Abstract
Background:
Specialty care remains a significant contributor to health care spending but largely unaddressed in novel payment models aimed at promoting value-based delivery. Bladder cancer, chiefly managed by subspecialists, is among the most costly. In 2005, Centers for Medicare and Medicaid Services (CMS) dramatically increased physician payment for office-based interventions for bladder cancer to shift care from higher cost facilities, but the impact is unknown. This study evaluated the effect of financial incentives on patterns of fee-for-service (FFS) bladder cancer care.
Methods:
Data from a 5% sample of Medicare beneficiaries from 2001–2013 were evaluated using interrupted time-series analysis with segmented regression. Primary outcomes were the effects of CMS fee modifications on utilization and site of service for procedures associated with the diagnosis and treatment of bladder cancer. Rates of related bladder cancer procedures that were not affected by the fee change were concurrent controls. Finally, the effect of payment changes on both diagnostic yield and need for redundant procedures were studied. All statistical tests were two-sided.
Results:
Utilization of clinic-based procedures increased by 644% (95% confidence interval [CI] = 584% to 704%) after the fee change, but without reciprocal decline in facility-based procedures. Procedures unaffected by the fee incentive remained unchanged throughout the study period. Diagnostic yield decreased by 17.0% (95% CI = 12.7% to 21.3%), and use of redundant office-based procedures increased by 76.0% (95% CI = 59% to 93%).
Conclusions:
Financial incentives in bladder cancer care have unintended and costly consequences in the current FFS environment. The observed price sensitivity is likely to remain a major issue in novel payment models failing to incorporate procedure-based specialty physicians.
The predominant model of payment in US health care remains fee-for-service (FFS). Nonetheless, FFS has been implicated in perpetuating incentives associated with the delivery of inefficient, low-value health care (1,2). There have been numerous efforts by several parties to control growth in health care costs through risk-shared arrangements aimed at aligning incentives to promote the delivery of high-value health care. Examples of payment reform include the development of Accountable Care Organizations (ACO) and the implementation of bundled or episode-based payments (3). However, many contend that FFS will likely remain an important determinant of physician payment even under risk-shared arrangements, particularly for specialty care services (4). Furthermore, despite the large proportion of health care spending attributed to surgical care, there has been little surgeon engagement in contemporary ACO leadership and development to date (5).
The cost of cancer care remains substantial, and it’s estimated to rise over the next half century for myriad reasons (6). Considering these escalating costs, cancer care delivery represents an opportunity for development and implementation of novel payment strategies. The Oncology Care Model, a bundled payment model introduced by Centers for Medicare and Medicaid Services (CMS), has a goal of tying 50% of traditional Medicare FFS payments to quality by 2018 (7). Yet this model is limited to the administration of chemotherapy, which is only one component of cancer care.
Bladder cancer is among the most common and expensive cancers to manage because of the ongoing need for invasive surveillance and treatment by surgical subspecialists (8). There remain considerable variations at the provider level in the management of both early- and higher-stage bladder cancers, leading to large differences in health care utilization absent concomitant variations in outcomes (9,10). Such variation has led to calls for better adherence to recommended treatment guidelines in order to optimize the value of bladder cancer care delivered to Americans (11,12).
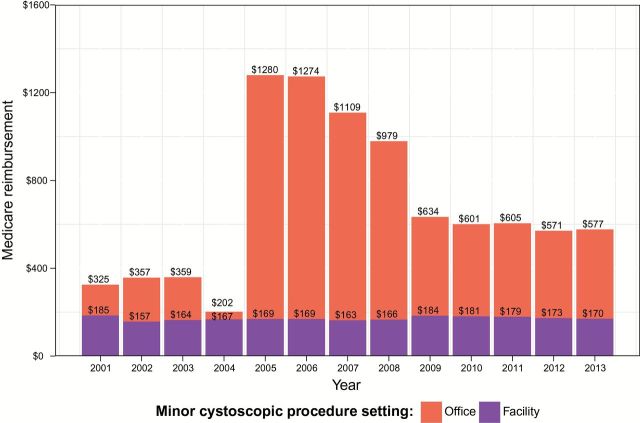
Alternative strategies to reduce growth in costs associated with the treatment of bladder cancer have focused on transitioning care from high-cost sites of service (hospital or ambulatory surgery center) to lower-cost sites of service (physician office) (13–15). In 2005, CMS dramatically increased physician fees for some office-based procedures associated with the diagnosis and/or treatment of bladder cancer to incentivize cost-efficient care while leaving fees unchanged for performing the same procedures in an operating room or ambulatory surgery center (Figure 1). Nonetheless, modifications to the fee schedule associated with bladder cancer care were not met with any shared risk agreements, resulting in the possibility of unrestrained spending absent any risk to the physician or physician organization.
Figure 1.
Average annual Medicare reimbursement for minor cystoscopic procedures in office−based and facility−based settings.
The objective of this study was to evaluate the impact of financial incentives on patterns of care for bladder cancer to inform policy that has the potential to impact an expensive component of healthcare that has been largely overlooked by contemporary payment reforms.
Methods
Study Population
Using a 5% Medicare sample from 2001 through 2013, we identified beneficiaries enrolled in FFS Medicare parts A and B age 66 years or above and enrolled in Medicare because of age.
Our main study groups of interest consisted of patients affected by changes in payment associated with relevant procedures (Table 1): those who underwent a minor cystoscopic procedure (MCP) in an office-based setting (procedure codes 52204-cystourethroscopy, with biopsy; 52214-cystourethroscopy, with fulguration; 52224-cystourethroscopy, with treatment of MINOR [<0.5 cm] lesion with or without biopsy) with an office place of service) and those who underwent an MCP in a facility-based setting (procedure codes 52204, 52214, 52224, and facility place of service including hospitals and ambulatory surgery centers). That is, the main comparison groups were office-based MCPs and facility-based MCPs. We also defined an additional comparison group that did not experience a change in payment, namely patients who underwent transurethral resection of bladder tumor (TURBT), regardless of site of service (procedure codes 52234, 52235, 52240).
Table 1.
Procedure codes grouped by study definition
| Procedure code | Minor cystoscopic procedure* |
|---|---|
| 52204 | Cystourethroscopy, with biopsy |
| 52214 | Cystourethroscopy, with fulguration |
| 52224 | Cystourethroscopy, with treatment of MINOR (<0.5cm) lesion with or without biopsy |
| Transurethral resection bladder tumor† | |
| 52234 | Cystourethroscopy, with resection of small tumor(s) (0.5 to 2.0cm) |
| 52225 | Cystourethroscopy, with resection of medium tumor(s) (2.0 to 5.0cm) |
| 52250 | Cystourethroscopy, with resection of large tumor(s) (>5cm) |
| Excluded cystoscopic procedure† | |
| 52000 | Cystourethroscopy |
* Affected by payment increase.
† Unaffected by payment increase.
It is important to note that the commonly used procedure code 52000 (cystourethroscopy) was not affected by the rule change and was not included in this analysis. This code is intended for inspection of the bladder and urethra and excludes interventions/treatments including biopsy, fulguration, or resection. That is, 52000 is used for surveillance of bladder tumor recurrence, and the other codes listed in Table 1 are utilized when a recurrence is identified and treated.
Prior to 2009, claims data contained only a quarter of service, and for consistency we used quarterly data throughout the 2001 to 2013 study period. Study and comparison groups were required to be continuously enrolled for four quarters prior to and during the quarter of the index procedure.
Outcomes
Our primary outcome was the rate of utilization of MCPs performed in office and facility settings as well as utilization of TURBT, each expressed as a rate per 10 000 Medicare eligible beneficiaries.
Secondary outcomes included quarterly diagnostic yield and rate of redundant procedures. Diagnostic yield represents the rate of incident bladder cancer (using ICD-9 code 188.xx, 233.7, or V10.51) using all procedure types (both MCP and TURBT) and suggests the efficiency with which these procedures identify bladder cancer. Redundant procedures were defined as the occurrence of more than one procedure for a given patient in a single quarter or in consecutive quarters. When a redundant procedure included both an MCP and a TURBT, the case was grouped with MCP, assuming that the MCP was the first procedure and TURBT was the second.
Statistical Analyses
We report average physician payment for MCPs performed in clinics and in facilities using the National Payment Amount from the Medicare Physician Fee Schedule. Prior to 2006, there was no established National Payment Amount. Payment data for Delaware was used for these years as this value closely approximates the National Payment Amount after this standard was established.
To evaluate the effect of changes in the Medicare fee schedule on utilization by site of service, we performed an interrupted time series (ITS) analysis using segmented regression (16). Models included two time periods of interest: the baseline period prior to the payment incentive from the first quarter 2001 to the fourth quarter 2004 and the period during and after payment increase from the first quarter 2005 to the end of the study. Utilization rates for office-based MCPs, facility-based MCPs, and TURBTs were evaluated in separate models. Time was allowed to be nonlinear using restricted cubic splines unless nonlinear terms failed to improve model fit. We assessed potential autocorrelation using the Durbin-Watson statistic.
We then identified three conceptual time periods of interest, during which we evaluated changes in utilization: the baseline period prior to the payment incentive from the first quarter 2001 to the fourth quarter 2004 (A), the period during and after payment increase from the first quarter 2005 to the peak of utilization in the third quarter 2007 (B), and the period during which CMS revised payment downward (see Figure 1) from the fourth quarter 2007 to the end of the study (C). We report absolute utilization changes between the end of A to the beginning of B (immediate level change with the payment incentive), the end of A to the end of B, and the end of A to the end of C. Absolute changes were constructed using model-based contrast tests and were evaluated using t-statistics.
For secondary outcomes, overall diagnostic yield, and rate of redundant procedures, we again performed ITS using segmented regression models containing the same two time periods of interest and evaluated changes between time points of interest using model-based contrast estimates and t-statistics.
Data management was conducted using SAS 9.4, and statistical analyses were performed using R 3.1.2 (http://www.r-project.org/). All tests were two-sided, and P values below .05 were considered statistically significant. This study was approved by the Vanderbilt University Institutional Review Board.
Results
The average physician payment for office-based MCPs increased from a low of $202 in period A to over $1200 during period B before decreasing to approximately $600 during period C. The amount for facility-based MCPs remained largely unchanged, at approximately $170 during all periods (Figure 1). Physician payment for TURBTs remained the same in both facility and office-based settings, at approximately $350 (data not shown).
The number of FFS Medicare A and B beneficiaries in our cohort ranged between approximately 1.1 million and 1.2 million across the study period, representing an underlying population ranging from 22.6 million to 24.7 million beneficiaries. Nineteen thousand six hundred sixty-six patients underwent an MCP (6832 prior to change in the Medicare fee schedule and 12 834 after changes to the Medicare fee schedule), and 16 881 patients underwent TURBTs (6393 prior to change in the Medicare fee schedule and 10 488 after changes to the Medicare fee schedule).
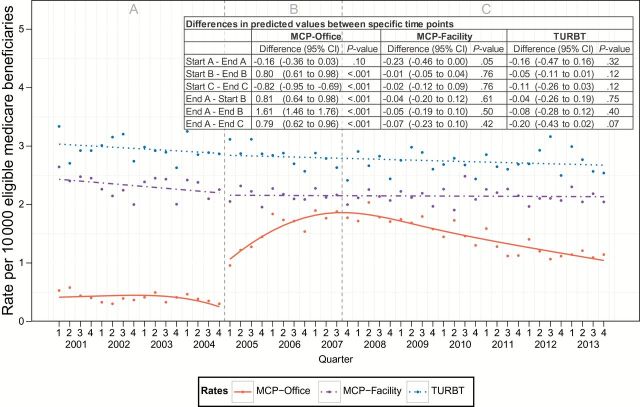
The baseline predicted rate of office-based MCP was 0.41 of every 10 000 beneficiaries, which remained stable throughout the duration of period A. There was a statistically significant increase in office-based MCP utilization immediately after modification to the Medicare fee schedule, which continued to rise to a peak in the third quarter of 2007, that was 644% (95% confidence interval [CI] = 584% to 704%) higher than the rate at the end of period A. The predicted rate declined from this peak, corresponding to a decrease in the financial incentive, but remained 316% (95% CI = 248% to 384%) higher than baseline. While there was a small decrease (9.5%; 95% CI = 18.9% to 0.00%) in the rate of facility-based MCPs across period A, there were no observed statistically significant changes in predicted rates of facility-based MCPs after the CMS fee change. There were also no observed changes in the rates for TURBTs, procedures unaffected by changes in the Medicare fee schedule (Figure 2).
Figure 2.
Trends in quarterly procedure rates by procedure type and location before and after payment incentive. Left dashed gray line represents change in fee schedule. Right dashed gray line represents peak utilization of minor cystoscopic procedure. Analyzed with interrupted time series analysis using segmented regression and two-sided Student’s t test. MCP = minor cystoscopic procedure; TURBT = transurethral resection of bladder tumor.
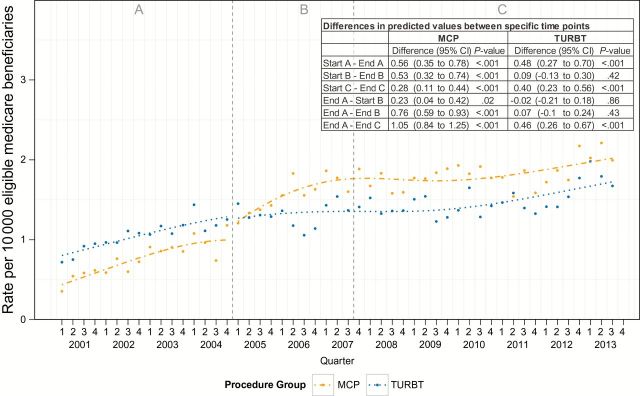
The rate of redundant procedures increased during periods A and C for both patients whose first procedure was a TURBT and those whose first procedure was an MCP, some of which may reflect changes in guideline-based recommendations for repeat resection under specific clinical circumstances. Nonetheless, during period B the rate of redundant procedures increased by 76.0% (95% CI = 59% to 93%) among patients undergoing initial MCPs, whereas there was no observed change (5.4%; 95% CI = -7.8% to 18.6%) among patients undergoing initial TURBT, suggesting unfavorable changes in efficiency of bladder cancer care coinciding with the change in reimbursement (Figure 3).
Figure 3.
Trends in quarterly redundant procedures before and after payment incentive. Left dashed gray line represents change in fee schedule. Right dashed gray line represents peak utilization of minor cystoscopic procedure. Analyzed with interrupted time series analysis using segmented regression and two-sided Student’s t test. MCP = minor cystoscopic procedure; TURBT = transurethral resection of bladder tumor.
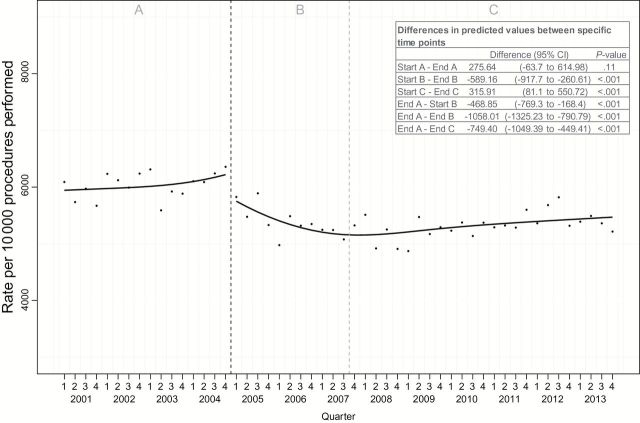
Diagnostic yield did not change statistically significantly during the baseline period (4.6%; 95% CI = -1.1% to 10.3%), with 62.2% of diagnostic procedures resulting in a new diagnosis of bladder cancer at the end of A. This decreased to a nadir of 17.0% lower (95% CI = 12.7% to 21.3%) after modification to the CMS fee schedule, and with reduction in reimbursement the diagnostic yield remained 12.5% (95% CI = 7.2% to 16.9%) lower than baseline (Figure 4).
Figure 4.
Trends in quarterly incident bladder cancer diagnosis rates for minor cystoscopic procedure (MCP) and transurethral resection of bladder tumor before and after payment incentive. Left dashed gray line represents change in fee schedule. Right dashed gray line represents peak utilization of MCP. Analyzed with interrupted time series analysis using segmented regression and two-sided Student’s t test. MCP = minor cystoscopic procedure; TURBT = transurethral resection of bladder tumor.
Discussion
The financial incentive created by dramatically increasing physician fees for office-based MCPs had the intended effect of increasing utilization in a lower-cost setting; however, we observed no reciprocal decrease in procedures performed in the facility setting. This suggests that rather than promoting a shift from a high-cost setting to a lower-cost environment, the policy change resulted in an overall increase in procedures, and ultimately, bladder cancer spending.
There are at least two possibilities to explain this behavior. First, it appears that physicians had a lower threshold to perform a biopsy after 2005, as the diagnostic yield per procedure declined. This finding may represent the clinical scenario in which a physician identifies an abnormality that previously would have been monitored either because of the low likelihood that it represented cancer or because of patient characteristics that made a formal surgical procedure undesirable (eg, high surgical risk because of comorbidity).
Second, patients were more likely to undergo a redundant procedure after an office-based intervention, suggesting that biopsies were being performed in the office for lesions that could not be adequately evaluated or managed in the office setting. It may be that benefits such as convenience to patients or physicians that are achievable through an office-based intervention are sufficiently enticing to attempt a procedure that may require a subsequent facility-based procedure if unsuccessful. Alternatively, the financial incentive to perform an MCP in the office may be so high that some may perform an MCP, even when a more extensive facility-based procedure, reimbursed at a lower professional fee, will ultimately be required.
Redundant procedures are not uniformly undesirable. Repeat resections are the standard of care for many cases of bladder cancer to ensure that proper staging and complete tumor removal have been achieved. Increases in redundant procedures that occurred in period A and C for both MCP and TURBT likely represent increased awareness and proper uptake of guideline-concordant repeat resections. Nonetheless, during period B, redundant procedures increased only among those procedures affected by change in Medicare reimbursement, suggesting an unbalanced effect of financial incentives.
The results of this study should be viewed in light of several limitations. The data for this study were obtained from FFS Medicare, and, therefore, our results are only directly applicable to this population. We cannot make conclusions about the utilization of office-based bladder cancer procedures under alternative payment models for Medicare Advantage beneficiaries or for those with commercial insurance.
We were limited to yearly quarters for dates, which affected the degree of specificity we could provide for the secondary outcomes. As such, we developed algorithms to define the sequence of events to enable calculation of diagnostic yield and rates of redundant procedures. Our analysis did not examine specific physicians, and there is likely substantial heterogeneity in practice patterns in different geographic regions and among individual physicians with some reacting differently to the financial incentives for these procedures. We cannot comment on provider characteristics that might influence changes at these levels of analysis.
Care must be taken not to react to these findings in a way that discourages the appropriate use of office-based MCPs to manage bladder cancer. Increasing evidence suggests that this strategy has the potential to be cost saving, at least in some specific settings (15). The larger problem is that there likely exists considerable heterogeneity in physician response to financial incentives in the context of a fee-for-service environment that permits unrestrained health care spending as has been previously reported with physician ownership of surgical centers (17–19). Indeed, there remain few contemporary payment models that confer financial risk to specialty physicians. Despite laudable efforts to expand the Medicare ACO programs, specialty physicians remain largely unaffected by these risk-shared arrangements, continuing to operate largely in a fee-for-service environment.
How, then, do we move forward to improve the value of care delivered to cancer patients? Certainly, there are multiple possible payment arrangements that would be expected to promote value-based cancer care. These include both traditional and inclusive shared savings, bundled payments, and performance-based payment among many others (20,21). While there are important differences between novel payment models for cancer care, there are common themes that merit mention. These include the transfer of (some degree of) financial risk from the payer to the provider organization and the use of quality measurement to guide payment (or penalty).
The transfer of financial risk from the payer to the provider organization theoretically serves to reduce overuse and encourage efficient, value-based practice. There remains considerable variation in the degree of financial capitation, and it remains unknown whether the degree of financial risk assumed by the provider organization maps to the degree of high-value cancer care delivered. Certainly, in the example of bladder cancer management, the use of either shared savings or bundled payment arrangements may discourage urologists from performing unnecessary procedures and may encourage urologists to perform the “right” procedure in the “right” setting to obtain the most clinical information at the lowest aggregate cost. Such models, however, may also have unintended consequences such as restricting timely access to needed services. As we continue to experiment with novel models of payment for health care services, implementing demonstration projects in specialty care settings will undoubtedly offer important lessons surrounding means to optimize specialist engagement in novel payment models.
There are few data that specifically evaluate the effect of payment reform on cancer care delivery. Colla et al. (22) specifically evaluated changes in spending and outcomes associated with participation in the Medicare Physician Group Practice Demonstration, a Medicare demonstration project. The study identified statistically significant reduction in cancer spending, largely attributable to reduction in inpatient payments, with no notable change in the intensity of cancer treatment (22). These data suggest that payment reform may indeed improve care coordination. However, there remains considerable uncertainty surrounding the ability of payment reform to modify physician practice.
Part and parcel to payment and delivery system reform is quality measurement. While the establishment of quality indicators for other disease states has flourished, there remains no established quality of care indicators for bladder cancer. Although several have been proposed, none address the potential inefficiency identified in the current study (23,24). Certainly, tracking the diagnostic yield for new cases of bladder cancer and monitoring the use of redundant procedures are measures for which benchmarks could be established. Given the paramount importance of meaningful quality measurement in cancer care, it is essential that we develop and rigorously evaluate quality measurement strategies. Doing so will ensure that emphasis on value does not jeopardize outcomes.
Finally, as financial incentives frequently have unpredictable and unintended consequences, systematic and timely evaluations of policy interventions are imperative. Prospectively planning studies of the impact of financial incentives in the Medicare program may have identified the undesired effect elaborated in this study years ago, potentially mitigating the incremental costs incurred.
Diagnosis and treatment of bladder cancer care are expensive and largely controlled by surgical subspecialists. Financial incentives in the current FFS model aimed at promoting cost-efficient behavior have resulted in unintended consequences that may ultimately be mitigated with risk-shared payment arrangements. These data serve to underscore the need for novel payment models aimed at promoting value-based specialty care.
Funding
The project was supported by Clinical and Translational Science Award No. UL1TR000445 from the National Center for Advancing Translational Sciences.
References
- 1. Arrow K, Auerbach A, Bertko J, et al. Toward a 21st-century health care system: recommendations for health care reform. Ann Intern Med. 2009;150(7):493–495. [DOI] [PubMed] [Google Scholar]
- 2. Avritscher EB, Cooksley CD, Grossman HB, et al. Clinical model of lifetime cost of treating bladder cancer and associated complications. Urology. 2006;68(3):549–553. [DOI] [PubMed] [Google Scholar]
- 3. Epstein AM, Jha AK, Orav EJ, et al. Analysis of early accountable care organizations defines patient, structural, cost, and quality-of-care characteristics. Health Aff (Millwood). 2014;33(1):95–102. [DOI] [PubMed] [Google Scholar]
- 4. Ginsburg PB. Fee-for-service will remain a feature of major payment reforms, requiring more changes in Medicare physician payment. Health Aff (Millwood). 2012;31(9):1977–1983. [DOI] [PubMed] [Google Scholar]
- 5. Dupree JM, Patel K, Singer SJ, et al. Attention to surgeons and surgical care is largely missing from early medicare accountable care organizations. Health Aff (Millwood). 2014;33(6):972–979. [DOI] [PubMed] [Google Scholar]
- 6. Yabroff KR, Lund J, Kepka D, et al. Economic burden of cancer in the United States: estimates, projections, and future research. Cancer Epidemiol Biomarkers Prev. 2011;20(10):2006–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oncology Care Model | Center for Medicare & Medicaid Innovation. http://innovation.cms.gov/initiatives/Oncology-Care/Accessed February 18, 2015.
- 8. Botteman MF, Pashos CL, Redaelli A, et al. The health economics of bladder cancer: a comprehensive review of the published literature. Pharmacoeconomics. 2003;21(18):1315–1330. [DOI] [PubMed] [Google Scholar]
- 9. Hollingsworth JM, Zhang YS, Miller DC, et al. Identifying better practices for early-stage bladder cancer. Med Care. 2011;49(12):1112–1117. [DOI] [PubMed] [Google Scholar]
- 10. Hollenbeck BK, Ye Z, Dunn RL, et al. Provider treatment intensity and outcomes for patients with early-stage bladder cancer. J Natl Cancer Inst. 2009;101(8):571–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Skolarus TA, Ye Z, Montgomery JS, et al. Use of restaging bladder tumor resection for bladder cancer among Medicare beneficiaries. Urology. 2011;78(6):1345–1349. [DOI] [PubMed] [Google Scholar]
- 12. Karl A, Adejoro O, Saigal C, et al. General adherence to guideline recommendations on initial diagnosis of bladder cancer in the United States and influencing factors. Clin Genitourin Cancer. 2014;12(4):270–277. [DOI] [PubMed] [Google Scholar]
- 13. Hollingsworth JM, Saigal CS, Lai JC, et al. Medicare payments for outpatient urological surgery by location of care. J Urol. 2012;188(6):2323–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hemani ML, Makarov DV, Huang WC, et al. The effect of changes in Medicare reimbursement on the practice of office and hospital-based endoscopic surgery for bladder cancer. Cancer. 2010;116(5):1264–1271. [DOI] [PubMed] [Google Scholar]
- 15. Al Hussein Al Awamlh B, Lee R, Chughtai B, et al. A Cost-effectiveness Analysis of Management of Low-risk Non-muscle-invasive Bladder Cancer Using Office-based Fulguration. Urology. 2015;85(2):381–387. [DOI] [PubMed] [Google Scholar]
- 16. Wagner AK, Soumerai SB, Zhang F, et al. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27(4):299–309. [DOI] [PubMed] [Google Scholar]
- 17. Strope SA, Daignault S, Hollingsworth JM, et al. Physician ownership of ambulatory surgery centers and practice patterns for urological surgery: evidence from the state of Florida. Med Care. 2009;47(4):403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hollingsworth JM, Ye Z, Strope SA, et al. Physician-ownership of ambulatory surgery centers linked to higher volume of surgeries. Health Aff (Millwood). 2010;29(4):683–689. [DOI] [PubMed] [Google Scholar]
- 19. Hollenbeck BK, Dunn RL, Suskind AM, et al. Ambulatory Surgery Centers and Their Intended Effects on Outpatient Surgery. Health Serv Res. 2015; In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bekelman JE, Epstein AJ, Emanuel EJ. Getting the next version of payment policy “right” on the road toward accountable cancer care. Int J Radiat Oncol Biol Phys. 2014;89(5):954–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schmidt H, Emanuel EJ. Lowering medical costs through the sharing of savings by physicians and patients: inclusive shared savings. JAMA Intern Med. 2014;174(12):2009–2013. [DOI] [PubMed] [Google Scholar]
- 22. Colla CH, Lewis VA, Gottlieb DJ, et al. Cancer spending and accountable care organizations: Evidence from the Physician Group Practice Demonstration. Healthc (Amst). 2013;1(3–4):100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Montgomery JS, Miller DC, Weizer AZ. Quality indicators in the management of bladder cancer. J Natl Compr Canc Netw. 2013;11(4):492–500. [DOI] [PubMed] [Google Scholar]
- 24. Cooperberg MR, Porter MP, Konety BR. Candidate quality of care indicators for localized bladder cancer. Urol Oncol. 2009;27(4):435–442. [DOI] [PubMed] [Google Scholar]