Abstract
Mounting evidence that alterations in brain-derived neurotrophic factor (BDNF) levels and signaling may be involved in the etiopathogenesis of Alzheimer’s disease (AD) has suggested that its blood levels could be used as a biomarker of the disease. However, higher, lower, or unchanged circulating BDNF levels have all been described in AD patients compared to healthy controls. Although the reasons for such different findings are unclear, methodological issues are likely to be involved. The heterogeneity of participant recruitment criteria and the lack of control of variables that influence circulating BDNF levels regardless of dementia (depressive symptoms, medications, lifestyle, lack of overlap between serum and plasma, and experimental aspects) are likely to bias result and prevent study comparability. The present work reviews a broad panel of factors, whose close control could help reduce the inconsistency of study findings, and offers practical advice on their management. Research directed at elucidating the weight of each of these variables and at standardizing analytical methodologies is urgently needed.
Keywords: Brain-derived neurotrophic factor, Alzheimer’s disease, Blood, Biomarker, Confounding variables
Introduction
Brain-derived neurotrophic factor (BDNF) is a neurotrophin family member that plays key physiological functions in both the peripheral and central nervous system (CNS) (Fig. 1) [1–3]. Growing evidence that changes in cerebral BDNF levels and in the BDNF-TrkB signaling pathway may be involved in the etiopathogenesis of Alzheimer’s disease (AD) [4] has suggested that blood BDNF could be used as a biomarker for AD diagnosis, prognosis, and treatment monitoring.
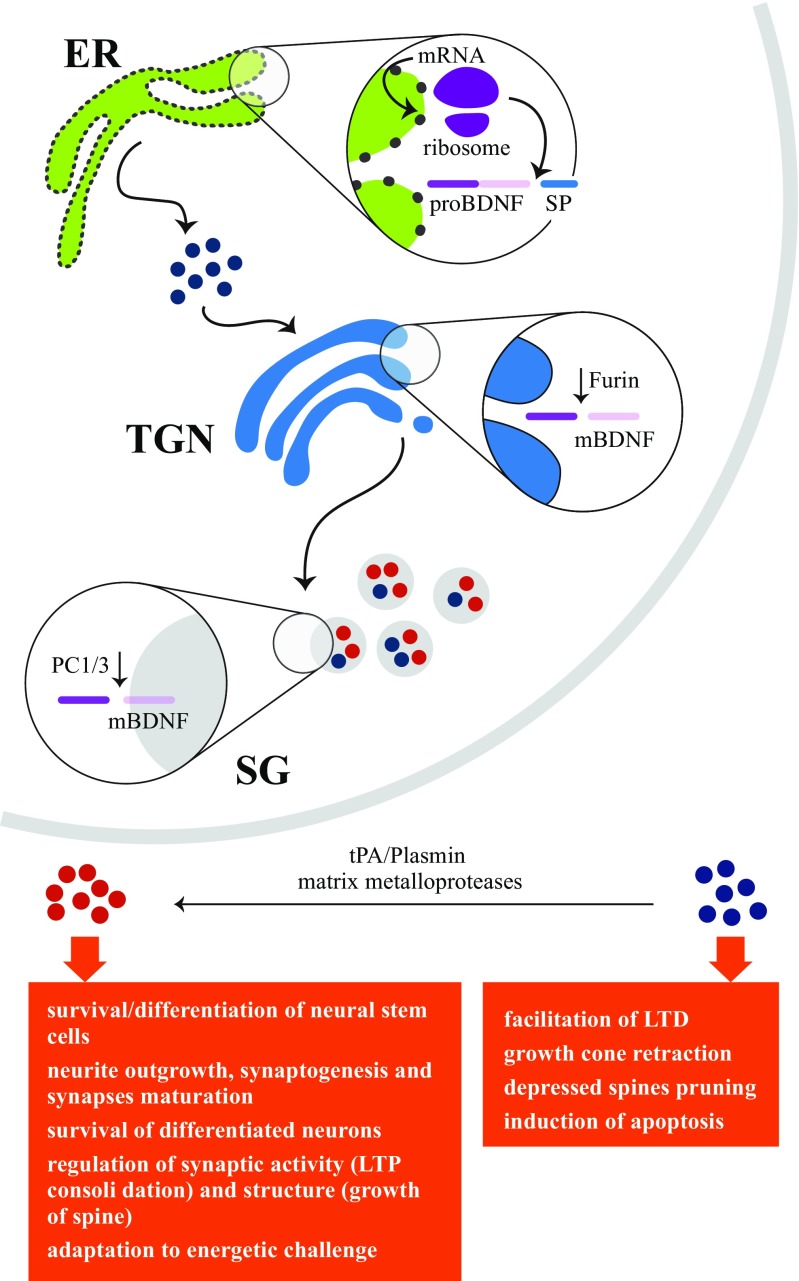
Fig. 1.
Sketch of brain-derived neurotrophic factor (BDNF) functions. BDNF, nerve growth factor, neurotrophin-3, and neurotrophin-4/5 belong to the neurotrophin family of closely related proteins. BDNF is synthesized as pre-proBDNF and converted to proBDNF by removal of the signal peptide in the endoplasmic reticulum (ER); proBDNF is then transformed to mature BDNF (mBDNF) by intracellular (in trans-Golgi network (TGN) and secretory granules (SG)) and extracellular cleavage events. Pro and mature BDNF are both active molecules playing different key roles: proBDNF through the p75NTR/sortilin complex and mBDNF through its receptor TrkB. Blue circles, proBDNF; red circles, mBDNF; LTD, long-term depression; LTP, long-term potentiation; tPA, tissue plasminogen activator
Circulating BDNF derives from both peripheral [5–7] and cerebral sources, since the blood brain barrier is permeable in both directions [8]. A correlation of circulating BDNF with brain levels as well as brain phenomena has been suggested by animal and human studies [9–15]. However, investigations of its value as a biomarker of AD have given inconclusive results, since higher [16, 17], lower [18–28], or similar levels of circulating BDNF [29–33] have all been described in AD patients compared to healthy controls. Although the reasons for the conflicting reports remain unclear, a recent meta-analysis has found that nonhomogenous recruitment criteria of demented subjects and healthy controls may induce a significant bias [34].
This work delves into the issue by reviewing a broad panel of factors that can influence circulating BDNF, compromising data comparability, and offers some practical advice on how to maximize their control.
AD Staging
Laske and colleagues [35] were the first to hypothesize that serum BDNF levels could be related to AD stage, i.e., that their early increase and subsequent reduction could depend on disease progression. Their initial upregulation has been hypothesized to be a compensatory mechanism, directed at counteracting β-amyloid accumulation, providing trophic support to offset the neuronal loss and/or promoting TAU dephosphorylation [36–41], or else a reflection of the increased choline acetyltransferase activity which characterizes the stage preceding neurodegeneration [42]. The view that different BDNF levels are found in different AD stages is shared by some researchers [43–45] and rejected by others [16, 17, 30]. Such dissimilar opinions are likely to depend on a number of factors that are discussed below. However, the inhomogeneous use of rating tools may also provide a contribution.
The Clinical Dementia Rating (CDR) scale and the Mini Mental State Examination (MMSE) are widely used tools, although the CDR scale is considered as the more reliable tool to stage AD patients [46]. The scale, which has been validated in 14 languages, assesses cognitive function using a semistructured interview for both patient and informant [47]. It evaluates a large number of domains including memory, orientation, judgment, problem solving, community affairs, household activities, hobbies, and personal care and assigns global scores that correspond to discrete levels of cognitive impairment [48]. Stringent application of the CDR would enhance comparability across studies. However, ad hoc investigations to clarify the link between AD progression and BDNF level are clearly required. Notably, patient enrolment based on strict staging criteria, i.e., mild, moderate, and severe AD (CDR 1, 2, and 3) or early, middle, and late AD (MMSE score ≥ 20, 20–10, and < 10), would ensure the recruitment of patients with similar disease stage and BDNF level, providing more homogeneous cohorts and more reliable data.
Demographic Characteristics of Healthy and AD Cohorts
An age-related reduction in circulating BDNF has been reported both in healthy individuals and in subjects with dementia [27, 45, 49–51]. Differences have also been described between women and men [33, 49, 51–54], as has a sexual dimorphic effect of the Val66Met polymorphism on susceptibility to AD [55]. Yet, in several studies, healthy subjects and demented patients are recruited without considering these differences, and the possible influence of age and/or gender composition on cohort homogeneity is not always considered [16, 20, 27, 29]. If age and gender are used as covariates in statistical analysis, they can affect the significance of BDNF comparisons [43]. As a general rule, the presence of confounding variables, i.e., variables that can influence the outcome of interest irrespective of the study design, should always be verified before performing the analysis [56], and dedicated statistical methods employed like, for instance, model adjustment for those variables [57].
Depressive Symptoms
Several lines of evidence indicate that cerebral BDNF is an effector of and a therapeutic target for depression [58]. BDNF signaling is significantly reduced in the hippocampus and prefrontal cortex of depressed subjects [59], and the ability of antidepressants and electroconvulsive shock treatment to induce neural plasticity appears to be closely related to BDNF signaling stimulation in these circuits [60]. Notably, the role of cerebral BDNF is area-specific, since an increase in BDNF levels in the brain reward system produces depressive symptoms [61]. With regard to the peripheral consequences of depression, a reduction in serum and plasma BDNF has repeatedly been reported [62, 63], even though its functional role is unclear. A reduction in circulating BDNF may reflect changes at the level of the CNS and/or depend on defective platelet release [64]. Since up to 50% of AD patients suffer from depression, making this disorder one of the most frequent comorbidities in such patients [65], it would be critical to discriminate depression-related from dementia-related BDNF changes.
This could be achieved in two steps. The first would involve excluding from AD cohorts those patients who suffer from clinical depression [16, 19, 20, 32, 33, 35, 43, 66] or by assessing its influence on BDNF levels [21, 45]. Indeed, whereas Lee and colleagues [21] have found no difference in serum BDNF between AD patients with and without severe depression, in another study [33], apathy associated to mild dysphoria symptoms, suggestive of subclinical depression, determined a significant reduction in serum BDNF in affected versus non-affected AD subjects. Furthermore, the difference in BDNF concentrations in platelet-rich plasma between AD patients and healthy subjects, found by Platenik and colleagues [45], has been confirmed in patients with depression, but not in those without it. Again, recruitment criteria play a critical role.
The second step would be the evaluation of depressive mood in healthy and AD cohorts. Even though this element may be a confounding variable, the few published data are not sufficient to draw conclusions, because they have been obtained with different methodological approaches. Most studies have used the Geriatric Depression Scale (GDS) [27, 31, 43, 45], but other diagnostic protocols have also been applied [16]. Moreover, whereas AD and healthy subjects have mostly been kept separate [16, 43, 45], in some studies they have been pooled [27, 31]. Here, too, uniformity is vital, especially where the protocol of depressive mood evaluation is concerned. Despite the existence of several depression rating scales [67], the GDS has several advantages. In fact, it was specifically designed to assess mood status and identify depressive symptoms in the elderly; moreover, it focuses on psychological aspects, avoiding symptoms that may overlap with medical disorders or aging. Although it had originally been based on 30 items, a 15-item version has subsequently been developed [68] and is the one used in most validation studies.
Medications
Several psychoactive drugs administered to AD patients influence circulating BDNF. Acetylcholinesterase inhibitors [20] and antidepressants [69] increase peripheral BDNF, whereas benzodiazepines reduce it [24, 70], even though it is unclear whether the changes are related to cerebral events or are rather epiphenomena. Despite their key importance, the effects of medications have been ignored in numerous studies [18, 19, 21–25, 27–30, 32, 35, 45]. The works that have considered them have adopted one of two approaches: (i) enrolment of naïve subjects or of patients in the washout phase [20, 33, 66] or (ii) evaluation of the consequences of medication use [16, 17, 24, 31, 43]. Although both approaches provide useful data, the latter sketches a clearer picture of the average condition of the population and could therefore be more useful to test the biomarker function of circulating BDNF in clinical practice. Besides psychoactive drugs, lipid-lowering drugs [51, 71] and antidiabetics [72] are also widely prescribed to the elderly and affect BDNF levels. These drugs should therefore be factored in, as should all medications influencing platelet activity, like nonsteroidal anti-inflammatories, anticoagulants, and antihypertensives (see section “Lack of Overlap Between Serum and Plasma BDNF”). Only a limited number of investigations have assessed psychoactive as well as nonpsychoactive medications [16, 20, 33, 43]. Moreover, some studies have examined medication use in AD as well as healthy elderly subjects, recognizing the fact that cognitively healthy elderly subjects are also often treated for hypertension, diabetes, coagulation defects, or dyslipidemia [16, 17, 33, 43, 66]. Clearly, all potentially interfering medications should be considered in patients and controls alike, for instance by a dichotomous analysis where the BDNF levels of medication users and nonusers in both groups are kept separate and/or combined.
Lifestyle
Daily habits like exercise, smoking, alcohol consumption, and eating behavior can influence circulating BDNF levels; yet, they are rarely and not exhaustively analyzed [16, 25, 32, 43, 45, 66].
Exercise, especially aerobic activity, is known to increase circulating BDNF [51, 73, 74], mainly through stimulation of its production in the brain [75–78], although release from peripheral sources is probably also stimulated [79, 80]. Yet, an inverse relationship with peripheral BDNF has been found when patients enrolled in studies have been examined for daily physical activity (not training programs) [81]. Although the reason for the discrepancy is unclear, improved cardiorespiratory fitness [82, 83] and energy metabolism [84] in spontaneously active persons may play a key role. It is reasonable to consider that healthy elderly subjects have different rates of physical activity compared to AD patients [25, 85] and that this influences BDNF levels irrespective of dementia. A simple and effective tool to assess daily physical activity in the aged is the Physical Activity Scale for the Elderly (PASE) [86]. The scale has been designed for epidemiological studies to rate leisure time, household work, and job tasks in individuals aged 65 years or older. Therefore, the PASE score of healthy and AD cohorts should always be compared and considered as a possible covariate.
The limited available evidence regarding the effect of smoking on circulating BDNF points at a different influence on its plasma and serum levels, which are respectively lower [87, 88] and higher [51, 89–91] in smokers than in nonsmokers. A single study has found opposite results, which the authors have been unable to explain [92]. A direct influence of nicotine on cerebral BDNF has been described. Chronic nicotine administration upregulates BDNF in the cortex and hippocampus [93, 94] and in dopaminergic brain areas including the nucleus accumbens, ventral tegmental area, and substantia nigra [95, 96], but it downregulates neurotrophin in the striatum [97]. Differences in the effect of nicotine are related to exposure duration—short-term administration reducing and long-term administration increasing hippocampal BDNF [98]—and to the amount of nicotine consumed, since high but not low chronic doses reduce BDNF in the dorsal striatum [99]. The modulation of peripheral BDNF by smoking may reflect cerebral phenomena, but influences from peripheral sources may be also crucial. Indeed, in vitro analyses have documented that cigarette smoke extracts induce a dose-dependent release of BDNF, indicating that abnormal BDNF trafficking from smokers’ platelets can contribute to changes in circulating BDNF [92]. Although an in-depth analysis of the functional relationship between smoking and BDNF is beyond the scope of this paper, the above data clearly show the vital importance of classifying subjects, both healthy individuals and AD patients, as current, former, and nonsmokers. In addition, given that a significant, positive correlation has been found among serum BDNF, years of smoking [89], and smoking intensity [51, 100, 101], smokers should also be evaluated for the number of cigarettes smoked and the age when the habit was acquired. Notably, since smoking cessation induces a significant increase in plasma BDNF [87, 88], a sufficient washout period enabling normalization should be considered when assessing former smokers: to date, they have only been studied at 12 weeks, and even though BDNF had already begun to decline after 4 weeks, at 3 months, it had not reached a steady state [87]. Considering the abstinence period as a confounding factor could be an acceptable compromise.
BDNF plays an acknowledged role in addiction [102]. Accordingly, the report of a functional relationship between cerebral BDNF and alcohol use is not surprising. Moderate alcohol consumption seems to upregulate BDNF—triggering a homeostatic mechanism aimed at suppressing further intake—whereas altered BDNF signaling due to chronic alcohol use may contribute to abuse behaviors [103]. Inconsistent data are available regarding the influence of alcohol on circulating BDNF. Compared to healthy controls, subjects with alcohol dependence have been found to have unchanged [104–106] or decreased [107] serum BDNF and unchanged [107], higher [108], or lower [109] plasma BDNF. Conflicting results have also been described on the effect of abstinence from alcohol, since withdrawal has been reported both to exert no influence on plasma [110, 111] and serum BDNF [105] or to increase its serum level [104, 106, 110]. Again, the factors that can be invoked to account for these discrepancies include the heterogeneity of patients’ clinical characteristics [107]; a familial predisposition to dependence [109], a widely underrated factor; and the lack of a standardized medication schedule during withdrawal [105]. Notably, the studies that have examined the effect of alcohol on circulating BDNF have largely considered male individuals and abuse behavior, whereas information on females and moderate, social drinking is scanty. Nonetheless, some basic rules to guide the clinician in sample assessment are easy to identify and apply: healthy subjects and AD patients should all be screened for alcohol abuse and addiction and these should be considered as exclusion criteria.
Several lines of evidence support the contribution of BDNF to the dys/regulation of eating behavior: BDNF and its receptor TrkB are localized in hypothalamic and hindbrain nuclei involved in energy homeostasis [112, 113], associations have been found between BDNF polymorphisms (Val66Met, -270C/T, 196G/A) and anorexia and bulimia nervosa [114–116], and animal models of BDNF alterations exhibit hyperphagia and obesity [117, 118]. Eating disorders affect circulating BDNF, but whereas patients with anorexia and bulimia nervosa show consistently lower serum BDNF [119–121], data from obese subjects are contrasting, since higher [119, 122–124], lower [125], or unchanged serum levels [126], and lower [127, 128] or unchanged levels of plasma BDNF [127, 129, 130] have been described in obese compared to normal-weight subjects. These inconclusive findings may partly be explained by the fact that obesity is a chronic, inflammatory condition with negative systemic effects that may include effects on platelet storage [122] and BDNF production by peripheral blood mononuclear cells [130], with consequences that may vary in relation to disease severity, comorbidities, and medications. The report by Heun and colleagues [131] that eating disorders are more frequent among AD patients than among healthy elderly controls suggests that severely under- and overweight subjects should not be enrolled. Another useful option would be to assess circulating BDNF in healthy and AD subjects divided according to the WHO body mass index (BMI) classes defined for the adult population (i.e., moderate/severe thinness, BMI ≤ 17 kg/m2; underweight, 17 < BMI < 18.5 kg/m2; normal weight, 18.5 ≤ BMI < 25.0 kg/m2; overweight, 25.0 ≤ BMI < 30.0 kg/m2; obesity, BMI ≥ 30.0 kg/m2; obesity class 3, BMI > 40.0 kg/m2) [132]. Interestingly, a correlation between BMI and circulating BDNF (positive for serum and negative for plasma) has been found in patients with eating and metabolic disorders [49, 121, 123, 124, 127], but little or no information is available for patients with dementia. The single study assessing the possible influence of BMI on plasma and serum BDNF in AD patients has found no effect [45]. The issue clearly deserves further analysis.
Lack of Overlap Between Serum and Plasma BDNF Levels
The average serum BDNF level is about 200-fold higher that of plasma, a difference that reflects the amount of BDNF that is stored in circulating platelets and released during clotting [5].
Platelets undergo extensive changes in AD, including hyperactivation [133], impairment of oxidative state [134], mitochondrial deficiencies [135], basal changes in membrane fluidity and cholesterol levels [136], and atypical amyloid precursor protein (APP) metabolism [137]. Some platelet dysfunctions, such as an elevated percentage of the coated subpopulation [138], changes in APP isoform ratio [139], and increased basal activation [140], have been related to AD progression. By influencing thrombocyte function, these phenomena may exert different and even opposite effects on serum and plasma BDNF; as a consequence, different results may be obtained from the two matrices [141]. Despite its potential importance, only two studies have analyzed the effect of platelet changes on plasma and serum BDNF in AD. Platenik and colleagues [45] showed that the reduced BDNF levels found in platelet-rich plasma in patients with moderate and severe AD were due to a reduced platelet number, not to reduced BDNF levels in thrombocytes, whereas Laske et al. [66] reported that serum BDNF levels in AD patients significantly correlated with the level of plasma β-thromboglobulin, a marker of platelet activation that is unrelated to plasma BDNF.
Additional factors besides dementia can differentially affect plasma and serum BDNF in AD patients, including proinflammatory cytokines [142] and medications [143–145]. The importance of caution when extending any conclusion has been confirmed by a recent meta-analysis [146] reporting reduced serum but unchanged plasma BDNF in AD compared to healthy subjects. This work, albeit informative, did not however consider key confounding factors such as dementia staging and lifestyle and assessed the possible influence of medications only in patients, not in healthy controls. Until fresh experimental data establish which peripheral matrix is the better mirror of cerebral BDNF, simultaneous evaluation of plasma and serum levels seems to be the safest approach, not only because it may offset the influence of platelet function but also because plasma and serum may provide different types of information. Indeed, whereas plasma BDNF is the “active” form of the molecule, i.e., the fraction that is available for crossing the blood brain barrier through a saturable transport system, serum BDNF, which reflects platelet amount, may rather provide a long-term marker [15, 54, 144].
Experimental Issues
To complete the analysis, here are some considerations on a number of methodological features that, albeit easier to control than the varied factors described above, are however underrated.
Two preanalytical conditions can be critical.
-
(i)
BDNF has been suggested to follow a circadian rhythm in humans, peak values being reached in the morning [147, 148]. Differences between plasma and serum and between genders have also been documented, with serum levels being more stable throughout the day in females [149]. In all studies, the subjects tested for daily variations were young or adult, involving that further work is needed to measure these data in the elderly. In all cases, the time of blood sampling should be consistent and carefully enforced.
-
(ii)
Little or no information tends to be reported about the duration of the sample storage period, even though Trajkovska and colleagues [150] reported that while BDNF levels in whole blood remain stable for up to 5 years at − 20 °C, serum levels are negatively affected by protracted storage. A significant correlation between lower serum BDNF and longer storage time has been confirmed by Bus et al. [89], who also demonstrated that a temperature of − 85 °C attenuates the phenomenon. Although immediate assays are likely to remove any possible bias, a limited, uniform storage time (i.e., a few months) should always be applied.
Materials and methods to detect the biomarker are also crucial. Circulating BDNF is easily measured using commercial ELISA kits that seem the best approach considering future clinical applications. Numerous kits are available, and comparing five of them, Polacchini et al. [151] evidenced that differences exist in their performances, especially regarding interassay variation and specificity for total rather than mature BDNF. This finding stressed that the definition/validation of adequate standards of reliability is extremely urgent. Unfortunately, up to date, we cannot suggest the best company(ies) as the analysis of the complete panel of all existing kits is lacking. An effort to fill this gap should be a priority with the involvement of different laboratories in a systemic program.
As noted above, statistical analysis may be critical. The distribution of the variable “blood BDNF” is not suitably considered. A nonnormal distribution has widely been reported [17, 43, 45, 50, 54, 105, 108, 119, 150], albeit with some exceptions [33, 52, 87]. The phenomenon may go undetected in small samples. Moreover, the assessment of variable distribution is not explicitly described in several studies, thus raising doubts on the use of parametric tests. Normality should always be verified, and if distribution is not normal, nonparametric statistics [152] or variable transformation [153] should be applied.
Conclusions
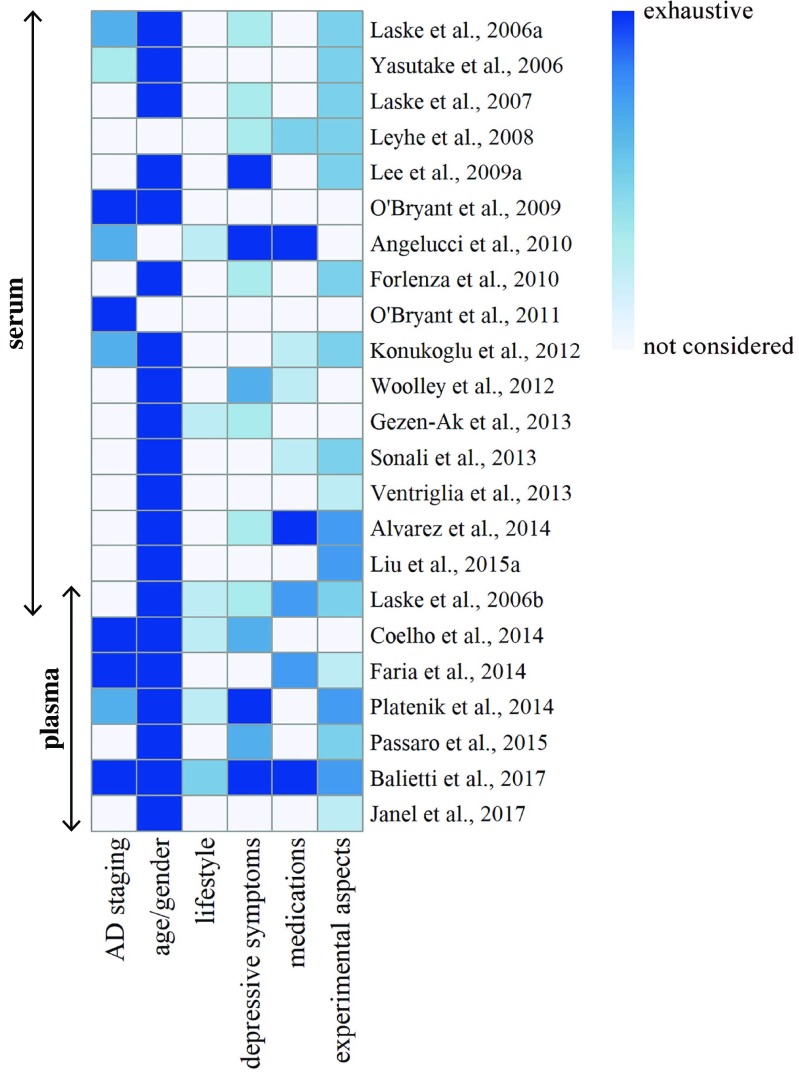
Figure 2 schematizes the degree of control (noncontrolled, partially controlled, completely controlled) that has been exerted on each potential confounding factor in the studies that have tested blood BDNF as a possible biomarker of AD. Lifestyle is by far the most underestimated area, since none of the studies have considered all four components (exercise and smoking, drinking and eating habits), and none of the components have been analyzed in depth. AD staging is also a thorny issue, since in more than half of the studies the patients’ cohort was not rigorously classified and the remaining investigations did not adopt homogenous criteria. The assessment of the influence of medications is insufficient, considering the limited attention given to polypharmacy in nondemented elderly people and to nonpsychoactive molecules in healthy and AD subjects. In contrast, there seems to be a greater awareness of the critical importance of depression and of the need for balancing age and gender in healthy and AD cohorts.
Fig. 2.
Representation of the degree of control exerted on the various factors. The heatmap outlines the level of control (indicated by the values of colors) that is exerted on each factor that may introduce a bias in the studies that have tested blood BDNF as a possible biomarker of AD. Colors range from noncontrolled (white) to completely controlled (dark blue) on the basis of the following categories: AD staging, (1) not considered; (2) defined by FAS (Functional Assessment Staging) score; (3) defined by MMSE (Mini Mental Stage Examination) score; (4) defined by CDR (Clinical Dementia Rating) score; age/gender, (1) differences between healthy and AD cohorts not adjusted; (2) healthy and AD cohorts without differences or with differences between healthy and AD cohorts adjusted; lifestyle, (1) not evaluated; (2) evaluation of one factor; (3) evaluation of two factors; (4) evaluation of three factors; (5) evaluation of four factors; depressive symptoms, (1) not evaluated; (2) exclusion of patients with a clinical diagnosis of depression or assessment of their weight; (3) evaluation of depressive symptoms; (4) exclusion of patients with a clinical diagnosis of depression or assessment of their weight and evaluation of depressive symptoms; medications, (1) not evaluated; (2) evaluation of psychoactive medications in AD patients; (3) evaluation of psychoactive and nonpsychoactive medications in AD patients; (4) evaluation of psychoactive medications in AD patients and healthy subjects; (5) evaluation of psychoactive and nonpsychoactive medications in AD patients and healthy subjects; experimental aspects, (1) not evaluated; (2) evaluation of data normality; (3) control of time of blood sampling; (4) evaluation of data normality and control of time of blood sampling; (5) evaluation of data normality, control of time of blood sampling, and information about storage duration
Where the experimental issues are concerned, the total lack of information about storage duration is maybe the most stunning aspect, even though this does not necessarily imply a real lack of standardization. Finally, the fact that a single study tested serum as well as plasma stresses the need for comparing the two matrices in the same cohorts, to clarify their different, possibly even complementary, nature.
The main conclusion that can be drawn from this review is that result comparability and extension are critically impaired by the lack of control on factors that can influence circulating BDNF irrespective of dementia. To shed light on the actual value and reliability of circulating BDNF as a biomarker of a complex condition such as AD, further work devoted to elucidating the weight of each factor and at standardizing methodologies is urgently required.
Acknowledgements
The authors are indebted to Drs. Daniele Marcotulli, Roberta Papa, and Paolo Fabbietti for their valuable advice.
Funding
This work was supported by the Italian Ministry of Health and Regione Marche [grant number 154/GR-2009-1584108].
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Marosi K, Mattson MP. BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol Metab. 2014;2:89–98. doi: 10.1016/j.tem.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mizui T, Ishikawa Y, Kumanogoh H, Kojima M. Neurobiological actions by three distinct subtypes of brain-derived neurotrophic factor: multi-ligand model of growth factor signaling. Pharmacol Res. 2016;105:93–98. doi: 10.1016/j.phrs.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 3.Park H, Poo MM. Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci. 2013;14:7–23. doi: 10.1038/nrn3379. [DOI] [PubMed] [Google Scholar]
- 4.Song JH, Yu JT, Tan L. Brain-derived neurotrophic factor in Alzheimer’s disease: risk, mechanisms, and therapy. Mol Neurobiol. 2015;52:1477–1493. doi: 10.1007/s12035-014-8958-4. [DOI] [PubMed] [Google Scholar]
- 5.Fujimura H, Altar CA, Chen R, Nakamura T, Nakahashi T, Kambayashi J, Sun B, Tandon NN. Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation. Thromb Haemost. 2002;87:728–734. [PubMed] [Google Scholar]
- 6.Kerschensteiner M, Gallmeier E, Behrens L, Leal VV, Misgeld T, Klinkert WE, Kolbeck R, Hoppe E et al (1999) Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation? J Exp Med 189:865–870 [DOI] [PMC free article] [PubMed]
- 7.Nakahashi T, Fujimura H, Altar CA, Li J, Kambayashi J, Tandon NN, Sun B. Vascular endothelial cells synthesize and secrete brain-derived neurotrophic factor. FEBS Lett. 2000;470:113–117. doi: 10.1016/s0014-5793(00)01302-8. [DOI] [PubMed] [Google Scholar]
- 8.Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology. 1998;37:1553–1561. doi: 10.1016/s0028-3908(98)00141-5. [DOI] [PubMed] [Google Scholar]
- 9.Erickson KI, Prakash RS, Voss MW, Chaddock L, Heo S, McLaren M, Pence BD, Martin SA et al (2010) Brain-derived neurotrophic factor is associated with age-related decline in hippocampal volume. J Neurosci 30:5368–5375. 10.1523/JNEUROSCI.6251-09.2010 [DOI] [PMC free article] [PubMed]
- 10.Hwang KS, Lazaris AS, Eastman JA, Teng E, Thompson PM, Gylys KH, Cole GM, Apostolova LG et al (2015) Plasma BDNF levels associate with Pittsburgh compound B binding in the brain. Alzheimers Dement (Amst) 1:187–193. 10.1016/j.dadm.2015.01.005 [DOI] [PMC free article] [PubMed]
- 11.Karege F, Schwald M, Cisse M. Postnatal developmental profile of brain-derived neurotrophic factor in rat brain and platelets. Neurosci Lett. 2002;328:261–264. doi: 10.1016/s0304-3940(02)00529-3. [DOI] [PubMed] [Google Scholar]
- 12.Klein AB, Williamson R, Santini MA, Clemmensen C, Ettrup A, Rios M, Knudsen GM, Aznar S. Blood BDNF concentrations reflect brain-tissue BDNF levels across species. Int J Neuropsychopharmacol. 2011;14:347–353. doi: 10.1017/S1461145710000738. [DOI] [PubMed] [Google Scholar]
- 13.Lang UE, Hellweg R, Seifert F, Schubert F, Gallinat J (2007) Correlation between serum brain-derived neurotrophic factor level and an in vivo marker of cortical integrity. Biol Psychiatry 62:530–535. 10.1016/j.biopsych.2007.01.002 [DOI] [PubMed]
- 14.Pillai A, Kale A, Joshi S, Naphade N, Raju MS, Nasrallah H, Mahadik SP. Decreased BDNF levels in CSF of drug-naive first-episode psychotic subjects: correlation with plasma BDNF and psychopathology. Int J Neuropsychopharmacol. 2010;13:535–539. doi: 10.1017/S1461145709991015. [DOI] [PubMed] [Google Scholar]
- 15.Sartorius A, Hellweg R, Litzke J, Vogt M, Dormann C, Vollmayr B, Danker-Hopfe H, Gass P. Correlations and discrepancies between serum and brain tissue levels of neurotrophins after electroconvulsive treatment in rats. Pharmacopsychiatry. 2009;42:270–276. doi: 10.1055/s-0029-1224162. [DOI] [PubMed] [Google Scholar]
- 16.Angelucci F, Spalletta G, di Iulio F, Ciaramella A, Salani F, Colantoni L, Varsi AE, Gianni W et al (2010) Alzheimer’s disease (AD) and mild cognitive impairment (MCI) patients are characterized by increased BDNF serum levels. Curr Alzheimer Res 7:15–20 [DOI] [PubMed]
- 17.Faria MC, Goncalves GS, Rocha NP, Moraes EN, Bicalho MA, Gualberto Cintra MT, Jardim de Paula J, Jose Ravic de Miranda LF et al (2014) Increased plasma levels of BDNF and inflammatory markers in Alzheimer’s disease. J Psychiatr Res 53:166–172. 10.1016/j.jpsychires.2014.01.019 [DOI] [PubMed]
- 18.Yasutake C, Kuroda K, Yanagawa T, Okamura T, Yoneda H. Serum BDNF, TNF-alpha and IL-1beta levels in dementia patients: comparison between Alzheimer’s disease and vascular dementia. Eur Arch Psychiatry Clin Neurosci. 2006;256:402–406. doi: 10.1007/s00406-006-0652-8. [DOI] [PubMed] [Google Scholar]
- 19.Laske C, Stransky E, Leyhe T, Eschweiler GW, Maetzler W, Wittorf A, Soekadar S, Richartz E et al (2007) BDNF serum and CSF concentrations in Alzheimer’s disease, normal pressure hydrocephalus and healthy controls. J Psychiatr Res 14:387–394. 10.1016/j.jpsychieres.2006.01 [DOI] [PubMed]
- 20.Leyhe T, Stransky E, Eschweiler GW, Buchkremer G, Laske C. Increase of BDNF serum concentration during donepezil treatment of patients with early Alzheimer’s disease. Eur Arch Psychiatry Clin Neurosci. 2008;258:124–128. doi: 10.1007/s00406-007-0764-9. [DOI] [PubMed] [Google Scholar]
- 21.Lee JG, Shin BS, You YS, Kim JE, Yoon SW, Jeon DW, Baek JH, Park SW et al (2009a) Decreased serum brain-derived neurotrophic factor levels in elderly Korean with dementia. Psychiatry Investig 6:299–305. 10.4306/pi.2009.6.4.299 [DOI] [PMC free article] [PubMed]
- 22.Forlenza OV, Diniz BS, Teixeira AL, Ojopi EB, Talib LL, Mondonca VA, Izzo G, Gattaz WF. Effect of brain-derived neurotrophic factor Val66Met polymorphism and serum levels on the progression of mild cognitive impairment. World J Biol Psychiatry. 2010;11:774–780. doi: 10.3109/15622971003797241. [DOI] [PubMed] [Google Scholar]
- 23.Gezen-Ak D, Dursun E, Hanağasi H, Bilgiç B, Lohman E, Araz ÖS, Atasoy IL, Alaylıoğlu M et al (2013) BDNF, TNFα, HSP90, CFH, and IL-10 serum levels in patients with early or late onset Alzheimer’s disease or mild cognitive impairment. J Alzheimers Dis 37:185–195. 10.3233/JAD-130497 [DOI] [PubMed]
- 24.Ventriglia M, Zanardini R, Bonomini C, Zanetti O, Volpe D, Pasqualetti P, Gennarelli M, Bocchio-Chiavetto L. Serum brain-derived neurotrophic factor levels in different neurological diseases. Biomed Res Int. 2013;2013:9D1D82. doi: 10.1155/2013/901082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coelho FG, Vital TM, Stein AM, Arantes FJ, Rueda AV, Camarini R, Teodoroy E, Santos-Galduroz RF. Acute aerobic exercise increases brain-derived neurotrophic factor levels in elderly with Alzheimer’s disease. J Alzheimers Dis. 2014;39:401–408. doi: 10.3233/JAD-131073. [DOI] [PubMed] [Google Scholar]
- 26.Liu YH, Jiao SS, Wang YR, Bu XL, Yao XQ, Xiang Y, Wang QH, Wang L et al (2015b) Associations between ApoEε4 carrier status and serum BDNF levels—new insights into the molecular mechanism of ApoEε4 actions in Alzheimer’s disease. Mol Neurobiol 51:1271–1277. 10.1007/s12035-014-8804-8 [DOI] [PubMed]
- 27.Passaro A, Dalla Nora E, Morieri ML, Soavi C, Sanz JM, Zurlo A, Fellin R, Zuliani G. Brain-derived neurotrophic factor plasma levels: relationship with dementia and diabetes in the elderly population. J Gerontol A Biol Sci Med Sci. 2015;70:294–302. doi: 10.1093/gerona/glu028. [DOI] [PubMed] [Google Scholar]
- 28.Janel N, Alexopoulos P, Badel A, Lamari F, Camproux AC, Lagarde J, Simon S, Feraudet-Tarisse C et al (2017) Combined assessment of DYRK1A, BDNF and homocysteine levels as diagnostic marker for Alzheimer’s disease. Transl Psychiatry 7:e1154. 10.1038/tp.2017.123 [DOI] [PMC free article] [PubMed]
- 29.O’Bryant SE, Hobson V, Hall JR, Waring SC, Chan W, Massman P, Lacritz L, Cullum CM et al (2009) Brain-derived neurotrophic factor levels in Alzheimer’s disease. J Alzheimers Dis 17:337–341. 10.3233/JAD-2009-1051 [DOI] [PMC free article] [PubMed]
- 30.O’Bryant SE, Hobson VL, Hall JR, Barber RC, Zhang S, Johnson L, Diaz-Arrastia R, Texas Alzheimer’s Research Consortium Serum brain-derived neurotrophic factor levels are specifically associated with memory performance among Alzheimer’s disease cases. Dement Geriatr Cogn Disord. 2011;31:31–36. doi: 10.1159/000321980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woolley JD, Strobl EV, Shelly WB, Karydas AM, Robin Ketelle RN, Wolkowitz OM, Miller BL, Rankin KP. BDNF serum concentrations show no relationship with diagnostic group or medication status in neurodegenerative disease. Curr Alzheimer Res. 2012;9:815–821. doi: 10.2174/156720512802455395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sonali N, Tripathi M, Sagar R, Vivekanandhan S. Val66Met polymorphism and BDNF levels in Alzheimer’s disease patients in North Indian population. Int J Neurosci. 2013;123:409–416. doi: 10.3109/00207454.2012.762515. [DOI] [PubMed] [Google Scholar]
- 33.Alvarez A, Aleixandre M, Linares C, Masliah E, Moessler H. Apathy and APOE4 are associated with reduced BDNF levels in Alzheimer’s disease. J Alzheimers Dis. 2014;42:1347–1355. doi: 10.3233/JAD-140849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim BY, Lee SH, Graham PL, Angelucci F, Lucia A, Pareje-Galeano H, Leyhe T, Turana Y et al (2016) Peripheral brain-derived neurotrophic factor levels in Alzheimer’s disease and mild cognitive impairment: a comprehensive systematic review and meta-analysis. Mol Neurobiol. 10.1007/s12035-016-0192-9 [DOI] [PubMed]
- 35.Laske C, Stransky E, Leyhe T, Eschweiler GW, Wittorf A, Richartz E, Bartels M, Buchkremer G et al (2006a) Stage-dependent BDNF serum concentrations in Alzheimer’s disease. J Neural Transm (Vienna) 113:1217–1224. 10.1007/s00702-005-0397-y [DOI] [PubMed]
- 36.Arancibia S, Silhol M, Mouliere F, Meffre J, Hollinger I, Maurice T, Tapia-Arancibia L. Protective effect of BDNF against beta-amyloid induced neurotoxicity in vitro and in vivo in rats. Neurobiol Dis. 2008;31:316–326. doi: 10.1016/j.nbd.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 37.Burbach GJ, Hellweg R, Haas CA, Del Turco D, Deicke U, Abramowski D, Jucker M, Staufenbiel M et al (2004) Induction of brain-derived neurotrophic factor in plaque-associated glial cells of aged APP23 transgenic mice. J Neurosci 24:2421–2430. 10.1523/JNEUROSCI.5599-03.2004 [DOI] [PMC free article] [PubMed]
- 38.Durany N, Michel T, Kurt J, Cruz-Sanchez FF, Cervas-Navarro J, Riederer P. Brain-derived neurotrophic factor and neurotrophin-3 levels in Alzheimer’s disease brains. Int J Dev Neurosci. 2000;18:807–813. [PubMed] [Google Scholar]
- 39.Elliott E, Atlas R, Lange A, Ginzburg I. Brain-derived neurotrophic factor induces a rapid dephosphorylation of tau protein through a PI-3 kinase signalling mechanism. Eur J Neurosci. 2005;22:1081–1089. doi: 10.1111/j.1460-9568.2005.04290.x. [DOI] [PubMed] [Google Scholar]
- 40.Kimura N, Takahashi M, Tashiro T, Terao K. Amyloid beta up-regulates brain-derived neurotrophic factor production from astrocytes: rescue from amyloid beta-related neuritic degeneration. J Neurosci Res. 2006;84:782–789. doi: 10.1002/jnr.20984. [DOI] [PubMed] [Google Scholar]
- 41.Poon WW, Blurton-Jones M, Tu CH, Feinberg LM, Chabrier MA, Harris JW, Jeon NL, Cotman CW. β-Amyloid impairs axonal BDNF retrograde trafficking. Neurobiol Aging. 2011;32:821–833. doi: 10.1016/j.neurobiolaging.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hachisu M, Konishi K, Hosoi M, Tani M, Tomioka H, Inamoto A, Minami S, Izuno T et al (2015) Beyond the hypothesis of serum anticholinergic activity in Alzheimer’s disease: acetylcholine neuronal activity modulates brain-derived neurotrophic factor production and inflammation in the brain. Neurodegener Dis 15:182–187. 10.1159/000381531 [DOI] [PubMed]
- 43.Balietti M, Giuli C, Fattoretti P, Fabbietti P, Papa R, Postacchini D, Conti F. Effect of a comprehensive intervention on plasma BDNF in patients with Alzheimer’s disease. J Alzheimers Dis. 2017;57:7–43. doi: 10.3233/JAD-161168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Konukoglu D, Andican G, Firtina S, Erkol KA. Serum brain-derived neurotrophic factor, nerve growth factor and neurotrophin-3 levels in dementia. Acta Neurol Belg. 2012;112:255–260. doi: 10.1007/s13760-012-0101-6. [DOI] [PubMed] [Google Scholar]
- 45.Platenik J, Fisar Z, Buchal R, Jirak R, Kitzlerova E, Zverova M, Raboch J. GSK3β, CREB, and BDNF in peripheral blood of patients with Alzheimer’s disease and depression. Prog Neuro-Psychopharmacol Biol Psychiatry. 2014;50:83–93. doi: 10.1016/j.pnpbp.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 46.Rikkert MG, Tona KD, Janssen L, Burns A, Lobo A, Robert P, Sartorius N, Stoppe N et al (2011) Waldemar G (2011) validity, reliability, and feasibility of clinical staging scales in dementia: a systematic review. Am J Alzheimers Dis Other Demen 26:357–365. 10.1177/1533317511418954 [DOI] [PMC free article] [PubMed]
- 47.Schafer KA, Tractenberg RE, Sano M, Mackell JA, Thomas RG, Gamst A, Thal LJ, Morris JC, Alzheimer’s Disease Cooperative Study Reliability of monitoring the clinical dementia rating in multicenter clinical trials. Alzheimer Dis Assoc Disord. 2004;18:219–222. [PMC free article] [PubMed] [Google Scholar]
- 48.Lanctôt KL, Hsiung GY, Feldman HH, Masoud ST, Sham L, Herrmann N. Assessing the validity of deriving clinical dementia rating (CDR) global scores from independently-obtained functional rating scale (FRS) scores in vascular dementia with and without Alzheimer’s disease. Int J Geriatr Psychiatry. 2009;24:1174–1176. doi: 10.1002/gps.2273. [DOI] [PubMed] [Google Scholar]
- 49.Lommatzsch M, Zingler D, Schuhbaeck K, Schloetcke K, Zingler C, Schuff-Werner P, Virchow JC. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol Aging. 2005;26:115–123. doi: 10.1016/j.neurobiolaging.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 50.Ziegenhorn AA, Schulte-Herbruggen O, Danker-Hopfe H, Malbranc M, Hartung HD, Anders D, Lang UE, Steinhagen-Thiessen E, Schaub RT, Hellweg R. Serum neurotrophins—a study on the time course and influencing factors in a large old age sample. Neurobiol Aging. 2007;28:1436–1445. doi: 10.1016/j.neurobiolaging.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 51.Weinstein G, Preis SR, Beiser AS, Kaess B, Chen TC, Satizabal C, Rahman F, Benjiamin EJ, Vasan RS, Seshadri S. Clinical and environmental correlates of serum BDNF: a descriptive study with plausible implications for AD research. Curr Alzheimer Res. 2017;14:722–730. doi: 10.2174/1567205014666170203094520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bus BA, Tendolkar I, Franke B, de Graaf J, den Heijer M, Buitelaar JK, Oude Voshaar RC. Serum brain-derived neurotrophic factor: determinants and relationship with depressive symptoms in a community population of middle-aged and elderly people. World J Biol Psychiatry. 2012;13:39–47. doi: 10.3109/15622975.2010.545187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Driscoll I, Martin B, An Y, Maudsley S, Ferrucci L, Mattson MP, Resnick SM. Plasma BDNF is associated with age-related white matter atrophy but not with cognitive function in older, non-demented adults. PLoS One. 2012;7:e35217. doi: 10.1371/journal.pone.0035217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Komulainen P, Pedersen M, Hanninen T, Bruunsgaard H, Lakka TA, Kivipelto M, Hassinen M, Rauramaa TH, Pedersen BK, Rauramaa R. BDNF is a novel marker of cognitive function in ageing women: the DR’s EXTRA study. Neurobiol Learn Mem. 2008;90:596–603. doi: 10.1016/j.nlm.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 55.Fukumoto N, Fujii T, Combarros O, Kamboh MI, Tsai SJ, Matsushita S, Nacmias B, Comings DE, Arboleda H, Inglsson M, Hyman BT, Akatsu H, Grupe A, Nishimura AL, Zatz M, Mattila KM, Rinne J, Goto Y, Asada T, Nakamura S, Kunugi H. Sexually dimorphic effect of the Val66Met polymorphism of BDNF on susceptibility to Alzheimer’s disease: new data and meta-analysis. Am J Med Genet B Neuropsychiatr Genet. 2010;153:235–242. doi: 10.1002/ajmg.b.30986. [DOI] [PubMed] [Google Scholar]
- 56.McDonald JH. Confounding variables. In: McDonald JH, editor. Handbook of biological statistics. 3. Baltimore: Sparky House Publishing; 2014. pp. 24–28. [Google Scholar]
- 57.Pourhoseingholi MA, Baghestani AR, Vahedi M. How to control confounding effects by statistical analysis. Gastroenterol Hepatol Bed Bench. 2012;5:79–83. [PMC free article] [PubMed] [Google Scholar]
- 58.Dwivedi Y. Involvement of brain-derived neurotrophic factor in late-life depression. Am J Geriatr Psychiatry. 2013;21:433–449. doi: 10.1016/j.jagp.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu H, Chen ZY. The role of BDNF in depression on the basis of its location in the neural circuitry. Acta Pharmacol Sin. 2011;32:3–11. doi: 10.1038/aps.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Castrén E, Rantamäki T. The role of BDNF and its receptors in depression and antidepressant drug action: reactivation of developmental plasticity. Dev Neurobiol. 2010;70:289–297. doi: 10.1002/dneu.20758. [DOI] [PubMed] [Google Scholar]
- 61.Eisch AJ, Bolaños CA, de Wit J, Simonak RD, Pudiak CM, Barrot M, Verhaagen J, Nestler EJ. Brain-derived neurotrophic factor in the ventral midbrain-nucleus accumbens pathway: a role in depression. Biol Psychiatry. 2003;54:994–1005. doi: 10.1016/j.biopsych.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 62.Brunoni AR, Lopes M, Fregni F. A systematic review and meta-analysis of clinical studies on major depression and BDNF levels: implications for the role of neuroplasticity in depression. In J Neuropsychopharmacol. 2008;11:1169–1180. doi: 10.1017/S1461145708009309. [DOI] [PubMed] [Google Scholar]
- 63.Molendijk ML, Spinhoven P, Pola M, Bus BA, Penninx BW, Elzinga BM. Serum BDNF concentrations as peripheral manifestations of depression: evidence from a systematic review and meta-analyses on 179 associations (N=9484) Mol Psychiatry. 2014;19:791–800. doi: 10.1038/mp.2013.105. [DOI] [PubMed] [Google Scholar]
- 64.Karege F, Bondolfi G, Gervasoni N, Schwald M, Aubry J, Bertschy G. Low brain-derived neurotrophic factor (BDNF) levels in serum of depressed patients probably results from lowered platelet BDNF release unrelated to platelet reactivity. Biol Psychiatry. 2005;57:1068–1072. doi: 10.1016/j.biopsych.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 65.Modrego PJ. Depression in Alzheimer’s disease. Pathophysiology, diagnosis, and treatment. J Alzheimers Dis. 2010;21:1077–1087. doi: 10.3233/jad-2010-100153. [DOI] [PubMed] [Google Scholar]
- 66.Laske C, Stransky E, Leyhe T, Eschweiler GW, Schott K, Langer H, Gawaz M. Decreased brain-derived neurotrophic factor (BDNF)- and beta-thromboglobulin (beta-TG)-blood levels in Alzheimer’s disease. Thromb Haemost. 2006;96:102–103. doi: 10.1160/TH06-03-0173. [DOI] [PubMed] [Google Scholar]
- 67.Novais F, Starkstein S. Phenomenology of depression in Alzheimer’s disease. J Alzheimers Dis. 2015;47:845–855. doi: 10.3233/JAD-148004. [DOI] [PubMed] [Google Scholar]
- 68.Sheikh JI, Yesavage JA. Geriatric depression scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165–173. [Google Scholar]
- 69.Polyakova M, Stuke K, Schuemberg K, Mueller K, Schoenknecht P, Schroeter ML. BDNF as a biomarker for successful treatment of mood disorders: a systematic & quantitative meta-analysis. J Affect Disord. 2015;174:432–440. doi: 10.1016/j.jad.2014.11.044. [DOI] [PubMed] [Google Scholar]
- 70.Huang TL, Hung YY. Lorazepam reduces the serum brain-derived neurotrophic factor level in schizophrenia patients with catatonia. Prog Neuro-Psychopharmacol Biol Psychiatry. 2009;33:158–159. doi: 10.1016/j.pnpbp.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 71.Zhang J, Mu X, Breker DA, Li Y, Gao Z, Huang Y. Atorvastatin treatment is associated with increased BDNF level and improved functional recovery after atherothrombotic stroke. Int J Neurosci. 2017;127:92–97. doi: 10.3109/00207454.2016.1146882. [DOI] [PubMed] [Google Scholar]
- 72.Allard JS, Perez EJ, Fukui K, Carpenter P, Ingram DK, de Cabo R. Prolonged metformin treatment leads to reduced transcription of Nrf2 and neurotrophic factors without cognitive impairment in older C57BL/6J mice. Behav Brain Res. 2016;301:1–9. doi: 10.1016/j.bbr.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Coelho FG, Gobbi S, Andreatto CA, Corazza DI, Pedroso RV, Santos-Galduroz RF. Physical exercise modulates peripheral levels of brain-derived neurotrophic factor (BDNF): a systematic review of experimental studies in the elderly. Arch Gerontol Geriatr. 2013;56:10–15. doi: 10.1016/j.archger.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 74.Zoladz JA, Pilc A. The effect of physical activity on the brain derived neurotrophic factor: from animal to human studies. J Physiol Pharmacol. 2010;61:533–541. [PubMed] [Google Scholar]
- 75.Berchtold NC, Chinn G, Chou M, Kesslak JP, Cotman CW. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience. 2005;133:853–861. doi: 10.1016/j.neuroscience.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 76.Rasmussen P, Brassard P, Adser H, Pedersen MV, Leick L, Hart E, Secher NH, Pedersen BK, Pilegaard H. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp Physiol. 2009;94:1062–1069. doi: 10.1113/expphysiol.2009.048512. [DOI] [PubMed] [Google Scholar]
- 77.Seifert T, Brassard P, Wissenberg M, Rasmussen P, Nordby P, Stallknech B, Adser H, Jakobsen AH, Pilegaard H, Nielsen HB, Secher NH. Endurance training enhances BDNF release from the human brain. Am J Physiol Regul Integr Comp Physiol. 2010;298:R372–R377. doi: 10.1152/ajpregu.00525.2009. [DOI] [PubMed] [Google Scholar]
- 78.Soya H, Nakamura T, Deocaris CC, Kimpara A, Iimura M, Fujikawa T, Chang H, McEwen BS, Nishijima T. BDNF induction with mild exercise in the rat hippocampus. Biochem Biophys Res Commun. 2007;358:961–967. doi: 10.1016/j.bbrc.2007.04.173. [DOI] [PubMed] [Google Scholar]
- 79.Brunelli A, Dimauro I, Sgrò P, Emerenziani GP, Magi F, Baldari C, Guidetti L, Di Luigi L, Parisi P, Caporossi D. Acute exercise modulates BDNF and pro-BDNF protein content in immune cells. Med Sci Sports Exerc. 2012;44:1871–1880. doi: 10.1249/MSS.0b013e31825ab69b. [DOI] [PubMed] [Google Scholar]
- 80.Cho HC, Kim J, Kim S, Son YH, Lee N, Jung SH. The concentrations of serum, plasma and platelet BDNF are all increased by treadmill VO2 max performance in healthy college men. Neurosci Lett. 2012;519:78–83. doi: 10.1016/j.neulet.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 81.Huang T, Larsen KT, Ried-Larsen M, Moller NC, Andersen LB. The effects of physical activity and exercise on brain-derived neurotrophic factor in healthy humans: a review. Scand J Med Sci Sports. 2014;24:1–10. doi: 10.1111/sms.12069. [DOI] [PubMed] [Google Scholar]
- 82.Currie J, Ramsbottom R, Ludlow H, Nevill A, Gilder M. Cardio-respiratory fitness, habitual physical activity and serum brain derived neurotrophic factor (BDNF) in men and women. Neurosci Lett. 2009;451:152–155. doi: 10.1016/j.neulet.2008.12.043. [DOI] [PubMed] [Google Scholar]
- 83.Jung SH, Kim J, Davis JM, Blair SN, Cho HC. Association among basal serum BDNF, cardiorespiratory fitness and cardiovascular disease risk factors in untrained healthy Korean men. Eur J Appl Physiol. 2011;111:303–311. doi: 10.1007/s00421-010-1658-5. [DOI] [PubMed] [Google Scholar]
- 84.Nofuji Y, Suwa M, Moriyama Y, Nakano H, Ichimiya A, Nishichi R, Sasaki H, Radak Z, Kumugai S. Decreased serum brain-derived neurotrophic factor in trained men. Neurosci Lett. 2008;437:29–32. doi: 10.1016/j.neulet.2008.03.057. [DOI] [PubMed] [Google Scholar]
- 85.Gagliardi C, Papa R, Postacchini D, Giuli C. Association between cognitive status and physical activity: study profile on baseline survey of the My Mind Project. Int J Environ Res Public Health. 2016;13:E585. doi: 10.3390/ijerph13060585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Washburn RA, McAuley E, Katula J, Mihalko SL, Boileau RA. The physical activity scale for the elderly (PASE): evidence for validity. J Clin Epidemiol. 1999;52:643–651. doi: 10.1016/s0895-4356(99)00049-9. [DOI] [PubMed] [Google Scholar]
- 87.Bhang SY, Choi SW, Ahn JH. Changes in plasma brain-derived neurotrophic factor levels in smokers after smoking cessation. Neurosci Lett. 2010;468:7–11. doi: 10.1016/j.neulet.2009.10.046. [DOI] [PubMed] [Google Scholar]
- 88.Kim TS, Kim DJ, Lee H, Kim YK. Increased plasma brain-derived neurotrophic factor levels in chronic smokers following unaided smoking cessation. Neurosci Lett. 2007;423:53–57. doi: 10.1016/j.neulet.2007.05.064. [DOI] [PubMed] [Google Scholar]
- 89.Bus BA, Molendijk ML, Penninx BJ, Buitelaar JK, Kenis G, Prickaerts J, Elzinga BM, Voshaar RC. Determinants of serum brain-derived neurotrophic factor. Psychoneuroendocrinology. 2011;36:228–239. doi: 10.1016/j.psyneuen.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 90.Jamal M, Van der Does W, Elzinga BM, Molendijk ML, Penninx BW. Association between smoking, nicotine dependence, and BDNF Val66Met polymorphism with BDNF concentrations in serum. Nicotine Tob Res. 2015;17:323–329. doi: 10.1093/ntr/ntu151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Umene-Nakano W, Yoshimura R, Yoshii C, Hoshuyama T, Hayashi K, Hori H, Katsuki A, Ikenouchi-Sugita A, Nakamura J. Varenicline does not increase serum BDNF levels in patients with nicotine dependence. Hum Psychopharmacol. 2010;25:276–279. doi: 10.1002/hup.1113. [DOI] [PubMed] [Google Scholar]
- 92.Amadio P, Baldassarre D, Sandrini L, Weksler BB, Tremoli E, Barbieri SS. Effect of cigarette smoke on monocyte procoagulant activity: focus on platelet-derived brain-derived neurotrophic factor (BDNF) Platelets. 2017;28:60–65. doi: 10.1080/09537104.2016.1203403. [DOI] [PubMed] [Google Scholar]
- 93.Andresen JH, Løberg EM, Wright M, Goverud IL, Stray-Pedersen B, Saugstad OD. Nicotine affects the expression of brain-derived neurotrophic factor mRNA and protein in the hippocampus of hypoxic newborn piglets. J Perinatal Med. 2009;37:553–560. doi: 10.1515/JPM.2009.081. [DOI] [PubMed] [Google Scholar]
- 94.Czubak A, Nowakowska E, Kus K, Burda K, Metelska J, Baer-Dubowska W, Cichocki M. Influences of chronic venlafaxine, olanzapine and nicotine on the hippocampal and cortical concentrations of brain-derived neurotrophic factor (BDNF) Pharmacol Rep. 2009;6:1017–1023. doi: 10.1016/s1734-1140(09)70163-x. [DOI] [PubMed] [Google Scholar]
- 95.Correll JA, Noel DM, Sheppard AB, Thompson KN, Li Y, Yin D, Brown RW. Nicotine sensitization and analysis of brain-derived neurotrophic factor in adolescent beta-arrestin-2 knockout mice. Synapse. 2009;63:510–519. doi: 10.1002/syn.20625. [DOI] [PubMed] [Google Scholar]
- 96.Kivinummi T, Kaste K, Rantamäki T, Castrén E, Ahtee L. Alterations in BDNF and phospho-CREB levels following chronic oral nicotine treatment and its withdrawal in dopaminergic brain areas of mice. Neurosci Lett. 2011;491:108–112. doi: 10.1016/j.neulet.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 97.Yeom M, Shim I, Lee HJ, Hahm DH. Proteomic analysis of nicotine-associated protein expression in the striatum of repeated nicotine-treated rats. Biochem Biophys Res Commun. 2005;326:321–328. doi: 10.1016/j.bbrc.2004.11.034. [DOI] [PubMed] [Google Scholar]
- 98.Kenny PJ, File SE, Rattray M. Acute nicotine decreases, and chronic nicotine increases the expression of brain-derived neurotrophic factor mRNA in rat hippocampus. Brain Res Mol Brain Res. 2000;85:234–238. doi: 10.1016/s0169-328x(00)00246-1. [DOI] [PubMed] [Google Scholar]
- 99.Ortega LA, Tracy BA, Gould TJ, Parikh V. Effects of chronic low- and high-dose nicotine on cognitive flexibility in C57BL/6J mice. Behav Brain Res. 2013;238:134–245. doi: 10.1016/j.bbr.2012.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang XY, Tan YL, Chen DC, Tan SP, Yang FD, Zunta-Soares GB, Soares JC. Effects of cigarette smoking and alcohol use on neurocognition and BDNF levels in a Chinese population. Psychopharmacology. 2016;233:435–445. doi: 10.1007/s00213-015-4124-6. [DOI] [PubMed] [Google Scholar]
- 101.Zhang XY, Xiu MH, Chen DC, Yang FD, Wu GY, Lu L, Kosten TA, Kosten TR. Nicotine dependence and serum BDNF levels in male patients with schizophrenia. Psychopharmacology. 2010;212:301–307. doi: 10.1007/s00213-010-1956-y. [DOI] [PubMed] [Google Scholar]
- 102.Koskela M, Bäck S, Võikar V, Richie CT, Domanskyi A, Harvey BK, Airayaara M. Update of neurotrophic factors in neurobiology of addiction and future directions. Neurobiol Dis. 2017;97:189–200. doi: 10.1016/j.nbd.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Logrip ML, Barak S, Warnault V, Ron D. Corticostriatal BDNF and alcohol addiction. Brain Res. 2015;1628:60–67. doi: 10.1016/j.brainres.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Costa MA, Girard M, Dalmay F, Malauzat D. Brain-derived neurotrophic factor serum levels in alcohol-dependent subjects 6 months after alcohol withdrawal. Alcohol Clin Exp Res. 2011;35:1966–1973. doi: 10.1111/j.1530-0277.2011.01548.x. [DOI] [PubMed] [Google Scholar]
- 105.Heberlein A, Muschler M, Wilhelm J, Frieling H, Lenz B, Gröschl M, Komhuber J, Bleich S, Hilemacher T. BDNF and GDNF serum levels in alcohol-dependent patients during withdrawal. Prog Neuropsychopharmacol Biol Psychiatr. 2010;34:1060–1064. doi: 10.1016/j.pnpbp.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 106.Huang MC, Chen CH, Chen CH, Liu SC, Ho CJ, Shen WW, Leu SJ. Alterations of serum brain-derived neurotrophic factor levels in early alcohol withdrawal. Alcohol Alcohol. 2008;43:241–245. doi: 10.1093/alcalc/agm172. [DOI] [PubMed] [Google Scholar]
- 107.Zanardini R, Fontana A, Pagano R, Mazzaro E, Bergamasco F, Romagnosi G, Gennarelli M, Bocchio-Chiavetto L, et al. Alterations of brain-derived neurotrophic factor serum levels in patients with alcohol dependence. Alcohol Clin Exp Res. 2011;35:1529–1533. doi: 10.1111/j.1530-0277.2011.01489.x. [DOI] [PubMed] [Google Scholar]
- 108.Lee BC, Choi IG, Kim YK, Ham BJ, Yang BH, Roh S, Choi J, Lee JS, Oh DY, Chai YG. Relation between plasma brain-derived neurotrophic factor and nerve growth factor in the male patients with alcohol dependence. Alcohol. 2009;43:265–269. doi: 10.1016/j.alcohol.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 109.Joe KH, Kim YK, Kim TS, Roh SW, Choi SW, Kim YB, Lee HJ, Kim DJ. Decreased plasma brain- derived neurotrophic factor levels in patients with alcohol dependence. Alcohol Clin Exp Res. 2007;31:1833–1888. doi: 10.1111/j.1530-0277.2007.00507.x. [DOI] [PubMed] [Google Scholar]
- 110.D’Sa C, Dileone RJ, Anderson GM, Sinha R. Serum and plasma brain-derived neurotrophic factor (BDNF) in abstinent alcoholics and social drinkers. Alcohol. 2012;4:253–259. doi: 10.1016/j.alcohol.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Reynolds PM, Mueller SW, MacLaren R. A comparison of dexmedetomidine and placebo on the plasma concentrations of NGF, BDNF, GDNF, and epinephrine during severe alcohol withdrawal. Alcohol. 2015;49:15–19. doi: 10.1016/j.alcohol.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 112.Conner JM, Lauterborn JC, Yan Q, Gall CM, Varon S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J Neurosci. 1997;17:2295–2313. doi: 10.1523/JNEUROSCI.17-07-02295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yan Q, Radeke MJ, Matheson CR, Talvenheimo J, Welcher AA, Feinstein SC. Immunocytochemical localization of TrkB in the central nervous system of the adult rat. J Comp Neurol. 1997;378:135–157. [PubMed] [Google Scholar]
- 114.Fox EA, Byerly MS. A mechanism underlying mature-onset obesity: evidence from the hyperphagic phenotype of brain-derived neurotrophic factor mutants. Am J Physiol Regul Integr Comp Physiol. 2004;286:R994–R1004. doi: 10.1152/ajpregu.00727.2003. [DOI] [PubMed] [Google Scholar]
- 115.Koizumi H, Hashimoto K, Itoh K, Nakazato M, Shimizu E, Ohgake S, Koike K, Okamura N, Matsushita S, Suzuki K, Murayama M, Higuchi S, Iyo M. Association between the brain-derived neurotrophic factor 196G/A polymorphism and eating disorders. Am J Med Genet B Neuropsychiatr Genet. 2004;127B:125–127. doi: 10.1002/ajmg.b.20153. [DOI] [PubMed] [Google Scholar]
- 116.Ribasés M, Gratacòs M, Fernández-Aranda F, Bellodi L, Boni C, Anderluh M, Cavallini MC, Cellini E, Di Bella D, Erzegovesi S, Foulon C, Gabrovsek M, Gorwood P, Hebebrand J, Hinney A, Holliday J, Hi X, Karwautz A, Kipman A, Komel R, Nacmias B, Remschmidt H, Ricca V, Sorbi S, Wagner G, Treasure J, Collier DA, Estivill X. Association of BDNF with anorexia, bulimia and age of onset of weight loss in six European populations. Hum Mol Genet. 2004;13:1205–1212. doi: 10.1093/hmg/ddh137. [DOI] [PubMed] [Google Scholar]
- 117.Kernie SG, Liebl DJ, Parada LF. BDNF regulates eating behaviour and locomotor activity in mice. EMBO J. 2000;19:1290–1300. doi: 10.1093/emboj/19.6.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Unger TJ, Calderon GA, Bradley LC, Sena-Esteves M, Rios M. Selective deletion of Bdnf in the ventromedial and dorsomedial hypothalamus of adult mice results in hyperphagic behaviour and obesity. J Neurosci. 2007;27:14265–14274. doi: 10.1523/JNEUROSCI.3308-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Monteleone P, Tortorella A, Martiadis V, Serritella C, Fuschino A, Maj M. Opposite changes in the serum brain-derived neurotrophic factor in anorexia nervosa and obesity. Psychosom Med. 2004;66:744–748. doi: 10.1097/01.psy.0000138119.12956.99. [DOI] [PubMed] [Google Scholar]
- 120.Nakazato M, Hashimoto K, Shimizu E, Kumakiri C, Koizumi H, Okamura N, Mitsumori M, Komatsu N, Iyo M. Decreased levels of serum brain-derived neurotrophic factor in female patients with eating disorders. Biol Psychiatry. 2003;54:485–490. doi: 10.1016/s0006-3223(02)01746-8. [DOI] [PubMed] [Google Scholar]
- 121.Saito S, Watanabe K, Hashimoto E, Saito T. Low serum BDNF and food intake regulation: a possible new explanation of the pathophysiology of eating disorders. Prog Neuropsychopharmacl Biol Psychiatry. 2009;33:312–316. doi: 10.1016/j.pnpbp.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 122.Kim KW, Won YL, Ko KS, Roh JW. Smoking habits and neuropeptides: adiponectin, brain-derived neurotrophic factor, and leptin levels. Toxicol Res. 2014;30:91–97. doi: 10.5487/TR.2014.30.2.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Roth CL, Elfers C, Gebhardt U, Müller HL, Reinehr T. Brain-derived neurotrophic factor and its relation to leptin in obese children before and after weight loss. Metabolism. 2013;62:226–234. doi: 10.1016/j.metabol.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 124.Suwa M, Kishimoto H, Nofuji Y, Nakano H, Sasaki H, Radak Z, Kumagai S. Serum brain-derived neurotrophic factor level is increased and associated with obesity in newly diagnosed female patients with type 2 diabetes mellitus. Metabolism. 2006;55:852–857. doi: 10.1016/j.metabol.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 125.El-Gharbawy AH, Adler-Wailes DC, Mirch MC, Theim KR, Ranzenhofer L, Tanofsky-Kraff M, Yanovski JA. Serum brain-derived neurotrophic factor concentrations in lean and overweight children and adolescents. J Clin Endocrinol Metab. 2006;91:3548–3552. doi: 10.1210/jc.2006-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lee IT, Wang JS, Fu CP, Lin SY, Sheu WH. Relationship between body weight and the increment in serum brain-derived neurotrophic factor after oral glucose challenge in men with obesity and metabolic syndrome: a prospective study. Medicine (Baltimore) 2016;95:e5260. doi: 10.1097/MD.0000000000005260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Araki S, Yamamoto Y, Dobashi K, Asayama K, Kusuhara K. Decreased plasma levels of brain-derived neurotrophic factor and its relationship with obesity and birth weight in obese Japanese children. Obes Res Clin Pract. 2014;8:e63–e69. doi: 10.1016/j.orcp.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 128.Krabbe KS, Nielsen AR, Krogh-Madsen R, Plomgaard P, Rasmussen P, Erikstrup C, Fischer CP, Lindegaard B, Petersen AM, Taudorf S, Secher NH, Pilegaard H, Bruunsgaard H, Pedersen BK. Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia. 2007;50:431–438. doi: 10.1007/s00125-006-0537-4. [DOI] [PubMed] [Google Scholar]
- 129.Corripio R, Gónzalez-Clemente JM, Jacobo PS, Silvia N, Lluis G, Joan V, Assumpta C. Plasma brain-derived neurotrophic factor in prepubertal obese children: results from a 2-year lifestyle intervention programme. Clin Endocrinol. 2012;77:715–720. doi: 10.1111/j.1365-2265.2012.04431.x. [DOI] [PubMed] [Google Scholar]
- 130.Huang CJ, Mari DC, Whitehurst M, Slusher A, Wilson A, Shibata Y. Brain-derived neurotrophic factor expression ex vivo in obesity. Physiol Behav. 2014;123:76–79. doi: 10.1016/j.physbeh.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 131.Heun R, Schoepf D, Potluri R, Natalwala A. Alzheimer’s disease and co-morbidity: increased prevalence and possible risk factors of excess mortality in a naturalistic 7-year follow-up. Eur Psychiatry. 2013;28:40–48. doi: 10.1016/j.eurpsy.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 132.WHO Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995;854:1–452. [PubMed] [Google Scholar]
- 133.Ciabattoni G, Porreca E, Di Febbo C, Di Iorio A, Paganelli R, Bucciarelli T, Pescara L, Del Re L, Giusti C, Falco A, Sau A, Patrono C, Davì G. Determinants of platelet activation in Alzheimer’s disease. Neurobiol Aging. 2007;28:336–342. doi: 10.1016/j.neurobiolaging.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 134.Kawamoto EM, Munhoz CD, Glezer I, Bahia VS, Caramelli P, Nitrini R, Gorjao R, Curi R, Scavone C, Marcourakis T. Oxidative state in platelets and erythrocytes in aging and Alzheimer’s disease. Neurobiol Aging. 2005;26:857–864. doi: 10.1016/j.neurobiolaging.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 135.Valla J, Schneider L, Niedzielko T, Coon KD, Caselli R, Sabbagh MN, Ahern GL, Baxter L, Alexander G, Walker DG, Reiman EM. Impaired platelet mitochondrial activity in Alzheimer’s disease and mild cognitive impairment. Mitochondrion. 2006;6:323–330. doi: 10.1016/j.mito.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Liu L, Zhang K, Tan L, Chen YH, Cao YP. Alterations in cholesterol and ganglioside GM1 content of lipid rafts in platelets from patients with Alzheimer disease. Alzheimer Dis Assoc Disord. 2015;29:63–69. doi: 10.1097/WAD.0000000000000041. [DOI] [PubMed] [Google Scholar]
- 137.Jelic V, Hagman G, Yamamoto NG, Teranishi Y, Nishimur T, Winblad B, Pavlov PF. Abnormal platelet amyloid-β protein precursor (AβPP) metabolism in Alzheimer’s disease: identification and characterization of a new AβPP isoform as potential biomarker. J Alzheimers Dis. 2013;35:285–295. doi: 10.3233/JAD-122122. [DOI] [PubMed] [Google Scholar]
- 138.Prodan CI, Ross ED, Vincent AS, Dale GL. Rate of progression in Alzheimer’s disease correlates with coated-platelet levels—a longitudinal study. Transl Res. 2008;152:99–102. doi: 10.1016/j.trsl.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 139.Baskin F, Rosenberg RN, Iyer L, Hynan L, Cullum CM. Platelet APP isoform ratios correlate with declining cognition in AD. Neurology. 2000;54:1907–1909. doi: 10.1212/wnl.54.10.1907. [DOI] [PubMed] [Google Scholar]
- 140.Stellos K, Panagiota V, Kogel A, Leyhe T, Gawaz M, Laske C. Predictive value of platelet activation for the rate of cognitive decline in Alzheimer’s disease patients. J Cereb Blood Flow Metab. 2010;30:1817–1820. doi: 10.1038/jcbfm.2010.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Serra-Millas M. Are the changes in the peripheral brain-derived neurotrophic factor levels due to platelet activation? World J Psychiatry. 2016;6:84–101. doi: 10.5498/wjp.v6.i1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chimienti G, Mezzapesa A, Rotelli MT, Lupo L, Pepe G. Plasma concentrations but not serum concentrations of brain-derived neurotrophic factor are related to pro-inflammatory cytokines in patients undergoing major abdominal surgery. Clin Biochem. 2012;45:631–636. doi: 10.1016/j.clinbiochem.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 143.Hochstrasser T, Ehrlich D, Sperner-Unterweger B, Humpel C. Antidepressants and anti-inflammatory drugs differentially reduce the release of NGF and BDNF from rat platelets. Pharmacopsychiatry. 2013;46:29–34. doi: 10.1055/s-0032-1314843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Serra-Millas M, Lopez-Vilchez I, Navarro V, Galan AM, Escolar G, Penades R, Catalan R, Fanasas L, Arias B, Gasto C. Changes in plasma and platelet BDNF levels induced by S-citalopram in major depression. Psychopharmacology. 2011;16:1–8. doi: 10.1007/s00213-011-2180-0. [DOI] [PubMed] [Google Scholar]
- 145.Stoll P, Plessow A, Bratke, Virchow JC, Lommatzsch M (2011) Differential effect of clopidogrel and aspirin on the release of BDNF from platelets. J Neuroimmunol 238:104–106. doi: 10.1016/j.jneuroim.2011.06.015 [DOI] [PubMed]
- 146.Qin XY, Cao C, Cawley NX, Liu TT, Yuan J, Lo YP, Cheng Y. Decreased peripheral brain-derived neurotrophic factor levels in Alzheimer’s disease: a meta-analysis study (N=7277) Mol Psychiatry. 2017;22:312–320. doi: 10.1038/mp.2016.62. [DOI] [PubMed] [Google Scholar]
- 147.Begliuomini S, Lenzi E, Ninni F, Casarosa E, Merlini S, Pluchino N, Valentino V, Luisi S, Luisi M, Genazzani AR. Plasma brain-derived neurotrophic factor daily variations in men: correlation with cortisol circadian rhythm. J Endocrinol. 2008;197:429–435. doi: 10.1677/JOE-07-0376. [DOI] [PubMed] [Google Scholar]
- 148.Pluchino N, Cubeddu A, Begliuomini S, Merlini S, Giannini A, Bucci F, Casarosa E, Luisi M, Cela V, Genazzani AR. Daily variation of brain-derived neurotrophic factor and cortisol in women with normal menstrual cycles, undergoing oral contraception and in postmenopause. Hum Reprod. 2009;24:2303–2309. doi: 10.1093/humrep/dep119. [DOI] [PubMed] [Google Scholar]
- 149.Choi SW, Bhang S, Ahn JH. Diurnal variation and gender differences of plasma brain-derived neurotrophic factor in healthy human subjects. Psychiatry Res. 2011;186:427–430. doi: 10.1016/j.psychres.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 150.Trajkovska V, Marcussen AB, Vinberg M, Hartvig P, Aznar S, Knudsen GM. Measurements of brain-derived neurotrophic factor: methodological aspects and demographical data. Brain Res Bull. 2007;73:143–149. doi: 10.1016/j.brainresbull.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 151.Polacchini A, Metelli G, Francavilla R, Baj G, Florean M, Mascaretti LG, Tongiorgi E. A method for reproducible measurements of serum BDNF: comparison of the performance of six commercial assays. Sci Rep. 2015;5:17989. doi: 10.1038/srep17989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nahm FS. Nonparametric statistical tests for the continuous data: the basic concept and the practical use. Korean J Anesthesiol. 2016;69:8–14. doi: 10.4097/kjae.2016.69.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bland JM, Altman DG. Transformations, means, and confidence intervals. BMJ. 1996;312:1079. doi: 10.1136/bmj.312.7038.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]