Abstract
Background
Chinese cancer patients often use Traditional Chinese Medicine (TCM) herbal medicine during or after active cancer treatments. However, little is known about how TCM herbal medicine impacts cancer outcomes. This study aimed to evaluate the association between TCM herbal therapy and survival outcomes in patients with stage II or III colorectal cancer.
Methods
We conducted an eight-center prospective cohort study in China among patients who had undergone radical resection for stage II and III colorectal cancer. All patients received comprehensive conventional treatments according to National Comprehensive Cancer Network (NCCN) guidelines, and follow-up visits were conducted over five years. We defined high exposure as a patient’s use of TCM individualized herbs for more than one year, ascertained via clinical interviews. The primary outcome was disease-free survival (DFS), with overall survival (OS) as a secondary outcome.
Results
Between April 2007 and February 2009, we enrolled 312 patients into the cohort; 166 (53.2%) met the definition of high exposure to TCM herbs. Adjusting for covariates, high exposure to TCM was associated with both better DFS (hazard ratio [HR] = 0.62, 95% confidence interval [CI] = 0.39 to 0.98) and OS (HR = 0.31, 95% CI = 0.14 to 0.68). In subgroup exploratory analysis, the effects demonstrated that the differences in outcomes were statistically significant in patients who had received chemotherapy.
Conclusion
Longer duration of TCM herbal use is associated with improved survival outcomes in stage II and III colorectal cancer patients in China. More research is needed to evaluate the effects and underlying mechanisms of herbal medicine on colorectal cancer outcomes.
Colorectal cancer (CRC) accounts for the third highest cancer incidence rate and the second highest cancer-related mortality rate worldwide (1,2). While the incidence of CRC is typically lower in developing countries, the incidence of CRC in China has increased substantially in the last five to 10 years due in large part to lifestyle changes, an aging population, and an increase in obesity (3–5). As a result, CRC is now China’s third most commonly diagnosed cancer in females and the fifth most commonly diagnosed cancer in males (6,7). Based on the US Surveillance, Epidemiology, and End Results (SEER) data, among all CRC patients, 39% have localized disease and 35% are regional cases (8). According to data from China, the proportions of postsurgery Dukes A and B stage CRC patients were 11% and 37.1%, respectively (9).
Postoperative recurrence and metastasis are the most important factors that affect CRC patients’ survival. In addition to adjuvant chemo- or radiotherapy, many Chinese patients also use Traditional Chinese Medicine (TCM) during or after active cancer treatments. TCM is deeply rooted in Chinese culture, where it has been used for thousands of years and is covered by most Chinese health insurance. A cross-sectional survey conducted in Hong Kong showed that 61% of Chinese CRC patients had utilized TCM as part of their treatments (10). Among the different TCM treatments, nearly 55% of cancer patients had previously used TCM herbal medicine, 7% had used tai chi and qigong, and only 1.3% had used acupuncture (11).
Despite wide use, the effects of TCM herbal therapy on survival outcomes remain elusive. Existing studies have only been published in the Chinese literature and have substantial methodological issues (12), leaving inadequate observational data to justify the initiation of a well-designed large clinical trial on TCM in patients with CRC. To address this gap in the literature, we evaluated the association between TCM herbal therapy and survival outcomes in postoperative patients with stage II and III CRC. We hypothesized that longer duration of TCM herbal therapy use would be associated with improved disease-free survival (DFS). Our secondary hypothesis was that longer duration of TCM herbal medicine use would be associated with better overall survival (OS) and lower cancer recurrence rate.
Methods
Study Design and Participants
We conducted a prospective cohort study in eight medical centers in China: the Department of Oncology, Xi-Yuan Hospital, China Academy of Chinese Medical Sciences; Department of Oncology, Guang-An-Men Hospital, China Academy of Chinese Medical Sciences; Department of Oncology, China-Japan Friendship Hospital, Ministry of Health; Cancer Hospital, Chinese Academy of Medical Sciences; Beijing Cancer Hospital; Beijing Chaoyang Hospital, Peking University First Hospital; and Henan Provincial Anyang Hospital. Each hospital’s independent ethics committee approved the protocol. All patients provided written informed consent.
The inclusion criteria were age 18 years or older, histologically confirmed adenocarcinoma of the colon or rectum, having undergone a complete resection of all lesions to R0 within six months prior to their enrollment date with no evidence of disease, and TNM classification of stage II or III disease according to the American Joint Committee on Cancer (13). The exclusion criteria were evidence of cancer recurrence or metastasis less than half a year after radical surgery and serious nonmalignant diseases such as heart, renal, and hepatic illnesses or the presence of other types of malignancies.
All enrolled patients received radical surgery between April 2007 and February 2009 and were treated by conventional adjuvant radiotherapy or chemotherapy according to the National Comprehensive Cancer Network (NCCN) guidelines (14). At the time of enrollment, some patients were undergoing chemo- or radiotherapy while some patients directly transitioned into the survivorship phase based on cancer stage and recurrence risk. Trained research staff performed interviews to collect data on herbal use and clinical outcomes and completed case report forms during the clinical visits. Patients received follow-up according to NCCN clinical guidelines, with clinical visits every three months for the first two years, every six months between years 3 and 5, and then yearly after year 5. For patients who occasionally missed clinical visits, trained research staff performed telephone follow-ups. Patients completed five years of follow-up by February 2014.
Exposure: TCM Herbal Therapy Use
We defined high exposure to TCM herbal therapy as 1) a patient having received a syndrome differentiation herbal decoction prescribed by a TCM physician for at least 12 months after radical surgery; and 2) in cases where recurrence and metastasis emerged within one year postsurgery, the patient used the syndrome differentiation herbal decoction for more than two-thirds of the disease-free survival period. We defined low exposure as those patients who received TCM herbal medicine for less than 12 months, including those who never received such treatment after radical surgery.
TCM syndrome differentiation herbal medicine decoction is the basic principle of TCM herbal medicine prescription. According to this principle, TCM physicians prescribe different herbs in a formula based on each patient’s symptoms and tongue and pulse diagnoses, which together form individualized TCM syndromes. In our study, TCM herbal medicine was based on general TCM principles, including nourishing spleen qi and kidney qi, soothing the liver qi, promoting blood circulation, and removing blood stasis. All of the TCM physicians in our study were board-certificated senior physicians who attended five-year medical schools and have many years of clinical training and experience. All of the TCM physicians who participated in our study received extensive training regarding the study protocol.
Outcome Measure
The primary end point was disease-free survival, defined as the time from surgery to disease recurrence or metastasis after the last outcome evaluation. The secondary end point included overall survival and rates of recurrence and metastasis for years 1 through 5. We collected outcome data with case report forms (CRF) at each follow-up, including information of cancer relapse and metastasis and survival outcomes.
Covariates
We obtained patients’ basic information, including age and sex, at the baseline assessment. We also collected patients’ disease and treatment information as confounders, including TNM stage (categorized by stage II and III) and whether they had undergone chemotherapy or not (categorized by yes or no), as well as cycles of chemotherapy completed.
Statistical Analysis
We compared baseline differences between high- and low-exposure groups. For continuous outcomes, we used the Student’s t test or the Wilcoxon rank-sum test when appropriate. For categorical data, we used the chi-square or Fisher’s exact test. For data with hierarchy, we applied the Wilcoxon rank-sum test.
To compare differences in DFS and OS between the TCM high- and low-exposure groups, we used Kaplan-Meier survival curves to display time to event data. We used the log-rank test to compare the difference between survival curves. We then performed Cox regression to adjust for confounders. Because stage and chemotherapy status were unbalanced between the two groups, we performed exploratory subgroup analysis stratified by stage and chemotherapy. In addition, we conducted sensitivity analysis among those patients receiving chemotherapy only, adjusting for cycles of chemotherapy in addition to other covariates.
We calculated the sample size based on the results of our prior study (15). We estimated that the three-year recurrence and metastasis rate was 13% in the high-exposure group and 25% in the low-exposure group. Including a 10% possibility for loss to follow-up, we needed 150 subjects each in the high- and low-exposure groups to detect the survival difference, with a power of 80% at a significance of .05.
All analyses were conducted using SPSS 17.0 ( New York, NY, USA). All tests were two-sided, with the statistical significance level set at .05. Considering all the secondary outcomes and subgroup analyses as exploratory, we did not adjust P values for multiple comparisons.
Results
Characteristics of Study Participants
Between April 2007 and February 2009, we enrolled 312 patients into the cohort (see Table 1 for baseline patient characteristics). The mean age was 58.1 years (SD = 12.8 years), and 137 (44.2%) were female. Among participants, 170 (54.4%) had colon cancer and 142 (45.5%) had rectal cancer; 151 (48.4%) presented at stage II, and 56.4% (176) received chemotherapy.
Table 1.
Baseline characteristics of participants (n = 312)
| High exposure No. (%) | Low exposure No. (%) | P* | |
|---|---|---|---|
| Age, y | |||
| Mean±SD | 58.5±12.6 | 56.5±12.9 | .18 |
| Sex | |||
| Male | 94 (56.6) | 81 (55.5) | .84 |
| Female | 72 (43.4) | 65 (44.5) | |
| Tumor location | .21 | ||
| Rectum | 81 (48.8) | 61 (41.8) | |
| Colon | 85 (51.2) | 85 (58.2) | |
| TNM stage | .008 | ||
| II | 92 (55.4) | 59 (40.4) | |
| III | 74 (44.6) | 87 (59.6) | |
| Chemotherapy regimen | |||
| 5-Fu+DDP | 0 | 1 (0.7) | .004 |
| FOLFIRI | 1 (0.6) | 0 | |
| FOLFOX | 76 (45.8) | 48 (32.9) | |
| Mayo Clinic | 0 | 1 (0.7) | |
| XELODA | 7 (4.2) | 3 (2.1) | |
| XELOX | 14 (8.4) | 5 (3.4) | |
| 5-fu+CF+LOHP | 9 (5.4) | 7 (4.8) | |
| Unknown | 3 (1.8) | 1 (0.7) | |
| None | 56 (33.7) | 80 (54.8) | |
| Stage and chemotherapy | <.001 | ||
| II with chemo | 51 (30.7) | 29 (19.9) | |
| II without chemo | 41 (24.7) | 30 (20.5) | |
| III with chemo | 59 (35.5) | 37 (25.3) | |
| III without chemo | 15 (9.0) | 50 (34.2) |
Chi-square test or Fisher’s exact test for categorical variables; t test for continuous variables.
Among participants, 166 (53.2%) were classified in the high-exposure group. There was no statistically significant difference between the two groups with respect to age, sex, or location. However, those in the high-exposure group were more likely to have stage II cancer than those in the low-exposure group (55.4% vs 43.8%, P = .01). In addition, more patients in the high-exposure group with stage III cancer used chemotherapy than those in the low-exposure group (79.7% vs 42.6%, P < .001) (see Table 1).
TCM Herbal Therapy and Disease-Free Survival
Over the course of the five-year follow-up period, 35 patients in the high-exposure group experienced a cancer relapse, compared with 49 patients in the low-exposure group (P = .016 by log-rank test) (Figure 2). Median disease-free survival was not reached in either group. The disease-free survival rates for the high-exposure group were 93.9% (year 1), 87.0% (year 2), 81.1% (year 3), 79.7% (year 4), and 75.4% (year 5). DFS rates for the low-exposure group were 88.3% (year 1), 77.1% (year 2), 72.1% (year 3), 66.1% (year 4), and 64.6% (year 5). After applying the Cox proportional hazards regression model to control for relevant covariates, TCM high exposure was associated with better disease-free survival (hazard ratio [HR] = 0.61, 95% confidential interval [CI] = 0.39 to 0.96) (Table 2).
Figure 2.
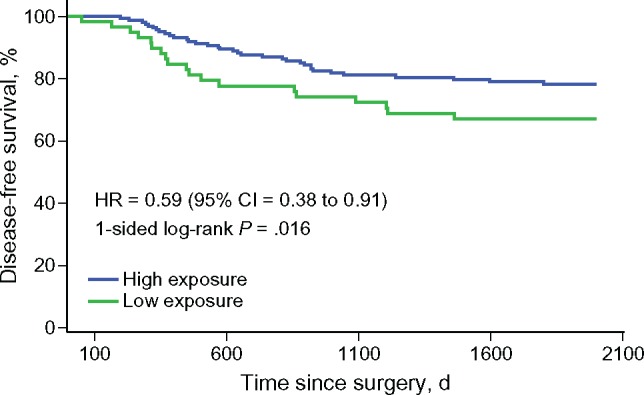
Disease-free survival by exposure to Traditional Chinese Medicine (TCM) herbal therapy. Log-rank test showed that the TCM herbal medicine high-exposure group had fewer events of cancer relapse than the low-exposure group (35 in 166 vs 49 in 146, P = .016). CI = confidence interval; HR = hazard ratio.
Table 2.
Multivariable Cox regression model of disease-free survival*
| Factors | Adjusted HR (95% CI) | P |
|---|---|---|
| High exposure | ||
| No | 1.00 (reference) | – |
| Yes | 0.62 (0.39 to 0.96) | .039 |
| Age, y | ||
| ≤65 | 1.00 (reference) | – |
| >65 | 1.13 (0.71 to 1.79) | .62 |
| Sex | ||
| Male | 1.00 (reference) | – |
| Female | 1.14 (0.74 to 1.74) | .57 |
| Location | ||
| Rectum | 1.00 (reference) | – |
| Colon | 0.76 (0.49 to 1.17) | .21 |
| TNM stage | ||
| II | 1.00 (reference) | – |
| III | 1.38 (0.88 to 2.17) | .17 |
| Chemotherapy | ||
| No | 1.00 (reference) | – |
| Yes | 0.97 (0.62 to 1.52) | .85 |
The model was adjusted by Traditional Chinese Medicine use exposure, age, sex, TNM stage, tumor site, and chemotherapy. CI = confidence interval; HR = hazard ratio.
Figure 1.
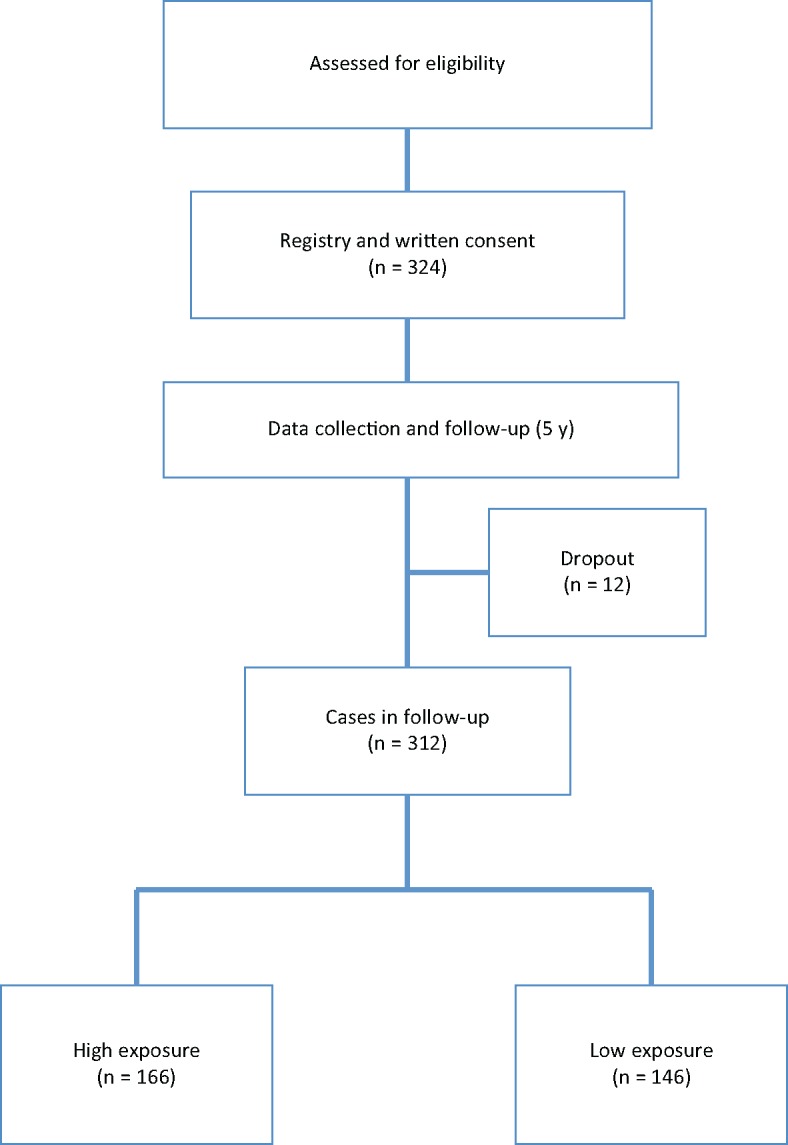
Study flow diagram. We enrolled 324 patients in this study. During the five-year follow up, 12 patients dropped out. Among the 312 who completed follow-up, 166 were in the Traditional Chinese Medicine (TCM) herbal medicine high-exposure group, and 146 were in the TCM low-exposure group.
TCM Herbal Therapy and Recurrence/Metastasis Rates
In the high-exposure cohort, the cancer recurrence and metastasis rates were 5.5% (year 1), 12.4% (year 2), 17.7% (year 3), 19.2% (year 4), and 23.5% (year 5). In the low-exposure cohort, recurrence and metastasis rates were 11.7% (year 1), 22.9% (year 2), 27.9% (year 3), 32.5% (year 4), and 34.0% (year 5). The recurrence and metastasis rates between the two cohorts were statistically significant (P = .01).
TCM Herbal Therapy and Overall Survival
Over the five-year follow-up period, nine participants in the high-exposure group died, compared with 24 participants in the low-exposure group (P = .01 by log-rank test) (Figure 3). Median survival was not reached in either group. Overall survival rates for the high-exposure group were 99.4% (year 1), 99.4% (year 2), 95.4% (year 3), 94.7% (year 4), and 93.2% (year 5). For the low-exposure group, overall survival rates were 99.3% (year 1), 95.1% (year 2), 87.8% (year 3), 84.0% (year 4), and 80.9% (year 5). After adjusting for covariates, the TCM high-exposure group had statistically significantly better overall survival (HR = 0.41, 95% CI = 0.21 to 0.83) (Table 3).
Figure 3.
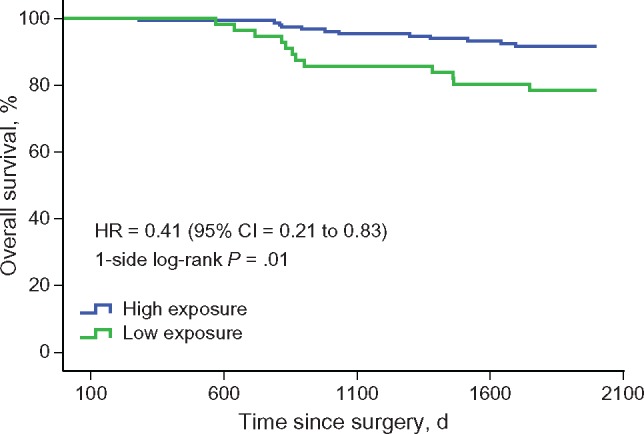
Overall survival by exposure to Traditional Chinese Medicine (TCM) herbal therapy. Log-rank test showed that the TCM herbal medicine high-exposure group had fewer events of all causes of death than the low-exposure group (9 in 166 vs 24 in 146, P = .01). CI = confidence interval; HR = hazard ratio.
Table 3.
Multivariable Cox regression model of overall survival
| Factors | Adjusted HR (95% CI) | P |
|---|---|---|
| High exposure | ||
| No | 1.00 (reference) | – |
| Yes | 0.40 (0.20 to 0.82) | .003 |
| Age, y | ||
| ≤65 | 1.00 (reference) | – |
| >65 | 1.92 (0.99 to 3.72) | .22 |
| Sex | ||
| Male | 1.00 (reference) | – |
| Female | 0.91 (0.47 to 1.75) | .99 |
| Location | ||
| Rectum | 1.00 (reference) | – |
| Colon | 0.69 (0.36 to 1.33) | .29 |
| TNM stage | ||
| II | 1.00 (reference) | – |
| III | 1.14 (0.58 to 2.22) | 1 |
| Chemotherapy | ||
| No | 1.00 (reference) | – |
| Yes | 1.06 (0.52 to 1.95) | .87 |
The model was adjusted by Traditional Chinese Medicine use exposure, age, sex, TNM stage, tumor site, and chemotherapy. CI = confidence interval; HR = hazard ratio.
Subgroup Analysis on Disease-Free Survival by Stage and Chemotherapy
Considering the baseline differences in TNM staging and chemotherapy use between the two groups, we conducted a further subgroup exploratory analysis stratified by these two variables (see Table 4). High-exposure cohorts had statistically significantly fewer events of cancer relapse than the low-exposure cohorts by log-rank test (P < .05 in both groups) for both stage II and III participants within the chemotherapy subgroups (see Table 4). In our exploratory sensitivity analysis, restricting to patients receiving chemotherapy only (n = 272), we adjusted for cycles for chemotherapy received in addition to other covariates and found that the association between high exposure and DFS was no longer statistically significant (HR = 0.63, 95% CI = 0.33 to 1.11). On the other hand, the association between high exposure and OS was even more robust (HR = 0.18, 95% CI = 0.62 to 0.53).
Table 4.
Events and survival rates (DFS) in subgroup analysis*
| DFS | Exposure cohort, No. | Year 1 Events (%) | Year 2 Events (%) | Year 3 Events (%) | Year 4 Events (%) | Year 5 Events (%) | P* |
|---|---|---|---|---|---|---|---|
| Stage II chemo | .02 | ||||||
| High | 51 | 0 (100) | 3 (93.7) | 3 (87.1) | 0 (87.1) | 0 (87.1) | |
| Low | 29 | 1 (96.5) | 5 (78.3) | 2 (70.5) | 0 (70.5) | 0 (70.5) | |
| Stage II no chemo | .43 | ||||||
| High | 41 | 2 (95.1) | 2 (90.0) | 4 (79.4) | 1 (76.6) | 1 (71.2) | |
| Low | 30 | 2 (93.3) | 3 (83.3) | 3 (73.3) | 2 (66.4) | 0 (66.4) | |
| Stage III chemo | .001 | ||||||
| High | 59 | 7 (88.1) | 5 (79.6) | 2 (76.0) | 1 (74.0) | 1 (69.9) | |
| Low | 37 | 5 (86.3) | 6 (69.3) | 0 (69.3) | 2 (63.4) | 1 (57.7) | |
| Stage III no chemo | .42 | ||||||
| High | 15 | 1 (93.3) | 1 (86.4) | 0 (86.4) | 0 (86.4) | 1 (72.0) | |
| Low | 50 | 9 (82.0) | 2 (78.0) | 2 (74.0) | 4 (65.7) | 0 (65.7) |
DFS = disease-free survival.
Discussion
Although CRC patients and survivors use TCM herbal therapy extensively in China (11), no strong evidence exists to demonstrate the safety and survival benefits of TCM herbal medicine combined with conventional Western medicine for CRC. In this multicenter prospective cohort study, we found that longer duration of Chinese herbal medicine use was associated with improved DFS and OS among postoperative stage II and III colorectal cancer survivors. In exploratory analyses, the effect of herb use on DFS was more evident for those patients who had undergone chemotherapy than for those who did not use chemotherapy. This multicenter prospective observational study provided the initial steps for evaluating TCM herbal medicine’s role in colorectal cancer care. These findings can be used to spearhead future observational and interventional studies to help improve survival outcomes of CRC patients.
Our study contributes to the very limited data of TCM herbal therapy’s impact on colorectal cancer survival outcomes. Our previous prospective two-center cohort study demonstrated that among postradical surgery stage II and III colorectal cancer patients, those who combined Chinese herbal medicine with conventional medicine had statistically significantly lower recurrence and metastasis rates than those who were treated with conventional medicine alone (15). In this study, we also found that longer duration of TCM herbal medicine use was associated with an increase in DFS and OS. Our study’s results provide similar but stronger evidence for OS and DFS benefits than two prior studies in patients with CRC (16,17).
Whether the patient had undergone chemotherapy is a major confounder in our study. In subgroup exploratory analysis, we found that longer duration of TCM use was associated with better survival outcomes for patients who had undergone chemotherapy after radical surgery. However, for patients who had not undergone chemotherapy, we did not find such significant differences. In exploratory sensitivity analysis restricted to patients undergoing chemotherapy only and adjusting for cycles of chemotherapy, we found that the magnitude of effect on DFS was approximately the same (although no longer statistically significant); however, the effect on OS was even stronger. Sufficient evidence has shown that chemotherapy-induced side effects contributed to significantly worse chemotherapy compliance among cancer patients (18,19). Failure to complete the recommended dose of chemotherapy could lead to a higher risk of cancer recurrence and cancer-related mortality (20–22). Several clinical studies have shown that TCM herbal medicine can reduce chemotherapy-induced side effects such as vomiting, diarrhea, and peripheral neuropathy for colorectal cancer patients (23–25). Thus, herbal medicine may indirectly benefit survival outcomes by helping patients adhere to their chemotherapy treatments. Future prospective clinical trials need to evaluate whether integrating Chinese herbal medicine can improve symptom control and adherence to chemotherapy treatment.
Other mechanisms of TCM herbal medicine’s potential role in improving survival outcomes of colorectal cancer patients are still largely unknown. Basic science indicates that certain herbal components in vivo may enhance the anticancer effect of 5-Fu for colon carcinoma (26,27). Recent basic studies have also found that some herbal components have direct anticancer effects by inducing apoptosis and cell cycle arrest that might help to eliminate residue cancer cells after active cancer treatments (28–31). Herbal medicine may also impact the tumor microenvironment by acting as an anti-inflammatory agent and inhibiting angiogenesis (32–34). Further, some herbs may reduce or regulate the colorectal cancer stem cell, which is important in tumor growth and drug resistance (35,36). To build on our observations, future research should focus on identifying specific herbal components or combinations that may have mechanistic targets for colorectal cancer.
We need to acknowledge several limitations. Because we conducted an observational study, we cannot establish a direct causal inference between TCM herbal medicine and CRC survival outcomes. Unmeasured covariates such as education and income, as well as other health behaviors, including smoking, obesity, and physical activity, may confound survival outcomes. In addition, we did not collect some disease information, like K-ras gene mutation status, because it was not part of standard care at the time that the study was designed. Our measures of duration of TCM exposure were not specific and may be subject to participant recall bias. Specific TCM herbal formulas or herbs used by the participants in our study were not recorded and analyzed. Thus, it is difficult to determine which herb or group of herbs could potentially benefit CRC patients. In our study, the high-exposure group had more early-stage patients and those who had undergone chemotherapy, which may bias results. However, we stratified chemotherapy and TNM stage in subgroup analysis.
Despite these limitations, this study has numerous strengths. To our knowledge, this is the first large multicenter prospective observational study to evaluate the association between TCM herbal medicine and outcomes in patients with colorectal cancer. The global collaboration in study design, conduct, analyses, interpretation, and writing is also novel in the setting of TCM oncology clinical research and builds a platform for future rigorous epidemiological and clinical investigation. Our research provides emerging evidence that longer duration of TCM herbal medicine use is associated with better clinical outcomes when combined with conventional cancer treatments for patients with early-stage CRC. This association was particularly evident among patients who had undergone chemotherapy. This observational data must be interpreted with caution and confirmed by a randomized controlled trial to establish the causal relationship between long-term TCM herbal use and survival outcomes. By creatively leveraging basic, translational, and clinical research, we may accelerate the discovery of evidence for the use of TCM herbal therapy for colorectal cancer patients around the globe.
Funding
This study was supported by the “11th Five-Year Plan” International Cooperation Project, funded by the Ministry of Science and Technology of the People’s Republic of China (Item Number: 2006BAI11B01). Dr. Mao is supported in part by the United States National Institutes of Health/National Cancer Institute Cancer Center Support Grant P30 CA008748 and the Laurance S. Rockefeller Fund. The funding agencies had no role in the design or conduct of the study.
Notes
This paper was presented at 11th annual meeting of the Society for Integrative Oncology, November 2014, Best of SIO.
We acknowledge Dr. Vinjar Fønnebø and Dr. Terje Alreak from the Norway National Research Centre in Complementary and Alternative Medicine and Dr. Vonen Barthold from the Northern Norway Regional Health Authority for supporting the design and completion of this study. We also acknowledge Dr. Xiaoshu Zhu from the University of Western Sydney and Juan Liao for language revision, as well as Congcong Wang and Qianyun Chai from Beijing University of Chinese Medicine for statistical analysis.
References
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;652:87–108. [DOI] [PubMed] [Google Scholar]
- 2. Cunningham D, Atkin W, Lenz HJ, et al. Colorectal cancer. Lancet. 2010;3759719:1030–1047. [DOI] [PubMed] [Google Scholar]
- 3. Zhang Y, Shi J, Huang H, Ren J, Li N, Dai M. [Burden of colorectal cancer in China.] Zhonghua Liu Xing Bing Xue Za Zhi. 2015;367:709–714. [PubMed] [Google Scholar]
- 4. Fang JY, Dong HL, Sang XJ, et al. Colorectal Cancer mortality characteristics and predictions in China, 1991-2011. Asian Pac J Cancer Prev. 2015;1617:7991–7995. [DOI] [PubMed] [Google Scholar]
- 5. Liu S, Zheng R, Zhang M, Zhang S, Sun X, Chen W. Incidence and mortality of colorectal cancer in China, 2011. Chin J Cancer Res. 2015;271:22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;662:115–132. [DOI] [PubMed] [Google Scholar]
- 7. Chen W. Cancer statistics: Updated cancer burden in China. Chin J Cancer Res. 2015;271:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. National Cancer Insititute, Surveillance, Epidemiology, and End Results Program. Cancer stat facts: Colon and rectum cancer. 2012. https://seer.cancer.gov/statfacts/html/colorect.html. Accessed May 21, 2016.
- 9. Li M, Gu J.. Changing patterns of colorectal cancer in China over a period of 20 years. World J Gastroenterol. 2005;1130:4685–4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lam YC, Cheng CW, Peng H, Law CK, Huang X, Bian Z. Cancer patients' attitudes towards Chinese medicine: A Hong Kong survey. Chin Med. 2009;4:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McQuade JL, Meng Z, Chen Z, et al. Utilization of and attitudes towards Traditional Chinese Medicine therapies in a Chinese cancer hospital: A survey of patients and physicians. Evid Based Complement Alternat Med. 2012;2012:504507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Luo L, Yang YF, Li PH.. [Cohort study on fuzheng capsule and quxie capsule in reducing relapse and metastasis of cancer in patients with stage II and III colorectal carcinoma after operation.] Zhongguo Zhong Xi Yi Jie He Za Zhi. 2006;268:677–680. [PubMed] [Google Scholar]
- 13. Greene FL, Trotti A, Fritz AG, Compton CC, Byrd DR, Edge SB, eds. AJCC Cancer Staging Handbook. 7th ed.Chicago: American Joint Committee on Cancer; 2010. [Google Scholar]
- 14. Desch CE, Benson AB 3rd, Somerfield MR, et al. Colorectal cancer surveillance: 2005 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2005;2333:8512–8519. [DOI] [PubMed] [Google Scholar]
- 15. Yang YF, Ge JZ, Wu Y, et al. Cohort study on the effect of a combined treatment of Traditional Chinese Medicine and Western medicine on the relapse and metastasis of 222 patients with stage II and III colorectal cancer after radical operation. Chin J Integr Med. 2008;144:251–256. [DOI] [PubMed] [Google Scholar]
- 16. Tao L, Zhu YJ, Lu, XM, et al. [Clinical study on survival benefit for elderly patients with resected stage II or III colorectal cancer based on Traditional Chinese Medicine syndrome differentiation and treatment.] Zhong Xi Yi Jie He Xue Bao. 2010;812:1159–1164. [DOI] [PubMed] [Google Scholar]
- 17. McCulloch M, Broffman M, van der Laan M, et al. Colon cancer survival with herbal medicine and vitamins combined with standard therapy in a whole-systems approach: Ten-year follow-up data analyzed with marginal structural models and propensity score methods. Integr Cancer Ther. 2011;103:240–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wells JS, Strickland OL, Dalton JA, et al. Adherence to intravenous chemotherapy in African American and white women with early-stage breast cancer. Cancer Nurs. 2015;382:89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Palli SR, Grabner M, Quimbo RA, et al. The impact of 5-hydroxytryptamine-receptor antagonists on chemotherapy treatment adherence, treatment delay, and nausea and vomiting. Cancer Manag Res. 2015;7:175–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Andre T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;35023:2343–2351. [DOI] [PubMed] [Google Scholar]
- 21. Andre T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;2719:3109–3116. [DOI] [PubMed] [Google Scholar]
- 22. Andre T, de Gramont A, Vernerey D, et al. Adjuvant fluorouracil, leucovorin, and oxaliplatin in stage II to III colon cancer: Updated 10-year survival and outcomes according to BRAF mutation and mismatch repair status of the MOSAIC Study. J Clin Oncol. 2015;3335:4176–4187. [DOI] [PubMed] [Google Scholar]
- 23. Kummar S, Copur MS, Rose M, et al. A phase I study of the chinese herbal medicine PHY906 as a modulator of irinotecan-based chemotherapy in patients with advanced colorectal cancer. Clin Colorectal Cancer. 2011;102:85–96. [DOI] [PubMed] [Google Scholar]
- 24. Mok TS, Yeo W, Johnson PJ, et al. A double-blind placebo-controlled randomized study of Chinese herbal medicine as complementary therapy for reduction of chemotherapy-induced toxicity. Ann Oncol. 2007;184:768–774. [DOI] [PubMed] [Google Scholar]
- 25. Taixiang W, Munro AJ, Guanjian L.. Chinese medical herbs for chemotherapy side effects in colorectal cancer patients. Cochrane Database Syst Rev. 20051:CD004540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Deng S, Hu B, An HM, et al. Teng-Long-Bu-Zhong-Tang, a Chinese herbal formula, enhances anticancer effects of 5-Fluorouracil in CT26 colon carcinoma. BMC Complement Altern Med. 2013;13:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xu Q, Brabham JG, Zhang S, et al. Chinese herbal formula, Bing De Ling, enhances antitumor effects and ameliorates weight loss induced by 5-fluorouracil in the mouse CT26 tumor model. DNA Cell Biol. 2005;247:470–475. [DOI] [PubMed] [Google Scholar]
- 28. Chu L, Zhao H, Fang J, et al. The Traditional Chinese Medicinal formula BDL301 suppresses tumor growth by inhibiting STAT3 pathway and inducing apoptosis in colorectal cancer cells. DNA Cell Biol. 2015;343:178–188. [DOI] [PubMed] [Google Scholar]
- 29. Mao F, Xiao B, Jiang Z, et al. Anticancer effect of Lycium barbarum polysaccharides on colon cancer cells involves G0/G1 phase arrest. Med Oncol. 2011;281:121–126. [DOI] [PubMed] [Google Scholar]
- 30. Kim DH, Hossain MA, Kang YJ, et al. Baicalein, an active component of Scutellaria baicalensis Georgi, induces apoptosis in human colon cancer cells and prevents AOM/DSS-induced colon cancer in mice. Int J Oncol. 2013;435:1652–1658. [DOI] [PubMed] [Google Scholar]
- 31. Mohammadi A, Mansoori B, Aghapour M, et al. The herbal medicine Utrica Dioica inhibits proliferation of colorectal cancer cell line by inducing apoptosis and arrest at the G2/M phase. J Gastrointest Cancer. 2016;472:187–195. [DOI] [PubMed] [Google Scholar]
- 32. Auyeung KK, Law PC, Ko JK.. Novel anti-angiogenic effects of formononetin in human colon cancer cells and tumor xenograft. Oncol Rep. 2012;286:2188–2194. [DOI] [PubMed] [Google Scholar]
- 33. Shen A, Lin J, Chen Y, et al. Pien Tze Huang inhibits tumor angiogenesis in a mouse model of colorectal cancer via suppression of multiple cellular pathways. Oncol Rep. 2013;304:1701–1706. [DOI] [PubMed] [Google Scholar]
- 34. Wang E, Bussom S, Chen J, et al. Interaction of a Traditional Chinese Medicine (PHY906) and CPT-11 on the inflammatory process in the tumor microenvironment. BMC Med Genomics. 2011;4:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ponnurangam S, Mammen JM, Ramalingam S, et al. Honokiol in combination with radiation targets notch signaling to inhibit colon cancer stem cells. Mol Cancer Ther. 2012;114:963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wei L, Chen P, Chen Y, et al. Pien Tze Huang suppresses the stem-like side population in colorectal cancer cells. Mol Med Rep. 2014;91:261–266. [DOI] [PubMed] [Google Scholar]


