Abstract
Background and objective
The risk of malignancy from “atypia of undetermined significance/follicular lesion of undetermined significance” (AUS/FLUS) is estimated to lie between 5% and 15%; however, some authors suggest that the risk of malignancy in AUS/FLUS depends upon specific clinical situations. This was a retrospective study which aimed to determine the incidence and risk of thyroid cancer (TC) based upon selected ultrasound features from patients with thyroid nodules (TN) classified as AUS/FLUS.
Methods
Univariate and multivariate logistic regression analyses were used to identify significant associations between ultrasound features and the risk of TC.
Results
Of 127 patients with TN classified as AUS/FLUS who underwent thyroidectomy, 114 (89.8%) had benign disease while 13 (10.2%) had TC. Univariate analysis identified several significant predictors for TC (all p<0.05), including microcalcifications, hypoechogenicity, the prevalence of irregular margins, a taller rather than a wide form, high vascularity, and fast tumor growth. Multivariate analysis further showed that microcalcifications (odds ratio =21.37; p=0.024) and fast growth (odds ratio =22.70; p=0.021) were significant and independent factors associated with the risk of developing TC.
Conclusion
Microcalcifications and fast growth of the TN could therefore be used as predictive factors for the development of TC in patients with AUS/FLUS.
Keywords: atypia/follicular lesion of undetermined significance, thyroid nodules, thyroid cancer
Introduction
The category referred to as “atypia of undetermined significance and follicular lesion of undetermined significance” (AUS/FLUS) was included in The Bethesda System for Reporting Thyroid Cytology (TBSRTC) and collectively for a third category of this classification which is known as “Bethesda III.”1 This is a rather heterogeneous group of thyroid lesions with borderline cellularity. Generally, the use of this TBSRTC classification during diagnosis minimizes the number of unnecessary thyroid surgeries and helps to avoid the unclear, long, and descriptive reports of material obtained from fine-needle aspiration biopsy (FNAB) procedures. TBSRTC therefore improves communication between cytologists, pathologists, endocrinologists, radiologists, and surgeons.
The risk of malignancy in the AUS/FLUS category is estimated to lie in the range of 5%–15%;2 however, some authors suggest that the risk of malignancy in this third category depends upon the specific clinical situation.3
The fourth category of the TBSRTC includes “follicular neoplasm (FN) and suspicious for a FN.” The risk of malignancy in this particular group lies in the range of 15%–30%.1 This category is used to diagnose nodules that might appear as cancer during routine histopathological examinations. Thus, the borderline between the third and fourth categories of the TBSRTC classification is not clear-cut.
Some studies that have adopted terminology and criteria from the Bethesda classification have revealed high concordance in ultrasound-guided fine-needle aspiration cytology with almost 95% of samples interpreted in a satisfactory manner. Approximately 55%–74% of these patients were defined as being definitively benign, while ~2%–5% were defined as being definitively malignant.4 The authors of this previous study classified the rest of the samples as “cytologically indeterminate.” This group contains the AUS/FLUS category, in which 2%–18% of patients have lesions, 2%–25% had FN in nodules, and 1%–6% were suspicious for malignancy. In a previous meta-analysis, Bongiovanni revealed significant variability in the probability of malignancy for each category of the Bethesda system.4 This was compatible with the range predicted by TBSRTC classification, but with the exception of the third category (AUS/FLUS), for which the risk of malignancy in some studies was significantly higher than predicted.4,5 In a further study, Hagag et al6 observed that palpable nodules had the same risk of malignancy as nonpalpable lesions when diagnosed by ultrasound examination. However, ultrasonography has been used to evaluate the risk of malignancy in thyroid nodules (TNs) and is considered to represent a decision-making tool for an indication of ultrasound-guided FNAB (UG-FNAB).
The most common ultrasound characteristics for thyroid malignancy are microcalcifications, hypoechogenicity, irregular margins, and a tall rather than a wide shape as measured on a transverse view. However, a recent study noted that the most specific characteristics for thyroid carcinoma are microcalcifications, irregular margins, and mentioned shape.7 Kwak et al8 used multivariable analyses to show that the risk of cancer is higher for nodules with either microlobulated margins or microcalcifications than for hypoechoic solid nodules without these features. The same authors noticed that macrocalcifications localized in the nodule with microcalcifications had the same oncological value as microcalcifications alone.
According to some studies which have adopted the TBSRTC classification, the risk of malignancy in the third and fourth category is ~15%,1 in the third category 5%–15% and in the fourth category from 15% to 30%. The recommended clinical management for patients of the third category is to repeat the diagnostic procedure (FNAB), while in the fourth category, the usual recommended management is lobectomy or thyroidectomy.1 Thus, the risk of malignancy in patients assigned to one of these 2 different categories might remain at the same level, although the recommendations for clinical management are completely different.
According to these observations, a very interesting question is very evident: are there any clinical characteristics of the TNs classified as AUS/FLUS, which form additional and helpful suggestions for repeat biopsy or surgery? Given the nuances of ultrasound pattern for different TNs, as well as the different cytological diagnoses of these lesions, we set out to evaluate whether malignancy risk stratification, based upon a multitude of ultrasound features, may help in determining the appropriate course of clinical management. In the absence of ultrasound features associated with a higher risk of malignancy in biopsied nodules categorized as AUS/FLUS in the TBSRTC classification, we could ascertain whether such lesions require further observation. On the other hand, the presence of these ultrasound patterns may allow us to direct patients with such nodules toward lobectomy or thyroidectomy. Moreover, the ability to distinguish between malignant TNs from benign ones in the AUS/FLUS category is extremely important, because, as some authors say, more tailored and minimally invasive treatment may be required.9
Materials and methods
Our study protocol was approved by the Bioethics Committee of Wroclaw Medical University (Reference number: KB-783/2017). We obtained verbal consent from the participants instead of written consent because the data were analyzed anonymously and retrospectively on the basis of medical records. The process used to obtain verbal consent was deemed to be acceptable and was approved by the Bioethics Committee of Wroclaw Medical University. The authors did not have access to any identifying patient information and did not have any direct access to the study participants.
We retrospectively reviewed all FNAB reports and histopathological diagnoses from 4,296 patients who were admitted and received surgery for thyroid tumors in The First Department and Clinic of General, Gastroenterological and Endocrine Surgery between January 1, 2008 and December 31, 2016. All analyzed FNAB results were reported using TBSRTC classification and patients defined as the third category of TBSRTC (“Bethesda III”) were evaluated. All patients admitted and surgically treated in our department received ultrasound examination of the thyroid gland and lymph nodes of the neck prior to treatment. We evaluated three thyroid ultrasound examinations of each patient which were performed 24, 12 and 1 month before hospital admission and surgical treatment. All ultrasound results which qualified for the study (categorized as AUS/FLUS) were retrospectively analyzed. All ultrasound patterns of biopsied nodules assigned to the third category (AUS/FLUS) of the TBSRTC system were evaluated and compared to the final histopathological diagnoses.
Statistical analysis
All statistical analyses were carried out using Statistica 13.0 software (StatSoft, Tulsa, OK, USA). Descriptive data were presented as numbers and percentages (for qualitative variables) or means, SD, and 95% CIs for quantitative variables. The distribution of the data was analyzed by the Shapiro–Wilk normality test. Differences between the 2 study groups were analyzed by the χ2 test, by Fisher’s exact test, or by the Student’s t-test for independent samples.
The stepwise method of multivariate logistic regression analysis was used to determine independent predictive factors that were associated with the presence of thyroid cancer (TC). Odds ratios (ORs) and 95% CIs were calculated. A p≥0.1 was considered an exclusion criterion for our univariate logistic regression analysis.
Because of the small number of cancer cases, p-values arising from logistic regression analyses were confirmed by Fisher’s exact test (using 2-way contingency tables). A 2-tailed p-value of <0.05 was considered statistically significant for all other analyses.
Results
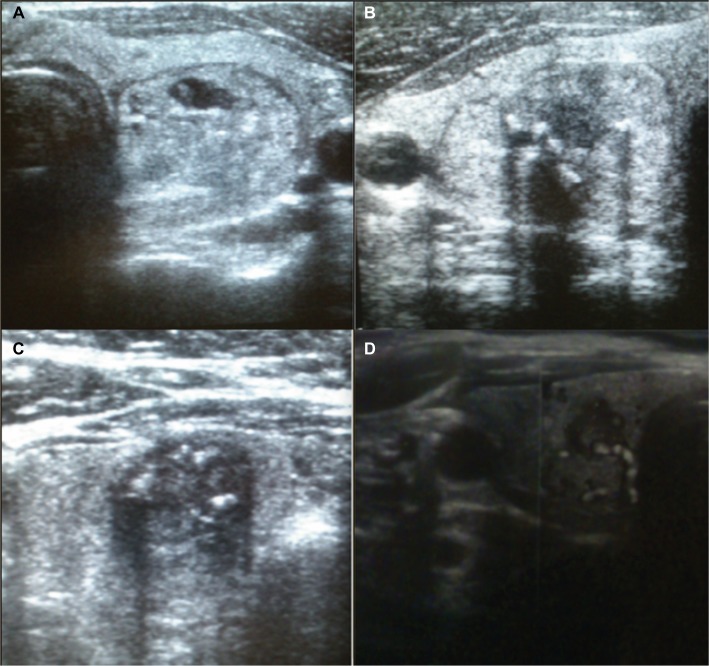
A total of 4,296 individuals were retrospectively reviewed. In this group of patients, 127 (2.95%) had TNs with a diagnosis of AUS/FLUS cytology. There were 105 (82.7%) females and 22 (17.3%) males with a mean age of 51.0±15.0 years. Resected nodules were classified histopathologically as follows: 57 (44.88%) patients had benign thyroid goiter, 12 (9.44%) patients had thyroiditis, 45 (35.43%) patients had adenoma, and 13 (10.23%) patients were diagnosed with TC. Radical surgery was performed in 9 (96.85%) patients, and a second round of surgery was required in 4 (3.14%) individuals with TC (Figure 1A–D). The characteristics of the total study group are shown in Table 1.
Figure 1.
Sonographic patterns of malignant TNs classified by cytology as in the AUS/FLUS category of TBSRTC.
Notes: (A) Solid, partially hypoechoic nodule with irregular margins, (B) microcalcifications, hyper- and hypoechoic nodule with irregular margins, (C) microcalcifications, hypoechoic nodule with irregular margins, and (D) hypoechoic nodule with high intranodular vascularity and irregular margins.
Abbreviations: AUS/FLUS, atypia of undetermined significance/follicular lesion of undetermined significance; TBSRTC, The Bethesda System for Reporting Thyroid Cytology; TN, thyroid nodule.
Table 1.
Demographic, clinical, and operative indications of patients with TN Bethesda category III
| Parameters | A: Total study group (n=127) | B: Noncancer (n=114) | C: Cancer (n=13) | p-value (B vs C) |
|---|---|---|---|---|
| Gender | 0.165 | |||
| Male | 22 (17.3) | 18 (15.8) | 4 (30.8) | |
| Female | 105 (82.7) | 96 (84.2) | 9 (69.2) | |
| Age (years) | 51.0±15.0 | 49.5±15.9 | 52.5±14.1 | 0.520 |
| Age | 1.000 | |||
| <45 years | 45 (35.4) | 41 (35.9) | 4 (30.8) | |
| ≥45 years | 82 (64.6) | 73 (64.1) | 9 (69.2) | |
| Final diagnosis | <0.0001a | |||
| Goiter | 57 (44.9) | 57 (50.0) | ||
| Thyroiditis | 12 (9.5) | 12 (10.5) | ||
| Adenoma | 45 (35.4) | 45 (39.5) | ||
| Cancer | 13 (10.2) | 0 (0.0) | 13 (100.0) | |
| Type of surgery | <0.0001a | |||
| Nonradical | 118 (92.9) | 114 (100.0) | 4 (30.8) | |
| Radical | 9 (7.1) | 0 (0.0) | 9 (69.2) | |
| Reoperation needed | <0.0001a | |||
| No | 123 (96.9) | 114 (100.0) | 9 (69.2) | |
| Yes | 4 (3.1) | 0 (0.0) | 4 (30.8) |
Notes:
Statistically significant at p<0.05. Descriptive data are presented as number (%) or mean ± SD.
Abbreviation: TN, thyroid nodule.
Demographic, clinical, and operative characteristics of the patient subgroups
A total of 127 (100%) patients with TNs assigned to category III of the TBSRTC classification (AUS/FLUS, “Bethesda III”) were divided into 2 subgroups: 114 (89.8%) patients with benign disease of the TNs and 13 (10.2%) patients with TC. The comparative characteristics of these two patient subgroups are shown in Table 1. There were no significant differences in terms of gender or age between these 2 subgroups (p>0.05). However, the rates of radical thyroid resection and necessity for reoperation were significantly higher in patients with cancer than in patients with benign thyroid disease (p<0.0001 for both).
Ultrasound features of the two subgroups of patients are shown in Table 2. There were statistically significant differences in the rates of microcalcifications, echogenicity, nodule proportions, and tumor growth rate when comparing patients with benign thyroid disease and those with TC (p<0.0001 for all). There was a tendency toward a significant difference in the rate of tumor vascularity when comparing the 2 patient subgroups (p=0.052).
Table 2.
Ultrasound features of patients with Bethesda category III TNs
| Parameters | A: Total study group (n=127) | B: Noncancer (n=114) | C: Cancer (n=13) | p-value (B vs C) |
|---|---|---|---|---|
| Microcalcification | <0.0001a | |||
| No | 99 (78.0) | 96 (84.2) | 3 (23.1) | |
| Yes | 28 (22.0) | 18 (15.8) | 10 (76.9) | |
| Echogenicity | <0.0001a | |||
| Hyperechoic | 79 (62.2) | 78 (68.4) | 1 (7.7) | |
| Hypoechoic | 48 (37.8) | 36 (31.6) | 12 (92.3) | |
| Irregular margin | <0.0001a | |||
| No | 83 (65.4) | 82 (71.9) | 1 (7.7) | |
| Yes | 44 (34.6) | 32 (28.1) | 12 (92.3) | |
| Taller than wide | <0.0001a | |||
| No | 91 (71.7) | 89 (78.1) | 2 (15.4) | |
| Yes | 36 (28.3) | 25 (21.9) | 11 (84.6) | |
| High vascularity | 0.052b | |||
| No | 61 (48.0) | 58 (50.9) | 3 (23.1) | |
| Yes | 66 (52.0) | 56 (49.1) | 10 (76.9) | |
| Fast growth | <0.0001a | |||
| No | 91 (71.7) | 89 (78.1) | 2 (15.4) | |
| Yes | 36 (28.3) | 25 (21.9) | 11 (84.6) | |
| Macrocalcification | 0.555 | |||
| No | 74 (58.3) | 65 (57.0) | 9 (69.2) | |
| Yes | 53 (41.7) | 49 (43.0) | 4 (30.8) |
Notes:
Statistically significant at p<0.05;
tendency toward statistical significance at 0.05<p<0.1. Descriptive data are presented as number (%).
Abbreviation: TN, thyroid nodule.
Ultrasound features as independent predictors for the risk of TC
Logistic regression analysis was used to investigate the association of gender, age, and ultrasound features with the risk of TC. The results are presented in Table 3. In univariate logistic regression analysis, microcalcifications, hypoechogenicity, irregular margins, a tall rather than wide nodule proportion, high vascularity, and fast tumor growth were all significantly related to the risk of cancer. Then, a multivariate logistic regression model showed that the risk of TC significantly increased with intranodular microcalcifications (Figure 1B and C) and fast tumor growth (p<0.05 for both). There was also a tendency for an association between tumors which were formed in a way that that were taller than they were wide and the risk of cancer (p=0.052).
Table 3.
Univariate and multivariate logistic regression analysis of the association between risk of TC (noncancer group vs cancer group) and demographic and ultrasound parameters in patients of Bethesda category III
| Parameters | Univariate analysis
|
Multivariate analysis
|
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | ±95% CI | p-value | |
| Gender: for “female” | 2.37 | 0.65–8.64 | 0.187 | – | – | – |
| Age: for “≥45” | 1.26 | 0.36–4.41 | 0.711 | – | – | – |
| Microcalcification | 17.77 | 4.39–71.98 | <0.0001a | 21.37 | 1.45–314.37 | 0.024b |
| Hypoechogenicity | 26.00 | 3.19–211.93 | 0.002a | 12.94 | 0.26–641.49 | 0.194 |
| Irregular margin | 30.75 | 3.76–251.33 | 0.001a | 17.88 | 0.40–794.72 | 0.132 |
| Taller than wide | 19.58 | 4.01–95.62 | 0.0002a | 18.40 | 0.94–360.62 | 0.053c |
| High vascularity | 3.45 | 0.89–13.38 | 0.070a | 4.70 | 0.28–79.91 | 0.279 |
| Fast growth | 19.58 | 4.01–95.63 | 0.0002a | 22.70 | 1.57–327.18 | 0.021b |
| Macrocalcification | 0.59 | 0.17–2.05 | 0.402 | – | – | – |
Notes:
Inclusion criterion (p<0.1) by univariate logistic regression analysis;
statistically significant at p<0.05;
tendency toward statistical significance at 0.05<p<0.1.
Abbreviations: OR, odds ratio; TC, thyroid cancer.
Discussion
Ultrasonography of the TNs is the most effective diagnostic tool for predicting malignancy and selecting these lesions for further evaluation.10 UG-FNAB is the most cost-effective procedure that provides useful diagnostic information relating to further clinical management.11 The clinical and practical purpose of this diagnostic tool is to reduce the number of unnecessary “diagnostic” surgical procedures in patients with benign nodules and detects those individuals in whom the risk of malignancy is high.
In 2007, the National Cancer Institute (NCI) hosted “The NCI Thyroid FNA State Science Conference” in Bethesda (Maryland), After this meeting, “The Bethesda Thyroid Atlas Project” was created and finally, the TBSRTC was formed.12 This classification contains 6 categories of thyroid cytology, and each of these categories has an implied risk of malignancy ranging from 0% to 3% (category II) to as high as 97%–99% (category VI). Following UG-FNAB, the use of TBSRTC reduced the number of unnecessary thyroid surgeries in patients with benign nodules, but also increased the number of patients qualifying for surgery due to malignant progression. However even this global and well-prepared classification is associated with clinical dilemmas. The TBSRTC classification has been used in our own clinic ever since 2007. Between 2008 and 2016, the classification served as a basic presurgical diagnostic criterion.
According to the TBSRTC classification, the third category is associated with a 5%–15% risk of cancer, and the usual clinically recommended management is to repeat UG-FNAB.¹ In our patients, of the 127 (2.95%; 127/4,296) individuals classified in the AUS/FLUS category, 13 were diagnosed with thyroid carcinoma and 114 with benign disease; thus, the risk of malignancy in our study group was estimated to be 10.2%. However, in our patients, the diagnosis of AUS/FLUS category was estimated in 127 (2.95%) individuals (127/4,296). Some authors recommend that the AUS/FLUS category should not constitute >7% of all TBSRTC reports.13 For example, Guo et al14 estimated that 3% of their study group (8/236) could be categorized as AUS/FLUS; however, other studies have claimed that 2%–18% of thyroid lesions are associated with the AUS/FLUS category.4
Generally, the most well-known and suspicious ultrasound features for the malignancy of TNs include hypoechogenicity, irregular margins, microcalcifications, and a taller than wide shape.10 Some authors suggest, however, that no single ultrasound pattern can represent a strong predictor for the risk of thyroid malignancy and that the combined use of a multitude of such features may provide higher diagnostic accuracy.15 In our present study, we observed statistically significant differences in the rates of microcalcifications, echogenicity, nodule proportions, and tumor growth rate between patients with benign thyroid disease and those with TC. A tendency for significant difference was also observed for the rate of tumor vascularity between patient subgroups. We also noticed that 52% of TNs within the AUS/FLUS category showed high intranodular vascularity (Table 1, Figure 1D). However, Moon et al16 suggested that this ultrasound pattern can be seen in 31% of benign TNs compared with 17% of malignant ones. Other studies suggest that microcalcifications and increased intranodular vascularity might be predictive for malignancy,17 but others were unable to confirm these observations.18 In our present study, 77% of patients in the AUS/FLUS category and with a final histopathological diagnosis of malignancy were shown to have high vascularity. However, in the group of cases in the AUS/FLUS category but with a benign final histopathological diagnosis, high vascularity was also observed in over 49% of individuals. This is why some authors have claimed that high intranodular vascularity is very useful in predicting TC,19 while others suggest that high vascularity does not predict thyroid malignancy.20 Chng et al10 assessed the higher number of malignant TNs with irregular margins, hypoechogenicity, and a taller than wide shape. However, these authors also noticed that the size of the nodule, microcalcifications, and intranodular vascularity were not significantly different when compared between malignant and benign thyroid lesions. Chng et al10 added that irregular margins had the highest positive predictive value for malignancy, because all of the nodules with this sonographic pattern were malignant in their study. The same observations were reported by Maia et al18 in a group of 80 patients with “indeterminate thyroid cytology” and surgically treated in a single institution. These authors reported that irregular margins had the highest positive predictive value for malignancy. In a meta-analysis by Brito et al,17 a taller than wide shape was estimated to the one ultrasound feature which has the highest diagnostic OR for predicting malignancy of the TNs when compared to other sonographic patterns. In our present study, we observed a tendency for association between taller than wide forms of tumor and the risk of cancer.
In accordance with the observations of other authors,21,22 we did not identify significant differences in either age or gender between patients with benign and malignant nodules.
Some authors describe a wide range in the risk of malignancy (6%–48%) in specimens categorized as AUS/FLUS.13,23,24 This suggests that the criteria used for this classification are inconsistent. Furthermore, previous authors have stated that the risk of malignancy in the AUS/FLUS category depends upon the clinical and cytological features of the TN cells and accounts for 38% for patients defined as “atypia, rule out papillary carcinoma.”23 These authors also stated that different types of follicular atypia have different risks of malignancy and this discrepancy should be communicated by the cytologist and clinicians.
Some authors have confirmed that the vast majority of malignant TNs are solid, and that this was observed in 82%–91% of all TCs.8 Other authors suggest that the presence of microcalcifications, even in a partially cystic TN, definitely increases the risk of malignancy.25 Besides this general agreement, that a purely cystic nodule (without a solid component) has a very low suspicion of malignancy and should be treated as a benign lesion with no need to UG-FNAB,17 in our study we had 2 patients with this type of ultrasound feature. These patients underwent biopsy and provided results which qualified for the AUS/FLUS category. The main indication for surgery in these patients was not the cytology diagnosis of the nodules or ultrasound patterns, but cosmetic reasons. In the histopathology diagnosis, these nodules were classified as a benign colloid goiter.
The incidence and risk of malignancy in the AUS/FLUS category is not very low. Nodules qualifying for the AUS/FLUS category of the TBSRTC system with a high suspicion of malignancy in the ultrasound patterns should undergo diagnostic or therapeutic surgery to either refute or confirm malignancy. However, in the absence of sonographic features associated with malignancy, another appropriate form of clinical management might be observation. Microcalcifications and fast tumor growth could be used as predictive factors for the development of TC in patients with TNs which qualify for the AUS/FLUS category.
Footnotes
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Cibas ES, Ali SZ. The Bethesda system for reporting thyroid cytopathology. Am J Clin Pathol. 2009;132(5):658–665. doi: 10.1309/AJCPPHLWMI3JV4LA. [DOI] [PubMed] [Google Scholar]
- 2.Ali SZ, Cibas ES. The Bethesda System for Reporting Thyroid Cytopathology. New York: Springer; 2009. [DOI] [PubMed] [Google Scholar]
- 3.Van der Laan PA, Marqusee E, Krane JF. Usefulness of diagnostic qualifiers for thyroid fine-needle aspirations: with atypia of undetermined significance. Am J Clin Pathol. 2011;136(4):572–577. doi: 10.1309/AJCPO0BQ2YSKPXXP. [DOI] [PubMed] [Google Scholar]
- 4.Bongiovanni M, Spitale A, Faquin WC, Mazzucchelli L, Baloch ZW. The Bethesda system for reporting thyroid cytopathology: a meta-analysis. Acta Cytol. 2012;56(4):333–339. doi: 10.1159/000339959. [DOI] [PubMed] [Google Scholar]
- 5.Ohori NP, Schoedel KE. Variability in the atypia of undetermined significance/follicular lesion of undetermined significance diagnosis in the Bethesda system for reporting thyroid cytopathology: sources and recommendations. Acta Cytol. 2011;55(6):492–498. doi: 10.1159/000334218. [DOI] [PubMed] [Google Scholar]
- 6.Hagag P, Strauss S, Weiss M. Role of ultrasound guided fine-needle aspiration biopsy in evaluation of nonpalpable thyroid nodules. Thyroid. 1998;8:989–995. doi: 10.1089/thy.1998.8.989. [DOI] [PubMed] [Google Scholar]
- 7.Haugen BR, Alexander EK, Bible KC, et al. American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwak JY, Han KH, Yoon JH, et al. Thyroid imaging reporting and data system for US features of nodules: a step in establishing better stratification of cancer risk. Radiology. 2011;260(3):892–899. doi: 10.1148/radiol.11110206. [DOI] [PubMed] [Google Scholar]
- 9.Wu W, Gong X, Zhou Q, Chen X, Chen X. Ultrasound-guided percutaneous microwave ablation for solid benign thyroid nodules: comparison of MWA versus control group. Inter J Endocrinol. 2017;2017:9724090. doi: 10.1155/2017/9724090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chng CL, Kurzawinski TR, Beale T. Value of sonographic features in predicting malignancy in thyroid nodules diagnosed as follicular neoplasm on cytology. Clin Endocrinol. 2015;83(5):711–716. doi: 10.1111/cen.12692. [DOI] [PubMed] [Google Scholar]
- 11.Kaliszewski K, Zubkiewicz-Kucharska A, Wojtczak B, Strutyńska-Karpińska M, Zaleska-Dorobisz U, Leśków E. Ultrasound guided fine-needle aspiration biopsy of thyroid nodules: does radiologist assistance decrease the rate of unsatisfactory biopsies? Adv Clin Exp Med. 2016;25(1):93–100. doi: 10.17219/acem/60084. [DOI] [PubMed] [Google Scholar]
- 12.Renuka IV, Saila Bala G, Aparna C, Kumari R, Sumalatha K. The Bethesda system for reporting thyroid cytopathology: interpretation and guidelines in surgical treatment. Indian J Otolaryngol Head Neck Surg. 2012;64(4):305–311. doi: 10.1007/s12070-011-0289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh RS, Wang HH. Eliminating the “Atypia of Undetermined Significance/Follicular Lesion of Undetermined Significance” category from the Bethesda system for reporting thyroid cytopathology. Am J Clin Pathol. 2011;136(6):896–902. doi: 10.1309/AJCPIX52MBOKTICP. [DOI] [PubMed] [Google Scholar]
- 14.Guo A, Kaminoh Y, Forward T, Schwartz FL, Jenkinson S. Fine needle aspiration of thyroid nodules using the Bethesda system for reporting thyroid cytopathology: an institutional experience in a rural setting. Inter J Endocrinol. 2017;2017:9601735. doi: 10.1155/2017/9601735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moon WJ, Jung SL, Lee JH, et al. Benign and malignant thyroid nodules: US differentiation–multicenter retrospective study. Radiology. 2008;247(3):762–770. doi: 10.1148/radiol.2473070944. [DOI] [PubMed] [Google Scholar]
- 16.Moon HJ, Kwak JY, Kim MJ, Son EJ, Kim EK. Can vascularity at power Doppler US help predict thyroid malignancy? Radiology. 2010;255(1):260–269. doi: 10.1148/radiol.09091284. [DOI] [PubMed] [Google Scholar]
- 17.Brito JP, Gionfriddo MR, Al Nofal A, et al. The accuracy of thyroid nodule ultrasound to predict thyroid cancer: systematic review and meta-analysis. J Clin Endocrinol Metab. 2014;99(4):1253–1263. doi: 10.1210/jc.2013-2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maia FF, Matos PS, Pavin EJ, Vassallo J, Zantut-Wittmann DE. Value of ultrasound and cytological classification system to predict the malignancy of thyroid nodules with indeterminate cytology. Endocr Pathol. 2011;22(2):66–73. doi: 10.1007/s12022-011-9159-6. [DOI] [PubMed] [Google Scholar]
- 19.Brunese L, Romeo A, Iorio S, et al. A new marker for diagnosis of thyroid papillary cancer: B-flow twinkling sign. J Ultrasound Med. 2008;27(8):1187–1194. doi: 10.7863/jum.2008.27.8.1187. [DOI] [PubMed] [Google Scholar]
- 20.Stacul F, Bartolotto M, De Gobbis F, et al. US, colour-Doppler US and fine-needle aspiration biopsy in the diagnosis of thyroid nodules. Radiol Med. 2007;112(5):751–762. doi: 10.1007/s11547-007-0178-9. [DOI] [PubMed] [Google Scholar]
- 21.Castro MR, Espiritu RP, Bahn RS, et al. Predictors of malignancy in patients with cytologically suspicious thyroid nodules. Thyroid. 2011;21(11):1191–1198. doi: 10.1089/thy.2011.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calo PG, Medas F, Santa Cruz R, et al. Follicular nodules (Thy3) of the thyroid: is total thyroidectomy the best option? BMC Surg. 2014;6:12. doi: 10.1186/1471-2482-14-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Renshaw AA. Does a repeated benign aspirate change the risk of malignancy after an initial atypical thyroid fine-needle aspiration? Am J Clin Pathol. 2010;134:788–792. doi: 10.1309/AJCPRA9Y2XQVFOFV. [DOI] [PubMed] [Google Scholar]
- 24.Nayar R, Ivanovic M. The indeterminate thyroid fine-needle aspiration: experience from an academic center using terminology similar to that proposed in the 2007 National Cancer Institute Thyroid Fine Needle Aspiration State of the Science Conference. Cancer. 2009;117(3):195–202. doi: 10.1002/cncy.20029. [DOI] [PubMed] [Google Scholar]
- 25.Kim DW, Lee EJ, In HS, Kim SJ. Sonographic differentiation of partially cystic thyroid nodules: a prospective study. AJNR Am J Neuroradiol. 2010;31(10):1961–1966. doi: 10.3174/ajnr.A2204. [DOI] [PMC free article] [PubMed] [Google Scholar]