Abstract
Introduction
Postmarketing pharmacovigilance reports have raised concerns about non-bleeding adverse events associated with direct oral anticoagulants (DOACs), but only limited results are available from large claims databases.
Objective
The aim of this study was to assess the potential association between DOAC initiation and the onset of four types of non-bleeding adverse events by sequence symmetry analysis (SSA).
Methods
SSA was performed using nationwide data from the French National Healthcare databases (Régime Général, 50 million beneficiaries) to assess a cohort of 386,081 DOAC new users for the first occurrence of four types of non-bleeding outcomes: renal, hepatic, skin outcomes identified by using hospitalization discharge diagnoses, and gastrointestinal outcomes by using medication reimbursement. Asymmetry in the distribution of each investigated outcome occurring before and after initiation of DOAC therapy was used to test the association between DOAC therapy and these outcomes. SSA inherently controls for time-constant confounders, and adjusted sequence ratios were computed after correcting for temporal trends. Negative (glaucoma) and positive (bleeding, depressive disorders) control outcomes were used and analyses were replicated on a cohort of 310,195 patients initiating a vitamin K antagonist (VKA).
Results
This study demonstrated the expected positive association between either DOAC or VKA therapy and hospitalised bleeding and initiation of antidepressant therapy, while no association was observed between either DOAC or VKA therapy and initiation of antiglaucoma medications. For DOAC therapy, signals were the associations with hepatic outcomes, including acute liver injury [for the 3-month time window, aSR3 = 2.71, 95% confidence interval (CI) 1.79–4.52]; gastrointestinal outcomes, including initiation of drugs for constipation and antiemetic drugs (aSR3 = 1.31, 95% CI 1.27–1.36; and 1.17, 95% CI 1.12–1.22, respectively); and kidney diseases (aSR3 = 1.33, 95% CI 1.29–1.37).
Conclusion
Results of this nationwide study suggest that DOACs are associated with rare but severe liver injury and more frequent gastrointestinal disorders. A low risk of kidney injury with DOAC therapy can also not be excluded.
Electronic supplementary material
The online version of this article (10.1007/s40264-018-0668-9) contains supplementary material, which is available to authorized users.
Key Points
| Results from this sequence symmetry analysis (SSA) using the French healthcare databases highlight the importance of non-bleeding adverse events in DOAC new users. |
| DOAC therapy may be associated with rare but severe liver injury and more frequent gastrointestinal disorders. |
| Channeling bias should be taken into account when interpreting results from SSA of a tested medication for which a therapeutic alternative exists. |
Introduction
The use of direct oral anticoagulants (DOACs) has increased dramatically worldwide over the last 5 years, with a corresponding economic burden on healthcare systems [1–4]. This rapid uptake of DOAC use is mainly related to the lifelong prescription of these agents for the prevention of stroke in patients with non-valvular atrial fibrillation (AF) as a more convenient, fixed-dose alternative to vitamin K antagonists (VKAs). Compared with VKAs, DOACs do not require regular laboratory monitoring of patients and allow once-daily (rivaroxaban, apixaban, edoxaban) or twice-daily (dabigatran) dosing instead of multiple dose adjustments [5, 6]. European guidelines have recently expressed a preference for DOACs over VKAs for stroke prevention in non-valvular AF patients [7]. Considering the important clinical role of oral anticoagulants (OACs), the declining use of VKAs, and the increasing prevalence of AF with ageing of the population, DOAC prescription is therefore expected to continue to increase over the next years [8]. Continuous and careful monitoring of their safety profile is therefore needed. Large administrative databases provide a timely opportunity to perform signal detection and investigate adverse reactions.
The relative safety of DOACs versus warfarin in non-valvular AF patients [9–11], or versus heparin in the treatment and prevention of recurrence of venous thromboembolism [12–14], has been demonstrated in large-scale randomized clinical trials (RCTs), and has been confirmed globally by observational studies [15, 16]; however, both clinical trials and observational studies have mainly focussed on bleeding adverse events to date [17]. Postmarketing data, including pharmacovigilance reports [18] and case reports, have raised concerns about other, albeit rarer, non-bleeding adverse events that could not be detected by RCTs due to intrinsic limitations, such as small sample size and short follow-up. Renal and hepatic adverse events are among the emerging DOAC safety issues [17]. Rare but severe skin adverse events, such as vasculitis, have also been reported in dabigatran-treated patients [19–21]. Furthermore, non-bleeding adverse events, including gastrointestinal symptoms such as dyspepsia, reported in dabigatran-treated patients [22–24], have been found to be one of the main reasons for permanent DOAC discontinuation.
At the request of the pharmacovigilance department of the French Medicines Agency (ANSM), we investigated a possible association between DOAC (dabigatran, rivaroxaban, apixaban) use and these potential non-bleeding adverse events by using data from the French Healthcare databases [25]. The sequence symmetry analysis (SSA) method [26, 27] has been shown to be a complementary tool to support other pharmacovigilance methods based on spontaneous reporting systems due to its high specificity for detecting adverse events using claims data [28, 29]. As this self-adjusted method is easy to process and allows concomitant investigation of different clinical entities within a limited timeframe, irrespective of the characteristics of the event and exposure [30], SSA was applied to test the potential association between DOAC initiation and the onset of four predefined types of non-bleeding adverse events.
Methods
Data Sources
French national health insurance (Assurance Maladie) covers the entire French population and is divided into several specific schemes according to beneficiaries’ occupational sector, with the largest scheme being ‘Régime général’ (approximately 50 million beneficiaries).
This study was conducted using data from the French health insurance system database (SNIIRAM, Système national d’information inter-régimes de l’assurance maladie) linked to the French hospital discharge database (PMSI, Programme de médicalisation des systèmes d’information). The SNIIRAM database contains individualised, anonymous, and comprehensive data on health spending reimbursements. Demographic data include date of birth, sex, and vital status. Drugs are coded according to the Anatomical Therapeutic Chemical (ATC) classification and packaging of each product is identified by means of a national specific pack identifier code providing information on the name of the product, active ingredient and dose in each pill, number of pills, and route of administration, but not the prescribed dose.
The PMSI database provides detailed medical information on all hospitalizations in France. The medical indication for drug reimbursements and the results of medical procedures or laboratory tests are not available in these databases. However, medical diagnosis information is available from two independent sources encoded according to the International Classification of Diseases, 10th edition (ICD-10): (1) diagnoses corresponding to patient eligibility for 100% reimbursement of severe and costly long-term diseases (LTDs) and disability, such as AF, coronary heart disease, certain debilitating diseases (such as multiple sclerosis, inflammatory bowel disease or rheumatoid arthritis), HIV infection, cancer, etc.; and (2) discharge diagnoses from hospitalization data. The SNIIRAM-PMSI databases also indicate medical procedures performed in the ambulatory setting or during a hospital stay.
The French healthcare databases have been previously described and used in epidemiology research, including pharmacoepidemiological studies [25, 31].
This research was authorised by the French Data Protection Agency (CNIL, Commission Nationale de l’Informatique et des Libertés).
Outcomes of Interest
Four groups of potential adverse events (renal, hepatic, skin, and gastrointestinal) were investigated. For each investigated group, outcomes were defined by using either hospitalization discharge diagnoses (renal, hepatic, and skin outcomes) or medication reimbursement (gastrointestinal outcomes) as proxies of these adverse events. Detailed definitions of these outcomes are provided in electronic supplementary material (ESM) 1.
Sequence Symmetry Analysis
Rationale
SSA is a case-only design based on the rationale that if a medication causes an adverse event, this medication will be prescribed more often before than after occurrence of this event [26, 27]. Asymmetry in the distribution of this outcome of interest before and after initiation of a tested medication is therefore used to assess the association between this medication and an outcome of interest. Outcomes can be identified either by medication prescription/reimbursement or hospitalization in healthcare databases. The advantages and pitfalls of this method have been recently described in detail [32].
Study Population
A cohort of patients who initiated treatment with dabigatran, rivaroxaban or apixaban between 1 January 2013 and 31 December 2015 (inclusion period) was identified from Régime général reimbursement data. Patients’ index date was the date of the first DOAC reimbursement (the date on which the prescription was filled) during this period.
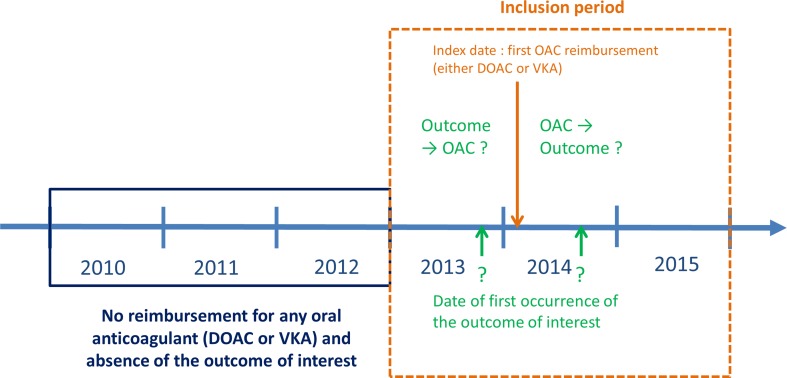
As usual in SSA, for each definition of each outcome of interest, only patients presenting both the outcome and initiating DOAC therapy were included, i.e. those meeting the following criteria: (1) having continuous Régime général health insurance coverage during the 2010–2015 period: at least one reimbursement each year related to Régime général coverage or death during this period; (2) being a DOAC new user: at least one reimbursement for DOACs (ATC: B01AE07 for dabigatran, B01AF01 for rivaroxaban, and B01AF02 for apixaban; edoxaban was not available in France during the inclusion period and was therefore not considered in this SSA) during the inclusion period and no reimbursement for any oral anticoagulant during the 2010–2012 period; and (3) presenting the outcome of interest: occurrence during the inclusion period and no occurrence during the 2010–2012 period. For each patient, only the date of the first time the outcome occurred during the inclusion period was considered in the analysis (Fig. 1).
Fig. 1.
Schematic representation of sequence symmetry analysis study design for a given outcome of interest. DOAC direct oral anticoagulant, OAC oral anticoagulant, VKA vitamin K antagonist
Only the first DOAC reimbursement was considered and the patient was assigned to the OAC group corresponding to this first reimbursement.
Data Analysis
A separate SSA was performed for each definition of the outcome of interest. Patients were classified according to the temporal sequence (outcome → DOAC or DOAC → outcome) between the DOAC first reimbursement and the date of occurrence of the first outcome of interest during the time window considered. Patients who experienced the outcome of interest on the same day as the prescription of DOAC therapy were excluded from the analysis. Three time windows (on either side of the index date) were tested to search for the adverse event: 3 months (90 days), 6 months (180 days), and 12 months (360 days) [27].
The asymmetry of sequences was measured by calculating a crude sequence ratio (cSR) as the ratio of the number of patients who initiated DOAC therapy before the outcome over the number of patients for whom the outcome occurred before the initiation of DOAC therapy. To take into account possible changes in both outcome and DOAC prescription trends in the background population during each time window that would confound the cSR, a null-effect sequence ratio (nSR) was then calculated, as described by Tsiropoulous et al. [27]. An adjusted sequence ratio (aSR) was then computed by dividing the cSR by the nSR. Ninety-five percent confidence intervals (95% CIs) were calculated for the aSR by using a normal approximation to the binomial distribution [33]. A positive association between DOAC exposure and the investigated outcome was considered as significant when the lower limit of the 95% CI of the aSR was > 1. Significant positive associations were considered valid when they were consistent over the three time windows investigated; results in which a significant positive association was observed when the length of the time window was extended were therefore not considered to be valid.
Analyses were performed for the entire cohort of DOAC new users, and separately for each of the three DOACs.
A subgroup analysis was carried out in patients with AF; patients with a history of deep vein thrombosis, pulmonary embolism or lower-limb orthopaedic procedures were excluded from the initial cohort.
All analyses were performed using SAS software version 9.2 (SAS Institute, Inc., Cary, NC, USA).
Sensitivity Analysis
Changes in underlying use and reimbursement trends were taken into account by computing an aSR. However, other possible sources of bias may exist with the SSA design, such as (1) detection bias that occurs when patients are more likely to receive DOAC therapy after having experienced the event of interest, as the disease requiring DOAC prescription may have been discovered on this occasion; and (2) situations in which events alter the probability of exposure, resulting in inverse causation bias [26, 32]. Two sensitivity analyses were therefore performed.
First, outcomes considered as ‘control’ outcomes were used to validate the method for OAC therapy [29]. As OAC therapy is known to cause bleeding, this outcome was used as a positive control outcome. As glaucoma appeared to be unrelated to the prescription of OACs, hospitalization and medication for glaucoma were used as negative control outcomes. Finally, as a relationship between cardiovascular drugs and depression was described in the first study using this method [26], analyses were replicated using major depressive disorders as another positive control outcome.
Second, as an alternative and active comparator to DOAC therapy, VKA therapy (ATC: B01AA, fluindione, warfarin, acenocoumarol) was used as an aid to better identify the source of potential bias in DOAC analyses [34]. An SSA was replicated for each definition of the four outcomes in a cohort of VKA new users by using the same design and study population definitions as described above. Detailed definitions of these control outcomes are provided in ESM 1.
Results
Baseline Characteristics of Eligible Patients
A total of 696,276 OAC new users were eligible for inclusion in the SSA. Their baseline characteristics are displayed on Table 1. DOAC new users were younger and less severe than VKA new users and DOAC therapy was more often prescribed by private cardiologists.
Table 1.
Baseline characteristics of eligible patients according to the oral anticoagulant treatment group
| Characteristics (%)a | VKA new users (N = 310,195) | DOAC new users | |||
|---|---|---|---|---|---|
| Total (N = 386,081) | Dabigatran (N = 67,889) | Rivaroxaban (N = 254,816) | Apixaban (N = 63,376) | ||
| Male sex | 142,434 (45.9) | 186,289 (48.2) | 33,293 (49.0) | 121,525 (47.7) | 31,471 (49.7) |
| Age, years [mean (SD)] | 71.3 (15.8) | 68.5 (14.1) | 71.0 (12.4) | 66.8 (14.7) | 72.7 (11.8) |
| Age groups, years | |||||
| < 65 | 88,564 (28.6) | 129,003 (33.4) | 18,287 (26.9) | 96,763 (38.0) | 13,953 (22.0) |
| 65–74 | 63,123 (20.4) | 108,884 (28.2) | 19,525 (28.8) | 70,934 (27.8) | 18,425 (29.1) |
| 75–79 | 42,210 (13.6) | 57,364 (14.9) | 11,474 (16.9) | 35,159 (13.8) | 10,731 (16.9) |
| ≥ 80 | 116,298 (37.5) | 90,830 (23.5) | 18,603 (27.4) | 51,960 (20.4) | 20,267 (32.0) |
| First prescriber’s specialty | |||||
| Private cardiologist | 30,448 (9.8) | 88,557 (23.0) | 17,244 (25.1) | 51,144 (20.1) | 20,169 (31.8) |
| Hospital practitioner | 150,214 (48.5) | 136,857 (35.5) | 24,673 (36.4) | 86,097 (33.8) | 26,087 (41.2) |
| General practitioner | 118,147 (38.1) | 116,865 (30.3) | 16,242 (23.9) | 88,175 (34.6) | 12,448 (19.7) |
| Other private specialists | 11,178 (3.6) | 43,502 (11.3) | 9675 (14.3) | 29,199 (11.4) | 4628 (7.3) |
| Comorbiditiesb | |||||
| At least one LTD | 222,287 (71.7) | 210,453 (54.5) | 40,453 (59.6) | 128,263 (50.3) | 41,737 (65.9) |
| Severe heart diseasesc | 58,261 (18.8) | 59,276 (15.4) | 13, 016 (19.2) | 31,979 (12.5) | 14,281 (22.5) |
| Coronary heart diseases | 39,515 (12.7) | 33,825 (8.8) | 6507 (9.6) | 19,629 (7.7) | 7689 (12.1) |
| Diabetes | 52,320 (16.9) | 51,059 (13.2) | 9889 (14.6) | 30,829 (12.1) | 10,341 (16.3) |
| Neoplasia | 52,866 (17.0) | 50,790 (13.2) | 9184 (13.5) | 32,191 (12.6) | 9415 (14.9) |
| Psychiatric disorders | 15,541 (5.0) | 14,779 (3.8) | 2202 (3.2) | 10,573 (4.1) | 2004 (3.2) |
DOAC direct oral anticoagulant, SD standard deviation, VKA vitamin K antagonist, LTD long-term disease
aDichotomous variables are expressed as N (%), and continuous variables are expressed as mean (SD)
bComorbidities were defined using information on severe and costly LTDs only (in France, a patient can only obtain LTD status when requested by a physician; the LTD registration allows patients to be fully reimbursed for health expenditures related to this LTD)
cSevere heart diseases: severe heart failure, cardiac arrhythmias, and congenital or valvular heart diseases
Potential Adverse Events
Table 2 summarizes the results of SSA for DOAC and VKA new users, providing, for each definition of each investigated outcome, the number of patients with ‘outcome → OAC’ or ‘OAC → outcome’ sequences and the resulting aSR. cSR and nSR estimates for all outcomes are reported in ESM 2.
Table 2.
Results from sequence symmetry analyses for patients initiating OAC therapy according to the type of OAC, definition of outcome and time-window exposure
| Types of outcomes | Type of OAC | Time windows | |||||
|---|---|---|---|---|---|---|---|
| 3 months | 6 months | 12 months | |||||
| OAC → Outcome/Outcome → OAC | aSR (95% CI)a | OAC → Outcome/Outcome → OAC | aSR (95% CI)a | OAC → Outcome/Outcome → OAC | aSR (95% CI)a | ||
| Control outcomes | |||||||
| Bleeding (hospitalizations) | DOACs | 2446/916 | 2.68 (2.49–2.90) | 3759/1395 | 2.71 (2.55–2.88) | 5657/2095 | 2.71 (2.58–2.85) |
| Dabigatran | 406/158 | 2.26 (1.90–2.74) | 675/228 | 2.31 (2.00–2.70) | 1106/316 | 2.22 (1.97–2.53) | |
| Rivaroxaban | 1741/591 | 2.95 (2.70–3.25) | 2618/894 | 2.93 (2.72–3.17) | 3886/1342 | 2.88 (2.71–3.07) | |
| Apixaban | 299/167 | 2.09 (1.74–2.54) | 466/273 | 2.29 (1.97–2.66) | 665/437 | 2.61 (2.32–2.95) | |
| VKAs | 4131/2629 | 1.54 (1.46–1.61) | 6237/3646 | 1.64 (1.57–1.71) | 9054/4691 | 1.79 (1.73–1.86) | |
| Glaucoma (hospitalizations) | DOACs | 44/45 | 1.01 (0.66–1.53) | 78/77 | 1.05 (0.77–1.44) | 128/139 | 0.95 (0.75–1.21) |
| Dabigatran | 9/11 | 0.75 (0.28–1.83) | 21/15 | 1.15 (0.60–2.38) | 31/20 | 1.03 (0.60–1.91) | |
| Rivaroxaban | 28/21 | 1.36 (0.78–2.51) | 46/42 | 1.13 (0.74–1.73) | 80/83 | 0.99 (0.72–1.34) | |
| Apixaban | 7/13 | 0.64 (0.20–1.51) | 11/20 | 0.76 (0.32–1.51) | 17/36 | 0.83 (0.43–1.43) | |
| VKAs | 26/43 | 0.60 (0.35–0.96) | 59/76 | 0.76 (0.54–1.07) | 100/120 | 0.79 (0.61–1.03) | |
| Glaucoma (medications) | DOACs | 1264/1347 | 0.96 (0.89–1.03) | 2536/2540 | 1.02 (0.97–1.08) | 4701/4772 | 1.01 (0.97–1.05) |
| Dabigatran | 267/269 | 0.90 (0.76–1.07) | 530/440 | 0.97 (0.86–1.11) | 1037/732 | 0.93 (0.84–1.02) | |
| Rivaroxaban | 756/813 | 0.94 (0.86–1.04) | 1575/1580 | 1.02 (0.95–1.09) | 3002/2978 | 1.02 (0.97–1.07) | |
| Apixaban | 241/263 | 1.08 (0.91–1.29) | 430/518 | 1.12 (0.99–1.28) | 661/1060 | 1.08 (0.98–1.19) | |
| VKAs | 1040/1103 | 0.93 (0.85–1.01) | 2081/2168 | 0.93 (0.88–0.99) | 3861/3951 | 0.92 (0.88–0.96) | |
| Major depressive disorders (medications) | DOACs | 4032/3221 | 1.29 (1.23–1.35) | 6882/5648 | 1.28 (1.24–1.33) | 11,112/9501 | 1.26 (1.23–1.30) |
| Dabigatran | 774/553 | 1.27 (1.14–1.42) | 1335/929 | 1.17 (1.08–1.27) | 2306/1382 | 1.13 (1.06–1.21) | |
| Rivaroxaban | 2611/2130 | 1.26 (1.19–1.34) | 4485/3717 | 1.26 (1.21–1.32) | 7318/6361 | 1.23 (1.19–1.27) | |
| Apixaban | 646/538 | 1.44 (1.28–1.61) | 1060/1002 | 1.48 (1.36–1.62) | 1485/1757 | 1.57 (1.47–1.69) | |
| VKAs | 4383/3816 | 1.15 (1.10–1.20) | 7418/6490 | 1.15 (1.11–1.19) | 11,471/10,267 | 1.12 (1.09–1.15) | |
| Renal outcomes (hospitalizations) | |||||||
| Kidney diseases | DOACs | 10,707/8264 | 1.33 (1.29–1.37) | 15,008/11,020 | 1.42 (1.39–1.46) | 20,401/14,783 | 1.48 (1.45–1.51) |
| Dabigatran | 2495/1632 | 1.37 (1.29–1.46) | 3445/2069 | 1.35 (1.28–1.43) | 4837/2563 | 1.29 (1.23–1.35) | |
| Rivaroxaban | 6057/4930 | 1.26 (1.21–1.30) | 8706/6645 | 1.37 (1.32–1.41) | 12,025/8944 | 1.43 (1.39–1.47) | |
| Apixaban | 2153/1698 | 1.51 (1.42–1.61) | 2854/2300 | 1.73 (1.64–1.83) | 3534/3270 | 1.97 (1.88–2.06) | |
| VKAs | 9986/12,698 | 0.78 (0.76–0.80) | 14,209/17,652 | 0.80 (0.79–0.82) | 19,267/23,123 | 0.83 (0.81–0.85) | |
| Acute kidney failure | DOACs | 308/157 | 2.36 (1.96–2.88) | 491/220 | 2.97 (2.54–3.5) | 754/304 | 3.65 (3.21–4.19) |
| Dabigatran | 43/19 | 2.86 (1.74–5.33) | 83/25 | 4.48 (2.99–7.53) | 149/35 | 5.52 (3.96–8.42) | |
| Rivaroxaban | 199/97 | 2.46 (1.94–3.17) | 305/142 | 2.85 (2.35–3.5) | 460/194 | 3.52 (2.99–4.19) | |
| Apixaban | 66/41 | 1.90 (1.30–2.87) | 103/53 | 2.63 (1.92–3.75) | 144/75 | 3.30 (2.52–4.43) | |
| VKAs | 903/1699 | 0.64 (0.59–0.70) | 1324/2183 | 0.82 (0.76–0.88) | 1903/2590 | 1.15 (1.08–1.22) | |
| Glomerular diseases | DOACs | 45/23 | 2.34 (1.46–4.10) | 89/30 | 3.90 (2.66–6.23) | 144/43 | 4.82 (3.52–7.05) |
| Dabigatran | 5/2 | 3.09 | 17/2 | 11.1 | 35/5 | 8.79 (4.26–54.54) | |
| Rivaroxaban | 33/20 | 1.95 (1.15–3.61) | 59/25 | 3.07 (1.99–5.21) | 91/33 | 3.97 (2.74–6.20) | |
| Apixaban | 7/1 | 7.90 | 13/3 | 5.42 | 18/5 | 5.35 (2.36–28.94) | |
| VKAs | 147/594 | 0.30 (0.25–0.36) | 203/696 | 0.40 (0.34–0.46) | 304/784 | 0.62 (0.54–0.70) | |
| Renal tubulo-interstitial diseases | DOACs | 378/554 | 0.79 (0.69–0.89) | 646/768 | 1.03 (0.93–1.14) | 1030/1025 | 1.30 (1.19–1.41) |
| Dabigatran | 65/74 | 0.95 (0.68–1.33) | 110/100 | 1.13 (0.86–1.49) | 191/131 | 1.28 (1.03–1.61) | |
| Rivaroxaban | 254/376 | 0.76 (0.65–0.89) | 429/530 | 0.96 (0.85–1.09) | 685/693 | 1.23 (1.11–1.37) | |
| Apixaban | 59/103 | 0.68 (0.48–0.92) | 107/137 | 1.06 (0.82–1.36) | 154/200 | 1.34 (1.09–1.66) | |
| VKAs | 811/1541 | 0.61 (0.56–0.66) | 1352/1982 | 0.84 (0.79–0.90) | 2093/2471 | 1.13 (1.07–1.20) | |
| Hepatic outcomes (hospitalizations) | |||||||
| Toxic liver disease | DOACs | 27/11 | 2.48 (1.32–5.94) | 37/23 | 1.60 (0.97–2.83) | 51/33 | 1.52 (0.99–2.42) |
| Dabigatran | 3/3 | 0.89 (0.10–7.97) | 7/3 | 1.81 (0.55–47.8) | 8/3 | 1.65 (0.54–64.45) | |
| Rivaroxaban | 17/7 | 2.44 (1.12–8.15) | 21/18 | 1.16 (0.61–2.26) | 33/25 | 1.28 (0.77–2.23) | |
| Apixaban | 7/1 | 8.24 | 9/2 | 6.00 | 10/5 | 3.39 (1.27–16.17) | |
| VKAs | 56/63 | 0.87 (0.60–1.25) | 81/93 | 0.83 (0.61–1.11) | 112/115 | 0.88 (0.68–1.14) | |
| Acute liver injury | DOACs | 70/26 | 2.71 (1.79–4.52) | 90/42 | 2.14 (1.51–3.18) | 133/53 | 2.47 (1.83–3.49) |
| Dabigatran | 9/5 | 1.59 (0.57–7.46) | 17/6 | 2.21 (0.99–8.78) | 24/6 | 2.49 (1.19–10.32) | |
| Rivaroxaban | 46/17 | 2.71 (1.64–5.26) | 54/29 | 1.85 (1.20–3.03) | 84/38 | 2.15 (1.50–3.27) | |
| Apixaban | 15/4 | 4.38 (1.80–41.76) | 19/7 | 3.61 (1.70–12.15) | 25/9 | 4.69 (2.40–12.81) | |
| VKAs | 129/150 | 0.84 (0.66–1.06) | 199/209 | 0.90 (0.74–1.10) | 308/268 | 1.04 (0.88–1.23) | |
| Disorders of the gallbladder and biliary tract | DOACs | 563/805 | 0.71 (0.63–0.79) | 1033/1171 | 0.89 (0.82–0.97) | 1698/1657 | 1.03 (0.96–1.10) |
| Dabigatran | 104/142 | 0.65 (0.50–0.83) | 175/198 | 0.69 (0.57–0.85) | 314/248 | 0.80 (0.68–0.95) | |
| Rivaroxaban | 364/513 | 0.72 (0.62–0.82) | 684/746 | 0.92 (0.83–1.02) | 1147/1061 | 1.08 (0.99–1.17) | |
| Apixaban | 95/150 | 0.74 (0.57–0.96) | 174/226 | 1.04 (0.85–1.26) | 237/347 | 1.18 (1.00–1.39) | |
| VKAs | 634/1461 | 0.43 (0.39–0.47) | 1111/1921 | 0.56 (0.52–0.60) | 1861/2462 | 0.70 (0.66–0.74) | |
| Skin outcomes (hospitalizations) | |||||||
| Skin diseases | DOACs | 405/387 | 1.05 (0.91–1.21) | 649/590 | 1.10 (0.98–1.23) | 1018/852 | 1.20 (1.09–1.31) |
| Dabigatran | 78/56 | 1.22 (0.87–1.74) | 127/87 | 1.12 (0.86–1.49) | 196/115 | 1.07 (0.86–1.36) | |
| Rivaroxaban | 267/276 | 0.97 (0.82–1.15) | 430/420 | 1.02 (0.89–1.17) | 690/601 | 1.14 (1.02–1.27) | |
| Apixaban | 60/54 | 1.30 (0.90–1.89) | 92/82 | 1.50 (1.12–2.03) | 132/135 | 1.68 (1.32–2.14) | |
| VKAs | 568/882 | 0.63 (0.56–0.70) | 879/1257 | 0.67 (0.61–0.73) | 1347/1636 | 0.76 (0.71–0.82) | |
| Vasculitis (composite) | DOACs | 91/84 | 1.10 (0.81–1.48) | 173/160 | 1.08 (0.87–1.34) | 299/258 | 1.14 (0.96–1.34) |
| Dabigatran | 23/18 | 1.14 (0.62–2.22) | 40/26 | 1.22 (0.75–2.07) | 63/36 | 1.10 (0.74–1.71) | |
| Rivaroxaban | 50/46 | 1.10 (0.73–1.65) | 102/99 | 1.03 (0.78–1.36) | 191/164 | 1.13 (0.92–1.39) | |
| Apixaban | 17/20 | 1.00 (0.50–1.92) | 30/35 | 1.13 (0.68–1.85) | 44/58 | 1.25 (0.83–1.85) | |
| VKAs | 98/171 | 0.56 (0.43–0.71) | 171/258 | 0.63 (0.52–0.76) | 307/363 | 0.76 (0.65–0.89) | |
| Vasculitis and unspecified arteritis | DOACs | 8/9 | 0.92 (0.31–2.51) | 14/16 | 0.92 (0.42–1.90) | 21/20 | 1.12 (0.60–2.12) |
| Dabigatran | 1/0 | – | 2/0 | 5/0 | |||
| Rivaroxaban | 5/7 | 0.74 (0.16–2.36) | 10/13 | 0.80 (0.32–1.84) | 13/16 | 0.86 (0.39–1.80) | |
| Apixaban | 2/2 | 1.2 (0.01–118.39) | 2/3 | 0.92 | 3/4 | 1.40 (0.12–7.23) | |
| VKAs | 18/33 | 0.55 (0.29–0.94) | 25/46 | 0.54 (0.32–0.86) | 34/56 | 0.61 (0.38–0.91) | |
| Urticaria and erythema | DOACs | 54/45 | 1.23 (0.83–1.84) | 89/62 | 1.48 (1.08–2.08) | 134/88 | 1.60 (1.23–2.12) |
| Dabigatran | 9/9 | 0.89 (0.33–2.42) | 21/15 | 1.12 (0.59–2.33) | 32/17 | 1.26 (0.73–2.47) | |
| Rivaroxaban | 35/30 | 1.19 (0.73–1.98) | 55/40 | 1.42 (0.95–2.17) | 82/59 | 1.45 (1.05–2.06) | |
| Apixaban | 10/6 | 1.98 (0.75–7.44) | 13/7 | 2.55 (1.08–8.35) | 20/12 | 2.97 (1.50–6.82) | |
| VKAs | 55/109 | 0.5 (0.35–0.68) | 79/143 | 0.54 (0.41–0.71) | 116/174 | 0.65 (0.51–0.82) | |
| Allergic purpura | DOACs | 14/10 | 1.46 (0.66–3.71) | 20/12 | 1.78 (0.90–4.09) | 34/16 | 2.33 (1.35–4.66) |
| Dabigatran | 5/3 | 1.52 (0.37–22.15) | 6/3 | 1.65 (0.46–31.63) | 8/5 | 1.09 (0.37–4.99) | |
| Rivaroxaban | 6/7 | 0.89 (0.24–2.84) | 11/9 | 1.30 (0.53–3.52) | 21/11 | 2.07 (1.05–4.97) | |
| Apixaban | 3/0 | – | 3/0 | – | 5/0 | – | |
| VKAs | 14/22 | 0.64 (0.30–1.23) | 27/32 | 0.86 (0.50–1.43) | 43/36 | 1.21 (0.78–1.91) | |
| Gastrointestinal outcomes (medications) | |||||||
| Composite | DOACs | 4131/5332 | 0.88 (0.85–0.92) | 6585/8950 | 0.92 (0.89–0.95) | 10,025/14,891 | 0.95 (0.92–0.97) |
| Dabigatran | 917/961 | 1.00 (0.91–1.09) | 1468/1568 | 0.95 (0.88–1.02) | 2272/2321 | 0.89 (0.84–0.95) | |
| Rivaroxaban | 2652/3669 | 0.82 (0.78–0.86) | 4275/6129 | 0.86 (0.83–0.90) | 6598/10,279 | 0.90 (0.87–0.93) | |
| Apixaban | 561/701 | 1.03 (0.92–1.15) | 840/1252 | 1.07 (0.98–1.17) | 1152/2288 | 1.18 (1.10–1.26) | |
| VKAs | 3152/5647 | 0.62 (0.59–0.64) | 4869/8831 | 0.65 (0.63–0.67) | 7286/13,539 | 0.69 (0.67–0.71) | |
| Composite without drugs for acid-related disorders | DOACs | 7119/6641 | 1.21 (1.17–1.25) | 11,556/11,460 | 1.23 (1.20–1.26) | 18,302/19,462 | 1.26 (1.24–1.29) |
| Dabigatran | 1436/1265 | 1.18 (1.1–1.28) | 2368/2071 | 1.14 (1.08–1.21) | 3987/3136 | 1.12 (1.07–1.17) | |
| Rivaroxaban | 4733/4365 | 1.22 (1.17–1.27) | 7673/7617 | 1.22 (1.18–1.26) | 12,153/13,064 | 1.24 (1.21–1.27) | |
| Apixaban | 949/1009 | 1.19 (1.09–1.3) | 1513/1769 | 1.33 (1.24–1.43) | 2158/3258 | 1.47 (1.39–1.55) | |
| VKAs | 6836/7380 | 1.02 (0.98–1.05) | 10,640/11,950 | 1.04 (1.01–1.06) | 15,955/19,087 | 1.05 (1.02–1.07) | |
| Antiemetic drugs | DOACs | 4947/4831 | 1.17 (1.12–1.22) | 8045/8304 | 1.20 (1.16–1.24) | 12,998/14,082 | 1.25 (1.22–1.28) |
| Dabigatran | 1002/913 | 1.19 (1.09–1.3) | 1618/1516 | 1.12 (1.05–1.20) | 2760/2270 | 1.11 (1.05–1.17) | |
| Rivaroxaban | 3293/3226 | 1.16 (1.1–1.21) | 5396/5599 | 1.18 (1.14–1.23) | 8770/9598 | 1.23 (1.20–1.27) | |
| Apixaban | 649/692 | 1.17 (1.05–1.3) | 1028/1187 | 1.33 (1.22–1.44) | 1464/2211 | 1.45 (1.36–1.55) | |
| VKAs | 5072/5738 | 0.98 (0.95–1.02) | 8016/9484 | 1.00 (0.97–1.03) | 12,500/15,199 | 1.05 (1.03–1.08) | |
| Drugs for constipation | DOACs | 8674/6947 | 1.31 (1.27–1.36) | 14,177/12,169 | 1.27 (1.23–1.3) | 22,592/20,520 | 1.25 (1.22–1.27) |
| Dabigatran | 1620/1190 | 1.27 (1.18–1.37) | 2749/1964 | 1.20 (1.13–1.27) | 4703/3009 | 1.13 (1.08–1.19) | |
| Rivaroxaban | 5791/4661 | 1.30 (1.25–1.36) | 9416/8183 | 1.24 (1.21–1.28) | 15,051/13,846 | 1.22 (1.20–1.25) | |
| Apixaban | 1260/1093 | 1.39 (1.29–1.51) | 2009/2019 | 1.42 (1.34–1.51) | 2833/3661 | 1.48 (1.41–1.56) | |
| VKAs | 8988/7999 | 1.15 (1.11–1.18) | 14,185/13,261 | 1.10 (1.08–1.13) | 21,625/21,093 | 1.07 (1.05–1.09) | |
aSR adjusted sequence ratio, CI confidence interval, OAC oral anticoagulant, DOAC direct oral anticoagulant, VKA vitamin K antagonist
aConfidence intervals are not given in the case of very small numbers of pairs
Valid and significant positive associations with DOAC therapy were observed in three of the four investigated outcome groups, i.e. in the renal, hepatic and gastrointestinal outcomes groups.
Hospitalizations for kidney diseases, acute kidney failure and glomerular diseases were more likely to occur after DOAC initiation than before (aSR3 = 2.36, 95% CI 1.96–2.88, for acute kidney failure). However, this significant positive association contrasted with the strong inverse association observed for VKA therapy (aSR3 = 0.64, 95% CI 0.59–0.70, for acute kidney failure).
Significant positive association between DOAC therapy and toxic liver disease was found with the 3-month time window (aSR3 = 2.48, 95% CI 1.32–5.94) and with acute liver injury irrespective of the time window considered (aSR3 = 2.71, 95% CI 1.79–4.52). On the contrary, no association was observed between VKA therapy initiation and these two hepatic outcomes (aSR3 = 0.87, 95% CI 0.60–1.25, for toxic liver disease; and aSR3 = 0.84, 95% CI 0.66–1.06, for acute liver injury). These temporal asymmetries were only found among rivaroxaban and apixaban new users.
After excluding drugs for acid-related disorders, such as proton pump inhibitors, the initiation of drugs for functional gastrointestinal disorders, such as antiemetic drugs or drugs for constipation, was significantly more likely after, rather than before, initiation of DOAC therapy. Overall, an almost 25% increased risk following DOAC initiation was consistently found for the three time windows. No asymmetry, or only slight asymmetry, was observed between VKA therapy and the initiation of these drugs.
A total of 168,807 DOAC and 122,925 VKA AF new users were eligible for inclusion in the subgroup analysis of patients with AF. Consistent results were obtained when analysis was restricted to AF patients with regard to the significant positive associations found in patients with no restriction of indication. However, the initiation of antiemetic drugs or drugs for constipation was also found to be significantly more likely after, rather than before, initiation of VKA therapy in AF patients (see ESM 3).
Control Outcomes
Results of SSA for the control outcomes are also reported in Table 2. Hospitalization for bleeding was consistently nearly threefold more likely to occur after DOAC initiation than before [aSR for the 3-month time window (aSR3) = 2.68, 95% CI 2.49–2.90] over the three time windows used. The same applies to VKA therapy, albeit to a lesser extent (aSR3 = 1.54, 95% CI 1.46–1.61).
No significant positive association was observed between DOACs and the initiation of antiglaucoma preparations, or with hospitalization for glaucoma (aSR3 = 0.96, 95% CI 0.89–1.03; and 1.01, 95% CI 0.66–1.53, respectively), while inverse associations were observed for VKA new users with hospitalization for glaucoma for the 3-month time window only (aSR3 = 0.6, 95% CI 0.35–0.96), and, to a lesser extent, with initiation of antiglaucoma preparations for the 6- and 12-month time windows (aSR6 = 0.93, 95% CI 0.88–0.99; and aSR12 = 0.92, 95% CI 0.88–0.96, respectively).
A significant positive association was observed between either DOAC or VKA therapy initiation and antidepressant initiation, irrespective of the type of OAC or the time window considered.
Discussion
Utilization of Sequence Symmetry Analysis on the French Healthcare Database for Monitoring Direct Oral Anticoagulant Adverse Events
This analysis, based on reimbursement data for approximately 390,000 DOAC new users and 310,000 VKA new users in 2013–2015, showed an association between OAC therapies and the well-known and specific increased bleeding risk, as well as an association between these cardiovascular drugs and depression, in line with the initial paper describing this method [26]. This suggests that this method can be used to monitor OAC adverse events in the French healthcare databases. However, if no association was observed between DOAC initiation and hospitalization for glaucoma, a negative control outcome, VKA therapy was associated with a tendency for reduction of hospitalization for glaucoma, and this association was significant when considering the 3-month time window (40% decreased risk of hospitalization for glaucoma). A weaker association was also observed between VKA therapy and bleeding compared with DOAC therapy. These unexpected results may reflect inverse causation as bleeding events may have altered the probability of OAC exposure in opposite directions for DOACs versus VKAs, which can be explained by channeling. Channeling bias occurs when drug therapies with similar therapeutic indications are preferentially prescribed to groups of patients with different baseline prognoses [35, 36]. Numerous studies, including studies based on French data [37], have indeed described the overall channeling of DOAC therapy over VKA therapy toward a younger and healthier population, with VKAs becoming the preferred OAC therapy for patients with a higher risk of stroke and bleeding [38–40]. Consequently, patients with a history of bleeding are more likely to receive VKAs than DOACs, increasing the number of sequences of ‘bleeding → OAC’ initiation among VKA new users compared with DOAC new users. In addition, as VKA patients were older and had more severe disease than DOAC new users, and were therefore more likely to be hospitalized than DOAC new users, results from the VKA cohort may be more sensitive to detection bias [32]. These biases are particularly likely for hospitalized renal outcomes; according to their respective summary of product characteristics, DOAC dose reduction may be necessary in patients with moderate-to-severe renal impairment. As the extent of anticoagulation by DOAC therapy cannot be monitored, it is likely that this factor led prescribers to preferentially prescribe a VKA to patients with a recent history of renal outcomes rather than a DOAC, as reported elsewhere [41]. This selection in clinical practice, combined with the use of hospitalization data to define renal outcomes, may explain the strong spurious protective association observed in VKA new users, and, reciprocally, the strong and positive association observed with DOAC therapy. However, a risk of kidney injury, albeit low, cannot be excluded with DOAC therapy [42–44].
Potential Adverse Events: Main Findings and Comparison with Postmarketing Literature
This study suggests that DOAC therapy is associated with rare but severe toxic liver diseases and more frequent non-bleeding gastrointestinal disorders. Channeling bias for OAC prescribing has been shown to be mostly related to the stroke and bleeding risks of patients. It is also unlikely that other sources of bias, particularly detection bias, could fully explain these associations. All DOACs undergo varying degrees of hepatic metabolism, with much lower hepatic metabolism for dabigatran [45]. Patients with acute liver disease were excluded from the landmark RCTs, but transient elevation of transaminase enzymes was reported to be uncommon during these trials [9–11]. A meta-analysis of RCT data concluded that DOACs were not associated with an increased risk of drug-induced liver injury (DILI) [46]. Data on the risk of DILI with DOACs are mostly derived from postmarketing experience, including case reports/case series [17, 47–49] and a pharmacovigilance study [50]. In a recent cohort study on the MarketScan Commercial and Medicare databases, dabigatran was associated with the lowest risk of liver injury compared with rivaroxaban and apixaban new users [51]. The results of this study are therefore consistent with the postmarketing literature [50, 52], which suggests that DOACs, especially rivaroxaban, can cause DILI. Overall, our results and the published literature advocate the monitoring of patients’ liver function in the first months following DOAC initiation, especially in the presence of pre-existing liver conditions or concomitant intake of hepatotoxic agents.
DOAC therapy was associated with the initiation of drugs for functional gastrointestinal disorders when drugs for acid-related disorders were excluded from the outcome definition. To date, non-bleeding gastrointestinal disorders have mostly been reported to be uncommon, especially with dabigatran use [22, 53]; however, dyspepsia and esophagitis are now increasingly recognized complications of dabigatran use [54]. It has been suggested that the tartaric acid component of dabigatran hard capsules is responsible for increased mucosal irritation; however, the report of a case of esophagitis dissecans superficialis with rivaroxaban that does not contain this excipient suggests a mechanism that might be common to all DOAC drugs [55]. Moreover, a recent review on the use of rivaroxaban for venous thromboembolism prophylaxis after total hip or knee replacement surgery found that pyrexia, vomiting, nausea and constipation were the most commonly reported treatment-emergent non-bleeding adverse events in rivaroxaban-treated patients [56]. Frequent gastrointestinal adverse effects should not be overlooked as they can be associated with considerable impairment of the patient’s quality of life, and may also lead to discontinuation of DOAC therapy, as previously described [23, 57–59], with the corresponding risk of subsequent thromboembolism. However, the signals observed for gastrointestinal outcomes in this SSA may also correspond to non-specific symptoms of various diseases. Moreover, the prevalent population taking OAC therapy corresponds to polymedicated elderly patients, and is therefore particularly vulnerable to nausea or constipation. Further field studies and registry research are therefore needed to investigate the prevalence and impact of this type of adverse event in DOAC-treated patients.
No valid and significant positive associations were observed for skin outcomes, despite cases having been reported for dabigatran-treated patients [21]. However, due to the lack of specific drug treatments, these rare adverse events could only be studied using hospitalization data, therefore limiting their identification, and claims data may not be relevant to properly study such outcomes.
Finally, a recent SSA performed in AF patients initiating dabigatran, rivaroxaban, or apixaban between 2011 and 2015 using the Danish registries found similar results in terms of potential adverse events associated with DOAC therapy for gastrointestinal symptoms, including nausea, vomiting or constipation, and acute renal failure [60].
Strengths and Limitations
In France, National Health Insurance covers the entire population and most people also subscribe to a private complementary health insurance plan. Selection bias related to the access of patients to more expensive DOAC therapy is therefore not an issue when using the French healthcare databases [31, 61]. In addition to being based on large nationwide healthcare databases, a major strength of this study is that SSA is able to overcome some of the pitfalls that can threaten the validity of other observational designs. The SSA design inherently controls for time-constant, patient-specific measured and unmeasured confounders, including sociodemographic characteristics, comorbidities, genetic and environmental factors, and healthcare-related behaviors [32]. The aSRs were corrected for time trends in the occurrence of exposure and events. For instance, for apixaban, the nSR estimates were smaller than 1, and this deviation grew with increasing length of the time window. In particular, this reflects the trend in apixaban prescribing because, in France, the indication of apixaban was extended to stroke prevention in January 2014, with a corresponding sharp increase in apixaban prescription during the period 2014 and 2015 of the inclusion period. However, for all DOACs, the nSR was close to 1 for most outcome definitions.
The definitions of the investigated outcomes were not strictly validated in our databases. However, recent studies assessing the quality of PMSI data, including ICD-10 codes, to identify healthcare outcomes have shown these data to be reliable for epidemiological purposes [62, 63].
Positive and negative control outcomes, and the use of the cohort of VKA new users as an active comparator, were used to guide interpretation of the results observed with DOACs in an attempt to identify results that could have been affected by bias, which is clearly a strength of this study. Replicating the analysis in VKA new users actually showed that the results of SSA in DOAC new users may be prone to channeling bias. This risk of channeling bias should particularly affect analyses on outcomes related to reasons for channeling, namely when prior knowledge on adverse events (for instance, bleeding) and their related risk factors (for instance, renal impairment) can affect the probability of OAC exposure in opposite ways between VKAs and DOACs. Results from hepatic, skin and non-hospitalized gastrointestinal outcomes may therefore be less sensitive to this type of bias. As illustrated by bleeding outcome, SSA estimates of VKAs could be biased toward lower values, and estimates of DOACs could be biased toward higher values. Although restricting analyses to more comparable patients, i.e. AF patients, providing consistent results in terms of the positive associations observed in the whole population, SSA remains an initial and easy-to-implement method of detecting adverse events by using claims data. More studies using other designs, either avoiding or less sensitive to channeling, are therefore needed to test the associations observed in this study. Analyses in which outcomes are defined using hospitalization data might be more prone to detection bias, as suggested by the weaker inverse association observed between VKA therapy and the initiation of antiglaucoma medications, compared with hospitalization for glaucoma.
To minimize time-varying confounding and detection bias that may affect the SSA design [27, 32], we restricted the time window to a maximum of 12 months, and did not consider the significant positive associations observed when the length of the time window was extended to be valid, such as certain results observed for kidney or skin outcomes groups. Consequently, we were unable to capture adverse events that only occur after the first year of treatment; however, this 1 year-period may ensure an acceptable sensitivity and positive predictive value [32]. In addition, due to the high discontinuation and switch rates observed in OAC patients [64], longer time windows may not have been relevant.
Conclusions
Based on nationwide health data for DOAC new users, results from this SSA highlight the importance of non-bleeding adverse events in OAC patients, and suggest that DOACs are associated with rare but severe liver injury and more frequent gastrointestinal disorders. A low risk of kidney injury associated with DOAC therapy can also not be excluded. Pending further field studies to confirm these results, patients initiating DOAC therapy should be informed and carefully monitored regarding the risk of hepatic and gastrointestinal adverse events.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Dr. Saul, medical translator, for assistance in writing the manuscript, and Dr. Emilie Sbidian, Department of Dermatology, Assistance Publique-Hôpitaux de Paris (APHP), for her contribution and expertise to defining skin outcomes on the French Healthcare databases.
Compliance with Ethical Standards
Funding
The authors received no funding.
Conflicts of interest
Géric Maura, Cécile Billionnet, Alain Weill and Anke Neumann are employees of the French National Health Insurance (Cnam); Joël Coste is employed by the French National Health Insurance (CNAM); Antoine Pariente belongs to the French National Institute of Health and Medical Research (Inserm). None of the authors have any conflicts of interest that are directly relevant to the content of this study.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s40264-018-0668-9) contains supplementary material, which is available to authorized users.
References
- 1.Desai NR, Krumme AA, Schneeweiss S, Shrank WH, Brill G, Pezalla EJ, et al. Patterns of initiation of oral anticoagulants in patients with atrial fibrillation: quality and cost implications. Am J Med. 2014;127(11):1075.e1–1082.e1. doi: 10.1016/j.amjmed.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 2.Lippi G, Mattiuzzi C, Cervellin G, Favaloro EJ. Direct oral anticoagulants: analysis of worldwide use and popularity using Google Trends. Ann Transl Med. 2017;5(16):322. doi: 10.21037/atm.2017.06.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirley K, Qato DM, Kornfield R, Stafford RS, Alexander GC. National trends in oral anticoagulant use in the United States, 2007 to 2011. Circ Cardiovasc Qual Outc. 2012;5(5):615–621. doi: 10.1161/CIRCOUTCOMES.112.967299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnes GD, Lucas E, Alexander GC, Goldberger ZD. National Trends in Ambulatory Oral Anticoagulant Use. Am J Med. 2015;128(12):1300.e2–1305.e2. doi: 10.1016/j.amjmed.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verheugt FWA, Granger CB. Oral anticoagulants for stroke prevention in atrial fibrillation: current status, special situations, and unmet needs. Lancet. 2015;386(9990):303–310. doi: 10.1016/S0140-6736(15)60245-8. [DOI] [PubMed] [Google Scholar]
- 6.Steinberg BA, Piccini JP. Anticoagulation in atrial fibrillation. BMJ. 2014;348:g2116. doi: 10.1136/bmj.g2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 8.Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, et al. Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation. 2014;129(8):837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus Warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 10.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus Warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 11.Granger CB, Alexander JH, McMurray JJV, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus Warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 12.The EINSTEIN-PE Investigators Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366(14):1287–1297. doi: 10.1056/NEJMoa1113572. [DOI] [PubMed] [Google Scholar]
- 13.Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369(9):799–808. doi: 10.1056/NEJMoa1302507. [DOI] [PubMed] [Google Scholar]
- 14.Schulman S, Kearon C, Kakkar AK, Mismetti P, Schellong S, Eriksson H, et al. Dabigatran versus Warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361(24):2342–2352. doi: 10.1056/NEJMoa0906598. [DOI] [PubMed] [Google Scholar]
- 15.Potpara TS, Lip GYH. Postapproval observational studies of non-vitamin K antagonist oral anticoagulants in atrial fibrillation. JAMA. 2017;317(11):1115. doi: 10.1001/jama.2017.1152. [DOI] [PubMed] [Google Scholar]
- 16.Raschi E, Bianchin M, Ageno W, De Ponti R, De Ponti F. Risk-benefit profile of direct-acting oral anticoagulants in established therapeutic indications: an overview of systematic reviews and observational studies. Drug Saf. 2016;39(12):1175–1187. doi: 10.1007/s40264-016-0464-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raschi E, Bianchin M, Ageno W, De Ponti R, De Ponti F. Adverse events associated with the use of direct-acting oral anticoagulants in clinical practice: beyond bleeding complications. Pol Arch Intern Med. 2016;126(7–8):552–561. doi: 10.20452/pamw.3529. [DOI] [PubMed] [Google Scholar]
- 18.VigiAccess. http://www.vigiaccess.org/. Accessed 31 Oct 2017.
- 19.Potolidis E, Mandros C, Kotsa K, Mitsiou E, Potolidis D, Fanourgiakis P. Dabigatran associated leukocytoclastic vasculitis. Case Rep Med. 2015;2015:1–2. doi: 10.1155/2015/616109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.An J, Garje R, Wanat K, Leone JP. Dabigatran-related leukocytoclastic vasculitis. BMJ Case Rep. 2017. pii:bcr2016217423. [DOI] [PMC free article] [PubMed]
- 21.Cakmak MA, Sahin S, Cinar N, Karsidag S. Adverse skin reaction caused by dabigatran. Eur Rev Med Pharmacol Sci. 2014;18(18):2595. [PubMed] [Google Scholar]
- 22.Hoffman A, Galle PR. Gastrointestinal disorders and dabigatran. Scand J Gastroenterol. 2013;48(1):9–16. doi: 10.3109/00365521.2012.706825. [DOI] [PubMed] [Google Scholar]
- 23.Beyer-Westendorf J, Ehlken B, Evers T. Real-world persistence and adherence to oral anticoagulation for stroke risk reduction in patients with atrial fibrillation. Europace. 2016;18(8):1150–1157. doi: 10.1093/europace/euv421. [DOI] [PubMed] [Google Scholar]
- 24.Hecker J, Marten S, Keller L, Helmert S, Michalski F, Werth S, et al. Effectiveness and safety of rivaroxaban therapy in daily-care patients with atrial fibrillation: results from the Dresden NOAC Registry. Thromb Haemost. 2016;115(5):939–949. doi: 10.1160/TH15-10-0840. [DOI] [PubMed] [Google Scholar]
- 25.Bezin J, Duong M, Lassalle R, Droz C, Pariente A, Blin P, et al. The national healthcare system claims databases in France, SNIIRAM and EGB: powerful tools for pharmacoepidemiology. Pharmacoepidemiol Drug Saf. 2017;26(8):954–962. doi: 10.1002/pds.4233. [DOI] [PubMed] [Google Scholar]
- 26.Hallas J. Evidence of depression provoked by cardiovascular medication: a prescription sequence symmetry analysis. Epidemiol Camb Mass. 1996;7(5):478–484. doi: 10.1097/00001648-199609000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Tsiropoulos I, Andersen M, Hallas J. Adverse events with use of antiepileptic drugs: a prescription and event symmetry analysis. Pharmacoepidemiol Drug Saf. 2009;18(6):483–491. doi: 10.1002/pds.1736. [DOI] [PubMed] [Google Scholar]
- 28.Wahab IA, Pratt NL, Wiese MD, Kalisch LM, Roughead EE. The validity of sequence symmetry analysis (SSA) for adverse drug reaction signal detection. Pharmacoepidemiol Drug Saf. 2013;22(5):496–502. doi: 10.1002/pds.3417. [DOI] [PubMed] [Google Scholar]
- 29.Pratt N, Chan EW, Choi N-K, Kimura M, Kimura T, Kubota K, et al. Prescription sequence symmetry analysis: assessing risk, temporality, and consistency for adverse drug reactions across datasets in five countries. Pharmacoepidemiol Drug Saf. 2015;24(8):858–864. doi: 10.1002/pds.3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takeuchi Y, Shinozaki T, Matsuyama Y. A comparison of estimators from self-controlled case series, case-crossover design, and sequence symmetry analysis for pharmacoepidemiological studies. BMC Med Res Methodol. 2018;18(1):4. doi: 10.1186/s12874-017-0457-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tuppin P, Rudant J, Constantinou P, Gastaldi-Ménager C, Rachas A, de Roquefeuil L, et al. Value of a national administrative database to guide public decisions: from the système national d’information interrégimes de l’Assurance Maladie (SNIIRAM) to the système national des données de santé (SNDS) in France. Rev Epidemiol Sante Publique. 2017;65(Suppl 4):S149–S167. doi: 10.1016/j.respe.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Lai ECC, Pratt N, Hsieh CY, Lin SJ, Pottegård A, Roughead EE, et al. Sequence symmetry analysis in pharmacovigilance and pharmacoepidemiologic studies. Eur J Epidemiol. 2017;32(7):567–582. doi: 10.1007/s10654-017-0281-8. [DOI] [PubMed] [Google Scholar]
- 33.Morris JA, Gardner MJ. Calculating confidence intervals for relative risks (odds ratios) and standardised ratios and rates. Br Med J Clin Res Ed. 1988;296(6632):1313–1316. doi: 10.1136/bmj.296.6632.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thacker EL, Schneeweiss S. Initiation of acetylcholinesterase inhibitors and complications of chronic airways disorders in elderly patients. Drug Saf. 2006;29(11):1077–1085. doi: 10.2165/00002018-200629110-00007. [DOI] [PubMed] [Google Scholar]
- 35.Petri H, Urquhart J. Channeling bias in the interpretation of drug effects. Stat Med. 1991;10(4):577–581. doi: 10.1002/sim.4780100409. [DOI] [PubMed] [Google Scholar]
- 36.Lobo FS, Wagner S, Gross CR, Schommer JC. Addressing the issue of channeling bias in observational studies with propensity scores analysis. Res Soc Adm Pharm. 2006;2(1):143–151. doi: 10.1016/j.sapharm.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 37.Maura G, Blotière P-O, Bouillon K, Billionnet C, Ricordeau P, Alla F, et al. Comparison of the short-term risk of bleeding and arterial thromboembolic events in nonvalvular atrial fibrillation patients newly treated with Dabigatran or Rivaroxaban versus vitamin K antagonists: a French Nationwide Propensity-Matched Cohort Study. Circulation. 2015;132(13):1252–1260. doi: 10.1161/CIRCULATIONAHA.115.015710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steinberg BA, Gao H, Shrader P, Pieper K, Thomas L, Camm AJ, et al. International trends in clinical characteristics and oral anticoagulation treatment for patients with atrial fibrillation: results from the GARFIELD-AF, ORBIT-AF I, and ORBIT-AF II registries. Am Heart J. 2017;194:132–140. doi: 10.1016/j.ahj.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 39.Olesen JB, Sorensen R, Hansen ML, Lamberts M, Weeke P, Mikkelsen AP, et al. Non-vitamin K antagonist oral anticoagulation agents in anticoagulant naive atrial fibrillation patients: Danish nationwide descriptive data 2011–2013. Europace. 2015;17(2):187–193. doi: 10.1093/europace/euu225. [DOI] [PubMed] [Google Scholar]
- 40.Lauffenburger JC, Farley JF, Gehi AK, Rhoney DH, Brookhart MA, Fang G. Factors driving anticoagulant selection in patients with atrial fibrillation in the United States. Am J Cardiol. 2015;115(8):1095–1101. doi: 10.1016/j.amjcard.2015.01.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Douros A, Renoux C, Coulombe J, Suissa S. Patterns of long-term use of non-vitamin K antagonist oral anticoagulants for non-valvular atrial fibrillation: Quebec observational study. Pharmacoepidemiol Drug Saf. 2017;26(12):1546–1554. doi: 10.1002/pds.4333. [DOI] [PubMed] [Google Scholar]
- 42.Abdulhadi B, Mulki R, Goyal A, Rangaswami J. Novel oral anticoagulant and kidney injury: apixaban-related acute interstitial nephritis. BMJ Case Rep. 2017. pii:bcr-2017-221641. [DOI] [PMC free article] [PubMed]
- 43.Kalaitzidis RG, Duni A, Liapis G, Balafa O, Xiromeriti S, Rapsomanikis PK, et al. Anticoagulant-related nephropathy: a case report and review of the literature of an increasingly recognized entity. Int Urol Nephrol. 2017;49(8):1401–1407. doi: 10.1007/s11255-017-1527-9. [DOI] [PubMed] [Google Scholar]
- 44.Caldeira D, Gonçalves N, Pinto FJ, Costa J, Ferreira JJ. Risk of renal failure with the non-vitamin K antagonist oral anticoagulants: systematic review and meta-analysis. Pharmacoepidemiol Drug Saf. 2015;24(7):757–764. doi: 10.1002/pds.3791. [DOI] [PubMed] [Google Scholar]
- 45.Heidbuchel H, Verhamme P, Alings M, Antz M, Diener H-C, Hacke W, et al. Updated European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace. 2015;17(10):1467–1507. doi: 10.1093/europace/euv309. [DOI] [PubMed] [Google Scholar]
- 46.Caldeira D, Barra M, Santos AT, de Abreu D, Pinto FJ, Ferreira JJ, et al. Risk of drug-induced liver injury with the new oral anticoagulants: systematic review and meta-analysis. Heart. 2014;100(7):550–556. doi: 10.1136/heartjnl-2013-305288. [DOI] [PubMed] [Google Scholar]
- 47.Cordeanu M, Lambert A, Gaertner S, Nouri S, Mirea C, Alt-Tebacher M, et al. Apixaban-induced hepatotoxicity. Int J Cardiol. 2016;204:4–5. doi: 10.1016/j.ijcard.2015.11.147. [DOI] [PubMed] [Google Scholar]
- 48.Rochwerg B, Xenodemetropoulos T, Crowther M, Spyropoulos A. Dabigatran-induced acute hepatitis. Clin Appl Thromb. 2012;18(5):549–550. doi: 10.1177/1076029611435840. [DOI] [PubMed] [Google Scholar]
- 49.Liakoni E, Rätz Bravo AE, Terracciano L, Heim M, Krähenbühl S. Symptomatic hepatocellular liver injury with hyperbilirubinemia in two patients treated with Rivaroxaban. JAMA Intern Med. 2014;174(10):1683. doi: 10.1001/jamainternmed.2014.3912. [DOI] [PubMed] [Google Scholar]
- 50.Raschi E, Poluzzi E, Koci A, Salvo F, Pariente A, Biselli M, et al. Liver injury with novel oral anticoagulants: assessing post-marketing reports in the US Food and Drug Administration adverse event reporting system: liver injury and novel oral anticoagulants. Br J Clin Pharmacol. 2015;80(2):285–293. doi: 10.1111/bcp.12611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alonso A, MacLehose RF, Chen LY, Bengtson LG, Chamberlain AM, Norby FL, et al. Prospective study of oral anticoagulants and risk of liver injury in patients with atrial fibrillation. Heart. 2017;103(11):834–839. doi: 10.1136/heartjnl-2016-310586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liakoni E, Rätz Bravo AE, Krähenbühl S. Hepatotoxicity of new oral anticoagulants (NOACs) Drug Saf. 2015;38(8):711–720. doi: 10.1007/s40264-015-0317-5. [DOI] [PubMed] [Google Scholar]
- 53.Chan P-H, Hai J-J, Huang D, Ho M-H, Chan EW, Cheung BM-Y, et al. Burden of upper gastrointestinal symptoms in patients prescribed dabigatran for stroke prevention. SAGE Open Med. 2016;4:205031211666241. doi: 10.1177/2050312116662414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang N, Liu XS, Li G, Liu T. Dabigatran-induced esophagitis: a frequently overlooked adverse effect. Int J Cardiol. 2016;212:358–359. doi: 10.1016/j.ijcard.2016.03.178. [DOI] [PubMed] [Google Scholar]
- 55.Cox R, Roche E, Fairley S. Novel oral anticoagulant drugs and severe oesophagitis dissecans. Intern Med J. 2016;46(12):1456–1457. doi: 10.1111/imj.13275. [DOI] [PubMed] [Google Scholar]
- 56.Duggan ST. Rivaroxaban: a review of its use for the prophylaxis of venous thromboembolism after total hip or knee replacement surgery. Am J Cardiovasc Drugs. 2012;12(1):57–72. doi: 10.2165/11208470-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 57.Beyer-Westendorf J, Forster K, Ebertz F, Gelbricht V, Schreier T, Gobelt M, et al. Drug persistence with rivaroxaban therapy in atrial fibrillation patients—results from the Dresden non-interventional oral anticoagulation registry. Europace. 2015;17(4):530–538. doi: 10.1093/europace/euu319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schulman S, Shortt B, Robinson M, Eikelboom JW. Adherence to anticoagulant treatment with dabigatran in a real-world setting. J Thromb Haemost. 2013;11(7):1295–1299. doi: 10.1111/jth.12241. [DOI] [PubMed] [Google Scholar]
- 59.Al-Khalili F, Lindström C, Benson L. The safety and persistence of non-vitamin-K-antagonist oral anticoagulants in atrial fibrillation patients treated in a well structured atrial fibrillation clinic. Curr Med Res Opin. 2016;32(4):779–785. doi: 10.1185/03007995.2016.1142432. [DOI] [PubMed] [Google Scholar]
- 60.Hellfritzsch M, Rasmussen L, Hallas J, Pottegård A. Using the symmetry analysis design to screen for adverse effects of non-vitamin K antagonist oral anticoagulants. Drug Saf. 2018 doi: 10.1007/s40264-018-0650-6. [DOI] [PubMed] [Google Scholar]
- 61.Steffen M. Universalism, responsiveness, sustainability: regulating the french health care system. N Engl J Med. 2016;374(5):401–405. doi: 10.1056/NEJMp1504547. [DOI] [PubMed] [Google Scholar]
- 62.Giroud M, Hommel M, Benzenine E, Fauconnier J, Béjot Y, Quantin C, et al. Positive predictive value of french hospitalization discharge codes for stroke and transient ischemic attack. Eur Neurol. 2015;74(1–2):92–99. doi: 10.1159/000438859. [DOI] [PubMed] [Google Scholar]
- 63.Sahli L, Lapeyre-Mestre M, Derumeaux H, Moulis G. Positive predictive values of selected hospital discharge diagnoses to identify infections responsible for hospitalization in the French national hospital database: PPV of hospitalization for infection codes in France. Pharmacoepidemiol Drug Saf. 2016;25(7):785–789. doi: 10.1002/pds.4006. [DOI] [PubMed] [Google Scholar]
- 64.Maura G, Pariente A, Alla F, Billionnet C. Adherence with direct oral anticoagulants in nonvalvular atrial fibrillation new users and associated factors: a French nationwide cohort study. Pharmacoepidemiol Drug Saf. 2017;26(11):1367–1377. doi: 10.1002/pds.4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.