the original method from the viewpoint of routine clinical use.
For this purpose, steady production of [18F]F2 is first essential. It is well known empirically that a short pre-irradiation step is essential before the main irradiation for 18F-labeling; however, systematic studies on [18F]F2 production have not been reported. A larger amount of carrier F2 in Ne target, for example 0.5% F2, has a benefit for the steady production of [18F]F2 but provides low molar activity, and the stoichiometric relationship in the fluorination of l-BPA with [18F]F2/[18F]AcOF should be considered carefully. For electrophilic fluorination of l-BPA, the original method used [18F]AcOF due to its higher selectivity than [18F]F2 [1], whereas the second and third methods employed [18F]F2 probably to avoid activity loss of activity during the conversion process from [18F]F2 to [18F]AcOF [25–27]. Regarding the formulation of l-[18F]FBPA, the most popular preparation method is purification by high-performance liquid chromatography (HPLC) using a reversed-phase column with 0.1% aqueous AcOH as the mobile phase followed by evaporation of the l-[18F]FBPA fraction and re-dissolution in physiological saline [1]. To avoid this time-consuming evaporation process, Vähätalo et al. separated l-[18F]FBPA by HPLC using physiological saline containing 1–2% EtOH and 0.01% AcOH as the eluent, and the l-[18F]FBPA fraction was used directly for injection in clinical studies [27]; however, the pH of this injection was not described, although it appeared to be below 4. Prior to this, Ishiwata et al. proposed HPLC separation with physiological saline alone without clinical use [30].
In the present study, we investigated (1) the importance of pre-irradiation for [18F]F2 production with an appropriate F2 carrier, (2) steady production of l-[18F]FBPA in relation to F2 content and l-BPA, (3) HPLC separation methods to provide injectable l-[18F]FBPA without any further formulation processing, (4) the optical purity of l-[18F]FBPA, and (5) analysis of residual trifluoroacetic acid (TFA) used as a solvent in radiosynthetic preparation of l-[18F]FBPA. To the best of our knowledge, no report on points (4) and (5) has been published previously. Findings for the three other points would also provide useful information for the radiosynthesis of l-[18F]FBPA using 18O-derived [18F]F2.
Labeled compounds and related terms are expressed according to the International Consensus Radiochemistry Nomenclature Guidelines recently recommended by an international Working Group on ‘Nomenclature in Radiopharmaceutical Chemistry and related areas’ [31, 32].
Materials and methods
l-BPA was purchased from Sigma-Aldrich Chemical (St Louis, MO). l-BPA, l-FBPA, and d-FBPA were kindly supplied by Stella Pharma (Osaka, Japan). 2-, 3-, and 4-fluoro-d,l-phenylalanine (2-, 3-, and 4-FPhe, respectively) were purchased from Tokyo Chemical Industry (Tokyo, Japan). Normal saline (500 and 1000 mL plastic bag), distilled water (20 ml plastic ampule and 500 ml plastic bottle) for injection, and sodium phosphate corrective injection 0.5 mmol/ml (pH 6.5, 20 ml plastic ampule) were purchased from Otsuka Pharmaceutical (Tokyo, Japan). Other chemical reagents were obtained from commercial sources.
Production of [18F]F2
An 18 MeV cyclotron (CYPRIS HM-18, 18 MeV protons and 9 MeV deuterons, Sumitomo Heavy Industries, Tokyo, Japan) was employed. Elemental [18F]F2 was produced via the 20Ne(d,α)18F reaction in Ne containing F2 in a cylindrical target chamber [30 mm inner diameter (i.d.) and 242 mm length] made of aluminum. The incident deuteron energy was 7.9 MeV. All deuteron irradiation processes were performed at a fixed current of 20 µA.
Conditioning production of [18F]F2
To determine a suitable pre-irradiation protocol before the main [18F]F2 production for 18F-labeling, three or four successive 5 min irradiations were conducted within 1–39 day intervals. The content of F2 in Ne was set in the same range in each experiment: 0.1% (v/v) (n = 7), 0.15% (n = 7), and 0.2% (n = 5) by mixing 5% F2-containing Ne and pure Ne gases. The final pressure was set at 380 kPa. However, the actual F2 concentrations calculated from the pressure were determined to have certain ranges. After the end of the 5 min irradiation, the [18F]F2 produced was recovered with a maximum flow rate by the target pressure and absorbed into a tandem column of soda lime (No.1, Wako Pure Chemicals, Osaka, Japan, 9 mm i.d. × 80 mm length) and activated charcoal (Granular, Wako Pure Chemicals, 9 mm i.d. × 80 mm length). The average flow rates from maximum pressure (ca. 380 kPa) to 100 kPa were in range of 756–845 ml/min. The activity absorbed in the tandem columns was estimated as the total activity recovered from [18F]F2 production, and corrected for decay to the end of cyclotron bombardment (EOB).
Synthesis of l-[18F]FBPA
l-[18F]FBPA (total 38 runs) was prepared by electrophilic fluorination with [18F]AcOF using a multipurpose synthesizer (CFN-MPS200, Sumitomo Heavy Industries) using a method that was a slightly modified method from previous reports [33, 34]. Two conditioning 5 min irradiations were performed, and a main irradiation for 18F-labeling was performed for 90–156 min (123 ± 17 min). In all three irradiations, the content of F2 in Ne was set at the same percentage of 0.10–0.30% (30–89 µmol: 0.10%, n = 3; 0.15%, n = 8; 0.20%, n = 23; 0.25%, n = 2; and 0.30%, n = 2). The [18F]F2 produced was passed through a column containing sodium acetate trihydrate or sodium acetate anhydrous (4 mm i.d. × 40 mm length), and the resultant [18F]AcOF was bubbled into 4 ml TFA containing l-BPA at room temperature with maximal flow rates: 426 ± 68 ml/min from the target pressure (ca. 380 kPa) to 100 kPa. To examine the effect of the l-BPA-to-F2 ratios on l-[18F]FBPA synthesis, the amount of l-BPA was varied in the range 14.2–33.3 mg (68–160 µmol: 14.2–14.8 mg, n = 2; 18.5 mg, n = 1; 25.1–25.3 mg, n = 3; and 29.3–33.3 mg, n = 32). The total (100%) recovered from [18F]F2 production was estimated as the summed activities of the sodium acetate column and the TFA solution. The radioactivity sensor using to monitor a reaction vial containing the TFA solution was calibrated according to the standard 18F-activity measured with a dose calibrator (CRC-15 PET, Capintec, Florham Park, NJ, USA).
The TFA solution was heated to 120 °C, and the TFA was removed using a 200 ml/min N2 flow. The residue was dissolved in 2 ml of the eluent used for preparative HPLC (described below). The solution was applied to HPLC separation, and the fraction with l-[18F]FBPA was obtained. The volumes of the l-[18F]FBPA fractions were estimated by weight (1.0 g = 1.0 ml), and the pH was measured using a pH meter (Laqua act, Horiba Scientific, Tokyo, Japan). In four of 38 runs, the l-[18F]FBPA fraction was collected through a 0.22 µm membrane filter (SLGVJ33RS, Merck Millipore, Darmstadt, Germany) for clinical purposes, and the sterility and apyrogenicity were examined. Filter integrity (> 150 kPa) was evaluated using a Millex/Sterivex integrity tester (Merck Millipore). The activity yields of l-[18F]FBPA at the end of synthesis (EOS) were normalized with respect to those produced by irradiation for 120 min.
HPLC separation of l-[18F]FBPA
The column used for HPLC separation of l-[18F]FBPA was YMC-Pack ODS-A (S-5 µm, 20 nm, 20 mm i.d. × 150 mm length, YMC, Kyoto, Japan) with a guard cartridge ODS-A (S-5 µm, 12 nm, 20 mm i.d. × 10 mm, YMC). The 10 different mobile phases investigated are summarized in Table 1 (eluents 1–10). Eluents of 10 and 5 mM phosphate-buffered saline (PBS) were prepared by mixing normal saline, sodium phosphate corrective injection 0.5 mmol/ml (pH 6.5), and distilled water to an isotonic ion strength of 0.15 mEq/ml. Eluent containing 1 mM PBS was prepared by adding 1/500 volume of sodium phosphate corrective injection 0.5 mmol/ml (pH 6.5) into normal saline. The flow rate was 10 ml/min, and the elution profile was monitored using an ultraviolet (UV, 260 nm) detector (UV 2715 Plus, Jasco, Tokyo Japan) and a radioactivity monitor (UG-PD1A, Universal Giken, Odawara, Japan). First, in the separation with eluent 1, based on previous reports [1, 27], a major radioactive peak, and later other minor radioactive peaks and shoulder components were fractionated, l-[18F]FBPA and three by-products of 2-, 3-, and 4-[18F]fluoro-l-phenylalanine were identified by comparison of their retention times with those of the authentic compounds (enantiomeric mixtures in the case of fluorophenylalanines) in the HPLC analysis described below.
Table 1.
Characteristics of 4-[10B]borono-2-[18F]fluoro-l-phenylalanine (l-[18F]FBPA) preparations separated by high-performance liquid chromatography using 10 different eluents
| Eluent | n | Volume | l-[18F]FBPAa | RCPb | l-BPAc | pH | |
|---|---|---|---|---|---|---|---|
| mL | GBq | GBq/mmol | % | % | |||
| (1) 0.1% AcOH water | 3 | 16.2 ± 1.8 | 1.21 ± 0.04 | 71.4 ± 7.2 | 97.2 ± 1.1 | 0.97 ± 0.36 | 3.3 ± 0.3 |
| (2) 0.01% AcOH water | 4 | 11.9 ± 1.2 | 1.06 ± 0.20 | 66.4 ± 6.4 | 98.5 ± 1.0 | 0.43 ± 0.17 | 3.9 ± 0.2 |
| (3) Saline | 2 | 22.3 | 1.32 | 66.4 | 98.7 | 0.97 | 6.0 |
| (4) 0.01% AcOH saline | 9 | 24.0 ± 3.0 | 1.18 ± 0.19 | 86.6 ± 23.8 | 98.9 ± 0.8 | 0.87 ± 0.71 | 3.8d |
| (5) 1% EtOH, 0.01% AcOH saline | 1 | 20.3 | 1.35 | 50.9 | 98.7 | 2.08 | 3.8 |
| (6) 0.01% AcOH, 5 mM phosphate-buffered saline | 3 | 13.8 ± 2.2 | 0.95 ± 0.05 | 83.0 ± 24.9 | 99.6 ± 0.1 | 0.26 ± 0.23 | 6.1 ± 0.1 |
| (7) 0.01% AcOH, 1 mM phosphate-buffered saline | 3 | 15.3 ± 0.7 | 1.22 ± 0.17 | 60.1 ± 12.3 | 99.5 ± 0.2 | 0.72 ± 0.40 | 4.4 ± 0.1 |
| (8) 10 mM phosphate-buffered saline | 2 | 14.1 | 1.51 | 72.5 | 97.0 | 0.55 | 6.8 |
| (9) 5 mM phosphate-buffered saline | 3 | 15.8 ± 1.3 | 1.14 ± 0.04 | 71.7 ± 1.3 | 98.0 ± 0.9 | 0.32 ± 0.06 | 6.8 ± 0.0 |
| (10) 1 mM phosphate-buffered saline | 8 | 13.2 ± 3.3 | 1.12 ± 0.14 | 92.8 ± 31.4 | 98.0 ± 0.5 | 0.41 ± 0.23 | 6.7 ± 0.0 |
Data are average ± standard deviation
al-[18F]FBPA obtained at the end of synthesis was normalized to that produced by 120-min irradiation
bRadiochemical purity (RCP) was determined based on HPLC analysis
cContamination (moles) of 4-[10B]borono-l-phenylalanine (l-BPA) is expressed as a percentage against the mass of l-FBPA.
dn = 2
HPLC analysis of l-[18F]FBPA
The column used was YMC-UltraHT Pro C18 S-2 µm (3.0 mm i.d. × 100 mm length, YMC). Four different mobile phases were investigated: (a) 50 mM AcOH/50 mM AcONH4 (1/1), (b) 50 mM NaH2PO4, (c) 0.1% AcOH, and (d) 0.8% AcOH containing 1 mM ethylenediaminetetraacetic acid (EDTA) and 1 mM sodium octylsulfate [similar eluents as described in Refs. 1, 23, 25] at a flow rate of 0.5 ml/min at 20–21 °C, and the elution profiled was monitored using a UV detector at 280 nm (SPD-20A Prominence UV/VIS detector, Shimadzu, Tokyo, Japan) and a radioactivity monitor (US-3000, Universal Giken). The retention times of l-BPA and l-FBPA were 6.0 and 8.5 min, 6.1 and 8.7 min, 5.5 and 7.7 min, and 4.4 and 6.1 min with eluents a, b, c, and d, respectively. The retention times of 2-, 3-, and 4-FPhe were 8.5, 12.5, and 12.7 min, and 12.5, 14.1, and 19.9 min with eluents a and d, respectively.
Optical purity of l-[18F]FBPA
A Crownpak CR (+) (4.0 mm i.d. × 150 mm length, Daicel, Tokyo, Japan) column was used with a mobile phase of HClO4 (pH 2.0) at a flow rate of 1.0 ml/min at 20–21 °C. The retention times of d-FBPA and l-FBPA were 6.5 and 9.9 min, respectively.
Measurement of TFA in l-[18F]FBPA
Gas chromatography-mass spectrometry (GC-MS) was applied for the analysis of residual TFA in eight l-[18F]FBPA preparations: eluents 1, n = 1; 4, n = 5; 9, n = 1; and 10, n = 1. 0.1 ml of concentrated H2SO4/MeOH (4/1) was added to a 0.5 ml l-[18F]FBPA sample, and the mixture was shaken vigorously for 30 s. 0.4 ml of CH2Cl2 was then added to the mixture followed by a 15 s extraction with methyl trifluoroacetate. The CH2Cl2 phase solution (1 µl) was applied to GC–MS analysis 1 min after the end of extraction. Standard aqueous TFA (0.1–100 ppm) was also treated in the same way, and a calibration curve of methyl trifluoroacetate was prepared.
A quadruple mass spectrometer (5975C, Agilent Technologies, Santa Clara, CA) in conjunction with a gas chromatograph (5890GC, Agilent Technologies) was used with a deactivated metal capillary column (0.25 mm i.d. × 30 m) with 1 µm film thickness of Ultra ALLOY-CW (Frontier Laboratories, Koriyama, Japan). The oven temperature was maintained at 40 °C for 3 min and then increased to 150 °C at 100°C/min and held there for 3 min. The split/splitless injector and the transfer line were kept at 200 °C. The flow rate of He carrier gas was 1.5 ml/min.
Results and discussion
Conditioning production of [18F]F2
Figure 1 shows a plot of the activity yields of [18F]F2 after each of three or four successive 5 min irradiations in experiments for the conditioning production of [18F]F2 together with the yields in each of two conditioning 5 min irradiations for the l-[18F]FBPA synthesis as a function of the intervals of each irradiation day. The calculated actual F2 concentrations for 0.10%, 0.15%, and 0.20% F2/Ne targets were 0.11 ± 0.01% (range 0.08–0.14%, n = 27), 0.15 ± 0.02% (0.10–0.19%, n = 38), and 0.20 ± 0.02% (0.17–0.25%, n = 65), respectively. The activity yields were variable after the first irradiation regardless of the F2 content or interval days. For 0.10% and 0.15% F2/Ne targets, the third irradiations produced almost steady-state yields. With 0.20% F2/Ne, the yields reached steady state with the second irradiation. It is noted that steady-state yields are useful from a practical perspective but did not mean the quantitative recovery of [18F]F2 produced with each irradiation even if a higher concentration of F2 was employed. The relationship between the molar mass of F2 set and that of recovered is shown later (Fig. 2d).
Fig. 1.
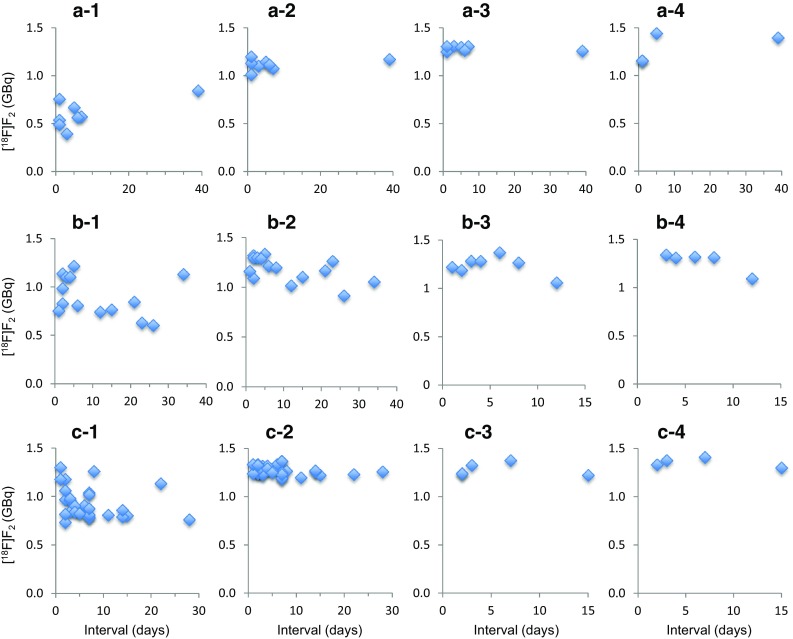
Effect of irradiation times and irradiation interval on the production of [18F]F2. a–c Show the activity yields (GBq) of [18F]F2 for 5 min irradiation of Ne containing 0.1%, 0.15%, and 0.2% F2, respectively, and –(1), -(2), -(3), and (4) indicate the first, second, third, and fourth 5 min irradiations, respectively
Fig. 2.
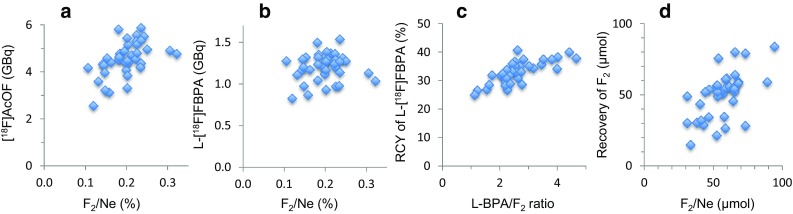
Effects of reaction conditions on the production of [18F]acetylhypofluorite ([18F]AcOF) and 4-[10B]borono-2-[18F]fluoro-l-phenylalanine (l-[18F]FBPA). a Relationship between F2 content (%) in Ne and activity yield of [18F]AcOF (GBq). b Relationship between F2 content (%) in Ne and activity yield of l-[18F]FBPA (GBq). c Relationship between 4-[10B]borono-l-phenylalanine (l-BPA)-to-F2 ratio (molar ratio) and radiochemical yield (RCY) of l-[18F]FBPA (%). d Relationship between F2 (mole) in Ne and F2 (mole) recovered as [18F]F2. The amount (mole) of [18F]F2 was calculated from the molar activity of l-[18F]FBPA and the total activity (summed activity absorbed in a sodium acetate column and recovered in a reaction vial as [18F]AcOF)
In an early study on [18F]F2 production, a carrier concentration of 0.1% F2 produced almost 95% of the theoretical yield of [18F]F2, and a part of [18F]F2 was adsorbed on a stainless steel tube in recovery from the target chamber [35]. The present study demonstrated the clear requirement of pre-irradiation in a range of 0.1–0.2% F2, and suggested that two pre-conditioning irradiations with these F2 concentrations were preferable for steady [18F]F2 production. It was also suggested that mixing 5% F2-containg Ne and pure Ne gases made accurate setting of the F2 concentration difficult.
Synthesis of l-[18F]FBPA using [18F]AcOF
The total activity of [18F]F2 (summed activities absorbed in a sodium acetate column and recovered in a reaction vial as [18F]AcOF) that was normalized as those produced by irradiation for 120 min, was 12.3 ± 1.9 GBq (n = 38). The activity yields of [18F]AcOF trapped in the reaction vial tended to increase with increasing the F2 percentage in Ne, and ≥ 0.15% F2 was preferable (Fig. 2a). The flow rates of [18F]F2 passing through a sodium acetate trihydrate column were slightly higher than those passing through a sodium acetate anhydrous column: 460 ± 89 ml/min (n = 23) vs. 363 ± 55 ml/min (n = 11, 4 data missing), and the respective radiochemical yields (RCYs) of [18F]AcOF were 38.8 ± 2.7% (n = 23) and 35.5 ± 1.9% (n = 11, 4 data missing) based on the total activity of [18F]F2 recovered. The difference in RCYs between the two cases was not large, but two other experiments using a sodium acetate anhydrous column with flow rates of 252 and 319 ml/min produced very low [18F]AcOF RCYs of 10.9 and 15.9%, respectively. These results suggested that low flow rates of [18F]F2 decreased the recovery of [18F]AcOF, and that sodium acetate trihydrate with larger particle sizes would be preferable compared with sodium acetate anhydrous with smaller particle sizes.
The activity yields of l-[18F]FBPA were variable and did not tend to increase with the F2 percentage in Ne (Fig. 2b). The averaged activity yield was 1200 ± 160 MBq (n = 38) at 31.6 ± 1.7 min from the EOB. The RCY of l-[18F]FBPA based on [18F]AcOF trapped in the reaction vial was 33.1 ± 3.8% (n = 38). The RCY increased with increasing l-BPA-to-F2 ratios (Fig. 2c), which suggested that the increased [18F]AcOF relative to l-BPA further fluorinated to produce 18F-difluorinated l-BPA as observed in the electrophilic fluorination of l-3-(hydroxy-4-pivaloyloxyphenyl)alanine with [18F]AcOF [36] and/or degraded l-[18F]FBPA. The preferred l-BPA-to-F2 ratio is > 2 for the steady production of l-[18F]FBPA. It is emphasized that the synthesis time (32 min) was the shortest compared with those in previous reports: 50 min [27], 72 min [26], 80 min [1], 88 min [25], and 110 min [24].
As previously described [1, 27], three by-products of 2-, 3-, and 4-[18F]fluoro-l-phenylalanine were tentatively identified by comparison with the retention times of authentic samples. Although baseline separation of 3- and 4-[18F]fluoro-l-phenylalanine could not be performed in all HPLC separations investigated, the relative amounts were in the order of 3- > 4- > 2-isomer (Fig. 3), and the summed RCYs of the three were constant at 11.6 ± 1.3% (n = 37). Electrophilic fluorination at the aromatic carbon 4 could explain undesired deboronation that leads to the 4-isomer; however, there is no known mechanism that produces the 2- and 3-isomers. Coenen et al. reported that the fluorination of l-phenylalanine in TFA with [18F]F2 produced 2- (72.5%), 3- (13.9%), and 4-[18F]fluoro-l-phenylalanine (13.6%) [37]. It is unlikely that l-phenylalanine produced after deboronation of l-BPA was fluorinated. We tried further identification of these byproducts by GC-MS as described for the determination of TFA using a deactivated metal capillary column (0.25 mm i.d. × 30 m) with 1 µm film thickness of Ultra ALLOY-1 (polydimethylsiloxane) (Frontier Laboratories), but could neither identify nor deny three byproducts to be 2-, 3-, and 4-[18F]fluoro-l-phenylalanine.
Fig. 3.
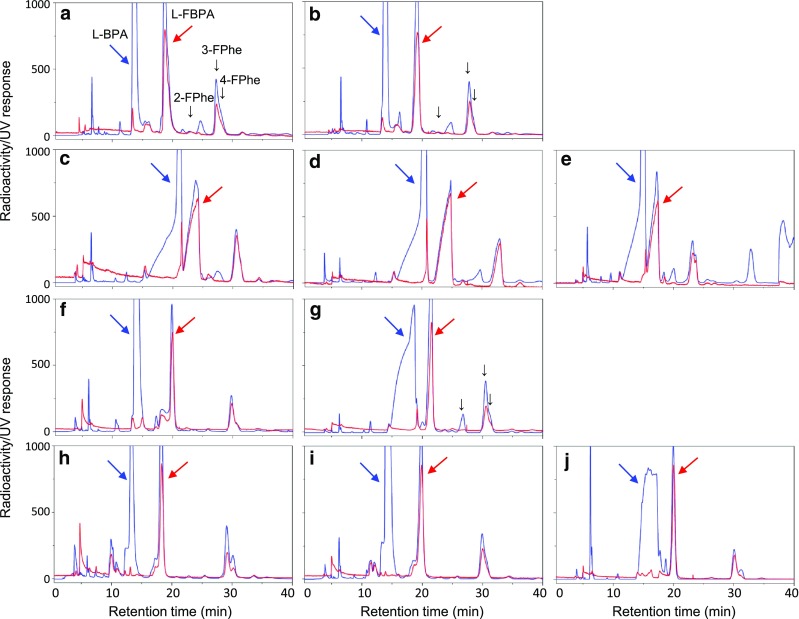
HPLC chromatograms of 4-[10B]borono-2-[18F]fluoro-l-phenylalanine (l-[18F]FBPA) separation using 10 different eluents. a–j Correspond to the chromatograms with eluents 1–10. Red and blue lines show the elution profiles of radioactivity and ultraviolet (UV, 260 nm) absorbance, respectively. The unit of the vertical axis are the millvolt output of the UV detector. Elution positions of 4-[10B]borono-l-phenylalanine (l-BPA), l-FBPA, and 2-, 3-, and 4-fluoro-d,l-phenylalanine (2-, 3-, and 4-FPhe, respectively) are indicated
The molar activities of l-[18F]FBPA synthesized using 0.1%, 0.15%, 0.2% 0.25%, and 0.3% F2 were 103.5 ± 9.5 (n = 3), 86.1 ± 24.4 (n = 8), 69.0 ± 7.3 (n = 23), 66.0 (n = 2), and 50.3 GBq/mmol (n = 2), respectively. It is reasonable that lower carrier F2 contents in [18F]F2 production resulted in higher molar activities of l-[18F]FBPA; however, no linear relationship was found between the F2 content and molar activity. Figure 2d shows the molar amounts of recovered [18F]F2 that were calculated from the molar activity of l-[18F]FBPA and the total activity. Large differences observed between the amounts of F2 set in Ne and the recovered F2 indicated that the carrier F2 added could not be recovered constantly, even after two pre-conditioning irradiations.
Discussion of the differences between the present and previous studies on l-[18F]FBPA synthesis using Ne-derived [18F]AcOF is difficult because the detailed reaction conditions were not described in the previous reports. However, the present activity yields (1200 ± 160 MBq) were much higher than in previous reports: 444–518 MBq (n = 10) [24] and 750 ± 250 MBq (n = 8) [33 figures not shown], and the molar activities were higher than those in some reports [1, 23, 24] but lower than that reported in [9] (130 GBq/mmol).
Fig. 3 shows HPLC separation patterns with 10 different eluents. Eluent 1 0.1% AcOH, a standard mobile phase used previously, gave a baseline separation (Fig. 3a); however, lower 0.01% AcOH (Fig. 3b) was preferable. Physiological saline (Fig. 3c) showed leading peaks of l-BPA and l-[18F]FBPA, but did not show baseline separation. The addition of AcOH to saline (Fig. 3d) improved the separation slightly. Further addition of EtOH (Fig. 3e) caused slightly faster elution but without improved separation. The addition of sodium phosphate corrective injection 0.5 mmol/ml (pH 6.5) to saline with/without AcOH improved the separation (Fig. 3f, h, i). Lower sodium phosphate eluted l-BPA more broadly, but l-[18F]FBPA was separated as an apparently single peak (Fig. 3g, j).
The characteristics of the 10 l-[18F]FBPA preparations are summarized in Table 1. First, the radiochemical purities (RCPs) of the four eluents for HPLC analysis were compared for five l-[18F]FBPA preparations. In analyses with eluents (a) 50 mM AcOH/50 mM AcONH4 (1/1) (Fig. 4a), (b) 50 mM NaH2PO4, (c) 0.1% AcOH, and (d) 0.8% AcOH containing 1 mM EDTA and 1 mM sodium octylsulfate, the RCPs were 97.7 ± 1.3%, 97.7 ± 1.4%, 98.6 ± 0.6%, and 98.4 ± 0.6%, respectively. Analyses with eluents (a) and (b) were similar, and preferable compared to eluents (c) and (d). Therefore, all subsequent analyses were conducted with eluent (a).
Fig. 4.
HPLC chromatograms of 4-[10B]borono-2-[18F]fluoro-l-phenylalanine (l-[18F]FBPA) analysis on a YMC-UltraHT Pro C18 S-2 µm and b Crownpak CR (+) columns. Red and blue lines show the elution profiles for radioactivity and ultraviolet (UV, 277 nm) absorbance, respectively. The units of the vertical axis are millivolt output of the UV detector. a In the stability test for 4 h, a radiochemical impurity appeared at the retention time indicated by the arrow. b The inset emphasizes the low levels of the chromatograms and the elution position of d-FBPA is indicated by the arrow
The RCPs were over 97% for all 10 l-[18F]FBPA preparations. The pH was below 4.0 in four of the 10 preparations. The volumes of l-[18F]FBPA separated with saline with/without 0.01% AcOH were over 20 ml, and the addition of sodium phosphate corrective injection reduced the volumes. Baseline separation could not be performed using eluents 3–5 and 1–2% contamination levels of l-BPA were obtained. Contamination levels of l-BPA in five other preparations with eluents 6–10 were very low. These five preparations were thus considered as acceptable for intravenous injection without any further processing. The l-[18F]FBPA eluted with 0.1% AcOH (eluent 1) was previously used directly in a clinical study after the addition of 25% ascorbic acid injection and 10% sodium chloride injection to manage the pH and ion strength, respectively [33]. Comparison of activity yields and molar activities in the 10 preparations shown in Table 2 may not be significant, because the F2 contents were randomly set.
Table 2.
Stability of 4-borono-2-[18F]fluoro-l-phenylalanine preparations separated by high-performance liquid chromatography using five different eluents
| Eluent | n | pH | Radiochemical purity (%)a | |||
|---|---|---|---|---|---|---|
| 0 h | 1 h | 2 h | 4 h | |||
| (3) Saline | 1 | 4.9 | 99.2 | 98.6 | 98.3 | 97.9 |
| (6) 0.01% AcOH, 5 mM phosphate-buffered saline | 2 | 6.1 | 99.6 | 97.9 | 97.6 | |
| (7) 0.01% AcOH, 1 mM phosphate-buffered saline | 3 | 4.4 ± 0.1 | 99.5 ± 0.2 | 99.3 ± 0.4 | 99.0 ± 0.5 | 99.1 ± 0.8 |
| (9) 5 mM phosphate-buffered saline | 3 | 6.8 ± 0.0 | 98.0 ± 0.9 | 97.1 ± 1.6 | 96.7 ± 0.7 | |
| (10) 1 mM phosphate-buffered saline | 4 | 6.7 ± 0.0 | 97.8 ± 0.9 | 97.4 ± 0.5 | 97.0 ± 0.5 | 96.7 ± 0.5 |
Data are average ± standard deviation
aThe time for the first analysis after the end of synthesis was defined as “0 h”, and then analyses were performed successively at approximately the indicated intervals until approximately 4 h
From these results and the stability of l-[18F]FBPA described later, eluent 10 was selected for routine clinical use because of the small amount of one additive in physiological saline and ease of eluent preparation. The clinical injection volumes of l-[18F]FBPA separated with eluent 10 (1120 MBq/13.2 ml, Table 2), were expected to be 2.6–3.9 ml/60 kg when the radioactive injection doses in l-[18F]FBPA PET were 3.7–5.55 MBq/kg [38, 39], and one l-[18F]FBPA preparation can be used for 2 subjects with one PET scanner or 3–4 subjects with two PET scanners. In four l-[18F]FBPA preparations separated with eluent 10 and collected through a 0.22 µm membrane filter, sterility and apyrogenicity (< 0.0029 EU/ml) of the l-[18F]FBPA injection and filter integrity (≥ 330 kPa) were confirmed. It is noted that no radionuclidic impurities were found in l-[18F]FBPA prepared using the present method [34].
In previous research to improve the activity yields or the molar activity of l-[18F]FBPA, l-BPA was fluorinated with [18F]F2 produced by the 18O(p,n)18F reaction [25–27]. We also fluorinated l-BPA with [18F]F2 and separated l-[18F]FBPA by HPLC with eluents 1, 6, and 8; however, the RCPs (n = 6) were lower than those of [18F]AcOF-derived l-[18F]FBPA separated using the same eluents. The higher reactivity of [18F]F2 than [18F]AcOF may result in more side reactions. Therefore, no further investigation was conducted for the synthesis using [18F]F2.
Stability
The stabilities of five l-[18F]FBPA preparations are summarized in Table 2. The l-[18F]FBPA separated by eluent 7 was the most stable over 4 h after EOS. In the four other preparations the RCPs decreased gradually but were maintained at over 96% for 4 h. Low pH with eluent 7 may contribute to the stability of l-[18F]FBPA; however, these five preparations without stabilizers such as EtOH [28] and ascorbate [33] were suitable for routine clinical use for at least 4 h. Preliminarily we found that the ascorbate was not effective for stability. In two l-[18F]FBPA preparations separated with 0.01% AcOH saline and 5 mM PBS, the addition of ascorbate injection (final concentration of 10 mg/ml) slightly decreased the RCPs from 98.4% and 97.7–93.2% and 91.3%, respectively, by approximately 4 h.
Optical purity
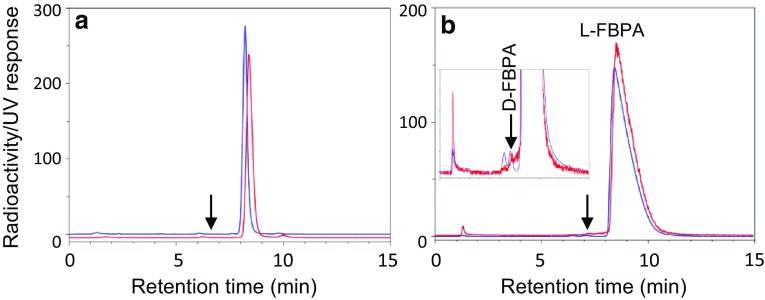
No signal associated with the d-isomer was found in l-[18F]FBPA preparations, probably because of the low activity concentrations (Table 1, 47–107 MBq/mL) and the sensitivity of the US-3000 radioactivity detector. However, in the l-[18F]FBPA samples concentrated by evaporation or only the peak fraction of l-[18F]FBPA from HPLC separation (270–970 MBq/ml), a very small amount of the d-isomer was present (Fig. 4b): < 0.1% (0.09 ± 0.04%, n = 4) compared with the l-isomer. The UV peak that corresponds to this activity peak was increased by the addition of small amounts of standard d-FBPA into the samples. It was noted that a temperature at 20–21 °C was critical in this analysis because d-[18F]FBPA was not separated from the radioactive impurities at higher temperature (25 °C).
A UV peak corresponding to d-FBPA was evident and the UV detection sensitivity was higher than that of radioactivity. However, UV signals could not be used to evaluate d-[18F]FBPA, because the retention times of authentic d-FBPA and l-BPA coincided. No UV peak that corresponded to the d-isomer was observed for the starting compound l-BPA (both from Sigma-Aldrich and Stella Pharma); therefore, we considered that epimerization of l-[18F]FBPA might occur in TFA solution in the radiosynthesis process, as l-amino acids such as l-phenylalanine were epimerized in acetic acid [40].
TFA analysis by GC-MS
The residual TFA in l-[18F]FBPA preparations was analyzed by GC-MS after methylation. The retention time of methyl trifluoroacetate in the GC stage was 2.5 min. Three ions were monitored in the SIM-EI+ mode of operation: m/z 59, 69, and 99. These are the most characteristic ions in the mass spectrum of methyl trifluoroacetate [41]. Because the signal at m/z 69 was much stronger than those at m/z 59 and 99; therefore, only the area of the m/z 69 peak was used for quantitative analysis. Methyl trifluoroacetate was degraded gradually by approximately 10% after 20 min from the end of extraction; therefore, the GC-MS analysis was started just 1 min after the end of extraction. The detection limit of methyl trifluoroacetate was determined to be 0.5 ppm [signal-to-nosise ratio (S/N) = 12], and the S/N of the 0.1 ppm standard sample was 8. In eight l-[18F]FBPA preparations the residual TFA was less than 0.5 ppm: 0.2 ± 0.1 ppm (range, 0.0–0.3 ppm) without evaporation processing for preparing the l-[18F]FBPA injection. It is noted that the LD50 values are 200 mg/kg in rats (oral administration) and 1200 mg/kg in mice (intravenous injection) (Hazardous Substances Data Bank, 2007: https://toxnet.nlm.nih.gov/cgi-bin/sis/search2/f?./temp/~TF1xcW:1).
Conclusion
Two 5 min pre-irradiations enabled the steady production of [18F]F2 for 18F-labeling by electrophilic fluorination. To achieve a high RCY of l-[18F]FBPA 0.15–0.2% carrier F2 in Ne and an l-BPA-to-F2 ratio > 2 were preferable. HPLC separation using 1 mM PBS provided injectable l-[18F]FBPA without any further formulation processing, which resulted in a 32-min synthesis period from EOB. The l-[18F]FBPA injection contained small amounts of d-enantiomer (< 0.1% of l-[18F]FBPA), l-BPA (< 1% of l-FBPA), and TFA (< 0.5 ppm).
References
- 1.Ishiwata K, Ido T, Mejia AA, Ichihashi M, Mishima Y. Synthesis and radiation dosimetry of 4-borono-2-[18F]fluoro-d,l-phenylalanine: a target compound for PET and boron neutron capture therapy. Appl Radiat Isot. 1991;42:325–328. doi: 10.1016/0883-2889(91)90133-L. [DOI] [PubMed] [Google Scholar]
- 2.Ishiwata K, Ido T, Kawamura M, Kubota K, Ichihashi M, Mishima Y. 4-Borono-2-[18F]fluoro-d,l-phenylalanine as a target compound for boron neutron capture therapy: tumor imaging potential with positron emission tomography. Nucl Med Biol. 1991;18:745–751. doi: 10.1016/0883-2897(91)90013-b. [DOI] [PubMed] [Google Scholar]
- 3.Ishiwata K, Ido T, Honda C, Kawamura M, Ichihashi M, Mishima Y. 4-Borono-2-[18F]fluoro-d,l-phenylalanine: a possible tracer for melanoma diagnosis with PET. Nucl Med Biol. 1992;19:311–318. doi: 10.1016/0883-2897(92)90116-g. [DOI] [PubMed] [Google Scholar]
- 4.Ishiwata K, Shiono M, Kubota K, Yoshino K, Hatazawa J, Ido T, Honda C, et al. A unique iv vivo assessment of 4-[10B]borono-l-phenylalanine in tumour tissues for boron neutron capture therapy of malignant melanoma using positron emission tomography and 4-borono-2-[18F]fluoro-l-phenylalanine. Melanoma Res. 1992;2:171–179. doi: 10.1097/00008390-199209000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Kubota R, Yamada S, Ishiwata K, Tada M, Ido T, Kubota K. Cellular accumulation of 18F-labelled boronophenylalanine depending on DNA synthesis and melanin incorporation: a double-tracer microautoradiographic study of B16 melanomas in vivo. Br J Cancer. 1993;67:701–705. doi: 10.1038/bjc.1993.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imahori Y, Ueda S, Ohmori Y, Yoshino E, Ono K, Kobayashi T, et al. A basic concept for PET-BNCT system. In: Mishima Y, et al., editors. Cancer neutron capture therapy. New York: Plenum Press; 1996. pp. 691–696. [Google Scholar]
- 7.Ueda S, Imahori Y, Ohmori Y, Yoshino E, Ono K, Kobayashi T, et al. Positron emission tomography and boron neutron capture therapy system to the patient with malignant brain tumor: the first clinical trial using 10B-BPA. In: Mishima Y, et al., editors. Cancer neutron capture therapy. New York: Plenum Press; 1996. pp. 823–827. [Google Scholar]
- 8.Mishima Y, Imahori Y, Honda C, Hiratsuka J, Ueda S, Ido T. In vivo diagnosis of human malignant melanoma with positron emission tomography using specific melanoma-seeking 18F-DOPA analogue. J Neuro Oncol. 1997;33:163–169. doi: 10.1023/A:1005746020350. [DOI] [PubMed] [Google Scholar]
- 9.Imahori Y, Ueda S, Ohmori Y, Kusuki T, Ono K, Fujii R, et al. Fluorine-18-labeled fluoroboronophenylalanine PET in patients with glioma. J Nucl Med. 1998;39:325–3. [PubMed] [Google Scholar]
- 10.Miyatake S, Kawabata S, Hiramatsu R, Kuroiwa T, Suzuki M, Kondo N, et al. Boron neutron capture therapy for malignant brain tumors. Neurol Med Chir (Tokyo) 2016;56:361–371. doi: 10.2176/nmc.ra.2015-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evangelista L, Jori G, Martini D, Sotti G. Boron neutron capture therapy and 18F-labelled borophenylalanine positron emission tomography: A critical and clinical overview of the literature. Appl Radiat Isot. 2013;74:91–101. doi: 10.1016/j.apradiso.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Nariai T, Ishiwata K. Analysis and imaging: PET. In: Sauerwein WAG, Wittig A, Moss R, Nakagawa Y, editors. Neutron capture therapy, principles and applications. Berlin: Springer; 2012. pp. 201–212. [Google Scholar]
- 13.Wittig A, Michel J, Moss RL, Stecher-Rasmussen F, Arlinghaus HF, Bendel P, Mauri PL, Altieri S, Hilger R, Salvadori PA, Menichetti L, Zamenhof R, Sauerwein WA. Boron analysis and boron imaging in biological materials for boron neutron capture therapy (BNCT) Crit Rev Oncol Hematol. 2008;68:66–90. doi: 10.1016/j.critrevonc.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka H, Sakurai Y, Suzuki M, Masunaga S, Kinashi Y, Kashino G, et al. Characteristics comparison between a cyclotron-based neutron source and KUR-HWNIF for boron neutron capture therapy. Nucl Instrum Methods Phys Res B. 2009;267:1970–1977. doi: 10.1016/j.nimb.2009.03.095. [DOI] [Google Scholar]
- 15.Tanaka H, Sakurai Y, Suzuki M, Masunaga S, Mitsumoto T, Fujita K, et al. Experimental verification of beam characteristics for cyclotron-based epithermal neutron source (C-BENS) Appl Radiat Isot. 2011;69:1642–1645. doi: 10.1016/j.apradiso.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 16.Yoshimoto M, Kurihara H, Honda N, Kawai K, Ohe K, Fujii H, et al. Predominant contribution of L-type amino acid transporter to 4-borono-2-18F-fluoro-phenylalanine uptake in human glioblastoma cells. Nucl Med Biol. 2013;40:625–629. doi: 10.1016/j.nucmedbio.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Hanaoka K, Watabe T, Naka S, Kanai Y, Ikeda H, Horitsugi G, et al. FBPA PET in boron neutron capture therapy for cancer: prediction of 10B concentration in the tumor and normal tissue in a rat xenograft model. EJNMMI Res. 2014;4:70. doi: 10.1186/s13550-014-0070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang FY, Chang WY, Li JJ, Wang HE, Chen JC, Chang CW. Pharmacokinetic analysis and uptake of 18F-FBPA-Fr after ultrasound-induced blood–brain barrier disruption for potential enhancement of boron delivery for neutron capture therapy. J Nucl Med. 2014;55:616–621. doi: 10.2967/jnumed.113.125716. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe T, Hattori Y, Ohta Y, Ishimura M, Nakagawa Y, Sanada Y, et al. Comparison of the pharmacokinetics between l-BPA and l-FBPA using the same administration dose and protocol: a validation study for the theranostic approach using [18F]-l-FBPA positron emission tomography in boron neutron capture therapy. BMC Cancer. 2016;16:859. doi: 10.1186/s12885-016-2913-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wingelhofer B, Kreis K, Mairinger S, Muchitsch V, Stanek J, Wanek T, et al. Preloading with l-BPA, l-tyrosine and l-DOPA enhances the uptake of [18F]FBPA in human and mouse tumour cell lines. Appl Radiat Isot. 2016;118:67–72. doi: 10.1016/j.apradiso.2016.08.026. [DOI] [PubMed] [Google Scholar]
- 21.Watabe T, Hanaoka K, Naka S, Kanai Y, Ikeda H, Aoki M, et al. Practical calculation method to estimate the absolute boron concentration in tissues using 18F-FBPA PET. Ann Nucl Med. 2017;31:481–485. doi: 10.1007/s12149-017-1172-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grunewald C, Sauberer M, Filip T, Wanek T, Stanek J, Mairinger S, et al. On the applicability of [18F]FBPA to predict l-BPA concentration after amino acid preloading in HuH-7 liver tumor model and the implication for liver boron neutron capture therapy. Nucl Med Biol. 2017;44:83–89. doi: 10.1016/j.nucmedbio.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 23.Watabe T, Ikeda H, Nagamori S, Wiriyasermkul P, Tanaka Y, Naka S, et al. 18F-FBPA as a tumor-specific probe of L-type amino acid transporter 1 (LAT1): a comparison study with 18F-FDG and 11C-Methionine PET. Eur J Nucl Med Mol Imaging. 2017;44:321–331. doi: 10.1007/s00259-016-3487-1. [DOI] [PubMed] [Google Scholar]
- 24.Wang HE, Liao AH, Deng WP, Chang PF, Chen JC, Chen FD, et al. Evaluation of 4-borono-2-18F-fluoro-l-phenylalanine-fructose as a probe for boron neutron capture therapy in a glioma bearing rat model. J Nucl Med. 2004;45:302–308. [PubMed] [Google Scholar]
- 25.Kabalka GW, Smith GT, Dyke JP, Reid WS, Longford CPD, Roberts TG, et al. Evaluation of fluorine-18-BPA-fructose for boron neutron capture treatment planning. J Nucl Med. 1997;38:1762–1767. [PubMed] [Google Scholar]
- 26.Mairinger S, Stanek J, Wanek T, Langer O, Kuntner C. Automated electrophilic radiosynthesis of [18F]FBPA using a modified nucleophilic GE TRACERlab FXFDG. Appl Radiat Isot. 2015;104:124–127. doi: 10.1016/j.apradiso.2015.06.034. [DOI] [PubMed] [Google Scholar]
- 27.Vähätalo JK, Eskola O, Bergman J, Forsback S, Lehikoinen P, Jääskeläinen J, et al. Synthesis of 4-dihydroxyboryl-2-[18F]fluorophenylalanine with relatively high-specific activity. J Label Compd Radiopharm. 2002;45:697–704. doi: 10.1002/jlcr.600. [DOI] [Google Scholar]
- 28.Havu-Aurén K, Kiiski J, Lehtiö K, Eskola O, Kulvik M, Vuorinen V, et al. Uptake of 4-borono-2-[18F]fluoro-l-phenylalanine in sporadic and neurofibromatosis 2-related schwannoma and meningioma studied with PET. Eur J Nucl Med Mol Imagimg. 2007;34:87–94. doi: 10.1007/s00259-006-0154-y. [DOI] [PubMed] [Google Scholar]
- 29.Honda N, Yoshimoto M, Mizukawa Y, Osaki K, Kanai Y, Kurihara H, et al. Radiosynthesis of 2-[18F]fluoro-4-borono-phenylaranine ([18F]FBPA) using copper mediated oxidative aromatic nucleophilic [18F]fluorination. J Label Compd Radiopharm. 2017;60(Suppl. 1):S512. [Google Scholar]
- 30.Ishiwata K, Ishii S, Senda M. HPLC using physiological saline for the quality control of radiopharmaceuticals used in PET studies. Appl Radiat isot. 1993;44:1119–1124. doi: 10.1016/0969-8043(93)90116-R. [DOI] [Google Scholar]
- 31.Coenen HH, Gee AD, Adam M, Antoni G, Cutler CS, Fujibayashi Y, et al. Consensus nomenclature rules for radiopharmaceutical chemistry. Nucl Med Biol. 2017;55:v–xi. doi: 10.1016/j.nucmedbio.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 32.Coenen HH, Gee AD, Adam M, Antoni G, Cutler CS, Fujibayashi Y, et al. Open letter to journal editors on: international consensus radiochemistry nomenclature guidelines. Ann Nucl Med. 2018;32:236–238. doi: 10.1007/s12149-018-1238-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakata M, Oda K, Toyohara J, Ishii K, Nariai T, Ishiwata K. Direct comparison of radiation dosimetry of six PET tracers using human whole-body imaging and murine biodistribution studies. Ann Nucl Med. 2013;27(3):285–296. doi: 10.1007/s12149-013-0685-9. [DOI] [PubMed] [Google Scholar]
- 34.Ishiwata K, Hayashi K, Sakai M, Kawauchi S, Hasegawa H, Toyohara J. Determination of radionuclides and radiochemical impurities produced by in-house cyclotron irradiation and subsequent radiosynthesis of PET tracers. Ann Nucl Med. 2017;31:84–92. doi: 10.1007/s12149-016-1134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Casella V, Ido T, Wolf AP, Fowler JS, MacGegor RR, Ruth TJ. Anhydrous F-18 labeled elemental fluorine for radiopharmaceutical preparation. J Nuci Med. 1980;21:750–757. [PubMed] [Google Scholar]
- 36.Hatano K, Ishiwata K, Yanagisawa T. Co production of 2,6-[18F]difluoroDOPA during electrophilic synthesis of 6-[18F]fluoro-l-DOPA. Nucl Med Biol. 1996;23:101–103. doi: 10.1016/S0969-8051(96)90004-9. [DOI] [PubMed] [Google Scholar]
- 37.Coenen HH, Franken K, Kling P, Stöcklin G. Direct electrophic radiofluorination of phenyl, tyrosine and dopa. Appl Radiat Isot. 1988;39:1243–1250. doi: 10.1016/0883-2889(88)90107-4. [DOI] [Google Scholar]
- 38.Ariyoshi Y, Shimahara M, Kimura Y, Ito Y, Shimahara T, Miyatake S, et al. Fluorine-18-labeled boronophenylalanine positron emission tomography for oral cancers: Qualitative and quantitative analyses of malignant tumors and normal structures in oral and maxillofacial regions. Oncol Lett. 2011;2:423–427. doi: 10.3892/ol.2011.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tani H, Kurihara H, Hiroi K, Honda N, Yoshimoto M, Kono Y, et al. Correlation of 18F-BPA and 18F-FDG uptake in head and neck cancers. Radiother Oncol. 2014;113:193–197. doi: 10.1016/j.radonc.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 40.Sato M, Tatsuno T, Matsuo H. Studies on the racemization of amino acids and their derivatives. IV. Structural relation of amino acids to their racemizability in acetic acid. Yakugaku Zasshi. 1970;9:1160–1163. doi: 10.1248/yakushi1947.90.9_1160. [DOI] [PubMed] [Google Scholar]
- 41.McLafferty FW, Stauffer DB. The Wiley/NBS registry of mass spectral data. New York: Wiley-Interscience; 1989. [Google Scholar]