Abstract
Purpose of Review
Physical activity is increasingly recommended for chronic pain. In this review, we briefly survey recent, high-quality meta-analyses on the effects of exercise in human chronic pain populations, followed by a critical discussion of the rodent literature.
Recent Findings
Most meta-analytical studies on the effects of exercise in human chronic pain populations describe moderate improvements in various types of chronic pain, despite substantial variability in the outcomes reported in the primary literature. The most consistent findings suggest that while greater adherence to exercise programs produces better outcomes, there is minimal support for the superiority of one type of exercise over another. The rodent literature similarly suggests that while regular exercise reduces hypersensitivity in rodent models of chronic pain, exercise benefits do not appear to relate to either the type of injury or any particular facet of the exercise paradigm. Potential factors underlying these results are discussed, including the putative involvement of stress-induced analgesic effects associated with certain types of exercise paradigms.
Summary
Exercise research using rodent models of chronic pain would benefit from increased attention to the role of stress in exercise-induced analgesia, as well as the incorporation of more clinically relevant exercise paradigms.
Keywords: Chronic pain, Exercise, Rodent, Treadmill, Voluntary, Stress
Introduction
Chronic pain represents an urgent global health problem [1] that incurs massive social and economic costs [2, 3]. Highly prevalent, chronic pain affects between 19 and 43% of the US population [4–8]. A substantial portion of the chronic pain population is comprised of those with bone/joint pain [7], where osteoarthritis (OA) and rheumatoid arthritis (RA) are considered to be among the most disabling of the chronic bone/joint diseases [9–11]. At the population level, over 20% of adults under 65 years old, and almost 50% of adults over 65, have some form of arthritis [12]. A substantial proportion of this population have restricted joint motion, muscle weakness, substantial activity limitations, and are physically inactive [12–19]. As such, arthritis is thought to be the main cause of disability in the USA, with a socio-economic impact approaching $200 billion annually as of 2007 [20].
Three main treatment modalities are available for bone/joint diseases such as OA and RA: surgical, pharmacological, and non-pharmacological [21, 22]. While surgical and pharmacological treatments can certainly be beneficial [23, 24], these approaches are not without risk and/or unpleasant secondary effects [22, 25, 26]. Indeed, referrals for orthopedic surgical interventions are often indicated only after less invasive treatment options have been exhausted [27]. As such, non-pharmacological approaches are increasingly recommended as first-line treatments for certain types of chronic pain, even prior to pharmacological interventions [28]. An increasingly popular non-pharmacological approach is exercise, which has been defined as “planned, structured, and repetitive bodily movements that are performed to improve or maintain one or more components of physical fitness” [29]. The beneficial effects of exercise are undeniable, both for maintaining health and for reducing the negative impacts of many chronic illnesses including cancer, type 2 diabetes, obesity, and depression [30–33]. In this brief review, our goal is to survey the current state of knowledge on the effects of exercise on chronic pain outcomes both in humans and in rodent models of chronic pain. Considering the number of high-quality reviews and meta-analyses that have critically appraised the literature on the effects of aerobic exercise in chronic bone/joint pain in humans, we will only outline their main conclusions. We will, however, discuss the limited rodent literature in more detail. Finally, we will discuss these rodent findings in light of the human studies.
Is Exercise Beneficial for Chronic Pain in Humans?
Habitual physical inactivity is one of the leading risk factors driving non-communicable diseases and death worldwide [34–37]. Sedentary lifestyles are associated with poorer health as well as reduced day-to-day functioning and quality-of-life [13, 38–40], where even intermittent bouts of vigorous activity appear to be unable to counteract the impacts of habitual physical inactivity [13, 39, 40]. Regular physical activity, however, can be effective in both prevention and treatment of many chronic diseases [30–32]. In healthy individuals, there is a long history of support for analgesic effects of regular physical activity, particularly highly aerobic exercises such as endurance running/cycling [41–43]. Additional benefits include the reduction of depression, anxiety, and stress [44–48]. Increasingly, guidelines state that exercise should be part of the core treatment for chronic pain [28, 49–52], generally advising low-to-moderate levels of physical activity that are increased incrementally (i.e. “Start low, go slow”) and monitored by a qualified health care professional. The effects of regular exercise for chronic pain and its comorbidities has been the subject of many primary research reports, reviews, and meta-analyses over the last 30 years. Rather than attempt to re-evaluate the surfeit of primary literature, we will instead briefly report the main conclusions from a few recent Cochrane reviews that have carefully collated this massive and diverse literature into a more digestible format.
Among the most up-to-date and comprehensive reviews is a Cochrane review from Geneen et al. [53••]. Geneen et al. assessed 21 previous Cochrane reviews, incorporating 264 primary reports and 19,642 participants, to determine the effectiveness of different physical exercise interventions in reducing pain stemming from various chronic pain syndromes. Overall, while exercise resulted in reduced pain severity and improved physical function in various forms of chronic pain, effects were small-to-moderate at best and were quite inconsistent across reviews. Exercise-induced effects on psychological function and quality of life were equally variable. Nonetheless, exercise was associated with few adverse events as well as improved pain severity, physical function, and quality of life. Similarly, in a 2014 Cochrane review assessing therapeutic exercise for hip OA in 9 trials (549 participants), Fransen et al. [54] reported high-quality evidence in support of exercise-induced improvements in pain and physical function in individuals with hip OA. In another Cochrane review from the same group based on 44 trials (3537 participants), Fransen et al. [55] reported that therapeutic exercise produced short-term improvements in pain and physical function in individuals with knee OA. However, the lack of blinding in most trials (i.e., participants being aware of their treatment group) may have contributed to the improvement.
In terms of the relative effects of different types or intensities of exercise, O’Connor et al. [56] assessed 17 studies on walking exercise in individuals with chronic low back pain, OA, or fibromyalgia. Overall, walking produced a small-to-medium improvement in pain and physical function in the short term, whereas longer-term effectiveness was uncertain. Regneaux et al. [57] included 6 reports comparing the effects of high- or low-intensity exercise in 656 participants experiencing hip or knee OA. Overall, high-intensity exercise did not seem to provide any clinical benefit over low-intensity exercise in terms of pain and physical function. Regneaux et al. [57] indicated that the paucity of studies comparing high- and low-intensity exercise programs in OA points to the need for research focusing on the minimal exercise intensity required for clinical effect as well as the highest intensity considered both safe and tolerable. In the same vein, Golightly et al. [58••] reviewed 39 studies focusing on the effects of aerobic and strength training exercise on OA-related pain and physical function. They showed that while both forms of exercise improved pain and function, there was no difference in effectiveness between the types of exercise programs studied (i.e., aerobic vs. strengthening regimens). Similarly, a recent meta-analysis of 48 randomized controlled trials on the effects of exercise on OA pain also showed an overall moderate benefit of exercise on pain, regardless of exercise type [59•]. However, Juhl et al. reported (i) that regular aerobic exercise (i.e., at least three times per week for 12 weeks) was more impactful than the intensity of aerobic exercise; (ii) that exercise programs using a single type of exercise (i.e., aerobic exercise, strength training) were more effective than programs mixing multiple types into the same exercise session [59•], but see [60]; and (iii) there was no evidence to support individualized exercise programs based on patient characteristics including radiographic severity of OA. In addition to these points, another important consideration is the duration of exercise-induced effects. A number of groups have indicated that exercise-induced OA benefits appear to fade once the exercise program is discontinued [56, 61, 62], underlining the importance of continued engagement in exercise programs in order to maintain beneficial effects. Indeed, improved adherence to exercise programs seems to be a stronger predictor of improvement in pain and physical function associated with knee OA than exercise frequency or intensity [63–65]. Taken together, while exercise appears to be beneficial for many types of chronic pain, a number of qualifications are in order: Firstly, no intensity or approach appears to be superior to another. Secondly, the available evidence does not appear to support individualized exercise programs. Thirdly, greater adherence to exercise programs yields better outcomes. Finally, benefits do not seem to last much longer than the duration of exercise program.
Is Exercise Beneficial for Mental Health Impacts of Chronic Pain?
Aerobic exercise can improve depression to a level similar in scope to either psychological or pharmacological therapies [33]. Considering that depression affects between 20 and 35% of the chronic pain population [7, 66, 67] and can be considered a consequence of chronic pain [68, 69], establishing the effectiveness of exercise against comorbid depression has clear clinical relevance. While some studies indicate that aerobic exercise improved depression comorbid with fibromyalgia [70, 71], only 5 of the 21 Cochrane reviews assessed in Geneen et al. [53] reported mental health/depression outcomes, with positive yet somewhat variable results. While the existing literature indicates that exercise for depression comorbid with chronic pain is at least moderately effective, more randomized controlled trials of high methodological quality are needed.
Mechanisms of Exercise-Induced Benefits in Chronic Pain
Activation of the endogenous opioid system has long been proposed to be the main biochemical mechanism underlying exercise-induced analgesia reviewed in [41, 65, 72, 73], as well as the euphoric state commonly referred to as ‘runners high’ [74]. However, these effects seem to occur acutely post-exercise and are largely dependent on exercise intensity, where a dose-response relationship exists between exercise intensity and reward/affective response [75] and perhaps also with anti-nociception [76]. Indeed, vigorous exercise (i.e., greater than 70% of the maximum aerobic capacity [VO2max], or the range required to improve cardiovascular fitness in healthy individuals) seems to be required to produce endogenous opioid release [77, 78], reviewed in [79] [80]. As such, benefits associated with lower intensity exercise (i.e., walking at an intensity substantially below 70% of VO2max) may involve longer-term engagement of the endogenous opioid system along with other endogenous systems. Another avenue by which exercise can reduce pain involves factors such as weight loss and other musculoskeletal benefits. Considering that excess weight plays an important structural role in OA pain [16, 81, 82], it is clear that weight loss can be beneficial [83, 84]. However, in terms of other musculoskeletal outcomes such as muscle function, the amount of benefit in pain does not necessarily correlate with the amount of benefit in these outcomes, suggesting that factors other than improved musculoskeletal function may be mediating pain relief [85]. Taken together, mechanisms of exercise-induced attenuation of persistent pain, especially pain relief associated with low-intensity exercise, remain unclear.
Is Exercise Beneficial in Rodent Models of Chronic Pain?
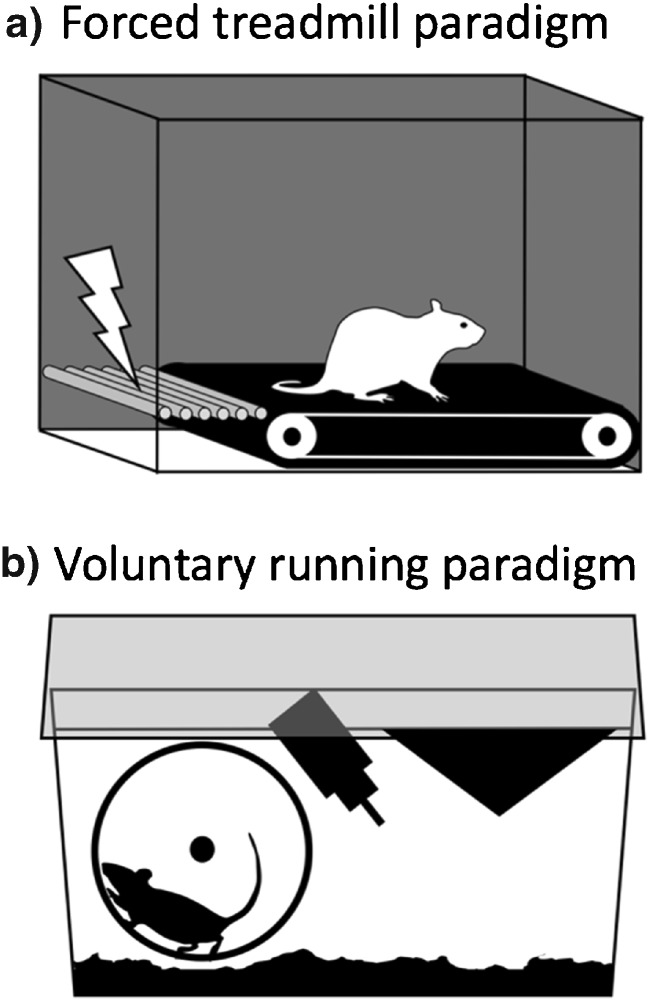
Given the variability and other challenges inherent in human clinical trials, the apparent difficulty in isolating factors underlying exercise-induced analgesia is perhaps not surprising. Rodent studies, however, allow precise control of biological and environmental factors, as well as experimental interventions. As such, they should be well placed to probe these questions. A careful search of the biomedical research repository Pubmed (https://www.ncbi.nlm.nih.gov/pubmed) was performed using the search terms “exercise” and “chronic pain” or “pain” in non-human animal research. Only studies incorporating land-based physical exercise (i.e., voluntary wheel running or treadmill running; Fig. 1) as a primary intervention were included. Studies in which access to exercise was not ensured for each animal (i.e., environmental enrichment paradigms with a single voluntary exercise wheel for numerous cage mates) were not included in this review. Review articles, studies not focused on rodent models of persistent pain, and studies lacking pain/hypersensitivity outcomes were also excluded from this assessment. This search strategy revealed no less than 43 studies focused on the effects of exercise in rodent models of persistent pain (Tables 1 and 2). In the context of these studies, we discuss the impact of a number of factors including the type of chronic pain model (neuropathic, osteoarthritis, etc), species (rat, mouse), gender (male, female), exercise modality (forced treadmill running, voluntary wheel running), and exercise intensity characteristics (i.e., duration, frequency and velocity). While most studies used exercise as a therapeutic intervention (i.e., main exercise paradigm initiated post-injury), a number used exercise preventatively (i.e., exercise initiated pre-injury) or integrated both pre- and post-injury exercise into a single paradigm. Given that preventative paradigms may influence not only the maintenance of persistent pain states, but also the development of pain, we considered preventative exercise paradigms (Table 1) separately from therapeutic exercise paradigms (Table 2).
Fig. 1.
Rodent exercise paradigms. a. Forced treadmill running involves placing the rodent in an inescapable enclosure, either with or without an electrical shock grid to reinforce running behavior. Speed and incline of the treadmill can be adjusted. b. Voluntary wheel running paradigms yield either restricted or unrestricted access to the running wheel. During access, the rodent is free to run as much or as little as desired
Table 1.
Preventative exercise in rodent models of chronic pain
| Reference | Pain model | Sex and Species | Exercise modality ± reinforcement | Pre-training | Exercise intensity | Maximal running velocity | Time delay between exercise and testing | Effective for mechanical hypersensitivity? | Effective for thermal hypersensitivity? | Other outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Cobianchi et al. 2010 | CCI (NP) | Male mice | Treadmill + shock | No | 60 min/day, 5 days/week, 2 weeks | 30 m/min | 3 days | No | n/a | n/a |
| Stagg et al. 2011 | SNL (NP) | Male rats | Treadmill + shock (some excluded for stress/not running) |
No | 10 min/day, 2 days/week, 2 weeks | 18 m/min | ~ 7 days | No | No | n/a |
| Sluka et al. 2013 | Muscle | Male mice | Voluntary | No | 24 h/day, 7 days/week, 5 days or 8 weeks | n/a | 6 days | Yes: 8 weeks, effect lost by 1 week No: 5 days |
n/a | Reduced phosphorylation of NMDA-NR1 in RVM |
| Gong et al. 2016 | Incision | Female rats (neonatal) | Treadmill + shock (excluded poor runners) | No | 30 min/day, 6 days/week, 3 weeks | 15 m/min | 4 days | No | No | n/a |
| Grace et al. 2016 | CCI (NP) | Male rats | Voluntary | No | 24 h/day 7 days/week, 6 weeks |
n/a | 1 day | Yes: effect lasted for 13 weeks post-exercise | n/a | Normalized IL-1b, GLT-1, P2X4R-BDNF. Reduced macrophage activity and CCL2 chemokine. Increased IL-10. |
| Kami et al. 2016A | PSNL (NP) | Male mice | Treadmill + prodding | No | 10–60 min/day, 5 d/week, 2 weeks | 7 m/min | 2 days | No | No | n/a |
| Kami et al. 2016B | PSNL (NP) | Male mice | Treadmill + shock + prodding | No | 10–60 min/day, 5 day/week, 2 weeks | 7 m/min | 2 dys | No | No | n/a |
| Leung et al. 2016 | Muscle | Male and female mice | Voluntary | No | 24 h/day 7 days/week, 8 weeks |
n/a | 6 days | Yes: Only tested 1 day post-injury. No gender differences. | n/a | Muscle macrophage and increasing IL-10 |
| Sabharwal et al. 2016 | Muscle | Male and female mice | Voluntary | No | 24 h/day 7 days/week, 5 days or 8 weeks |
n/a | 6 days | Yes: 5 days = 8weeks. Only tested 1 day post-injury. No gender differences. |
n/a | Prevented development of autonomic dysfunction (i.e., reduced HRV) |
| Wakaizumi et al. 2016 | PSNL (NP) | Male mice | Treadmill | No | 60 min/day, 5 days/week, 2 weeks |
6–12 m/min | 5 days | No | n/a | n/a |
| Safakhah et al. 2017 | CCI (NP) | Male rats | Treadmill | 5 days, 10 min/day, 10 m/min | 30 min/day, 5 days/week, 3 weeks |
16 m/min | 27 days | No | No | n/a |
CCI chronic constriction injury, PSNL partial sciatic nerve ligation, SNL spinal nerve ligation, Muscle muscle pain, Incision incision, NP neuropathic pain model, n/a not applicable, PID post-injury day, m/min meters per minute
Table 2.
Therapeutic exercise in rodent models of chronic pain
| Reference | Pain model | Sex and species | Exercise modality ± reinforcement | Pre-training | Exercise initiated | Exercise intensity | Maximal running velocity | Effective for mechanical hypersensitivity? | Effective for thermal hypersensitivity? | Other outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Hutchison et al. 2004 | SCI (NP) | Female rats | Treadmill + positive reinforcement | 1 week, 12 m/min | PID 4 | 25 min/day, 5 days/week, 7 weeks |
12 m/min | Yes | n/a | Restored BDNF signaling |
| Bement et al. 2005 | Muscle | Male rats | Treadmill (some excluded) | 3 days, 5 min/day, 3 m/min | PID 1 | 30 min/day, 5 days | 6 m/min | Yes | n/a | Opioid-dependent effect of exercise |
| Cobianchi et al. 2010 | CCI (NP) | Male mice | Treadmill + shock (some excluded) | 2 weeks, 60 min/day, 5 days/week, 30 m/min | PID 3 | 60 min/day, 5 days, or 8 weeks | 30 m/min | Yes 5 days No > 5 days |
n/a | Shorter duration reduced microglia/astrocyte expression, better nerve regeneration |
| Korb et al. 2010 | SNSR (NP) | Male rats | Treadmill | 4 days, 10 min/day, 5 m/min, then max test | PID 7 | 60 min/day, 5 days/week, 4 weeks |
9 m/min | Yes | n/a | Serotonin activity in the spinal cord |
| Sharma et al. 2010 | Muscle | Female mice | Treadmill + prodding | 3 days, 13 m/min | PID 5 | 45 min/day, 5 days/week, 3 weeks |
16 m/min | Yes | n/a | NT-3 expression increased |
| Stagg et al. 2011 | SNL (NP) | Male rats | Treadmill + shock (some excluded for stress/not running) |
2 weeks, 2 day/week, 10 min/day, 18 m/min, 8% grade | PID 7 or 28 | 30 min/day, 3 days/week or 5 days/week, 5 weeks |
10–16 m/min | Yes: 3 days/week = 5 days/week Yes: 7 days delay = 8 days delay. Yes: 16 m/min. No: 10 m/min In all cases, exercise effects lost ~ 5 days. |
Yes | Opioid-mediated effects in the RVM and the PAG |
| Shankarappa et al. 2011 | Diab (NP) | Male rats | Treadmill + shock | 5 days, 60 min/day, 18 m/min | n/a | 60 min/day, 5 days/week, 10 weeks |
18 m/min | Yes | No change in diabetic rats | Opioid-mediated. Ca2+-mediated changes in DRG neurons |
| Chen et al. 2012 | CCI (NP) | Male rats | Treadmill (no shock) | 3 days, 15 min/day, 20 m/min | PID 2 | 60 min/day, 5 days/week, 6 weeks | 30 m/min | Yes | Yes | Increased Hsp-72 and decreased IL-1b, TNF⍺ |
| Chen et al. 2013A | Diab (NP) | Male rats | Treadmill (some excluded) | n/a | Not indicated | 60 min/days, 7 days/week, 8 weeks | 20 m/min | Yes | Yes | Reduced blood glucose levels and increased Hsp-72 but no effect on IL-6, TNF⍺ |
| Chen et al. 2013B | Incision | Male rats | Treadmill | 3 days 15 min | PID 8 | 55 min/day, 5 days/week, 4 weeks | 18 m/min | Yes, partial reversal | n/a | Lower NMDA-NR1, TNF⍺, and IL-6 in the spinal cord |
| Cobianchi et al. 2013 | SNSR (NP) | Female rats | Treadmill + shock | n/a | PID 3 | 60 min/day, 5 days | 19 m/min | Yes, partial reversal | No | Improved axonal regeneration, reduced BDNF, NGF, and GDNF in DRG |
| Groover et al. 2013 | Diab (NP) | Male mice | Voluntary | n/a | PID 1 | 24 h/day 7 days/week, 12 weeks |
n/a | Yes: 6wks to take effect. | No change in diabetic rats | Reduced metabolic abnormalities. Improved innervation and neurotrophin levels (TrkA, NGF, BDNF) |
| Morimoto et al. 2013 | Cast | Male rats | Treadmill | 3 days | PID 3 | 30 min/day, 3 days/week, 2 weeks |
12 m/min | Yes | n/a | Range of motion and calf muscle atrophy improved |
| Chen et al. 2014 | Incision | Male rats | Treadmill + shock | n/a | PID 6 | 60 min/day, 5 days/week, 4 weeks | 18 m/min | Yes, partial reversal | n/a | Reduced substance P, IL-1b, IL-6, in DRG |
| Detloff et al. 2014 | SCI (NP) | Female rats | Automated running wheels (forced) | n/a | PID 5 | 20 min/day, 5 days/week, 5 weeks |
14 m/min | Yes: Immediately effective. | No | GDNF, artemin maintained at normal levels |
| Bobinski et al. 2015 | SNC (NP) | Male mice | Treadmill | 1 week, 10 min/day, 10 m/min | PID 3 | 30 min/day, 5 days/week, 2 weeks |
10 m/min | Yes | n/a | Brainstem serotonin increased, while TNF⍺ and IL-1b were reduced |
| Chen et al. 2015 | Diab (NP) | Male rats | Treadmill + prodding | 3 days, 15 min/day, 10 m/min | PID 3 | 60 min/day, 7 days/week, 4 weeks | 25 m/min | Yes | Yes | Increased IL-10 and decreased IL-6, TNF⍺, MDA |
| Chuganji et al. 2015 | Cast | Male rats | Treadmill | n/a | PID 0 | 30 min/day, 5 days/week, 8 weeks |
15 m/min | Yes | n/a | β-endorphin increased in PAG and hypothalamus |
| Lopez-Alvarez et al. 2015 | SNSR (NP) | Female rats | Treadmill | 1 h, 19 m/min | PID 3 | 60 min/day, 5 days or 10 days | 19 m/min | Yes | Yes | Prevented changes in peripheral innervation and disruption of Cl co-trans. Reduced NGF, BDNF |
| Kim et al. 2015 | CCI (NP) | Male rats | Treadmill | n/a | Not indicated | 30 min/day, 5 days/week, 4 weeks |
22 m/min | Yes | Yes, partial reversal | Reduced Mu opioid receptor expression in RVM and spinal cord |
| Luan et al. 2015 | Disc | Male rats | Treadmill | n/a | PID 14 | 40 min/day, 7 day/week, 1–8 weeks |
13 m/min | Yes | n/a | Improved degenerated discs and neurogenesis in and around disc |
| Sheahan et al. 2015 | SNI (NP) | Male mice | Voluntary | n/a | PID 8–10 | 2–12 h/day, 5–6 d/week, 1 or 4 weeks |
n/a | No | No | Ineffective in reversing muscle wasting or denervation. |
| Yoon et al. 2015 | Diab (NP) | Male rats | Treadmill | 2 days, 20 min/day, 5 m/min | PID 7 | 60 min/day, 5 days/week, 6 weeks |
10 m/min | Yes | Yes | Increased enkephalin, HSP70; decreased TNF⍺, IL-1b, TRPV1, TRPM8, pp38 in the DRG |
| Detloff et al. 2016 | SCI (NP) | Female rats | Automated running wheels (forced) | n/a | PID 14 or 28 | 20 min/day, 5 days/week, 5 weeks |
14 m/min | No | n/a | |
| Gong et al. 2016 | Incision | Female rats (neonatal) | Treadmill + shock (excluded poor runners) |
n/a | PID 22 | 30 min/day, 6 days/week, 2 weeks | 15 m/min | No | No | Reduced high P38 MAPK, 1L-1b, TNF⍺ (but not IL-6). |
| Grace et al. 2016 | CCI (NP) | Male rats | Voluntary | n/a | PID 0 or 14 | 24 h/d 7 days/week, 2 weeks or 11 weeks |
n/a | Yes: 2wks = 11wks | n/a | Normalized IL-1b, GLT-1, P2X4R-BDNF. Reduced macrophage activity and CCL2 chemokine. Increased IL-10. |
| Kami et al. 2016A | PSNL (NP) | Male mice | Treadmill + prodding | 10–60 min/day, 7 m/min, 2 weeks | PID 2 | 60 min/day, 5 day/week, 5 days |
7 m/min | Yes | Yes | Blocked decrease in GABA/GAD65/67 reductions in dorsal horn |
| Kami et al. 2016B | PSNL (NP) | Male mice | Treadmill + prodding | 10–60 min/day, 7 m/min, 2 weeks | PID 2 | 60 min/day, 5 day/week, 5 day | 7 m/min | Yes | Yes | Reduced histone deacetylase1+/CD11b+ microglia in spinal cord |
| Wakaizumi et al. 2016 | PSNL (NP) | Male Mice | Treadmill | 3 options: - None - 2 weeks, 1 h/day, 5 days/week, 6 m/min - 2 weeks, 1 h/day, 5 days/week, 12 m/min |
n/a | 60 min/day, 5 days/week, 1 week (fast) or 2 weeks (slow) |
6 m/min or 12 m/min | Yes: 2wks pre + 2wks post, slow. Yes: 2wks post, slow. Yes: 2wks pre + 1wk post, fast. No: 1wk post, fast. |
Yes: 2wks, slow | Mesolimbic dopamine activity |
| Allen et al. 2017 | MIA (OA) | Male Rats | Treadmill + shock | n/a | PID 10 | 30 min/day, 4 days/week, 4 weeks |
16 m/min | Yes | n/a | Opioid-mediated effects. Exercise improved trabecular bone microarchitecture. |
| Arbat-Plana et al. 2017 | SNSR (NP) | Female Rats | Treadmill + shock | n/a | PID 3 | 60 min/day, 5 days/week, 2 weeks | 18 m/min | Yes | n/a | BDNF/TrkB signaling maintaining functional spinal neuro-circuitry. |
| Bobinski et al. 2017 | SNC (NP) | Male Mice | Treadmill | n/a | PID 3 | 30 min/day, 5 days/week, 2 weeks |
10 m/min | Yes | n/a | Increased IL-4 |
| Cormier et al. 2017 | MIA (OA) | Male Rats | Voluntary | 1 or 3 weeks | PID 0 | 24 h/day 7 days/week, 3 weeks |
n/a | Yes: 3wks pre + 3wks post. No: 1wk pre + 3wks post. |
n/a | Improved trabecular bone microarchitecture. |
| Huang et al. 2017 | CCI (NP) | Male rats | Treadmill + prodding | n/a | PID 8 | 30 min/day, 7 days/week, 3 weeks |
16 m/min (8% grade) | Yes | Yes | Reduced IL-6 and TNF⍺ and increased IL-10 |
| Lopez-Alvarez et al. 2017 | SNSR (NP) | Female rats | Treadmill | 60 min, 19 m/min | PID 3 | 60 min/day, 12 day | 19 m/min | Yes | Yes | Monoaminergic (5HT) descending pathways, and BDNF and microglia in locus coeruleus |
| Pitcher et al. 2017 | CFA (OA) | Male rats | Voluntary | n/a | PID 3 | 2 h/day, 4 d/week, 3 weeks |
n/a | Yes | Yes | Decreased stress, increased HRV, no change in swelling. No association between running and benefit |
| Safakhah et al. 2017 | CCI (NP) | Male rats | Treadmill (some excluded) | 5 days, 10 min/day, 10 m/min | PID 5 | 30 min/day, 5 days/week, 3 weeks |
16 m/min | Yes | Yes | Decreased TNF⍺ and increased FRAP |
| Tsai et al. 2017 | CCI (NP) | Male rats | Treadmill + prodding | n/a | PID 6 | 30 min/day, 7 days/week, 3 weeks |
14–16 m/min (8% grade) | Yes: 8% incline better than 0%. | Yes: 8% incline better than 0%. | Decreased TNF⍺ and IL-6 and prevented downreg of IL-10 |
| Whitehead et al. 2017 | CCI (NP) | Male rats | Voluntary | 1wk, 60 min/d | PID 2–3 | 60 min/day, 7 days, 18 days |
n/a | No | n/a | |
| Yamaoka et al. 2017 | SNL (NP) | Female rats | Treadmill | n/a | PID 1 | 20 min/day, 5 days/week, 6 weeks |
20 m/min (10° incline) | Yes | Yes, partial reversal |
CCI chronic constriction injury, PSNL partial sciatic nerve ligation, SNL spinal nerve ligation, SNI spared nerve injury, SNSR sciatic nerve section and repair, SCI spinal cord contusion injury, SNC sciatic nerve crush, Diab diabetic neuropathy, MIA Mono-iodoacetate, CFA complete Freund’s adjuvant, Muscle muscle pain, Incision incision, Cast cast immobilization, Disc disc degeneration, OA osteoarthritis model, NP neuropathic pain model, n/a not applicable, PID post-injury day, m/min meters per minute
Preventative Exercise
A number of studies incorporated experiments that were designed specifically to assess the effectiveness of preventative exercise on the development of persistent pain in rodents (Table 1). Of the 11 studies employing preventative exercise paradigms, 7 used neuropathic pain models (64%), 3 used a model of chronic muscle pain (27%), and 1 study used a model of incisional pain (9%). A total of eight studies used male rodents (73%), whereas two studies used both males and females (18%) and one used only females (9%). Seven studies used mice (64%), while the remaining four used rats (36%). Seven studies used a forced treadmill running paradigm (64%), while the remaining four employed voluntary wheel running (36%). In terms of exercise characteristics, all of the studies employing voluntary running allowed rodents unrestricted access to running wheels for a duration between 5 days and 8 weeks. While the duration of forced treadmill running studies was fairly uniform (i.e., 2–3 weeks), their frequency ranged from 10 min per day twice a week to 1 h per day for 5 days a week. Maximal running velocity in studies using voluntary running wheels was not reported. However, the maximal running velocity used in forced treadmill studies ranged between 6 and 30 m/min.
A total of four studies reported that preventative exercise paradigms reduced mechanical hypersensitivity [86–88, 89••]. These studies all used voluntary exercise, where rodents had unrestricted access to running wheels. Moreover, three of the four voluntary exercise studies employed a muscle pain model [86, 88, 89••], whereas the fourth used a neuropathic pain model [87]. Exercise of longer duration, beginning 6–8 weeks prior to pain induction, appeared to be most effective [86–88] but see [89••]. In terms of the duration of exercise-induced benefits, while Grace et al. reported long-lasting effects of preventative exercise for neuropathic pain [87], the beneficial effects of exercise on chronic muscle pain lasted no longer than 3 days post-induction [86, 88, 89••]. In the two studies using both male and female rodents, no sex differences in the effects of exercise were observed [88, 89••]. Of the five studies assessing thermal hypersensitivity, all employed forced treadmill running paradigms. However, none found preventative exercise to be effective at reducing thermal hypersensitivity (Table 1). Overall, among the studies where preventative exercise was not effective at reducing hypersensitivity, all used forced treadmill running in models of neuropathic pain [90, 91•, 92–96]. As such, it appears that the exercise paradigm (i.e., voluntary exercise), the amount of running (i.e., unrestricted wheel access), and possibly the model of chronic pain, may all contribute to the effectiveness of preventative exercise paradigms in rodent models of chronic pain.
Therapeutic Exercise
A total of 40 studies initiated the main exercise program therapeutically, after the onset of experimental models of chronic pain in rodents (Table 2). Over half of these studies used therapeutic exercise that was preceded by some level of pre-training prior to injury (21/40, or 52.5%). None of these studies reported an analgesic effect of pre-training on early post-injury hypersensitivity. As such, these studies are considered in the context of therapeutic exercise.
Of the 40 studies employing therapeutic exercise paradigms, 29 used neuropathic pain models (72.5%), 3 used models of osteoarthritis (7.5%), 3 used incision models (7.5%), and 5 used other models (12.5%). A total of 30 studies used male rodents (75%), whereas 10 studies used females (25%). The majority of studies used rats (31 or 77.5%), while the remaining nine studies used mice (22.5%). In most cases, a forced running paradigm was used (34 studies, or 85%). In these studies, exercise was performed for between 20 and 60 min/day for 4–7 days/week. The six studies (15%) using voluntary wheel running generally allowed longer wheel access (2–24 h/day [87, 97, 98, 99••, 100••] but see [101]) for 4–7 days per week. However, it should be noted that the actual running time in voluntary exercise paradigms is unknown because rodents do not necessarily engage in constant running during periods of wheel access. The duration of the voluntary running paradigms ranged between 1 and 12 weeks, but most lasted between 2 and 4 weeks. Furthermore, only one study using voluntary exercise reported the average running velocity: Pitcher et al. [100••] showed that the average velocity of both sham and OA groups was approximately 45 m/min, which is comparable to other studies using voluntary wheel running [102•, 103]. On the other hand, the maximal velocity at which rodents were forced to run on treadmills ranged between 6 and 30 m/min. While it is certainly possible to train rodents to run on a treadmill at velocities of 30 m/min, maintaining this velocity for more than a few minutes appears to require substantial aerobic pre-training (8–10 weeks) at oxygen intake levels approaching maximal capacity (i.e., VO2max) [104]. In fact, some groups report difficulty in forcing rats to run faster than 20 m/min [102•], and approximately 25% of mice forced to run at 12 m/min on treadmills cease running by 10–15 min, and around 50% cease running by 20–25 min [105]. Considering that the forced running studies discussed here generally employed little or no pre-training prior to relatively high exercise intensities, it is somewhat surprising that rodents with painful hind paw injuries were able to complete the studies. Indeed, a number of studies reported that some rodents were excluded due to stress or refusal to run [90, 91•, 92, 96, 106, 107], while another reported that some mice discontinued running at the 12 m/min velocity during the therapeutic exercise phase [95]. Moreover, a number of studies indicated that electric shocks or physical encouragements were required to ensure continued running [90, 91•, 92–94, 108•, 109–113, 114••, 115–117]. Under these conditions, the vast majority of studies (36 or 90%) reported that at least some form of therapeutic exercise was effective in reducing or reversing mechanical hypersensitivity. While three of the four studies showing no beneficial effect of therapeutic exercise on mechanical hypersensitivity initiated exercise 8 or more days after injury [92, 98, 118], other studies using similar delays in initiation of exercise were effective [87, 91•, 114••, 116, 119, 120]. Similarly, while two of the four studies showing no beneficial effect of therapeutic exercise on mechanical hypersensitivity used voluntary exercise paradigms [98, 101], other studies using voluntary exercise were effective [87, 97, 99••, 100••]. Of the 36 studies showing that exercise was effective, a few indicate that some approaches were not as effective as others [91•, 95, 99••]. Specifically, Stagg et al. reported that 3 weeks at a slower treadmill speed of 10 m/min did not improve mechanical hypersensitivity, whereas the same duration at a higher speed of 16 m/min was effective [91•]. On the other hand, Wakaizumi et al. showed that while 2 weeks of slow running (6 m/min) was effective, 1 week of faster running (12 m/min) was not effective [95]. Finally, Cormier et al. indicated that while 3 weeks of pre-training followed by 3 weeks of therapeutic running resulted in beneficial effects, 1 week of pre-training followed by 3 weeks of therapeutic running was not beneficial [99••]. Importantly, in all of these cases, other studies using similar exercise parameters show effectiveness. Consequently, no particular factors appear to be consistently associated with either effectiveness or ineffectiveness of therapeutic exercise.
A total of 22 studies incorporated measures of thermal hypersensitivity (Table 2). Two of these studies, both using models of diabetes-induced pain, did not observe thermal hypersensitivity in diabetic rodents [97, 110], which is in contrast to other studies using diabetic models [107, 113, 121]. Of the remaining 20 studies, 16 showed that exercise was effective in reducing or reversing thermal hypersensitivity (80%). Of the four studies in which no effect of exercise was observed, three were conducted in female rats [92, 111, 122]. Nonetheless, three other studies also using female rats showed improved thermal hypersensitivity [123–125]. Similarly, while three of the four studies in which no effect of exercise was observed utilized neuropathic pain models [98, 111, 122], a number of other studies successfully employed therapeutic exercise against neuropathic pain-induced thermal hypersensitivity [123–125]. Therefore, among the studies considered here, therapeutic exercise appears to be an effective method of reducing thermal hypersensitivity. However, no specific factors appear to be related to exercise-induced improvements in thermal hypersensitivity.
Mechanisms of Exercise-Induced Benefits in Rodent Models of Chronic Pain
As illustrated in Tables 1 and 2, both voluntary wheel running and forced treadmill running promote favorable outcomes in a number of physiological systems impacted by persistent pain. Exercise improves measures of neurological function in the periphery and spinal cord [90, 94, 97, 111, 115, 119, 121, 123] as well as improved musculoskeletal outcomes [99••, 114••, 120, 126]. In addition, exercise improved neurotrophic receptor signaling in the spinal cord and periphery [87, 97, 108•, 109, 111, 115, 122–124]; restoration to pre-injury levels of cytokines and other neuroimmune products in the brainstem, spinal cord, and periphery [87, 88, 90, 93, 96, 112, 113, 116, 117, 119, 121, 123, 124, 127, 128]; and increased endogenous opioid activity in the rostroventral medulla (RVM), spinal cord, and dorsal root ganglia (DRG) [91•, 106, 110, 114••, 121, 129, 130]. Importantly, while endogenous opioid-mediated mechanisms can produce analgesia at acute post-exercise time points, some studies suggest that longer-term endogenous opioid-mediated effects also occur. Specifically, Stagg et al. [91•] and Allen et al. [114••] demonstrated that naloxone blocks exercise-induced analgesia even when injected at time points far beyond the potential acute effects of exercise. As such, regular exercise may induce long-term tonic changes in endogenous opioid tone that promote analgesia.
Does Exercise Alter Stress in Rodent Models of Chronic Pain?
Of the 43 studies discussed here, only two incorporated stress measures [89••, 100••], and both employed voluntary wheel running paradigms. Sabharwal et al. demonstrated that as little as 5 days and up to 8 weeks of unrestricted access to running wheels prior to injury prevented injury-induced reductions in heart rate variability (HRV), a measure of autonomic health known to be negatively impacted by stress [131–134], reviewed in [135] and chronic pain [136]. In the same vein, Pitcher et al. showed that following induction of a rat model of OA, 3 weeks of modest access to running wheels (2 h/day, 4 days/week) improved both HRV and plasma levels of the stress hormone corticosterone. Pitcher et al. also assessed the relationship between exercise intensity and pain and stress outcomes [100••]. Similar to studies in humans, exercise intensity (i.e., total distance, average velocity) was unrelated to the degree of benefit in both pain and stress, a finding that challenges the widely held belief that more exercise yields better outcomes. Others have shown a similar lack of accord between the intensity of voluntary running and measures of stress/reward [137••, 138–142]. Overall, relatively low levels of self-regulated exercise appear to be protective against persistent pain and persistent pain-induced stress.
Conclusions
The vast majority of rodent studies discussed here report beneficial effects of exercise in models of chronic pain. These benefits were accrued from both voluntary and forced exercise paradigms incorporating a diversity of exercise characteristics. To some extent, exercise-induced improvements in rodent models of chronic pain mirror the conclusions expressed in human chronic pain meta-analyses, where no intensity or approach appears to be superior to another, and exercise benefits do not seem to be related to any particular facet of either the type of injury or the exercise paradigm. In fact, there appears to be only one consistent factor among rodent studies showing exercise efficacy: regular exercise. In each study, rodents are exposed to exercise on a regular basis for a period of time. Similarly, regular exercise seems to be among the most important factors underlying exercise-induced benefits in the human literature. That said, this brief review of a relatively limited body of rodent literature was not meant to be meta-analytic (i.e., incorporating comparable data from multiple sources for statistical re-analysis). As such, it is possible that it was not sufficiently refined to detect an existing relationship between certain exercise characteristics and analgesic outcomes.
How might regular exercise exert its beneficial effects independently of other exercise-related factors? A number of rodent studies suggest that exercise-induced modulation of the immune/inflammatory response may play a role [87, 88, 90, 93, 96, 112, 113, 116, 117, 119, 121, 123, 124, 127, 128]. Indeed, stress-related changes in immune reactivity has been proposed as a major contributing factor in the development and maintenance of chronic pain [143]. Exercise, particularly regular aerobic exercise, appears well placed to reduce the impact of an altered immune/inflammatory responses in chronic diseases such as diabetes and obesity [144–146]. As such, it may be similarly effective in chronic pain states. Perhaps even more prominent than the immune response, the human literature emphasizes a role for endogenous opioids in exercise-induced analgesia reviewed in [41, 65, 72, 73]. However, the apparent intensity-dependence of acute exercise for activation of the endogenous opioid system [75–78], reviewed in [79, 80] seems to argue against its involvement in low-intensity exercises such as walking. In the rodent exercise literature, activity of the endogenous opioid system is also widely reported [91•, 106, 110, 114••, 121, 129, 130]. However, these studies employed forced treadmill running, which can be highly stressful [102•, 147–154]. Indeed, forced running is more stressful than voluntary exercise when both paradigms are compared directly [102•, 155, 156]. Stressors involving unpleasant, inescapable contexts (i.e., cold-water swim, restraint) and/or electric shock are commonly used to evoke stress-induced analgesia (SIA), a stress-induced reduction in pain sensitivity related to increased endogenous opioid or cannabinoid activity [157, 158]. By definition, the forced running paradigm is inescapable and often incorporates electric shock plates or other negative reinforcements to promote running (Fig. 1), and forced treadmill walking has been used by at least one research group as a model of stress-induced analgesia [159–162]. Of the 43 studies considered here, almost 80% employed forced running paradigms, the vast majority of which reported exercise-induced analgesia (Tables 1 and 2). Of these, 41% indicated that negative reinforcements, in the form of electric shock or physical stimuli such as manual prodding of the rodent, were used to promote running. Only one study stated explicitly that electrical shock was not used to reinforce running behavior [128], while another indicated that positive reinforcement (i.e., sweetened water) was used to reinforce treadmill running [108•]. The remaining forced running studies did not state whether or not negative reinforcement was used. Considering the clear potential for stress-induced analgesia in forced running paradigms, it is surprising that the only two rodent studies including stress outcomes both employed voluntary running paradigms [89••, 100••]. On the other hand, at least one study has reported that relatively intense forced treadmill running does not increase tail flick latencies, a common assay for stress-induced analgesia in rodents [163]. In addition, forced running paradigms can activate reward centers in the rodent brain if the animals are pre-trained appropriately [164], as well as produce beneficial physiological effects in some contexts [153, 165–167]. Nevertheless, given the absence of stress measures in the forced running studies, it is not possible to exclude the potential contribution of stress-induced analgesic effects in their results.
While voluntary running paradigms may avoid the influence of stress-induced analgesia, it is well known that rodents will often exhibit very high levels of running behavior when given unrestricted access to running wheels, where rats have been reported to attain peak velocities of approximately 160 m/min and mice up to 210 m/min for very brief bursts [168, 169]. As aforementioned, high-intensity activity triggers the endogenous opioid system [77, 78], reviewed in [79] [80], and voluntary wheel running can certainly increase endogenous opioid levels [138, 139, 170–174]. However, such high-intensity physical activity cannot be said to represent a clinically relevant therapeutic approach for most chronic pain patients. On the other hand, evidence in rodents suggests that regular exercise may also enhance tonic activity of the opioid system beyond acute post-exercise time points [91•] [114••]. As such, a better understanding of the effects of long-term exercise on tonic endogenous opioid activity is needed. Only three rodent studies assessed the effect of more modest levels of voluntary exercise [98, 100••, 101]. Of these, only one demonstrated that exercise was effective [100••], indicating that additional research directly assessing the role of stress in the analgesic effects of running is required, incorporating experimental paradigms that more accurately represent the human chronic pain population. Taken together, the human and rodent literature suggest that regular exercise, even at modest levels, can be beneficial for chronic pain. However, the current state of the literature precludes a nuanced understanding of optimal exercise parameters and putative biological mechanisms.
Compliance with Ethical Standards
Conflict of Interest
Mark Henry Pitcher declares no conflict of interest.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Footnotes
This article is part of the Topical Collection on Bone and Joint Pain
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major Importance
- 1.Goldberg DS, McGee SJ. Pain as a global public health priority. BMC Public Health 2011;11:770. [DOI] [PMC free article] [PubMed]
- 2.Demyttenaere K, Bruffaerts R, Lee S, Posada-Villa J, Kovess V, Angermeyer MC, Levinson D, de Girolamo G, Nakane H, Mneimneh Z, Lara C, de Graaf R, Scott KM, Gureje O, Stein DJ, Haro JM, Bromet EJ, Kessler RC, Alonso J, von Korff M. Mental disorders among persons with chronic back or neck pain: results from the world mental health surveys. Pain. 2007;129(3):332–342. doi: 10.1016/j.pain.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 3.Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012;13(8):715–724. doi: 10.1016/j.jpain.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Johannes CB, Le TK, Zhou XL, Johnston JA, Dworkin RH. The prevalence of chronic pain in United States adults results of an internet-based survey. J Pain. 2010;11(11):1230–1239. doi: 10.1016/j.jpain.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Kennedy J, Roll JM, Schraudner T, Murphy S, McPherson S. Prevalence of persistent pain in the US adult population: new data from the 2010 National Health Interview Survey. J Pain. 2014;15(10):979–984. doi: 10.1016/j.jpain.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Nahin RL. Estimates of pain prevalence and severity in adults: United States, 2012. J Pain. 2015;16(8):769–780. doi: 10.1016/j.jpain.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsang A, Von Korff M, Lee S, Alonso J, Karam E, Angermeyer MC, et al. Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disorders. J Pain. 2008;9(10):883–891. doi: 10.1016/j.jpain.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Institute of Medicine (U.S.) Committee on Advancing Pain Research Care and Education. Relieving Pain in America: A blueprint for transforming prevention, care, education, and research. Washington (DC): national academies press; 2011. [PubMed]
- 9.Cross M, Smith E, Hoy D, Carmona L, Wolfe F, Vos T, Williams B, Gabriel S, Lassere M, Johns N, Buchbinder R, Woolf A, March L. The global burden of rheumatoid arthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(7):1316–1322. doi: 10.1136/annrheumdis-2013-204627. [DOI] [PubMed] [Google Scholar]
- 10.Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, Bridgett L, Williams S, Guillemin F, Hill CL, Laslett LL, Jones G, Cicuttini F, Osborne R, Vos T, Buchbinder R, Woolf A, March L The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis 2014;73(7):1323–1330. [DOI] [PubMed]
- 11.Langley P, Muller-Schwefe G, Nicolaou A, Liedgens H, Pergolizzi J, Varrassi G. The impact of pain on labor force participation, absenteeism and presenteeism in the European Union. J Med Econ. 2010;13(4):662–672. doi: 10.3111/13696998.2010.529379. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease C, Prevention Prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation—United States, 2010-2012. MMWR Morb Mortal Wkly Rep. 2013;62(44):869–873. [PMC free article] [PubMed] [Google Scholar]
- 13.Lee J, Dunlop D, Ehrlich-Jones L, Semanik P, Song J, Manheim L, Chang RW. Public health impact of risk factors for physical inactivity in adults with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64(4):488–493. doi: 10.1002/acr.21582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hootman JM, Macera CA, Ham SA, Helmick CG, Sniezek JE. Physical activity levels among the general US adult population and in adults with and without arthritis. Arthritis Rheum. 2003;49(1):129–135. doi: 10.1002/art.10911. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan MS, Huguet N, Newsom JT, McFarland BH. Characteristics of physically inactive older adults with arthritis: results of a population-based study. Prev Med. 2003;37(1):61–67. doi: 10.1016/s0091-7435(03)00059-8. [DOI] [PubMed] [Google Scholar]
- 16.Mehrotra C, Chudy N, Thomas V. Obesity and physical inactivity among Wisconsin adults with arthritis. WMJ. 2003;102(7):24–28. [PubMed] [Google Scholar]
- 17.Fontaine KR, Heo M, Bathon J. Are US adults with arthritis meeting public health recommendations for physical activity? Arthritis Rheum. 2004;50(2):624–628. doi: 10.1002/art.20057. [DOI] [PubMed] [Google Scholar]
- 18.Shih M, Hootman JM, Kruger J, Helmick CG. Physical activity in men and women with arthritis National Health Interview Survey. 2002 Am J Prev Med. 2006;30(5):385–393. doi: 10.1016/j.amepre.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Badley EM, Ansari H. Arthritis and arthritis-attributable activity limitations in the United States and Canada: a cross-border comparison. Arthritis Care Res (Hoboken). 2010;62(3):308–315. doi: 10.1002/acr.20100. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease C, Prevention National and state medical expenditures and lost earnings attributable to arthritis and other rheumatic conditions—United States, 2003. MMWR Morb Mortal Wkly Rep. 2007;56(1):4–7. [PubMed] [Google Scholar]
- 21.Buttgereit F, Burmester GR, Bijlsma JW. Non-surgical management of knee osteoarthritis: where are we now and where do we need to go? RMD Open. 2015;1(1):e000027. [DOI] [PMC free article] [PubMed]
- 22.Bijlsma JWJ, Berenbaum F, Lafeber FPJG. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377(9783):2115–2126. doi: 10.1016/S0140-6736(11)60243-2. [DOI] [PubMed] [Google Scholar]
- 23.Shan L, Shan B, Graham D, Saxena A. Total hip replacement: a systematic review and meta-analysis on mid-term quality of life. Osteoarthr Cartil. 2014;22(3):389–406. doi: 10.1016/j.joca.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 24.Kroenke K, Krebs EE, Bair MJ. Pharmacotherapy of chronic pain: a synthesis of recommendations from systematic reviews. Gen Hosp Psychiat. 2009;31(3):206–219. doi: 10.1016/j.genhosppsych.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Pedersen L, Fredheim OMS. Opioids for chronic noncancer pain: still no evidence for superiority of sustained-release opioids. Clin Pharmacol Ther. 2015;97(2):114–115. doi: 10.1002/cpt.26. [DOI] [PubMed] [Google Scholar]
- 26.Paolucci T, Saraceni VM, Piccinini G. Management of chronic pain in osteoporosis: challenges and solutions. J Pain Res. 2016;9:177–186. doi: 10.2147/JPR.S83574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthr Cartilage. 2014;22(3):363–388. doi: 10.1016/j.joca.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Qaseem A, Wilt TJ, McLean RM, Forciea MA. Clinical guidelines Committee of the American College of P. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166(7):514–530. doi: 10.7326/M16-2367. [DOI] [PubMed] [Google Scholar]
- 29.Howley ET. Type of activity: resistance, aerobic and leisure versus occupational physical activity. Med Sci Sport Exer. 2001;33(6):S364–S3S9. doi: 10.1097/00005768-200106001-00005. [DOI] [PubMed] [Google Scholar]
- 30.Durstine JL, Gordon B, Wang ZZ, Luo XJ. Chronic disease and the link to physical activity. J Sport Health Sci. 2013;2(1):3–11. [Google Scholar]
- 31.Dobson JL, McMillan J, Li L. Benefits of exercise intervention in reducing neuropathic pain. Front Cell Neurosci. 2014;8 [DOI] [PMC free article] [PubMed]
- 32.Flynn MAT, McNeil DA, Maloff B, Mutasingwa D, Wu M, Ford C, et al. Reducing obesity and related chronic disease risk in children and youth: a synthesis of evidence with 'best practice' recommendations. Obes Rev. 2006;7:7–66. doi: 10.1111/j.1467-789X.2006.00242.x. [DOI] [PubMed] [Google Scholar]
- 33.Cooney GM, Dwan K, Greig CA, Lawlor DA, Rimer J, Waugh FR, McMurdo M, Mead GE, Cochrane Common Mental Disorders Group Exercise for depression. Cochrane Db Syst Rev 2013(9). [DOI] [PMC free article] [PubMed]
- 34.Global status report on noncommunicable diseases . Geneva: World Health Organization; 2014.
- 35.Cecchini M, Sassi F, Lauer JA, Lee YY, Guajardo-Barron V, Chisholm D. Tackling of unhealthy diets, physical inactivity, and obesity: health effects and cost-effectiveness. Lancet. 2010;376(9754):1775–1784. doi: 10.1016/S0140-6736(10)61514-0. [DOI] [PubMed] [Google Scholar]
- 36.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, AlMazroa MA, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker-Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan-Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng ATA, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FGR, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD, III, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang YH, Khatibzadeh S, Khoo JP, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Memish ZA, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Hanafiah KM, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Nolla JM, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CDH, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D, Pope CA, III, Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez-Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez-Riera L, Sanman E, Sapkota A, Seedat S, Shi P, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJC, Steenland K, Stöckl H, Stovner LJ, Straif K, Straney L, Thurston GD, Tran JH, van Dingenen R, van Donkelaar A, Veerman JL, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CJL, Ezzati M. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380(9859):2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beaglehole R, Bonita R, Horton R, Adams C, Alleyne G, Asaria P, Baugh V, Bekedam H, Billo N, Casswell S, Cecchini M, Colagiuri R, Colagiuri S, Collins T, Ebrahim S, Engelgau M, Galea G, Gaziano T, Geneau R, Haines A, Hospedales J, Jha P, Keeling A, Leeder S, Lincoln P, McKee M, Mackay J, Magnusson R, Moodie R, Mwatsama M, Nishtar S, Norrving B, Patterson D, Piot P, Ralston J, Rani M, Reddy KS, Sassi F, Sheron N, Stuckler D, Suh I, Torode J, Varghese C, Watt J. Priority actions for the non-communicable disease crisis. Lancet. 2011;377(9775):1438–1447. doi: 10.1016/S0140-6736(11)60393-0. [DOI] [PubMed] [Google Scholar]
- 38.Dunlop DD, Song J, Arnston EK, Semanik PA, Lee J, Chang RW, et al. Sedentary time in US older adults associated with disability in activities of daily living independent of physical activity. J Phys Act Health. 2015;12(1):93–101. doi: 10.1123/jpah.2013-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Semanik PA, Lee J, Song J, Chang RW, Sohn MW, Ehrlich-Jones LS, Ainsworth BE, Nevitt MM, Kwoh CK, Dunlop DD. Accelerometer-monitored sedentary behavior and observed physical function loss. Am J Public Health. 2015;105(3):560–566. doi: 10.2105/AJPH.2014.302270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sohn MW, Manheim LM, Chang RW, Greenland P, Hochberg MC, Nevitt MC, Semanik PA, Dunlop DD. Sedentary behavior and blood pressure control among osteoarthritis initiative participants. Osteoarthr Cartil. 2014;22(9):1234–1240. doi: 10.1016/j.joca.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janal MN, Colt EW, Clark WC, Glusman M. Pain sensitivity, mood and plasma endocrine levels in man following long-distance running: effects of naloxone. Pain. 1984;19(1):13–25. doi: 10.1016/0304-3959(84)90061-7. [DOI] [PubMed] [Google Scholar]
- 42.Drury DG, Greenwood K, Stuempfle KJ, Koltyn KF. Changes in pain perception in women during and following an exhaustive incremental cycling exercise. J Sports Sci Med. 2005;4(3):215–222. [PMC free article] [PubMed] [Google Scholar]
- 43.Hoffman MD, Lee J, Zhao H, Tsodikov A. Pain perception after running a 100-mile ultramarathon. Arch Phys Med Rehabil. 2007;88(8):1042–1048. doi: 10.1016/j.apmr.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 44.Morgan WP. Affective beneficence of vigorous physical activity. Med Sci Sports Exerc. 1985;17(1):94–100. [PubMed] [Google Scholar]
- 45.Rhyner KT, Watts A. Exercise and depressive symptoms in older adults: a systematic meta-analytic review. J Aging Phys Act. 2016;24(2):234–246. doi: 10.1123/japa.2015-0146. [DOI] [PubMed] [Google Scholar]
- 46.Sciolino NR, Holmes PV. Exercise offers anxiolytic potential: a role for stress and brain noradrenergic-galaninergic mechanisms. Neurosci Biobehav Rev. 2012;36(9):1965–1984. doi: 10.1016/j.neubiorev.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salam JN, Fox JH, Detroy EM, Guignon MH, Wohl DF, Falls WA. Voluntary exercise in C57 mice is anxiolytic across several measures of anxiety. Behav Brain Res. 2009;197(1):31–40. doi: 10.1016/j.bbr.2008.07.036. [DOI] [PubMed] [Google Scholar]
- 48.Greenwood BN, Loughridge AB, Sadaoui N, Christianson JP, Fleshner M. The protective effects of voluntary exercise against the behavioral consequences of uncontrollable stress persist despite an increase in anxiety following forced cessation of exercise. Behav Brain Res. 2012;233(2):314–321. doi: 10.1016/j.bbr.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Physical activity guidelines for Americans Okla Nurse. 2008;53(4):25. [PubMed] [Google Scholar]
- 50.Richmond J, Hunter D, Irrgang J, Jones MH, Snyder-Mackler L, Van Durme D, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on the treatment of osteoarthritis (OA) of the knee. J Bone Joint Surg Am. 2010;92(4):990–993. doi: 10.2106/JBJS.I.00982. [DOI] [PubMed] [Google Scholar]
- 51.Richmond J, Hunter D, Irrgang J, Jones MH, Levy B, Marx R, Snyder-Mackler L, Watters WC, 3rd, Haralson RH, 3rd, Turkelson CM, Wies JL, Boyer KM, Anderson S, St Andre J, Sluka P, McGowan R, American Academy of Orthopaedic Surgeons Treatment of osteoarthritis of the knee (nonarthroplasty) J Am Acad Orthop Surg. 2009;17(9):591–600. doi: 10.5435/00124635-200909000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Osteoarthritis: National Clinical Guideline for Care and Management in Adults. National Institute for Health and Clinical Excellence: Guidance. London2008.
- 53.Geneen LJ, Moore RA, Clarke C, Martin D, Colvin LA, Smith BH. Physical activity and exercise for chronic pain in adults: an overview of Cochrane reviews. Cochrane Database Syst Rev. 2017;4:CD011279. doi: 10.1002/14651858.CD011279.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fransen M, McConnell S, Hernandez-Molina G, Reichenbach S. Exercise for osteoarthritis of the hip. Cochrane Database Syst Rev. 2014;4:CD007912. doi: 10.1002/14651858.CD007912.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fransen M, McConnell S, Harmer AR, Van der Esch M, Simic M, Bennell KL. Exercise for osteoarthritis of the knee: a Cochrane systematic review. Br J Sports Med. 2015;49(24):1554–1557. doi: 10.1136/bjsports-2015-095424. [DOI] [PubMed] [Google Scholar]
- 56.O'Connor SR, Tully MA, Ryan B, Bleakley CM, Baxter GD, Bradley JM, McDonough SM Walking exercise for chronic musculoskeletal pain: systematic review and meta-analysis. Arch Phys Med Rehabil 2015;96(4):724–34 e3. [DOI] [PubMed]
- 57.Regnaux JP, Lefevre-Colau MM, Trinquart L, Nguyen C, Boutron I, Brosseau L, et al. High-intensity versus low-intensity physical activity or exercise in people with hip or knee osteoarthritis. Cochrane Database Syst Rev. 2015;(10):CD010203. [DOI] [PMC free article] [PubMed]
- 58.Golightly YM, Allen KD, Caine DJ. A comprehensive review of the effectiveness of different exercise programs for patients with osteoarthritis. Phys Sportsmed. 2012;40(4):52–65. doi: 10.3810/psm.2012.11.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.• Juhl C, Christensen R, Roos EM, Zhang W, Lund H. Impact of exercise type and dose on pain and disability in knee osteoarthritis: a systematic review and meta-regression analysis of randomized controlled trials. Arthritis Rheumatol. 2014;66(3):622–36. Juhl et al. is an excellent systematic review and meta-regression analysis of randomized controlled trials on the effects of exercise intensity and type in knee osteoarthritis.–36. [DOI] [PubMed]
- 60.Uthman OA, van der Windt DA, Jordan JL, Dziedzic KS, Healey EL, Peat GM, Foster NE. Exercise for lower limb osteoarthritis: systematic review incorporating trial sequential analysis and network meta-analysis. BMJ. 2013;347:f5555. doi: 10.1136/bmj.f5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ettinger WH, Jr, Burns R, Messier SP, Applegate W, Rejeski WJ, Morgan T, et al. A randomized trial comparing aerobic exercise and resistance exercise with a health education program in older adults with knee osteoarthritis. The fitness arthritis and seniors trial (FAST) JAMA. 1997;277(1):25–31. [PubMed] [Google Scholar]
- 62.van Baar ME, Dekker J, Oostendorp RA, Bijl D, Voorn TB, Bijlsma JW. Effectiveness of exercise in patients with osteoarthritis of hip or knee: nine months’ follow up. Ann Rheum Dis. 2001;60(12):1123–1130. doi: 10.1136/ard.60.12.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beinart NA, Goodchild CE, Weinman JA, Ayis S, Godfrey EL. Individual and intervention-related factors associated with adherence to home exercise in chronic low back pain: a systematic review. Spine J. 2013;13(12):1940–1950. doi: 10.1016/j.spinee.2013.08.027. [DOI] [PubMed] [Google Scholar]
- 64.Wang SY, Olson-Kellogg B, Shamliyan TA, Choi JY, Ramakrishnan R, Kane RL. Physical therapy interventions for knee pain secondary to osteoarthritis: a systematic review. Ann Intern Med. 2012;157(9):632–644. doi: 10.7326/0003-4819-157-9-201211060-00007. [DOI] [PubMed] [Google Scholar]
- 65.Kroll HR. Exercise therapy for chronic pain. Phys Med Rehabil Clin N Am. 2015;26(2):263–281. doi: 10.1016/j.pmr.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 66.Currie SR, Wang JL. Chronic back pain and major depression in the general Canadian population. Pain. 2004;107(1–2):54–60. doi: 10.1016/j.pain.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 67.Miller LR, Cano A. Comorbid chronic pain and depression: who is at risk? J Pain. 2009;10(6):619–627. doi: 10.1016/j.jpain.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 68.Bair MJ, Wu JW, Damush TM, Sutherland JM, Kroenke K. Association of depression and anxiety alone and in combination with chronic musculoskeletal pain in primary care patients. Psychosom Med. 2008;70(8):890–897. doi: 10.1097/PSY.0b013e318185c510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arnow BA, Hunkeler EM, Blasey CM, Lee J, Constantino MJ, Fireman B, Kraemer HC, Dea R, Robinson R, Hayward C. Comorbid depression, chronic pain, and disability in primary care. Psychosom Med. 2006;68(2):262–268. doi: 10.1097/01.psy.0000204851.15499.fc. [DOI] [PubMed] [Google Scholar]
- 70.Kaleth AS, Saha CK, Jensen MP, Slaven JE, Ang DC. Effect of moderate to vigorous physical activity on long-term clinical outcomes and pain severity in fibromyalgia. Arthritis Care Res (Hoboken). 2013;65(8):1211–1218. doi: 10.1002/acr.21980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Harden RN, Song S, Fasen J, Saltz SL, Nampiaparampil D, Vo A, Revivo G. Home-based aerobic conditioning for management of symptoms of fibromyalgia: a pilot study. Pain Med. 2012;13(6):835–842. doi: 10.1111/j.1526-4637.2012.01384.x. [DOI] [PubMed] [Google Scholar]
- 72.Wildmann J, Kruger A, Schmole M, Niemann J, Matthaei H. Increase of circulating beta-endorphin-like immunoreactivity correlates with the change in feeling of pleasantness after running. Life Sci. 1986;38(11):997–1003. doi: 10.1016/0024-3205(86)90233-x. [DOI] [PubMed] [Google Scholar]
- 73.Koltyn KF, Brellenthin AG, Cook DB, Sehgal N, Hillard C. Mechanisms of exercise-induced hypoalgesia. J Pain. 2014;15(12):1294–1304. doi: 10.1016/j.jpain.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Boecker H, Sprenger T, Spilker ME, Henriksen G, Koppenhoefer M, Wagner KJ, Valet M, Berthele A, Tolle TR. The runner’s high: opioidergic mechanisms in the human brain. Cereb Cortex. 2008;18(11):2523–2531. doi: 10.1093/cercor/bhn013. [DOI] [PubMed] [Google Scholar]
- 75.Saanijoki T, Tuominen L, Tuulari JJ, Nummenmaa L, Arponen E, Kalliokoski K, Hirvonen J. Opioid release after high-intensity interval training in healthy human subjects. Neuropsychopharmacology. 2018;43(2):246–254. doi: 10.1038/npp.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Naugle KM, Naugle KE, Fillingim RB, Samuels B, Riley JL., 3rd Intensity thresholds for aerobic exercise-induced hypoalgesia. Med Sci Sports Exerc. 2014;46(4):817–825. doi: 10.1249/MSS.0000000000000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Koltyn KF, Garvin AW, Gardiner RL, Nelson TF. Perception of pain following aerobic exercise. Med Sci Sports Exerc. 1996;28(11):1418–1421. doi: 10.1097/00005768-199611000-00011. [DOI] [PubMed] [Google Scholar]
- 78.Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66(6):355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 79.Daenen L, Varkey E, Kellmann M, Nijs J. Exercise, not to exercise, or how to exercise in patients with chronic pain? Applying science to practice. Clin J Pain. 2015;31(2):108–114. doi: 10.1097/AJP.0000000000000099. [DOI] [PubMed] [Google Scholar]
- 80.Bender T, Nagy G, Barna I, Tefner I, Kadas E, Geher P. The effect of physical therapy on beta-endorphin levels. Eur J Appl Physiol. 2007;100(4):371–382. doi: 10.1007/s00421-007-0469-9. [DOI] [PubMed] [Google Scholar]
- 81.Felson DT, Anderson JJ, Naimark A, Walker AM, Meenan RF. Obesity and knee osteoarthritis. The Framingham Study Ann Intern Med. 1988;109(1):18–24. doi: 10.7326/0003-4819-109-1-18. [DOI] [PubMed] [Google Scholar]
- 82.Sturmer T, Gunther KP, Brenner H. Obesity, overweight and patterns of osteoarthritis. the Ulm Osteoarthritis Study J Clin Epidemiol. 2000;53(3):307–313. doi: 10.1016/s0895-4356(99)00162-6. [DOI] [PubMed] [Google Scholar]
- 83.Messier SP, Loeser RF, Miller GD, Morgan TM, Rejeski WJ, Sevick MA, Ettinger WH, Pahor M, Williamson JD. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the arthritis, diet, and activity promotion trial. Arthritis Rheum. 2004;50(5):1501–1510. doi: 10.1002/art.20256. [DOI] [PubMed] [Google Scholar]
- 84.Richette P, Poitou C, Garnero P, Vicaut E, Bouillot JL, Lacorte JM, Basdevant A, Clement K, Bardin T, Chevalier X. Benefits of massive weight loss on symptoms, systemic inflammation and cartilage turnover in obese patients with knee osteoarthritis. Ann Rheum Dis. 2011;70(1):139–144. doi: 10.1136/ard.2010.134015. [DOI] [PubMed] [Google Scholar]
- 85.Mannion AF, Caporaso F, Pulkovski N, Sprott H. Spine stabilisation exercises in the treatment of chronic low back pain: a good clinical outcome is not associated with improved abdominal muscle function. Eur Spine J. 2012;21(7):1301–1310. doi: 10.1007/s00586-012-2155-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sluka KA, O'Donnell JM, Danielson J, Rasmussen LA. Regular physical activity prevents development of chronic pain and activation of central neurons. J Appl Physiol (1985) 2013;114(6):725–733. doi: 10.1152/japplphysiol.01317.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Grace PM, Fabisiak TJ, Green-Fulgham SM, Anderson ND, Strand KA, Kwilasz AJ, Galer EL, Walker FR, Greenwood BN, Maier SF, Fleshner M, Watkins LR. Prior voluntary wheel running attenuates neuropathic pain. Pain. 2016;157(9):2012–2023. doi: 10.1097/j.pain.0000000000000607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Leung A, Gregory NS, Allen LA, Sluka KA. Regular physical activity prevents chronic pain by altering resident muscle macrophage phenotype and increasing interleukin-10 in mice. Pain. 2016;157(1):70–79. doi: 10.1097/j.pain.0000000000000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sabharwal R, Rasmussen L, Sluka KA, Chapleau MW. Exercise prevents development of autonomic dysregulation and hyperalgesia in a mouse model of chronic muscle pain. Pain. 2016;157(2):387–398. doi: 10.1097/j.pain.0000000000000330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cobianchi S, Marinelli S, Florenzano F, Pavone F, Luvisetto S. Short- but not long-lasting treadmill running reduces allodynia and improves functional recovery after peripheral nerve injury. Neuroscience. 2010;168(1):273–287. doi: 10.1016/j.neuroscience.2010.03.035. [DOI] [PubMed] [Google Scholar]
- 91.• Stagg NJ, Mata HP, Ibrahim MM, Henriksen EJ, Porreca F, Vanderah TW, et al. Regular exercise reverses sensory hypersensitivity in a rat neuropathic pain model: role of endogenous opioids. Anesthesiology. 2011;114(4):940–8. Stagg et al. is a thorough assessment of the effects of various exercise parameters in rodent model of neuropathic pain.–8. [DOI] [PMC free article] [PubMed]
- 92.Gong X, Jiang J, Zhang M. Exercise preconditioning reduces neonatal incision surgery-induced enhanced hyperalgesia via inhibition of P38 mitogen-activated protein kinase and IL-1beta, TNF-alpha release. Int J Dev Neurosci. 2016;52:46–54. doi: 10.1016/j.ijdevneu.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 93.Kami K, Taguchi S, Tajima F, Senba E. Histone acetylation in microglia contributes to exercise-induced hypoalgesia in neuropathic pain model mice. J Pain. 2016;17(5):588–599. doi: 10.1016/j.jpain.2016.01.471. [DOI] [PubMed] [Google Scholar]
- 94.Kami K, Taguchi Ms S, Tajima F, Senba E. Improvements in impaired GABA and GAD65/67 production in the spinal dorsal horn contribute to exercise-induced hypoalgesia in a mouse model of neuropathic pain. Mol Pain. 2016;12:174480691662905. doi: 10.1177/1744806916629059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wakaizumi K, Kondo T, Hamada Y, Narita M, Kawabe R, Narita H, Watanabe M, Kato S, Senba E, Kobayashi K, Kuzumaki N, Yamanaka A, Morisaki H, Narita M. Involvement of mesolimbic dopaminergic network in neuropathic pain relief by treadmill exercise: a study for specific neural control with Gi-DREADD in mice. Mol Pain. 2016;12:174480691668156. doi: 10.1177/1744806916681567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Safakhah HA, Moradi Kor N, Bazargani A, Bandegi AR, Gholami Pourbadie H, Khoshkholgh-Sima B, et al. Forced exercise attenuates neuropathic pain in chronic constriction injury of male rat: an investigation of oxidative stress and inflammation. J Pain Res. 2017;10:1457–1466. doi: 10.2147/JPR.S135081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Groover AL, Ryals JM, Guilford BL, Wilson NM, Christianson JA, Wright DE. Exercise-mediated improvements in painful neuropathy associated with prediabetes in mice. Pain. 2013;154(12):2658–2667. doi: 10.1016/j.pain.2013.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sheahan TD, Copits BA, Golden JP, RWt G. Voluntary exercise training: analysis of mice in uninjured, inflammatory, and nerve-injured pain states. PLoS One. 2015;10(7):e0133191. doi: 10.1371/journal.pone.0133191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cormier J, Cone K, Lanpher J, Kinens A, Henderson T, Liaw L, Bilsky EJ, King T, Rosen CJ, Stevenson GW. Exercise reverses pain-related weight asymmetry and differentially modulates trabecular bone microarchitecture in a rat model of osteoarthritis. Life Sci. 2017;180:51–59. doi: 10.1016/j.lfs.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pitcher MH, Tarum F, Rauf IZ, Low LA, Bushnell C. Modest amounts of voluntary exercise reduce pain- and stress-related outcomes in a rat model of persistent hind limb inflammation. J Pain. 2017;18(6):687–701. doi: 10.1016/j.jpain.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Whitehead RA, Lam NL, Sun MS, Sanchez J, Noor S, Vanderwall AG, Petersen TR, Martin HB, Milligan ED. Chronic sciatic neuropathy in rat reduces voluntary wheel-running activity with concurrent chronic mechanical allodynia. Anesth Analg. 2017;124(1):346–355. doi: 10.1213/ANE.0000000000001662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Leasure JL, Jones M. Forced and voluntary exercise differentially affect brain and behavior. Neuroscience. 2008;156(3):456–465. doi: 10.1016/j.neuroscience.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 103.Rodnick KJ, Reaven GM, Haskell WL, Sims CR, Mondon CE. Variations in running activity and enzymatic adaptations in voluntary running rats. J Appl Physiol (1985). 1989;66(3):1250–1257. doi: 10.1152/jappl.1989.66.3.1250. [DOI] [PubMed] [Google Scholar]
- 104.Hoydal MA, Wisloff U, Kemi OJ, Ellingsen O. Running speed and maximal oxygen uptake in rats and mice: practical implications for exercise training. Eur J Cardiovasc Prev Rehabil. 2007;14(6):753–760. doi: 10.1097/HJR.0b013e3281eacef1. [DOI] [PubMed] [Google Scholar]
- 105.Wojewoda M, Kmiecik K, Ventura-Clapier R, Fortin D, Onopiuk M, Jakubczyk J, Sitek B, Fedorowicz A, Majerczak J, Kaminski K, Chlopicki S, Zoladz JA. Running performance at high running velocities is impaired but V'O((2)max) and peripheral endothelial function are preserved in IL-6(−)/(−) mice. PLoS One. 2014;9(2):e88333. doi: 10.1371/journal.pone.0088333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bement MK, Sluka KA. Low-intensity exercise reverses chronic muscle pain in the rat in a naloxone-dependent manner. Arch Phys Med Rehabil. 2005;86(9):1736–1740. doi: 10.1016/j.apmr.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 107.Chen YW, Hsieh PL, Chen YC, Hung CH, Cheng JT. Physical exercise induces excess hsp72 expression and delays the development of hyperalgesia and allodynia in painful diabetic neuropathy rats. Anesth Analg. 2013;116(2):482–490. doi: 10.1213/ANE.0b013e318274e4a0. [DOI] [PubMed] [Google Scholar]
- 108.Hutchinson KJ, Gomez-Pinilla F, Crowe MJ, Ying Z, Basso DM. Three exercise paradigms differentially improve sensory recovery after spinal cord contusion in rats. Brain. 2004;127(Pt 6):1403–1414. doi: 10.1093/brain/awh160. [DOI] [PubMed] [Google Scholar]
- 109.Sharma NK, Ryals JM, Gajewski BJ, Wright DE. Aerobic exercise alters analgesia and neurotrophin-3 synthesis in an animal model of chronic widespread pain. Phys Ther. 2010;90(5):714–725. doi: 10.2522/ptj.20090168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shankarappa SA, Piedras-Renteria ES, Stubbs EB., Jr Forced-exercise delays neuropathic pain in experimental diabetes: effects on voltage-activated calcium channels. J Neurochem. 2011;118(2):224–236. doi: 10.1111/j.1471-4159.2011.07302.x. [DOI] [PubMed] [Google Scholar]
- 111.Cobianchi S, Casals-Diaz L, Jaramillo J, Navarro X. Differential effects of activity dependent treatments on axonal regeneration and neuropathic pain after peripheral nerve injury. Exp Neurol. 2013;240:157–167. doi: 10.1016/j.expneurol.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 112.Chen YW, Tzeng JI, Lin MF, Hung CH, Wang JJ. Forced treadmill running suppresses postincisional pain and inhibits upregulation of substance P and cytokines in rat dorsal root ganglion. J Pain. 2014;15(8):827–834. doi: 10.1016/j.jpain.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 113.Chen YW, Chiu CC, Hsieh PL, Hung CH, Wang JJ. Treadmill training combined with insulin suppresses diabetic nerve pain and cytokines in rat sciatic nerve. Anesth Analg. 2015;121(1):239–246. doi: 10.1213/ANE.0000000000000799. [DOI] [PubMed] [Google Scholar]
- 114.•• Allen J, Imbert I, Havelin J, Henderson T, Stevenson G, Liaw L, et al. Effects of treadmill exercise on advanced osteoarthritis pain in rats. Arthritis Rheumatol. 2017;69(7):1407–17. Allen et al. is one of the few rodent studies assessing the effects of exercise on trabecular bone microarchitecture in a rat model of osteoarthritis. In addition, innovative approaches of assessing persistence of pain are used (i.e. Conditioned place preference).–17. [DOI] [PMC free article] [PubMed]
- 115.Arbat-Plana A, Cobianchi S, Herrando-Grabulosa M, Navarro X, Udina E. Endogenous modulation of TrkB signaling by treadmill exercise after peripheral nerve injury. Neuroscience. 2017;340:188–200. doi: 10.1016/j.neuroscience.2016.10.057. [DOI] [PubMed] [Google Scholar]
- 116.Huang PC, Tsai KL, Chen YW, Lin HT, Hung CH. Exercise combined with ultrasound attenuates neuropathic pain in rats associated with downregulation of IL-6 and TNF-alpha, but with upregulation of IL-10. Anesth Analg. 2017;124(6):2038–2044. doi: 10.1213/ANE.0000000000001600. [DOI] [PubMed] [Google Scholar]
- 117.Tsai KL, Huang PC, Wang LK, Hung CH, Chen YW. Incline treadmill exercise suppresses pain hypersensitivity associated with the modulation of pro-inflammatory cytokines and anti-inflammatory cytokine in rats with peripheral nerve injury. Neurosci Lett. 2017;643:27–31. doi: 10.1016/j.neulet.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 118.Detloff MR, Quiros-Molina D, Javia AS, Daggubati L, Nehlsen AD, Naqvi A, Ninan V, Vannix KN, McMullen MK, Amin S, Ganzer PD, Houlé JD. Delayed exercise is ineffective at reversing aberrant nociceptive afferent plasticity or neuropathic pain after spinal cord injury in rats. Neurorehabil Neural Repair. 2016;30(7):685–700. doi: 10.1177/1545968315619698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen YW, Lin MF, Chen YC, Hung CH, Tzeng JI, Wang JJ. Exercise training attenuates postoperative pain and expression of cytokines and N-methyl-D-aspartate receptor subunit 1 in rats. Reg Anesth Pain Med. 2013;38(4):282–288. doi: 10.1097/AAP.0b013e31828df3f9. [DOI] [PubMed] [Google Scholar]
- 120.Luan S, Wan Q, Luo H, Li X, Ke S, Lin C, Wu Y, Wu S, Ma C. Running exercise alleviates pain and promotes cell proliferation in a rat model of intervertebral disc degeneration. Int J Mol Sci. 2015;16(1):2130–2144. doi: 10.3390/ijms16012130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yoon H, Thakur V, Isham D, Fayad M, Chattopadhyay M. Moderate exercise training attenuates inflammatory mediators in DRG of type 1 diabetic rats. Exp Neurol. 2015;267:107–114. doi: 10.1016/j.expneurol.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 122.Detloff MR, Smith EJ, Quiros Molina D, Ganzer PD, Houle JD. Acute exercise prevents the development of neuropathic pain and the sprouting of non-peptidergic (GDNF- and artemin-responsive) c-fibers after spinal cord injury. Exp Neurol. 2014;255:38–48. doi: 10.1016/j.expneurol.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lopez-Alvarez VM, Modol L, Navarro X, Cobianchi S. Early increasing-intensity treadmill exercise reduces neuropathic pain by preventing nociceptor collateral sprouting and disruption of chloride cotransporters homeostasis after peripheral nerve injury. Pain. 2015;156(9):1812–1825. doi: 10.1097/j.pain.0000000000000268. [DOI] [PubMed] [Google Scholar]
- 124.Lopez-Alvarez VM, Puigdomenech M, Navarro X, Cobianchi S. Monoaminergic descending pathways contribute to modulation of neuropathic pain by increasing-intensity treadmill exercise after peripheral nerve injury. Exp Neurol. 2018;299(Pt A):42–55. doi: 10.1016/j.expneurol.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 125.Yamaoka S, Oshima Y, Horiuchi H, Morino T, Hino M, Miura H, et al. Altered gene expression of RNF34 and PACAP possibly involved in mechanism of exercise-induced analgesia for neuropathic pain in rats. Int J Mol Sci. 2017;18(9) [DOI] [PMC free article] [PubMed]
- 126.Morimoto A, Winaga H, Sakurai H, Ohmichi M, Yoshimoto T, Ohmichi Y, Matsui T, Ushida T, Okada T, Sato J. Treadmill running and static stretching improve long-lasting hyperalgesia, joint limitation, and muscle atrophy induced by cast immobilization in rats. Neurosci Lett. 2013;534:295–300. doi: 10.1016/j.neulet.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 127.Bobinski F, Ferreira TAA, Cordova MM, Dombrowski PA, da Cunha C, Santo C, et al. Role of brainstem serotonin in analgesia produced by low-intensity exercise on neuropathic pain after sciatic nerve injury in mice. Pain. 2015;156(12):2595–2606. doi: 10.1097/j.pain.0000000000000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen YW, Li YT, Chen YC, Li ZY, Hung CH. Exercise training attenuates neuropathic pain and cytokine expression after chronic constriction injury of rat sciatic nerve. Anesth Analg. 2012;114(6):1330–1337. doi: 10.1213/ANE.0b013e31824c4ed4. [DOI] [PubMed] [Google Scholar]
- 129.Chuganji S, Nakano J, Sekino Y, Hamaue Y, Sakamoto J, Okita M. Hyperalgesia in an immobilized rat hindlimb: effect of treadmill exercise using non-immobilized limbs. Neurosci Lett. 2015;584:66–70. doi: 10.1016/j.neulet.2014.09.054. [DOI] [PubMed] [Google Scholar]
- 130.Kim YJ, Byun JH, Choi IS. Effect of exercise on micro-opioid receptor expression in the rostral ventromedial medulla in neuropathic pain rat model. Ann Rehabil Med. 2015;39(3):331–339. doi: 10.5535/arm.2015.39.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dekker JM, Schouten EG, Klootwijk P, Pool J, Swenne CA, Kromhout D. Heart rate variability from short electrocardiographic recordings predicts mortality from all causes in middle-aged and elderly men. The Zutphen study. Am J Epidemiol. 1997;145(10):899–908. doi: 10.1093/oxfordjournals.aje.a009049. [DOI] [PubMed] [Google Scholar]
- 132.Kuo TB, Lin T, Yang CC, Li CL, Chen CF, Chou P. Effect of aging on gender differences in neural control of heart rate. Am J Phys. 1999;277(6 Pt 2):H2233–H2239. doi: 10.1152/ajpheart.1999.277.6.H2233. [DOI] [PubMed] [Google Scholar]
- 133.Laing ST, Gluckman TJ, Weinberg KM, Lahiri MK, Ng J, Goldberger JJ. Autonomic effects of exercise-based cardiac rehabilitation. J Cardiopulm Rehabil Prev. 2011;31(2):87–91. doi: 10.1097/HCR.0b013e3181f1fda0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tsuji H, Venditti FJ, Jr, Manders ES, Evans JC, Larson MG, Feldman CL, et al. Reduced heart rate variability and mortality risk in an elderly cohort. The Framingham heart study. Circulation. 1994;90(2):878–883. doi: 10.1161/01.cir.90.2.878. [DOI] [PubMed] [Google Scholar]
- 135.Thayer JF, Ahs F, Fredrikson M, Sollers JJ, 3rd, Wager TD. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci Biobehav Rev. 2012;36(2):747–756. doi: 10.1016/j.neubiorev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 136.Meeus M, Goubert D, De Backer F, Struyf F, Hermans L, Coppieters I, et al. Heart rate variability in patients with fibromyalgia and patients with chronic fatigue syndrome: a systematic review. Semin Arthritis Rheum. 2013;43(2):279–287. doi: 10.1016/j.semarthrit.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 137.Greenwood BN, Foley TE, Day HE, Campisi J, Hammack SH, Campeau S, et al. Freewheel running prevents learned helplessness/behavioral depression: role of dorsal raphe serotonergic neurons. J Neurosci. 2003;23(7):2889–2898. doi: 10.1523/JNEUROSCI.23-07-02889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Greenwood BN, Foley TE, Le TV, Strong PV, Loughridge AB, Day HE, et al. Long-term voluntary wheel running is rewarding and produces plasticity in the mesolimbic reward pathway. Behav Brain Res. 2011;217(2):354–362. doi: 10.1016/j.bbr.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Belke TW, Wagner JP. The reinforcing property and the rewarding aftereffect of wheel running in rats: a combination of two paradigms. Behav Process. 2005;68(2):165–172. doi: 10.1016/j.beproc.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 140.Kanarek RB, Gerstein AV, Wildman RP, Mathes WF, D'Anci KE. Chronic running-wheel activity decreases sensitivity to morphine-induced analgesia in male and female rats. Pharmacol Biochem Behav. 1998;61(1):19–27. doi: 10.1016/s0091-3057(98)00059-8. [DOI] [PubMed] [Google Scholar]
- 141.Smith MA, Lyle MA. Chronic exercise decreases sensitivity to mu opioids in female rats: correlation with exercise output. Pharmacol Biochem Behav. 2006;85(1):12–22. doi: 10.1016/j.pbb.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 142.Smith MA, Yancey DL. Sensitivity to the effects of opioids in rats with free access to exercise wheels: mu-opioid tolerance and physical dependence. Psychopharmacology. 2003;168(4):426–434. doi: 10.1007/s00213-003-1471-5. [DOI] [PubMed] [Google Scholar]
- 143.Woda A, Picard P, Dutheil F. Dysfunctional stress responses in chronic pain. Psychoneuroendocrinology. 2016;71:127–135. doi: 10.1016/j.psyneuen.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 144.Hopps E, Canino B, Caimi G. Effects of exercise on inflammation markers in type 2 diabetic subjects. Acta Diabetol. 2011;48(3):183–189. doi: 10.1007/s00592-011-0278-9. [DOI] [PubMed] [Google Scholar]
- 145.You T, Arsenis NC, Disanzo BL, Lamonte MJ. Effects of exercise training on chronic inflammation in obesity : current evidence and potential mechanisms. Sports Med. 2013;43(4):243–256. doi: 10.1007/s40279-013-0023-3. [DOI] [PubMed] [Google Scholar]
- 146.Shephard RJ, Shek PN. Autoimmune disorders, physical activity, and training, with particular reference to rheumatoid arthritis. Exerc Immunol Rev. 1997;3:53–67. [PubMed] [Google Scholar]
- 147.Dishman RK. Brain monoamines, exercise, and behavioral stress: animal models. Med Sci Sports Exerc. 1997;29(1):63–74. doi: 10.1097/00005768-199701000-00010. [DOI] [PubMed] [Google Scholar]
- 148.Griesbach GS, Tio DL, Vincelli J, McArthur DL, Taylor AN. Differential effects of voluntary and forced exercise on stress responses after traumatic brain injury. J Neurotrauma. 2012;29(7):1426–1433. doi: 10.1089/neu.2011.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ke Z, Yip SP, Li L, Zheng XX, Tong KY. The effects of voluntary, involuntary, and forced exercises on brain-derived neurotrophic factor and motor function recovery: a rat brain ischemia model. PLoS One. 2011;6(2):e16643. doi: 10.1371/journal.pone.0016643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Li JY, Kuo TB, Yen JC, Tsai SC, Yang CC. Voluntary and involuntary running in the rat show different patterns of theta rhythm, physical activity, and heart rate. J Neurophysiol. 2014;111(10):2061–2070. doi: 10.1152/jn.00475.2013. [DOI] [PubMed] [Google Scholar]
- 151.Mello PB, Benetti F, Cammarota M, Izquierdo I. Effects of acute and chronic physical exercise and stress on different types of memory in rats. An Acad Bras Cienc. 2008;80(2):301–309. doi: 10.1590/s0001-37652008000200008. [DOI] [PubMed] [Google Scholar]
- 152.Melo SF, Lunz W, Fontes EP, Dias CM, Carneiro MA, Jr, Moura AG, et al. Different levels of Hsp72 in female rat myocardium in response to voluntary exercise and forced exercise. Arq Bras Cardiol. 2009;93(5):456–462. doi: 10.1590/s0066-782x2009001100004. [DOI] [PubMed] [Google Scholar]
- 153.Moraska A, Deak T, Spencer RL, Roth D, Fleshner M. Treadmill running produces both positive and negative physiological adaptations in Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol. 2000;279(4):R1321–R1329. doi: 10.1152/ajpregu.2000.279.4.R1321. [DOI] [PubMed] [Google Scholar]
- 154.Contarteze RV, Manchado Fde B, Gobatto CA, De Mello MA. Stress biomarkers in rats submitted to swimming and treadmill running exercises. Comp Biochem Physiol A Mol Integr Physiol. 2008;151(3):415–422. doi: 10.1016/j.cbpa.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 155.Moraska A, Deak T, Spencer RL, Roth D, Fleshner M. Treadmill running produces both positive and negative physiological adaptations in Sprague-Dawley rats. Am J Physiol-Reg I. 2000;279(4):R1321–R13R9. doi: 10.1152/ajpregu.2000.279.4.R1321. [DOI] [PubMed] [Google Scholar]
- 156.Noble EG, Moraska A, Mazzeo RS, Roth DA, Olsson MC, Moore RL, Fleshner M. Differential expression of stress proteins in rat myocardium after free wheel or treadmill run training. J Appl Physiol (1985). 1999;86(5):1696–1701. doi: 10.1152/jappl.1999.86.5.1696. [DOI] [PubMed] [Google Scholar]
- 157.Koolhaas JM, Bartolomucci A, Buwalda B, de Boer SF, Flugge G, Korte SM, et al. Stress revisited: a critical evaluation of the stress concept. Neurosci Biobehav Rev. 2011;35(5):1291–1301. doi: 10.1016/j.neubiorev.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 158.Butler RK, Finn DP. Stress-induced analgesia. Prog Neurobiol. 2009;88(3):184–202. doi: 10.1016/j.pneurobio.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 159.Furuta S, Onodera K, Kumagai M, Honma I, Miyazaki S, Sato T, Sakurada S. Involvement of adenosine A1 receptors in forced walking stress-induced analgesia in mice. Methods Find Exp Clin Pharmacol. 2003;25(10):793–796. doi: 10.1358/mf.2003.25.10.793327. [DOI] [PubMed] [Google Scholar]
- 160.Onodera K, Sakurada S, Furuta S, Yonezawa A, Hayashi T, Honma I, Miyazaki S. Age-related differences in forced walking stress-induced analgesia in mice. Drugs Exp Clin Res. 2001;27(5–6):193–198. [PubMed] [Google Scholar]
- 161.Nakagawasai O, Tadano T, Tan No K, Niijima F, Sakurada S, Endo Y, et al. Changes in beta-endorphin and stress-induced analgesia in mice after exposure to forced walking stress. Methods Find Exp Clin Pharmacol. 1999;21(7):471–476. [PubMed] [Google Scholar]
- 162.Sakurada S, Onodera K, Katsuyama S, Yonezawa A, Arai K, Hayashi T, Furuta S, Sato T, Kisara K. Effects of forced walking stress on formalin-induced paw licking in mice. Methods Find Exp Clin Pharmacol. 1999;21(7):467–470. [PubMed] [Google Scholar]
- 163.Blustein JE, McLaughlin M, Hoffman JR. Exercise effects stress-induced analgesia and spatial learning in rats. Physiol Behav. 2006;89(4):582–586. doi: 10.1016/j.physbeh.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 164.Herrera JJ, Fedynska S, Ghasem PR, Wieman T, Clark PJ, Gray N, Loetz E, Campeau S, Fleshner M, Greenwood BN. Neurochemical and behavioural indices of exercise reward are independent of exercise controllability. Eur J Neurosci. 2016;43(9):1190–1202. doi: 10.1111/ejn.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hayes K, Sprague S, Guo M, Davis W, Friedman A, Kumar A, Jimenez DF, Ding Y. Forced, not voluntary, exercise effectively induces neuroprotection in stroke. Acta Neuropathol. 2008;115(3):289–296. doi: 10.1007/s00401-008-0340-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kinni H, Guo M, Ding JY, Konakondla S, Dornbos D, 3rd, Tran R, et al. Cerebral metabolism after forced or voluntary physical exercise. Brain Res. 2011;1388:48–55. doi: 10.1016/j.brainres.2011.02.076. [DOI] [PubMed] [Google Scholar]
- 167.O'Callaghan RM, Ohle R, Kelly AM. The effects of forced exercise on hippocampal plasticity in the rat: a comparison of LTP, spatial- and non-spatial learning. Behav Brain Res. 2007;176(2):362–366. doi: 10.1016/j.bbr.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 168.Layne JN, Benton AH. Some speeds of small mammals. J Mammal. 1954;35(1):104–105. [Google Scholar]
- 169.Garland T. The relation between maximal running speed and body mass in terrestrial mammals journal of zoology. London. 1983;199:157–170. [Google Scholar]
- 170.de Oliveira MS, da Silva Fernandes MJ, Scorza FA, Persike DS, Scorza CA, da Ponte JB, et al. Acute and chronic exercise modulates the expression of MOR opioid receptors in the hippocampal formation of rats. Brain Res Bull. 2010;83(5):278–283. doi: 10.1016/j.brainresbull.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 171.Lett BT, Grant VL, Koh MT. Naloxone attenuates the conditioned place preference induced by wheel running in rats. Physiol Behav. 2001;72(3):355–358. doi: 10.1016/s0031-9384(00)00427-3. [DOI] [PubMed] [Google Scholar]
- 172.Lett BT, Grant VL, Koh MT, Flynn G. Prior experience with wheel running produces cross-tolerance to the rewarding effect of morphine. Pharmacol Biochem Behav. 2002;72(1–2):101–105. doi: 10.1016/s0091-3057(01)00722-5. [DOI] [PubMed] [Google Scholar]
- 173.McLachlan CD, Hay M, Coleman GJ. The effects of exercise on the oral consumption of morphine and methadone in rats. Pharmacol Biochem Behav. 1994;48(2):563–568. doi: 10.1016/0091-3057(94)90572-x. [DOI] [PubMed] [Google Scholar]
- 174.Sisti HM, Lewis MJ. Naloxone suppression and morphine enhancement of voluntary wheel-running activity in rats. Pharmacol Biochem Behav. 2001;70(2–3):359–365. doi: 10.1016/s0091-3057(01)00624-4. [DOI] [PubMed] [Google Scholar]