Abstract
The public health and medical response to a radiological or nuclear incident requires the capability to sort, assess, treat, triage and to ultimately discharge, refer or transport people to their next step in medical care. The size of the incident and scarcity of resources at the location of each medical decision point will determine how patients are triaged and treated. This will be a rapidly evolving situation impacting medical responders at regional, national and international levels. As capabilities, diagnostics and medical countermeasures improve, a dynamic system-based approach is needed to plan for and manage the incident, and to adapt effectively in real time. In that the concepts and terms can be unfamiliar and possibly confusing, resources and a concept of operations must be considered well in advance. An essential underlying tenet is that medical evaluation and care will be managed by healthcare professionals with biodosimetry assays providing critical supporting data.
INTRODUCTION
There was substantial progress made over the last decade that is still ongoing in the public health and medical planning and response to a nuclear detonation and radiological incident(1, 2). This includes investment, research and development in understanding the pathophysiology of radiation injury, developing medical countermeasures (MCMs) to mitigate injury post-exposure and exploring a range of diagnostic tests to assist the medical decision-makers. These efforts are largely supported by the Radiation and Nuclear Medical Countermeasures programme of the National Institute of Allergy and Infectious Diseases (NIAID)(3) and the Office of Emergency Management (OEM)(4) and the Chemical, Biological, Nuclear and Radiological Threat Program of the Biomedical Advanced Research and Development Authority (BARDA)(5) in the Office of the Assistant Secretary for Preparedness and Response (ASPR) within the Department of Health and Human Services. There is also significant investment in research and development by industry and substantial voluntary efforts by the Radiation Injury Treatment Network (RITN)(6).
Since a nuclear detonation will be a resource-constrained environment, critical triage decisions must be made that are fair,(7) widely used and ensure care is provided in a manner that will save the most lives. The role of clinical diagnostics will differ depending on the location, and the goals of triage and care will evolve over time. The clinical diagnostics will be used for all aspects of triage and management, which will be trauma assessment first, then radiation-focused triage, then ongoing evaluation of patients’ medical management and later epidemiology and long-term risk assessment.
The purpose of this report is to (a) provide an overview of the resources and tools now available that were developed by the ASPR along with government and non-government partners for planning and response to a nuclear detonation and large-scale radiological incident, (b) address ongoing issues and concepts related to biodosimetry and laboratory support that would benefit from clarification based on our experience in presentations and discussions and (c) present ideas under consideration that could further enhance national and international preparedness and response. This is not intended to be a detailed review of this complex and evolving subject.
CONCEPTS IN BIODOSIMETRY
The essential concept in medical evaluation and care is that patients will be managed by healthcare professionals. Laboratory data are critical to support decision-making, but the data themselves are not the final adjudicator of medical and public health assessment, triage, treatment and medical management. Assessment in the chaotic aftermath of a large-scale nuclear incident will require that any and all useful information be judiciously utilised, recognising that the fidelity of the information will improve as resources and personnel arrive. Initially, there will be a ‘scarce resources’ setting(8, 9) with the degree of scarcity varying by location and time. The importance of ‘fairness’ in triage and management has been discussed elsewhere(7). Together, all of the above concepts will impact the overall response including decisions for resource allocation, with a focus on medical and public health considerations as top priorities and decision drivers(2).
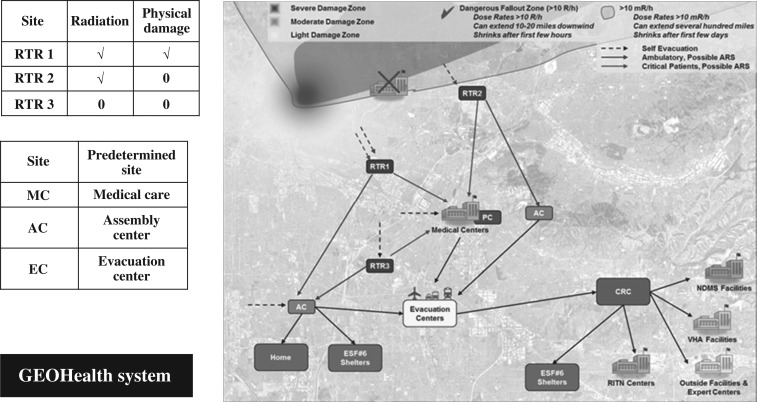
The number and type of casualties will vary by the size, scope and location of the incident,(8) and any nuclear incident or large-scale radiological incident will be accompanied by fear of radiation and some reluctance of responders to enter a potentially contaminated environment. The general concept of operations (CONOPS) used by the US government includes the RTR (Radiation TRiage, TReatment and TRansport) System(10) to help responders and decision-makers describe the presence of physical damage and radiation. This medical and geographical information systems (GIS)-based approach is currently being used for planning and during exercises; more specific guidance and technical resources are under development by a team lead by ASPR and the Federal Emergency Management Agency(11). Figure 1 illustrates the RTR medical response system and its medical and GIS-based response systems-based approach.
Figure 1.
The RTR system and medical response, GIS systems approach. The response to a nuclear or large radiological disaster requires accounting for the physical destruction and the presence of radiation. The damage zones include severe, moderate and light damage, dangerous fallout zones and a zone where time in the zone is monitored. The RTR system(10) includes sites that will form and be determined spontaneously, RTR1, 2 and 3, and others that are predetermined sites (e.g. assembly centres, medical centres [Veterans Administration (VA), National Disaster Medical System (NDMS) and RITN], evacuation centres, community reception centres (CRCs), and Emergency Support Function (ESF) shelters). The coordination of information is accomplished using GeoHEALTH, which is the GIS approach developed by ASPR(12). (Figure reproduced with permission from Hrdrina et al.(10))
Dose versus biodose
This distinction between dose and biodose is critical as it not only leads to confusion for responders and the public, but has been the source of debate among experts because the unit ‘Gray (Gy)’ is generally applied to describe the dose and the biodose. Dose is a physical measure of radiation absorbed. The Unit is Gray which is the absorption of one joule of energy per kilogram of matter(13). The National Institute of Standards and Technology (NIST) establishes standards for physical measurement and for calibration of instruments(13, 14). Properly measuring physical dose will provide the same result in any well-calibrated laboratory. Physical dosimetry is available during a radiological/nuclear incident including devices held by responders, attached to vehicles and located at a range of fixed locations. The information from physical dose measurements and the time of the measurement following the incident will be very helpful in constructing physical plots of dose. With radioactive decay following a nuclear detonation, the dose rate will rapidly decline over time.
Biodose is a measurement obtained by exposing living systems, albeit cells, tissues or animals, to a known amount of radiation (dose) and examining a biological change, which might be a chromosomal, molecular, proteomic or other physiological response. A number of assays and parameters may be used in clinical application and methods are complementary(15, 16). The biological changes are assessed over a range of relevant doses using a population of subjects (e.g. mice or non-human primates), and a ‘curve’ of specific biological change versus administered dose is produced. There may be a family of curves for different populations of people (e.g. male versus female, age-related, diabetics versus non-diabetics, etc.). Following an incident, the biological change is assessed by the assay, and by using the curve(s) of specific change versus dose, a ‘dose’ is estimated for that person. This is a biodose. It is not a specific measure of what the person received, but it takes a biological change, reads this change versus dose on a predetermined curve and then provides a ‘number’ to be used for triage and medical management. The unit generally used is also the Gray.
The unit ‘Sievert’ is a measure of the health effect of low levels of ionising radiation on the human body accounting for different types of radiation and their effects on the tissue. For example, the same dose of neutron radiation may produce a larger biological effect than photons and the effect of neutrons compared to photon may differ for different parts of the body. Thus, for neutrons there is a tissue weighting factor that indicates the relative higher amount of damage compared to photons(17, 18). These weighting factors are used primarily in the lower levels of exposure. The impact of the mix of type of radiation from a nuclear detonation may be relevant as it may impact ultimate outcome. Not to dismiss their potential impact, it is generally felt that for a ground burst nuclear detonation the impact of the weighting factor for neutrons is relatively small compared to that of the total dose from photons, A discussion of the need to account for neutron exposure is beyond the scope of this paper. Some of the biodosimetry assays may reflect the mixed exposure. While the initial sorting may employ a ‘biodose’ as part of the assessment, the clinician will be cognizant of the possibility that there may be forms of radiation that will have a greater biological effect such as neutrons or charged particles, the latter if they are in contact with skin. As further data are obtained in reconstructing individual people's exposure the unit Sievert may be used to account for the quality of the radiation.
While a physical dose will essentially be identical using NIST-traceable standards(14) and techniques, the biodose assigned to a person/patient may vary based on the specific assay used, variability among laboratories and by unknown biological/medical factors that might impact how one person's body responds to radiation exposure compared to another's. The laboratories conducting the assays should be checked (and possibly credentialled) to see how their results compare to one another using known and unknown radiated samples sent to them. Inter-laboratory comparison and standardisation are critical. Various international standards are often required before an assay is acceptable for clinical use such as those by the International Organization for Standardization (ISO)(19). In the USA, a laboratory providing results for clinical use are generally CLIA-certified (Clinical Laboratory Improvement Amendments) by the Centers for Medicare & Medicaid Services(20).
The biodose number is given in Gray, and this number is provided to the healthcare professional for helping to assess, triage and treat the patient. The number by itself does not determine medical management. There is some confusion among experts in the radiological/nuclear community that the US government triage system is ‘dose-based’ which is not correct. The approach is to use all available information for initial sorting and triage including estimates of physical exposure, for example, knowing that someone was in an area that had little to no prompt radiation and fallout will be extraordinarily useful as it can rule out the need to assess radiation injury. The ‘biodose’ result from the laboratory is used along with consideration for trauma and/or burn (called combined injury) and in the context of pre-existing and concurrent medical conditions to help in the initial sorting and triage. That the unit from the biodosimetry assays and physical dosimetry is ‘Gray’ underlies some of this confusion.
Repeat assessments
The critical tenet, that medical evaluation and care are managed by healthcare professionals with biodosimetry tools providing critical supporting data, is exemplified in the approach employing repeat assessments and adaptive management as espoused by the emergency medical services community. For example, the steps in SALT Mass Casualty Triage Algorithm (Sort, Assess, Lifesaving Interventions, Treatment/Transport)(21) were adapted for use during a mass casualty radiation emergency. In essence, a person's assigned specific triage category (Immediate, Delayed, Minimal, Expectant) may change as a person's medical condition worsens or improves, and will also depend on resource scarcity(22). A single biodose number may be helpful in determining the course of treatment (e.g. a completely normal blood count for 2–3 d following an incident or a very abnormal blood count within the first 24 h can help determine if there was radiation exposure). It will allow initial sorting into a group of people who may not need immediate attention versus a group who needs referral for further diagnostic evaluation. Nonetheless, the ultimate assessment of a person's medical condition is determined following repeated assessments, intervention and examination of the response to medical intervention as is done in all aspects of medical care. In this regard, the biodose-based approach that underlies the US planning and response is fully consistent with the METREPOL (MEdical TREatment ProtocOLs for Radiation Accident Victims) approach established in Europe(23) in that the medical decisions by knowledgeable professionals determine the clinical course. These include the judicious use of biomarkers of radiation exposure which may utilise standard medical blood tests such as a complete blood count and specialised biodosimetry assays, which the medical management team consider.
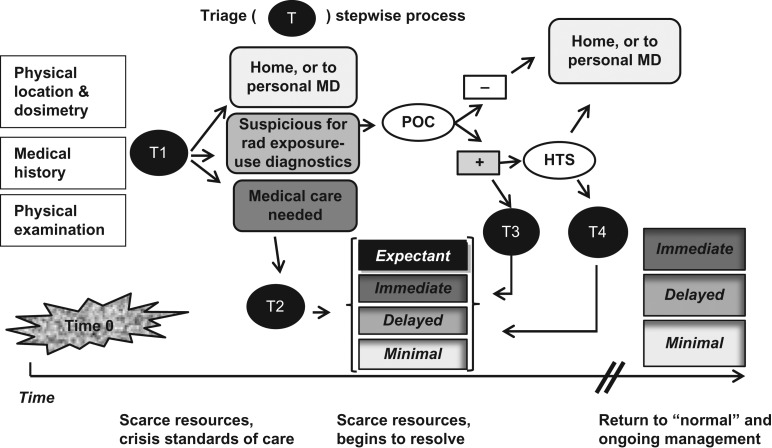
Sullivan et al.(15), analysed the biodosimetry techniques available and illustrated how these would be part of serial assessment. In Figure 2, the steps in triage, which depend on the observation of the physical infrastructure, patient examination, estimated physical dose ,and use of diagnostic tests are shown. The importance of an integrated clinical diagnostics system to provide laboratory surge capacity and diagnostic information is described below.
Figure 2.
Stepwise triage process after a nuclear detonation. Given that there may be hundreds of thousands of casualties and a scarce resources setting, serial determinations are important for determining who needs further assessment, whether or not medical interventions are needed and, if so, where can the person be sent based on an estimate of the severity of the injury. Serial analysis helps match the medical condition with the resources and expertise needed. T – Triage (T1 is first triage, T2, second, etc.). POC – Point of Care. HTS – High Throughput Screening: − means normal, + means further evaluation or treatment necessary. Triage tiles (shaded boxes) represent categories in Coleman et al.(22) (Figure reproduced with permission from Coleman et al.(22))
Partial body- and organ-specific biodosimetry
The need to assess partial body exposure as part of triage during an emergency response is a recurring issue. While beyond the scope of this paper, in general the models of radiation injury indicate that for a ground burst detonation it will be very uncommon for a victim to only receive prompt radiation that leaves a part of the body unexposed to any radiation. Furthermore, the vast majority of radiation exposure will be from fallout so that the person is exposed to whole body radiation, although it may well be heterogeneous. Should a segment of the marrow receive no or very low dose, this will result in a marrow autograft in which the estimate of that person's exposure may be an overestimate of the severity of their radiation injury. In this instance, the person would have been over-triaged (i.e. triaged to a worse category compared to the actual severity of their injury), but this will play out as a more favourable clinical course; therefore, the approach used in triage would have been conservative by bringing this person to medical attention. Caution must be exercised by the clinician to consider that some patients may be ‘over triaged’ so as to avoid the possibility of triaging a patient into the ‘Expectant’ category (e.g. to receive only palliative care) when they would actually benefit from additional intervention and should instead be triaged into the ‘Immediate’ or ‘Delayed’ category depending on the resources available. This caution emphasises the important need and purpose for the repeated triage and regular reassessment of the patient.
That dose heterogeneity is not a major consideration in the initial triage does not mean that biomarkers that can rapidly and accurately provide information on heterogenous exposure are not potentially useful. Currently, under study is the possibility of having biomarkers for organ-specific injury (e.g. marrow, vascular system, heart, lung, gastrointestinal tract, liver and kidney). Such an assessment, if possible, would help in medical management such that organ-specific interventions if developed would be utilised at some future point. Organ-specific biomarkers would also help in the reconstruction of heterogeneous exposure.
CONCEPTS IN DECISION SUPPORT
Tools for decision-makers
Recognising the complexity and scope of a nuclear detonation, ASPR continually works with federal, state and local partners, academia and global partners to develop tools and informational documents for decision-makers, planners and responders. The Radiation Emergency Medical Management (REMM) website(24) was developed by ASPR and the National Library of Medicine (NLM) to assist with education, training, planning and incident- and patient-management. A ‘Medical-Decision’ model(25) was proposed based on experience with the Fukushima Daiichi radiation emergency that emphasised the need for subject matter experts to work closely with the overall incident decision-maker who is likely to be a government leader without formal training in medicine and radiation science. The capability to engage appropriate subject matter experts to advise leadership includes the FDA/CDC-led Advisory Team for Environment, Food and Health (A-Team)(26), Department of Energy's Radiation Emergency Assistance Center/Training Site (REAC/TS)(27), ASPR's developing Subject Matter Advisory Resource Team (SMART Team), Veteran's Administration (VA) Medical Emergency Radiological Response Team (MERRT)(28) and RITN6.
To further facilitate the ability of the decision-maker to rapidly understand key concepts and components of a nuclear or radiological incident, Medical Planning and Response Manual for a Nuclear Detonation: A Practical Guide was prepared(29) and published in a summary form(30). In addition, a dynamic management tool will soon be available on the ASPR website (personal communication). Finally, a playbook prepared specifically for state and local planners and responders is also available(31).
Nuclear Incident Management Enterprise
Working with a broad range of experts and collaborators, a number of capabilities, concepts, tools, and documents were developed, and is named Nuclear Incident Management Enterprise (NIME)(1). The integrated clinical diagnostics system and biodosimetry tools and capacity are critical components of NIME, and development is ongoing.
Evolution of an Integrated Clinical Diagnostics System - biodosimetry and mass casualty triage come together
After the Goiânia, Brazil radiation incident in 1987, there was a broad consensus among science, medical and emergency management professionals that coordinated radiation biodosimetry surge was necessary to manage mass casualty radiation incidents and to rapidly assess and triage potential and actual casualties for exposure and medical intervention. Since then, international biodosimetry networks were established and include the World Health Organization's BioDoseNet,(32) the European Realizing the European Network of Biodosimetry (RENEB)(33) and MULTIBIODOSE(34) projects, as well as, numerous other national reference and surge laboratory systems across the globe. All of these efforts recognise the need for multi-parametric analysis coupled with rigorous quality assurance/quality control, speed in sample collection and analysis, high-throughput technology for sample preparation and analysis, strong linkage with medical triage and treatment surge, and robust information management systems.
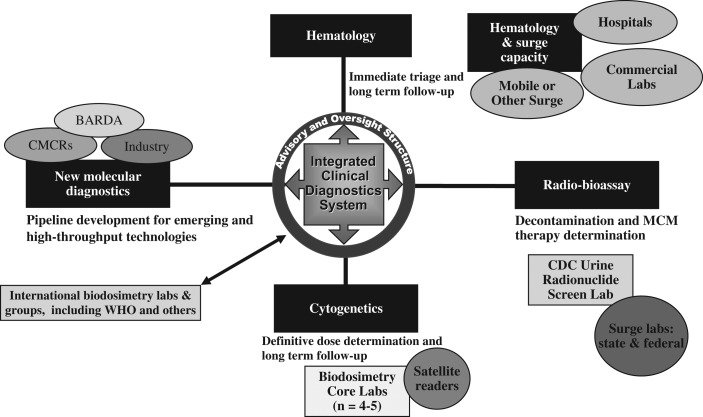
In addition to these efforts and following similar principles, in 2005 the US government laid the scientific and policy groundwork for developing US biodosimetry surge capacity in the event of nuclear detonation. In 2009, the concept for a Radiation Laboratory Network (Rad-LN) was formulated, and in 2010 a Biodosimetry Architecture was proposed to incorporate the US strategy for medical countermeasures development, as well as to link the US planning and response with global partners based on previous experience in radiation incidence and expertise available from partner countries. The Fukushima Daiichi radiation emergency in 2011 reinforced the global need for biodosimetry surge and provided important lessons for the US scientists and planners to incorporate mass casualty triage and a novel Integrated Clinical Diagnostics System (ICDS)(1) to assess radiation injury via numerous parameters into the development of a national CONOPS for nuclear detonation. Figure 3 illustrates how an ICDS integrates with the national emergency response.
Figure 3.
Integrated clinical diagnostic system. Illustrated is the concept of how a biodosimetry architecture might be utilised at various times, locations and stages following an incident. Details of the specific components are in NIME(1) with this overall system and some of the components in development while others, such as novel molecular diagnostics, are in research laboratories.
Following a nuclear detonation or large radiation incident, hundreds of thousands of people are likely to require assessment, diagnosis, and medical treatment for trauma and/or radiation exposure. In this scarce-resources setting, casualty assessment criteria and triage will take place early in the incident in the field or at healthcare facilities close to the detonation. The primary focus of the casualty assessment and triage will be on physical trauma, burns, emergent medical conditions and various acute exposures (e.g. chemical, smoke inhalation, etc.), but exposure to radiation is an important consideration as well. Biodosimetry tools, including those for rapid use at the point-of-care (POC), are being developed for use in the first 24–48 h to help in triage and also in determining medical countermeasure use. After the first few days and at a further distance from the incident, radiation assessment and triage for surviving casualties will be more accurate and help in patient management, and also in determining who may need additional follow-up for radiation exposure. These technologies are discussed in greater detail in other reports from the 2015 BioDose conference.
In this resource-constrained environment, critical triage decisions must be made that are fair, widely used, and ensure care is provided in a manner that will save the most lives(7). To support triage decisions and the provision of care, laboratory surge including standard laboratory tests and biodosimetry will be required at as many POCs as possible. Since these POCs will be distributed within the impacted area, regionally and across the Nation, a national CONOPS(1) will facilitate not only that appropriate diagnostics and triage capabilities are ready and in place, but also help ensure that personnel, countermeasures, facilities and supplies are properly co-located. As described in NIME,(1) a User-Managed Inventory (UMI) model(35) was proposed and a pilot project was implemented in the New York Metropolitan Area. UMI, along with other inventory strategies such as vendor managed inventory, will be critical to ensuring that adequate amounts of certain countermeasures (e.g. cytokines) are available for distribution under a national CONOPs. This overall national approach to resource distribution and stockpiling is in the conceptual and early planning stages.
The proposed ICDS is an amalgam of the RTR functional model with a consensus biodosimetry architecture(15) to support the massive surge for assays such as lymphocyte depletion kinetics (complete blood count and differential), dicentric analysis (cytogenetics), radiobioassay and other novel assays and diagnostics under development by NIAID and BARDA (Figure 3). Importantly, the national CONOPs and ICDS systems recognise that the role of clinical diagnostics will differ depending on the location of the medical assessment relative to the incident (local, regional or at a distant medical centre) and also the time after the incident.
In addition to the specific assays, critical components of the ICDS include capabilities that are useful to first responders, intermediate/stabilising care and definitive care. These capabilities include data management with integrated dose estimation and medical management guidance; assay and data validation; privacy requirements; and mapping and modelling capabilities from the Interagency Modeling and Atmospheric Assessment Center (IMAAC)(36) and Federal Radiological Monitoring and Assessment Center (FRMAC)(37). These critical components necessitate the development of a coordinated information technology (IT) solution for local capabilities analysis that includes various parameters of locally available diagnostic devices (e.g. make, model, type, normal reagent reserve, estimated throughput and geographical location) with data associated with GIS coordinates to integrate into the overall GIS platforms. Public/private partnerships will be needed to conduct physical surveys of existing locations, types and capabilities of analysers in an area to be plotted in GIS systems integrated with real-time weather, plume, people movement routing, infrastructure damage or other layers(12). An IT architecture for data collection and dissemination with a simple, common cloud-based interface that leverages existing robust, web-based data management systems (e.g. RITN centres, VA facilities, REMM(24) and commercial blood laboratories) is desired. It would include patient-specific fields, allow downstream patient access to data, and would also incorporate automated assay-based dose estimators linked to medical management guidance. Finally, operating procedures are necessary to ensure consistent application of best practices for local/regional patient data and sample management, user guidance for component tools, and data validity and quality assurance.
Taken together, the ICDS framework allows for the use of a systems approach that incorporates recognised requirements for biodosimetry, triage systems, prudent medical management, UMI, operational realities and a national approach to radiation response. It would be logically supported by and integrated with international projects, and utilise a scalable, flexible, evidence-based, sustainable approach based partly on existing capabilities. Consistent with the overall all-hazards planning and response by the US government, the approach and capability developed for a nuclear incident would be useful for other large-scale disasters. When applied to medical, operational and strategic decision support needs, implementation of this framework fills a major gap in medical and public health response to a major radiation incident and has the potential to improve outcomes for a large proportion of patients.
An overview of sorting and triage
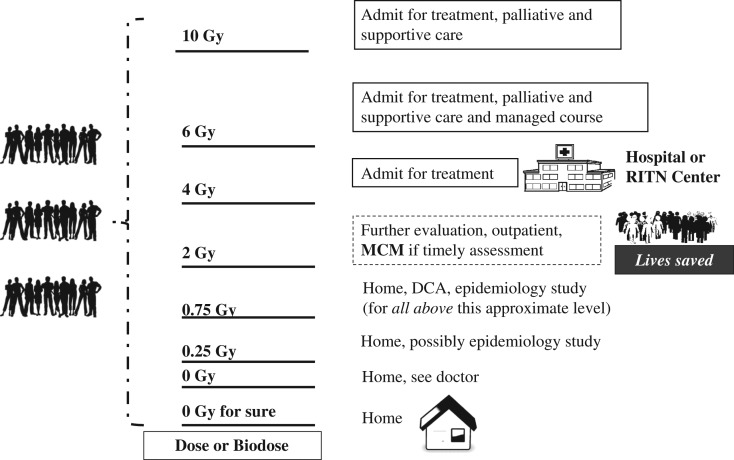
Figure 4 illustrates how the use of knowledge of a person's physical dose or an assay of their biodose will enable medical decision-makers to sort, assess, triage and treat people. By being able to make effective decisions, resources can be allocated to those who need them. Therefore, given the size and capacity of the US healthcare system, collaborations with experts, and the national and international planning and coordination of resources, it may be possible to deliver very high quality care to many hundreds of thousands of people after a nuclear incident, thereby saving lives whose outcome depends on the use of appropriate triage and medical management.
Figure 4.
Medical decision-makers are responsible for treatment. An assessment of an individual's dose is made as in Figure 1. In the approach illustrated any person with a dose >2 Gy would be considered in need of immediate medical assessment and possible intervention. Those with doses above 4 Gy would require hospitalisation. For doses above ~0.75 Gy but below 2 Gy one would likely be considered for a dicentric chromosome analysis at some point to estimate additional lifetime risk of radiation-induced cancer. Those without medical conditions that need immediate attention would be sent from the acute medical management areas to ‘home’ so that those in immediate need of care can receive attention. Home may be their own home or some temporary shelter or other accommodation. People with minor injuries, even broken bones and some injury may be sent to non-acute medical care so that resources are used to address life-threatening injuries.
CONCLUSIONS
The public health and medical response to a radiological and/or nuclear incident requires the capability to sort, assess, treat, triage and to ultimately discharge, refer or transport people to their next step in medical or definitive care. The response will be a dynamic situation likely characterised by a scarcity of resources for which preplanning is needed. It will be a challenge to effectively operationalise a response on a regional, national, and international level, therefore the national CONOPS and ICDS are critical components.
The use of the unit ‘Gray’ to specify both physical dose and also the output of biodosimetry assays can lead to some confusion; however, the biological response of the person (i.e. their ‘biodose’) is what will help guide medical management. An understanding of the requirements for biodosimetry, triage and treatment decisions, and massive public health and medical response informs and modifies the overall large-scale operational response. The bioassays need to be reproducible within and between laboratories, and some standardisation, inter-laboratory comparison and credentialing are necessary as the ‘biodose’ number will be an extraordinarily important measurement. Nonetheless, as is true in medical care in general, no single number by itself determines the course of action. Medical evaluation and care will be managed by healthcare professionals with biodosimetry tools providing critical supporting data. Further refining and expanding the currently available methods and developing new measures, potentially including assessment of organ-specific injury, will add to the ability to effectively manage the incident and provide the best care.
The biodosimetry tools and assays in development will enhance the utility of the ‘biodose’ and also likely guide development of new classes of MCMs. The science and technology developed for radiation incidents can both benefit from and enhance ongoing research in cancer care and in understanding the consequences of environmental hazards. This dual-utility concept enhances investment by government supported research and development and product development by academia and industry.
DISCLAIMER
The ideas, views and opinions expressed in this paper are strictly those of the authors and do not necessarily represent the views of the Office of the Assistant Secretary for Preparedness and Response, National Cancer Institute, Department of Health and Human Services, or the United States Government. No endorsement of products is implied.
ACKNOWLEDGEMENTS
The authors thank the many contributors to the development of the products, tools and knowledge contained in this manuscript. Dr Denis Fitzgerald provided editorial recommendations. Alicia A. Livinski from the NIH Library provided editorial review and assistance with manuscript preparation.
REFERENCES
- 1. Coleman C. N., et al. Public health and medical preparedness for a nuclear detonation: the nuclear incident medical enterprise. Health Phys. 108, 149–160 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Homeland Security Council Interagency Policy Coordination Subcommittee for Preparedness and Response to Radiological and Nuclear Threats Planning Guidance for Response to a Nuclear Detonation, second edn (Washington, DC: Homeland Security Council; ) (2010) http://www.remm.nlm.gov/PlanningGuidanceNuclearDetonation.pdf. [Google Scholar]
- 3. National Institutes of Health, National Institute of Allergy and Infectious Diseases Radiation and Nuclear Countermeasures Program. http://www.niaid.nih.gov/topics/radnuc/pages/default.aspx.
- 4. U.S. Department of Health and Human Services, Office of the Assistant Secretary for Preparedness and Response Office of Emergency Management. http://www.phe.gov/about/oem/Pages/default.aspx.
- 5. U.S. Department of Health and Human Services, Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority Chemical, Biological, Radiological, and Nuclear (CBRN) Threat Programs (2013). https://www.medicalcountermeasures.gov/barda/cbrn.
- 6. Radiation Injury Treatment Network http://www.ritn.net/.
- 7. Caro J. J., DeRenzo E. G., Coleman C. N., Weinstock D. M. and Knebel A. R.. Resource allocation after a nuclear detonation incident: unaltered standards of ethical decision making. Disaster Med. Public Health Prep. 5, S46–S53 (2011). [DOI] [PubMed] [Google Scholar]
- 8. Knebel A. R., et al. Allocation of scarce resources after a nuclear detonation: setting the context. Disaster Med. Public Health Prep. 5, S20–S31 (2011). [DOI] [PubMed] [Google Scholar]
- 9. Coleman C N., et al. Scarce resources for nuclear detonation: project overview and challenges. Disaster Med. Public Health Prep. 5, S13–S19 (2011). [DOI] [PubMed] [Google Scholar]
- 10. Hrdina C. M., Coleman C. N., Bogucki S., Bader J. L., Hayhurst R. E., Forsha J. D., Marcozzi D., Yeskey K. and Knebel A. R.. The ‘RTR’ medical response system for nuclear and radiological mass-casualty incidents: a functional TRiage-TReatment-TRansport medical response model. Prehosp. Disaster Med. 24, 167–178 (2009). [DOI] [PubMed] [Google Scholar]
- 11. Federal Emergency Management Agency http://www.fema.gov/.
- 12. Office of the Assistant Secretary for Preparedness and Response GeoHEALTH. http://geohealth.hhs.gov/arcgis/home/.
- 13. National Institute of Standards and Technology Physical Measurement Laboratory High-Dose Dosimetry. http://www.nist.gov/pml/div682/grp02/high-dose-dosimetry.cfm.
- 14. National Institute of Standards and Technology http://www.nist.gov/.
- 15. Sullivan J. M., Prasanna P. G., Grace M. B., Wathen L. K., Wallace R. L., Koerner J. F. and Coleman C. N.. Assessment of biodosimetry methods for a mass-casualty radiological incident: medical response and management considerations. Health Phys. 105, 540–554 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Blumenthal D. J., Sugarman S. L., Christensen D. M., Wiley A. L., Livingston G. K., Glassman E. S., Koerner J. F., Sullivan J. M. and Hinds S.. Role of dicentric analysis in an overarching biodosimetry strategy for use following a nuclear detonation in an urban environment. Health Phys. 106, 516–522 (2014). [DOI] [PubMed] [Google Scholar]
- 17. Wrixon A. D. New ICRP recommendations. J. Radiol. Prot. 28, 161–168 (2008). [DOI] [PubMed] [Google Scholar]
- 18. ICRP The 2007 Recommendations of the International Commission on Radiological Protection. ICRP Publication 103. Ann. ICRP 37, 2–4 (2007). [DOI] [PubMed] [Google Scholar]
- 19. International Organization for Standardization http://www.iso.org/iso/home.htm.
- 20. Centers for Medicare & Medicaid Services Clinical Laboratory Improvement Amendments (CLIA). https://www.cms.gov/Regulations-and-Guidance/Legislation/CLIA/index.html?redirect=/clia/.
- 21. National Institutes of Health, National Library of Medicine, Office of the Assistant Secretary for Preparedness and Response SALT Mass Casualty Triage Algorithm (Sort, Assess, Lifesaving Interventions, Treatment/Transport) — Adapted for a Very Large Radiation Emergency. http://www.remm.nlm.gov/salttriage.htm.
- 22. Coleman C. N., Weinstock D. M., Casagrande R., Hick J. L., Bader J. L., Chang F., Nemhauser J. B. and Knebel A. R.. Triage and treatment tools for use in a scarce resources-crisis standards of care setting after a nuclear detonation. Disaster Med. Public Health Prep. 5, S111–S121 (2011). [DOI] [PubMed] [Google Scholar]
- 23. Fliedner T. M, Powles R., Sirohi B. and Niederwieser D.. Radiologic and nuclear events: the METREPOL severity of effect grading system. Blood 111, 5757–5759 (2008). [DOI] [PubMed] [Google Scholar]
- 24. National Institutes of Health, National Library of Medicine (2015) In: Radiation Emergency Medical Management: REMM. Bader J. L., Ed. (Bethesda, MD: National Library of Medicine; ) http://remm.nlm.gov. (23 June 2016, date last accessed). [Google Scholar]
- 25. Koerner J. F., Coleman C. N., Murrain-Hill P., FitzGerald D. J. and Sullivan J. M.. The medical decision model and decision maker tools for management of radiological and nuclear incidents. Health Phys. 106, 645–651 (2014). [DOI] [PubMed] [Google Scholar]
- 26. Advisory Team for Environment, Food and Health https://crcpd.org/ATeam/Ateam.htm.
- 27. Radiation Emergency Assistance Center/Training Site (REAC/TS) https://orise.orau.gov/reacts/.
- 28. Veterans Health Administration Medical Emergency Radiological Response Team (MERRT). http://www.va.gov/VHAEMERGENCYMANAGEMENT/CEMP/CEMP_MERRT.asp.
- 29. U.S. Department of Health and Human Services, Office of the Assistant Secretary for Preparedness and Response Medical planning and response manual for a nuclear detonation: a practical guide 146, (Washington, D.C.: U.S. Department of Health and Human Services) (2012). http://www.phe.gov/Preparedness/planning/nuclearresponsemanual/Documents/medplanresmannucdet-guide-final.pdf. [Google Scholar]
- 30. Coleman C N., et al. Medical planning and response for a nuclear detonation: a practical guide. Biosecur Bioterror 10, 346–371 (2012). [DOI] [PubMed] [Google Scholar]
- 31. Murrain-Hill P., et al. Medical response to a nuclear detonation: creating a playbook for state and local planners and responders. Disaster Med. Public Heal Prep. 5, S89–S97 (2011). [DOI] [PubMed] [Google Scholar]
- 32. World Health Organization WHO BioDoseNet. http://www.who.int/ionizing_radiation/a_e/biodosenet/en/.
- 33. Realizing the European Network of Biodosimetry (RENEB) http://reneb.eu/.
- 34. Multibiodose http://www.multibiodose.eu/.
- 35. Coleman C. N., et al. User-managed inventory: an approach to forward-deployment of urgently needed medical countermeasures for mass-casualty and terrorism incidents. Disaster Med. Public Health Prep. 6, 408–414 (2012). [DOI] [PubMed] [Google Scholar]
- 36. U.S. Department of Homeland Security Interagency Modeling and Atmospheric Assessment Center (IMAAC). http://www.dhs.gov/imaac.
- 37. National Nuclear Security Administration Federal Radiological Monitoring and Assessment Center. http://www.nnsa.energy.gov/aboutus/ourprograms/emergencyoperationscounterterrorism/respondingtoemergencies-0-1.